Abstract
Background
Titanium dioxide (TiO2) is considered as an inert and safe material and has been used in many applications for decades. However, with the development of nanotechnologies TiO2 nanoparticles, with numerous novel and useful properties, are increasingly manufactured and used. Therefore increased human and environmental exposure can be expected, which has put TiO2 nanoparticles under toxicological scrutiny. Mechanistic toxicological studies show that TiO2 nanoparticles predominantly cause adverse effects via induction of oxidative stress resulting in cell damage, genotoxicity, inflammation, immune response etc. The extent and type of damage strongly depends on physical and chemical characteristics of TiO2 nanoparticles, which govern their bioavailability and reactivity. Based on the experimental evidence from animal inhalation studies TiO2 nanoparticles are classified as “possible carcinogenic to humans” by the International Agency for Research on Cancer and as occupational carcinogen by the National Institute for Occupational Safety and Health. The studies on dermal exposure to TiO2 nanoparticles, which is in humans substantial through the use of sunscreens, generally indicate negligible transdermal penetration; however data are needed on long-term exposure and potential adverse effects of photo-oxidation products. Although TiO2 is permitted as an additive (E171) in food and pharmaceutical products we do not have reliable data on its absorption, distribution, excretion and toxicity on oral exposure. TiO2 may also enter environment, and while it exerts low acute toxicity to aquatic organisms, upon long-term exposure it induces a range of sub-lethal effects.
Conclusions
Until relevant toxicological and human exposure data that would enable reliable risk assessment are obtained, TiO2 nanoparticles should be used with great care.
Keywords: titanium dioxide, nanoparticles, toxicity, applications, safety
Introduction
Titanium dioxide (titania, TiO2) is chemically inert, semiconducting material that also exhibits photocatalytic activity in the presence of light with an energy equal to or higher than its band-gap energy. These characteristics offer a wide range of applications. For these reasons, and because of the relatively low price of the raw material and its processing, titania has gained widespread attention over recent decades.
TiO2 has been classified in humans and animals as biologically inert1,2, and is widely considered to be a “natural” material, which at least partially contributes to its relatively positive acceptance by the public. In fact, most TiO2 has been synthesized from the mineral illmenite, FeTiO3, using the “sulphate” or “chloride” process for nearly 100 years. The annual worldwide production of titania powder in 2005 has been estimated to be around 5 million tons3, provoking the question as to its abundance in the environment. The proportion of nano-sized titania is estimated to have been approximately 2.5 % in 2009, increasing to 10 % by 20154, with an exponential increase over the past decade.
During recent decades, TiO2 powders have begun to appear in many applications, mainly due to their ability to confer whiteness and opacity on various products, such as paints, papers and cosmetics. Its high technological attractiveness originates from its light-scattering properties and very high refractive index, which mean that relatively low levels of the pigment are required to achieve a white, opaque coating. The range of light that is scattered depends on the particle size. Numerous technological improvements, based on nano-sized TiO2, have been introduced that enable its use for antifogging and self-cleaning coatings on glass, for building facades, in confectionary, in the plastics industry, and so on. Furthermore, TiO2 is accepted as a food and pharmaceutical additive.5 In the United States it is included in the Food and Drug Administration (FDA) Inactive Ingredients Guide for dental paste, oral capsules, suspensions, tablets, dermal preparations and in non-parenteral medicines.
The increasing production of nano-sized TiO2 powder has led to growing concerns about the consequences of exposure of humans and the environment.6 In the present paper we review and discuss the latest findings on potential hazard of exposure to nano-sized TiO2 for humans and environment, in regard to the particle size and the crystal structure of TiO2, the route of exposure as well as the effect of ultraviolet (UV) irradiation-induced photocatalysis.
Chemical and physical properties of TiO2 nanoparticles
Nanoparticles (NPs) are generally defined as particles having at least one dimension smaller than 100 nm. Accordingly, particles with different morphologies, from equi-axial shapes, whiskers, and nano-tubes to nanorods, need to be considered. Although micron-sized and nano-sized TiO2 powders are, in general, chemically identical, due to their significantly higher specific surface area, nano-powders may exhibit physical and chemical properties that differ from those of the coarser grades, and so should not be treated in the same way. In a recent paper7 the size-dependent properties of a variety of inorganic NPs were reviewed and it was suggested that they are likely to be of concern due to the appearance of unique properties when they have diameters of ≤ 30 nm. In this size range, many particles undergo dramatic changes in behaviour that enhance their interfacial reactivity. While less than 20 % of the constituent atoms are at the surface of 30 nm NPs, approximately 35–40 % of the atoms are localized at the surface of a 10 nm particle.
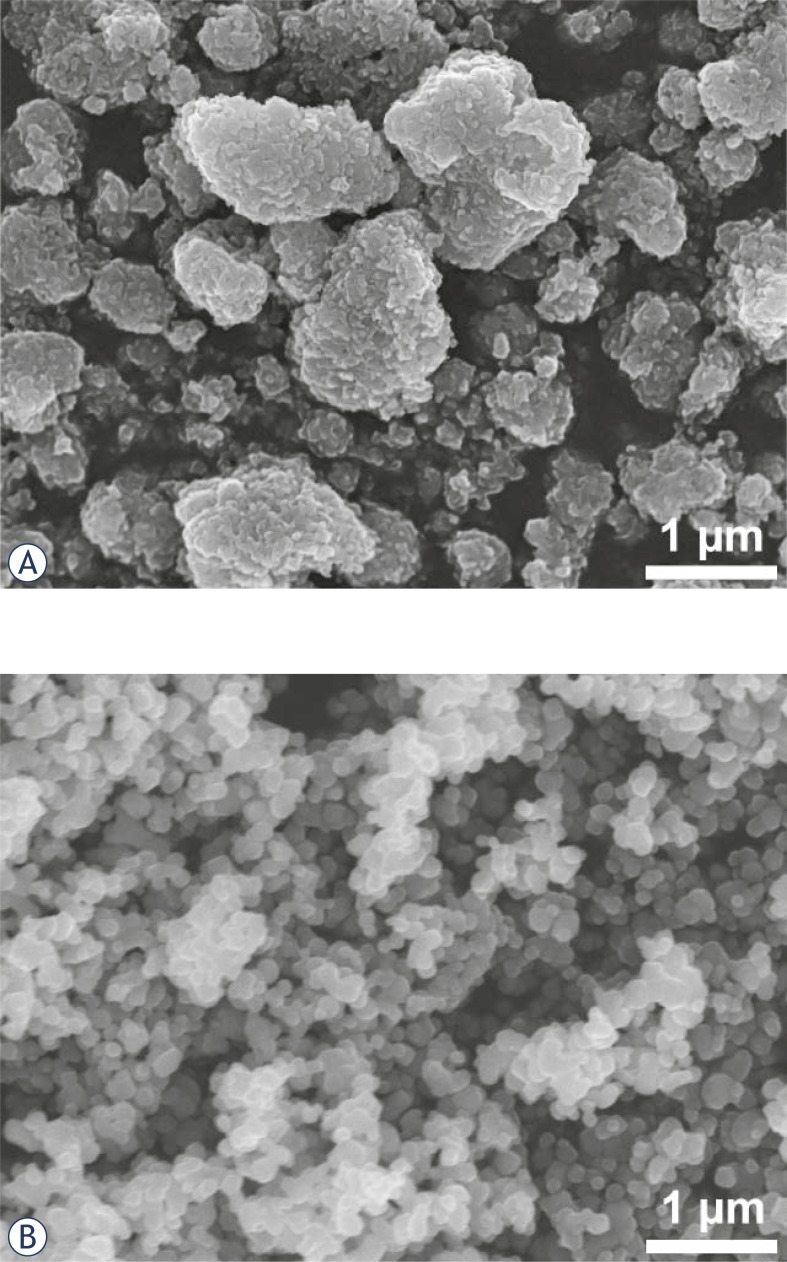
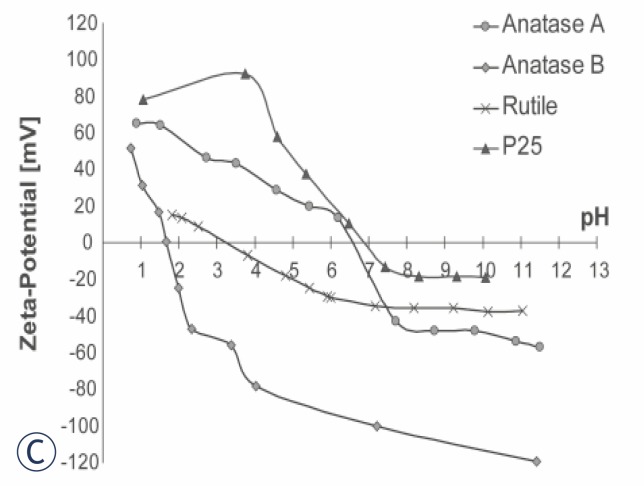
In practice, it is difficult to draw a clear borderline between nano- and submicron-sized particles. Submicron-sized powders always contain a certain proportion of nano-sized particles and, conversely, NPs tend to associate to form relatively strongly bonded aggregates (Figure 1A) or soft agglomerates (Figure 1B). The latter can usually be disintegrated easily in a liquid; however, their dispersion depends strongly on the zeta-potential. As illustrated in Figure 1C, the zeta-potential of TiO2 powders may differ significantly over a wide range of pH values. The reported isoelectric points for TiO2 powders range from pH 3.5 to 8 8 which may greatly affect the bioavailability in the region of physiological pH values. The effective size of particles and their zeta-potential have been neglected almost completely in most of the studies of the interaction of TiO2 NPs with biological systems.
FIGURE 1.
Field emission electron micrographs of different TiO2 powders: A) Anatase A (Sigma 637254), B) Anatase B (Sigma T8141); C) Zeta-potential of these two powders, Rutile (Sigma 637262) and P25 (Degussa).
Crystalline TiO2 occurs naturally in three polymorphs – anatase, rutile and brookite – among which rutile is the most stable. A powder with an average particle size of 230 nm scatters visible light, while its counterpart, with an average size of 60 nm, scatters UV light and reflects visible light. Under UV, TiO2 exhibits photocatalytic activity, which is a consequence of the electronic structure of the titania, and is, to a large extent, more characteristic of anatase than of rutile and brookite. In the presence of light with energy equal to or higher than the TiO2 band-gap energy, an electron is promoted from the valence band to the conduction band, leaving behind a positive hole. The extrapolated optical absorption gaps of anatase and rutile are 3.2 and 3.0 eV at room temperature, which correspond to wavelengths of around 413 nm and 387 nm. Consequently, the photo-activation of nano-TiO2 can be achieved by irradiation with UV-A, B and C, visible, fluorescent light, and X-ray radiation. The photocatalytic activity results in formation of highly reactive radicals, that are capable of reacting with most of the surrounding organic substances.9–12
Mechanisms of TiO2 NPs toxicity
As already discussed, the physicochemical properties of particles depend on their size, so that, at the nanometre level, the material is chemically more reactive. This can be exploited as a desirable property, e.g., as a catalyst. However, at the same time, the material may possess biological activities that can be either desirable (e.g., carrier capacity for therapeutics, penetration of cellular barriers for drug delivery) or undesirable (e.g., toxicity, induction of oxidative stress or cellular dysfunction), or a mix of the two.
Cellular uptake of TiO2 NPs
From a toxicological point of view the important characteristics of NPs are their size, surface area, surface chemistry and charge, crystallinity, shape, solubility and agglomeration/aggregation state. Surface groups may render NPs hydrophilic or hydrophobic, lipophilic or lipophobic, catalytically active or passive. Cellular uptake, subcellular localization, and ability to cause toxic effects depend on these properties of NPs.13 The two main pathways of NP uptake in the cell are active uptake by endocytosis, and passive uptake by free diffusion. Phagocytosis is an actin-dependent, endocytic mechanism, typical of “professional” phagocytes like macrophages. Geiser et al.14 reported that, in rats exposed to TiO2 powders by inhalation, alveolar macrophages effectively cleared micron-sized (3–6 μm) but not nano-sized (20 nm) TiO2 particles. This is important, since phagocytes generally remove particulate matter >500 nm 15 and, as they are unable to phagocytose smaller particles, the latter are retained in the tissue, leading to a sustained burden on other tissues and cells. It was demonstrated that the uptake of 50 nm nano-TiO2, by endocytosis with alveolar A549 epithelial cells, was limited to aggregated particles.16 After inhalation exposure of rats to TiO2 NPs, free particles were found within the cytoplasm of epithelial and endothelial cells and fibroblasts.17 Rothen-Rutishauser et al.18, used an in vitro airway wall model, and found membrane-bound aggregates (>200 nm) of TiO2 as well as smaller unbound aggregates within the cell cytoplasm. In an in vitro study Kocbek et al.19 demonstrated the endocytotic uptake of 25 nm-sized anatase TiO2 by human keratinocytes. They observed highly aggregated NPs within early and late endosomes and in amphisomes, confirming endocytotic uptake. Experiments with red blood cells, which lack phagocytic receptors18, revealed that TiO2 NP aggregates smaller than 200 nm are able to enter red blood cells, while larger particles were only found attached to the cell’s surface. Xia et al.20 showed that fluorescence-labelled TiO2 NPs (11 nm) were taken up and localized in late endosomal and caveolar compartments in phagocytic RAW 246.7 and lung endothelial BEAS-2B cells.
