Abstract
Background
The main treatments for cancers are still chemotherapy and radiotherapy for intermediate-stage cancer and terminal cancer. However, the therapeutic methods often result in a decreased neutrophilic granulocyte count and other side effects. In this study, in order to improve the neutrophilic granulocyte levels in the blood after radiotherapy and chemotherapy, we developed a sustained-release granulocyte colony–stimulating factor (G-CSF) microsphere formulation using a novel solid-in-oil-in-oil-in-water (S/O/O/W) emulsification method.
Methods
G-CSF was loaded into dextran nanoparticles by freezing-induced phase separation, and then the G-CSF–loaded nanoparticles were encapsulated into sustained-release poly(lactic-co- glycolic acid) microspheres using S/O/O/W emulsification. The control microspheres were also prepared through W/O/W emulsification. The performance of the two microsphere formulations was investigated both in vitro and in vivo.
Results
The microspheres for the controlled release of G-CSF in a zero-order or near-zero-order pattern were provided for 2 weeks. The in vitro cumulative G-CSF release kept over 90% of its bioactivity, which was proved by a NFS-60 cell line growth assay. The microspheres of the control group fabricated by W/O/W emulsification maintained less then half of its bioactivity. The in vivo efficacy of microspheres made using the S/O/O/W method was higher than those using the W/O/W method.
Conclusion
These results suggested that the microspheres prepared by the S/O/O/W method had increased neutrophil activity compared to those prepared by W/O/W.
Keywords: microspheres, bioactivity, G-CSF, stability, sustained release
Introduction
Granulocyte colony–stimulating factor (G-CSF)1 has been investigated and found able to promote granulocyte precursors and neutrophil differentiation, proliferation, and maturation, and control neutrophil dynamics in the blood.2 A reduction in neutrophilic granulocyte count often results from melanoma radiotherapy, chemotherapy, or various other unknown conditions. Some drugs have been developed and used in clinical therapy. However G-CSF is still the first-line drug for clinically increasing the neutrophil count in the blood.3–6 Due to its short-life in blood, G-CSF has to be provided by frequent injection, which is inconvenient and painful for patients. To solve the problem, a different administration route or formulation development, including intranasal administration,7–9 pulmonary delivery,10–12 and oral administration,13–15 is needed. There are studies focusing on the above administration routes, but the bioavailability shows that the efficacy is very low, so these cannot meet with therapeutic success.16–18 A long-acting administration route or formulation can reduce administration frequency; therefore, the long-acting fusion protein polyethylene glycol (PEG)ylated G-CSF, and sustained-release or controlled-release poly(lactic-co-glycolic acid) (PLGA) microspheres have been investigated also.18–20 However, fusion protein and PEGylation often result in the bioactivity reduction of G-CSF and produce other side effects; Sustained- or controlled-release microspheres suffer from bioactivity loss, conformation changes, and immunogenicity increase, because the preparation methods cannot avoid producing water–air or water–oil interfaces of water-in-oil-in- water (w/o/w), water-in-oil-in-oil (w/o/o), or water-in-oil (w/o) emulsion.21–24 To avoid the interfaces, solid-in-oil-in-water (s/o/w) emulsion has also been studied.24,27 However, the protein microparticles or nanoparticles can still partly contact with the water continuous phase and be dissolved during the preparation processes, resulting in loss of protein bioactivity and increase in side effects.
We investigated several novel methods for overcoming creation of these interfaces: a low-temperature-induced phase separation and a stable water–water phase emulsion method for protein microencapsulated into polysaccharide particles.25 These protein polysaccharide particles can be further applied to fabricate different long-action protein formulations.26–32 For example, the polysaccharide particles were used to prepare coat stents, scaffolds, and other drug devices.24,31 We developed a scaffold that enhances post-chemotherapy neutrophilic granulocyte levels after tumor resection.33 However, patients with intermediate-stage cancer, terminal cancer, or blood cancer often cannot receive surgery to remove tumors and can only undergo chemotherapy and radiotherapy, so a novel formulation increasing the neutrophilic granulocyte count is essential to enhance their compliance.
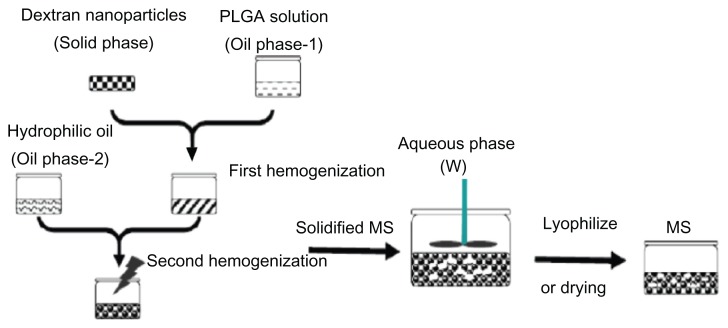
The aim of this investigation was to develop a long-acting microsphere formulation with higher loading efficiency, preserved G-CSF bioactivity, and enhanced long-acting treatment effect. The G-CSF–dextran nanoparticles were microencapsulated into PLGA microsphere using a novel solid-in-oil-in-oil-in-water (S/O/O/W) method. A schematic drawing of the microsphere preparation is shown in Figure 1. The microsphere was evaluated by size-exclusion chromatography– high-pressure liquid chromatography (SEC-HPLC), scanning electron microscopy (SEM), release measurement, an NSF-60 cell-growth assay, and in vivo and in vitro efficacy determination.
Figure 1.
Schematic drawing of microsphere preparation.
Abbreviations: PLGA, poly(lactic-co-glycolic acid); MS, microspheres.
Materials and methods
PEG (molecular weight 6000, biochemical reagent), dichloromethane (DCM, analytical reagent), dextran (molecular weight 64,000–76,000, biochemical reagent), polyvinyl alcohol (PVA, biochemical reagent, average molecular weight 9000–10,000, 80% hydrolyzed), glycerol (analytical reagent), and ethylene glycol (EG, analytical reagent) were purchased from Sigma-Aldrich (St Louis, MO). A human G-CSF enzyme-linked immunosorbent assay (ELISA) kit was acquired from R&D Systems (Minneapolis, MN). G-CSF was obtained from Jinsai Yaoye (Changchun, China). A bipotential murine hemopoietic cell line (NFS-60) was supplied from the American Type Culture Collection (Manassas, VA). PLGA (50:50, molecular weight 20 kDa) came from SurModics Pharmaceuticals (Birmingham, AL).
Animals
Adult male 200-g Sprague Dawley (SD) rats were kept under standard cultured environments at 23°C ± 2°C. The animal experiments abided by the laboratory animal care principles and were approved by the Institutional Animal Care and Utilization Committee of Shanghai Jiao Tong University.
