Abstract
Purpose.
The purpose of our study was to test the prediction that if the tear film thins due to evaporation, rather than tangential flow, a high concentration of fluorescein in the tear film would show a greater reduction in fluorescent intensity compared to a low concentration of fluorescein due to self-quenching at high concentrations.
Methods.
Tear film thickness, thinning rate, and fluorescent intensity were measured continuously and simultaneously with a modified spectral interferometer in 30 healthy subjects with two different concentrations (2% followed by 10%) of 1 μL of liquid fluorescein on the eye. Measurements of fluorescein self-quenching (fluorescent efficiency as a function of fluorescein concentration) are described in an Appendix and are reported in arbitrary units.
Results.
Under low and high fluorescein concentration conditions, there were no differences in tear film thickness (P = 0.09) or thinning rates (P = 0.76). While the mean initial fluorescent intensity was similar between groups (637.47 ± 381.47 vs. 672.09 ± 649.72, P = 0.55), the mean rate of fluorescent decay was 4-fold faster in the high (16.57 ± 29.34) than in the low (4.11 ± 6.78) concentration group (P < 0.01).
Conclusions.
The large difference in the rate of fluorescent decay between groups can be explained by the effects of evaporation and self quenching of fluorescein; the latter is expected to be greater for high than for low fluorescein concentration. Fluorescence decay due to tangential flow would be expected to be similar at high and low fluorescein concentrations. This supports previous evidence that evaporation has the primary role in normal tear thinning between blinks.
Understanding the mechanisms of tear film thinning is critical as it will yield basic insights into future understandings of dry eye disease states. This work has shown via exploitation of fluorescent quenching, that tear film thinning is due largely to evaporation.
Introduction
The tear film is in a constant state of flux, thinning when the eye is open and restructuring itself with every blink.1 Changes in the distribution or flow of the tear film, either from evaporation or from low tear production, can be associated with dry eye disease.2–6 Dry eye disease is multifactorial and has the potential to lead to damage of the ocular surface via tear film instability, hyperosmolarity, and inflammation.7 Dry eye disease has two primary classifications.8–11 Evaporative dry eye (EDE) is due to excessive tear film loss from the ocular surface in the presence of normal lacrimal fluid production and can involve etiologies, like blepharitis, including meibomian gland dysfunction, contact lens wear, and congenital meibomian gland loss, among other disorders. Aqueous-deficient dry eye (ADDE) is due to inadequate lacrimal production (e.g., Sjögren's syndrome).12 Tear evaporation is thought to have a significant role in EDE and ADDE.2–6
While it commonly is thought that tear stability is important in dry eye disease, relatively few studies have addressed the core mechanisms associated with tear film structure instability. While tear film instability is attributed most often to evaporation in pathologic circumstances, information is lacking as to the contribution evaporation makes to tear film thinning under normal conditions. Some studies have concluded that evaporation has only a minor role if the superficial lipid layer remains intact.13,14 Other studies suggest evaporation is not quick enough to make a significant contribution to tear film thinning rates under normal conditions.15,16 A review by Mathers in 2004 summarized the findings of 18 studies of the pre-corneal tear film evaporation rate and concluded that the mean reported evaporation rate was 0.75 μL per minute.1,17 King-Smith et al. found that these averaged values could account for only 20 to 25 percent of the thinning rates they observed using spectral interferometry in free-air conditions.6 This discrepancy may be explained methodologically, that is evaporation measurements have been performed most typically using closed-air chambers covering the ocular surface, as opposed to open-air systems. Experimental measurements of tear film evaporation rates using ventilated chambers have been relatively similar to tear film thinning rates obtained using spectral interferometry, and generally report tear film evaporation at approximately 2.36 μL/min.18
Tear break-up time (TBUT) with fluorescein is one of the most common clinical tests used to evaluate tear film stability.19,20 However, interpretation of the meaning of the test is controversial. Sodium fluorescein is a polar molecule at physiologic pH and has an octanol/aqueous partition ratio near 0.6, implying that it is reasonably soluble in the aqueous and in the lipid phase of the tear film; it also can penetrate cell membranes.21,22 When using fluorescein to perform a TBUT test, a small quantity is applied to the ocular surface (usually using a wetted dye impregnated strip), and the subject is asked to blink and hold their eyes open, and the examiner records the time to tear film breakup. Tear film breakup generally is accepted as the moment when the first black spot appears, which might occur when the tear thickness is reduced to zero and the superficial lipid layer comes into contact with the glycocalyx.6 A TBUT value of 6 to 10 seconds generally is considered the cut-off to identify dry eye from normal patients, although a battery of tests usually is recommended for the final dry eye diagnosis.11,23,24
A notable characteristic of fluorescein is its “self-quenching” property. If the dye concentration is above a critical concentration, there is a reduction in fluorescent efficiency (see Appendix). Webber and Jones measured fluorescent intensity from a thin film of fluorescein as a function of its concentration.25 They did not measure self-quenching directly because their results also were affected by the variation of absorbance (fraction of incident light absorbed) as a function of concentration. Therefore a more direct measurement of self-screening was performed and is described in the Appendix. Fluorescent efficiency is independent of fluorescein concentration at low concentrations, but at high concentrations efficiency falls as the inverse square of the concentration (Fig. A1). The boundary between these two laws (independence of concentration and inverse square law) is the “critical concentration” of approximately 0.2%. This is important because aspects of self-quenching may relate to the interpretation of the tear breakup time test. For example, tear film breakup, or the appearance of a dark spot in the film or low levels of fluorescence, may be attributed to either a reduction in tear film thickness with an absence of fluorescein itself or to excessively high concentrations of fluorescein with a reduction in fluorescence itself due to quenching.