Oxidative stress induced by TiO2 NPs
Oxidative stress is thought to be a key mechanism responsible for adverse biological effects exerted by NPs.21,22 The role of oxidative stress in TiO2-induced adverse effects has been confirmed by evidence that it induces an increase in reactive oxygen species (ROS) production and oxidative products (i.e., lipid peroxidation), as well as the depletion of cellular antioxidants.23–29
TiO2 mediates oxidative stress under UV irradiation as well as without it. Uchino et al.30 showed that, under UV irradiation, the TiO2 NPs of different crystalline structures and sizes produces different amounts of hydroxyl radicals, and that cytotoxicity against Chinese hamster ovary cells correlates with the production of radicals. Dodd and Jha31 confirmed that hydroxyl radicals are the primary damaging species produced by UV irradiated nano-sized TiO2, and react to give carboxyl radicals. A number of studies have shown photo-activated anatase TiO2 to induce higher cytotoxicity and genotoxicity than similarly activated rutile TiO2. These differences could arise from the fact that anatase particles possess a wider absorption gap and a smaller electron effective mass, resulting in the higher mobility of the charge carriers and the greater generation of ROS. On the other hand, there is evidence that TiO2 also induces ROS formation and the associated adverse effects in the absence of photo-activation. For instance, Gurr et al.24 found that anatase TiO2 NPs and mixtures of anatase and rutile TiO2 NPs induced oxidative damage in human bronchial epithelial (BEAS-2B) cells, and Petkovi et al.32 reported that in human hepatoma cells (HepG2), non-irradiated anatase nano-TiO2 induced significantly higher levels of intracellular ROS than the corresponding rutile-TiO2, and only anatase nano-TiO2 caused oxidative DNA damage. Recently, Petkovi et al.33 compared cytotoxicity and genotoxicity of non-irradiated and UV pre-irradiated anatase TiO2 of two sizes (<25 nm and >100 nm). They showed that non-irradiated TiO2 particles did not affect survival of the cells; they caused slight increase in number of DNA strand breaks, while only TiO2 NPs caused increase in oxidative DNA damage. After pre-irradiation with UV both sizes of anatase TiO2 particles reduced cell viability, induced DNA strand breaks and oxidative DNA damage. This is an important finding that, for the first time, showed that photo-activated TiO2 particles retained higher cytotoxic and genotoxic potential also when UV irradiation was discontinued and that it was not particle size dependent.
ROS are also important signalling modulators, therefore exposure of cells to NPs may, via elevated ROS formation, affect cellular signalling cascades that control processes such as cell proliferation, inflammation and cell death.34 The role of oxidative stress in TiO2-induced inflammation has recently been confirmed by Kang et al.35 In the mouse peritoneal macrophage cell line RAW 246.7 exposed to nano-TiO2, ROS production was associated with the activation of pro-inflammatory cascade, as indicated by extracellular signal-regulated kinases ERK1/2 phosphorylation, tumour necrosis factor TNFα production and macrophage inflammatory protein MIP-2 secretion.
Taken together, these studies indicate that the high level of oxidative stress that is related to an exposure to a high concentration of TiO2 NPs leads to cell damage-associated responses, whereas at moderate levels of oxidative stress, inflammatory responses may be stimulated by the activation of ROS-sensitive signalling pathways.
Genotoxicity of TiO2 NPs
Several studies show that nano-TiO2 induces ge-notoxic effects, including DNA damage, and micronuclei formation that is indicative of chromosomal aberrations in different cell lines32, 35–38 The studies also showed that genotoxic effects elicited NPs strongly depended upon their size by TiO2 and form. For instance, Gurr et al.24 showed that NPs up to 20 nm in size induced an anatase TiO2 increase in micronuclei formation, while 200 nm did not. Zhu et al.39 anatase or 200 nm rutile TiO2 demonstrated clear differences in the cytotoxicity and the extent of DNA strand scission, together with the formation of 8-hydroxy-2-deoxyguanosine (8-OHdG) adducts in isolated DNA, after a treatment with different types of TiO2 NPs in the order 10-20 nm anatase > 50-60 nm anatase > 50-60 nm rutile. At the molecular level it has been shown that the exposure of peripheral human lymphocytes to TiO2 NPs caused the activation of DNA damage check points and the accumulation of tumour suppressor protein p53, the main regulator of the cellular response to DNA damage.40 Exposure of human hepatoma HepG2 cells under similar conditions led to the elevated expression of tumor suppressor p53 mRNA and its downstream regulated DNA damage response genes (cyclin-dependent kinase inhibitor p21, growth arrest and DNA damage-inducible gene GADD45a and the E3 ubiquitin ligase MDM2).32
On the other hand, TiO2 NPs were devoid of mutagenic activity in microbial mutation assays (with Salmonella typhimurium) and in chromosomal aberration the in Chinese hamster ovary cells.41 Similarly, Theogaraj et al.42 reported that nano-sized TiO2 (eight different anatase and rutile forms) at concentrations up to 5 mg/ml did not induce any increase in the chromosomal aberration frequency in Chinese hamster ovary cells, in either the absence or the presence of UV light. However, in this study only a short-term, 3-hour, and no continuous (i.e., 20 hours) exposure, was performed.
In an early study Driscoll et al.43 reported that in rats intratracheal instillation of TiO2 NPs (100 mg/kg BW) induced increased HPRT mutation frequency in alveolar cells. They also showed that mutagenicity in alveolar cells was associated with inflammation. Trouiller et al.44 recently reported that oral exposure of mice to TiO2 NPs through drinking water (50–500 mg/kg BW/day for 5 days) induced oxidative DNA damage, micronuclei formation and γ-H2AX foci, the indicators of DNA double strand breaks. Since also high-level gene expression of pro-inflammatory cytokines was also observed, the authors suggested the inflammatory effects were responsible for the induction of genotoxic effects.
The in vitro and in vivo genotoxicity studies using different experimental models indicate that nano-TiO2 may cause genotoxic effects via secondary mechanisms that include oxidative stress and inflammation.32,38,40,43,44 However, there is some evidence that nano-sized TiO2 can locate in nuclei 17, and recently Li et al.45 reported the presence of anatase nano-sized TiO2 in DNA extracted from the liver of mice exposed intraperitoneally to these NPs (5–150 mg/kg BW/day for 14 days). The authors showed that Ti inserted between DNA base pairs or bound to DNA nucleotides, in such a way that it altered the conformation of the DNA and, at higher doses, caused DNA cleavage. These findings indicate that TiO2 may also induce genetic damage by a direct interaction with the DNA.
Immunotoxic effects of TiO2 NPs
Depending on physicochemical properties of NPs, they are recognized and taken up by immune cells, such as macrophages, monocytes, platelets, leuko-cytes and dendritic cells, and can trigger an inflammatory response. In a human monoblastoid cell line (U937) exposure to TiO2 NPs induced apoptosis and necrosis in concentrations corresponding to those found in blood, plasma, or in tissues surrounding Ti implants 46. Palomäki et al.47 reported that rutile TiO2 NPs and silica-coated rutile TiO2 NPs induced the enhanced expression of a variety of proinflammatory cytokines in murine dendritic cells (bm-DC) and in murine macrophages (RAW 246.7). The particles were for dendritic cells more toxic than for macrophages. In dendritic cells nano-sized TiO2 led to an upregulation of maturation markers and activated the NLRP3 inflammasome, a multiprotein complex within the cytoplasm of antigen-presenting cells, leading to significant IL 1β-secretion. It was demonstrated for neutrophils that the short-term exposure of neutrophils to nano-anatase TiO2 induces changes in their morphology, indicating its potential to activate these cells, while longer exposure resulted in the inhibition of apoptosis and cytokine production, confirming that in vitro TiO2 exerts neutrophil agonist properties.
Immunomodulating effects after exposure to TiO2 NPs have been observed also in in vivo studies. Larsen et al.48 showed that in ovalbumin immunized mice intraperitoneal exposure to TiO2 NPs promoted a T-helper type 2 cells mediated dominant immune response with high levels of oval-bumin-specific immunoglobulins IgE and IgG1 in serum and influx of eosinophils, neutrophils and lymphocytes in bronchoalveolar lavage fluid. Airway inflammation and immune adjuvant activity in ovalbumin immunized mice was observed also after intranasal exposure to TiO2 NPs 48,49 indicating that airborne exposure to TiO2 NPs may induce respiratory allergy, where the possible mechanism could be an adjuvant-like activity of NPs on allergic sensitization. Associated with the impairment of the immune response, recently Moon et al.50 showed that the intraperitoneal exposure of mice to TiO2 NPs enhanced the growth of subcutaneously implanted B16F10 melanoma through the immunomodulation of B- and T-lymphocytes, macrophages, and natural killer cells.
Neurotoxic effects of TiO2 NPs
It has been reported that inhaled NPs can translocate to the central nervous system through the olfactory pathway22 and by crossing the blood-brain barrier.51,52 In vitro studies of non-irradiated TiO2 NPs (Degussa P25) showed that they cause oxidative stress in the brain microglia BV2 cell line 53 that was associated with the up-regulation of genes involved in the inflammation, apoptosis, and the cell cycle, and down-regulation of genes involved in energy metabolism.25 While Degussa P25 NPs stimulated ROS formation in BV2 microglia, they were nontoxic to isolated N27 neurons. However, in complex brain cultures the Degussa P25 particles rapidly damaged neurons, plausibly through microglial generated ROS. In contrast, Liu et al.54 reported that, in the neuronal cell line PC12, exposure to nano-TiO2 induced dose-dependent oxidative stress and apoptosis that was partly prevented by pre-treatment with a ROS scavenger. Surprisingly, it was shown recently 55 that TiO2 NPs (rutile TiO2 coated with SiO2; 80–100 nm) might be an inducer of the differentiation of (mouse) neural stem cells towards neurons. These results indicate that the responses may be cell-type dependent and oxidative stress-mediated.
Recently Scuri et al.56 reported that inhalation exposure of newborn (2-day-old) and weanling (2-week-old), but not adult, rats to TiO2 NPs (12 mg/m3; 5.6 h/day for 3 days) up-regulates the expression of lung neurotrophins, key regulatory elements of neuronal development and responsiveness that play a critical role in the pathophysiology of childhood asthma. The effect was associated with the development of airway hyperreactivity (AHR) and mild airway inflammation. These results suggest the presence of a critical window of vulnerability in the earlier stages of lung development, which may lead to a higher risk of developing asthma.
TiO2 NPs in everyday life
Nano-sized TiO2 in various forms is used widely in everyday life in a variety of products, such as anti-fouling paints, household products, plastic goods, medications, cosmetics, sunscreens, pharmaceutical additives and food colorants, and many new applications are under development or already in pilot production. In the following sections we consider the main entry ports of nano-sized TiO2 into the human body and potential adverse effects.
