Abstract
Background and Aims
Although tension wood formation and the structure of gelatinous fibres (G-fibres) have been widely investigated, studies of the influence of the reaction phenomenon on phloem fibres have been few and incomplete in comparison with those of xylem wood fibres. This study was undertaken to clarify the influence of stem inclination on phloem fibres using several Japanese hardwood species that produce different G-fibre types in tension wood.
Methods
Eight hardwood species were inclined at 30–45° at the beginning of April. Specimens were collected in July and December. The cell-wall structure and lignin distribution of phloem fibres on both the tension and opposite sides were compared by light microscopy, ultraviolet microscopy, confocal laser scanning microscopy after staining with acriflavine, and transmission electron microscopy after staining with potassium permanganate.
Key Results
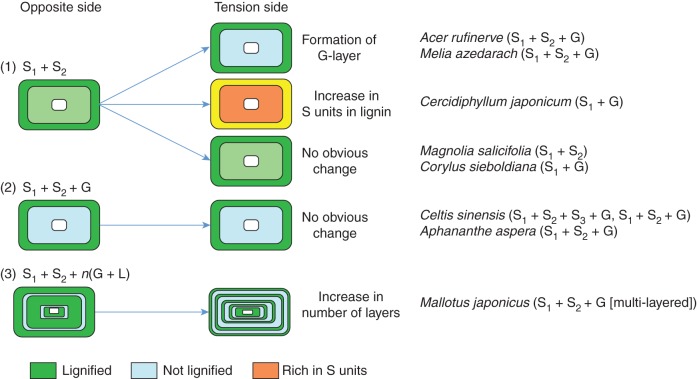
Three types of changes were found in tension-side phloem fibres: (1) increases in the proportion of the syringyl unit in lignin in the S1 and S2 layers and compound middle lamella (Cercidiphyllum japonicum), (2) formation of unlignified gelatinous layers (Melia azedarach and Acer rufinerve) and (3) increases in the number of layers (n) in the multi-layered structure of S1 + S2 + n (G + L) (Mallotus japonicus). Other species showed no obvious change in cell-wall structure or lignin distribution.
Conclusions
Phloem fibres of the tree species examined in our study showed three types of changes in lignin distribution and cell-wall structure. The reaction phenomenon may vary with tree species and may not be closely related to G-fibre type in tension wood.
Keywords: Phloem fibre, lignification, reaction phloem, Cercidiphyllum japonicum, Melia azedarach, Acer rufinerve, Mallotus japonicus, Celtis sinensis, Aphananthe aspera, Corylus sieboldiana, Magnolia salicifolia
INTRODUCTION
Trees produce special tissue in xylem, referred to as tension wood in angiosperms, when their stems are inclined (Bamber, 2001; Clair et al., 2005). Tension wood is generally formed on the upper side of the stems of leaning trees, the trunks of which display eccentric growth on the upper side and exhibit specific structural changes in cell-wall layers (Onaka, 1949; Robards, 1966). In some species, tension wood consists of gelatinous wood fibres (G-fibres) with a gelatinous layer (G-layer) containing highly oriented crystalline cellulose. These G-fibres have been classified into three types, namely S1 + G, S1 + S2 + G and S1 + S2 + S3 + G (Wardrop and Dadswell, 1955; Saiki and Ono, 1971), although more than half of species contain no G-fibres (Onaka, 1949; Fisher and Stevenson, 1981; Yoshizawa et al., 2000; Clair et al., 2006).
Most trees have phloem fibres in bark that have thick, lignified secondary walls. The formation of phloem fibres is known to be affected by soil drought in flax (Chemikosova et al., 2006). Although tension wood formation and G-fibre structure in relation to mechanical stress have been well studied in xylem, research on the influence of the reaction phenomenon on phloem fibres has been relatively sparse and incomprehensive. Phloem fibres of dicotyledonous woods have been classified into three types, S1 + S2, S1 + S2 + G and S1 + S2 + n (G + L) (Nanko, 1979), where L refers to the lignified cell-wall layer and n is the number of repetitions of G and L. The value of n varies with individual phloem fibre. Dadswell and Wardrop (1955) reported that phloem fibres on the tension side (T side) of the stem in Eucalyptus were similar in structure to tension wood fibres but different from those on the opposite side (O side) of the stem. Scurfield and Wardrop (1962) reported that G-layers developed within reaction phloem fibres and G-fibres of Acacia acuminata xylem. Scurfield (1964) reported that microfibril orientation of S2 layers in primary phloem fibres changed toward a more axial position on the upper sides of bent stems in Lagunaria pattersoni, which did not produce a G-layer in tension wood fibres. Krishnamurthy et al. (1997) examined reaction phloem in ten species of Leguminosae and found that phloem fibres either were exclusively gelatinous or were both gelatinous and normal, except in Prosopis juliflora, in which G-fibres in phloem could be seen irrespective of whether they were located on the T or normal side of the stem. Hsu et al. (2005) studied the anatomical characteristics of secondary phloem in branches of Zelkova serrata and found an increased proportion of G-fibres on the upper side as compared with the lower side. Toghraie et al. (2006) reported that reaction phloem fibres in Eucalyptus gunnii had G-layers. However, detailed information about the formation or lignification of phloem fibres is not available because anatomical variability is much greater in hardwood phloem than in xylem.