Preparation of G-CSF–loaded dextran nanoparticles
A 2.5-mL mixture solution of G-CSF (0.05%, w/w), dextran (0.45%, w/w), and PEG (4.5%, w/w) was prepared in 5-mL vials.25,37 The mixture was stirred at 2000 rpm for 20–30 seconds at 0°C, and then the mixture solution was kept in the refrigerator at −80°C for up to 12 hours. The samples were freeze-dried with Christ (Osterode, Germany) Alpha 1–2 laboratory freeze-drying equipment at 5.25 × 10−3 Pa pressure for 24 hours. The freeze-dried samples were resuspended and dissolved PEG in 6 mL of DCM, followed by centrifugation at 12,000 rpm for 6 minutes (5415D; Eppendorf, Hamburg, Germany) to remove the PEG solution. The above experiments were repeated five times, and then the dextran nanoparticles were collected through being evaporated at 25°C ± 2°C and 1.33 Pa for 36 hours with a vacuum dryer (DZF-3; Shanghai Fuma, Shanghai, China) to remove the DCM residue. The nanoparticles containing less than 0.5% (w/w) PEG were obtained after the PEG-removal process.24
Preparation of dextran nanoparticle–loaded microspheres
Ten-mg dextran nanoparticles were suspended in 0.9 g of 10% PLGA solution and dissolved in dichloromethane (forming oil phase 1). After the mixture was placed on vortex and stirred at 5000 rpm for 1 minute, the suspension was further placed into a 5.5-mL hydrophilic oil phase (the hydrophilic oil phase was prepared using mixture of 72.7% [w/w] glycerol, 18.2% [w/w] EG, 9.1% [w/w] water containing 1% [w/w] PVA and 5% [w/w] NaCl) and emulsified under stirring with a Fluko (Shanghai, China) FA25 homogenizer at 2000 rpm for 30 seconds to obtain primary emulsion droplets. To extract the organic solvent and solidify the PLGA microspheres, this emulsion was immediately transferred into 1000 mL water at 0°C–4°C containing 5% (w/w) NaCl at stirring (100 rpm) with an electromotive stirrer (JJ-1; Jintan Xinhang, Jintan, China) for 3 hours, after which the system was up to room temperature. The solidified microspheres were washed three times using distilled water and then freeze-dried again before storage (Figure 1).37
Preparation for G-CSF–loaded PLGA microspheres using W/O/W
As control, G-CSF was microencapsulated into PLGA microspheres through a conventional water-in-oil-in-water (W/O/W) method. The 0.1-mL G-CSF water solution (containing 0.25% w/w G-CSF) was added into 0.9 mL PLGA DCM solution (containing 10% w/w PLGA) under stirring with a Fluko FA25 homogenizer at 5000 rpm to prepare a water-in-oil primary emulsion. Then, this emulsion was transferred to in 4 mL water solution containing 1% (w/w) PVA and 5% (w/w) NaCl under stirring at 1000 rpm for 2 minutes. Finally, the processed sample was the same as the above section.24
Scanning electron microscopy
PLGA microspheres and SEM images of dextran nanoparticles were characterized using a Sirion 200 SEM (FEI, Hillsboro, OR). Before scanning, these nanoparticles or microspheres were sprayed using gold vapors under argon atmospheres and then the images were detected at 5–10 keV under high vacuum.
G-CSF dextran nanoparticle (dextran nanoparticle) size distribution
The G-CSF-loaded dextran nanoparticle (dextran nanoparticle) size distribution and average nanoparticle size were performed using a Particle Size and Particle Shape Analyzer (CIS-100; Ankersmid, Edegem, Belgium). Ten-mg dry dextran nanoparticles were dispersed in the quartz cell.
G-CSF encapsulation efficiency
The PLGA microspheres were resuspended and dissolved by PLGA in 10 mL DCM. The suspension was centrifuged at 12,500 rpm for 6 minutes to remove supernatant liquid. The processes were repeated five times, and these nanoparticles were obtained. The recovered nanoparticles were then redissolved in phosphate-buffered saline (PBS, pH 7.4) and the obtained solution was diluted according to the requirement of detection with a G-CSF ELISA kit. The microsphere encapsulation efficiency was measured with the follow equation:
where W was G-CSF total weight from PLGA microspheres and Wt was that obtained from feeding G-CSF into the microspheres. The standard deviation for encapsulation efficiency of G-CSF was calculated according to the five repeated experiments.
Size-exclusion chromatography
SEC-HPLC was performed with an HPLC system equipped with a TSK G2000SWXL size exclusion column (Shimadzu, Tokyo, Japan). Running elution was done using a peristaltic pump with 50 mM PBS (pH 7.4). The running-elution flow rate was 1.0 mL/min1 at 25°C ± 2°C. Absorbance peak was determined at a wavelength of 214 nm for each sample. The G-CSF solution was filtered through a 0.22-μm film before the filtered solution was placed into the SCE-HPLC. The ratio of monomer G-CSF was calculated through the peak area percentage of the total peak area.
In vitro release profile
The PLGA microspheres (20 mg) were suspended in release vials containing 2 mL of PBS (100 mM, pH 7.4) at 37°C, and the vials were shaken at 150 rpm.37 The release solution was substituted for fresh release solution every day, and the G-CSF concentration was measured with a G-CSF ELISA kit. For the two release microspheres, the release experiments were repeated four times. The release-dynamics profiles of the microspheres were obtained according to the average of the four experiments.
In vitro release activity study
The NFS-60 cells were cultivated in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Invitrogen, Carlsbad, CA) with 5 ng/mL G-CSF and 10% fetal calf serum. Before the measurement experiments, exponentially growing NFS-60 cells were suspended and washed using PBS solution free of G-CSF. Then, the cell suspension was centrifuged at 1200 rpm at 4°C ± 2°C for 5 minutes and the supernatant liquid was removed to obtain the cells to be assayed. The cells were resuspended in RPMI 1640 culture solution containing 5% fetal calf serum. A total of 5 × 104 cells were cultured in 100 μL RPMI 1640 containing release solution of microspheres with 10% fetal calf serum. After 2 days, the proliferation of NFS-60 cells was measured with the MTT approach.26
In vivo release of SD rat study
Six male SD rats were subcutaneously injected with the two microspheres and original G-CSF water solution at a dose of 0.15 μg/g. A 0.4-mL blood sample was collected with a syringe containing heparin of each rat at 24, 48, 72, 96, 120, 144, 168, 192, 240, 288, 360, 432, and 504 hours after injection. These plasma samples were obtained through the blood samples being centrifuged at 12,000 rpm at 4°C ± 1°C for 21 minutes, followed by collection of the supernatant liquid (plasma samples). The obtained levels of plasma G-CSF were measured with an ELISA kit.