Dermal exposure to TiO2 NPs
TiO2 NPs as a component of the sunscreen-technology revolution
During recent decades, skin cancer has become the most frequent neoplastic disease among the Caucasian population in Europe, North America and Australia, and its incidence has reached epidemic proportions.57 As a consequence, the trend in sun protection in daily cosmetics is towards increased use of organic and inorganic UV filters. It is estimated that worldwide use of nano-sized TiO2 in sunscreens is around 1000 tons per year.51
TiO2 has been used in sunscreens since 1952, however the Food and Drug Administration (FDA) approved the use of TiO2 in sunscreens in 1999.58,59 Currently it is not required to label sunscreens as containing nano-TiO2. 60 The situation could change if the European Union (EU) commission adopts a proposed new regulation within EU Cosmetic Directive, under which all cosmetics that contain more than 1 % w/w of NPs will have to declare it on the packaging. Since TiO2 is considered as low- irritating, it is the only inorganic UV filter allowed by European legislation in concentrations as high as 25 %.61,62 There is also some confusion regarding the classification of sunscreens. In the EU they are classified as cosmetics, while in the USA, they are classified as over the counter (OTC) drugs.63
The average size of the TiO2 particles in sun-screens ranges between 10 and 100 nm, while some products contain particles down to 5 nm or up to 500 nm.64 TiO2 particles in the size range between 200 and 500 nm are opaque and act as a true sun-block when applied to the skin.61,65,66 However, this opacity is lost when much finer particles are used. Such sunscreens are more transparent, less viscous, and blend into the skin more easily. Therefore, the optimum size of TiO2 particles was suggested to be around 50 nm, which provides good protection against UV light, while the dispersion of visible light is such that sunscreens do not appear white on the skin.67
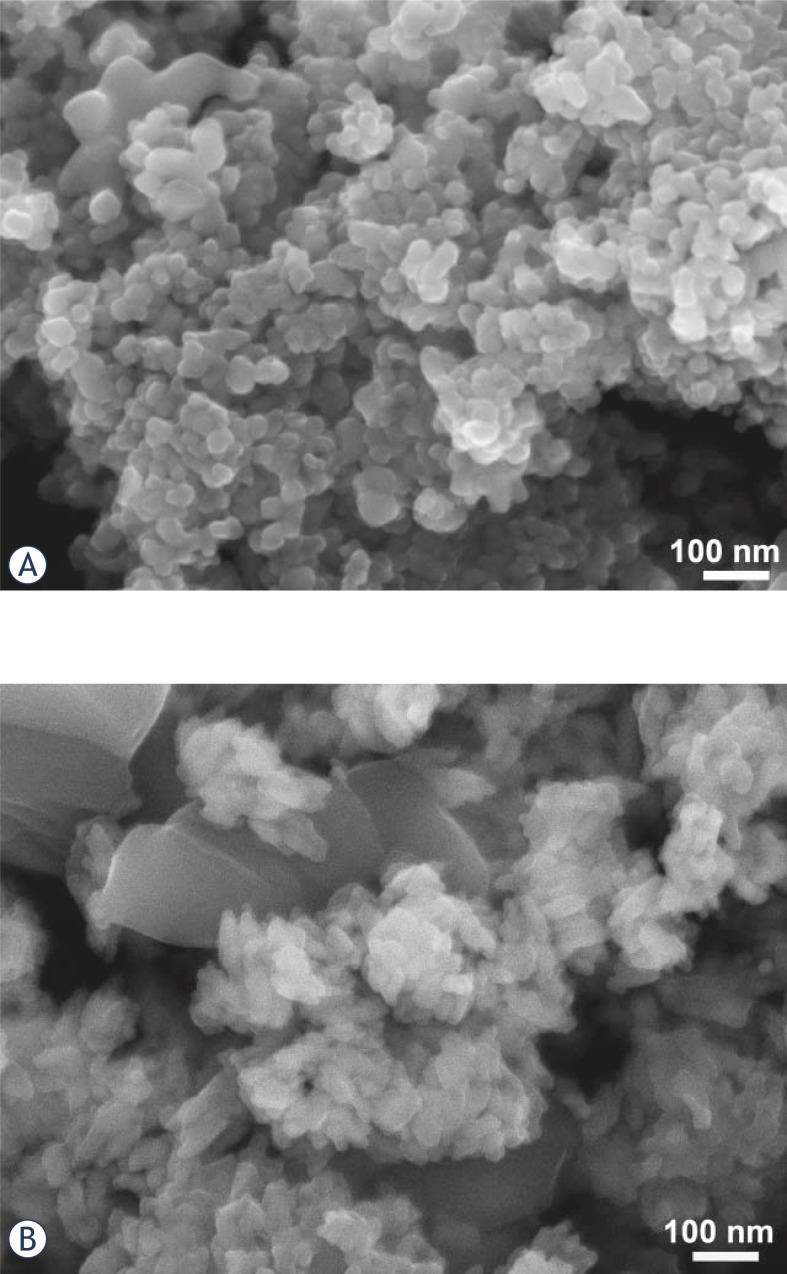
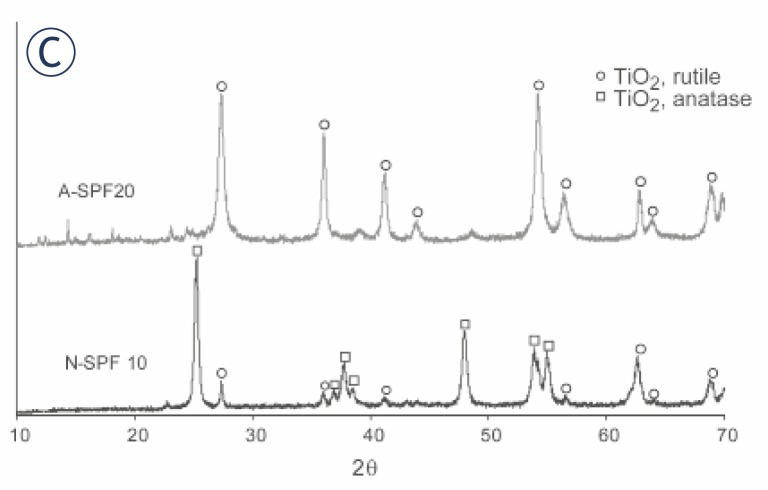
Sunscreens typically, but not exclusively, contain rutile TiO2 powder, which is less photo-active than the anatase TiO2. Micrographs of the powders extracted from two commercial sunscreens from different producers are shown in Figures 2A and B. From the X-ray diffractograms (Figure 2C) it is evident that the TiO2 powder in the sunscreen “N-SPF10” is predominantly in the anatase form, with an estimated particle size of around 50 nm (Figure 2A, C), while the powder in the sunscreen “A-SPF20” contained rutile TiO2 with two size populations (Figure 2B, C). (The original commercial names of the products were adapted for this study.)
FIGURE 2.
Field emission electron micrographs of the powders from two commercial sunscreens: A-SPF 20 (A) and N-SPF 10 (B), and their XRD diffraction (C).
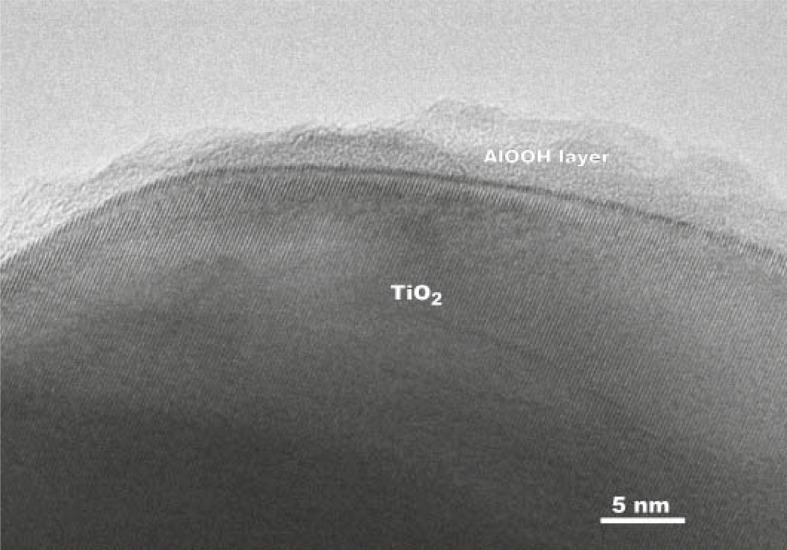
To minimize the harmful effects of photo-active nano-TiO2, various coatings such as magnesia, silica, alumina or zirconia68–71 were introduced. However, certain coating materials may have side effects, such as aluminium-based ones (Figure 3), and it is also not clear how stable the coatings are and what is the lifetime of the “inert” particle released from sunscreens.
FIGURE 3.
Transmission electron micrograph of an AlOOH-coated TiO2 NP (Courtesy of dr. G. Dražić).
Cytotoxic and genotoxic effects of TiO2 NPs in dermal cells and skin models
Different dermal cell types have been reported to differ in their sensitivity to nano-sized TiO2 . Kiss et al.72 exposed human keratinocytes (HaCaT), human dermal fibroblast cells, sebaceous gland cells (SZ95) and primary human melanocytes to 9 nm-sized TiO2 particles at concentrations from 0.15 to 15 μg/cm2 for up to 4 days. The particles were detected in the cytoplasm and perinuclear region in fibroblasts and melanocytes, but not in kerati-nocytes or sebaceous cells. The uptake was associated with an increase in the intracellular Ca2+ concentration. A dose- and time-dependent decrease in cell proliferation was evident in all cell types, whereas in fibroblasts an increase in cell death via apoptosis has also been observed. Anatase TiO2 in 20–100 nm-sized form has been shown to be cytotoxic in mouse L929 fibroblasts.73 The decrease in cell viability was associated with an increase in the production of ROS and the depletion of glutathione. The particles were internalized and detected within lysosomes. In human keratinocytes exposed for 24 h to non-illuminated, 7 nm-sized anatase TiO2, a cluster analysis of the gene expression revealed that genes involved in the “inflammatory response” and “cell adhesion”, but not those involved in “oxidative stress” and “apoptosis”, were up-regulated.73 The results suggest that non-illuminated TiO2 particles have no significant impact on ROS-associated oxidative damage, but affect the cell-matrix adhesion in keratinocytes in extracellular matrix remodelling. In human keratinocytes, Kocbek et al.19 investigated the adverse effects of 25 nm-sized anatase TiO2 (5 and 10 μg/ml) after 3 months of exposure and found no changes in the cell growth and morphology, mitochondrial function and cell cycle distribution. The only change was a larger number of nanotubular intracellular connections in TiO2-exposed cells compared to non-exposed cells. Although the authors proposed that this change may indicate a cellular transformation, the significance of this finding is not clear. On the other hand, Dunford et al.23 studied the genotoxicity of UV-irradiated TiO2 extracted from sunscreen lotions, and reported severe damage to plasmid and nuclear DNA in human fibroblasts. Manitol (antioxidant) prevented DNA damage, implying that the genotoxicity was mediated by ROS.
Recently, Yanagisawa et al.74 reported that the transdermal exposure (mimicking skin-barrier dysfunction or defect) of NC/Nga mice to TiO2 NPs (15, 50, or 100 nm), in combination with allergen, aggravated atopic dermatitis-like lesions through a T-helper type 2 (Th2) dominant immune response. The study also indicated that TiO2 NPs can play a role in the initiation and/or progression of skin diseases, since histamine was released, even in the absence of allergen.
Skin-penetration studies
The skin of an adult person is, in most places, covered with a relatively thick (∼10 μm) barrier of keratinised dead cells. One of the main questions is still whether TiO2 NPs are able to penetrate into the deeper layers of the skin.75 The majority of studies suggest that TiO2 NPs, neither uncoated nor coated (SiO2, Al2O3 and SiO2/Al2O3) of different crystalline structures, penetrate normal animal or human skin.76,77–82 However, in most of these studies the exposures were short term (up to 48 h); only few long-term or repeated exposure studies have been published. Wu et al.83 have shown that dermal application of nano-TiO2 of different crystal structures and sizes (4–90 nm) to pig ears for 30 days did not result in penetration of NPs beyond deep epidermis. On the other hand, in the same study the authors reported dermal penetration of TiO2 NPs with subsequent appearance of lesions in multiple organs in hairless mice, that were dermal exposed to nano-TiO2 for 60 days. However, the relevance of this study for human exposure is not conclusive because hairless mice skin has abnormal hair follicles, and mice stratum corneum has higher lipid content than human stratum corneum, which may contribute to different penetration. Recently Sadrieh et al.84 performed a 4 week dermal exposure to three different TiO2 particles (uncoated submicron-sized, uncoated nano-sized and coated nano-sized) in 5 % sunscreen formulation with minipigs. They found elevated titanium levels in epidermis, dermis and in inguinal lymph nodes, but not in precapsular and submandibular lymph nodes and in liver. With the energy dispersive X-ray spectrometry and transmission electron microscopy (TEM) analysis the authors confirmed presence of few TiO2 particles in dermis and calculated that uncoated nano-sized TiO2 particles observed in dermis represented only 0.00008 % of the total applied amount of TiO2 particles. Based on the same assumptions used by the authors in their calculations it can be calculated that the total number of particles applied was 1.8 × 1013 /cm2 and of these 1.4 x107/cm2 penetrated. The surface area of skin in humans is around 1.8 m2 85 and for sun protection the cream is applied over whole body, which would mean that 4 week usage of such cream with 5 % TiO2 would result in penetration of totally 2.6 × 1010 particles. Although Sadrieh et al.84 concluded that there was no significant penetration of TiO2 NPs through intact normal epidermis, the results are not completely confirmative.
TiO2 NPs intake by food
TiO2 has been well accepted in the food industry and can be found as the E171 additive in various food products, mainly for whitening and texture. It is present in some cottage and Mozzarella cheeses, horseradish cream and sauces, lemon curd, and in low-fat products such as skimmed milk and ice-cream. Even if the product is labelled as containing E171, no information is usually given about the quantity, particle size and particle structure. FDA claims that TiO2 may be safely used as a colour additive for colouring foods in quantities up to 1 % by weight of the food.86 Interestingly, TiO2 is frequently declared as a “natural colouring agent” and is therefore well accepted by consumers.
TiO2 is also used in oral pharmaceutical formulations5, and the Pharmaceutical Excipients handbook considers nano-sized TiO2 a non-irritant and non-toxic excipient. Despite the fact that TiO2 submicron- and nano-sized particles are widely used as food and pharmaceutical additives, information on their toxicity and distribution upon oral exposure is very limited.
Potential hazards of oral exposure to TiO2 NPs
The gastrointestinal tract is a complex barrier/exchange system, and is the most important route by which macromolecules can enter the body. The main absorption takes place through villi and microvilli of the epithelium of the small and large intestines, which have an overall surface of about 200 m2. Already in 1922, it was recognized by Kumagai87, that particles can translocate from the lumen of the intestinal tract via aggregation of intestinal lymphatic tissue (Peyer’s patch, containing M-cells (phagocytic enterocytes)). Uptake can also occur via the normal intestinal enterocytes. Solid particles, once in the sub-mucosal tissue, are able to enter both the lymphatic and blood circulation.
In an early study Jani et al.88 administred rutile TiO2 (500 nm) as a 0.1 ml of 2.5 % w/v suspension (12.5 mg/kg BW) to female Sprague Dawley rats, by oral gavage daily for 10 days and detected presence of particles in all the major gut associated lymphoid tissue as well as in distant organs such as the liver, spleen, lung and peritoneal tissue, but not in heart and kidney. The distribution and toxicity of nano- (25 nm, 80 nm) and submicron-sized (155 nm) TiO2 particles were evaluated in mice administered a large, single, oral dosing (5 g/kg BW) by gavage.89 In the animals that were sacrificed two weeks later, ICP-MS analysis showed that the particles were retained mainly in liver, spleen, kidney, and lung tissues, indicating that they can be transported to other tissues and organs after uptake by the gastrointestinal tract. Interestingly, although an extremely high dose was administrated, no acute toxicity was observed. In groups exposed to 80 nm and 155 nm particles, histopathological changes were observed in the liver, kidney and in the brain. The biochemical serum parameters also indicated liver, kidney and cardiovascular damage and were higher in mice treated with nano-sized (25 or 80 nm) TiO2 compared to submicron-sized (155 nm) TiO2. However, the main weaknesses of this study are the use of extremely high single dose and insufficient characterisation of the particles.