The purpose of this study was to clarify the influence of stem inclination on secondary phloem fibres using several Japanese hardwood species that produce different types of G-fibres or no G-fibres in their tension wood to gain a better understanding of the mechanism of the reaction phenomenon associated with inclination of the stem. Changes in cell-wall structure and lignin distribution in reaction phloem fibres were investigated. Moreover, the relationship between cell-wall formation in tension wood fibres and that in reaction phloem fibres is discussed.
MATERIALS AND METHODS
Plant materials
To examine the relationship between changes in tension wood fibres and reaction phloem fibres, 12 trees from eight hardwood species with different types of phloem fibres that produce different types of G-fibres in tension wood were selected. The tree species used are listed in Table 1.
TABLE 1.
Species used in this study
| No. | Family | Species | Sampling date | Height (cm) | DBH (cm) | SW structure of TW fibre |
|---|---|---|---|---|---|---|
| 1 | Betulaceae | Corylus sieboldiana | 2008.7.15 | 425 | 2·36 | S1 + G |
| 2 | 2008.12.1 | 536 | 2·87 | |||
| 3 | Cercidiphyllaceae | Cercidiphyllum japonicum | 2008.7.11 | 430 | 3·82 | S1 + G |
| 4 | Aceraceae | Acer rufinerve | 2008.7.10 | 460 | 4·14 | S1 + S2 + G |
| 5 | Euphorbiaceae | Mallotus japonicus | 2008.7.9 | 512 | 3·79 | S1 + S2+ G (multi-layered) |
| 6 | 2008.12.2 | 530 | 3·50 | |||
| 7 | Meliaceae | Melia azedarach | 2008.7.14 | 560 | 4·87 | S1 + S2 + G |
| 8 | 2008.12.1 | 279 | 1·59 | |||
| 9 | Ulmaceae | Aphananthe aspera | 2008.12.2 | 360 | 2·23 | S1 + S2 + G |
| 10 | Celtis sinensis | 2008.7.8 | 550 | 6·37 | S1 + S2 + S3 + G and S1 + S2 + G | |
| 11 | 2008.12.1 | 420 | 2·61 | |||
| 12 | Magnoliaceae | Magnolia salicifolia | 2008.7.2 | 309 | 2·23 | S1 + S2 |
Abbreviations: DBH, diameter of breast height; SW, secondary wall; TW, tension wood.
Samples were collected in Japan at the Kitashirakawa Experimental Station of the Field Science Education and Research Center of Kyoto University, the Botanical Garden of the Faculty of Science, Kyoto University, and Hieidaira (Shiga, 35°N, 136°E; 400 m a.s.l.). At the beginning of cambial growth in April 2007 or 2008, sample trees were inclined artificially to about 30–45° from vertical. To prevent upward bending of stems, a rope was fixed to the lower side of each inclined stem. Sample trees were cut down during active xylem and phloem formation in July 2008 and after completion of xylem and phloem formation in December 2008 (Table 1). Stem portions showing eccentric growth in tension wood were cut into discs and stored in 70 % ethanol. Hand sections were made from portions of these discs and stained with chlorozinc-iodine solution to confirm the distribution of G-fibres in xylem. Two discs were selected, one for light microscopy and the other for embedding.
Light microscopy
Two blocks containing xylem and phloem were collected from the T and O sides of the disc. Transverse sections 20–30 µm thick were cut from the T- and O-side blocks with a sliding microtome and stained with safranin and astra blue to observe the presence of non-lignified cell walls in xylem and phloem. The width of current-year xylem and phloem was then measured on both the T and O sides to evaluate the degree of eccentric growth due to tension wood formation. An example is shown in Fig. 1.
Fig. 1.
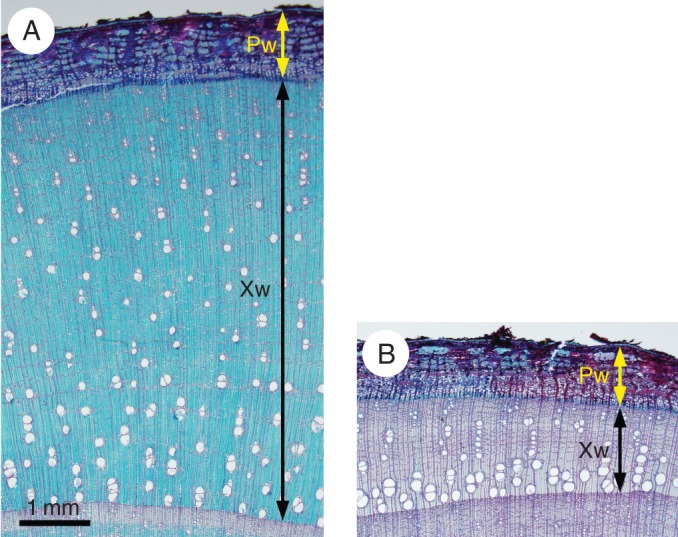
Light micrographs of transverse sections of Mallotus japonicus collected in December (tree 6) after staining with safranin and astra blue on the tension (A) and opposite (B) sides. Xw, width of xylem produced in the current year; Pw, whole phloem width.
Sections were also stained with Wiesner and Mäule reagents to observe the distribution of cinnamaldehyde groups and syringyl units in lignin, respectively. For the Wiesner reaction, sections were treated with 2 % phloroglucin in ethanol and then mounted with 6 m hydrochloric acid. For the Mäule reaction, sections were treated with 1 % potassium permanganate for 5 min, washed with water, treated with 2 m hydrochloric acid for 5 min, washed again with water, and then mounted in concentric ammonium hydroxide. The sections were observed soon after application of the reagents because the Wiesner and Mäule colour reactions are not stable.