In vivo efficacy of SD rat study
In order to evaluate the efficacy study in vivo of SD rats, 6-week-old SD rats were subcutaneously injected with original G-CSF solution at 0.15 μg/g – the prepared microspheres using the S/O/O/W method, and the other microspheres by the W/O/W method – and physiological saline. A 0.2-mL blood sample was collected from each rat with a syringe containing heparin for each rat at 24, 48, 72, 96, 120, 144, 168, 192, 240, 288, 360, 432, and 504 hours after injection. Neutrophil levels were measured as reported previously.27 The neutrophil percentage increase was measured with a complete blood count (Beckman Coulter, Fullerton, CA), and per-μL blood neutrophil ratio increase was calculated based on the percentage of neutrophils from G-CSF multiplied by 100% and divided by the percentage of neutrophils from physiological saline.
Statistical analysis
All statistical data analyses were done with SPSS software (v 19.0; IBM, Armonk, NY). The statistical data were indicated as the average ± standard deviation. Significant differences were sought with Student’s t-test. P < 0.05 was viewed as significant.
Results
Size distribution and morphology
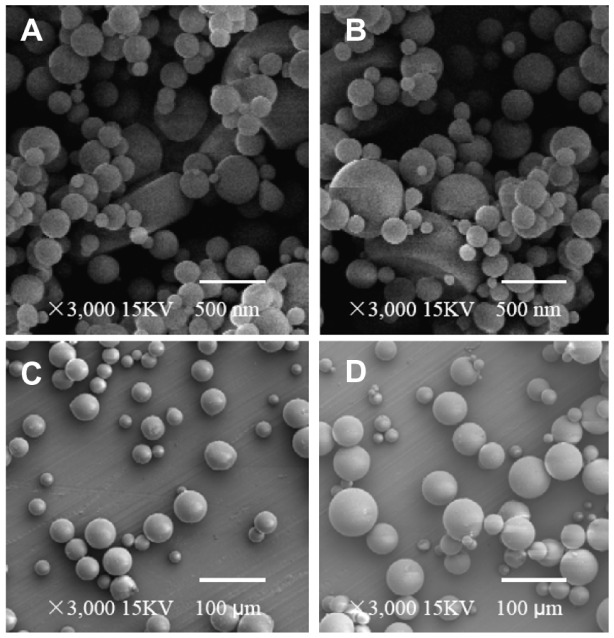
The morphology of microspheres was evaluated by SEM. Figure 2 showed the morphology of the dextran nanoparticles from different processes and microspheres with different preparation methods. We found that the surfaces of microspheres and dextran nanoparticles were smooth. The dextran nanoparticles possessed spherical shapes and diameters ranging between 80 nm and 200 nm. The microspheres were also spherical, and their size distribution ranged from 40 to 100 μm in diameter. The images of nanoparticles recovered from the prepared microspheres using the S/O/O/W method and nanoparticles alone are shown as Figure 2A and B. The results indicate that the nanoparticle surfaces of both recovered from the microspheres and alone were the same in terms of nonporosity and smoothness, and these nanoparticles also had the same morphology and size.31 These results suggested that when the dextran nanoparticles suffered from the preparation processes, the nanoparticles did not show any changes.
Figure 2.
(A–D) Scanning electron micrographs of different samples. (A) Dextran nanoparticles; (B) dextran nanoparticles recovered from the prepared dextran nanoparticle–loaded microspheres using S/O/O/W; (C) control microspheres (prepared microspheres using W/O/W); (D) prepared dextran nanoparticle–loaded microspheres using S/O/O/W.
Abbreviations: S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
G-CSF encapsulation efficiency study of microspheres and nanoparticles
Microsphere encapsulation efficiency was determined with an ELISA kit of G-CSF. The encapsulation efficiency of nanoparticles was about 98% (w/w).25 The encapsulation efficiency from the PLGA microspheres was above 85% (w/w), but the encapsulation efficiency of the control microspheres (PLGA microspheres prepared using the W/O/W method) was below 60%. This suggested that the prepared protein nanoparticles or microparticles might be prevented from protein dissolving in local water droplets during the preparation of microsphere processes when forming the first emulsion droplets using S/O/O/W. If the protein microparticles or nanoparticles were dissolved locally in their water droplets during the preparation processes, the protein macromolecules might be exposed to oil–water interfaces again. These dissolved protein molecules would adsorb onto the oil–water interfaces and result in both aggregation and decrease of protein encapsulation efficiency.24 When protein– water solution was microencapsulated into PLGA microspheres by W/O/W, protein was directly exposed to oil–water interfaces. Besides, protein–water solution produced higher osmotic pressure than the continuous water phase. These oil– water interfaces and high osmotic pressure directly resulted in protein aggregation and diffusion into the continuous water phase. While the second oil phase of S/O/O/W emulsion was inability to dissolve proteins and dextran, it was used only to extract organic solvent and solidify the microsphere.34 The solid nanoparticles could not produce high osmotic pressure and dissolved to produce protein molecules being exposed to oil–water interfaces. Therefore, this method might have reduced the amount of protein aggregation and diffusion to the water continuous phase and increased the encapsulation efficiency of microspheres prepared by S/O/O/W.
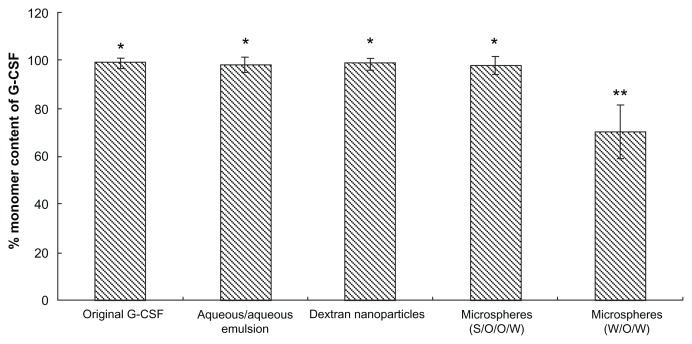
Aggregation study of G-CSF during different preparation processes
Protein aggregates of formulation may show integrity of protein during the whole preparation step.30 Therefore, to evaluate any change in G-CSF during the different preparation processes and methods, we measured the samples from these processes using SEC-HPLC. The G-CSF aggregation results after different preparation methods and each microencapsulation step are shown as Figure 3. The G-CSF monomer percentage did not change during the whole preparation process compared with native G-CSF, while the G-CSF monomer percentage of the control group (prepared microspheres using the W/O/W method) reduced by 30%. The results indicated that the PLGA microsphere fabrication with the present investigated method was an effective approach for injectable long-acting microspheres without G-CSF aggregation.
Figure 3.
Monomer content of recovery G-CSF from different samples during preparation processes by SEC-HPLC (n = 5).
