Summary
Skeletal muscle disturbances are commonly reported in patients with Fabry disease. Whether they derive from cardiac dysfunction or direct muscle involvement is still unclear. Clinical, noninvasive, and invasive cardiac and muscle studies, including an endomyocardial and muscle biopsy, were obtained in 12 patients (mean age, 42.1 ± 12.6 years; range, 24-58 years) with Fabry disease. In the youngest patients (group A, 4 men aged <35 years), results of cardiac and skeletal noninvasive studies were normal, except for reduced velocities in tissue Doppler imaging. Histologic examination indicated that muscle myocytes were unaffected, whereas muscle vessels showed the presence of mild glycosphingolipid accumulation in endothelial and smooth muscle cells. In the heart, cardiomyocytes and endothelial and smooth muscle cells of intramural cardiac vessels were involved by the disease. The oldest patients (group B, 6 men and 2 women aged >35 years) showed ultrasound muscle disarray and electromyography signs of myopathy, increased left ventricular mass, and normal cardiac function. Histologic examination showed that muscle myocytes contained mild glycosphingolipid accumulation compared with severe engulfment of cardiomyocytes. Moreover, similar infiltration of myocardial and muscle intramural vessels, causing lumen narrowing and fibrofatty tissue replacement, was observed. Direct muscle involvement occurs in patients with Fabry disease. It is milder and delayed compared with that in the heart. The difference in organ function and the need of residual α-galactosidase A activity are the likely causes.
Keywords: Cardiomyopathy, Fabry disease, Muscles, Pathology
1. Introduction
Fabry disease (FD) is an X-linked disorder caused by the deficiency of lysosomal enzyme α-galactosidase A, resulting in progressive intracellular accumulation of glycosphingolipids in different tissues including the skin, kidneys, vascular endothelium, ganglion cells of the peripheral nervous system, and the heart [1]. Cardiac involvement is characterized by progressive left ventricular (LV) hypertrophy that mimics the morphologic and clinical features of hypertrophic cardiomyopathy [2-4], with diastolic LV dysfunction and preserved LV ejection fraction until the end stage of the disease [5].
Patients with FD often complain of muscular pain, fatigability, and asthenia, which may involve deambulation and motor performance of the superior limbs. Heart failure and diastolic dysfunction can be responsible for exercise intolerance and muscle fatigue, inducing secondary skeletal muscle abnormalities [6,7]. On the other hand, peripheral nervous system involvement by FD can influence motor performance [8]. In the present study, we sought to investigate whether primary muscle involvement can be responsible for the muscle disturbances observed in patients with FD and compared the pathologic pattern of skeletal and cardiac myopathy.
2. Materials and methods
2.1. Patient population
The study population included 12 patients with FD, with ages ranging from 24 to 58 years. In all patients, the diagnosis of FD was based on the identification of α-galactosidase A mutations and, in male patients, on the detection of low α-galactosidase A activity in peripheral leukocytes [9]. Our patient population was divided into 2 groups on the basis of an age cutoff of 35 years because cardiac involvement becomes evident as LV hypertrophy after 35 years of age [1].
Four patients were younger than 35 years (group A, 4 men; age, 27.0 ± 2.6 years) and were symptomatic for palpitation and/or angina and muscular cramps, whereas 8 were older than 35 years (group B, 6 men, 2 women; age, 49.6 ± 7.2 years) and were symptomatic for angina and dyspnea on effort, as well as for pain and fatigability of limb-girdle muscles during exercise. At the time of the study, no patients were on enzyme replacement therapy.
2.2. Cardiac studies
Extensive clinical examination, including the assessment of FD systemic manifestations, and noninvasive cardiac studies (resting electrocardiogram, echocardiography with tissue Doppler analysis, cardiac magnetic resonance) were performed in all patients as previously described [10]. Invasive cardiac studies were performed after patient written informed consent and approval by the ethics committees of our institution and included cardiac catheterization, selective coronary angiography, LV angiography, and biventricular or LV endomyocardial biopsy. Serum levels of cardiac troponin I, total creatine phosphokinase, and cardiac (creatine kinase MB) and skeletal muscle (creatine kinase MM) isoenzymes were assessed in all patients.
2.3. Muscle evaluation
The voluntary contraction for the deltoid, biceps brachii, quadriceps femoris, and tibilias anterior muscles was measured according to the British Medical Research Council Scale [11]. For the assessment of the maximum voluntary isometric contraction, each movement was performed bilaterally and held for 3 to 5 seconds, with 6 to 12 seconds of rest between each movement. The previously mentioned muscles were also studied with needle electromyography to evaluate the muscular activity and with ultrasound to obtain images in longitudinal and transversal views. In addition, needle muscle biopsies of the vastus lateralis were performed using a Bard Biopsy instrument (Bard, Covington, GA) with a 14-gauge needle as described [12].
