Abstract
Background and Aims
Latewood formation in conifers occurs during the later part of the growing season, when the cell division activity of the cambium declines. Changes in temperature might be important for wood formation in trees. Therefore, the effects of a rapid decrease in temperature on cellular morphology of tracheids were investigated in localized heating-induced cambial reactivation in Cryptomeria japonica trees and in Abies firma seedlings.
Methods
Electric heating tape and heating ribbon were wrapped on the stems of C. japonica trees and A. firma seedlings. Heating was discontinued when 11 or 12 and eight or nine radial files of differentiating and differentiated tracheids had been produced in C. japonica and A. firma stems, respectively. Tracheid diameter, cell wall thickness, percentage of cell wall area and percentage of lumen area were determined by image analysis of transverse sections and scanning electron microscopy.
Key Results
Localized heating induced earlier cambial reactivation and xylem differentiation in stems of C. japonica and A. firma as compared with non-heated stems. One week after cessation of heating, there were no obvious changes in the dimensions of the differentiating tracheids in the samples from adult C. japonica. In contrast, tracheids with a smaller diameter were observed in A. firma seedlings after 1 week of cessation of heating. Two or three weeks after cessation of heating, tracheids with reduced diameters and thickened cell walls were found. The results showed that the rapid decrease in temperature produced slender tracheids with obvious thickening of cell walls that resembled latewood cells.
Conclusions
The results suggest that a localized decrease in temperature of stems induces changes in the diameter and cell wall thickness of differentiating tracheids, indicating that cambium and its derivatives can respond directly to changes in temperature.
Keywords: Cambial activity, conifers, latewood formation, morphology of tracheids, rapid decrease in temperature
INTRODUCTION
The date of annual transition from earlywood to latewood formation is a conspicuous developmental process in conifers. Earlywood is characterized by thin-walled tracheids with a large radial diameter, while latewood is composed of tracheids with a smaller radial diameter and thick cell walls (Larson, 1964; Denne and Dodd, 1981; Schweingruber, 1988). Thus, the induction of latewood is associated with changes in cellular morphology. Formation of latewood is generally induced during the later part of the growing season, when cell division in cambium declines. The amount of latewood that is formed controls wood quality through changes in wood density and reflects the amount of CO2 fixed by trees. Latewood formation is, therefore, of considerable interest and has been studied in detail (Larson, 1964; Funada et al., 1987, 1990; Schweingruber, 1988). The cited studies suggested that changes in environmental conditions and/or endogenous factors might be important for the formation of latewood.
A reduction in radial diameter and/or an increase in the thickness of the cell wall of tracheids results in latewood formation, and these changes are controlled by internal and external factors (Larson, 1964; Wodzicki, 1971; Denne and Dodd, 1981; Timell, 1986). Larson (1964) proposed that the formation of earlywood is associated with active shoot growth in conjunction with high levels of auxin, while latewood formation follows cessation of shoot growth and a decline in the transport of auxin to the cambium in Pinus resinosa. In addition, evidence has been presented to suggest that, during latewood formation, the decrease in radial diameter of tracheids occurs as a result of decreased levels of auxin, and increased thickening of secondary walls results from the increased availability of assimilates. The transition from earlywood to latewood in Abies balsamea and Pinus densiflora was associated with a decrease in the total amount of endogenous auxin in the cambial region after the level of auxin had peaked (Sundberg et al., 1987; Funada et al., 2001). In contrast, Uggla et al. (2001), studying Pinus sylvestris, concluded that there was no clear correlation between changes in seasonal levels of auxin in cambial cells and latewood formation. Details of the physiological regulation of the reduction in tracheid diameter and increases in the thickness of tracheid cell walls during latewood formation remain to be clarified.