Duan et al.90 administered 125 mg/kg BW or 250 mg/kg BW of anatase TiO2 (5 nm) intragastrically to mice continuously for 30 days. The exposed mice lost body weight, whereas the relative liver, kidney, spleen and thymus weights increased. Particles seriously affected the haemostasis of the blood and the immune system. The decrease in the immune response could be the result of damage to the spleen, which is the largest immune organ in animals and plays an important role in the immune response. Powel et al.91 demonstrated that TiO2 NPs may trigger immune reactions of the intestine after oral intake. They showed that TiO2 NPs conjugated with bacterial lipopolysaccharide, but not TiO2 NPs or lipopolysaccharide alone, trigger the immune response in human peripheral blood mononuclear cells and in isolated intestinal tissue. This indicates that TiO2 NPs may be important mediators in overcoming normal gut-cell hyporesponsiveness to endogenous luminal molecules, which may be particularly relevant to patients with inflammatory bowel disease, which is characterized by an abnormal intestinal permeability.
The National Cancer Institute tested TiO2 for possible carcinogenicity by the oral route of exposure by feeding rats and mice with TiO2 (size not specified) at doses 25,000 or 50,000 ppm TiO2 for 103 weeks. They concluded that TiO2 was not carcinogenic.92 Also, the study with rats fed diets containing up to 5 % TiO2 coated mica for 130 weeks showed no treatment-related carcinogenicity.93 Since the size and other TiO2 properties were not specified or determined, we cannot generalize this conclusion and we have to take into account other possible outcomes of this scenario in different exposure conditions (other size/crystalline structure of TiO2 etc.).
It should also be considered that due to the low pH in the stomach, the increased dissolution of the TiO2 particles may increase its bioavailability and may facilitate the entry of titanium ions into the blood circulation.94 Despite the relatively large consumption of TiO2 as a food additive, no studies on the effect of pH on its absorption and bioavailability have been found in the literature. This can be attributed to a general belief that TiO2is completely insoluble. However, this is not completely true, as TiO2 particles show a certain degree of solubility.33
Exposure to TiO2 NPs by inhalation
Inhalation exposure to TiO2 particles occurs pre-dominantly in occupational settings during production of TiO2 powders and manufacturing the products containing TiO2.95 The highest levels of exposure occur during packing, milling and site cleaning however, the empirical data regarding air-borne TiO2 particle concentrations in occupational settings is very limited. Fryzek et al.96 reported that packers, micronizers and addbackes had the highest TiO2 exposure levels measuring 6.2±9.4 mg/m3, whereas ore handlers had lower TiO2 exposure level of 1.1±1.1 mg/m3. Boffetta et al.97 reported that the yearly averaged estimated exposure to TiO2 dust in EU factories varied from 0.1 to 1.0 mg/m3, and the average levels ranged up to 5 mg/m3 for individual job categories. However, in these studies the particle size distribution has not been determined. Nevertheless, the data indicate that in certain jobs categories the exposure exceed the values of time-weighted average (10 h TWA) concentrations of 2.4 mg/m3 for submicron-sized TiO2 and 0.3 mg/m3 for nano-sized TiO2, which are recommended as exposure limits by National Institute for Occupational Safety and Health (NIOSH).98
Potential hazards of inhalation exposure to TiO2 NPs
The lung consists of about 2300 km of airways and 300 million alveoli. The epithelium of airways is protected by a viscous layer of mucus, and is a relatively robust barrier. In alveoli, the barrier between the alveolar wall and the capillaries is very thin, about 0.5 μm. Thus, the large surface area of the alveoli and the intense air-blood contact in this region makes the alveoli less protected against environmental damage than other parts of the respiratory system.75 The clearance of particles from the upper airways is achieved through the mucociliary escalator, while clearance from the deep lung is supposed to be achieved predominantly by macrophage phagocytosis. Deposited particles can lead to the activation of cytokine production and inflammation by macrophages and epithelial cells. It has been reported that besides the pulmonary and systemic inflammation, inhaled insoluble NPs can also accelerate atherosclerosis and alter the cardiac autonomic function.99–102
Following administration of nano-sized TiO2 to rats by inhalation the particles were detected in the cytoplasm of all lung-cell types in a non-membrane bound manner.17 Ferin et al.103 reported that 20 nm- sized TiO2 particles penetrate more easily into the pulmonary interstitial space of rats than 250 nm-sized TiO2 particles. Three-month inhalation exposure in rats demonstrated that the clearance of 20 nm TiO2 particles was significantly slower than that of 200 nm TiO2 particles, and more particles translocated to interstitial sites and regional lymph nodes.104 Geiser et al.14 confirmed that alveolar macrophages were not primarily responsible for the uptake and clearance of TiO2 NPs. These findings are in agreement with the known size limitations of uptake processes such as phagocytosis, which is thought to be restricted to particles that are 1 to 5 μm in size, while NPs might escape macrophage phagocytosis.101,105
Inhaled TiO2 NPs can enter the alveoli of the lung and consequently the blood circulation106,107 and can then translocate to other organs.102,108,109 In addition to several reports on the absence of toxicity following the inhalation of TiO2 NPs in rodents, the majority of lung-inhalation and instillation studies have pointed out obvious toxic effects, like inflammation and damage to pulmonary epithelium.110 The studies also showed that TiO2 NPs induced greater pulmonary inflammation and tissue damage than an equal dose of submicron-sized TiO2 particles. The greater toxicity of TiO2 NPs has been explained as being related to their larger surface area and their increased internalization.111 Multiple studies showed the reversibility of the inflammatory response after cessation of the exposure to TiO2 particles. After a single instillation exposure to different types of submicron- and nano-sized TiO2, acute inflammatory response returned to control levels within one week112 or 90 days113 after the instillation. In mice that were exposed to TiO2 NPs (2–5 nm) by whole body inhalation (0.77 or 7.22 mg/m3 4 h/day 10 days) the recovery was observed during the third week after exposure.114
Pulmonary toxicity studies suggest that, besides the particle size and surface area, crystal structure and surface treatment are also important parameters. Warheit et al.115 demonstrated higher pulmonary toxicity of anatase than rutile TiO2 NPs. These observations were confirmed in a recent study by Roursgaard et al.116 who showed that the intrat-racheal instillation of submicron- and nano-sized rutile, nano-sized anatase, or amorphous TiO2 to mice induced a dose-dependent acute inflammation, while subchronic inflammation was apparent only in mice exposed to nano-sized rutile and amorphous TiO2.
Recently, toxicogenomic studies were published that may contribute to a better understanding of the mechanisms of TiO2-mediated pulmonary toxicity. In mice exposed to a single intratracheal dose (0.1 or 0.5 mg/kg BW) of TiO2 with an average particle size of 20 nm Chen et al.117 showed that changes in the morphology and histology of the lungs were associated with the differential expression of hundreds of genes, including those involved in cell cycle regulation, apoptosis, chemokines, and complement cascades. In particular, TiO2 NPs upregulated the expression of the placenta growth factor and other chemokines that are associated with pulmonary emphysema and alveolar epithelial cell apoptosis. Park et al.118 showed that exposure of mice to nano-sized TiO2 (5–50 mg /kg BW) by a single intratracheal instillation can, in addition to chronic inflammation, also trigger an autoimmune response. They found that many classes of genes related to antigen presentation and the induction of chemotaxis of immune cells were over-expressed.
The studies have shown that submicron-sized TiO2 119 and nano-sized TiO2 120,121 induce lung tumors in chronically exposed rats. TiO2 NPs induced a significantly increased number of lung tumors during inhalation exposure to 10 mg/m3 (18 h/day, 2 years), while submicron-sized TiO2 increased the number of lung tumors at exposure to 250 mg/ m3 (6 h/day 2 years). In contrast, no tumours were observed in similarly exposed mice and hamsters.121,122 These apparent species differences suggest that the experimentally induced lung tumours may be a rat-specific, threshold phenomenon, depending on lung overloading accompanied by chronic inflammation to exert the observed tumorigenic response. Comparative toxicological studies of the development and possible progression of the lung response in rats, mice and hamsters exposed to a range of concentrations of submicron- or nano-sized TiO2 over a period of 90 days showed distinct species differences in the lung responses. Rats and mice had similar lung burdens and clearance rates, while hamsters showed higher clearance rates. At high lung-particle burdens, rats showed a marked progression of the histopathological lesions during the post-exposure period, while mice and hamsters showed minimal initial lesions with apparent recovery during the post-exposure period.123,124 It has been thus argued that the dose response data from inhalation studies in rats should not be used when extrapolating the cancer risk to humans.95 However, clearance of insoluble particles is in humans slower than in rats.125 In addition, it has been shown that the lung-tumour response to exposure to non-soluble particles can be predicted by the particle surface area dose without the need to account for overloading.98 Therefore, for workers with a high dust exposure the doses that cause overloading in rats may be relevant for estimating the health risk for humans.
Animal studies showed also other adverse effects after inhalation exposure to TiO2 particles. Nurkiewicz et al.109 showed that exposure to TiO2 particles may cause cardiovascular effects at concentrations below those causing adverse pulmonary effects. In rats exposed to submicron-sized TiO2 m) or nano-sized TiO2 (<1 μ (21 nm) at airborne exposures aimed at achieving similar particle mass deposition in the lungs (nano-sized: 1.5–12 mg/ m3, 240–720 min; submicron-sized: 3–15 mg/m3, 240–480 min) they observed systemic microvessel dysfunction in the absence of pulmonary inflammation or lung damage. The effect was related to the adherence of polymorphonuclear leukocytes to the microvessel walls and the production of ROS in the microvessels. As already described previously, inhalation exposure to TiO2 NPs may cause immune responses and neurotoxic effects that may lead to respiratory allergy and higher risk of developing asthma, respectively.
It has been reported that TiO2 NPs can translocate to the central nervous system following nasal instillation, potentially via the olfactory bulb, and accumulate mainly within the cerebral cortex, thalamus and hippocampus.22,29,126 The absorption appears to occur via neuronal transport, bypassing the blood-brain barrier.29,126 The main target is the hippocampus, where TiO2 NPs caused morphological alteration and the loss of neurones. In addition, TiO2 induced oxidative stress and an inflammatory response within the whole brain, with anatase nano-TiO2 inducing a stronger inflammatory response than rutile. However, from these studies it is not clear to what extent large local doses during nasal instillation reflect inhalation exposure.
Human epidemiological studies
Several case reports described adverse health effects in workers with potential TiO2 exposure that later lead to epidemiological studies of a relationship between occupational exposure and observed cases.98 The lung particle analyses indicated that workers exposed to respirable TiO2 had particle retention in their lungs that included TiO2, silica, and other minerals, sometimes years after cessation of exposure. In most cases of tissue-deposited TiO2 was associated with a local macrophage response and fibrosis that was generally mild. In one case papillary adenocarcinoma and TiO2 associated pneumoconiosis was reported in the lung of a 53-year-old male who had been engaged in packing TiO2 for about 13 years and had 40-year smoking history.127 The cohort epidemiological studies undertaken in the USA 96,128 did not report excess risks of lung cancer; nor did a Canadian population-based case-control study.129 The retrospective cohort lung cancer mortality study130, which included workers in the TiO2 production industry in six European countries, showed a small but significant elevation in lung cancer mortality among male TiO2 workers when compared to the general population. However, the data did not suggest an exposure-response relation.
TiO2 has been classified by the International Agency for Research on Cancer (IARC) as an IARC Group 2B carcinogen, “possibly carcinogenic to humans” by inhalation.131 Although the IARC working group concluded that the epidemiological studies on TiO2 provide inadequate evidence of carcinogenicity, they considered that the results from animal studies of inhalation and intratracheal instillation provide sufficient evidence to classify TiO2 in Group 2B.132 Also NIOSH98 has recently classified TiO2 NPs as a potential occupational carcinogen but considered that there is insufficient evidence at this time to classify also submicron-sized TiO2 as a potential occupational carcinogen. NIOSH also recommended new exposure limits at 2.4 mg/m3 for submicron-sized TiO2 and 0.3 mg/m3 for nano-sized TiO2, as time-weighted average concentrations for up to 10 hours per day during a 40-hour work week.
Exposure to TiO2 NPs through body implants
A few-nanometres-thick layer of amorphous TiO2 is commonly formed on the surface of orthopaedic and dental implants made of titanium metal or its alloys. In non-moving implants (hip stems, plates, screws, etc.) this does not appear to represent the same kind of risk for the body as free TiO2 NPs discussed in previous sections. However, this is not the case for wear-exposed implants, such as hip and knee joints. There are many reports proving that under mechanical stress or altered physiological conditions, Ti-based implants can release biologically relevant amounts of debris, in both the micrometre and nanometre ranges, that can migrate to the surrounding tissues. During the wear process, a thin amorphous oxide layer is continuously being created and removed, resulting in large numbers of titanium particles. It is increasingly being suggested that they are associated with major inflammation and systemic diseases.133 Furthermore, increasing numbers of reports indicate that the delayed hypersensitivity to titanium and its oxides may constitute a health risk for individuals with higher susceptibility.134–136
The effects of the TiO2 particles released from implants were investigated by Wang et al.137 in rats by intra-articular injection of 0.2 to 20 mg of anatase nano-TiO2 per kg BW. Their results demonstrate that particles can potentially affect major organs like the heart, lung and liver. Generally, the maximum diameter of particles that move across the synovial capillary wall was suggested to be 50 nm. The released TiO2 NPs resulted in synovial hypotrophy, lymphocyte and plasma infiltration, and fibroblast proliferation in the knee joint. Oxidative stress and lipid peroxidation was detected in exposed synovial fluid. Seven days after the initial exposure a brown particulate deposit was observed in vascular endothelial cells and in alveolar macrophages. Similar results have been reported by Urban et al.138, who found TiO2 particles in the liver and in the spleen of the patients with implants. TiO2 NPs were observed in joint simulators and in joint periprosthetic tissues. Margevicius et al.139 characterized the debris around the total hip joint prosthesis and found up to 140.109 particles/g dry weight, in diameters ranging from 0.58 to 100 μm. Agins et al.140 found concentration of wear particles in the tissue adjacent to a prosthesis in the range between 56 μg/g and 3.7 mg/g dry weight. Thus, due to the natural tendency of titanium to oxidise, Ti-based implants should not be neglected as a possible source of TiO2 exposure.