2.4. Evaluation of peripheral nerve function
Sensory and motor neurographies (sural, peroneal, median, and ulnar nerves) according to conventional methods were performed [13]. The following parameters were assessed: motor conduction velocity, distal latencies, compound muscle action potential amplitudes, sensory conduction velocity, and sensory action potential amplitude. Standardized distal distances for motor and sensory studies were used.
2.5. Histopathologic and ultrastructural studies
Endomyocardial and skeletal muscle samples were processed for routine histologic and histochemical analyses including Sudan black, Masson trichrome, and elastic van Gieson stainings. Additional samples were fixed in 2% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.3) and embedded in Epon resin; semithin sections were processed for Azur II staining, and ultrathin sections were stained with uranyl acetate and lead hydroxide for transmission electron microscopy [10].
2.6. Morphometry
The cardiomyocyte diameter at the nuclear level in transversely cut cells [10] and skeletal myocyte cross-sectional diameter in transverse sections were computed [14] and compared with normal biopsies in 5-μm-thick paraffin-embedded histologic sections stained with hematoxylin and eosin. The cell area occupied by storage vacuoles was evaluated as the percentage of the total cardiomyocyte or skeletal myocyte area measured by electron microscopy in at least 25 cells from 10 micrographs [9].
Histologic sections stained with Masson trichrome were examined at ×400 magnification, with a reticule containing 42 sampling points (105844; Wild Heerbrugg Instruments, Gals, Switzerland) to determine the percent area occupied by fibrous tissue [10]. The structure of intramural coronary and skeletal muscle arteries was examined on elastic van Gieson–stained paraffin sections, and the examination was confined to those that were viewed in a cross section without obvious obliquity and those that did not appear to be branches of another intramural vessel [15]. The ratio of external diameter to luminal diameter was computed (E/L ratio) and used as a quantitative index for the degree of luminal narrowing [15]. Nikon Nis Elements BR software (Nikon Instruments Inc., Melville, NY) was used.
Controls for morphometric analysis consisted of surgical LV endomyocardial biopsies from patients with mitral stenosis and quadriceps muscle biopsies from healthy controls. In particular, the endomyocardial fragments, although not obtained from healthy individuals, were derived from a chamber without overload and were considered the samples nearest to normal endomyocardial tissue.
2.7. Statistical analysis
Normal distribution of the explored variables was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Continuous variables were presented as mean ± SD. Categorical variables were presented as absolute frequencies or percentages. Between-group comparisons of variables showing normal distribution and homogeneous variance (as assessed by the Levene test) were performed using a 1-way analysis of variance; in the case of between-group significant differences, a post hoc analysis was performed using the Scheffé test. Between-group comparisons of variables not showing normal distribution were performed using the Kruskal-Wallis test; in the case of overall between-group significant differences, direct comparisons were performed using the Mann-Whitney test. In case of multiple comparisons, Bonferroni correction was applied to control the experiment with respect to type I error probability. Significance between 2 groups was determined using the Student t test for continuous variables. A 2-tailed P ≤ .05 was considered statistically significant.
3. Results
3.1. Patient population and clinical studies
Clinical, echocardiographic, genetic, cardiac, and skeletal muscle morphometric characteristics of the 12 patients are shown in Table 1. In Table 2, the comparison between group A and B patients and healthy subjects (group C) is presented.
Table 1.
