Abstract
Previous evidence suggests soy genistein may be protective against prostate cancer, but whether this protection involves an estrogen receptor (ER)-dependent mechanism is unknown. To test the hypothesis that phytoestrogens may act through ERα or ERβ to play a protective role against prostate cancer, we bred transgenic mice lacking functional ERα or ERβ with transgenic adenocarcinoma of mouse prostate (TRAMP) mice. Dietary genistein reduced the incidence of cancer in ER wild-type (WT)/transgenic adenocarcinoma of mouse prostate mice but not in ERα knockout (KO) or ERβKO mice. Cancer incidence was 70% in ERWT mice fed the control diet compared with 47% in ERWT mice fed low-dose genistein (300 mg/kg) and 32% on the high-dose genistein (750 mg/kg). Surprisingly, genistein only affected the well differentiated carcinoma (WDC) incidence but had no effect on poorly differentiated carcinoma (PDC). No dietary effects have been observed in either of the ERKO animals. We observed a very strong genotypic influence on PDC incidence, a protective effect in ERαKO (only 5% developed PDC), compared with 19% in the ERWT, and an increase in the incidence of PDC in ERβKO mice to 41%. Interestingly, immunohistochemical analysis showed ERα expression changing from nonnuclear in WDC to nuclear in PDC, with little change in ERβ location or expression. In conclusion, genistein is able to inhibit WDC in the presence of both ERs, but the effect of estrogen signaling on PDC is dominant over any dietary treatment, suggesting that improved differential targeting of ERα vs. ERβ would result in prevention of advanced prostate cancer.
Estrogen therapy, specifically diethylstilbesterol treatment, has been used in prostate cancer treatment as early as 1941 (1–5). Initially, this was believed to be an indirect inhibition, involving negative feedback on the hypothalamic-pituitary-gonadal axis to shut down androgen synthesis and thereby the growth of prostate tissue. However, the direct effect of estrogens and estrogen receptors (ER) on the prostate has been recognized both in organ development (6) and disease (7).
The potential for phytoestrogens to act as natural selective estrogen receptor modulators and affect prostate disease has been considered. Dietary consumption of soy products has long been associated with reduced incidence of various diseases. Considerable epidemiological evidence supports the observation that soy foods promote health and reduce chronic ailments, including cardiovascular disease and osteoporosis (8, 9). Soy genistein has been implicated as being cancer preventative through epidemiological studies (10, 11). The cancer incidence for breast (12) and prostate (13) cancer is significantly lower in cultures with high soy consumption (14) and correspondingly high serum genistein concentrations. We and others have previously reported an inhibitory growth effect of genistein on prostate cancer cells, LNCaP and PC3 (15, 16), as well as in the transgenic adenocarcinoma of mouse prostate (TRAMP)-C2 cell line (17). Genistein has a multitude of documented mechanisms of action (8, 17, 18). It has been shown to affect protein tyrosine kinase, hedgehog signaling, and estrogen signaling. Genistein binds to both ERs in the low nanomolar range, with a 7-fold greater specificity to ERβ (19).
The ERs are expressed in the prostate epithelium and stroma (20, 21), and neonatal estrogen exposure has profound effects on prostate development both in the rodent (22–26) and man (27). There is evidence that not only can estrogen signaling affect prostate cancer but that ERα and ERβ may have opposing effects in this regard. Work with receptor knockout (KO) mice showed that diethylstilbesterol treatment led to squamous metaplasia of prostatic epithelium in wild-type (WT) and ERβKO mice but not in ERαKO mice (28).
In this article, we investigate to what degree the cancer protective effects of genistein could be attributed to estrogenic activity. By using either ERαKO/TRAMP or ERβKO/TRAMP mice compared with ERWT/TRAMP mice, we demonstrate that both receptors are required for genistein to have an effect in preventing prostate carcinogenesis and that ERα promotes and ERβ prevents aggressive prostate cancer.
Materials and Methods
TRAMP mouse studies
The TRAMP model was developed by placing the simian virus 40 large and small T-antigen genes under the control of the androgen-regulated rat probasin promoter (PBTag transgene), which has been shown to be highly and specifically expressed in the mouse prostate epithelium (29, 30). The expression of simian virus 40 T-antigens disrupts function of p53 and Rb proteins, tumor suppressors important in prostate cancer pathology (31, 32), leading to spontaneous development of prostate tumors by 3 months of age. The biology of the TRAMP model makes it a useful tool to study the progression of the disease, as well as prevention and treatment options.
Male TRAMP mice on a C57BL6/J background were raised in-house as described earlier (17). All University of Missouri institutional guidelines for animal care and use were followed.
To generate the ERα/βKO-TRAMP mice, female C57Bl/6J mice, heterozygous for the ERα or ERβ gene and positive for the PBTag transgene (TRAMP), were crossed with male C57Bl/6J mice that were heterozygous for either the ERα or ERβ gene and negative for the PBTag transgene. All breeder pairs were maintained on a casein-based diet as previously described (33). ERWT, ERαKO, and ERβKO offspring of this breeding scheme that were positive for the PBTag transgene were used in this study.
The mice were fed the casein diet until weaning and then randomly assigned to three groups fed the casein diet or the casein diet, to which 300 or 750 mg of genistein/kg (LC Laboratories, Woburn, MA) had been added. The mice were maintained on these diets from 5 to 6 wk until 5 months of age (18–22 mice per treatment group). The concentration of genistein was selected after analyzing serum from mice consuming a range of diets from 0 to 500 mg genistein/kg diet in 100 mg increments (data not shown). The goal was to provide a concentration of dietary genistein that resulted in serum concentrations lower than 1 μm range. These concentrations are below the threshold for inhibition of tyrosine kinase (34) but are sufficient to saturate both ERs. We also used another estrogenic phytoestrogen in soy, daidzein, which lacks tyrosine kinase inhibitory activity.
At 5 months of age, mice were euthanized and tissues collected. The reproductive tract (testes, vas deferens, urinary bladder, seminal vesicles, and prostate lobes) was removed and weighed. A portion of the dorsal lobe and the ventral prostate were fixed in neutral buffered formalin and paraffin embedded for histological analysis and the remainder snap frozen in liquid nitrogen and stored at −80 C for future studies. Tissues sections were stained with hematoxylin and eosin and examined by light microscopy for assessment of cancer stages (35). All lobes of the prostates were scanned by trained veterinary pathologists, who were unaware of the treatment groups, and the dorsal prostate was staged as either 1) normal, 2) hyperplasia (HYP), 3) prostatic intraepithelial neoplasia (PIN), 4) well differentiated carcinoma (WDC), 5) moderately differentiated carcinoma (MDC), or 6) poorly differentiated carcinoma (PDC), “neuro-endocrine-like carcinoma,” although identification of PDC in any lobe of the prostate prompted a stage of PDC to be assigned to the animal regardless of the status of the dorsal prostate. This protocol follows a procedure described previously (36, 37).