Inter-blink tear film thinning could result from the following three “local” factors: Ocular surface absorption, evaporation, or tangential flow.26 Other “distant” factors, such as tear drainage through the canaliculi, also can affect tear film thinning, but they do so by altering these local factors. Thus, tear thinning at any position is determined completely by these local factors. The contribution of ocular surface absorption to tear film thinning likely is insignificant as there is no measurable thickening of the corneal epithelium as the tear film thins.6,26 Furthermore, any evaporation, causing a hyperosmotic tear film, would be expected to create an osmotic gradient that would cause osmotic flow out of the corneal epithelium, reducing its thickness. Therefore, absorption can be considered as a minor contributor to tear film thinning.
Besides ocular surface absorption, which most would agree does not seem plausible as a contributor to inter-blink tear film thinning, the other two potential primary mechanisms that need further study are tangential flow and evaporation. Figure 1 illustrates differences in these processes. Tangential flow results from surface tension gradients and is parallel flow of the tear film along the ocular surface. Surface tension forces reportedly are greater in dry eye patients than in normal cases.27 These tension gradients can create localized tear thinning with a corresponding tear thickening occurring elsewhere; tangential flow affects tear distribution but not total tear volume. Tangential flow has been associated with tear film break-up in certain circumstances, including meniscus-induced thinning, in the presence of corneal elevations, in small areas of thickened lipid layer, during partial blinks, and immediately following complete blinks.28–31 However, tangential flow apparently is too slow to explain the observed thinning rate of the tear film as a whole.32
Figure 1. .
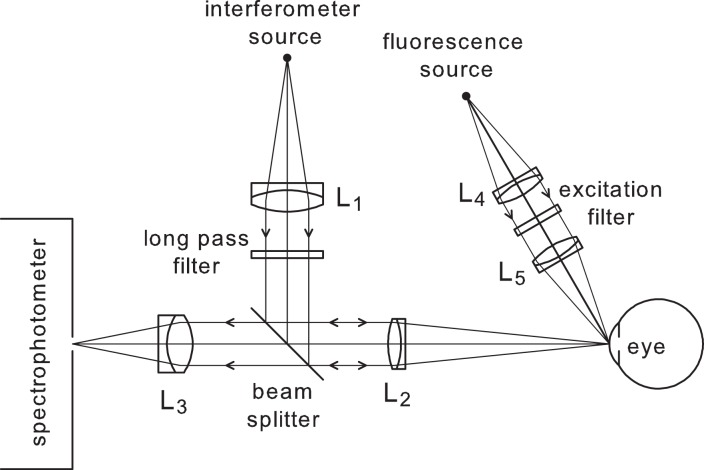
Optical system for combined fluorescence and tear film thickness measurements. Tungsten filament sources are used. The long pass filter of the spectral interferometer passes wavelengths above 630 nm, whereas the excitation filter for fluorescence passes wavelengths from 455 to 495 nm. The spectrophotometer covers the wavelength range 450 to 1050 nm and so records spectral oscillations (for tear thickness) at longer wavelengths, fluorescence in a middle spectral range (approximately 500–600 nm) and excitation light scattered from the cornea at shorter wavelengths (455–495 nm). Details of the eye alignment system are given by Nichols et al.26
Evaporation is the outward flow of aqueous across the entire corneal surface into the surrounding air.6 Unlike tangential flow, evaporation results in a decrease of aqueous volume (Fig. 1) and increases the osmolarity of the tear film.33,34 The lipid layer is important in reducing evaporation rates as its removal results in a significant increase in tear film evaporation rates.35–37 Patients with dry eye and meibomian gland dysfunction have increased rates of evaporation due to alterations in lipid layer thickness and composition.2,5,14,38–42
It is hypothesized that evaporation and tangential flow are the most plausible mechanisms of tear film thinning under normal conditions. The relative contribution of these two possible mechanisms can be studied by taking advantage of the “self-quenching” property of fluorescein. The use of high and low concentrations of sodium fluorescein, as well as simultaneous measurement of tear thickness and fluorescent intensity, allows for an investigation into the mechanisms associated with inter-blink tear film thinning. If tear film thinning is due to evaporation, the aqueous loss and subsequent increase in fluorescein concentration will cause fluorescent decay as a result of quenching during tear film thinning, which will be greater for the high concentration condition. If tear film breakup is due to tangential flow, fluorescein concentration would remain relatively stable during tear film thinning due to a redistribution of the tear film, and fluorescence decay would be similar for low and high concentration conditions. The purpose of this work is to test these predictions.
Methods
This research protocol was approved by an Institutional Review Board in accordance with the Declaration of Helsinki. An informed consent was obtained from each subject at study enrollment. Participants were required to be noncontact lens wearers of at least 18 years of age. Subjects who currently were pregnant or breastfeeding, as determined through self-report, were excluded from participation. Each subject participated in one study visit lasting approximately 30 minutes. Subjects were asked to complete a demographic form and the Ocular Surface Disease Index (OSDI; Allergan Inc., Irvine, CA) to verify subjective dry eye status for subjects.43 All OSDI scores were computed using the reported OSDI scoring guidelines. All subjects were essentially otherwise healthy individuals. No other testing was conducted to characterize the participants. Subjects attended their study visit at any time during the day, and no controls for diurnal variations in tear parameters were implemented.