Evaluation of the Mäule reaction staining intensity
Visible light absorption spectra measured after the Mäule colour reaction indicated strong absorption at 520 nm (Yoshinaga et al., 1989; Takabe et al., 1992). Transverse sections (30 µm thick) of blocks of Cercidiphyllum japonicum from both T and O sides collected in July were photographed at 520 nm with a microspectrophotometer (UMSP-80; Carl Zeiss, Oberkochen, Germany) after the Mäule colour reaction. Photographs were taken within 5 min of the reaction. The absorption in various morphological regions was evaluated by image analysis. The grayscale images obtained were inverted, and brightness profiles were analysed along lines between the centres of the lumen of adjacent phloem fibres by Image J (National Institutes of Health, Bethesda, MD, USA). This evaluation was conducted on the first tangential band of phloem fibres that were produced in the current year and influenced by the bending stem.
Resin embedding
Both T- and O-side blocks were cut into small pieces that included bark, cambium and xylem. They were then dehydrated through an ethanol series and embedded in epoxy resin or LR White resin.
Fluorescence and polarizing light microscopy
Transverse sections (5 µm thick) were prepared from epoxy resin-embedded specimens with a rotary microtome (Porter-Blum JB-4; Sorvall, Thermo Scientific, Rockford, IL, USA). Sections were then stained with 0·001 % acriflavine, which stains lignified tissue with a green fluorescence (530 nm) (Donaldson et al., 2001), for 1 h at room temperature and observed under a fluorescence microscope (BX-50; Olympus, Tokyo, Japan). To observe a wider area at higher resolution, 30-μm-thick transverse sections were also stained with 0·001 % acriflavine and observed under a confocal laser scanning microscope (Fluoview FV300; Olympus).
To investigate the relationship between lignin distribution and arrangement of cellulose microfibrils in different cell-wall layers of phloem fibres, the same sections (5 µm thick) used for fluorescence microscopy were observed under a polarizing microscope with polarizer and analyser filters in crossed positions. Using polarizing microscopy of transverse sections, cell walls with a steep helix of cellulose microfibrils (e.g. G-layers) showed no birefringence, whereas cell walls with a flat helix of microfibrils (e.g. S1 layers) showed birefringence.
Ultraviolet and transmission electron microscopy
For ultraviolet (UV) microscopy, 3-μm-thick transverse sections were prepared from specimens embedded in epoxy resin, mounted on quartz slides with glycerine, and covered with quartz coverslips. UV photomicrographs were taken at 280 nm using a microspectrophotometer (UMSP-80; Carl Zeiss) and recorded on Fuji Neopan SS film. The bandwidth of the illuminating monochrometer was set at 15 nm. Photo negatives were scanned with a film scanner (DimageScan Multi; Minolta, Tokyo, Japan) to produce digital images.
For transmission electron microscopic (TEM) analysis, ultrathin sections were prepared from specimens embedded in LR White resin. Sections were stained with 1 % KMnO4 and 0·1 % sodium citrate aqueous solution. Stained sections were observed by TEM (JEM-1220 or JEM 1400; JEOL, Tokyo, Japan) at 100 kV.
RESULTS
Differences in phloem width between T and O sides
The widths of xylem produced in the current year and the whole phloem width are shown in Table 2. The ratio of T-side to O-side widths was also calculated for both xylem and phloem. Because it was difficult to distinguish current-year from older phloem, the entire phloem width was measured. The T/O ratio was quite high in xylem, indicating eccentric growth during tension wood formation in eight hardwood species. Except for Melia azedarach collected in December (tree 8) and Magnolia salicifolia (tree 12), the T/O ratio of phloem was also >1, but was lower than that of xylem.
TABLE 2.
The width of phloem and xylem
| No. | Species | Month | Width of phloem (mm) |
Width of xylem produced in current year (mm) |
||||
|---|---|---|---|---|---|---|---|---|
| T | O | T/O | T | O | T/O | |||
| 1 | Corylus sieboldiana | July | 0·82 | 0·82 | 1·00 | 1·69 | 0·53 | 3·19 |
| 2 | December | 0·67 | 0·50 | 1·34 | 2·00 | 0·61 | 3·28 | |
| 3 | Cercidiphyllum japonicum | July | 1·32 | 1·30 | 1·02 | 3·07 | 1·23 | 2·50 |
| 4 | Acer rufinerve | July | 2·59 | 2·29 | 1·13 | 2·06 | 1·03 | 2·00 |
| 5 | Mallotus japonicus | July | 0·95 | 0·80 | 1·19 | 5·31 | 1·63 | 3·26 |
| 6 | December | 0·88 | 0·85 | 1·04 | 5·29 | 1·43 | 3·70 | |
| 7 | Melia azedarach | July | 1·31 | 1·16 | 1·13 | 9·61 | 3·39 | 2·83 |
| 8 | December | 0·63 | 0·82 | 0·77 | 1·36 | 0·20 | 6·80 | |
| 9 | Aphananthe aspera | December | 1·18 | 1·10 | 1·07 | 5·69 | 1·70 | 3·35 |
| 10 | Celtis sinensis | July | 0·89 | 0·86 | 1·03 | 0·95 | 0·61 | 1·56 |
| 11 | December | 0·96 | 0·82 | 1·17 | 0·49 | 0·17 | 2·88 | |
| 12 | Magnolia salicifolia | July | 1·10 | 1·21 | 0·91 | 1·51 | 0·89 | 1·70 |
Cell-wall structure and lignin distribution of reaction phloem fibres
The reaction phenomenon observed in cell-wall structure and lignin distribution in phloem fibres induced by stem inclination differed among tree species.