Serum measurements
Serum concentrations of total genistein were analyzed by HPLC-multiple ion-monitoring mass spectrometry (34). Serum testosterone was separated by HPLC before measurement by RIA. Serum estradiol was similarly measured in samples from ERWT and ERαKO female mice fed control or 1 g genistein per kg casein diet for 34 wk.
Immunohistochemistry (IHC)
Slides were prepared using heat-mediated antigen retrieval by immersing and heating in 0.1 m citrate buffer (pH 6.0). IHC reactions were performed using the DakoCytomation Autostainer (Dako, Carpinteria, CA). Primary antibodies were used in the following dilutions: androgen receptor (AR) RG-21 1:50 (Upstate, Billerica, MA), ERα MC-20 1:300 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and ERβ PPG5/10 1:50 (Serotec, Raleigh, NC). Sixty-six slides (which included 39 ERWT mice, 11 ERαKO mice, and 16 ERβKO mice) were evaluated for AR staining, 49 slides (which included 32 ERWT mice and 17 ERβKO mice) were evaluated for ERα staining, and 45 slides (which included 34 ERWT mice and 11 ERβKO mice) were evaluated for ERα staining. We also looked at 12 B6FVBF1 non-TRAMP mice as a control for ER expression in normal prostate epithelium.
Dorsal prostate was examined, and the percent of immunopositive cells was estimated for 1) normal, 2) HYP, 3) PIN, 4) WDC, 5) MDC, and 6) PDC, using the following scale: 1 = 0% staining, 2 = 1–25% staining, 3 = 25–50% staining, 4 = 50–75% staining, 5 = 75–99% staining, and 6 = 100% staining.
Histological images were captured using DP2-BSW software with a DP72 camera (Olympus Corp., Center Valley, PA) attached to an Eclipse E6000 (Nikon, Tokyo, Japan) microscope.
Statistical analysis
The stages of tumor incidence were classified into noncancer stages (normal, HYP, and PIN) and cancer stages (WDC, MDC, and PDC or neuro-endocrine-like carcinoma). Tumor incidence data were analyzed as a 2 × 2 factorial with genotype (ERαWT or ERαKO) and diet (casein or genistein) as main effects using Fisher's exact test. Values that achieved two-tailed P < 0.05 were considered to be statistically significantly different. Comparison within the same tumor stage among different dietary groups and genotypes has also been done. Body weight, reproductive tract weight, testicular weight, prostate weight, and IHC scores were analyzed using a two-sample t test assuming unequal variance or a one-way ANOVA with Tukey post hoc test. GraphPad Prism4 software was used to perform the analyses (GraphPad, La Jolla, CA).
Results
Genistein's effect on cancer incidence in TRAMP
Genistein significantly reduced overall cancer incidence in ERWT animals compared with control diet in a dose-dependent manner (Table 1 and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Specifically, PIN incidence increased with genistein treatment, and WDC incidence was dramatically decreased from 50% in the control to 19% with low-dose (300 mg/kg) and 8% with high-dose (750 mg/kg) genistein (Table 1). Although there was a slight increase in PDC incidence in the genistein treatment groups, the change was not statistically significant, nor was an effect of genistein seen on tumor stage in the ERαKO or ERβKO animals.
Table 1.
Effect of diet and genotype on the incidence of prostate cancer in 5-month-old TRAMP mice
| Genotype | Diet | n | Tumor stage |
|||||
|---|---|---|---|---|---|---|---|---|
| Noncancer |
Cancer |
|||||||
| Normal | HYP | PIN | WDC | MDC | PDC | |||
| ERWT | Casein | 175 | 2 (1%) | 10 (6%)a | 41 (23%)c | 88 (50%)g | 0 | 34 (20%)k |
| ERWT | Genistein 300 mg/kg | 81 | 1 (1%) | 13 (16%)b | 29 (36%)d | 15 (19%)h | 1 (1%) | 22 (27%)k,l |
| ERWT | Genistein 750 mg/kg | 25 | 0 | 0a | 17 (68%)e | 2 (8%)i | 0 | 6 (24%)k,l |
| ERαKO | Casein | 80 | 0 | 3 (4%)a | 4 (5%)f | 68 (85%)j | 1 (1%) | 4 (5%)m |
| ERαKO | Genistein 300 mg/kg | 25 | 0 | 0a | 1 (4%)f | 23 (92%)j | 1 (4%) | 0m |
| ERβKO | Casein | 51 | 0 | 0a | 13 (26%)c,d | 18 (35%)g | 0 | 20 (39%)l |
| ERβKO | Genistein 300 mg/kg | 23 | 0 | 0a | 5 (22%)c,d | 8 (35%)g,h | 0 | 10 (43%)l |
Incidence of tumor stage were analyzed using Fisher's exact test two-tailed P value. Both doses of genistein had an extremely significant (P < 0.0001) effect on WDC incidence within the ERWT mice but not in ERKO mice. There was also an extremely significant genotypic effect on WDC between ERWT and ERαKO but not quite significant (P = 0.0572) compared with ERβKO mice. There was a very significant (P < 0.005) genotype effect in the observed PDC incidence, between both, ERαKO and ERβKO, compared with ERWT. However, there was no significant dietary effect on PDC incidence. Tumor incidences within columns are being compared with each other; different superscript letters indicate a statistically significant (P < 0.05) difference between groups.
Daidzein is often used as a control for genistein due both to lack of tyrosine kinase inhibition and lower estrogenic activity (38). In separate animals, we also tested daidzein, in ERWT and ERαKO mice, and found a nonsignificant decrease in overall cancer incidence (Supplemental Table 2).
ER status and cancer incidence
Approximately 70% of TRAMP mice WT for both ER developed cancer by 5 months of age (Supplemental Table 1), with WDC incidence of 50% and PDC incidence of 19% (Table 1). ERαKO TRAMP mice had higher WDC incidence at 85% but reduced PDC at 5%. ERβKO TRAMP mice had moderate levels of PDC incidence at 39%, indicating a possible protective role for ERβ in tumorigenesis, or a tumorigenic role of ERα, or a combination of both (Table 1).