The optical system used for simultaneous spectral interferometry and fluorescence recording is shown in Figure 2. This was used to measure simultaneously and continuously the tear film thickness, tear film thinning rate, and fluorescent intensity (with the addition of liquid fluorescein to the ocular surface). A point source located behind a filter emits light above 630 nm, which then passes through an aperture stop, which is focused on the front of the central cornea at normal incidence while the subject fixated a cross-hair target. Light is reflected from the various interfaces associated with the ocular surface, passes back through the optical system, and is captured by a spectrophotometer.
Figure 2. .
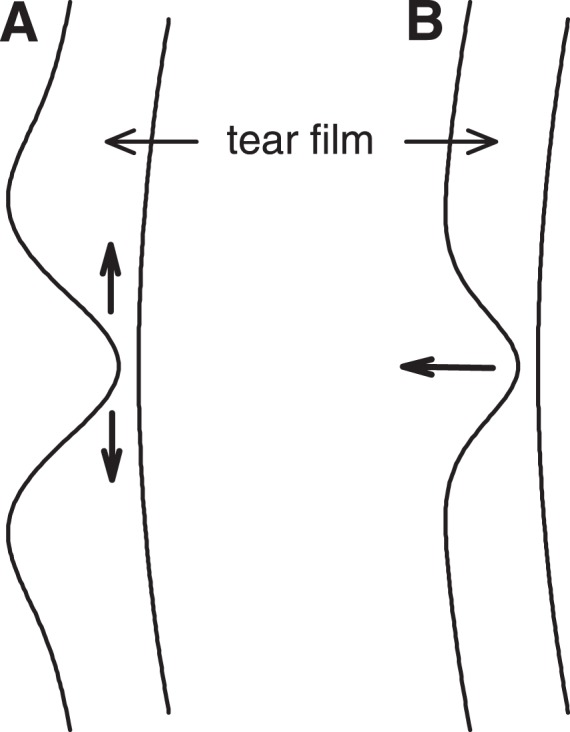
Schematic illustrating the impact of (A) tangential flow and (B) evaporation on the distribution of the tear film. Note that the tangential flow (A) has caused a thicker rim surrounding the thin region so that the total tear volume is unchanged; evaporation (B) reduces the total tear volume.
The light that is captured in the spectrometer is the result of reflections from a number of different surfaces, including the air-tear interface and the tear-cornea interface. The intensity of reflected light is dependent on the interference between these two reflections, which depends on the wavelength of light. This interference pattern at different wavelengths is termed “spectral oscillations.” The thickness of the tear film is proportional to the frequency of these oscillations, and tear film thinning rate can be determined if the measurement is captured continuously over a period of time. For this application, the optical system was modified such that a cobalt blue light was projected simultaneously obliquely onto the cornea. The light emitted from the fluorescein was determined by measuring the intensity of light in the range 500 to 600 nm with the spectrograph. The measurement area for fluorescence was 768 × 25 μm, while the measurement area for the interferometry measures was 33 × 25 μm.
Two different concentrations of non-preserved liquid fluorescein were used in our study: a low concentration composed of 2% fluorescein and a high concentration composed of 10% fluorescein (Leiter's Pharmacy, San Jose, CA). A total of four spectral interferometry measurements was obtained from the right eye of each subject. Two recordings separated by two minutes using one dose of 1 μL of 2% fluorescein were captured first followed by two recordings separated by two minutes using one dose of 1 μL of 10% fluorescein. If one assumes a 7 μL tear volume, the initial tear film concentration after instillation of the 2% fluorescein solution should be approximately 2.5 mg/ml. A micropipette with a special nonstick tip was used to apply the fluorescein to the ocular surface. After application, the subject was instructed to close his or her eye and roll it in a clockwise fashion to ensure that the fluorescein had been distributed across the ocular surface. Approximately one second after the start of the 20-second recording, the subject was instructed to blink, and then hold the eye open and steady for the remaining 19 seconds. After a rest period of two minutes, a second recording was obtained; however, no fluorescein was added to the eye. Following the low concentration experimental recordings, 1 μL of 10% fluorescein was applied and the procedure described above was repeated.
Data from each reflection spectrum were analyzed starting two seconds after the subject blinked. This was to avoid the transient 2-second upward drift of the lipid and aqueous layers immediately following the blink.32 A previously described computer program was created to translate the raw data supplied by the spectrometer into the tear film and fluorescent outcome measures.26
The statistical analyses were performed using Microsoft Excel (Microsoft Corp., Seattle, WA) and SPSS V16 software (IBM, Chicago, IL). Prior studies have shown that tear film thickness and thinning rates do not follow a normal distribution, so nonparametric analyses were conducted using the Wilcoxon Sign Rank test.26 For the analyses, the second trial of the low concentration (2%) fluorescein was compared to the first trial of the high concentration (10%) fluorescein. The analysis was conducted in this fashion to compare the fluorescein decay rate to the tear film decay rate between the least concentrated and most concentrated trials.
Results
A total of 30 individuals participated in the study, and data were analyzed from all 30 subjects. The mean (± SD) age of this sample was 34.3 ± 13.1 years, and 57% were female. The mean OSDI score was 9.86 ± 9.09. Five of the 30 subjects scored over 22 on the OSDI, indicating possible dry eye. All subjects were included in the final data analyses as their exclusion did not produce statistically different results. The mean temperature within the room was 22.1°Celsius, and the mean humidity was 48%.
