Abstract
Although recent studies report a high prevalence of vitamin D deficiency in HIV-infected adults similar to that in the general population, metabolic complications of vitamin D deficiency may be worsened with HIV infection and remain insufficiently characterized. We conducted a retrospective cross-sectional cohort study to determine prevalence and correlates of vitamin D deficiency and hyperparathyroidism among HIV-infected patients attending an urban clinic. Vitamin D deficiency was defined as 25(OH)-vitamin D <20 ng/ml and insufficiency as 20 to <30 ng/ml, and hyperparathyroidism as parathyroid-hormone >65 pg/ml. We used the X2 test to compare proportions and logistic regression to assess for associations. Among 463 HIV-infected patients, the prevalence of vitamin D deficiency was 59%. The prevalence of hyperparathyroidism was 30% among patients with vitamin D deficiency, 23% among those with insufficiency, and 12% among those with sufficient vitamin D levels. Vitamin D deficiency was associated with increased odds of hyperparathyroidism. Severe vitamin D deficiency was associated with elevated alkaline phosphatase, a marker for increased bone turnover. Although efavirenz use was associated with vitamin D deficiency, and protease inhibitor use with decreased odds of vitamin D deficiency, there was no statistical difference in rates of hyperparathyroidism stratified by combination antiretroviral therapy (cART) use. Given the increased risk of osteopenia with HIV infection and cART use, vitamin D supplementation for all HIV-infected patients on cART should be prescribed in accordance with the 2011 Endocrine Society guidelines. In HIV-infected patients with severe vitamin D deficiency or hyperparathyroidism, screening for osteomalacia and osteopenia may be warranted.
Introduction
HIV infection and combination antiretroviral therapy (cART) increase the risk of metabolic complications such as bone disorders (i.e., osteopenia, osteoporosis, and avascular necrosis), dyslipidemia, and glucose intolerance. 1–5 Use of cART increased the odds of osteopenia by 2.5-fold,6 and initiation of cART decreased bone mineral density (BMD) by 2–6% within the first 2 years.4,7 A meta-analysis of cross-sectional cohorts found that HIV infection increased the odds of osteopenia by 6-fold and of osteoporosis by nearly 4-fold.6 In addition to osteopenia and osteoporosis, HIV infection was associated with higher rates of fractures, including vertebral, hip, and wrist fractures in several studies,8–11 though not among premenopausal women.12
Recent studies report a high prevalence of vitamin D deficiency in HIV-infected adults.13–15,17 Analysis of data from the Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN), a large, prospective observational cohort study, found 70% prevalence of vitamin D insufficiency or deficiency among 672 HIV-infected adults.16 Although adults surveyed in the U.S. National Health and Nutrition Examination Survey (NHANES) had a similar prevalence of vitamin D deficiency,16 in HIV-infected adults the metabolic complications of vitamin D deficiency may be worsened by additive or synergistic effects with HIV infection and cART use.
The range of complications associated with vitamin D deficiency overlaps with known metabolic complications of HIV infection and cART use. Vitamin D deficiency results in secondary hyperparathyroidism, osteomalacia, osteoporosis, muscle weakness, and increased fracture risk in adults.18,19 In addition, vitamin D deficiency may also play a role in insulin resistance, the metabolic syndrome, and immunomodulation.18,20 Furthermore, metabolic complications (e.g., decreased BMD) associated with use of cART may be mediated in part by alterations in vitamin D metabolism through the effect of cART on the cytochrome P450 system.21 The use of efavirenz (EFV) in HIV-infected adults has been demonstrated to decrease 25(OH)-vitamin D levels and increase the risk of vitamin D deficiency,16,22–25 while the use of protease inhibitors (PIs), in contrast, was associated with increased 25(OH)-vitamin D levels.6,16 The spectrum and severity of metabolic complications associated with vitamin D deficiency in HIV-infected adults remain to be better characterized.
We conducted a retrospective cross-sectional cohort study to determine prevalence, associated risk factors, and complications associated with vitamin D deficiency in an HIV clinic population in New York City. We compared proportions of patients with hyperparathyroidism stratified by level of vitamin D deficiency, and evaluated current use of cART as a risk factor for vitamin D deficiency and hyperparathyroidism.
Materials and Methods
The study included HIV-infected persons age ≥18 years who received routine HIV care at Bellevue Virology Clinic from October 2009 through March 2010, and who had serum 25(OH)-vitamin D measured as part of a clinic-wide quality-improvement project initiated to assess for prevalence of vitamin D deficiency and to determine whether a therapeutic intervention was warranted for the clinic population. Patients with ≥ stage 3 chronic kidney disease (CKD, defined as estimated GFR <60 ml/min/1.73m2) were excluded, as CKD is known to interfere with vitamin D metabolism. We extracted data on patient demographics, current medications including nutritional supplements, duration of antiretroviral use, HIV viral load, CD4 cell count, serum 25(OH)-vitamin D, parathyroid hormone (PTH), and other concurrent laboratory results. Study approval was received from the NYU School of Medicine, Bellevue Hospital Center and the central office of the New York City Health and Hospital Corporation.