Notes: Samples: original G-CSF (G-CSF:dextran = 1.4:5.0 ± 0.3 mg) solution; G-CSF from dextran aqueous phase/polyethyene glycol aqueous phase emulation; G-CSF from dextran nanoparticles; G-CSF from prepared microspheres using the S/O/O/W method (PLGA 50/50 2A: 100.0 ± 0.5 mg PLGA, dextran nanoparticles [G-CSF:dextran, 1:4] = 10.0 ± 0.3 mg); G-CSF from control microspheres (prepared microspheres using the W/O/W method) (PLGA 50/50 2A: 100.0 ± 0.5 mg, G-CSF solution [G-CSF:dextran = 1:4] = 10.0 ± 0.3 mg). *P > 0.05; **P < 0.05.
Abbreviations: G-CSF, granulocyte colony–stimulating factor; SEC-HPLC, size-exclusion chromatography–high-pressure liquid chromatography; PLGA, polylactic-co-glycolic acid; S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
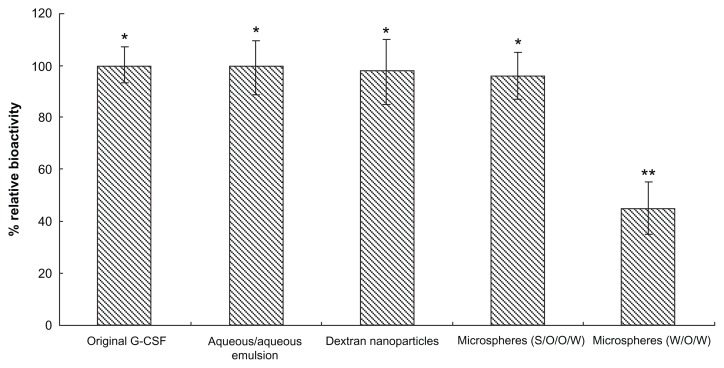
In vitro bioactivity from different preparation processes
To measure the different sample G-CSF bioactivities from different preparation processes, G-CSF was obtained from these samples using the encapsulation efficiency measurement method. G-CSF bioactivity was evaluated by proliferation of the NFS-60 cell line. The bioactivity of G-CSF from dextran nanoparticles, from the microspheres, from the control microspheres using W/O/W, and the native G-CSF solution, was respectively shown as Figure 4. The results showed that the bioactivity of the native G-CSF solution was almost the same as the samples for the original G-CSF solution (P > 0.05), while the G-CSF bioactivity in the control group (W/O/W method) decreased by 30% (P < 0.05). The bioactivities of the recovered samples indicated that the nanoparticles could protect G-CSF well during the whole fabrication. This was possibly because the conversion of protein drugs into solid particles may be an effective approach to endow protein molecules with immobility and resistance to organic solvent exposure, to storage stress, and other stress.30,38,39 The dextran nanoparticles also provided an environment of sugars that stabilized the protein molecule against degradation during further process and storage.40 Once G-CSF was microencapsulated into the dextran nanoparticles, these nanoparticles might have prevented the protein molecule directly from contacting organic solvent such as DCM and also endowing protein molecules with immobility and resistance to organic solvents.24 This suggested that the dextran nanoparticles were an effective protein stabilizer for preserving bioactivity of G-CSF in both hydrated and dry states.24
Figure 4.
Bioactivity from different samples during preparation processes (n = 5).
Notes: Samples: original G-CSF (G-CSF:dextran = 1.4:5.0 ± 0.3 mg) solution; G-CSF from dextran aqueous phase/polyethylene glycol aqueous phase emulation; G-CSF from dextran nanoparticles; G-CSF from prepared microspheres using the S/O/O/W method (PLGA 50/50 2A: 100.0 ± 0.5 mg PLGA, dextran nanoparticles [G-CSF:dextran, 1:4] = 10.0 ± 0.3 mg); G-CSF from control microspheres (prepared microspheres using the W/O/W method) (PLGA 50/50 2A: 100.0 ± 0.5 mg, G-CSF solution [G-CSF:dextran = 1:4] = 10.0 ± 0.3 mg). *P > 0.05; **P < 0.05.
Abbreviations: G-CSF, granulocyte colony–stimulating factor; PLGA, polylactic-co-glycolic acid; S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
In vitro release kinetics study
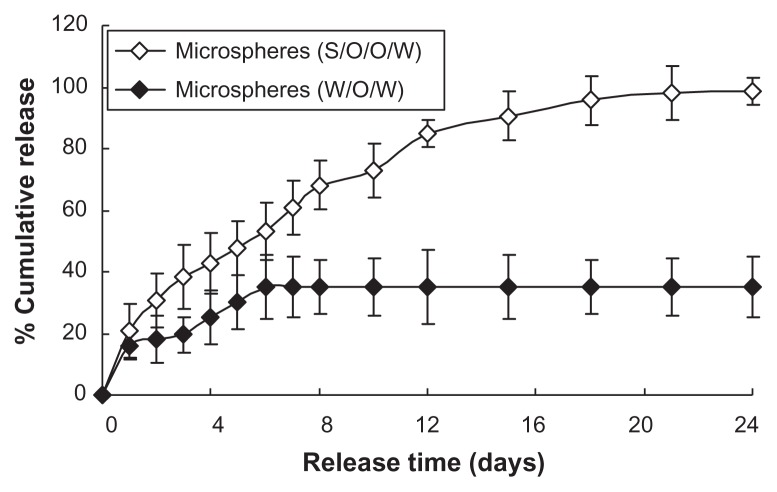
Figure 5 shows the G-CSF release profiles of the prepared microspheres using the S/O/O/W and W/O/W methods. At the beginning of release, the initial release of two microspheres showed a burst release and then slow release for a long time (Figure 5). The release profiles for prepared microspheres using S/O/O/W indicated a near zero–order release kinetics profile (release kinetics equations: QA = 5.621t + 16.664, R2 = 0.9338, from day 2 to day 14 and slow release up to day 24; Q represents the percentage of cumulative release). The control microspheres also showed a kinetic profile (release kinetics equations: QB = 5.0273t + 5.4639, R2 = 0.9146, from hour 24 to hour 144 and no further G-CSF release, after 6 days; Q represents the percentage of cumulative release). The cumulative release of the microspheres using S/O/O/W (the cumulative release percentage was above 90%) was much higher than that of the control microspheres (the cumulative release percentage was only 38%), with the release rate much higher. This was possibly because of many air–water and oil–water interfaces generated during the preparation, leading to loss of bioactivity, G-CSF aggregation, G-CSF absorption onto the interfaces, and no G-CSF release.24 Further reasons are suggested in the Discussion section.
Figure 5.
In vitro release profiles of microspheres (n = 5, P < 0.05).
Notes: ⋄: Prepared microspheres using the S/O/O/W method (PLGA 50/50 2A: 100.0 ± 0.5 mg PLGA, dextran nanoparticles (G-CSF:dextran, 1:4) = 10.0 ± 0.3 mg); ♦: control microspheres (prepared microspheres using W/O/W method) (PLGA 50/50 2A: 100.0 ± 0.5 mg, G-CSF solution [G-CSF:dextran = 1:4] = 10.0 ± 0.3 mg).