Results showed that both conditions had similar initial tear film thicknesses (P = 0.09) and thinning rates (P = 0.76), despite differences in the concentration of fluorescein (Table 1).
Table 1. .
Mean Tear Film Thickness and Thinning Rates
|
|
2% Fluorescein |
10% Fluorescein |
P Value |
| Mean initial thickness (μm) | 3.82 ± 0.86 | 4.40 ± 1.68 | 0.09 |
| Mean thinning rate (μm/s) | 0.05 ± 0.08 | 0.07 ± 0.13 | 0.91 |
| Mean thinning rate (μm/min) | 3.09 ± 4.69 | 4.34 ± 7.82 | 0.91 |
| Mean percent thinning rate (%/s) | 1.58 ± 2.54 | 1.83 ± 3.04 | 0.76 |
As shown in Table 2, the mean initial fluorescent intensity was similar between groups (P = 0.55). However, the mean rate of fluorescent decay was 4-fold faster in the high concentration group compared to the low concentration group (P = 0.003, Table 2).
Table 2. .
Mean Fluorescein Intensity and Decay Rates (Arbitrary Fluorescent Units)
|
|
2% Fluorescein |
10% Fluorescein |
P Value |
| Mean initial fluorescein intensity | 637.47 ± 381.47 | 672.09 ± 649.72 | 0.55 |
| Mean fluorescein decay rate per s | 4.11 ± 6.78 | 16.57 ± 29.34 | 0.003 |
| Mean % fluorescein decay rate per s | 0.50 ± 1.46 | 3.09 ± 3.55 | 0.0005 |
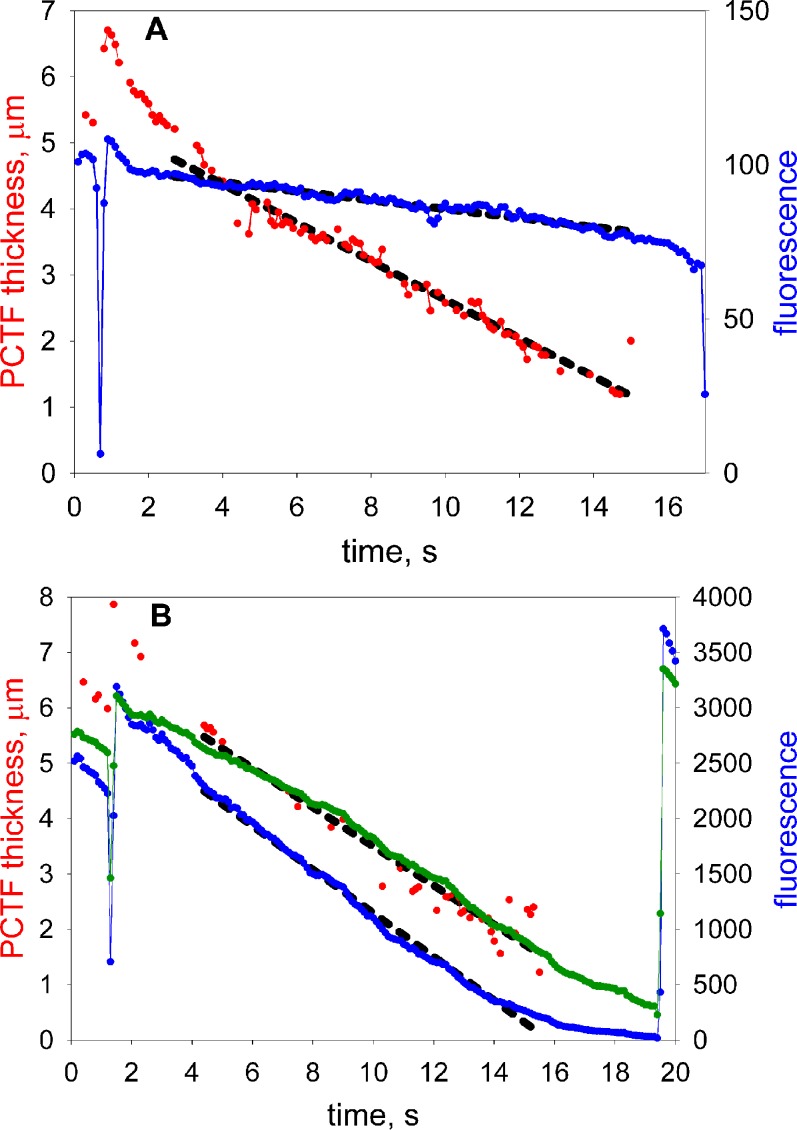
Figure 3 shows a representative result illustrating fluorescent decay and tear thinning rates from a subject in low and high concentration conditions. Regression lines are fit for data starting 2 seconds after the blink. Note that the rates of tear thinning are similar for both concentrations of fluorescein. However, the rate of fluorescein decay is more rapid at higher concentrations of fluorescein.
Figure 3. .
Tear film thinning and fluorescence measurements for one subject with (A) low and (B) high fluorescein concentrations. Red: tear thickness. Blue: fluorescent intensity. Black dashed lines: regression lines fitted to these data, starting 2 seconds after a blink. The timing of blinks is indicated by dips in the fluorescence response. The green curve in (B) is proportional to the square root of fluorescence (see text for discussion).