Certain environmental conditions can induce the formation of latewood, For example, latewood formation was observed when red pine trees were exposed to severe drought conditions (Zahner et al., 1964), and an 18 h photoperiod caused the formation of large diameter tracheids, while an 8 h photoperiod resulted in the formation of narrow tracheids (Larson, 1964, 1969a). Tracheids of conifers grown at lower temperatures had thicker walls than those grown at higher temperatures, and the thickness of cell walls decreased at increasing temperature (Richardson, 1964; Larson, 1967; Denne, 1971). Localized heating of Picea sitchensis stems increased tracheid diameter but had no effect on the thickness of cell walls (Richardson, 1966). Variations in daytime and night-time temperatures are important for the radial expansion of tracheids (Richardson and Dinwoodie, 1960; Richardson, 1964; Dünisch, 2010), and Dünisch (2010) reported that low night-time temperatures were responsible for reductions in the radial expansion of tracheids in seedlings of Podocarpus latifolius. Thus, low temperatures might play a major role in xylem differentiation.
Localized heating of stems during cambial dormancy hastens cambial reactivation and xylem differentiation in conifers (Savidge and Wareing, 1981; Barnett and Miller, 1994; Oribe and Kubo, 1997; Oribe et al., 2001; Gričar et al., 2006; Begum et al., 2010a) and in a hardwood hybrid poplar (Begum et al., 2007). In all cases, the patterns of heat-induced cambial reactivation and earlywood formation were almost identical to those of natural systems. Therefore, it was postulated that artificial heating of stems might provide a good model system for investigating the biology of cambium because it would allow the direct analysis of changes in cambial activity and earlywood formation over relatively short periods of time. Such an artificial system provides only a localized effect in stems and does not affect the physiology of a whole tree (Oribe et al., 2001; Begum et al., 2007, 2010a).
The main purpose of the present study was to determine whether localized changes in temperature can induce latewood formation in conifers to test the hypothesis that low temperatures are responsible for reductions in the radial expansion of tracheids. The effects of a rapid decrease in temperature on the induction of latewood formation were examined by using a localized heating-induced cambial reactivation and earlywood formation system in stems of adult Cryptomeria japonica and in Abies firma seedlings, both of which provide good models for studies of cambial biology and xylogenesis. The localized heating of stems was discontinued when earlywood formation occurred in order to analyse the effects of ambient low temperature on differentiating tracheids. We discuss our results in the context of the role of a decrease in temperature in the formation of latewood.
MATERIALS AND METHODS
Plant materials
Two adult Cryptomeria japonica D.Don trees, which were 71 and 93 years old respectively, and 56 three-year-old seedlings of Abies firma Siebold & Zucc. that were growing in the field nursery of the Tokyo University of Agriculture and Technology in Fuchu, Tokyo (35°40′N, 139°29′E; 40 m above sea level) were used in the present study. The adult trees and seedlings were subjected to sequential observations of cambial activity and the differentiation of phloem and xylem during localized heating and after cessation of heating.
Heat treatment
Electric heating tape (Silicone-Rubber Heater; O & M Heater, Nagoya, Japan; 50 cm long and 30 cm wide) was affixed to one side of the main stem of each C. japonica tree at breast height which was treated as the heated stem (Fig. 1A; Oribe et al., 2001, 2003; Begum et al., 2007, 2010a). The opposite side of each heated area was used as the non-heated stem (Fig. 1A). Electric heating ribbon (Nippon Heater Co., Ltd, Tokyo, Japan; 6 m long and 0·5 cm wide) was wrapped around the entire stem at the base of each A. firma seedling, at 2–3 cm above the soil level, one by one, such that 3–4 cm of the stem of each seedling was covered with tape, which was treated as the heated stem (Fig. 1B). The seedlings around which electric heating ribbon was not wrapped were used as the non-heated stem. An alternating current was passed through the heating tape and the heating ribbon at a potential of 100 V to warm the surface of each stem. The temperature between the outer bark and the heating tape was recorded with a thermometer and, at the site at which each stem was heated, the temperature was adjusted to between 20 and 22 °C with a thermostat. There is the possibility of creating a very small electric or magnetic field by the heating system, which could have minor effects on cell activity. However, no injured cells were observed after the treatment.
Fig. 1.
(A, B) Electric heating tape affixed to one side of the main stem of adult Cryptomeria japonica trees (A) and electric heating ribbon wrapped around the entire base of stems of seedling of Abies firma in series (B).