Abbreviations: G-CSF, granulocyte colony–stimulating factor; PLGA, polylactic-co-glycolic acid; S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
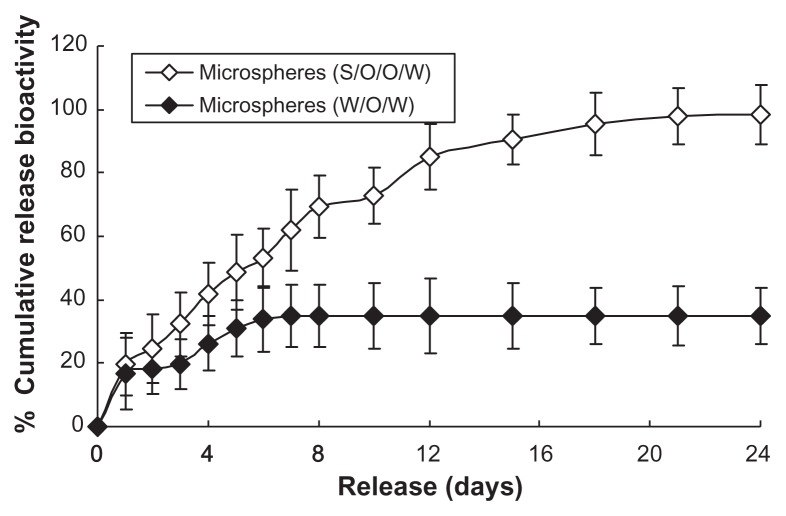
In vitro release bioactivity study
The release bioactivity of microspheres was studied using the proliferation of NFS-60 cells. The release bioactivity of the prepared microspheres using the S/O/O/W method indicated higher efficiency than that of W/O/W ones (Figure 6). The initial G-CSF release bioactivity amount was only 20% of the cumulative release amount. The cumulative release bioactivity percentage of the microspheres was above 90% of the bioactivity from the microspheres during the release period of 24 days. While the native G-CSF solution was microencapsulated into PLGA microspheres using the W/O/W method, the cumulative release bioactivity percentage was about 38%. This was possibly because of many air–water and oil–water interfaces generated in the preparation process, leading to G-CSF adsorption onto the interfaces, aggregation and more hydrophobicity.24 Further reasons are suggested in the Discussion section.
Figure 6.
In vitro release relative bioactivity of microspheres (n = 5, P < 0.05).
Notes: ⋄: Prepared microspheres using the S/O/O/W method (PLGA 50/50 2A: 100.0 ± 0.5 mg PLGA, dextran nanoparticles [G-CSF:dextran, 1:4] = 10.0 ± 0.3 mg); ♦: control microspheres (prepared microspheres using the W/O/W method) (PLGA 50/50 2A: 100.0 ± 0.5 mg, G-CSF solution [G-CSF:dextran = 1:4] = 10.0 ± 0.3 mg).
Abbreviations: G-CSF, granulocyte colony–stimulating factor; PLGA, polylactic-co- glycolic acid; S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
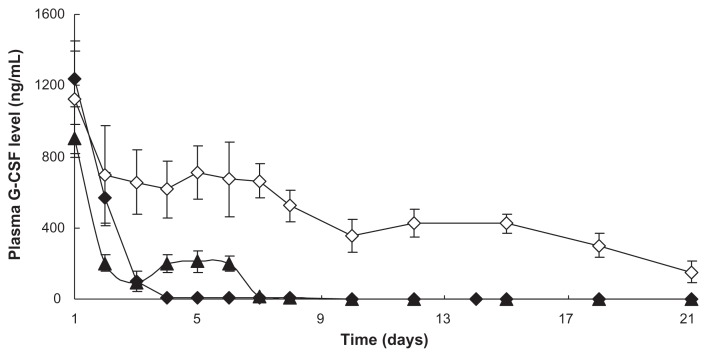
In vivo release study
In order to study in vivo release effects of micropsheres, we measured the G-CSF release of the prepared dextran nanoparticle–loaded microspheres using S/O/O/W and the control microspheres (the prepared microspheres using W/O/W) in vivo. The release profiles of plasma G-CSF concentration time for the prepared microspheres using W/O/W and the prepared microspheres using S/O/O/W are shown in Figure 7. The results indicated that the G-CSF in plasma concentration had a stable level from day 1 (small burst release) to 14, followed by slow G-CSF release up to day 24 of the prepared microspheres using S/O/O/W. However, the prepared microspheres using W/O/W did not indicate that G-CSF in plasma concentration was not at a stable level, and had quick G-CSF release from hour 24 to hour 144, and no release of G-CSF thereafter. It was confirmed that the prepared microspheres using S/O/O/W were more effective than the prepared microspheres using W/O/W.
Figure 7.
In vivo release plasma G-CSF level of PLGA microspheres (n = 5, P < 0.05).
Notes: ▲: Original G-CSF (G-CSF:dextran = 1.4:5.0 ± 0.3 mg) solution; ⋄: G-CSF from prepared microspheres using the S/O/O/W method (PLGA 50/50 2A: 100.0 ± 0.5 mg PLGA, dextran nanoparticles [G-CSF:dextran, 1:4] = 10.0 ± 0.3 mg); ♦: G-CSF from control microspheres (prepared microspheres using the W/O/W method) (PLGA 50/50 2A: 100.0 ± 0.5 mg, G-CSF solution [G-CSF:dextran = 1:4] = 10.0 ± 0.3 mg).
Abbreviations: G-CSF, granulocyte colony–stimulating factor; PLGA, polylactic-co-glycolic acid; S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
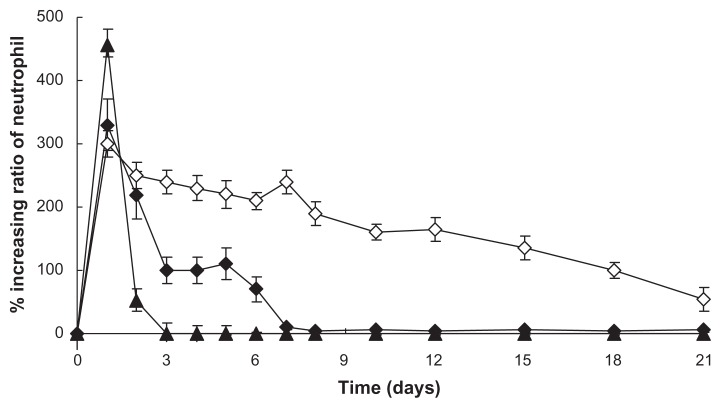
In vivo efficacy study of SD rats
To compare the in vivo efficacy of the prepared dextran nanoparticle–loaded microspheres using S/O/O/W with original G-CSF alone and the prepared control microspheres using W/O/W, SD rats were subcutaneously injected with the microspheres, the control microspheres, or original G-CSF at 0.15 μg/g. The percentages of neutrophil level increase was measured for 21 days. Figure 8 indicates that the microspheres, the control microspheres, and the original G-CSF solution obtained a great increase in neutrophil percentage for a prolonged time period, but the increases in neutrophil ratio and the prolonged period of the prepared microspheres using S/O/O/W were more than the prepared control microspheres using W/O/W. This suggested that S/O/O/W afforded an effective sustained-release delivery of G-CSF and enhanced neutrophil count therapy.