Characteristics of the 12 study patients with FD
| Characteristics | Group A |
Group B |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Age (y) | 24 | 26 | 30 | 28 | 40 | 58 | 43 | 53 | 57 | 53 | 52 | 40 |
| Sex | M | M | M | M | M | F | M | M | F | M | M | M |
| Genotype | c946delG | c946delG | G328R | Q279K | Q279K | Q279K | c946delG | N215S | c946del G | Y216C | c427delACTT | R220X |
| Troponin I (ng/mL)a | 0.05 | 0.03 | 1.03 | 0.08 | 0.04 | 1.34 | 0.09 | 1.65 | 1.20 | 2.00 | 1.00 | 0.09 |
| Cardiac symptoms | P | P | P | P | A, D, P | A, D, P | A, D | A | A, P | A, D, P | D, P | D, P |
| Muscle symptoms | C | C | C | C | P, F, C | P, F | P, F | F, C | F, C | P, F | P, F, C | F, C |
| Echocardiography | ||||||||||||
| MWT (mm) | 12.0 | 11.8 | 11.5 | 12.0 | 18.5 | 17.0 | 21.0 | 16.5 | 19.0 | 23.0 | 21.0 | 17.0 |
| EF (%) | 70 | 56 | 60 | 65 | 65 | 68 | 57 | 60 | 70 | 67 | 55 | 59 |
| Cardiac morphometry | ||||||||||||
| Cardiomyocyte diameter (μm) | 20.5 | 21.3 | 20.2 | 19.8 | 28.0 | 36.0 | 37.8 | 35.2 | 35.9 | 35.5 | 34.2 | 28.7 |
| Glycolipid deposit area (%)b | 37.3 | 32.3 | 26.7 | 30.3 | 48.1 | 50.2 | 55.0 | 59.3 | 50.1 | 48.5 | 63.3 | 43.1 |
| Fibrosis (%) | 2.9 | 4.4 | 4.2 | 3.5 | 6.1 | 15.0 | 8.3 | 9.2 | 14.0 | 18.6 | 16.0 | 5.3 |
| Interstitial | 1.9 | 3.8 | 3.5 | 3.0 | 4.1 | 6.0 | 5.0 | 4.0 | 6.0 | 9.0 | 5.5 | 3.2 |
| Perivascular | 0.2 | 0.6 | 0.5 | 0.3 | 0.5 | 3.8 | 1.0 | 1.2 | 3.6 | 4.4 | 4.0 | 0.8 |
| Replacement | 0.8 | 0.0 | 0.2 | 0.2 | 1.5 | 5.2 | 2.3 | 4.0 | 5.4 | 5.2 | 6.5 | 1.3 |
| E/L ratioc | 2.0 | 1.5 | 1.8 | 1.7 | 2.7 | 4.0 | 2.8 | 3.2 | 3.3 | 5.3 | 4.3 | 2.6 |
| Muscle morphometry | ||||||||||||
| Myocyte diameter (μm) | 59.6 | 60.3 | 58.9 | 62.3 | 75.3 | 73.4 | 63.2 | 66.3 | 59.4 | 65.2 | 70.3 | 65.4 |
| Glycolipid deposit area (%)b | 0.0 | 0.0 | 0.0 | 0.0 | 12.1 | 11.3 | 16.6 | 25.1 | 15.4 | 22.0 | 19.4 | 9.5 |
| Fibrosis (%) | 1.5 | 1.0 | 2.0 | 1.0 | 2.8 | 4.3 | 4.0 | 3.8 | 4.5 | 5.2 | 6.2 | 4.0 |
| Interstitial | 1.3 | 0.8 | 1.2 | 0.6 | 1.1 | 1.5 | 1.5 | 1.3 | 1.6 | 1.3 | 1.5 | 1.5 |
| Perivascular | 0.2 | 0.2 | 0.8 | 0.4 | 0.2 | 0.6 | 0.5 | 0.9 | 0.7 | 1.4 | 1.4 | 1.1 |
| Replacement | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 2.2 | 2.0 | 1.6 | 2.2 | 2.5 | 3.3 | 1.4 |
| E/L ratioc | 1.6 | 1.1 | 1.4 | 1.6 | 3.0 | 3.9 | 3.2 | 3.0 | 3.5 | 4.3 | 4.0 | 2.6 |
Abbreviations: A, angina; D, dyspnea; P, palpitation (cardiac) or pain (muscle); F, fatigability; C, cramps; MWT, maximal wall thickness; EF, ejection fraction; M, male; F, female.
Troponin I reference value <0.15 ng/mL.
Calculated as the percentage of the total cell area, excluding nuclear area.
External/lumen ratio of intramural arteries, average value.
Table 2.
Comparison of clinical, echocardiographic, and morphometric data among patients with FD younger than 35 years (group A), older than 35 years (group B), and healthy controls (group C)
| Characteristics | Group A (n = 4) | Group B (n = 8) | Group C (n = 10) | Pa |
|---|---|---|---|---|
| Age (y) | 27.0 ± 2.6 | 49.6 ± 7.2 | 41.4 ± 8.4 | <.001 A vs B <.001 A vs C |
| Echocardiography | ||||
| MWT (mm) | 11.8 ± 0.2 | 19.1 ± 2.3 | 9.9 ± 1.0 | <.001 B vs A <.001 B vs C |
| EF (%) | 62.8 ± 6.1 | 62.6 ± 5.6 | 61.2 ± 4.6 | NS |
| E/A ratio | 1.3 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.2 | NS |
| Tissue Doppler velocities | <.001 A vs C <.001 B vs C |
|||