The localized heat treatment of adult C. japonica trees and A. firma seedlings was initiated on 8 January 2007 and 13 January 2010, respectively. Heating was discontinued on 29 January 2007 and on 1 February 2010 when 11 or 12 and eight or nine radial files of tracheids had formed in the C. japonica and A. firma stems, respectively, according to the pre-planned experimental design to observe the effects of a rapid decrease in temperature on differentiating and differentiated tracheids. Samples were collected from heated and non-heated portions of stems until 27 February 2007 and 1 March 2010, respectively.
Collection of samples
Samples were taken at 3–4 d intervals from heated stems and non-heated stems of adult C. japonica trees that were growing under natural conditions throughout the sampling period. A series of small blocks (2 × 2 × 1 cm3), which contained phloem, cambium and some xylem cells, were removed with a disposable scalpel and chisel in zigzag fashion, to minimize the effects of wounding, from the heated stems and non-heated stems under natural conditions. On each sampling date, two blocks from two heated stems and two blocks from two non-heated stems were collected from two adult C. japonica trees. Thus, two blocks were collected from each tree in every sampling period. Each block was cut into 2 mm thick samples immediately after removal from the tree.
In the case of A. firma, samples were collected at 1 day intervals until 1 February 2010 and then at 1 week intervals until 1 March 2010. On each sampling date, two heated seedlings and two intact non-heated seedlings were collected. Samples were taken from the same region of stems, 2–3 cm above the base in heated and non-heated stems. Heated and non-heated portions of stems were cut into 2 mm thick samples immediately after removal from the seedlings.
Preparation of samples for light microscopy
All samples were fixed in 4 % glutaraldehyde in 0·1 m phosphate buffer (pH 7·3), under a vacuum, for 1 h at room temperature. Fixed samples were washed in 0·1 m phosphate buffer (pH 7·3) and trimmed to 3 mm in length for subsequent fixation in 1 % osmium tetroxide in 0·1 m phosphate buffer for 2 h at room temperature. After washing in phosphate buffer, specimens were dehydrated in a graded ethanol series and embedded in epoxy resin. A similar fixation process was used in previous studies in which the dimensions of phloem, cambium and xylem cells were observed properly, indicating that this fixation system was successful and there were no fixation artefacts affecting the dimensions of cambial cells and differentiating cells during seasons of active and dormant cambium (Begum et al., 2007, 2008, 2010a). Transverse sections were cut at a thickness of approx, 1 µm with a glass knife on an ultramicrotome (Ultracut N; Reichert, Vienna, Austria) for sequential observations of cambial reactivation and earlywood formation. Sections were stained with a solution of 1 % safranin in water for observations of cambial reactivation (the presence of new cell plates) and earlywood formation, and then examined under a light microscope (Axioscop; Carl Zeiss, Oberkochen, Germany, Murakami et al., 1999; Nakaba et al., 2006).
Preparation of samples for scanning electron microscopy (SEM)
Plane transverse surfaces were prepared from samples of wood that had been fixed in 4 % glutaraldehyde in 0·1 m phosphate buffer (pH 7·3) at room temperature. Fixed samples were washed three times with distilled water and then water-saturated samples were cut with a razor blade. Trimmed samples were then cleaned with distilled water, dehydrated with a graded ethanol series and dried at room temperature for 2–3 d. Finally, samples were attached to mounting stubs and sputter-coated with a 10 nm layer of gold (Sano et al., 1999). Prepared specimens were examined under a scanning electron microscope (NeoScope JCM-5000; JEOL, Tokyo, Japan) at an accelerating voltage of 10 kV for observations of differentiating tracheids.