On the other hand, the man-made (crystalline) TiO2 coatings on the surfaces of pure Ti or Ti alloys are reported to be able to modulate protein absorption, cell adhesion, osseointegration and bone mineralization at the bone-biomaterial interface, both in vivo and in vitro.141,142 For this reason, the development of a more stable crystalline titania coating on Ti-based implants is in progress.143
Environmental pollution by TiO2 NPs
Toxic effects of TiO2 NPs on aquatic organisms
The trend in the production of NPs is likely to lead to increasing amounts of nano-powders in the air, water and soil, which will consequently affect living organisms. Labielle et al.68 demonstrated that 25 % of Al(OH)3-coated TiO2 particles from sunscreens are dispersed as a stable colloid and become available to microorganisms and filter-feeders, while the remaining 75 % are probably incorporated into geogenic sediments, where they could become available to benthic fauna. Solar UV iradiation may penetrate as far as 20 m in the water column 144 and therefore photo-activate the dispersed particles, which may have an adverse effect on various aquatic organisms.
Freshwater algae show low-to-moderate susceptibility to TiO2 exposure, with more pronounced toxic effects in the presence of UV irradiation. It has also been shown that nano-sized TiO2 is significantly more toxic to algae Pseudokirchneriella sub-capitata than submicron-sized TiO2.145 Hund-Rinke and Simon 146 reported that UV irradiated 25 nm TiO2 NPs are more toxic to green freshwater algae Desmodesmus subspicatus than UV irradiated 50 nm particles, which is in agreement with Hartmann et al.147 UV irradiated TiO2 NPs also inactivated other algae species such as Anabaena, Microcystis, Melsoira148 and Chroococcus.149 It was demonstrated that smaller particles have a greater potential to penetrate the cell interior than submicron-sized particles and larger aggregates. Studies have shown that the amount of TiO2 adsorbed on algal cells can be up to 2.3 times their own weight.142
Nano-sized TiO2 generally shows low or no acute toxicity in both invertebrates146 and vertebrates.150 However, exposure of Daphnia magna to 20 ppm TiO2 for 8 consecutive days was found to cause 40 % mortality.151 Zhu et al.152 showed minimal toxicity to D. magna after 48 h exposure, while upon chronic exposure for 21 days, D. magna suffered severe growth retardation and mortality. A significant amount of nano-sized TiO2 was found also accumulated in the body of the animals. Similar findings with coated nano-sized TiO2 (T-Lite™ SF, T-Lite™ SF-S and T-Lite™ MAX; BASF SE) were reported by Wiench et al.153 Biochemical measurements showed that exposure to TiO2 NPs induces significant concentration-dependent antioxidant enzyme activities in D. magna154. Lee et al.155 showed that 7 and 20 nm-sized TiO2 induced no genotoxic effect in D. magna and in the larva of the aquatic midge Chironomus riparius.
No acute effects of nano-sized TiO2 were observed in Danio rerio (zebrafish) embryos.156 Exposure of rainbow trout to TiO2 NPs triggered lipid peroxidation, influence on the respiratory tract, disturbance in the metabolism of Cu and Zn, induction of intestinal erosion 157 and accumulation in kidney tissue.158 Linhua et al.159 exposed juvenile carp to 100 and 200 mg/ml of particles and TiO2 observed no mortality. However, the fish suffered from oxidative stress and pathological changes in gill and liver. In the infaunal species Arenicola marina, exposure to TiO2 NPs in sediment caused sub-lethal effects including decrease in casting rate and increase in cellular and DNA damage.160 Aggregated particles were visible in the lumen of the gut, but no uptake through the gut or the skin was observed.
Zhu et al.161 were the first to provide evidence that TiO2 NPs (21 nm) can transfer from daphnia to zebrafish by dietary exposure. Hence, dietary intake could be a major route of exposure to NPs for high trophic level aquatic organisms. Ecological research should therefore focus, not only on the concentration of NPs in the environment, but also on its bioconcentration, bioaccumulation and biomagnification. In addition it has been shown that TiO2 NPs can increase accumulation of other environmental toxicants: enhanced accumulation of cadmium (Cd) and arsenic (As) was found in carp in the presence of TiO2 NPs.162,163 The strong adsorption capacity for Cd and As was explained by the large specific surface area and strong electrostatic attraction of TiO2 NPs that contribute to facilitated transport into different organs.
In vitro, in the hemocytes of the marine mussel Mytilus hemocytes, suspension of TiO2 NPs (Degussa P25, 10 μg/ml) stimulated immune and inflammatory responses, such as lysozyme release, oxidative burst and nitric oxide production.164 Vevers and Jha165 demonstrated the intrinsic genotoxic and cytotoxic potential of TiO2 NPs on a fish-cell line derived from rainbow-trout gonadal tissue (RTG-2 cells) after 24 h of exposure to 50 μg/ml. Reeves et al.166 demonstrated a significant increase in the level of oxidative DNA damage in goldfish cells, and suggested that damage could not repaired by DNA repair mechanisms. Another suggestion from the mentioned study was that hydroxyl radicals are generated also in the absence of UV light. It has been shown that fish cells are generally more susceptible to toxic/oxidative injury than mammalian cells.
Toxic effects of TiO2 NPs on soil organisms
Drobne et al.167 used the terrestrial arthropod Porcellio scaber as a test organism for determining the cytotoxic effect of TiO2 NPs (anatase). The animals were exposed to TiO2 NPs of two different sizes (25 nm and 75 nm) in the concentration range 10–1000 μg TiO2/g dry food for 3 to 14 days. No adverse effects, such as mortality, body weight changes or reduced feeding, were observed. In fact, quite the opposite, an enhanced feeding rate, food absorption efficiency and increase in catalase activity were observed. The intensity of these responses appeared to be time- but not dose-dependent. It should also be noted that the concentrations tested in this study were much higher than the predicted concentration (4.8 μg/g soil) at high emission scenario of nano-sized TiO2.168 Using the same test organism another group169 showed that exposure to TiO2 NPs induced destabilization of cell membrane in the epithelium of digestive glands isolated from exposed animals. They also showed that this effect can be observed after just 30 minutes of exposure.
TiO2 NPs appeared to be more toxic to nematode Caenorhabditis elegans than submicron-sized TiO2. 170 At a concentration of 1 mg/l, 7 nm particles affected its fertility and survival rate and were more toxic than 20 nm anatase particles.171 Similarly, Hu et al.172 showed that rutile particles (10–20 nm), at concentrations above 1 g/kg soil, can be bio-accumulated in earthworms, where they induce oxidative stress, inhibit the activity of cellulase and induce DNA and mitochondrial damage.
The effects of TiO2 NPs in plants
In addition to the toxic effects of TiO2 NPs, discussed in previous chapters, these NPs have been also shown to promote photosynthesis and nitrogen metabolism, resulting in the enhanced growth of spinach.173–175 It increases the absorption of light and accelerates the transfer and transformation of the light energy.176 It was also found that treatment with nano-sized TiO2 significantly increased the level of antioxidant enzymes, and decreased the ROS accumulation and malonyldialdehyde content in spinach chloroplasts under visible and UV irradiation.177 TiO2 NPs also increased the superoxide dismutase activity of germinating soybean, enhanced its antioxidant ability, and promoted seed germination and seedling growth.178
Potential desirable effects of TiO2 NPs
The same properties of nano-sized TiO2 that are associated with undesirable, harmful effects can be exploited for certain useful applications. The antimicrobial effect of photo-activated TiO2 NPs has been known since 1985179 and since then numerous reports have described its potential antimicrobial activity against numerous microorganisms.180 As expected, the antimicrobial effect increases with smaller particle sizes181; however, powder agglomeration may obscure this effect.151 When submitted to UV-C irradiation, TiO2 depresses the photo-activation and dark repair of DNA in bacteria, which increases the bactericidal efficiency of UV-C irradiation.182
TiO2 NPs have potential application in removing or minimizing the effect of the red tides183 that are associated with the harmful algae K.brevis that produces neurotoxic brevetoxin (PbTxs). Further, it can be used for disinfecting water, air and surfaces, with possible applications of TiO2 in form of solid films or free particles. Given its use for eradicating toxins, pollutants and spores from water and air, it can be classified as a broad-spectrum oxidizing/cleaning substance. However, an informed balance between the benefits of such a cleaning system and its potential adverse effects needs to be maintained.
NPs are offering new possibilities for in medicine either for diagnostic or therapeutic purposes. For instance recent studies indicate that magnetic NPs may be used in cancer treatment for targeted drug delivery.184 Several recent studies indicated that also cultured cancer cells are more sensitive to TiO2 NPs than normal cells.. Photo-activated TiO2 exhibited selective cytotoxicity against highly malignant breast-cancer cells MDA-MB-468, in comparison with non-malignant MCF-7 cells.185 Similarly, UV-irradiated Degussa P25 TiO2 NPs reduced viability of sarcoma cells but were not toxic to cultured fibroblasts MCR-5.186 In addition, UV-C photo-activated TiO2 particles inhibited aggregation of sarcoma cells with human platelets, thus preventing the formation of metastases. Cai et al.187 found that photo-activated (50 μg/ml), but not non-irradiated nano-sized TiO2, was lethal for HeLa cells in vitro and suppressed the growth of HeLa tumours in nude mice. Photo-activated TiO2 also showed antitumour activity in vivo against murine skin tumours.188 The potential usefulness of nano-sized TiO2 in cancer cell therapy has also been reported by other research groups.189–192 Cytotoxicity against different cancer cell lines appears to depend on the cell type, the particle concentration and the surface chemistry.
The appearance of multidrug-resistant tumour cells is a major obstacle to the success of chemotherapy. Song et al.193 reported an enhanced effect of nano-sized TiO2 on drug uptake by drug-resistant leukaemia cells under UV irradiation. Very promising is also the finding that cancer cells can be effectively destroyed by the use of X-ray irradiated nano-sized TiO2.194 A combination of monoclonal antibody conjugated nano-sized TiO2 with photoinduction195 and electroporation196 have also been proposed for selective cancer treatment. The monoclonal antibodies would enable selective targeting of cancer cells, photoinduction would trigger local generation of radicals and electroporation would accelerate the delivery of nano-sized TiO2 into the cancer cells. A novel possibility of cancer treatment was recently suggested197, in which TiO2 NPs and folic acid were coupled and shown to be internalized by HeLa cells via the folate receptor.
Where we are and where to go
The mechanistic toxicological studies showed that TiO2 NPs induced adverse effects are predominantly mediated by oxidative stress, which may lead to cell damage, genotoxic effects, inflammatory responses and changes in cell signalling. The studies also showed that these effects strongly depend on numerous chemical and physical characteristics of the TiO2 particles: size, crystal structure, specific surface area, particle shape, purity, surface charge, solubility, agglomeration rate, photo-activation, etc. TiO2 particles are without doubt associated with the hazardous properties, and the risk for human health and environment depends on the route and extent of exposure.
Based on the widespread use of creams with SPF based on nano-sized TiO2, human exposure to TiO2 NPs by dermal applications is apparently enormous. In vitro studies with skin models showed that TiO2 NPs are taken up by keratinocytes, fibroblasts, and melanocytes, in which they cause toxic effects that are not different from the effects observed in other cell types. Current experimental evidence indicates that TiO2 NPs do not penetrate through healthy skin and thus do not reach viable skin cells and distribute to other organs and tissues. However, the data on TiO2 NPs skin penetration during long-term or repeated exposure and in the presence of UV, which is actually characteristic for real life exposure, are insufficient. Therefore, there is no simple answer to the question regarding safety of the use of TiO2 NPs in sunscreens. The safety of the use of TiO2 in cosmetics is often argumented by the claim, that it has been used for decades without observing any adverse effects on human health. This, however, is not completely true, as no monitoring and post market health surveillance has been conducted, neither for submicron-sized nor for nano-sized TiO2 in sunscreens. Such surveillance is currently impossible, since current legislation does not require labelling whether the products contain nano-sized TiO2, which is also incorrect to customers who have no possibility to make a choice whether to use or not the sunscreen containing nano-sized TiO2. In our opinion dermal applications of TiO2 NPs as sunscreen should be limited until appropriate long-term experimental studies confirm their harmlessness. It is undeniable that long-term sun exposure can induce skin cancer. It is questionable, however, whether people are, by using sunscreens, actually encouraged to expose themselves to the sun instead of avoiding it, and if the benefit provided by TiO2 as a protection from UV compensates for the potential harm.
The available data on absorption, distribution, elimination or any consequent adverse effects after oral exposure to specific TiO2 NPs are extremely limited. TiO2 NPs have been shown to be absorbed from gastrointestinal tract and distributed to other organs, however this was observed at extremely high, for human exposure, irrelevant doses. On the other hand, it has been shown that at lower concentrations TiO2 may induce different adverse effects. TiO2 is an approved food additive with the limit set at 1 % by weight of the food; however, neither the size nor the structure is defined. It has been estimated that the average daily exposure to TiO2 from food, medicines and toothpaste is around 5 mg/individual (i.e., about 0.07 mg/kg BW)198, which is a much lower dose than those that showed adverse effects in experimental animals. Currently there is no data if, and what proportion of TiO2 NPs is absorbed at doses relevant for human exposure, and how different food matrices affect behaviour and absorption of TiO2 NPs. However, even if very small portion of consumed nano-sized TiO2 is absorbed from gastrointestinal tract and distributed to distant organs, this brings into question accumulation of TiO2 NPs that may, through a constant lifetime oral exposure, reach concentrations that would trigger adverse effects. Another important question, which should not be neglected is, whether low exposure may trigger symptoms in subjects with an underlying susceptibility. Before in vivo toxicokinetic data for nano-sized TiO2 are available, no conclusion about the risk of nano-sized TiO2 by oral exposure is possible. Therefore, it should be seriously reconsidered if the use of TiO2 NPs in nutrition and pharmacy just to shade or stabilise the products is justified at all.