Plasma 25(OH)-vitamin D was measured using liquid chromatography, tandem mass spectrometry (Quest Diagnostics, Madison, NJ). PTH was measured using solid-phase chemiluminescent immunoassay (Immulite 2000, Siemens Healthcare Diagnostics, Deerfield, IL). HIV viral load was measured using the Amplicor HIV-1 Monitor v1.5 Ultrasensitive Assay (Roche Diagnostics, Branchburg, NJ).
The primary endpoint was vitamin D deficiency, defined as 25(OH)-vitamin D < 20 ng/ml.18 Secondary endpoints included vitamin D insufficiency, defined as 25(OH)-vitamin D 20 to <30 ng/ml, and vitamin D sufficiency, defined as 25(OH)-vitamin D ≥30 ng/ml.18 Severe vitamin D deficiency was defined as 25(OH)-vitamin D <12 ng/ml, a level of deficiency associated with increased risk for adverse effects on bone health.26
Hyperparathyroidism was defined as PTH greater than 65 pg/ml, elevated alkaline phosphatase as serum alkaline phosphatase greater than 100 U/liter, and hypocalcemia as serum albumin-adjusted calcium level of less than 9.0 mg/dl. In accordance with American Diabetes Association 2011 guidelines, prediabetes was defined as having glycated hemoglobin A(1c) (HbA1c) 5.7–6.4%, and diabetes as having HbA1c ≥6.5% or being on antihyperglycemics.27 HIV viral load cut-offs were defined at 200 copies/ml, in accordance to the 2011 DHHS HIV Guidelines, and at 50 copies/ml, the lower limit of detection of the assay.28
We calculated the prevalence of vitamin D deficiency, severe deficiency, insufficiency, and sufficiency in our study population. Data were analyzed using SPSS 19 statistical software. Comparisons of proportions were made using chi-square testing. Student's t-test, ANOVA with Bonferroni and Tukey's post-hoc tests, and linear regression were used to assess for correlates of 25(OH)-vitamin D level, and univariate logistic regression to assess for factors associated with vitamin D deficiency. Variables with a p-value ≤0.10 in univariate analysis were included in multivariate logistic regression, and adjusted odds ratios (aORs) and corresponding 95% confidence intervals (CIs) were calculated.
Results
Baseline characteristics
Of 507 patients, 44 were excluded due to evidence of stage ≥3 CKD, and 463 were included in the analysis. Seventy-six percent (N=351) were male, 48% (N=221) were black, and the median age was 46 years [interquartile range (IQR) 38–53 years] (Table 1). Eighty-two percent of patients were currently on cART (N=381). Among patients not on cART, 52% (43/82) were cART-naive and 48% (39/82) were self-reported nonadherent to cART in the medical record. Sixty-four percent of patients had a CD4 T cell count >350 cells/mm3 (N=296), and 72% (N=333) had a viral load ≤200 copies/ml, including 65% (N=300) with a viral load <50 copies/ml (Table 2). Mean and median CD4 T cell counts were 455 cells/mm3 and 433 cells/mm3, respectively (IQR 365–617 cells/mm3).
Table 1.
Baseline Characteristics by Level of Vitamin D Deficiency
| Variable | All N=463 | Vitamin D sufficient N=77 | Vitamin D insufficient N=111 | Vitamin D deficient N=275 |
|---|---|---|---|---|
| Age, years** | ||||
| Median (IQR) | 46 (38–53) | 48 (43–56) | 47 (40–52) | 44 (36–53) |
| Age in years by strata, no. (%)‡ | ||||
| 18–34 | 84 (18) | 4 (5) | 13 (12) | 64 (24) |
| 35–44 | 125 (27) | 21 (27) | 33 (30) | 71 (26) |
| 45–54 | 152 (33) | 30 (39) | 41 (37) | 81 (30) |
| ≥55 | 101 (22) | 22 (29) | 23 (21) | 56 (20) |
| Sex, no. (%) | ||||
| Male | 351 (76) | 53 (69) | 85 (77) | 213 (78) |
| Race or ethnicity, no. (%)‡ | ||||
| White | 26 (6) | 7 (9) | 6 (5) | 13 (5) |
| Black | 221 (48) | 26 (34) | 42 (38) | 153 (56) |
| Hispanic | 160 (35) | 34 (44) | 44 (40) | 82 (30) |
| Asian | 40 (9) | 6 (8) | 13 (12) | 21 (8) |
| Other | 16 (4) | 4 (5) | 6 (5) | 6 (2) |
| Season, no. (%) | ||||
| Fall | 46 (10) | 13 (17) | 4 (4) | 29 (11) |
| Winter | 401 (87) | 61 (79) | 104 (94) | 236 (86) |
| Spring | 15 (3) | 3 (4) | 3 (3) | 9 (3) |
| Weight, kg* | ||||
| Median (IQR) | 74 (66–86) | 72 (63–86) | 73 (66–82) | 75 (66–86) |
| Current medications, no. (%) | ||||
| Anticonvulsants | 17 (4) | 4 (5) | 4 (4) | 9 (3) |
| Antimycobacterial | 8 (2) | 1 (1) | 2 (2) | 5 (2) |
| MVI‡ | 156 (34) | 41 (53) | 55 (50) | 60 (22) |
| Vitamin D‡ | 17 (4) | 8 (10) | 7 (6) | 2 (1) |
| Current antiretroviral therapy, no. (%)‡ | ||||
| None | 82 (17.7) | 9 (11.7) | 16 (14.4) | 57 (20.7) |
| EFV-based >1 year | 110 (23.8) | 15 (19.5) | 26 (23.4) | 69 (25.1) |
| EFV-based <1 year | 30 (6.5) | 3 (3.9) | 6 (5.4) | 21 (7.6) |
| PI-based >1 year | 145 (31.3) | 36 (46.8) | 38 (34.2) | 71 (25.8) |
| PI-based <1 year | 56 (12.1) | 9 (11.7) | 10 (9.0) | 37 (13.5) |
| Other | 40 (8.6) | 5 (6.5) | 15 (13.5) | 20 (7.3) |
| Current NRTI ≥12 months, no. (%) | ||||
| Abacavir | 42 (9.1) | 10 (13) | 10 (9.0) | 22 (8.0) |
| Emtricitabine | 203 (44) | 37 (48) | 51 (46) | 115 (42) |
| Lamivudine | 71 (15) | 13 (17) | 22 (20) | 36 (13) |
| Tenofovir | 235 (51) | 44 (57) | 58 (52) | 133 (48) |
*p<0.10 and **p<0.05 by t-test, linear regression, or ANOVA as appropriate for 25(OH)-vitamin D level.