Air temperatures during experiments
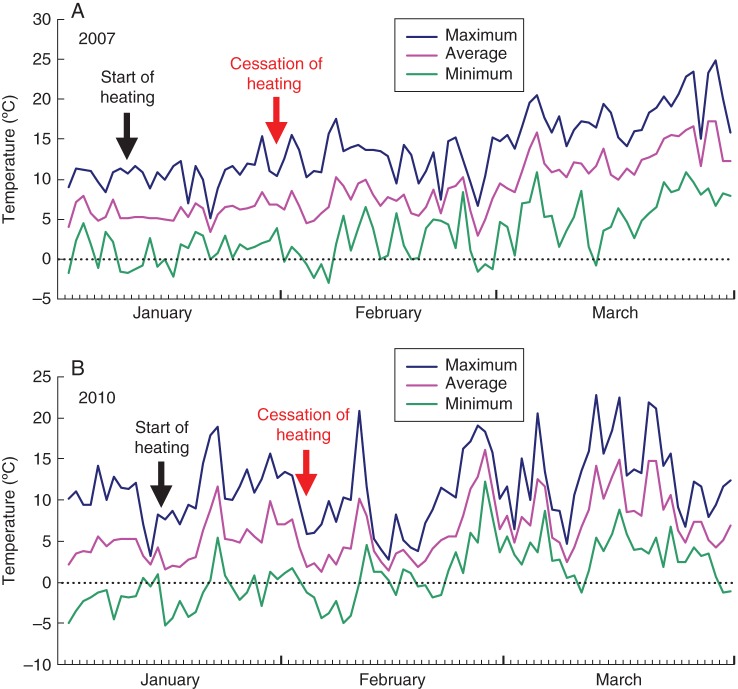
Daily maximum, average and minimum air temperatures during each experimental growth period were obtained from the Japan Meteorological Agency in Fuchu, Tokyo (Fig. 2). After cessation of heating, on 29 January 2007, the minimum temperature was 3·8 °C, the maximum temperature was 10·4 °C and the average temperature was 6·9 °C (Fig. 2A). On 1 February 2010, the minimum temperature was 0·3 °C, the maximum temperature was 9·5 °C and the average temperature was 4·9 °C (Fig. 2B).
Fig. 2.
Air temperatures at the experimental site in Fuchu, Tokyo in (A) 2007 and (B) 2010.
Anatomical measurements of cell morphology
The anatomical parameters of the tracheids, namely the radial diameters of the tracheid and cell wall thickness, were measured and the percentage of cell wall area (per unit area) and percentage of lumen area (per unit area) were calculated using transverse sections and image analysis software (ImageJ; National Institutes of Health, MD, USA) in adult C. japonica and A. firma seedlings. Tracheid characteristics were assessed 3 weeks after cessation of heating from two heated stems by using eight microscopic images per species. In the current year's xylem, from the annual ring boundary towards the cambial zone, 14 and 11 radial layers of differentiating and differentiated tracheids were selected for measurements of anatomical parameters in adult C. japonica and A. firma seedlings, respectively. The cambial cells and differentiating tracheids that were deformed and had tortuous cell walls were discarded from this calculation. In this study, the definition of latewood that was proposed by Mork (1928) was used, according to which tracheids with a common wall that is twice or more as wide as the width of the lumen are regarded as latewood cells (the percentage of cell wall area of tracheids is >50 %).
Statistical analysis
Statistical analysis was performed among tracheids 3 weeks after cessation of heating in C. japonica and A. firma stems. The raw data of radial diameters of tracheids (μm) and cell wall thicknesses of tracheids (μm) were compiled by taking the means of eight microscopic images from two heated stems of C. japonica. In A. firma, the numbers of samples were the same as in C. japonica. The means were subjected to further statistical analysis. Mean, standard deviation and variance were analysed statistically using StatGraphics Plus 5·1 software (Stat Point, Inc., Warrenton, VA, USA). The statistical significance of differences in the tracheid characteristics was tested by Fisher's least significant difference (LSD) test. The LSD test was used to compare mean diameters of tracheids and mean cell wall thicknesses of tracheids in the current year's xylem from the annual ring boundary towards the cambial zone 3 weeks after cessation of heating. Differences were taken as significant when P < 0·05.