Cercidiphyllum japonicum
The phloem fibres in Cercidiphyllum japonicum were of the S1 + S2 cell-wall type. T-side phloem fibres stained a stronger reddish-purple colour in the Mäule reaction than did O-side fibres due to the presence of more syringyl units in lignin (Fig. 2). To evaluate staining intensity in the Mäule reaction, photographs were taken at 520 nm with a microspectrophotometer (Fig. 3A, B), and absorption was evaluated as the brightness of inverted images. The brightness profiles of inverted images of phloem fibres from the T and O sides are shown in Fig. 3C and D. Brightness was higher in S1, S2 and compound middle lamella on the T side than on the O side, indicating that the proportion of syringyl units in lignin was greater in the S1 and S2 layers and compound middle lamella on the T side.
Fig. 2.
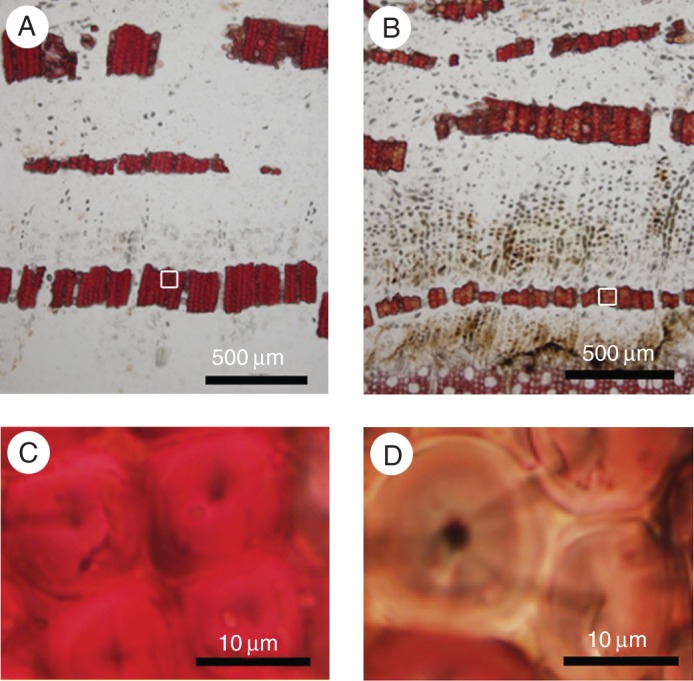
Light micrographs of transverse sections of Cercidiphyllum japonicum after the Mäule reaction. Phloem fibres from the tension side (A, C) showed a more pronounced reddish-purple colour than those from the opposite side due to the presence of more syringyl units in lignin (B, D). (C) The rectangular area in (A) at higher magnification; (D) the rectangular area in (B) at higher magnification.
Fig. 3.
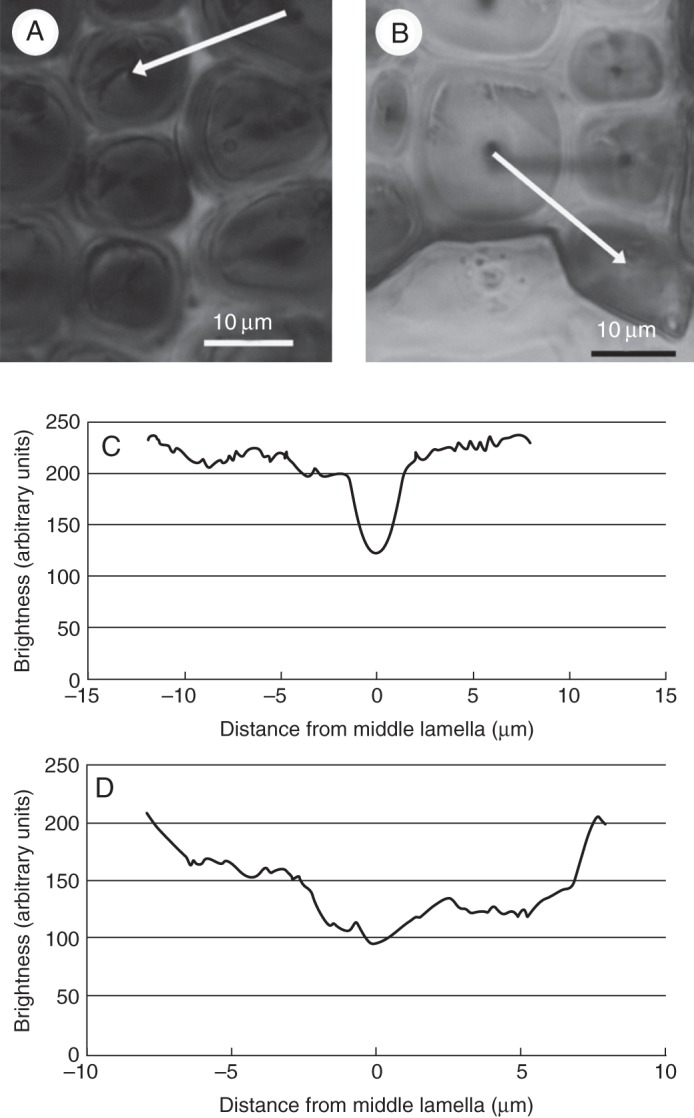
Light micrographs of Cercidiphyllum japonicum taken at 520 nm after the Mäule reaction on the tension (A) and opposite (B) sides. (C) Brightness profile of the tension side along the white line in (A) from an inverted image; (B) brightness profile of the opposite side along the white line in (B) from an inverted image.