| Septal Sa (cm/s) | 7.8 ± 0.6 | 5.9 ± 0.8 | 14.5 ± 1.7 | |
| Septal Ea (cm/s) | 8.4 ± 0.5 | 5.5 ± 0.5 | 15.2 ± 1.8 | |
| Septal Aa (cm/s) | 8.6 ± 0.6 | 6.2 ± 0.5 | 10.4 ± 1.3 | |
| Septal Ea/Aa (cm/s) | 0.98 ± 0.02 | 0.89 ± 0.06 | 1.5 ± 0.1 | |
| Septal E/Ea (cm/s) | 9.7 ± 1.1 | 13.6 ± 2.7 | 5.5 ± 0.7 | |
| Lateral Sa (cm/s) | 8.3 ± 0.5 | 8.8 ± 0.5 | 14.2 ± 1.6 | |
| Lateral Ea (cm/s) | 8.8 ± 0.5 | 6.1 ± 0.4 | 14.8 ± 1.6 | |
| Lateral Aa (cm/s) | 8.9 ± 0.5 | 6.4 ± 0.4 | 10.2 ± 1.3 | |
| Lateral Ea/Aa (cm/s) | 0.99 ± 0.02 | 0.96 ± 0.03 | 1.5 ± 0.3 | |
| Lateral E/Ea (cm/s) | 9.2 ± 0.9 | 12.2 ± 1.8 | 5.6 ± 0.8 | |
| Cardiac morphometry | ||||
| Cardiomyocytes diameter (μm) | 20.5 ± 0.6 | 33.9 ± 3.6 | 12.0 ± 2.8 | <.001 B vs A <.001 B vs C .001 A vs C |
| Glycolipid area (%)b | 31.5 ± 4.4 | 52.2 ± 6.6 | – | <.001 B vs A |
| Fibrosis (%) | 3.8 ± 0.7 | 11.6 ± 5.0 | 3.7 ± 2.0 | .003 B vs A <.001 B vs C |
| Interstitial | 3.1 ± 0.8 | 5.4 ± 1.8 | 3.3 ± 1.9 | |
| Perivascular | 0.4 ± 0.2 | 1.4 ± 1.7 | 0.4 ± 0.2 | |
| Replacement | 0.3 ± 0.3 | 3.9 ± 2.0 | 0.0 ± 0.0 | |
| E/L ratioc | 1.8 ± 0.2 | 3.5 ± 0.9 | 1.6 ± 0.2 | <.001 B vs A <.001 B vs C |
| Muscle morphometry | ||||
| Myocyte diameter (μm) | 60.3 ± 1.5 | 67.3 ± 5.3 | 61.3 ± 7.3 | NS |
| Glycolipid area (%)b | – | 16.4 ± 5.5 | – | |
| Fibrosis (%) | 1.4 ± 0.5 | 4.4 ± 1.0 | 1.3 ± 0.5 | .02 B vs A .001 B vs C |
| Interstitial | 1.0 ± 0.3 | 1.4 ± 0.5 | 1.0 ± 0.4 | |
| Perivascular | 0.4 ± 0.3 | 0.9 ± 0.4 | 0.3 ± 0.2 | |
| Replacement | 0.0 ± 0.0 | 2.1 ± 0.6 | 0.0 ± 0.0 | |
| E/L ratioc | 1.4 ± 0.2 | 3.4 ± 0.6 | 1.4 ± 0.2 | <.001 B vs A <.001 B vs C |
Abbreviations: MWT, maximal wall thickness; EF, ejection fraction; NS, not statistically significant; Sa, systolic myocardial velocity at the mitral annulus; Ea, early diastolic myocardial relaxation velocity at the mitral annulus; Aa, myocardial velocity associated with atrial contraction at the mitral annulus.
P values refer to the comparison between groups.
Calculated as the percentage of the total cell area, excluding nuclear area.
External/lumen ratio of intramural arteries, average value.
Patients were from 7 different families. Patients 1, 2, 7, and 9 belonged to the same family. Patients 4, 5, and 6 belonged to another family. The remaining 5 patients were from different families. Patient 8 was the only one affected by the cardiac variant of FD.
Troponin I was elevated in 5 of the 12 patients (1 from group A and 5 from group B, reference value <0.15 ng/mL), whereas creatine phosphokinase and creatine kinase MB and creatine kinase MM fraction were normal in all patients. None of them smoked, had hypercholesterolemia, or had diabetes or hypertension. Cardiac magnetic resonance confirmed the morphologic and functional data observed by echocardiography and showed late-gadolinium enhancement in none of group A patients, in 5 (62%) of 8 group B patients, and in all cases localized in the basal or basal-medium segments of the inferolateral LV wall.
In 4 patients in group A, the results of muscle clinical evaluation, skeletal muscle ultrasound (Fig. 1A), and electromyography were normal. In group B, clinical muscular evaluation showed mild muscle weakness and pain, and the skeletal muscle echographic findings revealed a moderate to severe muscular disarray of deltoid and quadriceps femoris (Fig. 2A), whereas electromyography showed mild signs of myopathy.