p<0.05 by Chi-square test comparing proportion with and without vitamin D deficiency.
IQR, interquartile range; MVI, multivitamin; EFV, efavirenz; PI, protease inhibitor; NRTI, nucleoside reverse transcription inhibitor.
Table 2.
Metabolic Complications, Viral Load, and Immunologic Status by Level of Vitamin D Deficiency
| Variable | All N=463 | Vitamin D sufficient N=77 | Vitamin D insufficient N=111 | Vitamin D deficient N=275 |
|---|---|---|---|---|
| Parathyroid hormone level, pg/ml** | ||||
| Median (IQR) | 46 (31–65) | 40 (26–57) | 45 (29–61) | 49 (34–73) |
| Hyperparathyroidism,‡ no. (%) | ||||
| PTH >65 pg/ml | 104 (26) | 8 (12) | 23 (23) | 73 (30) |
| PTH ≤65 pg/ml | 301 (74) | 57 (88) | 76 (77) | 168 (70) |
| Elevated alkaline phosphatase, hypocalcemia, no. (%) | ||||
| Alk phos >100 U/liter | 165 (36) | 27 (35) | 32 (29) | 106 (39) |
| Adjusted calcium | 188 (41) | 30 (39) | 43 (39) | 115 (42) |
| <9.0 mg/dl | ||||
| Prediabetes or diabetes, no. (%)‡ | ||||
| HbA1c ≥5.7%‡ | 162 (35) | 18 (25) | 38 (37) | 106 (41) |
| Current viral load, no. (%)‡ | ||||
| <50 copies/ml | 300 (65) | 59 (77) | 84 (76) | 157 (57) |
| 50–200 copies/ml | 33 (7) | 4 (5) | 9 (8) | 20 (7) |
| >200 copies/ml | 130 (28) | 14 (18) | 18 (16) | 98 (36) |
| Current CD4 T cell count, no. (%)‡ | ||||
| >350 cells/mm3 | 296 (64) | 52 (68) | 78 (70) | 166 (60) |
| 200–350 cells/mm3 | 94 (20) | 16 (21) | 23 (21) | 55 (20) |
| <200 cells/mm3 | 73 (16) | 9 (12) | 10 (9.0) | 54 (20) |
**p<0.05 by t-test, linear regression, or ANOVA as appropriate for 25(OH)-vitamin D level.
p<0.05 by Chi-square test comparing proportion with and without vitamin D deficiency.
IQR, interquartile range; PTH, parathyroid hormone; Alk phos, alkaline phosphatase.
Thirty percent (N=140) of patients were on an EFV-based regimen, of which 88% (123/140) had a viral load ≤200 copies/ml and 79% (110/140) had been on EFV ≥12 months. Forty-three percent (N=201) were on a protease inhibitor (PI)-based regimen, of which 97% (195/201) were on ritonavir-boosted PI (rPI), 84% (168/201) had a viral load ≤200 copies/ml, and 70% (141/201) had been on an rPI-based regimen ≥12 months (Table 1). Thirty-four percent (N=156) of patients were taking multivitamins (MVI), and 4% (N=17) vitamin D supplementation. Among patients on MVI, 12% (18/156) had detectable vitamin D2, the form of vitamin D associated with supplementation, compared to 5% (14/306) in patients not on MVI (p=0.005). Among patients on vitamin D supplementation, 29% (5/17) had detectable vitamin D2, compared to 7% (30/445) among those not on vitamin D supplementation (p=0.005). Use of MVI or vitamin D supplementation was associated with age ≥35 years (p<0.001), use of EFV-based cART (p=0.005), and use of PI-based cART (p=0.009). The use of other medications known to interfere with vitamin D metabolism, such as anticonvulsants (4%, N=17), antimycobacterial drugs (2%, N=8; i.e., izoniazid, ethambutol, pyrazinamide, or rifamycins), and corticosteroids (0.4%, N=2), was minimal. Eighty-seven percent (N=401) of patients had vitamin D levels measured during the winter season, from December through February. Nine percent (N=40) of patients also had chronic hepatitis B infection (i.e., hepatitis B surface antigen or RNA positivity), and 22% (N=102) were coinfected with hepatitis C (i.e., hepatitis C antibody positivity).