Figure 8.
Increasing ratio of in vivo neutrophil levels (n = 5, P < 0.05).
Notes: ▲: Original G-CSF (G-CSF:dextran = 1.4:5.0 ± 0.3 mg) solution; ⋄: G-CSF from prepared microspheres using the S/O/O/W method (PLGA 50/50 2A: 100.0 ± 0.5 mg PLGA, dextran nanoparticles [G-CSF:dextran, 1:4] = 10.0 ± 0.3 mg); ♦: G-CSF from control microspheres (prepared microspheres using the W/O/W method) (PLGA 50/50 2A: 100.0 ± 0.5 mg, G-CSF solution [G-CSF:dextran = 1:4] = 10.0 ± 0.3 mg).
Abbreviations: G-CSF, granulocyte colony–stimulating factor; PLGA, polylactic-co-glycolic acid; S/O/O/W, solid-in-oil-in-oil-in-water; W/O/W, water-in-oil-in-water.
Discussion
Generally speaking, when protein and peptide drugs were microencapsulated into PLGA microspheres, they often released in a multiphasic manner. At the beginning of release, an initial burst release of drug close to the surface of the microspheres was shown, and then a slower release period was observed principally with the rate of degradation of the PLGA. If the proteins denatured and aggregated during the preparation formulation and release process, the proteins in PLGA microspheres often resulted in adsorption on PLGA matrix and incomplete release.41 In order to address both these problems, some hydrophilic polymers (such as polysaccharide and PEG) and proteins were microencapsulated into PLGA microspheres, because the hydrophilic polymers could decrease adsorption of protein on PLGA matrix and incomplete release of proteins from the microspheres due to the increased PLGA matrix hydrophilicity.41 Although the control microspheres (the prepared microspheres using the W/O/W method) were mixed with hydrophilic protein stabilizers (dextran), the denatured and aggregated factors would still generate many air–water and oil–water interfaces during the preparation process.24 When the protein is loaded into dextran nanoparticles, the nanoparticles might avoid the protein molecule directly contacting with organic solvent such as DCM and also endowing protein molecules with immobility and resistance to organic solvents.24 Furthermore, the S/O/O/W method provided no oil–water and air–water surfaces before the solidifying and hardening of PLGA microspheres, because the second oil phase cannot dissolve proteins and dextran.34 These factors contributed to improve encapsulation efficiency, release rate, and bioactivity of G-CSF in PLGA microspheres. 34
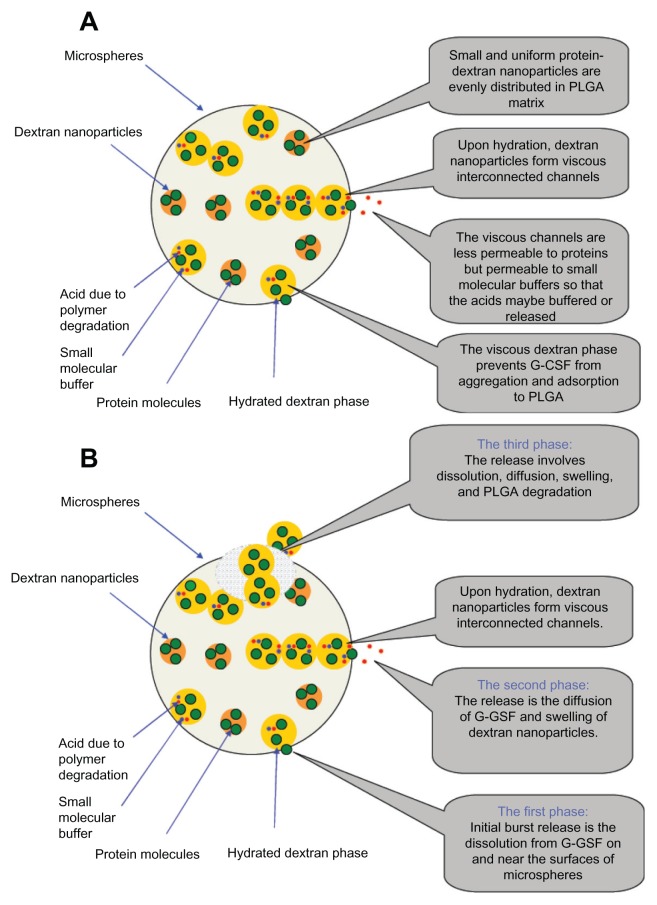
During release of PLGA microspheres, acid produced in the PLGA degradation process may have accumulated inside the PLGA microspheres, leading to G-CSF denaturation and aggregation.42 To improve the protein-release profile and decrease the concentration of acid inside the microspheres, preloading proteins into dextran microparticles or nanoparticles before the microencapsulation process might be a good method, because the dispersed dextran phase could be maintained in the microsphere matrix longer than protein retained in the matrix during the period of sustained release.43 This was probably because the dispersed dextran phase was kept in the PLGA matrix and could protect proteins for longer during the whole sustained-release period. Furthermore, when the dextran nanoparticle loadings increased, dextran nanoparticles started to touch each other and form a lot of diffusion channels in hydrated state.44 Dextran nanoparticles could also absorb water and swelled in volume during the course of release, so as to touch each other easily and produce more diffusion channels inside the PLGA microspheres. These diffusion channels made the acid produced by PLGA degradation diffuse out of microspheres and decreased the acid inside the microspheres. Fluorescent dextran was also microencapsulated in PLGA microspheres, and dextran release was confirmed to be slower than erythropoietin protein drug release by Bittner et al.45,46 This might make the protein molecular always in dextran solution which can protect the protein conformation. At the same time, PLGA started to degrade and the protein diffused distance to the outside of the microspheres also became shorter and shorter. These factors contributed to enhancement of the G-CSF release rate and improved bioactivity from the prepared microspheres using S/O/O/W (Figure 9).35,36
Figure 9.
(A and B) The released mechanism diagram from the PLGA microsphere. (A) Mechanism diagram stability of G-CSF; (B) Released mechanism diagram of G-CSF.
Abbreviations: G-CSF, granulocyte colony–stimulating factor; PLGA, polylactic-co-glycolic acid.