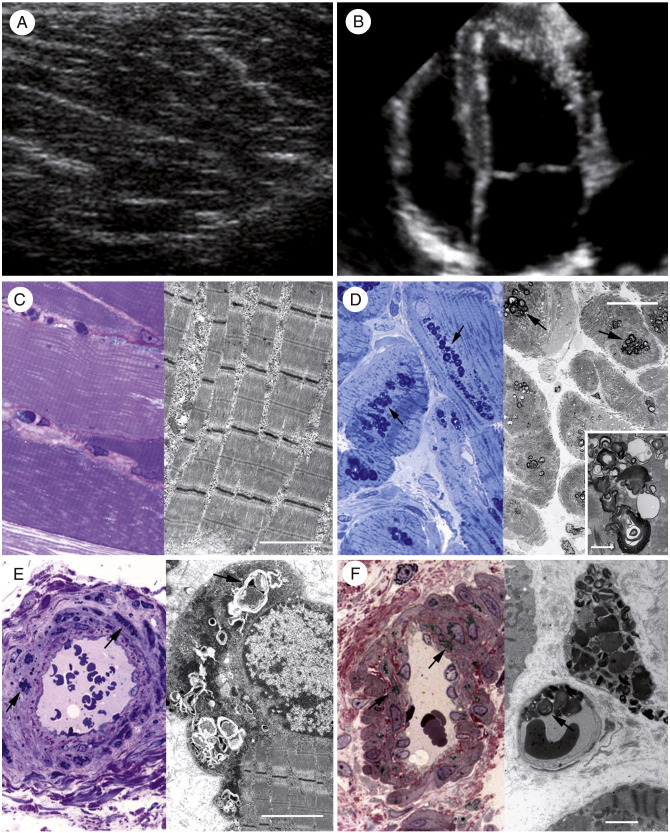
Fig. 1.
Comparison between skeletal (left column) and cardiac (right column) muscles in a 26-year-old man with early FD (patient 2). Echo findings are normal for both skeletal (A) and cardiac (B) muscles. Histology and electron microscopy of skeletal myocytes (C; left and right panels, respectively) fail to document storage material, whereas histologic examination and electron microscopy of cardiomyocytes (D; left and right panels, respectively) show appreciable glycosphingolipid deposits (arrows). Both skeletal (E) and myocardial vessels (F) show equal degree of smooth muscle cell (E and F; left panel, arrows) and endothelial cell (E and F; right panel, arrows) infiltration without lumen narrowing. C, Left panel: Azur II, ×600 magnification; right panel: scale bar corresponds to 2 μm. D, Left panel: Azur II, ×400 magnification; right panel: scale bar corresponds to 10 μm, and inset scale bar corresponds to 1 μm. E, Left panel: Azur II, ×600 magnification; right panel: scale bar corresponds to 2 μm. F, Left panel: Azur II, ×600 magnification; right panel: scale bar corresponds to 2 μm.
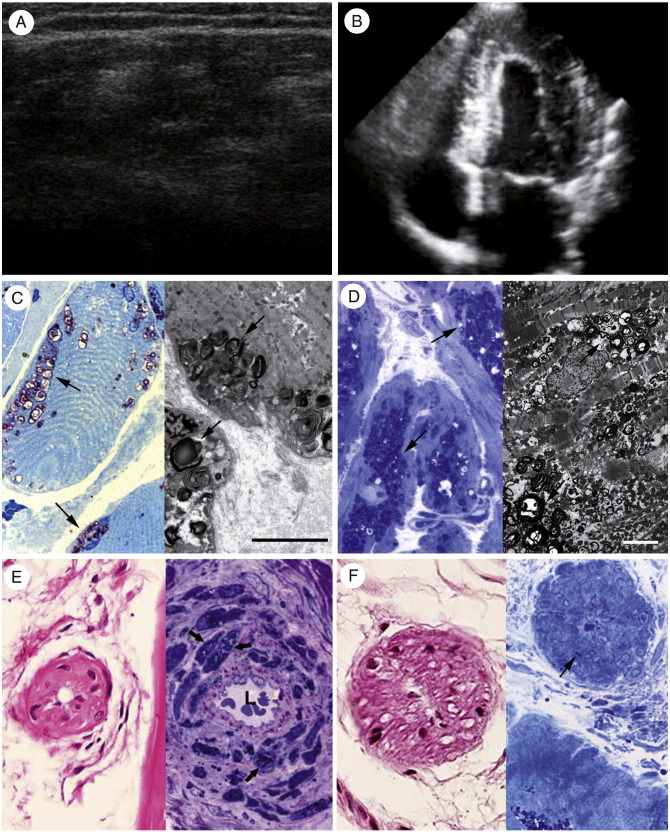
Fig. 2.