According to the analysis of self-quenching in the Appendix, at high fluorescein concentrations, fluorescent efficiency (and hence fluorescent intensity) should be inversely proportional to the square of fluorescein concentration. If tear thinning is due to evaporation, fluorescein concentration is expected to vary inversely with tear thickness. Combining these observations, fluorescent intensity would be expected to be proportional to the square of tear thickness and, conversely, tear thickness would be expected to be proportional to the square root of fluorescent intensity. In correspondence with this prediction, the green curve in Figure 3B (high fluorescein concentration condition) is proportional to the square root of fluorescent intensity and is seen to be a reasonable fit to the tear thinning data (ignoring the first two seconds after the blink when upward tangential flow contributes to tear film thickness and fluorescence changes). It may be noted that this may allow estimation of the thickness of thinner tear films, down to approximately 0.6 μm in Figure 3B, than is possible with spectral interferometry (limit approximately 1 μm for the precorneal tear film).
Discussion
By using simultaneous measures of tear thickness and fluorescent intensity, our study provided evidence that the primary mechanism of normal, inter-blink tear film thinning is evaporation. The two concentrations of fluorescein used in this experiment resulted in similar tear thicknesses and rates of tear thinning, suggesting that the administered volume of fluorescein did not fundamentally alter these parameters. However, the rate of fluorescent decay was much faster in subjects who received the high concentration of fluorescein on the eye, as opposed to when they received the low concentration on the eye. This difference in fluorescent decay under the two concentration conditions is explained by proposing that evaporation causes increasing fluorescein concentration which, in turn, causes considerably more quenching in the high concentration compared to the low concentration condition. This supports the suggestion that evaporation is the primary cause of tear film thinning between blinks.
The rate of tear thinning found in the presence of two fluorescein concentrations in our study (3.1 μm/min low, 4.3 μm/min high) is similar to previous reports that measured tear thinning rates in normal tears using spectral interferometry of 3.8 μm/min.26
A theoretical concern of our study's design is that it did not directly measure evaporation from the ocular surface. The methodology used here measured the reduction in tear film thickness over time, or tear film thinning. Therefore, to determine the primary mechanisms of tear film thinning, a series of studies (including this one) have been conducted to help provide insight into the core mechanisms of tear film thinning. As discussed before, we have shown that tear film thinning is not caused by absorption into the ocular surface.26 Likewise, we also have shown that tangential flow of the lipid layer occurs for the first 2 to 3 seconds following the blink, which also follows an exponential decay; conversely, tear film thinning, as is measured here and in other studies, is a very linear process.26 Thus, tangential flow might not be considered a primary mechanism causing tear film thinning.
We have shown evidence here, in addition to one other study, that evaporation appears to be the primary mechanism associated with tear film thinning. In a prior study, subjects wore air-tight goggles, raising the humidity inside the goggle to nearly 100%, which was followed by measurement of tear film thinning.44 The results showed that a substantial (over 95%) reduction in tear film thinning occurred, which would be predicted if evaporation were the primary mechanisms of tear film thinning. In the present study, we used indirect evidence associated with varying concentrations of fluorescein, quenching and predictions associated with fluorescent intensity during tear film thinning to provide further evidence that evaporation is the primary mechanism of tear film thinning. During evaporation, the aqueous phase of the tear film is lost to the atmosphere. The evidence of our present study shows quenching under a high concentration of fluorescein in the tear film (due to loss of aqueous from evaporation), which supports aqueous evaporation as the primary mechanism of tear thinning. This finding also challenges the traditional interpretation of fluorescein tear breakup time. The formation of black spots on the cornea may not be from an absence of the fluorescein due to a thin film, indicating an exposed corneal surface. Rather, it may be the result of fluorescent quenching indicating a highly concentrated area of fluorescein in a thin tear film layer, that is the lack of fluorescence does not indicate that the amount of fluorescein (per unit area) is reduced.
Appendix. Fluorescence Self Quenching of Fluorescein
To understand the dimming of fluorescence after a blink and its contribution to tear film breakup, it is important to understand the effect of fluorescein concentration, c, on fluorescent intensity, I(c). Fluorescence intensity from a film is proportional to two factors: (1) the illuminant absorptance (fraction of the incident light absorbed, thus causing excitation of fluorescein molecules), A(c), and (2) the fluorescent efficiency (fraction of excited fluorescein molecules that emit a photon of fluorescent light), E(c). Thus
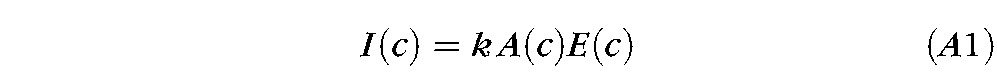 |
where k is a constant that is independent of concentration. While absorptance, A(c), increases to a maximum of unity at high concentrations, efficiency, E(c), falls to low levels at high concentration due to the phenomenon of self quenching.45
For monochromatic illumination, absorptance is given by Beer's Law
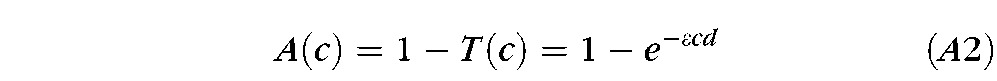 |
where T(c) is transmittance, ε is the molar extinction coefficient, c is molar concentration of fluorescein and d is film thickness.45 Combining Equations A1 and A2 gives
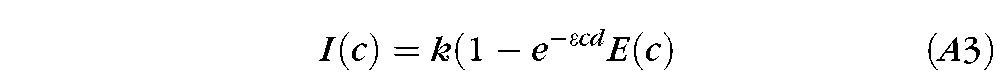 |
(For a broad band illumination source, this equation should be integrated over wavelength, including the effects of energy distribution of the source, wavelength variation in extinction coefficient and spectral sensitivity of the detector.)