Melia azedarach and Acer rufinerve
The phloem fibres in Melia azedarach and Acer rufinerve were of the S1 + S2 cell-wall type. The Wiesner and Mäule reactions showed some phloem fibres with unlignified cell-wall layers (G-like layer) on the T side, similar to the G-fibres in tension wood (Fig. 4). Fluorescence microscopy revealed that this G-like layer contained less lignin than the phloem fibres on the O side (Fig. 5C, D). Polarizing light microscopy also showed that this layer did not display birefringence in transverse section, suggesting that microfibrils were arranged almost parallel to the fibre axis in these G-like layers (Fig. 5E, F). The same pattern is seen in G-layers of tension wood. In these specimens, therefore, G-layers were formed on the T side and contained microfibrils arranged almost parallel to the fibre axis and less lignin, similar to the G-layers in tension wood fibres. G-layers were thinner in Melia azedarach collected in July (tree 7 in Table 1) than in Melia azedarach collected in December (tree 8 in Table 1).
Fig. 4.
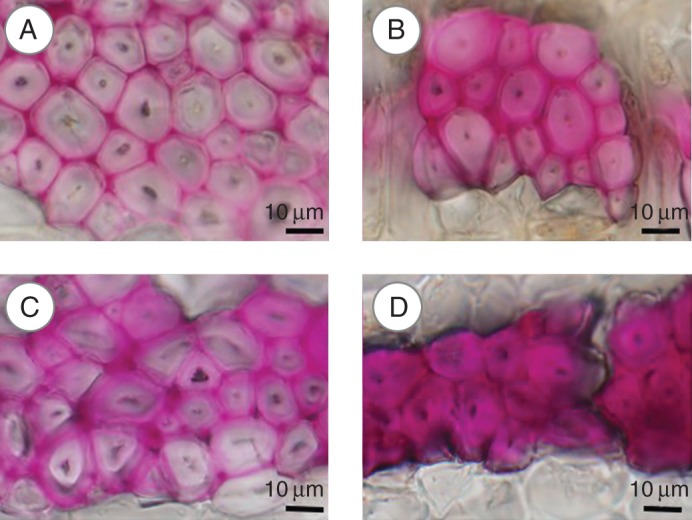
Light micrographs of transverse sections after the Wiesner reaction in Acer rufinerve (A, B) and Melia azedarach (C, D) collected in July. (A, C) Tension side; (B, D) opposite side.
Fig. 5.
Light micrographs of transverse sections of Melia azedarach collected in July. (A, B) Phloem fibres stained with Azur II and methylene blue showing the presence of gelatinous (G)-layers on the tension side (A). (C, D) Fluorescence micrographs of transverse sections of the same phloem fibres shown in (A) and (B) after staining with acriflavine. G-layers contained less lignin (C). (E, F) Polarizing light micrographs of transverse sections of the same phloem fibres shown in (A) and (B). Microfibrils in G-layers arranged almost parallel to the fibre axis (E). (A, C, E) Tension side; (B, D, F) opposite side. G, G-layers; L, lignified layers.
Mallotus japonicus
In Mallotus japonicus, the Wiesner and Mäule reactions showed a multi-layered structure in which thick and thin layers were alternatively arranged on both sides, but with more on the T side. Given the thinness of the thin layers, it was not clear whether these layers were stained in the Wiesner and Mäule reactions. UV microscopy and confocal laser scanning microscopy after staining with acriflavine clearly showed that the cell-wall type of the phloem fibres was multi-layered (Fig. 6), with alternating unlignified G-layers and lignified L-layers. TEM also confirmed that the cell-wall structure was S1 + S2 + n (G + L) (Fig. 7). The number of layers (n) was lower in tree 5, collected in July, than in tree 6, collected in December.
Fig. 6.
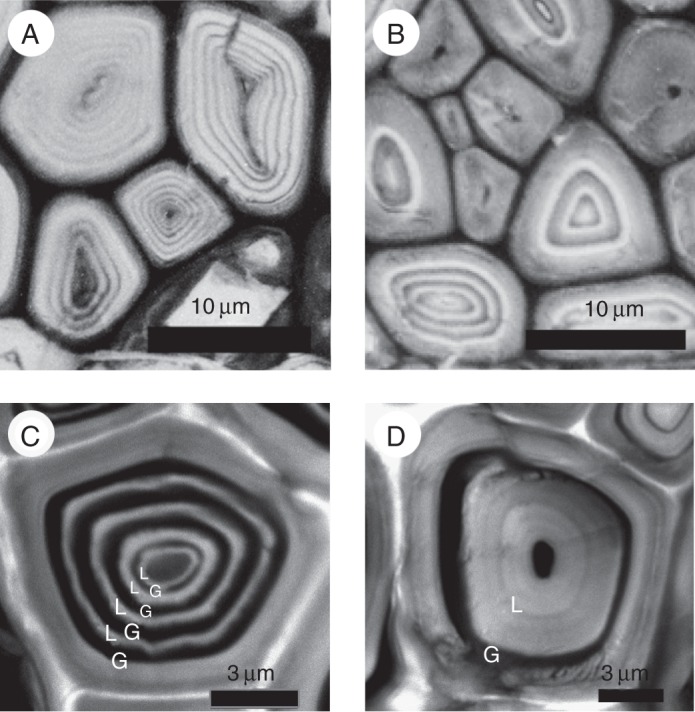
(A, B) Ultraviolet micrographs of transverse sections of phloem fibres in Mallotus japonicus collected in December, taken at 280 nm. (C, D) Confocal images of phloem fibres stained with acriflavine. (A, C) Tension side; (B, D) opposite side.