ERα, ERβ and AR profile changes with cancer progression
To have a better understanding of the involvement of ER in cancer progression, we performed IHC analyses of hormone receptor expression in the prostate from TRAMP mice. IHC analysis for ERα, ERβ, and AR was performed on a total of 66 representative mice from the above mentioned studies.
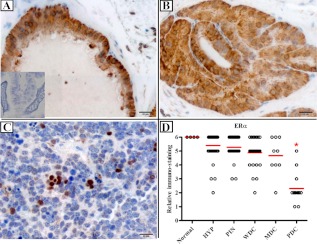
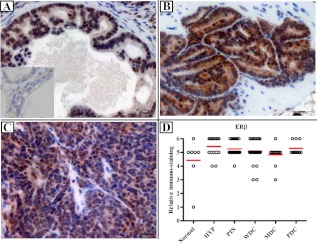
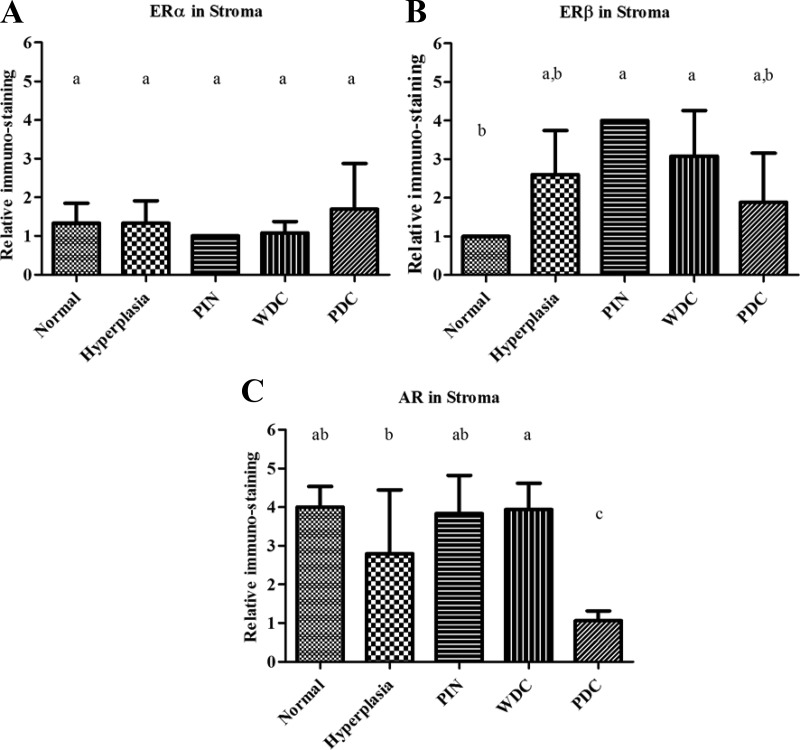
The ERα IHC score in PDC epithelium was significantly reduced compared with other stages of prostate adenocarcinoma (Fig. 1). Just as striking was the change in distribution of ERα, from a nonnuclear location in the more benign cells to an essentially exclusive nuclear presence in PDC lesions. No significant difference in the percent of ERβ positive epithelial cells was observed, although there was a predilection for nuclear staining in normal/HYP/PIN/WDC/MDC lesions and predominately cytoplasmic staining in PDC (Fig. 2). In addition, although there was no change in low stromal ERα expression across different stages of prostate cancer, the expression of stromal ERβ was significantly lower in normal prostate (see figure 4 below). Neither diet nor genotype affected stromal expression of ERα or ERβ (Supplemental Fig. 1).
Fig. 1.
ERα immunostaining in TRAMP mice. Mice were euthanized at 5 months, and prostates were analyzed for ERα immunostaining. Normal prostates from non-TRAMP mice were stained as control. A, PIN lesion. B, WDC lesion. C, PDC lesion. D, Plot of IHC scores. n = 45, from studies 1 and 5–7 (see Supplemental Table 2). ERα expression decreases with cancer progression. In PDC tumors, even though quantitatively lower, the expression shifts from mainly nonnuclear to nuclear. Scale bar, 20 μm. Inset in A shows negative control with specific blocking peptide. *, P < 0.05.
Fig. 2.
ERβ immunostaining in TRAMP mice. Mice were euthanized at 5 months, and prostates were analyzed for ERβ immunostaining. Normal prostates from non-TRAMP mice were stained as control. A, PIN lesion. B, WDC lesion. C, PDC lesion. D, Plot of IHC scores. n = 49, from studies 1, 2, 6, and 7 (see Supplemental Table 2). ERβ expression did not significantly change with tumor progression. Scale bar, 20 μm. Inset in A shows negative control with specific blocking peptide.
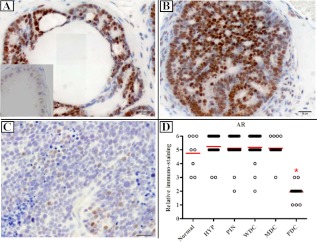
AR expression in the epithelium was stable across HYP, PIN, WDC, and MDC and comparable with normal prostates from non-TRAMP mice. A significant decrease in AR was observed in PDC samples (Fig. 3), which correlates with the loss of responsiveness to androgen. In addition, AR decreased in the stroma of PDC compared with other stages (Fig. 4C).
Fig. 3.
AR immunostaining in TRAMP mice. Mice were euthanized at 5 months, and prostates were analyzed for AR immunostaining. Normal prostates from non-TRAMP mice were stained as control. A, PIN lesion. B, WDC lesion. C, PDC lesion. D, Plot of IHC scores. n = 49, from studies 1 and 5–7 (see Supplemental Table 2). There is a very significant reduction of AR expression in PDC tumors. Scale bar, 20 μm. Inset in A shows negative control without antibody. *, P < 0.05.
Fig. 4.
Stromal immunostaining in TRAMP mice. Mice were euthanized at 5 months, and prostate stroma was analyzed for AR (A), ERα (B), and ERβ (C) immunostaining. Relative immunostaining was noted for prostates with a weighted lesion stage. Normal prostates from non-TRAMP mice were stained as control. Error bars show sd. Statistical significance (P < 0.05) between different stages is noted with differing letters.
No significant difference in AR expression due to genotype or ER expression was found among the diet groups (Supplemental Figs. 1 and 2).