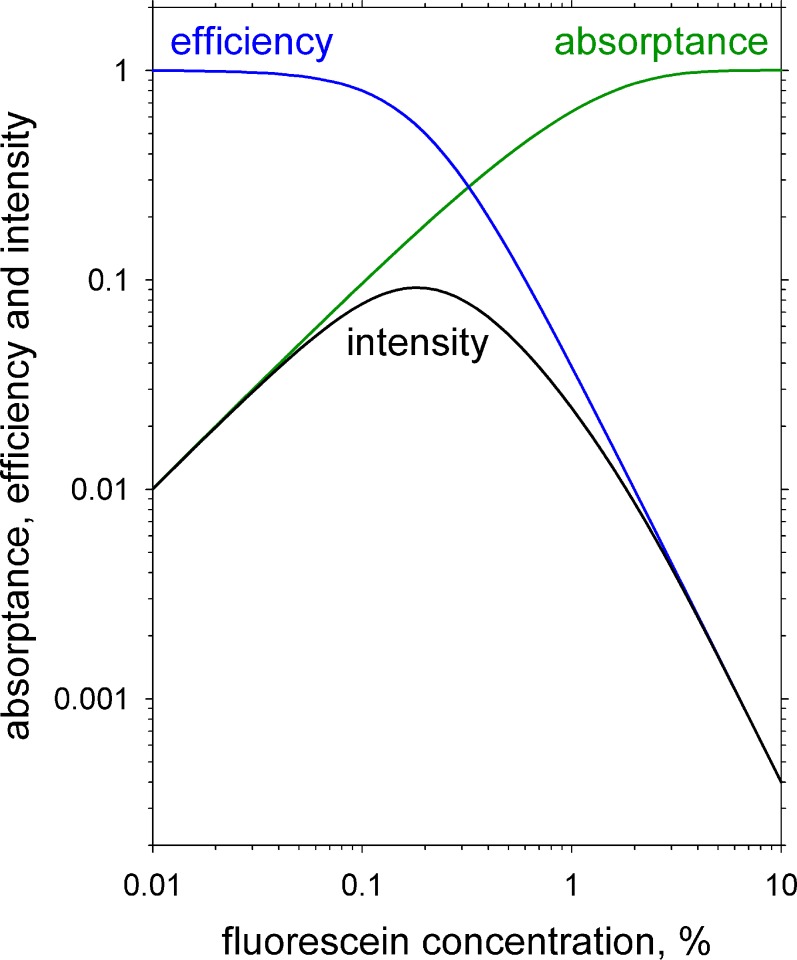
A log-log plot of calculated absorptance, efficiency and intensity of fluorescence as a function of fluorescein concentration is shown in Figure A2 for a 5 μm thickness of buffered saline at pH 7.4 and illumination wavelength of 490 nm. Molar extinction coefficient was assumed to be 76,000 cm−1 M−1 and molecular weight of sodium fluorescein 476.46 Efficiency of fluorescence was assumed to be reduced by the phenomenon of Resonance Energy Transfer (RET)45 using the formula:
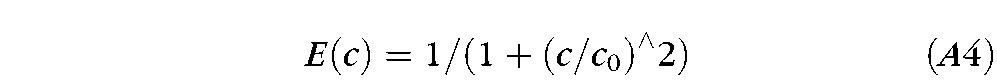 |
where the “critical concentration,” c0, was taken to be 0.2%. Evidence for this assumption is given below.
Figure A1. .
Calculated illumination absorptance, fluorescent efficiency, and intensity of fluorescence as a function of sodium fluorescein concentration. Intensity is calculated as the product of absorptance and efficiency (k = 1 in Equation A1). See text for details.
Figure A2. .