Inhalation seems to be the most vulnerable entrance point of the TiO2 NPs and the toxic effects of inhalation exposure are therefore by far the most studied. Animal studies showed that on inhalation exposure the particles deposit in the lung, where they may cause chronic inflammation and lung-tissue damage, which can lead to lung-tumour development. The important finding is that inhalation exposure to nano-sized TiO2 represents a higher health risk than exposure to submicron-sized TiO2 particles. Experimental data indicate that on inhalation exposure nano-sized TiO2 may translocate to distant organs and tissues, which may be associated with systemic effects, such as allergy, asthma and cardiovascular effects, however further studies are needed to confirm these observations and to clarify if they are associated with increased risk for humans. In the scientific community there is still a debate whether the data from in vivo rodent toxicity studies are reliable enough to predict the effects in humans in particular regarding mode of exposure (instillation vs. inhalation exposure) and the differences in susceptibility between different experimental species. Nevertheless, the experimental evidence, although not clearly supported by human epidemiological data, was considered to be sufficient to classify TiO2 (unrespectable to particle size and form) as “possible human carcinogen” upon inhalation exposure by IARC. Recently also NIOSH classified nano-sized, but not submicron-sized TiO2 as occupational carcinogen, and accordingly established different limit values for occupational inhalation exposure for nano-sized (0.3 mg/m3) and submicron-sized (2.4 mg/m3) TiO2. At present, through environmental air pollution general population is probably not at risk. However, occupational exposure should be controlled and protective measures applied, not only in TiO2 production industries, but also in certain areas of TiO2 applications; for instance when removing paints or destroying TiO2 containing materials the workers may be exposed to high concentrations of TiO2. Thus, accurate, portable, and cost effective measurement techniques should be developed and applied for effective exposure control and protection.
TiO2 can also be released within the human body as a result of the wear of Ti-based implants. The released particles cause local inflammation, but even more importantly they distribute over the body and can potentially cause systemic effects. Generally the benefit provided by the implant compensates for the potential harm, in particular in the cases where there is no better alternative to the Ti-based implants available. However, although there is no direct experimental evidence that released TiO2 can be deposited in the body or can cause systemic effects, it can be postulated from other exposure studies and mechanistic data that at least for individuals with hypersensitivity to titanium such exposure may represent a permanent health threat. Thus, it should be obligatory to test the patients for titanium hypersensitivity prior to implantation of titanium based implants.
Due to the widespread use TiO2 can enter aquatic and terrestrial environment and potentially affect the indigenous organisms. Although data from acute ecotoxicity tests in crustaceans, fish and algae indicate a low toxic potential of TiO2 NPs for aquatic species, when chronic exposure was applied TiO2 NPs induced a range of sub-lethal adverse effects. In addition it has been shown that nano-sized TiO2 can enter the freshwater food chain, which means that it can be transferred from lower to higher trophic organisms, including humans.
Taken together, the overall exposure of an average individual TiO2 NPs is not known; there are still opened questions regarding toxicokinetics and specific organ toxicity of TiO2 NPs, in particular at oral and dermal exposure, and thus it is impossible to make a reliable quantitative risk assessment. One of the main observations of this review is that, due to the versatility of the TiO2 NPs in terms of particle size, shape, crystal structure, dispersion in biological surroundings (bioavailability) and UV-induced photocatalytic activity, no single conclusion can be drawn, since different forms of TiO2 may act very differently. Until we know more, in our opinion TiO2 NPs should be used with great care, in particular in food and cosmetics. The least that should be done for the consumer is that a declaration of nano-sized TiO2 in these products is obligatory, so that we will have the choice whether to use it or not.
Acknowledgments
The study was supported financially by the Slovenian Research Agency within the research programmes “Nanostructured Materials” (P2-0084) and “Ecotoxicology, Toxicological Genomics and Carcinogenesis (P1-0245). We thank Prof. Roger Pain and Assist. Prof. Paul McGuiness for critical reading of the manuscript. Authors would also like to thank Dr. Zoran Samardžija for field-emission-gun scanning electron microscopy observations of the TiO2 powders.
References
- 1.Ophus EM, Rode L, Gylseth B, Nicholson DG, Saeed K. Analysis of titanium pigments in human lung tissue. Scand J Work Environ Health. 1979;53:290–6. doi: 10.5271/sjweh.3104. [DOI] [PubMed] [Google Scholar]
- 2.Lindenschmidt RC, Driscoll KE, Perkins MA, Higgins JM, Maurer JK, Belfiore KA. The comparison of a fibrogenic and two nonfibrogenic dusts by bronchoalveolar lavage. Toxicol Appl Pharmacol. 1990;102:268–81. doi: 10.1016/0041-008x(90)90026-q. [DOI] [PubMed] [Google Scholar]
- 3.Backus R. Lighting up time for TiO2. Industrial Minerals. 2007;473:28–39. [Google Scholar]
- 4.Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol. 2009;43:4227–33. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- 5.Rowe RC, Sheskey PJ, Weller PJ. Fourth ed. London: Pharmaceutical Press, London, United Kingdom, and the American Pharmaceutical Association; 2003. Handbook of pharmaceutical excipients. [Google Scholar]
- 6.Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, et al. Nanomaterials in the environment: behaviour, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27:1825–51. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 7.Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–41. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 8.Kosmulski M. The pH-dependent surface charging and points of zero charge V. Update. J Colloid Interface Sci. 2011;353:1–15. doi: 10.1016/j.jcis.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Tang H, Prasad K, Sanjinbs R, Schmid PE, Levy F. Electrical and optical properties of Ti02 anatase thin films. J Appl Phys. 2004;75:2042–7. [Google Scholar]
- 10.Augustynski J. The role of the surface intermediates in the photoelectrochemical behaviour of anatase and rutile TiO2. Electrochimica Acta. 1993;38:43–6. [Google Scholar]
- 11.Hewitt JP. Titanium dioxide: a different kind of sunshield. Drug Cosmet Ind. 1992;151:26–32. [Google Scholar]
- 12.Fujishima A, Zhang X, Tryk DA. TiO2 photocatalysis and related surface phenomena. Surf Sci Rep. 2008;63:515–82. [Google Scholar]
- 13.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;68:1794–807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 14.Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am J Respir Cell Mol Biol. 2008;38:371–6. doi: 10.1165/rcmb.2007-0138OC. [DOI] [PubMed] [Google Scholar]
- 15.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 16.Stearns RC, Paulauskis JD, Godleski JJ. Endocytosis of ultrafine particles by A549 cells. Am J Respir Cell Mol Biol. 2001;24:108–15. doi: 10.1165/ajrcmb.24.2.4081. [DOI] [PubMed] [Google Scholar]
- 17.Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–60. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothen-Rutishauser BM, Schurch S, Haenni B, Kapp N, Gehr P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ Sci Technol. 2006;40:4353–9. doi: 10.1021/es0522635. [DOI] [PubMed] [Google Scholar]
- 19.Kocbek P, Teskac K, Kreft ME, Kristl J. Toxicological aspects of long-term treatment of keratinocytes with ZnO and TiO2 nanoparticles. Small. 2010;6:1908–17. doi: 10.1002/smll.201000032. [DOI] [PubMed] [Google Scholar]
- 20.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi HB, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. Acs Nano. 2008;2:2121–34. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson K, Stone V, Clouter A, Renwick L, MacNee W. Ultrafine particles. Occup Environ Med. 2001;58:211–6. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 23.Dunford R, Salinaro A, Cai LZ, Serpone N, Horikoshi S, Hidaka H, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. Febs Lett. 1997;418:87–90. doi: 10.1016/s0014-5793(97)01356-2. [DOI] [PubMed] [Google Scholar]
- 24.Gurr JR, Wang ASS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Persp. 2007;115:1631–7. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu N, Zhu Z, Zhao X, Tao R, Yang X, Gao Z. Nano titanium dioxide photocatalytic protein tyrosine nitration: a potential hazard of TiO2 on skin. Biochem Biophys Res Commun. 2008;370:675–80. doi: 10.1016/j.bbrc.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Park EJ, Yi J, Chung YH, Ryu DY, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 2008;180:222–9. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 28.Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, et al. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92:174–85. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 29.Wang JX, Chen CY, Liu Y, Jiao F, Li W, Lao F, et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183:72–80. doi: 10.1016/j.toxlet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Uchino T, Tokunaga H, Ando M, Utsumi H. Quantitative determination of OH radical generation and its cytotoxicity induced by TiO2-UVA treatment. Toxicol in Vitro. 2002;16:629–35. doi: 10.1016/s0887-2333(02)00041-3. [DOI] [PubMed] [Google Scholar]
- 31.Dodd NJ, Jha AN. Titanium dioxide induced cell damage: a proposed role of the carboxyl radical. Mutat Res. 2009;660:79–82. doi: 10.1016/j.mrfmmm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Petković J, Žegura B, Stevanović M, Drnovšek N, Uskoković D, Novak S, et al. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology. 2011;5:341–53. doi: 10.3109/17435390.2010.507316. [DOI] [PubMed] [Google Scholar]
- 33.Petković J, Küzma T, Rade K, Novak S, Filipič M. Pre-irradiation of anatase TiO2 particles with UV enhances their cytotoxic and genotoxic potential in human hepatoma HepG2 cells. J Hazard Mater. 2011 doi: 10.1016/j.jhazmat.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Barthel A, Klotz LO. Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biol Chem. 2005;386:207–16. doi: 10.1515/BC.2005.026. [DOI] [PubMed] [Google Scholar]
- 35.Kang JL, Moon C, Lee HS, Lee HW, Park EM, Kim HS, et al. Comparison of the biological activity between ultrafine and fine titanium dioxide particles in RAW 264.7 cells associated with oxidative stress. J Toxicol Environ Health, Part A. 2008;71:478–85. doi: 10.1080/15287390801906675. [DOI] [PubMed] [Google Scholar]
- 36.Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, et al. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Persp. 2002;110:797–800. doi: 10.1289/ehp.02110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JJ, Sanderson BJS, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res-Gen Tox En. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Xu A, Chai YF, Nohmi T, Hei TK. Genotoxic responses to titanium dioxide nanoparticles and fullerene in gpt delta transgenic MEF cells. Part Fibre Toxicol. 2009;6:3. doi: 10.1186/1743-8977-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu RR, Wang SL, Chao J, Shi DL, Zhang R, Sun XY, et al. Bio-effects of Nano-TiO2 on DNA and cellular ultrastructure with different polymorph and size. Mat Sci Eng C-Bio S. 2009;29:691–6. [Google Scholar]
- 40.Kang SJ, Kim BM, Lee YJ, Chung HW. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 41.Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Theogaraj E, Riley S, Hughes L, Maier M, Kirkland D. An investigation of the photo-clastogenic potential of ultrafine titanium dioxide particles. Mutat Res-Gen Tox En. 2007;634:205–19. doi: 10.1016/j.mrgentox.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG, Bertram TA. Effects of particle exposure and particle-elicited inflammatory cells on mutation in rat alveolar epithelial cells. Carcinogenesis. 1997;18:423–30. doi: 10.1093/carcin/18.2.423. [DOI] [PubMed] [Google Scholar]
- 44.Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;69:8784–9. doi: 10.1158/0008-5472.CAN-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li N, Ma LL, Wang J, Zheng L, Liu J, Duan YM, et al. Interaction between nano-anatase TiO2 and liver DNA from mice in vivo. Nanoscale Res Lett. 2010;51:108–15. doi: 10.1007/s11671-009-9451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vamanu CI, Cimpan MR, Hol PJ, Sornes S, Lie SA, Gjerdet NR. Induction of cell death by TiO2 nanoparticles: Studies on a human monoblastoid cell line. Toxicol in Vitro. 2008;22:1689–96. doi: 10.1016/j.tiv.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Palomaki J, Karisola P, Pylkkanen L, Savolainen K, Alenius H. Engineered nanomaterials cause cytotoxicity and activation on mouse antigen presenting cells. Toxicology. 2010;267:125–31. doi: 10.1016/j.tox.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 48.Larsen ST, Roursgaard M, Jensen KA, Nielsen GD. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol. 2010;106:114–7. doi: 10.1111/j.1742-7843.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36:1469–79. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- 50.Moon EY, Yi GH, Kang JS, Lim JS, Kim HM, Pyo S. An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J Immunotoxicol. 2011;81:56–67. doi: 10.3109/1547691X.2010.543995. [DOI] [PubMed] [Google Scholar]