The ideal release kinetics, such as almost-initial burst release, incomplete release, and a release profile of near zero order, was easily obtained through dextran nanoparticle– loaded PLGA microspheres using S/O/O/W method also. Dextran nanoparticle–loaded PLGA microspheres could easily be applied to enhance neutrophilic levels during radiotherapy or chemotherapy for intermediate-stage cancer, terminal cancer, or blood cancer patients not able to undergo surgery to remove tumors who would have to rely on chemotherapy and radiotherapy.
Conclusion
Clinically available PLGA and dextran reagents were used in a microsphere formulation. We demonstrated that the prepared dextran nanoparticle–loaded PLGA microspheres using the S/O/O/W method could achieve sustained-release G-CSF delivery. This G-CSF release mechanism was through diffusion channels inside the microspheres to make the dextran nanoparticle hydrated and swell so as to protect protein in the PLGA microspheres from absorbing on the PLGA matrix. The method might provide a simple yet effective method for developing sustained-release G-CSF formulation. The microspheres have the potential to be applied to increasing neutrophilic counts in chemotherapy or radiotherapy for intermediate-stage cancer, terminal cancer, or blood cancer patients.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (nos 81170292 and 81100223) and the PhD Programs Foundation of the Ministry of Education of China (no 20090073120085) for financial support. We are also grateful for the generous support from the Instrumental Analysis Centre (IAC) of Shanghai Jiao Tong University.
Footnotes
Disclosure
The authors declare no competing financial interests in this work.
References
- 1.Kamachi S, Motojima H, Oheda M, et al. Properties of natural granulocyte colony-stimulating factor (G-CSF) Kiso to Rinsho Clin Rep. 1991;25:311–316. [Google Scholar]
- 2.Shochat E, Rom-Kedar V, Segel LA. G-CSF control of neutrophils dynamics in the blood. Bull Math Biol. 2007;69:2299–2338. doi: 10.1007/s11538-007-9221-1. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Estey E, O’Brien S, et al. Granulocyte colony-stimulating factor supportive treatment following intensive chemotherapy in acute lymphocytic leukemia in first remission. Cancer. 1993;72:2950–2955. doi: 10.1002/1097-0142(19931115)72:10<2950::aid-cncr2820721015>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Felici A, Carlini P, Ruggeri EM, et al. Bi-weekly chemotherapy with cisplatin, epirubicin, folinic acid and 5-fluororacil continuous infusion plus G-CSF in advanced gastric cancer: a multicentric phase II study. Cancer Chemother Pharmacol. 2006;57:59–64. doi: 10.1007/s00280-005-0032-5. [DOI] [PubMed] [Google Scholar]
- 5.Russell H, Shohe JM. Pediatric oncology: G-CSF counteracts chemotherapy toxicity in neuroblastoma. Nat Rev Clin Oncol. 2011;8:6–8. doi: 10.1038/nrclinonc.2010.195. [DOI] [PubMed] [Google Scholar]
- 6.Crea F, Giovannetti E, Zinzani PL, Danesi R. Pharmacologic rationale for early G-CSF prophylaxis in cancer patients and role of pharmacogenetics in treatment optimization. Crit Rev Oncol Hematol. 2009;72:21–44. doi: 10.1016/j.critrevonc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Machida M, Sano K, Arakawa M, Hayashi M, Awazu S. Absorption of recombinant human granulocyte colony-stimulating factor (rhG-CSF) from rat nasal mucosa. Pharm Res. 1993;10:1372–1377. doi: 10.1023/a:1018990318090. [DOI] [PubMed] [Google Scholar]
- 8.Nomura H, Akamisaka S, Maruyama K. Effects of a dosing solution on the nasal absorption of non-glycosylated recombinant human granulocyte colony-stimulating factor in rats. Biol Pharm Bull. 1996;19:1490–1493. doi: 10.1248/bpb.19.1490. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Kikuchi R, Kiriyama M, et al. Increase in total blood leukocyte count following intranasal administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) in rabbits with cyclophosphamide-induced leucopenia. Biol Pharm Bull. 1995;18:1084–1088. doi: 10.1248/bpb.18.1084. [DOI] [PubMed] [Google Scholar]
- 10.Machida M, Hayashi M, Awazu S. The effects of absorption enhancers on the pulmonary absorption of recombinant human granulocyte colony-stimulating factor (rhG-CSF) in rats. Biol Pharm Bull. 2000;23:84–86. doi: 10.1248/bpb.23.84. [DOI] [PubMed] [Google Scholar]
- 11.Machida M, Hayashi M, Awazu S. Pulmonary absorption of recombinant human granulocyte colony-stimulating factor (rhG-CSF) after intratracheal administration to rats. Biol Pharm Bull. 1996;19:259–262. doi: 10.1248/bpb.19.259. [DOI] [PubMed] [Google Scholar]
- 12.Niven RW, Whitcomb KL, Shaner L, Ip AY, Kinstler OB. The pulmonary absorption of aerosolized intratracheally instilled rhG-CSF and monoPEGylated rhG-CSF. Pharm Res. 1995;12:1343–1349. doi: 10.1023/a:1016281925554. [DOI] [PubMed] [Google Scholar]
- 13.Eiamtrakarn S, Itoh Y, Kishimoto J, et al. Gastrointestinal mucoadhesive patch system (GI-MAPS) for oral administration of G-CSF, a model protein. Biomaterials. 2002;23:145–152. doi: 10.1016/s0142-9612(01)00089-8. [DOI] [PubMed] [Google Scholar]
- 14.Takaya T, Ikeda C, Imagawa N, Niwa K, Takada K. Development of a colon delivery capsule and the pharmacological activity of recombinant human granulocyte colony stimulating factor (rhG-CSF) in beagle dogs. J Pharm Pharmacol. 1995;47:474–478. doi: 10.1111/j.2042-7158.1995.tb05834.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen-Pippo KE, Whitcomb KL, DePrince RB, Ralph L, Habberfield AD. Enteral bioavailability of human granulocyte colony-stimulating factor conjugated with poly(ethyleneglycol) Pharm Res. 1996;13:102–107. doi: 10.1023/a:1016089503186. [DOI] [PubMed] [Google Scholar]
- 16.Shen WC. Oral peptide and protein delivery: unfulfilled promises? Drug Discov Today. 2003;8:607–608. doi: 10.1016/s1359-6446(03)02692-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ. Protein drug oral delivery: the recent progress. Arch Pharm Res. 2002;25:572–584. doi: 10.1007/BF02976925. [DOI] [PubMed] [Google Scholar]