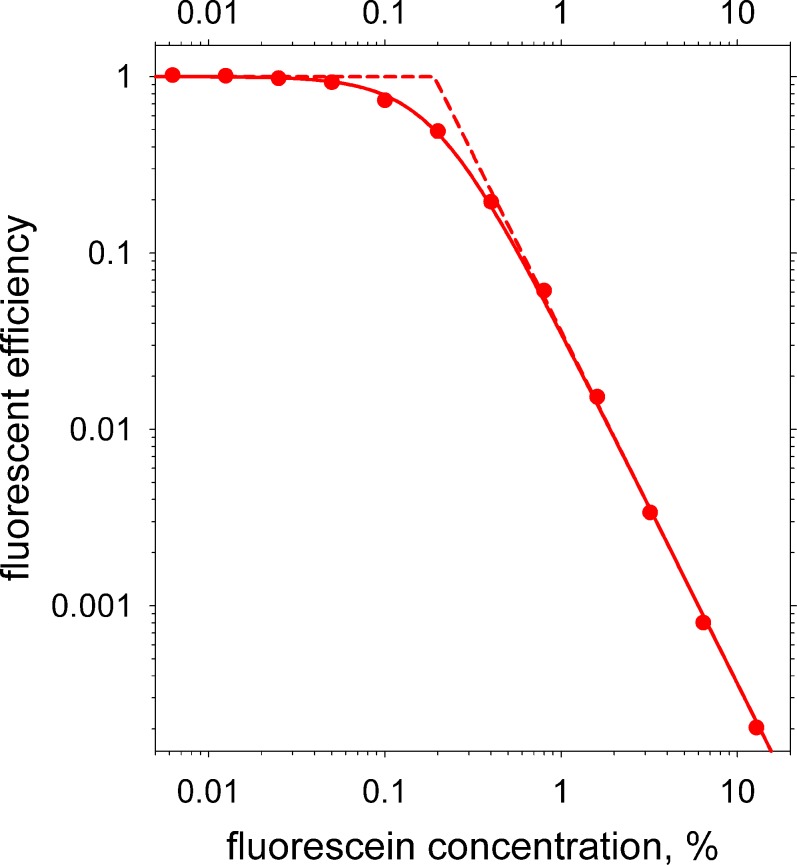
Filled circles are measured values of fluorescent efficiency as a function of fluorescein concentration (log scales). The solid curve is a least squares fit based on Equation A4 for RET. Dashed curves are the two asymptotes, which intersect at the “critical concentration” (c0 in Equation A4) of 0.19%.
Webber and Jones measured fluorescence intensity as a function of fluorescein concentration in a 5 μm thick layer.47 They obtained a curve qualitatively similar to the intensity curve of Figure A2 with a peak intensity near 0.2% concentration, also similar to Figure A2. They did not analyze their results in terms of contributions from absorptance and fluorescent efficiency (i.e., self quenching).
To measure the effect of self quenching on fluorescent efficiency, it may be noted that absorptance can be made independent of concentration by using a relatively thick container (in this case, A(c) = 1). We, therefore, repeated measurements similar to those of Webber and Jones but using a 1 cm thick container, that is 2000 times (3.3 log units) thicker. Thus, from Equation A2, the absorptance curve in Figure A2 would be moved 3.3 log units to the left and would have a constant value of unity for the plotted range.
Fluorescein was dissolved in tris buffered saline with a pH of 7.4. An initial 12.8% solution was made by dissolving 12.8 g of sodium fluorescein to make 100 mL of solution; additional solutions were prepared by dilution in steps of 2 with the buffered saline, taking care to ensure thorough mixing, to a final concentration of 0.00625%. The solutions were illuminated at normal incidence through a narrow band, 490 nm interference filter and the images recorded on a monochrome CCD camera viewing through a broad band interference filter (central wavelength 535 nm, bandwidth 45 nm) at an angle of 40 degrees.
Results are shown in the log-log plot of fluorescent efficiency versus fluorescein concentration in Figure A2, where filled circles show measured values. The solid curve is a least squares fit to the data based on Equation A4 for RET. A good correlation between log fitted and log measured efficiency was observed, r2 = 0.9995, indicating that self quenching of fluorescein is mainly or entirely due to RET, in agreement with evidence from other methods.48 The two asymptotes of slope 0 and −2 are shown by dashed lines; their intersection gives an estimate of the critical concentration of fluorescein, c0 in Equation A4, of 0.19%.
Footnotes
Supported by NEI Grants EY0015519 (KKN, JJN) and EY017951 (PEKS).
Disclosure: J.J. Nichols, None; P.E. King-Smith, None; E.A. Hinel, None; M. Thangavelu, None; K.K. Nichols, Alcon (C), Allergan (C), B&L (C), Pfizer (C), Tearlab (C), Sarcode (C), ISTA (C), Eleven Biotherapeutics (C)
References
- 1.Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78:389–394 [DOI] [PubMed] [Google Scholar]
- 2.Rolando M, Refojo MF, Kenyon KR. Increased tear evaporation in eyes with keratoconjunctivitis sicca. Arch Ophthalmol. 1983;101:557–558 [DOI] [PubMed] [Google Scholar]
- 3.Tsubota K, Yamada M. Tear evaporation from the ocular surface. Invest Ophthalmol Vis Sci. 1992;33:2942–2950 [PubMed] [Google Scholar]
- 4.Mathers WD, Daley TE. Tear flow and evaporation in patients with and without dry eye. Ophthalmology. 1996;103:664–669 [DOI] [PubMed] [Google Scholar]
- 5.Goto E, Matsumoto Y, Kamoi M, et al. Tear evaporation rates in Sjögren syndrome and non-Sjogren dry eye patients. Am J Ophthalmol. 2007;144:81–85 [DOI] [PubMed] [Google Scholar]
- 6.King-Smith PE, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom Vis Sci. 2008;85:623–630 [DOI] [PubMed] [Google Scholar]
- 7.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:93–107 [DOI] [PubMed] [Google Scholar]
- 8.Introduction to the Report of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:69–70 [Google Scholar]
- 9.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92 [DOI] [PubMed] [Google Scholar]
- 10.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13 [DOI] [PubMed] [Google Scholar]
- 14.Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351 [DOI] [PubMed] [Google Scholar]
- 15.Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525 [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, Ruckenstein E. Mechanism of tear film rupture and its implications for contact lens tolerance. Am J Optom Physiol Opt. 1985;62:246–253 [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson A, Doane MG, McFadyen A. Inputs and outputs of the lacrimal system: review of production and evaporative loss. Ocul Surf. 2009;7:186–198 [DOI] [PubMed] [Google Scholar]