Comparison between skeletal (left column) and cardiac (right column) muscles in a 43-year-old man with advanced FD (patient 7). Muscle echography (A) shows disarray of the muscular structure (reduction of hyperechogenic and increase of hypoechogenic areas), and echocardiography (B) shows a remarkable increase in LV wall thickness. Muscle histologic examination and electron microscopy (C; left and right panels, respectively) reveal mild glycosphingolipid accumulation in the subsarcolemmal region (arrows) compared with severe cardiomyocyte engulfment by storage material (D; left panel [histology], right panel [electromicroscopy], arrows). Conversely, a similar degree of muscular (E) and myocardial vessel (F) endothelial and smooth muscle cell involvement (arrows) determining lumen narrowing (L) and increased perivascular fibrosis is observed. C, Left panel: Azur II, ×600 magnification; right panel: scale bar corresponds to 5 μm. D, Left panel, Azur II, ×600 magnification; right panel: scale bar corresponds to 2 μm. E, Left panel, hematoxylin and eosin, ×200 magnification; right panel: Azur II, ×600 magnification. F, Left panel: hematoxylin and eosin, ×400 magnification; right panel: Azur II, ×600 magnification.
All group B patients had LV hypertrophy, documented by basal electrocardiogram and echocardiography (Table 1; Fig. 1B); whereas in group A, the thickness of cardiac walls was within the upper normal limit (Table 1; Fig. 2B).
All nerve conduction studies in the patients with FD showed latencies, amplitudes, and conduction velocities within normal limits.
3.2. Histologic, morphometric, and ultrastructural studies
Both cardiac and muscle biopsy procedures were well tolerated in all patients, and no periprocedural complications were registered.
Skeletal myocytes from group A patients did not show the presence of glycosphingolipid vacuoles either histologically or by electron microscopy (Fig. 1C). Glycosphingolipid deposits were present only in the endothelial and smooth muscle cells of intramural vessels that showed normal thickness (Fig. 1E). Conversely, in cardiomyocytes of group A patients, histologic examination showed the presence of perinuclear vacuoles containing a material that, on frozen section, stained positively with periodic acid–Schiff stain and Sudan-Black, suggesting the accumulation of glycosphingolipids. Electron microscopy revealed that these vacuoles consisted of concentric lamellar figures in single membrane–bound vesicles (myelin bodies), diagnostic for FD (Fig. 1D). Intramural arteries showed endothelial and smooth muscle cell glycosphingolipid infiltration without lumen narrowing (Fig. 1F).
Skeletal myocytes from group B patients showed the presence of glycosphingolipid vacuoles localized in the subsarcolemmal region, often in the perinuclear area, consisting of typical myelin bodies (Fig. 2C). Myocyte diameter was slightly and not significantly increased compared with healthy controls, and glycosphingolipid vacuoles occupied, on average, less than 20% of the cell area (Tables 1 and 2). Muscle fibrosis was increased. Lumen narrowing of muscle arterioles with glycosphingolipid deposits either in endothelial and smooth muscle cells or in perivascular areas of fibrofatty replacement were also detected (Fig. 2E). Cardiomyocytes were bigger, and the percentage of the cell area occupied by glycosphingolipids was wider compared with group A (Tables 1 and 2; Fig. 2D). Moreover, morphometric analysis showed an increase in interstitial and replacement fibrosis in group B compared with group A and healthy controls (Table 2). Most (>50%) of the intramyocardial arteries from patients with FD in group B showed lumen narrowing with wall thickening due to hypertrophy and proliferation of smooth muscle cells, both engulfed by glycosphingolipids, and due to increased fibrosis of the medial and intimal layer (Fig. 2F). Myocardial replacement fibrosis surrounding severely narrowed intramural coronary arteries was often present.
Typically, in the female patient, the cardiac and the muscle involvement showed a patchy distribution because of the pattern of inactivation of the X chromosome [4].
4. Discussion
Muscle involvement in FD patients with frequent complaints of fatigability and pain of the proximal limb muscles is not completely clarified because these symptoms are often considered a consequence of diastolic heart failure or related to peripheral nerve involvement. Muscle function and pathology have been described mostly in single-case reports [16-19] and in a small study [20], without any comparison with cardiac pathology. Our study analyzed the clinical, functional, and structural findings of the skeletal muscles from a cohort of FD patients with various degrees of muscle disturbances, showing that the disease causes direct skeletal muscle infiltration with milder and delayed compromise compared with cardiac involvement.
4.1. Muscle involvement in FD
At variance with the involvement of cardiomyocytes observed in both the patient populations studied, skeletal myocytes appeared to be involved only in the older patients, showing intracellular glycosphingolipid accumulation mainly located in the subsarcolemmal region. In addition, even in cases with an advanced disease, this infiltration was limited in extent, involving no more than 20% of the cell area. This is in accordance with the normal dimension of myocytes and the preserved muscle volume observed. Interestingly, the endothelial and smooth muscle cells of intramuscular vessels were always affected, confirming the predominant role of vessel involvement in FD. Indeed, renal, coronary, and brain vessels are an early and important target of the disease, resulting in coronary microvascular dysfunction [15], progressive renal failure, and cryptogenic stroke, respectively, where FD accounts for 7% of cases in the young (<40 years) population [21].