Fig. 7.
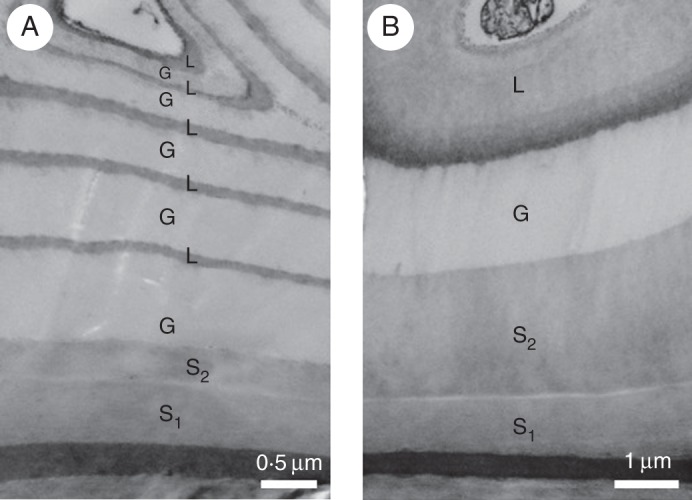
Transmission electron micrographs of transverse sections of Mallotus japonicus phloem fibres stained with KMnO4. (A) Tension side; (B) opposite side.
The frequency distribution (number of cells) of n in the multi-layered structure of S1 + S2 + n (G + L) in phloem fibres of both the T and O sides was examined for more than 80 cells using a confocal laser scanning microscope. The number of layers (n) was significantly (P < 0·01) greater on the T side than on the O side (Fig. 8). The thickness of the innermost L-layers, which were relatively thick on the O side (Figs 6D and 7B), tended to decrease as the number of G- and L-layers increased on the T side. Therefore, the L-layer area percentage tended to decrease with increasing proportion of G-layer area in the phloem fibres on the T side.
Fig. 8.
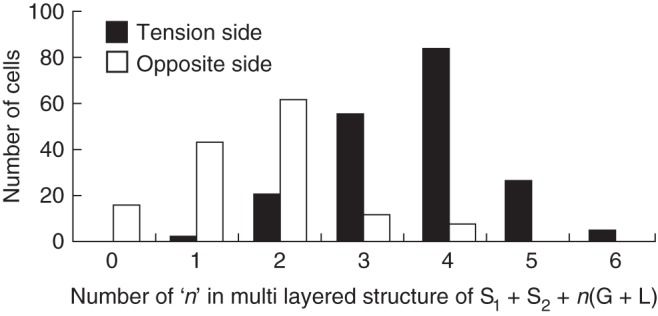
Frequency distribution (number of cells; n) in multi-layered structures of S1 + S2 + n (G + L) in phloem fibres of Mallotus japonicus. Tension side and opposite side as indicated in the key.
Celtis sinensis, Aphananthe aspera, Corylus sieboldiana and Magnolia salicifolia
The phloem fibres of Celtis sinensis and Aphananthe aspera were of the S1 + S2 + G cell-wall type, and those of Corylus sieboldiana and Magnolia salicifolia were of the S1 + S2 type. These species showed no obvious change in cell-wall structure or lignin distribution with stem inclination. In Celtis sinensis, G-layers were already formed in tree 10 collected in July. In Corylus sieboldiana, phloem fibres with S1 + S2 layers were already formed in tree 1 collected in July and no obvious change was observed between tree 1 and tree 2, collected in December.
DISCUSSION
In half of the tree species examined in the present study, the cell-wall structure and lignin distribution in phloem fibres differed between the upper and lower sides of inclined tree stems. Except for Melia azedarach collected in July and Magnolia salicifolia, the T/O ratio of phloem width was slightly greater than 1 and much lower than that of xylem (Table 2). This result was due to our use of measurements of whole phloem width, which, in addition to including phloem that was affected by stem inclination, also included phloem that was formed before the tree stems were placed at an angle. Particularly in Melia azedarach collected in July (tree 7 in Table 1), the tree was naturally inclined in the opposite direction before artificial inclination and the phloem contained both tension phloem produced in the current year and opposite phloem produced before artificial inclination. Although the rate of cell production was much lower in phloem than in xylem, our results suggest that the rate of cell production in phloem was higher on the T side than on the O side. This result suggests that cambial activity was promoted by stem inclination, even in tree species that showed no difference in cell-wall structure or lignin distribution of phloem fibres, to ensure against mechanical stress by increased amounts of xylem and phloem on the T side. In contrast to xylem, secondary phloem contains non-lignified tissues (sieve tube members, phloem parenchyma cells) that play important physiological roles in trees. Secondary xylem contains lignified tissues that are better able to ensure mechanical stress than secondary phloem. Therefore, our results also suggest that secondary phloem on the T side may play an auxiliary role, increasing the content of lignified tissues by promoting cambial activity.