- 18.Liu DT, Di Pascuale MA, Sawai J, Gao YY, Tseng SC. Tear film dynamics in floppy eyelid syndrome. Invest Ophthalmol Vis Sci. 2005;46:1188–1194 [DOI] [PubMed] [Google Scholar]
- 19.Korb DR. Survey of preferred tests for diagnosis of the tear film and dry eye. Cornea. 2000;19:483–486 [DOI] [PubMed] [Google Scholar]
- 20.Nichols KK, Nichols JJ, Zadnik K. Frequency of dry eye diagnostic test procedures used in various modes of ophthalmic practice. Cornea. 2000;19:477–482 [DOI] [PubMed] [Google Scholar]
- 21.Mokhtarzadeh M, Casey R, Glasgow BJ. Fluorescein punctate staining traced to superficial corneal epithelial cells by impression cytology and confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson G, Ren H, Laurent J. Corneal epithelial fluorescein staining. J Am Optom Assoc. 1995;66:435–441 [PubMed] [Google Scholar]
- 23.Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:108–152 [DOI] [PubMed] [Google Scholar]
- 24.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285 [DOI] [PubMed] [Google Scholar]
- 25.Webber WR, Jones DP. Continuous fluorophotometric method of measuring tear turnover rate in humans and analysis of factors affecting accuracy. Med Biol Eng Comput. 1986;24:386–392 [DOI] [PubMed] [Google Scholar]
- 26.Nichols JJ, Mitchell GL, King-Smith PE. Thinning rate of the precorneal and prelens tear films. Invest Ophthalmol Vis Sci. 2005;46:2353–2361 [DOI] [PubMed] [Google Scholar]
- 27.Tiffany JM, Winter N, Bliss G. Tear film stability and tear surface tension. Curr Eye Res. 1989;8:507–515 [DOI] [PubMed] [Google Scholar]
- 28.McDonald JE, Brubaker S. Meniscus-induced thinning of tear films. Am J Ophthalmol. 1971;72:139–146 [DOI] [PubMed] [Google Scholar]
- 29.Brown SI. Further studies on the pathophysiology of keratitis sicca of Rollet. Arch Ophthalmol. 1970;83:542–547 [DOI] [PubMed] [Google Scholar]
- 30.Berger RE, Corrsin S. A surface tension gradient mechanism for driving the pre-corneal tear film after a blink. J Biomech. 1974;7:225–238 [DOI] [PubMed] [Google Scholar]
- 31.King-Smith PE, Fink BA, Hill RM. Evaporation from the human tear film studied by interferometry. Adv Exp Med Biol. 2002;506:425–429 [DOI] [PubMed] [Google Scholar]
- 32.King-Smith PE, Fink BA, Nichols JJ, Nichols KK, Braun RJ, McFadden GB. The contribution of lipid layer movement to tear film thinning and breakup. Invest Ophthalmol Vis Sci. 2009;50:2747–2756 [DOI] [PubMed] [Google Scholar]
- 33.Farris RL. Tear osmolarity—a new gold standard? Adv Exp Med Biol. 1994;350:495–503 [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–3679 [DOI] [PubMed] [Google Scholar]
- 35.Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45 [DOI] [PubMed] [Google Scholar]
- 36.Iwata S, Lemp MA, Holly FJ, Dohlman CH. Evaporation rate of water from the precorneal tear film and cornea in the rabbit. Invest Ophthalmol. 1969;8:613–619 [PubMed] [Google Scholar]
- 37.King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51:2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto E, Endo K, Suzuki A, Fujikara Y, Matsumoto Y, Tsubota K. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–539 [DOI] [PubMed] [Google Scholar]
- 39.Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119 [DOI] [PubMed] [Google Scholar]
- 40.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 41.Yamada K, Hayasaka S, Setogawa T. Test results in patients with Sjögren's syndrome defined by the Japanese criteria. Acta Ophthalmol (Copenh). 1990;68:80–86 [DOI] [PubMed] [Google Scholar]
- 42.Craig JP, Singh I, Tomlinson A, Morgan PG, Efron N. The role of tear physiology in ocular surface temperature. Eye. 2000;14:635–641 [DOI] [PubMed] [Google Scholar]
- 43.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621 [DOI] [PubMed] [Google Scholar]
- 44.Kimball SH, King-Smith PE, Nichols JJ. Evidence for the major contribution of evaporation to tear film thinning between blinks. Invest Ophthalmol Vis Sci. 2010;51:6294–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakowicz JR, Chowdhury MH, Ray K, et al. Plasmon-controlled fluorescence: a new detection technology. Proc Soc Photo Opt Instrum Eng. 2006;6099:609909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mota P, Carvalho MC, Ramalho J, Leite E. Spectrophotometric analysis of sodium fluorescein aqueous solutions. Determination of molar absorption coefficient. Int Ophthalmol. 1991;15:321–326 [DOI] [PubMed] [Google Scholar]
- 47.Webber WR, Jones DP. Continuous fluorophotometric method of measuring tear turnover rate in humans and analysis of factors affecting accuracy. Med Biol Eng Comput. 1986;24:386–392 [DOI] [PubMed] [Google Scholar]
- 48.Viger ML, Live LS, Therrien OD, Boudreau D. Reduction of self-quenching in fluorescent silica-coated silver nanoparticles. Plasmonics. 2008;3:33–40 [Google Scholar]