Involvement of the skeletal muscle was reflected in our patients as muscular cramps in the early phase and as muscular pain, fatigability, and asthenia, involving deambulation and motor performance and constraining patients' everyday activity and quality of life in the advanced disease.
Instrumental analysis was normal in the younger group, whereas the older group was characterized by the presence of muscle disarray and hyperechogenic areas during echography and mild signs of myopathy in electromyography, mainly localized in the proximal limb muscles (deltoid and quadriceps femoris). The presence of fibrofatty replacement surrounding the intramural narrowed arteries and the global increase in muscular fibrosis are most likely the consequences of both myocyte infiltration and, possibly, myocyte death due to local ischemia, followed by reparative phenomena.
All patients had normal sensory and motor neurography, similarly to what has been reported in previous studies, despite the clinical and histologic alterations observed in the peripheral nervous system [1]. Most likely, this is because motor and sensory nerve conduction studies evaluate mainly large myelinated fibers and not the small and unmyelinated nerve fibers that are most affected in FD [20].
4.2. Correlation between cardiac and skeletal muscle involvement
Both cardiac and skeletal muscle involvement in FD are progressive and minimally symptomatic in the early phase of the disease. Indeed, the patients with early FD cardiomyopathy had normal clinical, echographic, and functional skeletal muscle parameters. Skeletal muscle ultrastructural findings in these cases revealed only mild glycosphingolipid accumulation in smooth muscle and endothelial cells, without narrowing of intramural vessels. In the heart, the perinuclear vacuoles occupied up to 30% of the cardiomyocyte area, and the intramural vessels did not show lumen obstruction.
In the 8 cases with advanced FD cardiomyopathy, there was a more severe muscle involvement, but the amount of the intramyocyte glycosphingolipid accumulation was higher in the heart (up to 50% of the cell area) than in the muscle (up to 20%). A possible explanation could be related to the difference in the activity of the quadriceps femoris, a voluntary muscle that contracts mainly in response to stimuli, and the heart that contracts spontaneously and continuously. Likewise, the influence of cell activity on glycosphingolipid accumulation in FD has been already documented in low-stress, not-affected veins compared with higher-stress affected arteries [22]. Moreover, the predominant postmitotic status of cardiomyocytes can prevent their replacement by enzyme-supplied precursors [23], whereas in the skeletal muscle, resident stem cells supplied by the exogenous enzyme can actively contribute to regeneration of normal or nearly normal myofibers [24].
Intramural vessel narrowing showed a similar severity in the heart and in the skeletal muscle. This is consistent with the recently demonstrated presence, in patients with FD, of a circulating smooth muscle cell–proliferating factor [25] that can act in intramural vessels of the heart and the skeletal muscle independently from the severity of the intracellular deposits.
5. Conclusions
The skeletal muscle is a target of FD, which is affected even in young and minimally symptomatic subjects. Muscle symptoms are independent from cardiac disease and result from skeletal myocyte and vessel involvement.
Footnotes
This work was supported by the Telethon Grant No. GGP08167.
The authors have nothing to disclose.
References
- 1.Desnick R.J., Ioannou Y.A., Eng C.M. Alpha-galactosidase a deficiency: Fabry disease. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Kinzler K.E., Vogelstein B., editors. The metabolic and molecular bases of inherited disease. McGraw-Hill; New York: 2001. pp. 3733–3774. [Google Scholar]
- 2.Nakao S., Takenaka T., Maeda M. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- 3.Sachdev B., Takenaka T., Teraguchi H., Lee P., McKenna W.J., Elliott P.M. Prevalence of Anderson-Fabry disease in male patients with late-onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–1411. doi: 10.1161/01.cir.0000012626.81324.38. [DOI] [PubMed] [Google Scholar]
- 4.Chimenti C., Pieroni M., Morgante E. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation. 2004;110:1047–1053. doi: 10.1161/01.CIR.0000139847.74101.03. [DOI] [PubMed] [Google Scholar]