25(OH)-vitamin D levels and vitamin D deficiency
Median 25(OH)-vitamin D level was 17 ng/ml (IQR 10–25 ng/ml). Fifty-nine percent (N=275) of patients were vitamin D deficient, 24% (N=111) vitamin D insufficient, and 17% (N=77) vitamin D sufficient (Table 1). Among patients with vitamin D deficiency, 49% (135/275) had severe vitamin D deficiency [i.e., 25(OH)-vitamin D <12 ng/ml].
Factors associated with increased odds of vitamin D deficiency included younger age (p=0.001) and black race/ethnicity [odds ratio (OR) 2.18, 95% confidence interval (CI): 1.49-–3.19] (Tables 1 and 3). Hyperparathyroidism (OR 1.86, 95% CI: 1.16–3.01) and having prediabetes or diabetes (OR 1.514, 95% CI: 1.01–2.27) were associated with increased odds of vitamin D deficiency (Tables 2 and 3). Additionally, current CD4 T cell count <200 cells/mm3 (OR 2.26, 95% CI: 1.26–3.94) and current viral load >200 copies/ml (OR 2.79, 95% CI: 1.76–4.41) were associated with increased odds of vitamin D deficiency (Tables 2 and 3); serum 25(OH)-vitamin D levels directly correlated with CD4 T cell count (p=0.002) and inversely correlated with HIV viral load (p=0.001).
Table 3.
Characteristics Associated with Vitamin D Deficiency
| Variable | Odds ratio for vitamin D deficiency (95% CI) | p-valuea | Adjusted odds ratio for vitamin D deficiencyb(95% CI) | p-valuec |
|---|---|---|---|---|
| Age, years | ||||
| 18–34 | Ref | Ref | Ref | Ref |
| 35–44 | 0.33 (0.18, 0.63) | <0.01 | 0.41 (0.20, 0.83) | 0.01 |
| 45–54 | 0.29 (0.16, 0.54) | <0.01 | 0.35 (0.18, 0.71) | <0.01 |
| ≥55 | 0.32 (0.16, 0.61) | <0.01 | 0.50 (0.24, 1.06) | 0.07 |
| Black race | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 2.18 (1.49, 3.19) | <0.01 | 2.26 (1.45, 3.54) | <0.01 |
| Weight | ||||
| 1st quartile | Ref | Ref | Ref | Ref |
| 2nd quartile | 0.82 (0.49, 1.37) | 0.44 | 0.80 (0.44, 1.46) | 0.47 |
| 3rd quartile | 1.23 (0.73, 2.08) | 0.45 | 1.22 (0.66, 2.27) | 0.52 |
| 4th quartile | 1.19 (0.70, 2.00) | 0.52 | 1.07 (0.58, 1.97) | 0.84 |
| Current medications | ||||
| MVI | 0.27 (0.18, 0.40) | <0.01 | 0.27 (0.17, 0.42) | <0.01 |
| Vitamin D | 0.08 (0.02, 0.37) | <0.01 | 0.13 (0.03, 0.64) | 0.01 |
| Current CD4 T cell count, cells/mm3 | ||||
| >350 | Ref | Ref | Ref | Ref |
| 200–350 | 1.10 (0.69, 1.77) | 0.68 | 0.77 (0.45, 1.33) | 0.35 |
| <200 | 2.23 (1.26, 3.94) | 0.01 | 1.50 (0.76, 2.93) | 0.24 |
| Current viral load, copies/mL | ||||
| <50 | Ref | Ref | Ref | Ref |
| 50–200 | 1.40 (0.67, 2.92) | 0.37 | 1.13 (0.49, 2.60) | 0.77 |
| >200 | 2.79 (1.76, 4.41) | <0.01 | 2.97 (1.48, 5.97) | <0.01 |
| Current antiretroviral therapy, no. (%) | ||||
| None | Ref | Ref | Ref | Ref |
| EFV-based | 0.79 (0.44, 1.42) | 0.43 | 3.06 (1.32, 7.13) | 0.01 |
| PI-based | 0.51 (0.30, 0.88) | 0.02 | 1.50 (0.69, 3.29) | 0.31 |
| Other | 0.44 (0.20, 0.96) | 0.04 | 1.82 (0.65, 5.07) | 0.25 |
Univariate analysis of association with vitamin D deficiency.
Variables associated with vitamin D deficiency with p≤0.10 were included in multivariate logistic regression (i.e., age, black race/ethnicity, current use of MVI, current use of vitamin D supplements, current CD4 count, current viral load, current antiretroviral therapy).
Multivariate analysis of association with vitamin D deficiency.