- 51.Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40:4346–52. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 54.Liu SC, Xu LJ, Zhang T, Ren GG, Yang Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010;267:172–7. doi: 10.1016/j.tox.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Liu XY, Ren XF, Deng XY, Huo YA, Xie J, Huang H, et al. A protein interaction network for the analysis of the neuronal differentiation of neural stem cells in response to titanium dioxide nanoparticles. Biomaterials. 2010;31:3063–70. doi: 10.1016/j.biomaterials.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 56.Scuri M, Chen BT, Castranova V, Reynolds JS, Johnson VJ, Samsell L, et al. Effects of titanium dioxide nanoparticle exposure on neuroimmune responses in rat airways. J Toxicol Environ Health, Part A. 2010;73:1353–69. doi: 10.1080/15287394.2010.497436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nohynek GJ, Schaefer H. Benefit and risk of organic ultraviolet filters. Regul Toxicol Pharmacol. 2001;333:285–99. doi: 10.1006/rtph.2001.1476. [DOI] [PubMed] [Google Scholar]
- 58.FDA . US Rockville, MD: 2000. Sunscreen drug products for over-the-counter human use, Final Monograph, Federal Register 64 27666. [PubMed] [Google Scholar]
- 59.Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61:685–92. doi: 10.1016/j.jaad.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 60.Breggin L, Falkner R, Jaspers N, Pendergrass J, Porter R. London: Affairs RIoI; 2009. Securing the promise of nanotechnologies towards transatlantic regulatory cooperation. [Google Scholar]
- 61.Serpone N, Dondi D, Albini A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg Chim Acta. 2007;360:794–802. [Google Scholar]
- 62.Salvador A, Chisvert A. Sunscreen analysis - A critical survey on UV filters determination. Anal Chim Acta. 2005;537:1–14. [Google Scholar]
- 63.Nohynek GJ, Antignac E, Re T, Toutain H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010;243:239–59. doi: 10.1016/j.taap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;373:251–77. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- 65.Salinaro A, Emeline AV, Zhao J, Hidaka H, Ryabchuk V, Serpone KN. Terminology, relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. Part II: Experimental determination of quantum yields (Technical Report) Pure Appl Chem. 1999;71:321–6. [Google Scholar]
- 66.Serpone N, Salinaro A, Hidaka H, Horikoshi S, Knowland J, Dunford R. Solar engineering. In: Morehouse JM, Hogan RE, editors. New York: ASME; 1998. [Google Scholar]
- 67.Jaroenworaluck A, Sunsaneeyametha W, Kosachan N, Stevens R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf Interface Anal. 2006;38:473–7. [Google Scholar]
- 68.Labiele J, Feng J, Botta C, Borschneck D, Sammut M, Cabie M, et al. Agging of TiO2 nanocomposites used in sunscreens. Dispersion and fate of the degradation products in aqueous environment. Environ Pollut. 2010;158:1–8. doi: 10.1016/j.envpol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Mills A, Le Hunte S. An overview of semiconductor photocatalysis. J Photoch Photobio A. 1997;108:1–35. [Google Scholar]
- 70.Wakefield G, Lipscomb S, Holland E, Knowland J. The effects of manganese doping on UVA absorption and free radical generation of micronised titanium dioxide and its consequences for the photostability of UVA absorbing organic sunscreen components. Photochem Photobiol Sci. 2004;37:648–52. doi: 10.1039/b403697b. [DOI] [PubMed] [Google Scholar]
- 71.Pan Z, Lee W, Slutsky L, Clark RA, Pernodet N, Rafailovich MH. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small. 2009;54:511–20. doi: 10.1002/smll.200800798. [DOI] [PubMed] [Google Scholar]
- 72.Kiss B, Biro T, Czifra G, Toth BI, Kertesz Z, Szikszai Z, et al. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp Dermatol. 2008;17:659–67. doi: 10.1111/j.1600-0625.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 73.Jin CY, Zhu BS, Wang XF, Lu QH. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem Res Toxicol. 2008;219:1871–7. doi: 10.1021/tx800179f. [DOI] [PubMed] [Google Scholar]
- 74.Yanagisawa R, Takano H, Inoue K, Koike E, Kamachi T, Sadakane K, et al. Titanium Dioxide Nanoparticles Aggravate Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. Exp Biol Med. 2009;234:314–22. doi: 10.3181/0810-RM-304. [DOI] [PubMed] [Google Scholar]
- 75.Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J Nanobiotechnology. 2004;21:12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tyner KM, Wokovich AM, Godar DE, Doub WH, Sadrieh N. The state of nano-sized titanium dioxide (TiO2) may affect sunscreen performance. Int J Cosmetic Sci. 2010;33:234–44. doi: 10.1111/j.1468-2494.2010.00622.x. [DOI] [PubMed] [Google Scholar]
- 77.Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, et al. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol. 1999;12:247–56. doi: 10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- 78.Pflucker F, Wendel V, Hohenberg H, Gartner E, Will T, Pfeiffer S, et al. The human stratum corneum layer: an effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacol Appl Skin Physiol. 2001;14:92–7. doi: 10.1159/000056396. [DOI] [PubMed] [Google Scholar]
- 79.Schulz J, Hohenberg H, Pflucker F, Gartner E, Will T, Pfeiffer S, et al. Distribution of sunscreens on skin. Adv Drug Deliv Rev. 2002;54:S157–63. doi: 10.1016/s0169-409x(02)00120-5. [DOI] [PubMed] [Google Scholar]
- 80.Schilling K, Bradford B, Castelli D, Dufour E, Nash JF, Pape W, et al. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci. 2010;9:495–509. doi: 10.1039/b9pp00180h. [DOI] [PubMed] [Google Scholar]
- 81.Senzui M, Tamura T, Miura K, Ikarashi Y, Watanabe Y, Fujii M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. J Toxicol Sci. 2010;35:107–13. doi: 10.2131/jts.35.107. [DOI] [PubMed] [Google Scholar]
- 82.Tan MH, Commens CA, Burnett L, Snitch PJ. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas J Dermatol. 1996;37:185–7. doi: 10.1111/j.1440-0960.1996.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 83.Wu J, Liu W, Xue C, Zhou S, Lan F, Bi L, et al. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Letters. 2009;191:1–8. doi: 10.1016/j.toxlet.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 84.Sadrieh N, Wokovich AM, Gopee NV, Zheng JW, Haines D, Parmiter D, et al. Lack of Significant Dermal Penetration of Titanium Dioxide from Sunscreen Formulations Containing Nano- and Submicron-Size TiO(2) Particles. Toxicol Sci. 2010;115:156–66. doi: 10.1093/toxsci/kfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 86.FDA [Internet] US Rockville, MD: 2010. Food and drugs chapter I, Listing of color additives exempt from certification. Federal Register 21CFR73. [cited 2011 October 14]. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=73&showFR=1. [Google Scholar]
- 87.Kumagai K. Uber den Resorptionvergang der corpuscularen Bestandteile im Darm. 192:429–31. [Google Scholar]
- 88.Jani PU, McCarthy DE, Florence AT. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int J Pharm. 1994;105:157–68. [Google Scholar]
- 89.Wang JX, Zhou GQ, Chen CY, Yu HW, Wang TC, Ma YM, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168:176–85. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Duan Y, Liu J, Ma L, Li N, Liu H, Wang J, et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials. 2010;31:894–9. doi: 10.1016/j.biomaterials.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Powell JJ, Harvey RSJ, Ashwood P, Wolstencroft R, Gershwin ME, Thompson RPH. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14:99–105. doi: 10.1006/jaut.1999.0342. [DOI] [PubMed] [Google Scholar]
- 92.National Cancer Institute. Washington, DC: U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health; 1979. Bioassay of titanium dioxide for possible carcinogenicity. [Google Scholar]
- 93.Bernard BK, Osheroff MR, Hofmann A, Mennear JH. Toxicology and carcinogenesis studies of dietary titanium dioxide-coated mica in male and female Fischer 344 rats. J Toxicol Env Health. 1990;29:417–29. doi: 10.1080/15287399009531402. [DOI] [PubMed] [Google Scholar]
- 94.Okazaki Y, Gotoh E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials. 2005;26:11–21. doi: 10.1016/j.biomaterials.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 95.Hext PM, Tomenson JA, Thompson P. Titanium dioxide: inhalation toxicology and epidemiology. Ann Occup Hyg. 2005;49:461–72. doi: 10.1093/annhyg/mei012. [DOI] [PubMed] [Google Scholar]
- 96.Fryzek JP, Chadda B, Marano D, White K, Schweitzer S, McLaughlin JK, et al. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J Occup Environ Med. 2003;45:400–9. doi: 10.1097/01.jom.0000058338.05741.45. [DOI] [PubMed] [Google Scholar]
- 97.Boffetta P, Soutar A, Cherrie JW, Granath F, Andersen A, Anttila A, et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control. 2004;157:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 98.NIOSH . 2011. Occupational Exposure to Titanium Dioxid Department Of Health And Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. [Google Scholar]
- 99.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 100.Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349:1582–7. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 101.McGuinnes C, Duffin R, Brown S, Mills NL, Megson IL, MacNee W, et al. Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro. Toxicol Sci. 2011;119:359–68. doi: 10.1093/toxsci/kfq349. [DOI] [PubMed] [Google Scholar]
- 102.Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ Health Perspect. 2001;109:523–27. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferin J, Oberdorster G, Penney DP. Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Cell Mol Biol. 1992;65:535–42. doi: 10.1165/ajrcmb/6.5.535. [DOI] [PubMed] [Google Scholar]
- 104.Oberdörster G, Ferin J, Lehnert BE. Correlation between particle-size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102:173–9. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Renwick LC, Donaldson K, Clouter A. Impairment of alveolar macrophage phagocytosis by ultrafine particles. Toxicol Appl Pharmacol. 2001;172:119–27. doi: 10.1006/taap.2001.9128. [DOI] [PubMed] [Google Scholar]
- 106.van Ravenzwaay B, Landsiedel R, Fabian E, Burkhardt S, Strauss V, Ma-Hock L. Comparing fate and effects of three particles of different surface properties: nano-TiO2, pigmentary TiO2 and quartz. Toxicol Lett. 2009;186:152–9. doi: 10.1016/j.toxlet.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 107.Kapp N, Studer D, Gehr P, Geiser M. Electron energy-loss spectroscopy as a tool for elemental analysis in biological specimens. Methods Mol Biol. 2007;369:431–47. doi: 10.1007/978-1-59745-294-6_21. [DOI] [PubMed] [Google Scholar]
- 108.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164:1665–8. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 109.Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, et al. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim HW, Ahn EK, Jee BK, Yoon HK, Lee KH, Lim Y. Nanoparticulate-induced toxicity and related mechanism in vitro and in vivo. J Nanopart Res. 2009;111:55–65. [Google Scholar]
- 111.Oberdorster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- 112.Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006;91:227–36. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- 113.Rehn B, Seiler F, Rehn S, Bruch J, Maier M. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol Appl Pharmacol. 2003;189:84–95. doi: 10.1016/s0041-008x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 114.Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology. 2007;230:90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 116.Roursgaard M, Jensen KA, Poulsen SS, Jensen NEV, Poulsen LK, Hammer M, et al. Acute and subchronic airway inflammation after intratracheal instillation of quartz and titanium dioxide agglomerates in mice. Scientific World Journal. 2011;11:801–25. doi: 10.1100/tsw.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen HW, Su SF, Chien CT, Lin WH, Yu SL, Chou CC, et al. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006;20:2393–5. doi: 10.1096/fj.06-6485fje. [DOI] [PubMed] [Google Scholar]
- 118.Park EJ, Yoon J, Choi K, Yi J, Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009;260:37–46. doi: 10.1016/j.tox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 119.Lee KP, Trochimowicz HJ, Reinhardt CF. Pulmonary Response of Rats Exposed to Titanium-Dioxide (TiO2) by Inhalation for 2 Years. Toxicol Appl Pharmacol. 1985;79:179–92. doi: 10.1016/0041-008x(85)90339-4. [DOI] [PubMed] [Google Scholar]
- 120.Borm PJA, Hohr D, Steinfartz Y, Zeittrager I, Albrecht C. Chronic inflammation and tumor formation in rats after intratracheal instillation of high doses of coal dusts, titanium dioxides, and quartz. Inhal Toxicol. 2000;12:225–31. doi: 10.1080/08958378.2000.11463217. [DOI] [PubMed] [Google Scholar]
- 121.Heinrich U, Fuhst R, Rittinghausen S, Creutzenberg O, Bellmann B, Koch W, et al. Chronic inhalation exposure of Wistar rats and 2 different strains of mice to diesel-engine exhaust, carbon-black, and titanium-dioxide. Inhal Toxicol. 1995;74:533–56. [Google Scholar]
- 122.Muhle H, Bellmann B, Creutzenberg O, Koch W, Dasenbrock C, Ernst H, et al. Pulmonary response to toner, TiO2 and crystalline silica upon chronic inhalation exposure in Syrian golden hamsters. Inhal Toxicol. 1998;10:699–729. [Google Scholar]
- 123.Bermudez E, Mangum JB, Asgharian B, Wong BA, Reverdy EE, Janszen DB, et al. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol Sci. 2002;70:86–97. doi: 10.1093/toxsci/70.1.86. [DOI] [PubMed] [Google Scholar]
- 124.Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77:347–57. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- 125.Hsieh TH, Yu CP. Two-phase pulmonary clearance of insoluble particles in mammalian species. Inhal Toxicol. 1998;102:121–30. [Google Scholar]
- 126.Wang JX, Liu Y, Jiao F, Lao F, Li W, Gu YQ, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology. 2008;254:82–90. doi: 10.1016/j.tox.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 127.Yamadori I, Ohsumi S, Taguchi K. Titanium dioxide deposition and adenocarcinoma of the lung. Acta Pathol Jpn. 1986;36:783–90. doi: 10.1111/j.1440-1827.1986.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 128.Chen JL, Fayerweather WE. Epidemiologic-study of workers exposed to titanium-dioxide. J Occup Environ Med. 1988;30:937–42. doi: 10.1097/00043764-198812000-00011. [DOI] [PubMed] [Google Scholar]