Weight differences
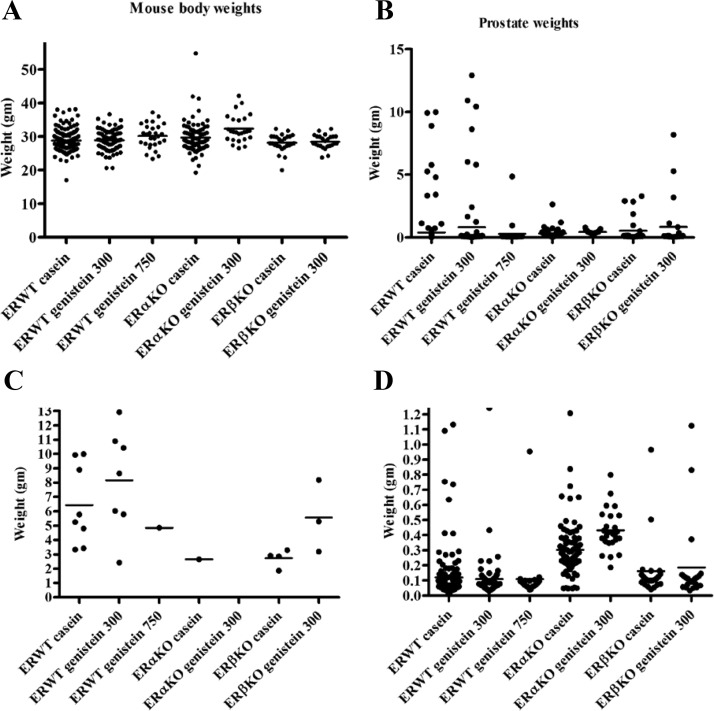
WT and ERαKO mice fed the high genistein diet had increased total body weight compared with those fed casein (Fig. 5).
Fig. 5.
Mouse body and prostate weights from all studies combined. A, ERWT mice fed the high genistein diet and ERαKO mice fed the low genistein diet had significantly increased total body weight compared with those fed the casein diet. When combined as in B, there are no significant differences in prostate weights. When we applied a cut-off of 1.5 g for very large prostate, which were exclusively PDC, we obtained a more differentiated pattern for prostate weight above 1.5 g (C) and under 1.5 g (D).
ERαKO mice had significantly lower testes weights, which corresponds with previous reports (39), but no difference between ERWT and ERβKO mice (Supplemental Fig. 3).
Total prostate weights did not vary between groups due to significant variance in tumor size, specifically within the PDC tumors (Fig. 5B and Supplemental Table 3). To clarify the data, we used a cut-off weight of 1.5 g to define two groups. The separation by weight was used to determine whether there were differences between groups within the same cancer stage. Prostates over 1.5 g, which were exclusively PDC, were most highly represented in the ERWT casein group (8/175) and in the ERWT low genistein group (7/81). The largest prostates were found in the ERWT low genistein group, followed by ERWT casein, and ERWT high genistein (1/25) and ERβKO genistein (3/23). It is interesting to note that even though ERβKO mice had a significantly higher PDC incidence relative to WT mice, their prostate weights were not higher. ERαKO mice had only one prostate above 1.5 g corresponding to the near absence of PDC in ERαKO (n = 105) (Fig. 5C). Within prostates under 1.5 g, ERαKO mice had significant higher weights compared with the other genotypes (Fig. 5D), corresponding to the increased WDC incidence in ERαKO mice (Table 1).
Serum hormone concentrations
Serum testosterone concentrations were measured, and no significant differences in serum testosterone were found due to genotype (in αKO casein vs. αKO genistein, 13,540 ± 11,090 vs. 9570 ± 3120 pg/ml) or diet (WT casein vs. WT genistein, 7670 ± 3440 vs. 16,440 ± 8350 pg/ml). Serum was also analyzed for total genistein concentration in a subset of mice (n = 11 for each treatment group). Serum concentrations for ERαWT and ERαKO mice were not significantly different (0.44 ± 0.11 and 0.64 ± 0.15 μm, respectively). Genistein in the diet was associated with a decrease in serum estradiol, although the effect was not statistically significant due to small number of animals tested (Supplemental Fig. 4).
Concentrations of genistein in the serum of mice not fed genistein were not detected.
Discussion
We present here the cumulative results from seven studies with a total of 460 ERWT, ERα, and ERβKO TRAMP mice (Supplemental Table 2). This work found opposing roles for the two ERs in carcinogenesis (Table 1). ERαKO animals had almost no PDC incidence (5%), whereas ERβKO mice had approximately double the PDC incidence (39%) compared with WT mice (19%). This is in agreement with previous suggestions that ERα increases and ERβ decreases prostate cancer risk (28). Recently, a polymorphism in ERα has been correlated with increased prostate cancer incidence in a north Indian population (40), supporting the idea of ERα being the “bad” ER.
It is likely that the genotypic effect of ERKO that we observed is dominant over any dietary treatment. In our many dietary studies in TRAMP mice, we found no dietary effect on PDC in ERαKO animals compared with animals WT for both receptors. This work supports the conclusion that genistein is protective against prostate tumorigenesis (Table 1), although contrary to a previous report, it did not affect PDC incidence (41). Although the percent of mice with PDC was slightly greater in the genistein group, the change is not statistically significant. Furthermore, if we look at the individual studies (Supplemental Table 2), we can see that there are several instances where the incidence of PDC is lower in the genistein treatment groups. The higher incidence of PIN in mice fed genistein may indicate that genistein may delay progression of early prostate cancer. This may imply that genistein would be more appropriate as a preventative or perhaps as a therapy in early prostate cancer.
To exclude the developmental effect of mice lacking ERα or ERβ, studies are planned to repeat this work with conditional KO animals whose ER are turned off at puberty. An alternative approach would be to perform the study with selective ERα and ERβ ligands to recreate the KO results. Examples of such compounds include the such compounds the ERβ-specific agonist 2,3-bis (4-hydroxyphenyl) propionitrile (DPN), to mimic an ERαKO, and the ERα-specific agonist 4,4′,4″-(propyl-[(1)H]-pyrazole-1,3,5-triyl) trisphenol (PPT), to mimic an ERβKO, or the methoxychlor metabolite 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE), which is an ERα agonist, ERβ antagonist, and AR antagonist (42), which would silence any confounding effects through AR. The difficulty with using these compounds is their current high cost and that their selectivity for the specific receptor is between 70- and 300-fold for 2,3-bis (4-hydroxyphenyl) propionitrile (DPN) (43, 44), 400-fold for 4,4′,4″-(propyl-[(1)H]-pyrazole-1,3,5-triyl) trisphenol (PPT) (45), and 20-fold for 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) (46), which will require careful titrating and monitoring of the uptake of the ligands in the prostate.