Current use of MVI (OR 0.27, 95% CI: 0.18–0.40), vitamin D supplements (OR 0.08, 95% CI: 0.02–0.37), and PI-based cART regimen (OR 0.51, 95% CI: 0.30–0.88) was associated with decreased odds of vitamin D deficiency (Tables 1 and 3). There was insufficient sample size to evaluate the effects of any individual PI. Current use of EFV-based cART was not significantly associated with vitamin D deficiency (OR 0.79, 95%CI: 0.44–1.42) in univariate analysis. Analysis by type of nucleoside reverse transcriptase inhibitor used for ≥12 months did not find an association of vitamin D deficiency with use of abacavir (N=42, p=0.324), emtricitabine (N=203, p=0.288), lamivudine (N=71, p=0.107), or tenofovir (N=235, p=0.213). Vitamin D deficiency was not associated with chronic hepatitis B (p=0.958) or hepatitis C infection (p=0.773).
Multivariate analysis
We included age, black race, weight by quartiles, current use of MVI, current use of vitamin D supplements, CD4 count, viral load, and current antiretroviral therapy in our multivariate logistic regression model (Table 3). Black race [adjusted OR (aOR) 2.26, 95% CI: 1.45–3.54] was independently associated with significantly higher odds of vitamin D deficiency. Age 35–54 years (p<0.05), current use of MVI (aOR 0.27, 95% CI: 0.17–0.42), and current use of vitamin D supplements (aOR 0.13, 95% CI: 0.03–0.64) were independently associated with lower odds of vitamin D deficiency. Current CD4 cell count <200 cells/mm3 was not significant (p=0.241), but current viral load >200 copies/ml (aOR 2.97, 95% CI: 1.48–5.97) remained independently associated with increased odds of vitamin D deficiency. Current use of EFV-based cART (aOR 3.06, 95% CI: 1.32–7.13) was an independent risk factor for vitamin D deficiency, while current use of PI-based cART (p=0.308) was not significantly associated.
Hyperparathyroidism
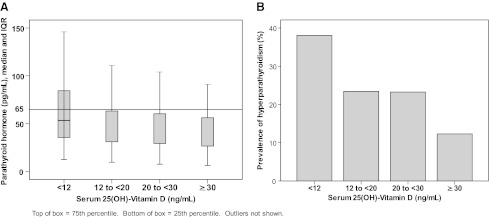
Eighty-seven percent (405/463) of patients had serum PTH measured concurrently with serum 25(OH)-vitamin D. Of patients with documented serum PTH, the prevalence of hyperparathyroidism was 30% (73/241) among patients with vitamin D deficiency, 23% (23/99) among those with vitamin D insufficiency, and 12% (8/65) among those vitamin D sufficient (Table 2 and Fig. 1). Among patients with severe vitamin D deficiency, 38% (43/113) had hyperparathyroidism. Mean and median 25(OH)-vitamin D levels among patients with hyperparathyroidism were 15.1 ng/ml and 13.0 ng/ml, respectively (IQR 8.0–21.0 ng/ml). Vitamin D deficiency doubled the odds of hyperparathyroidism (OR 1.86, 95% CI: 1.16, 3.01). After adjusting for vitamin D deficiency, current use of EFV-based (p=0.91) or PI-based cART (p=0.12) did not increase the risk of hyperparathyroidism when compared with no cART. Compared to current use of PI-based cART, current use of EFV-based cART had increased odds of hyperparathyroidism (OR 1.66, 95% CI: 0.993, 2.785; p=0.053), though this difference was not statistically significant. After adjusting for vitamin D deficiency, there was no difference between use of PI-based or EFV-based cART in odds of hyperparathyroidism (p=0.111). Current use of tenofovir ≥12 months (p=0.09) was not associated with hyperparathyroidism.
FIG. 1.
(A) Median serum parathyroid hormone (PTH) (pg/ml) by serum 25(OH)-vitamin D subgroups. (B) Prevalence of hyperparathyroidism (PTH >65 pg/ml) by serum 25(OH)-vitamin D subgroups.
We assessed alkaline phosphatase and albumin-adjusted calcium concentrations as indicators of increased bone turnover associated with vitamin D deficiency and hyperparathyroidism. Severe vitamin D deficiency (OR 1.63, 95% CI: 1.08, 2.45; p=0.02), but not vitamin D deficiency (p=0.12), was associated with elevated alkaline phosphatase. Hyperparathyroidism increased the odds of having elevated alkaline phosphatase (OR 1.57, 95% CI: 0.996, 2.475; p=0.052), though this association did not reach statistical significance. Neither vitamin D deficiency (p=0.32) nor severe vitamin D deficiency (p=0.21) was associated with albumin-adjusted calcium concentration.
Glucose intolerance
Vitamin D deficiency was associated with being prediabetic or diabetic (p=0.044, OR 1.51, 95% CI: 1.01, 2.27). In our cohort, being in the top quartile by weight (≥86 kg) was associated with being prediabetic or diabetic (p=0.002). After adjusting for weight by quartile, there remained a trend toward increased odds of being prediabetic or diabetic (p=0.053, aOR 1.50, 95% CI: 0.995, 2.256) with vitamin D deficiency.