- 5.Takenaka T., Teraguchi H., Yoshida A. Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol. 2008;51:50–59. doi: 10.1016/j.jjcc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Coirault C., Guellich A., Barbry T., Samuel J.L., Riou B., Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1009–H1017. doi: 10.1152/ajpheart.00438.2006. [DOI] [PubMed] [Google Scholar]
- 7.Lavietes M.H., Gerula C.M., Fless K.G., Cherniack N.S., Arora R.R. Inspiratory muscle weakness in diastolic dysfunction. Chest. 2004;126:838–844. doi: 10.1378/chest.126.3.838. [DOI] [PubMed] [Google Scholar]
- 8.Lacomis D., Roeske-Anderson L., Mathie L. Neuropathy and Fabry's disease. Muscle Nerve. 2005;31:102–107. doi: 10.1002/mus.20130. [DOI] [PubMed] [Google Scholar]
- 9.Frustaci A., Chimenti C., Ricci R. Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose infusion therapy. N Engl J Med. 2001;345:25–32. doi: 10.1056/NEJM200107053450104. [DOI] [PubMed] [Google Scholar]
- 10.Chimenti C., Hamdani N., Boontje N.M. Myofilament degradation and dysfunction of human cardiomyocytes in Fabry disease. Am J Pathol. 2008;172:1482–1490. doi: 10.2353/ajpath.2008.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James M.A. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am. 2007;32:154–156. doi: 10.1016/j.jhsa.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Coté A.M., Jiménez L., Adelman L.S., Munsat T.L. Needle muscle biopsy with the automatic Biopty instrument. Neurology. 1992;42:2212–2213. doi: 10.1212/wnl.42.11.2212. [DOI] [PubMed] [Google Scholar]
- 13.Mondelli M., Baldasseroni A., Aretini A., Ginanneschi F., Padua L. Prevalent involvement of thenar motor fibres in vineyard workers with carpal tunnel syndrome. Clin Neurophysiol. 2010;121:1251–1255. doi: 10.1016/j.clinph.2010.02.150. [DOI] [PubMed] [Google Scholar]
- 14.Wang J.F., Forst J., Schröder S., Schröder J.M. Correlation of muscle fiber type measurements with clinical and molecular genetic data in Duchenne muscular dystrophy. Neuromuscul Disord. 1999;9:150–158. doi: 10.1016/s0960-8966(98)00114-x. [DOI] [PubMed] [Google Scholar]
- 15.Chimenti C., Morgante E., Tanzilli G. Angina in Fabry disease reflects coronary small vessels disease. Circ Heart Fail. 2008;1:161–169. doi: 10.1161/CIRCHEARTFAILURE.108.769729. [DOI] [PubMed] [Google Scholar]
- 16.Uchino M., Uyama E., Kawano H. A histochemical and electron microscopic study of skeletal and cardiac muscle from a Fabry disease patient and carrier. Acta Neuropathol. 1995;90:334–338. doi: 10.1007/BF00296520. [DOI] [PubMed] [Google Scholar]
- 17.Pellissier J.F., Van Hoof F., Bourdet-Bonerandi D., Monier-Faugere M.C., Toga M. Morphological and biochemical changes in muscle and peripheral nerve in Fabry's disease. Muscle Nerve. 1981;4:381–387. doi: 10.1002/mus.880040506. [DOI] [PubMed] [Google Scholar]
- 18.Tomé F.M., Fardeau M., Lenoir G. Ultrastructure of muscle and sensory nerve in Fabry's disease. Acta Neuropathol. 1977;38:187–194. doi: 10.1007/BF00688064. [DOI] [PubMed] [Google Scholar]
- 19.Macovei M., Alexianu M., Cardaş M., Petrovici A., Hălălău F. Fabry's disease (angiokeratoma corporis diffusum). Report of a case with ultrastructural study of the skeletal muscle. Neurol Psychiatr (Bucur) 1977;15:221–226. [PubMed] [Google Scholar]
- 20.Gomes I., Nora D.B., Becker J. Nerve conduction studies, electromyography and sympathetic skin response in Fabry's disease. J Neurol Sci. 2003;214:21–25. doi: 10.1016/s0022-510x(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 21.Rolfs A., Böttcher T., Zschiesche M. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366:1794–1796. doi: 10.1016/S0140-6736(05)67635-0. [DOI] [PubMed] [Google Scholar]
- 22.Chimenti C., Morgante E., Critelli G., Russo M.A., Frustaci A. Coronary artery bypass grafting for Fabry's disease: veins more suitable than arteries? Hum Pathol. 2007;38:1864–1867. doi: 10.1016/j.humpath.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Keslová-Veselíková J., Hůlková H., Dobrovolný R. Replacement of α-galactosidase A in Fabry disease: effect on fibroblast cultures compared with biopsied tissues of treated patients. Virchows Arch. 2008;452:651–665. doi: 10.1007/s00428-008-0586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Grand F., Rudnicki M.A. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbey F., Brakch N., Linhart A. Cardiac and vascular hypertrophy in Fabry disease: evidence for a new mechanism independent of blood pressure and glycosphingolipid deposition. Arterioscler Thromb Vasc Biol. 2006;26:839–844. doi: 10.1161/01.ATV.0000209649.60409.38. [DOI] [PubMed] [Google Scholar]