A surprising finding of the current study was that genistein does not act solely through ERβ. Because genistein had no effect on total cancer incidence in either ERαKO or ERβKO animals, it may be that genistein requires both ERs to exert its protective action. Alternatively, genistein could be acting via a non-ER pathway. This effect is likely not via genistein's action on tyrosine kinases, because the genistein serum concentrations achieved in the mice were 10-fold lower than the reported Ki (inhibitory binding affinity) of genistein for tyrosine kinases (34). The phytoestrogen, daidzein, had no significant effect on cancer incidence. Daidzein has a 10-fold lower binding affinity to mouse uterine cytosol than does genistein (47) and, in our study, was able to decrease WDC incidence, although to a smaller degree than did genistein. This implies that the conformation of ER when bound to daidzein is different than when bound to genistein, resulting perhaps in altered interactions with necessary coregulatory proteins. Regardless, genistein's effects are nullified or overshadowed by the stronger effects of both ERs.
We observed that a diet containing a high dose of genistein significantly increased total body weights in the WT and ERαKO mice (Fig. 5 and Supplemental Table 3). This is consistent with previous reports that genistein contributes to increased lean body mass in neutered cats (48) and food intake and total body mass in quail (49).
Comparing prostate weights by tumor stage among the groups, we observed an interesting phenomenon. Besides having an increased WDC incidence (Table 1), which accounts for the overall increase in cancer in the ERαKO group, when looking at prostates under 1.5 g, the ERαKO mice had significantly higher prostate weights compared with both ERWT and ERβKO mice (Fig. 5D). Interestingly, although ERαKO mice had a more rapidly growing cancer, they did not acquire the PDC stage within the time frame of the study. Additionally, aged ERαKO mice did not develop PDC even after 8 months (data not shown).
ERβKO/TRAMP mice had fewer tumors over 1.5 g than ERWT, even though they had twice the PDC incidence (Fig. 5). It would be interesting to determine whether the PDC tumors differed in other aspects, such as expression of neuroendocrine or proliferation markers, or had a different metastatic potential. We plan to examine this question using allograft of PDC tumors from different ER genotypes implanted in young ERWT TRAMP mice. Differing biological conditions in the WDC and PDC tumors might be possible if they arose from different types of cells within the prostate epithelial cell population. This idea has been proposed by others (50) and is supported by our observations. The difference in cell origin for WDC and PDC may be part of the reason why there was an increased incidence of WDC in the ERαKO groups, opposite of what was seen with PDC.
In addition, the PIN and WDC are likely more androgen responsive than is PDC, given the difference in AR expression (Fig. 3D). The ERαKO mice had slightly higher serum testosterone levels than the WT mice, and an increased androgen signaling in this group may have favored WDC growth, although the differences in testosterone concentrations were not statistically significant. Mentor-Marcel et al. (41) reported in WT/TRAMP mice a dose-dependent effect of genistein treatment at stage 6 (PDC) but not at any lower stages. An explanation for why this differed from our study may have been the different genetic background of the TRAMP mice and duration (7-month vs. our 5-month end date) of genistein administration.
The IHC analysis of the tumors is in agreement with the hypothesis of the importance of ERα and ERβ in cancer progression. The noncancerous, hyperplastic prostate expresses both ERs, with ERα being predominately nonnuclear. It also expresses AR, corresponding with the initial responsiveness to androgens in the prostate. In PDC tumors, however, the expression pattern changes to low but mostly nuclear expression of ERα. The role of ERα in the developing prostate and its association with metaplasia of prostate epithelium may fit with its shift in localization from nonnuclear in the benign adult prostate epithelium to a nuclear location (51). This may indicate a change in function of ERα in PDC, which could be consistent with a dedifferentiated cell line having ER characteristics similar to embryonic prostate tissue (7).
Nonnuclear signaling of ER has been reported and is recognized as a means of rapid estrogenic response in tissues (52–54). The ER has been identified tethered to lipid rafts at the cell membrane, where it can interact with components of other signaling pathways and affect, and potentially be affected by, these other pathways (55). In addition to being localized in the nucleus or cell membrane, ERs have also been found in the mitochondria (56), where they may affect energy metabolism or apoptosis. One group saw ERα in the cytoplasm and the nucleus of normal human prostate stem/progenitor cells (57). This group also noticed that these stem/progenitor cells have a nuclear receptor expression profile more similar to androgen-independent cell lines, such as PC3, than the androgen-responsive cell line, LNCaP.
The hypothesis of protective ERβ and tumorigenic ERα (58, 59) is supported by our data. The associations of PDC incidence in the ERβKO and ERαKO mice may be due to an alteration of estrogen signaling from membrane or cytoplasmic ERα (and conversely, nuclear ERβ) in PIN and WDC to nuclear (and cytoplasmic) in PDC, which may result not only in changes in the expression of expression of estrogen response element-containing genes, but also in G protein and other membrane and cytoplasmic cellular signals that may affect cell growth, hormone response, and cell adhesion (60, 61). We thus speculate that the nonnuclear localized receptors in TRAMP are not necessarily inactive; they may in fact be interacting with other pathways.
We did not observe any changes in ERα, ERβ, or AR expression associated with genistein diets, nor with genotype (Supplemental Fig. 2). We did, however, see a significant decrease in AR at the PDC (Fig. 3D). This decrease in AR expression, along with a loss of androgen responsiveness, has been seen by others in the TRAMP mice (62, 63). Considering that both AR and ERα change so dramatically in PDC, we attribute the effects of genistein on PDC to ERα rather than AR. The affinity constant of genistein for AR is 400 μm (64), and the average serum genistein concentration that we measured was four orders of magnitude below this, which is still well above genistein's binding affinity for ERα (64). Therefore, it is unlikely that genistein is acting directly through AR in these studies. In a study of Japanese men, testosterone concentrations were inversely correlated with soy intake (65), possibly indicating indirect action via negative feedback on the hypothylamic-pituitary axis, or genistein inhibition of aromatase activity (66), but we did not see genistein affecting serum testosterone in our mice. Serum estradiol tended to be higher in the ERαKO compared with the ERWT mice and was slightly decreased in both groups by feeding genistein (Supplemental Fig. 4). However, due to small amounts of serum available, estradiol concentrations were measured in only a few animals. As reported previously, elevated estrogen concentrations in the ERαKO mice are presumed to occur due to the absence of negative feedback from the ERs (67). It is possible that a genistein-bound ERα or ERβ may affect androgen signaling. Interestingly, genistein decreased AR protein concentration and PSA expression in LNCaP cells (which naturally lack ERα) (68). This effect was relieved by cotreatment with ICI, an ER antagonist. And because LNCaP cells lack ERα, it is possible that genistein's effect on AR in the aforementioned example was via ERβ. There have been other accounts of genistein modulating AR expression. Wang et al. (69) reported genistein causing a decrease in ER and increase in AR expression. Another lab had earlier shown genistein treatment of LNCaP cells resulted in decreased AR mRNA with a concurrent increase in AR protein (70). And yet others have seen genistein have no affect on AR concentration (71).