Discussion
Consistent with the published literature, vitamin D insufficiency and deficiency were highly prevalent (combined prevalence 83%) in our cohort, and black race/ethnicity increased the risk of vitamin D deficiency, while current use of MVI and vitamin D supplements decreased the risk.13,14,16,21 Older age is traditionally a risk factor for vitamin D deficiency; however, we found younger age was associated with vitamin D deficiency, which was also reported in the SUN cohort.16 In our study, this finding may be partly explained by higher rates of MVI use and vitamin D supplementation in patients aged ≥35 years, which was corroborated by a higher proportion with detectable serum vitamin D2, the form of vitamin D associated with dietary supplementation (data not shown). There was no significant difference in race/ethnicity by age group (data not shown).
Vitamin D deficiency doubled the odds of hyperparathyroidism in our cohort, and the prevalence of hyperparathyroidism increased with severity of vitamin D deficiency.
The prevalence of hyperparathyroidism was 30% among patients with vitamin D deficiency and 23% among those with vitamin D insufficiency. Among patients with severe vitamin D deficiency, 38% had hyperparathyroidism, and severe vitamin D deficiency was associated with elevated levels of alkaline phosphatase, a marker for increased bone turnover. These findings are consistent with known biological pathways linking vitamin D deficiency with secondary hyperparathyroidism and increased bone resorption. Serum 25(OH)-vitamin D less than 30 ng/ml is associated with increased PTH; PTH activates osteoblasts, which in turn catalyzes the maturation of preosteoclasts into osteoclasts, which resorb bone by dissolving its mineralized matrix.18 The high prevalence of vitamin D deficiency in HIV-infected patients, its association with hyperparathyroidism, and its association with elevated alkaline phosphatase in the setting of severe vitamin D deficiency are concerning, as it suggests that vitamin D deficiency may exacerbate the risk for osteopenia, osteoporosis, and fractures with HIV infection and cART use. The 2011 Endocrine Society clinical practice guidelines recommend that all HIV-infected patients on cART should be given at least two to three times more vitamin D for their age group.1 Furthermore, in HIV-infected patients with vitamin D deficiency and hyperparathyroidism, or with severe vitamin D deficiency, screening for osteomalacia (i.e., serum and urinary phosphate) and osteopenia (i.e., bone scan) and aggressive vitamin D repletion may be warranted.4
EFV use was associated with vitamin D deficiency in multivariate analysis, but not in univariate analysis. The higher proportion of MVI and vitamin D supplementation among patients on EFV compared with patients not on cART (39% vs. 21%, p=0.005) may have mitigated the effects of EFV on vitamin D deficiency in univariate analysis. Conversely, PI use was associated with decreased odds of vitamin D deficiency in univariate analysis, but not in multivariate analysis, which may be partially explained by a higher proportion of MVI and vitamin D supplementation among patients on PI (37%). We were unable to detect any significant difference in black race/ethnicity based on current cART use.
Our findings that EFV use was correlated with lower vitamin D levels, and PI use with higher vitamin D levels are consistent with the published literature.16,21,24,29–31 Efavirenz is thought to induce CYP24 of the cytochrome P450 system, which may lead to increased metabolism of 25(OH)-vitamin D and 1,25(OH)2-vitamin D to inactive metabolites.21,32,33 Since 1,25(OH)2-vitamin D is thought to inhibit PTH secretion, use of efavirenz may lead to an increase in PTH and hyperparathyroidism. Protease inhibitors are associated with increased 25(OH)-vitamin D levels and paradoxically with decreased BMD, and one potential mechanism may be its inhibition of 1α-hydroxylase, an enzyme responsible for converting 25(OH)-vitamin D to the biologically active 1,25(OH)2-vitamin D.21 Use of PIs may decrease conversion of 25(OH)-vitamin D to 1,25(OH)2-vitamin D, leading to increased PTH and hyperparathyroidism, decreased calcium absorption, and increased rates of osteopenia and osteoporosis.18,21 However, in our study, we were not able to detect a statistical difference in rates of hyperparathyroidism among those not on cART, on EFV-based ART, or on PI-based ART. Of the nucleoside/nucleotide analogues, tenofovir has been associated with loss of BMD.4,21,34 Tenofovir use was associated with vitamin D deficiency in the SUN study, and two studies reported an association of tenofovir use with hyperparathyroidism in the setting of vitamin D deficiency.16,35,36 In contrast, we were not able to find an association between tenofovir use and either vitamin D deficiency or hyperparathyroidism.
In our study, vitamin D deficiency trended toward increased odds of being prediabetic or diabetic in HIV-infected patients, after controlling for weight. Vitamin D deficiency has been associated with glucose intolerance and insulin resistance, and the large prospective Nurse’ Health Study found that vitamin D supplementation reduced the risk for type 2 diabetes.18,20,37–39 Although vitamin D receptors have been found on pancreatic β-islet cells, and the biologically active form of vitamin D [1,25(OH)2-vitamin D] was shown to stimulate insulin production, the precise mechanism by which vitamin D affects insulin production and risk of diabetes remains unknown. The risk of diabetes mellitus in HIV-infected patients is unclear; it was found to be higher among HIV-infected patients in the Multicenter AIDS Cohort Study, but not in other studies.5,40–42 Whether and how HIV infection and vitamin D deficiency may have additive or synergistic effects on insulin production and glucose tolerance are unknown.