Three kinds of changes were found in lignin distribution and cell-wall structure in phloem fibres of leaning stems. The first change was an increase in the proportion of syringyl units in lignin of Cercidiphyllum japonicum, although no change in cell-wall structure (S1 and S2) was observed (Figs 2 and 3). This increase in syringyl units may have strengthened the cell walls of the fibres. This phenomenon seems to be a reaction against stem inclination in phloem fibres. Akiyama et al. (2003) investigated tension wood lignin in Liriodendron tulipifera and found that the methoxyl content and proportion of erythro form in β-O-4 linkage were higher in tension wood than in opposite wood. As Liriodendron tulipifera produces tension wood fibres without G-layers, an increase in the syringyl units in lignin might be another reaction phenomenon in response to stem inclination.
The second change was the formation of G-layers, which was similar to the reaction phenomenon in the tension wood fibres in response to stem inclination (Figs 4 and 5). Fang et al. (2008) suggested that G-layers play the most important role in high growth-stress generation. This may be explained by the hypothesis that the tensile stress of microfibrils governs the longitudinal tensile stress in tension wood (Okuyama et al., 1994). Clair et al. (2011) found an increase in cellulose lattice spacing during tension wood formation in poplar, and suggested that the G-layer directly generates and supports tensile maturation stress in poplar tension wood. Secondary phloem contains both hard tissues (phloem fibres, fibre sclereids and sclereids) with lignified secondary walls and soft tissues (sieve tube members and phloem parenchyma cells) with unlignified primary walls. Therefore, the contribution of phloem fibres to the generation of tensile stress is unclear. Clair et al. (2006) also showed that some species without the G-layer are able to produce higher stress than other species with fibres containing a G-layer. However, it is reasonable to expect that G-layers produced in phloem fibres may play some role in growth-stress generation.
The third change was an increase in the number of layers in multi-layered structures (Fig. 6). This was found in Mallotus japonicus, which originally has a multi-layered structure. This species responds to the inclination stimulus by increasing the number of repetitions of G- and L-layers, i.e. by increasing the proportion of normal G-layers to generate more tensile stress. Nanko et al. (1982) reported that the cell-wall type of secondary phloem fibres in Populus euramericana was S1 + S2, whereas the secondary walls of reaction phloem fibres were of the S1 + S2 + n (G + L) type. Similar multi-layered cell-wall structures have been reported in tension wood fibres of tropical hardwoods (Clair et al., 2006; Ruelle et al., 2007). Ruelle et al. (2007) reported that the residual growth strain of tension wood with the multi-layered structure was in the upper range of reported values. The multi-layered structure of phloem fibres may play some role in growth-stress generation. Additional research to clarify this cell-wall formation process is required to better understand this multi-layer-type cell-wall structure. In Mallotus japonicus, a peculiar multi-layered structure [S1 + S2 + G + n (L + G); n = 2, L = very thin lignified layer] was found in the tension wood fibre. This suggests that Mallotus japonicus forms a multi-layered structure of both xylem and phloem to ensure mechanical stress.
In conclusion, phloem fibres of the eight studied tree species showed three types of cell-wall structure: S1 + S2, S1 + S2 + G and a multi-layered type. Three changes induced by stem inclination were observed in the T side phloem fibres of four species, namely an increase in the proportion of syringyl units in lignin, the formation of G-layers and an increase in the number of layers in multi-layered structures. The four other species exhibited no obvious change in cell-wall structure or lignin distribution (Fig. 9), although they showed promoted cambial activity on the T side. Our results suggest that secondary phloem on the T side may play an auxiliary role in ensuring mechanical stress by increasing lignified tissue content and promoting cambial activity. The influence of the reaction phenomenon on the cell-wall organization and lignin distribution of phloem fibres might vary among species depending on the degree of the secondary phloem auxiliary role. In addition, changes in the cell walls of reaction phloem fibres were weakly related to the cell-wall organization of tension wood fibres (Table 1), namely S1 + G, S1 + S2 + G, S1 + S2 + S3 + G and S1 + S2 (no G-layer). The multi-layered phloem fibre structure in Mallotus japonicus was similar to the cell-wall structure of tension wood fibres with a multi-layered structure.
Fig. 9.
Schematic of the three types of change in phloem fibres caused by stem inclination: (1) an increase in the proportion of syringyl units in lignin, (2) formation of gelatinous layers and (3) an increase in the number of layers in multi-layered structures. Parentheses after scientific names indicate the type of cell-wall structure in the tension wood fibres.
Further research on the structural variation in reaction phloem fibres in various plants is needed to better understand the reaction phenomenon in phloem fibres.
ACKNOWLEDGEMENTS
We are grateful to Prof. Minoru Fujita for providing samples of Cercidiphyllum japonicum. We also thank members of the Laboratory of Tree Cell Biology of Kyoto University for their kind support.