The importance of stroma in hormonal responsiveness of prostate epithelium has been shown by several groups (71–73). In one striking example, proliferation of prostate epithelium in response to varying dihydrotestosterone to estradiol ratios was seen when the cells were cocultured with prostate stromal cells, but this growth response was lost when the epithelial cells were grown without stromal cells (71). We saw low expression of ERα in the stroma, which did not change between tumor stages. However, we did see lower ERβ in normal prostate stroma and a decrease of AR expression in PDC stroma, when comparing different stages with each other. Others have noted a decrease in stromal AR in association with advanced prostate cancer, although it is not known whether a low AR stroma is a more favorable environment for PDC tumors or if the PDC is silencing the expression of AR in the surrounding stroma. Microarray analysis of normal and reactive prostate stroma showed differential expression of many genes (73), including Gli2, a key mediator of hedgehog signaling, which has been implicated in advanced prostate cancer (74–77). Potentially, these changes in ERβ and AR expression in the stroma of the TRAMP prostate are important in the development of prostate cancer.
As mentioned above, the fact that genistein treatment was associated with a decrease in cancer incidence in the WT mice, but not in either of the KO groups, suggests the possibility that genistein requires both ER present to enact its protective action. This is supported by observations that knocking down ERα and/or ERβ abrogated the effects of genistein in vitro (78–80). The IHC results are consistent with the idea that a heterodimer may be important in the role that ER plays in prostate cancer. If a heterodimer is protective, then the localization of ERα to the nucleus would leave ERβ to form a homodimer in other areas of the cell. Levin and co-workers (81) previously found the ER heterodimer to be present and active in the membrane of breast cancer and endothelial cells.
Another player in the prostate in addition to estradiol is the dihydrotestosterone metabolite, 5-androstene-3β, 17β-diol, which is able to bind to both ERs in vitro (19), with affinities of 6 nm for ERα and 2 nm for ERβ. However, this metabolite was reported to preferentially activate ERβ (82). We speculate that the protective effects seen in ERαKO mice against PDC are mediated through 5-androstene-3β, 17β-diol via ERβ. In support of this idea, Dondi et al. (83) recently demonstrated that treatment with 5-androstene-3β, 17β-diol decreased proliferation of DU145 and PC3 cells in vitro and PC3 xenografts in vivo. Previously, the Poletti lab had shown that 5-androstene-3β, 17β-diol inhibited the migratory effects of DU145 (negative for ERα and positive for ERβ) and that this inhibitory effect could be counteracted with ICI or an ERβ-selective antagonist (84). In a mouse model, 5-androstene-3β, 17β-diol treatment resulted in decreased proliferation of prostate epithelium (82). However, the effect of the compound was not evident in ERβKO mice. This inhibitory effect on prostate growth by a natural ERβ ligand is one way in which removal of the “good” receptor in the ERβKO mice leads to a doubling of PDC incidence and is an additional reason to consider ERs as potential targets for human prostate cancer therapy.
In summary, we propose that ERβ has a protective role in prostate tumorigenesis, and/or ERα has a tumorigenic influence. This report offers a unique insight into the effects of ER signaling on prostate carcinogenesis. Many questions remain to be answered. However, the comprehensive data demonstrate clearly the dualistic involvement of ERα and ERβ in the aggressive PDC phenotype and suggests that targeting both ERα and ERβ would be useful in preventing and treating prostate cancer.
Supplementary Material
Acknowledgments
We thank Norman Greenberg for initially supplying TRAMP breeder mice and Leslie G. Newton for help in managing the care and treatment of the TRAMP mice in several of the above studies.
Present address for A.Ś.: Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, Missouri 65212.
Present address for J.K.D.: Director, Operations Quality and Compliance, Miraca Life Sciences, Irving, Texas 75039.
This work was made possible by Grant Number P50AT006273 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health. This work was also supported by Missouri Center for Phytonutrient and Phytochemical Studies, National Institutes of Health (NIH) Grant P01-ES510535, Department of Defense Grant DAMD 17-98-1-8529, and partially by NIH Training Grant T32-RR007004.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- ER
- estrogen receptor
- HYP
- hyperplasia
- IHC
- immunohistochemistry
- KO
- knockout
- MDC
- moderately differentiated carcinoma
- PDC
- poorly differentiated carcinoma
- PIN
- prostatic intraepithelial neoplasia
- TRAMP
- transgenic adenocarcinoma of mouse prostate
- WDC
- well differentiated carcinoma
- WT
- wild type.