In our study, vitamin D deficiency was independently associated with increased odds of having an HIV viral load >200 copies/mL, after adjusting for current use of antiretroviral therapy. Data from the Women's Interagency HIV Study found a similar effect inversely, in which an undetectable HIV viral load was independently associated with lowered odds of vitamin D deficiency.31 Published data on the association of vitamin D deficiency with HIV disease progression are conflicting.16,17,29,43,44 Two large studies have reported an association of vitamin D deficiency with HIV disease progression and all-cause mortality, but not with CD4 count or viral load.29,44 A potential biological mechanism for the role of vitamin D deficiency in HIV disease progression may be alteration in a type of programmed cell death called autophagy. Autophagy allows for clearance of intracellular organisms in innate immunity and assists with antigen presentation in adaptive immunity.45 The biologically active form of vitamin D [1,25(OH)2-vitamin D] induces autophagy in cells by binding to the vitamin D receptor, which initiates a molecular cascade that eventually leads to fusion of autophagosomes with lysosomes and clearance of intracellular organisms.45 Because HIV infection alters and blocks autophagy in infected macrophages and lymphocytes as a mechanism to increase intracellular HIV viral replication,45 vitamin D deficiency may augment HIV viral replication by further dampening autophagy in infected cells, and, hence, increase plasma viral load and worsen HIV disease progression.
This study has several limitations. We were unable to account for the degree of outdoors exposure to sunlight in our patients, although most had vitamin D levels measured during the winter season. We were unable to account for the body mass index (BMI) in our patients. Low BMI in HIV-infected patients is known to be a risk factor for vitamin D deficiency and osteopenia,16 and high BMI is associated with insulin resistance. Data on gamma-glutamyltransferase (GGT) to assess whether the elevated alkaline phosphatase was of liver origin rather than bone was not available, and most patients did not have bone scan results available. HbA1c levels may have underestimated the prevalence of prediabetes and of diabetes in our study due to the effects of HIV infection and medication on the sensitivity of HbA1c as a measure of glycemic control.46–49 Although we assessed for risk factors and complications associated with vitamin D deficiency, this analysis was based on cross-sectional data, and correlation does not imply a causal relationship.
In summary, vitamin D deficiency was highly prevalent in our clinic cohort of HIV-infected adults, and was associated with efavirenz use as well as known risk factors for vitamin D deficiency. Use of protease inhibitors, MVI, and vitamin D supplementation reduced the odds of vitamin D deficiency. The prevalence of hyperparathyroidism was 30% among patients with vitamin D deficiency and 23% among those with vitamin D insufficiency, and vitamin D deficiency was associated with increased odds of hyperparathyroidism. Severe vitamin D deficiency was associated with elevated levels of alkaline phosphatase, a marker for increased bone turnover. In light of the increased risk of osteopenia in HIV-infected patients on cART, vitamin D supplementation for all HIV-infected patients on cART should be prescribed in accordance with the 2011 Endocrine Society guidelines.1 Screening for osteomalacia and osteopenia in HIV-infected patients with severe vitamin D deficiency or hyperparathyroidism may be warranted. In our cohort of HIV-infected patients, vitamin D deficiency was associated with increased odds of being prediabetic or diabetic, and with increased odds of having an unsuppressed viral load. These findings corroborate the limited amount of published literature supporting the role of vitamin D in glucose control, immune function, and HIV disease progression. Further studies are needed to better characterize the spectrum and severity of complications associated with vitamin D deficiency in HIV infection.
Acknowledgments
We thank Edgar T. Overton for his insightful comments on early drafts of the manuscript. This work was supported in part by NIAID grant 5 U01 AI069532.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Holick MF. Binkley NC. Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 2.Aberg JA. Kaplan JE. Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 3.Hoy J. Bone, fracture and frailty. Curr Opin HIV AIDS. 2011;6(4):309–314. doi: 10.1097/COH.0b013e3283478741. [DOI] [PubMed] [Google Scholar]
- 4.McComsey GA. Tebas P. Shane E, et al. Bone disease in HIV infection: A practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown TT. Cole SR. Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Brown TT. Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 7.Brown TT. McComsey GA. King MS. Qaqish RB. Bernstein BM. da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 8.Collin F. Duval X. Le Moing V, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23(8):1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triant VA. Brown TT. Lee H. Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93(9):3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womack JA. Goulet JL. Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6(2):e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young B. Dao CN. Buchacz K. Baker R. Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 12.Yin MT. Lu D. Cremers S, et al. Short-term bone loss in HIV-infected premenopausal women. J Acquir Immune Defic Syndr. 2010;53(2):202–208. doi: 10.1097/QAI.0b013e3181bf6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasserman P. Rubin DS. Highly prevalent vitamin D deficiency and insufficiency in an urban cohort of HIV-infected men under care. AIDS Patient Care STDS. 2010;24(4):223–227. doi: 10.1089/apc.2009.0241. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez M. Daniels B. Gunawardene S. Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses. 2009;25(1):9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 15.Mueller NJ. Fux CA. Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24(8):1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 16.Dao CN. Patel P. Overton ET, et al. Low vitamin D among HIV-infected adults: Prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52(3):396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 17.Stein EM. Yin MT. McMahon DJ, et al. Vitamin D deficiency in HIV-infected postmenopausal Hispanic and African-American women. Osteoporos Int. 2011;22(2):477–487. doi: 10.1007/s00198-010-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Stechschulte SA. Kirsner RS. Federman DG. Vitamin D: Bone and beyond, rationale and recommendations for supplementation. Am J Med. 2009;122(9):793–802. doi: 10.1016/j.amjmed.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D: Extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381–400. doi: 10.1016/j.ecl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Overton ET. Yin MT. The rapidly evolving research on vitamin D among HIV-infected populations. Curr Infect Dis Rep. 2011;13(1):83–93. doi: 10.1007/s11908-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 22.Welz T. Childs K. Ibrahim F, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24(12):1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 23.Van Den Bout-Van Den Beukel CJ. Fievez L. Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: Effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24(11):1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 24.Brown TT. McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15(3):425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 25.Wohl D. Doroana M. Orkin C. Pilotto JH. Sungkanuparph S. Yeni P. Vanveggel S. Deckx H. Boven K. Change in vitamin D levels smaller and risk of development of severe vitamin D deficiency lower among HIV-1-infected, treatment-naive adults receiving TMC278 compared with EFV: 48-week results from the Phase III ECHO Trial. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 26.IOM. Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine; Washington, DC: 2011. [Google Scholar]
- 27.ADA: Standards of medical care in diabetes––2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DHHS: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [May 27;2011 ]. pp. 1–166.http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 29.Viard JP. Souberbielle JC. Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: Results from the EuroSIDA study. AIDS. 2011;25(10):1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 30.Fox J. Peters B. Prakash M. Arribas J. Hill A. Moecklinghoff C. Improvement in vitamin D deficiency following antiretroviral regime change: Results from the MONET trial. AIDS Res Hum Retroviruses. 2011;27(1):29–34. doi: 10.1089/aid.2010.0081. [DOI] [PubMed] [Google Scholar]
- 31.Adeyemi OM. Agniel D. French AL, et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr. 2011;57(3):197–204. doi: 10.1097/QAI.0b013e31821ae418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzmann C. Arasteh K. Efavirenz-induced osteomalacia. AIDS. 2009;23(2):274–275. doi: 10.1097/QAD.0b013e32831f4685. [DOI] [PubMed] [Google Scholar]
- 33.Fabbriciani G. De Socio GV. Efavirenz and bone health. AIDS. 2009;23(9):1181. doi: 10.1097/QAD.0b013e32832bab0f. [DOI] [PubMed] [Google Scholar]
- 34.Stellbrink HJ. Orkin C. Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 35.Rosenvinge MM. Gedela K. Copas AJ, et al. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr. 2010;54(5):496–499. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- 36.Childs KE. Fishman SL. Constable C, et al. Inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses. 2010;26(8):855–859. doi: 10.1089/aid.2009.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu KC. Chu A. Go VL. Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 38.Pittas AG. Sun Q. Manson JE. Dawson-Hughes B. Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33(9):2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittas AG. Dawson-Hughes B. Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 40.Brar I. Shuter J. Thomas A. Daniels E. Absalon J. A comparison of factors associated with prevalent diabetes mellitus among HIV-infected antiretroviral-naive individuals versus individuals in the National Health and Nutritional Examination Survey cohort. J Acquir Immune Defic Syndr. 2007;45(1):66–71. doi: 10.1097/QAI.0b013e318031d7e3. [DOI] [PubMed] [Google Scholar]
- 41.Butt AA. McGinnis K. Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50(5):499–505. doi: 10.1097/QAI.0b013e31819c291b. [DOI] [PubMed] [Google Scholar]
- 43.Barber Y. Rubio C. Fernandez E. Rubio M. Fibla J. Host genetic background at CCR5 chemokine receptor and vitamin D receptor loci and human immunodeficiency virus (HIV) type 1 disease progression among HIV-seropositive injection drug users. J Infect Dis. 2001;184(10):1279–1288. doi: 10.1086/324000. [DOI] [PubMed] [Google Scholar]
- 44.Mehta S. Giovannucci E. Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5(1):e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spector SA. Vitamin D and HIV: Letting the sun shine in. Top Antivir Med. 2011;19(1):6–10. [PMC free article] [PubMed] [Google Scholar]
- 46.Diop ME. Bastard JP. Meunier N, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retroviruses. 2006;22(12):1242–1247. doi: 10.1089/aid.2006.22.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polgreen PM. Putz D. Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis. 2003;37(4):e53–e56. doi: 10.1086/376633. [DOI] [PubMed] [Google Scholar]
- 48.Glesby MJ. Hoover DR. Shi Q, et al. Glycated haemoglobin in diabetic women with and without HIV infection: Data from the Women's Interagency HIV Study. Antivir Ther. 2010;15(4):571–577. doi: 10.3851/IMP1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckhardt B. Holzman R. Kwan CK. Baghdadi J. Aberg J. Glycated hemoglobin A1C as screening for diabetes mellitus in HIV-infected individuals; Conference for Retroviruses and Opportunistic Infections. Vol Poster Abstract #849; Boston, MA. 2011. [Google Scholar]