- 18.Choi SH, Lee H, Park TG. PEGylation of G-CSF using cleavable oligolactic acid linkage. J Control Release. 2003;89:271–284. doi: 10.1016/s0168-3659(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 19.Halpern W, Riccobene TA, Agostini H, et al. Albugranin, a recombinant human granulocyte colony stimulating factor (G-CSF) genetically fused to recombinant human albumin induces prolonged myelopoietic effects in mice and monkeys. Pharm Res. 2002;19:1720–1729. doi: 10.1023/a:1020917732218. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Satake-Ishikawa R, Ishikawa M, Matsuki S, Asano K. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor conjugated to polyethylene glycol in rats. Cancer Res. 1991;51:3710–3714. [PubMed] [Google Scholar]
- 21.Choi SH, Park TG. G-CSF loaded biodegradable PLGA nanoparticles prepared by a single oil-in-water emulsion method. Int J Pharm. 2006;311:223–228. doi: 10.1016/j.ijpharm.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Maeda H, Nakagawa T, Adachi N, et al. Design of long-acting formulation of protein drugs with a double-layer structure and its application to rhG-CSF. J Control Release. 2003;91:281–297. doi: 10.1016/s0168-3659(03)00247-5. [DOI] [PubMed] [Google Scholar]
- 23.Gibaud S, Rousseau C, Weingarten C, et al. Polyalkylcyanoacrylate nanoparticles as carriers for granulocyte-colony stimulating factor (G-CSF) J Control Release. 1998;52:131–139. doi: 10.1016/s0168-3659(97)00194-6. [DOI] [PubMed] [Google Scholar]
- 24.Jin T, Zhu J, Wu F, Yuan W, Geng LL, Zhu H. Preparing polymer-based sustained-release systems without exposing proteins to water-oil or water-air interfaces and cross-linking reagents. J Control Release. 2008;128:50–59. doi: 10.1016/j.jconrel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Yuan W, Geng Y, Wu F, et al. Preparation of polysaccharide glassy nanoparticles with stabilization of proteins. Int J Pharm. 2009;366:154–159. doi: 10.1016/j.ijpharm.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Yuan W, Wu F, Guo M, Jin T. Development of protein delivery microsphere system by a novel s/o/o/w multi-emulsion. Eur J Pharm Sci. 2009;36:212–218. doi: 10.1016/j.ejps.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Geng Y, Yuan W, Wu F, Chen J, He M, Jin T. Formulating erythropoietin- loaded sustained-release PLGA microspheres without protein aggregation. J Control Release. 2008;130:259–265. doi: 10.1016/j.jconrel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Shirafuji N, Asano S, Matsuda S, Watari K, Takaku F, Nagata S. A new bioassay for human granulocyte colony stimulating factor (G-CSF) using murine myeloblastic NFS-60 cells as targets and estimation of its level in sera from normal healthy persons and patients with infections and hematological disorders. Exp Hematol. 1989;17:116–119. [PubMed] [Google Scholar]
- 29.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;21:77–89. [PubMed] [Google Scholar]
- 30.Cleland JL, Jones JS. Stable formulations of recombinant human growth hormone and interferon-gamma for microencapsulation in biodegradable microspheres. Pharm Res. 1996;13:1464–1475. doi: 10.1023/a:1016063109373. [DOI] [PubMed] [Google Scholar]
- 31.Yuan W, Liu Z. Surgical wound healing using hemostatic gauze scaffold loaded with nanoparticles containing sustained-release granulocyte colony-stimulating factor. Int J Nanomedicine. 2011;6:3139–3149. doi: 10.2147/IJN.S26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwendeman SP. Recent advances in the stabilization of protein encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 33.Ricci MS, Sarkar CA, Fallon EM, Lauffenburger DA, Brems DN. pH dependence of structural stability of interleukin-2 and granulocyte colony-stimulating factor. Protein Sci. 2003;12:1030–1038. doi: 10.1110/ps.0230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan W, Zhang Y, Wu F, et al. Preparation of protein-loaded sustained- release microspheres via ‘solid-in-oil-in-hydrophilic oil-in-ethanol (S/O/hO/E)’ emulsification. Colloids Surf B Biointerfaces. 2010;79:326–333. doi: 10.1016/j.colsurfb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Kim TH, Park TG. Critical effect of freezing/freeze-drying on sustained release of FITC-dextran encapsulated within PLGA microspheres. Int J Pharm. 2004;271:207–214. doi: 10.1016/j.ijpharm.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Yuan W, Liu Z. Controlled-release and preserved bioactivity of proteins from (self-assembled) core-shell double-walled microspheres. Int J Nanomedicine. 2012;7:257–270. doi: 10.2147/IJN.S27621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Morita T, Horikiri Y, Yamahara H, Suzuki T, Yoshino H. Formation and isolation of spherical fine protein microparticles through lyophilization of protein-poly(ethylene glycol) aqueous mixture. Pharm Res. 2000;17:1367–1373. doi: 10.1023/a:1007526301331. [DOI] [PubMed] [Google Scholar]
- 39.Johnson OL, Jaworowicz W, Cleland JL, et al. The stabilization and encapsulation of human growth hormone into biodegradable microspheres. Pharm Res. 1997;14:730–735. doi: 10.1023/a:1012142204132. [DOI] [PubMed] [Google Scholar]
- 40.Wang B, Tchessalov S, Warne NW, Pikal MJ. Impact of sucrose level on storage stability of proteins in freeze-dried solids: I. Correlation of protein-sugar interaction with native structure preservation. J Pharm Sci. 2009;98:3131–3144. doi: 10.1002/jps.21621. [DOI] [PubMed] [Google Scholar]
- 41.Jagota SK, Ramano MV, Dutta RSM. Beta-galactosidase of Streptococcus cremoris H. J Food Sci. 1981;46:161–168. [Google Scholar]
- 42.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly(lactide-co-glycolide) Nat Biotechnol. 2000;18:52–55. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 43.Leelarasamee N, Howard SA, Malanga CJ, et al. Kinetics of drug release from polylactic acid-hydrocortisone microspheres. J Microencapsul. 1986;3:171–179. doi: 10.3109/02652048609031571. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W, Swenderman SP. Stabilization and controlled release of bovine serum albumin encapsulated in poly(D,L-lactide) and poly(ethylene glycol) microsphere blends. Pharm Res. 2001;18:878–885. doi: 10.1023/a:1011009117586. [DOI] [PubMed] [Google Scholar]
- 45.Bittner B, Morlock M, Koll H, Winter G, Kissel T. Recombinant human erythropoietin (rhEPO) loaded poly(lactide-co-glycolide) microspheres: influence of the encapsulation technique and polymer purity on microsphere characteristics. Eur J Pharm Biopharm. 1998;45:295–305. doi: 10.1016/s0939-6411(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 46.Xu D, Hu A, Su J, Wu F, Yuan W. Micro and Nanotechnology for Intracellular Delivery Therapy Protein. Nano-Micro Lett. 2012;4(2):118–123. [Google Scholar]