References
- 1. Mukamel E, Nissenkorn I, Servadio C. 1980. Early combined hormonal and chemotherapy for metastatic carcinoma of prostate. Urology 16:257–260 [DOI] [PubMed] [Google Scholar]
- 2. Huggins C, Hodges CV. 2002. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 168:9–12 [DOI] [PubMed] [Google Scholar]
- 3. Karr JP, Wajsman Z, Kirdani RY, Murphy GP, Sandberg AA. 1980. Effects of diethylstilbestrol and estramustine phosphate on serum sex hormone binding globulin and testosterone levels in prostate cancer patients. J Urol 124:232–236 [DOI] [PubMed] [Google Scholar]
- 4. Klugo RC, Farah RN, Cerny JC. 1981. Bilateral orchiectomy for carcinoma of prostate. Response of serum testosterone and clinical response to subsequent estrogen therapy. Urology 17:49–50 [DOI] [PubMed] [Google Scholar]
- 5. Kemp HA, Read GF, Riad-Fahmy D, Pike AW, Gaskell SJ, Queen K, Harper ME, Griffiths K. 1981. Measurement of diethylstilbestrol in plasma from patients with cancer of the prostate. Cancer Res 41:4693–4697 [PubMed] [Google Scholar]
- 6. Jarred RA, Cancilla B, Prins GS, Thayer KA, Cunha GR, Risbridger GP. 2000. Evidence that estrogens directly alter androgen-regulated prostate development. Endocrinology 141:3471–3477 [DOI] [PubMed] [Google Scholar]
- 7. Prins GS, Korach KS. 2008. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 73:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Setchell KD, Cassidy A. 1999. Dietary isoflavones: biological effects and relevance to human health. J Nutr 129:758S–767S [DOI] [PubMed] [Google Scholar]
- 9. Knight DC, Eden JA. 1996. A review of the clinical effects of phytoestrogens. Obstet Gynecol 87:897–904 [PubMed] [Google Scholar]
- 10. Jian L. 2009. Soy, isoflavones, and prostate cancer. Mol Nutr Food Res 53:217–226 [DOI] [PubMed] [Google Scholar]
- 11. Steiner C, Arnould S, Scalbert A, Manach C. 2008. Isoflavones and the prevention of breast and prostate cancer: new perspectives opened by nutrigenomics. Br J Nutr 99(E Suppl 1):ES78–ES108 [DOI] [PubMed] [Google Scholar]
- 12. Iwasaki M, Inoue M, Otani T, Sasazuki S, Kurahashi N, Miura T, Yamamoto S, Tsugane S. 2008. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: a nested case-control study from the Japan public health center-based prospective study group. J Clin Oncol 26:1677–1683 [DOI] [PubMed] [Google Scholar]
- 13. Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. 2007. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev 16:538–545 [DOI] [PubMed] [Google Scholar]
- 14. Hamilton-Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. 2008. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer 60:7–13 [DOI] [PubMed] [Google Scholar]
- 15. Shenouda NS, Zhou C, Browning JD, Ansell PJ, Sakla MS, Lubahn DB, Macdonald RS. 2004. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer 49:200–208 [DOI] [PubMed] [Google Scholar]
- 16. Peterson G, Barnes S. 1993. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 22:335–345 [DOI] [PubMed] [Google Scholar]
- 17. Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. 2010. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res 70:3382–3390 [DOI] [PubMed] [Google Scholar]
- 18. Banerjee S, Li Y, Wang Z, Sarkar FH. 2008. Multi-targeted therapy of cancer by genistein. Cancer Lett 269:226–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- 20. Chang WY, Prins GS. 1999. Estrogen receptor-β: implications for the prostate gland. Prostate 40:115–124 [DOI] [PubMed] [Google Scholar]
- 21. Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, Birch L. 1998. Estrogen receptor-β messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology 139:874–883 [DOI] [PubMed] [Google Scholar]
- 22. Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. 2001. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Cancer Res 61:6089–6097 [PubMed] [Google Scholar]
- 23. Prins GS, Birch L. 1995. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology 136:1303–1314 [DOI] [PubMed] [Google Scholar]
- 24. Prins GS, Birch L. 1997. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology 138:1801–1809 [DOI] [PubMed] [Google Scholar]
- 25. Prins GS, Woodham C, Lepinske M, Birch L. 1993. Effects of neonatal estrogen exposure on prostatic secretory genes and their correlation with androgen receptor expression in the separate prostate lobes of the adult rat. Endocrinology 132:2387–2398 [DOI] [PubMed] [Google Scholar]
- 26. Prins GS. 1992. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology 130:2401–2412 [DOI] [PubMed] [Google Scholar]
- 27. Driscoll SG, Taylor SH. 1980. Effects of prenatal maternal estrogen on the male urogenital system. Obstet Gynecol 56:537–542 [PubMed] [Google Scholar]
- 28. Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G, Wong YZ. 2001. Evidence that epithelial and mesenchymal estrogen receptor-α mediates effects of estrogen on prostatic epithelium. Dev Biol 229:432–442 [DOI] [PubMed] [Google Scholar]
- 29. Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. 1995. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 92:3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, et al. 1994. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol 8:230–239 [DOI] [PubMed] [Google Scholar]
- 31. Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, Morrissey C, Zhang X, Comstock CE, Witkiewicz AK, Gomella L, Knudsen ES, Nelson PS, Knudsen KE. 2010. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J Clin Invest 120:4478–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navone NM, Troncoso P, Pisters LL, Goodrow TL, Palmer JL, Nichols WW, von Eschenbach AC, Conti CJ. 1993. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst 85:1657–1669 [DOI] [PubMed] [Google Scholar]
- 33. Guo JY, Li X, Browning JD, Jr, Rottinghaus GE, Lubahn DB, Constantinou A, Bennink M, MacDonald RS. 2004. Dietary soy isoflavones and estrone protect ovariectomized ERαKO and wild-type mice from carcinogen-induced colon cancer. J Nutr 134:179–182 [DOI] [PubMed] [Google Scholar]
- 34. Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262:5592–5595 [PubMed] [Google Scholar]
- 35. Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. 2004. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the mouse models of human cancer consortium prostate pathology committee. Cancer Res 64:2270–2305 [DOI] [PubMed] [Google Scholar]
- 36. Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. 2003. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 55:219–237 [DOI] [PubMed] [Google Scholar]
- 37. Shenouda NS, Sakla MS, Newton LG, Besch-Williford C, Greenberg NM, MacDonald RS, Lubahn DB. 2007. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine 31:72–81 [DOI] [PubMed] [Google Scholar]
- 38. Yu L, Blackburn GL, Zhou JR. 2003. Genistein and daidzein downregulate prostate androgen-regulated transcript-1 (PART-1) gene expression induced by dihydrotestosterone in human prostate LNCaP cancer cells. J Nutr 133:389–392 [DOI] [PubMed] [Google Scholar]
- 39. Couse JE, Mahato D, Eddy EM, Korach KS. 2001. Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod Fertil Dev 13:211–219 [DOI] [PubMed] [Google Scholar]
- 40. Gupta L, Thakur H, Sobti RC, Seth A, Singh SK. 2010. Role of genetic polymorphism of estrogen receptor-α gene and risk of prostate cancer in north Indian population. Mol Cell Biochem 335:255–261 [DOI] [PubMed] [Google Scholar]
- 41. Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. 2001. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res 61:6777–6782 [PubMed] [Google Scholar]
- 42. Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. 2000. Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: structure-activity studies. Mol Pharmacol 58:852–858 [PubMed] [Google Scholar]