LITERATURE CITED
- Akiyama T, Matsumoto Y, Okuyama Y, Meshitsuka G. Ratio of erythro and threo forms of beta-O-4 structures in tension wood lignin. Phytochemistry. 2003;64:1157–1162. doi: 10.1016/s0031-9422(03)00509-0. [DOI] [PubMed] [Google Scholar]
- Bamber RK. A general theory for the origin of growth stresses in reaction wood: how trees stay upright. IAWA Journal. 2001;22:205–212. [Google Scholar]
- Chemikosova SB, Pavlencheva NV, Gur'yanov OP, Gorshkova TA. The effect of soil drought on the phloem fiber development in long-fiber flax. Russian Journal of Plant Physiology. 2006;53:656–662. [Google Scholar]
- Clair B, Gril J, Baba K, Thibaut B, Sugiyama J. Precautions for the structural analysis of the gelatinous layer in tension wood. IAWA Journal. 2005;26:189–195. [Google Scholar]
- Clair B, Ruelle J, Beauchêne J, Prévost MF, Fournier M. Tension wood and opposite wood in 21 tropical rain forest species. 1. Occurrence and efficiency of the G-layer. IAWA Journal. 2006;27:329–338. [Google Scholar]
- Clair B, Alméras T, Pilate G, Jullien D, Sugiyama J, Riekel C. Maturation stress generation in poplar tension wood studied by synchrotron radiation microdiffraction. Plant Physiology. 2011;155:562–570. doi: 10.1104/pp.110.167270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadswell HE, Wardrop AB. The structure and properties of tension wood. Holzforschung. 1955;9:97–104. [Google Scholar]
- Donaldson LA, Hague J, Snell R. Lignin distribution in coppice poplar, linseed and white straw. Holzforschung. 2001;55:379–385. [Google Scholar]
- Fang CH, Clair B, Gril J, Liu SQ. Growth stresses are highly controlled by the amount of G-layer in poplar tension wood. IAWA Journal. 2008;29:237–246. [Google Scholar]
- Fisher JB, Stevenson JW. Occurrence of reaction wood in branches of dicotyledons and its role in tree architecture. Botanical Gazette. 1981;142:82–95. [Google Scholar]
- Hsu YS, Chen SJ, Lee CM, Kuo-Huang LL. Anatomical characteristics of the secondary phloem in branches of Zelkova serrata Makino. Botanical Bulletin of Academia Sinica. 2005;46:143–149. [Google Scholar]
- Krishnamurthy KV, Venugopal N, Nandagopalan V, Hariharan Y, Sivakumari A. Tension phloem in some legumes. Journal of Plant Anatomy and Morphology. 1997;7:20–23. [Google Scholar]
- Nanko H. Studies on the development cell wall structure of sclerenchymatous elements in the secondary phloem of woody dicotyledons , conifers. Japan (in English): Kyoto University; 1979. PhD Thesis. [Google Scholar]
- Nanko H, Saiki H, Harada H. Structural modification of secondary phloem fibers in the reaction phloem of Populus euramericana. Mokuzai Gakkaishi. 1982;28:202–207. [Google Scholar]
- Okuyama T, Yamamoto H, Yoshida M, Hattori Y, Archer RR. Growth stresses in tension wood: role of microfibrils and lignification. Annals of Forest Science. 1994;51:291–300. [Google Scholar]
- Onaka F. Studies on compression and tension wood. Wood Research. 1949;1:1–88. [Google Scholar]
- Robards AW. The application of the modified sine rule to tension wood production and eccentric growth in the stem of crack willow (Salix fragilis L.) Annals of Botany. 1966;30:513–523. [Google Scholar]
- Ruelle J, Yoshida M, Clair B, Thibaut B. Peculiar tension wood structure in Laetia procera (Poepp.) Eichl. (Flacourtiaceae) Trees. 2007;21:345–355. [Google Scholar]
- Saiki H, Ono K. Cell wall organization of gelatinous fibers in tension wood. Bulletin of Kyoto University Forests. 1971;42:210–220. [Google Scholar]
- Scurfield G. The nature of reaction wood. IX. Anomalous cases of reaction anatomy. Australian Journal of Botany. 1964;12:173–184. [Google Scholar]
- Scurfield G, Wardrop AB. The nature of reaction wood. VI. The reaction anatomy of seedlings of woody perennials. Australian Journal of Botany. 1962;10:93–105. [Google Scholar]
- Takabe K, Miyauchi S, Tsunoda R, Fukazawa K. Distribution of guaiacyl and syringyl lignins in Japanese beech (Fagus crenata): variation within an annual ring. IAWA Bulletin New Series. 1992;13:105–112. [Google Scholar]
- Toghraie N, Parsapajouh D, Ebrahimzadeh H, Thibaut B, Gril J, Moghadam Y. Tension wood in Eucalypt trees. Journal of Science (University of Tehran) 2006;32:13–22. [Google Scholar]
- Wardrop AB, Dadswell HE. The nature of reaction wood. Variations in cell wall organization of tension wood fibres. Australian Journal of Botany. 1955;3:177–189. [Google Scholar]
- Yoshinaga A, Fujita M, Saiki H. Evaluation of varieties of lignins in wood and bamboo cell walls by Mäule color reaction coupled with microscopic spectrophotometry. Bulletin of the Kyoto University Forests. 1989:276–284. No. 61: [Google Scholar]
- Yoshizawa N, Inami A, Miyake S, Ishiguri F, Yokota S. Anatomy and lignin distribution of reaction wood in two Magnolia species. Wood Science Technology. 2000;34:183–196. [Google Scholar]