- 129.Ramanakumar AV, Parent ME, Latreille B, Siemiatycki J. Risk of lung cancer following exposure to carbon black, titanium dioxide and talc: results from two case-control studies in Montreal. Int J Cancer. 2008;122:183–9. doi: 10.1002/ijc.23021. [DOI] [PubMed] [Google Scholar]
- 130.Boffetta P, Soutar A, Cherrie JW, Granath F, Andersen A, Anttila A, et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 131.IARC . International Agency for Research on Cancer; Lyon, France: 2006. Carbon black, titanium dioxide, and talc. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 93. [PMC free article] [PubMed] [Google Scholar]
- 132.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006;74:295–6. doi: 10.1016/s1470-2045(06)70651-9. [DOI] [PubMed] [Google Scholar]
- 133.Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening-Current concepts. J Biomed Mater Res A. 2009;91:1252–62. doi: 10.1002/jbm.a.32625. [DOI] [PubMed] [Google Scholar]
- 134.Sargeant A, Goswami T. Hip implants - Paper VI - Ion concentrations. Mater Design. 2007;28:155–71. [Google Scholar]
- 135.Valentine-Thon E, Schiwara HW. Validity of MELISA (R) for metal sensitivity testing. Neuroendocrinol Lett. 2003;241:57–64. [PubMed] [Google Scholar]
- 136.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–36. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 137.Wang JX, Fan YB, Gao Y, Hu QH, Wang TC. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials. 2009;30:4590–600. doi: 10.1016/j.biomaterials.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 138.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457–76. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 139.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994;76:1664–75. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 140.Agins HJ, Alcock NW, Bansal M, Salvati EA, Wilson PD, Pellicci PM, et al. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. J Bone Joint Surg Am. 1988;70:347–56. [PubMed] [Google Scholar]
- 141.Giavaresi G, Ambrosio L, Battiston GA, Casellato U, Gerbasi R, Finia M, et al. Histomorphometric, ultrastructural and microhardness evaluation of the osseointegration of a nanostructured titanium oxide coating by metal-organic chemical vapour deposition: an in vivo study. Biomaterials. 2004;25:5583–91. doi: 10.1016/j.biomaterials.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 142.Cui C, Liu H, Li Y, Sun J, Wang R, Liu S, et al. Fabrication and biocompatibility of nano-TiO2/titanium alloys biomaterials. Mater Lett. 2005;59:3144–48. [Google Scholar]
- 143.Drnovsek N, Daneu N, Recnik A, Mazaj M, Kovac J, Novak S. Hydrothermal synthesis of a nanocrystalline anatase layer on Ti6A4V implants. Surf Coat Tech. 2009;203:1462–68. [Google Scholar]
- 144.Tedetti M, Sempere R. Penetration of ultraviolet radiation in the marine environment. A review. Photochem Photobiol. 2006;82:389–97. doi: 10.1562/2005-11-09-IR-733. [DOI] [PubMed] [Google Scholar]
- 145.Aruoja V, Dubourguier H-C, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ. 2009;407:1461–68. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 146.Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut Res Int. 2006;134:225–32. doi: 10.1065/espr2006.06.311. [DOI] [PubMed] [Google Scholar]
- 147.Hartmann NB, Von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A. Algal testing of titanium dioxide nanoparticles--Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. 2010;269:190–7. doi: 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 148.Kim S-C, Lee D-K. Preparation of TiO2-coated hollow glass beads and their application to the control of algal growth in eutrophic water. Microchem J. 2005;80:227–32. [Google Scholar]
- 149.Hong J, Ma H, Otaki M. Controlling algal growth in photo-dependent decolorant sludge by photocatalysis. J Biosci Bioeng. 2005;99:592–7. doi: 10.1263/jbb.99.592. [DOI] [PubMed] [Google Scholar]
- 150.Velzeboer I, Hendriks AJ, Ragas AMJ, Van de Meent D. Aquatic ecotoxicity tests of some nanomaterials. Environ Toxicol Chem. 2008;27:1942–47. doi: 10.1897/07-509.1. [DOI] [PubMed] [Google Scholar]
- 151.Adams LK, Lyon DY, Alvarez PJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–32. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 152.Zhu X, Chang Y, Chen Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere. 2010;78:209–15. doi: 10.1016/j.chemosphere.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 153.Wiench K, Wohlleben W, Hisgen V, Radke K, Salinas E, Zok S, et al. Acute and chronic effects of nano- and non-nano-scale TiO(2) and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere. 2009;76:1356–65. doi: 10.1016/j.chemosphere.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 154.Kim KT, Klaine SJ, Cho J, Kim SH, Kim SD. Oxidative stress responses of Daphnia magna exposed to TiO(2) nanoparticles according to size fraction. Sci Total Environ. 408:2268–72. doi: 10.1016/j.scitotenv.2010.01.041. [DOI] [PubMed] [Google Scholar]
- 155.Lee S-W, Kim S-M, Choi J. Genotoxicity and ecotoxicity assays using the freshwater crustacean Daphnia magna and the larva of the aquatic midge Chironomus riparius to screen the ecological risks of nanoparticle exposure. Environ Toxicol Phar. 2009;281:86–91. doi: 10.1016/j.etap.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 156.Zhu X, Zhu L, Duan Z, Qi R, Li Y, Lang Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;433:278–84. doi: 10.1080/10934520701792779. [DOI] [PubMed] [Google Scholar]
- 157.Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;844:415–30. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 158.Scown TM, van Aerle R, Johnston BD, Cumberland S, Lead JR, Owen R, et al. High Doses of Intravenously Administered Titanium Dioxide Nanoparticles Accumulate in the Kidneys of Rainbow Trout but with no Observable Impairment of Renal Function. Toxicol Sci. 2009;109:372–80. doi: 10.1093/toxsci/kfp064. [DOI] [PubMed] [Google Scholar]
- 159.Linhua H, Zhenyu W, Baoshan X. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histpathological changes in Juvenile Carp (Cyprinus carpio) J Environ Sci. 2009;21:1459–66. doi: 10.1016/s1001-0742(08)62440-7. [DOI] [PubMed] [Google Scholar]
- 160.Galloway T, Lewis C, Dolciotti I, Johnston BD, Moger J, Regoli F. Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environ Pollut. 2010;158:1748–55. doi: 10.1016/j.envpol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 161.Zhu X, Wang J, Zhang X, Chang Y, Chen Y. Trophic transfer of TiO(2) nano-particles from Daphnia to zebrafish in a simplified freshwater food chain. Chemosphere. 2010;79:928–33. doi: 10.1016/j.chemosphere.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 162.Zhang XZ, Sun HW, Zhang ZY, Niu Q, Chen YS, Crittenden JC. Enhanced bioaccumulation of cadmiumincarp in the presence of titanium dioxide nano-particles. Chemosphere. 2007;67:160–66. doi: 10.1016/j.chemosphere.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 163.Sun HW, Zhang XZ, Niu Q, Chen YS, Crittenden JC. Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles. Water Air Soil Pollut. 2007;178:245–54. [Google Scholar]
- 164.Canesi L, Ciacci C, Vallotto D, Gallo G, Marcomini A, Pojana G. In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat Toxicol. 2010;96:151–8. doi: 10.1016/j.aquatox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 165.Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;175:410–20. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- 166.Reeves JF, Davies SJ, Dodd NJF, Jha AN. Hydroxyl radicals (OH) are associatedwithtitaniumdioxide(TiO2)nanoparticle-inducedcytotoxicityandoxidative DNA damage in fish cells. Mutat Res-Fund Mol M. 2008;640:113–22. doi: 10.1016/j.mrfmmm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 167.Drobne D, Jemec A, Pipan Tkalec Z. In vivo screening to determine hazards of nanoparticles: nanosized TiO2. Environ Pollut. 2009;157:1157–64. doi: 10.1016/j.envpol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 168.Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol. 2008;421:4447–53. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- 169.Valant J, Drobne D, Sepcic K, Jemec A, Kogej K, Kostanjsek R. Hazardous potential of manufactured nanoparticles identified by in vivo assay. J Hazard Mater. 2009;171:160–5. doi: 10.1016/j.jhazmat.2009.05.115. [DOI] [PubMed] [Google Scholar]
- 170.Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut. 2009;157:1171–7. doi: 10.1016/j.envpol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 171.Roh J-Y, Park Y-K, Park K, Choi J. Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Phar. 2010;29:167–72. doi: 10.1016/j.etap.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 172.Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem. 2010;42:586–91. [Google Scholar]
- 173.Yang F, Hong F, You W, Liu C, Gao F, Wu C, et al. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res. 2006;110:179–90. doi: 10.1385/bter:110:2:179. [DOI] [PubMed] [Google Scholar]
- 174.Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, et al. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res. 2006;111:239–53. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- 175.Gao F, Liu C, Qu C, Zheng L, Yang F, Su M, et al. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase? BioMetals. 2008;212:211–17. doi: 10.1007/s10534-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 176.Su MY, Liu C, Qu CX, Zheng L, Chen L, Huang H, et al. Nano-anatase relieves the inhibition of electron transport caused by linolenic acid in chloroplasts of spinach. Biol Trace Elem Res. 2008;122:73–81. doi: 10.1007/s12011-007-8055-x. [DOI] [PubMed] [Google Scholar]
- 177.Lei Z, Mingyu S, Xiao W, Chao L, Chunxiang Q, Liang C, et al. Antioxidant Stress is Promoted by Nano-anatase in Spinach Chloroplasts Under UV-B Radiation. Biol Trace Elem Res. 2008;121:69–79. doi: 10.1007/s12011-007-8028-0. [DOI] [PubMed] [Google Scholar]
- 178.Lu CM, Zhang CY, Wen JQ, Wu GR. Soybean Sci. 2002;21:168–71. (in Chinese) [Google Scholar]
- 179.Matsunaga T, Tomoda R, Nakajima T, Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29:211–4. [Google Scholar]
- 180.Ibáńez JA, Litter MI, Pizarro RA. Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae: Comparative study with other Gram (-) bacteria. J Photochem Photobiol, A. 2003;157:81–5. [Google Scholar]
- 181.Jang HD, Kim S-K, Kim S-J. Effect of particle size and phase composition of titanium dioxide nanoparticles on the photocatalytic properties. J Nanopart Res. 2001;3:141–7. [Google Scholar]
- 182.Shang C, Cheung LM, Ho C-M, Zeng M. Repression of photoreactivation and dark repair of coliform bacteria by TiO2-modified UV-C disinfection. App Catal B. 2009;89:536–42. [Google Scholar]
- 183.Khan U, Benabderrazik N, Bourdelais AJ, Baden DG, Rein K, Gardinali PR, et al. UV and solar TiO2 photocatalysis of brevetoxins (PbTxs) Toxicon. 2010;55:1008–16. doi: 10.1016/j.toxicon.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Prijic S, Sersa G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol Oncol. 2011;45:1–16. doi: 10.2478/v10019-011-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lagopati N, Kitsiou PV, Kontos AI, Venieratos P, Kotsopoulou E, Kontos AG, et al. Photo-induced treatment of breast epithelial cancer cells using nanostructured titanium dioxide solution. J Photochem Photobiol, A. 2010;214:215–23. [Google Scholar]
- 186.Stefanou E, Eyangelou A, Falaras P. Effects of UV-irradiated titania nanoparticles on cell proliferation, cancer metastasis and promotion. Catal Today. 2010;151:58–63. [Google Scholar]
- 187.Cai R, Kubota Y, Shuin T, Sakai H, Hashimoto K, Fujishima A. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992;52:2346–8. [PubMed] [Google Scholar]
- 188.Fujishima A, Hashimoto K, Watanabe T. Tokyo: BKC, Inc; 1999. TiO2 Photocatalysis: Fundamentals and Applications. [Google Scholar]
- 189.Fujishima A, Call RX, Otsuki J, Hashimoto K, Iron K, Yamashita T, et al. Biochemical application of photoelectrochemistry: photokilling of malignant cells with TiO2 powder. Electrochim Acta. 1993;38:153–7. [Google Scholar]
- 190.Kalbacova M, Macak MJ, Schmidt-Stein F, Mierke CT, Schmuki P. Phys. Status Solidi (RRL) 2008;2:194–8. [Google Scholar]
- 191.Kubota Y, Shuin T, Kawasaki C, Hosaka M, Kitamura H, Cai R, et al. Photokilling of T-24 Human Bladder-Cancer Cells with Titanium-Dioxide. Br J Cancer. 1994;70:1107–11. doi: 10.1038/bjc.1994.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thevenot P, Cho J, Wavhal D, Timmons RB, Tang L. Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomedicine. 2008;43:226–36. doi: 10.1016/j.nano.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Song M, Zhang RY, Dai YY, Gao F, Chi HM, Lv G, et al. The in vitro inhibition of multidrug resistance by combined nanoparticulate titanium dioxide and UV irradition. Biomaterials. 2006;27:4230–38. doi: 10.1016/j.biomaterials.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 194.Schmidt-Stein F, Hahn R, Gnichwitz JF, Song YY, Shrestha NK, Hirsch A, et al. X-ray induced photocatalysis on TiO2 and TiO2 nanotubes: Degradation of organics and drug release. Electrochem Commun. 2009;1111:2077–80. [Google Scholar]
- 195.Matsui K, Segawa M, Tanaka T, Kondo A, Ogino C. Antibody-immobilized TiO2 nanoparticles for cancer therapy. J Biosci Bioeng. 2009;108:S36–S37. [Google Scholar]
- 196.Xu J, Sun Y, Huang JJ, Chen CM, Liu GY, Jiang Y, et al. Photokilling cancer cells using highly cell-specific antibody-TiO2 bioconjugates and electroporation. Bioelectrochem. 2007;712:217–22. doi: 10.1016/j.bioelechem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 197.Lai T-Y, Lee W-C. Killing of cancer cell line by photoexcitation of folic acid-modified titanium dioxide nanoparticles. J Photochem Photobiol, A. 2009;204:148–53. [Google Scholar]
- 198.Lomer MCE, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RPH, et al. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Brit J Nutr. 2004;92:947–55. doi: 10.1079/bjn20041276. [DOI] [PubMed] [Google Scholar]