- 43. Carroll VM, Jeyakumar M, Carlson KE, Katzenellenbogen JA. 2012. Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands. J Med Chem 55:528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. 2001. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]
- 45. Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. 2000. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43:4934–4947 [DOI] [PubMed] [Google Scholar]
- 46. Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. 1999. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors α and β. Endocrinology 140:5746–5753 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. 1999. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr 129:399–405 [DOI] [PubMed] [Google Scholar]
- 48. Cave NJ, Backus RC, Marks SL, Klasing KC. 2007. Oestradiol, but not genistein, inhibits the rise in food intake following gonadectomy in cats, but genistein is associated with an increase in lean body mass. J Anim Physiol Anim Nutr 91:400–410 [DOI] [PubMed] [Google Scholar]
- 49. Onderci M, Sahin K, Sahin N, Gursu MF, Doerge D, Sarkar FH, Kucuk O. 2004. The effect of genistein supplementation on performance and antioxidant status of Japanese quail under heat stress. Arch Anim Nutr 58:463–471 [DOI] [PubMed] [Google Scholar]
- 50. Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. 2008. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol 172:236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. 2010. Estrogen receptor β2 and β5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer 17:675–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kelly MJ, Wagner EJ. 1999. Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab 10:369–374 [DOI] [PubMed] [Google Scholar]
- 53. Aronica SM, Kraus WL, Katzenellenbogen BS. 1994. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA 91:8517–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Razandi M, Pedram A, Greene GL, Levin ER. 1999. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- 55. Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. 2002. ERβ has nongenomic action in caveolae. Mol Endocrinol 16:938–946 [DOI] [PubMed] [Google Scholar]
- 56. Pedram A, Razandi M, Wallace DC, Levin ER. 2006. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell 17:2125–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, Kajdacsy-Balla A, Prins GS. 2011. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology 152:2150–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ellem SJ, Risbridger GP. 2009. The dual, opposing roles of estrogen in the prostate. Ann NY Acad Sci 1155:174–186 [DOI] [PubMed] [Google Scholar]
- 59. McPherson SJ, Ellem SJ, Simpson ER, Patchev V, Fritzemeier KH, Risbridger GP. 2007. Essential role for estrogen receptor β in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology 148:566–574 [DOI] [PubMed] [Google Scholar]
- 60. Jia S, Zhang X, He DZ, Segal M, Berro A, Gerson T, Wang Z, Casale TB. 2011. Expression and function of a novel variant of estrogen receptor-ER-α36 in mouse airway. Am J Respir Cell Mol Biol 45:1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, Agyin JK, Brann D, Sun LZ, Yeh IT, Lee FY, Tekmal RR, Kumar R, Vadlamudi RK. 2010. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res 70:4092–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. 1997. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res 57:4687–4691 [PubMed] [Google Scholar]
- 63. Bono AV, Montironi R, Pannellini T, Sasso F, Mirone V, Musiani P, Iezzi M. 2008. Effects of castration on the development of prostate adenocarcinoma from its precursor HGPIN and on the occurrence of androgen-independent, poorly differentiated carcinoma in TRAMP mice. Prostate Cancer Prostatic Dis 11:377–383 [DOI] [PubMed] [Google Scholar]
- 64. Beck V, Unterrieder E, Krenn L, Kubelka W, Jungbauer A. 2003. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol 84:259–268 [DOI] [PubMed] [Google Scholar]
- 65. Nagata C, Inaba S, Kawakami N, Kakizoe T, Shimizu H. 2000. Inverse association of soy product intake with serum androgen and estrogen concentrations in Japanese men. Nutr Cancer 36:14–18 [DOI] [PubMed] [Google Scholar]
- 66. Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. 1998. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ Health Perspect 106:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. 1997. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 31:232–243 [DOI] [PubMed] [Google Scholar]
- 68. Bektic J, Berger AP, Pfeil K, Dobler G, Bartsch G, Klocker H. 2004. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor β. Eur Urol 45:245–251; discussion 251 [DOI] [PubMed] [Google Scholar]
- 69. Wang J, Eltoum IE, Lamartiniere CA. 2007. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maggiolini M, Vivacqua A, Carpino A, Bonofiglio D, Fasanella G, Salerno M, Picard D, Ando S. 2002. The mutant androgen receptor T877A mediates the proliferative but not the cytotoxic dose-dependent effects of genistein and quercetin on human LNCaP prostate cancer cells. Mol Pharmacol 62:1027–1035 [DOI] [PubMed] [Google Scholar]
- 71. Bhattacharyya RS, Krishnan AV, Swami S, Feldman D. 2006. Fulvestrant (ICI 182,780) down-regulates androgen receptor expression and diminishes androgenic responses in LNCaP human prostate cancer cells. Mol Cancer Ther 5:1539–1549 [DOI] [PubMed] [Google Scholar]
- 72. King KJ, Nicholson HD, Assinder SJ. 2006. Effect of increasing ratio of estrogen: androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate 66:105–114 [DOI] [PubMed] [Google Scholar]
- 73. Dakhova O, Ozen M, Creighton CJ, Li R, Ayala G, Rowley D, Ittmann M. 2009. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res 15:3979–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. 2004. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431:707–712 [DOI] [PubMed] [Google Scholar]
- 75. Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. 2004. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology 145:3961–3970 [DOI] [PubMed] [Google Scholar]
- 76. Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. 2004. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA 101:12561–12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. 2004. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Su Y, Simmen RC. 2009. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates β-catenin signaling in mammary epithelial cells. Carcinogenesis 30:331–339 [DOI] [PubMed] [Google Scholar]
- 79. Matsumura K, Tanaka T, Kawashima H, Nakatani T. 2008. Involvement of the estrogen receptor β in genistein-induced expression of p21(waf1/cip1) in PC-3 prostate cancer cells. Anticancer Res 28:709–714 [PubMed] [Google Scholar]
- 80. Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. 2008. Estrogen receptors α and β as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol 22:1032–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. 2004. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol 18:2854–2865 [DOI] [PubMed] [Google Scholar]
- 82. Weihua Z, Lathe R, Warner M, Gustafsson JA. 2002. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β,17β-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA 99:13589–13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dondi D, Piccolella M, Biserni A, Della Torre S, Ramachandran B, Locatelli A, Rusmini P, Sau D, Caruso D, Maggi A, Ciana P, Poletti A. 2010. Estrogen receptor β and the progression of prostate cancer: role of 5α-androstane-3β,17β-diol. Endocr Relat Cancer 17:731–742 [DOI] [PubMed] [Google Scholar]
- 84. Guerini V, Sau D, Scaccianoce E, Rusmini P, Ciana P, Maggi A, Martini PG, Katzenellenbogen BS, Martini L, Motta M, Poletti A. 2005. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor β subtype. Cancer Res 65:5445–5453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.