RESULTS
Dormant cambium
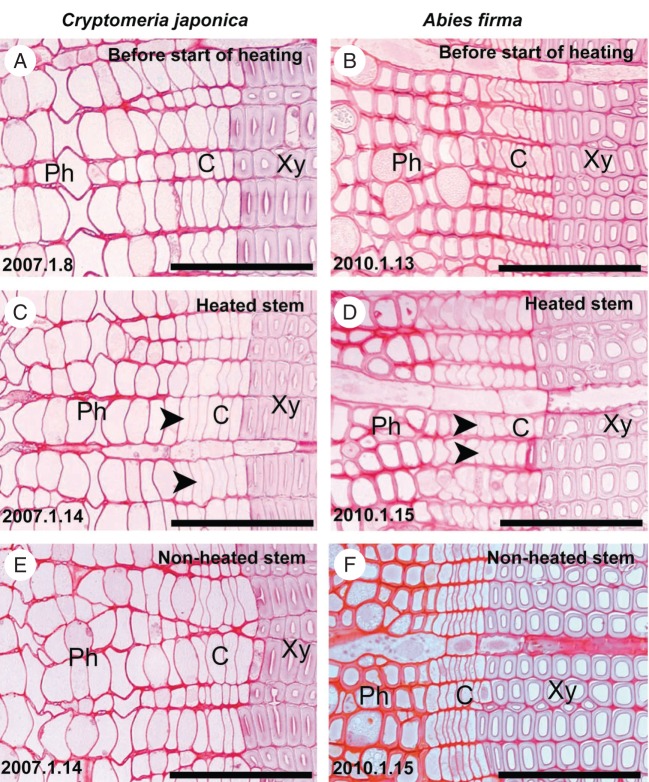
Before the start of heating, no division of fusiform cambial cells and ray cambial cells was detected in the samples of cambium from C. japonica and A. firma that had been collected on 8 January 2007 and 13 January 2010, respectively, confirming that the cambium was dormant (Fig. 3A, B). The cambium was located between the previous year's sieve cells and the narrow-diameter thick-walled latewood tracheids that had been formed during the previous growing season. During dormancy, the cambium consisted of five or six radial layers of radially narrow and compactly arranged cells (Fig. 3A, B).
Fig. 3.
Light micrographs showing transverse views of cambium collected from heated and non-heated stems of Cryptomeria japonica and Abies firma. (A, B) Before the start of heating, cambial cells were arranged very compactly and the cambial zone consisted of four or five layers of fusiform cambial cells in stems of C. japonica (A) and in A. firma (B), respectively. (C, D) New cell plates (arrowheads) were evident in cambial cells on 14 January 2007 (C) and on 15 January 2010 (D) in the respective species. (E, F) On the same dates, the cambium was dormant in non-heated stems of C. japonica (E) and Abies firma (F), respectively. Abbreviations: C, cambium; Ph, phloem; Xy, xylem. Scale bars = 100 µm.
Timing of cambial reactivation and earlywood formation in locally heated stems
The formation of the first new cell plates in the cambium from late winter to early spring was referred to as cambial reactivation. In heated stems of C. japonica and A. firma, cambial reactivation occurred after 6 and 2 d of heating, on 14 January 2007 and 15 January 2010, respectively (Fig. 3C, D). On these days, the cambium was dormant in non-heated stems of C. japonica and A. firma (Fig. 3E, F). The start of radial expansion of cambial cells was referred to as earlywood formation. Earlywood formation started after 14 and 9 d of heating, on 22 January 2007 and 22 January 2010, in C. japonica and A. firma, respectively (Fig. 4A, B). On the same days, 22 January 2007 and 22 January 2010, the cambium was still dormant in non-heated stems of C. japonica and A. firma (Fig. 4C, D). Heating was discontinued on 29 January 2007 and on 1 February 2010 when 11 or 12 and eight or nine radial files of differentiating and differentiated tracheids had been produced in stems of C. japonica and A. firma, respectively (Fig. 4E, F).
Fig. 4.
Light micrographs showing transverse views of cambium collected from heated and non-heated stems of Cryptomeria japonica and Abies firma. (A, B) Earlywood formation started on 22 January 2007 (A) and 22 January 2010 (B) in the heated stems of the respective species. (C, D) On the same dates, the cambium was still dormant in non-heated stems of C. japonica (C) and A. firma (D). (E, F) Heating was discontinued on 29 January 2007 and on 1 February 2010 when 11 or 12 and eight or nine radial files of differentiating and differentiated tracheids were evident in stems of C. japonica (E) and in A. firma (F), respectively. Abbreviations: C, cambium; Ph, phloem; Xy, xylem; Nxy, new xylem. Scale bars = 100 µm.
There were no differences in terms of the timing of cambial reactivation and earlywood formation, respectively, between the two heated stems of C. japonica and the two A. firma saplings sampled on the respective dates.
Effects of a rapid decrease in temperature on differentiating tracheids after cessation of heating
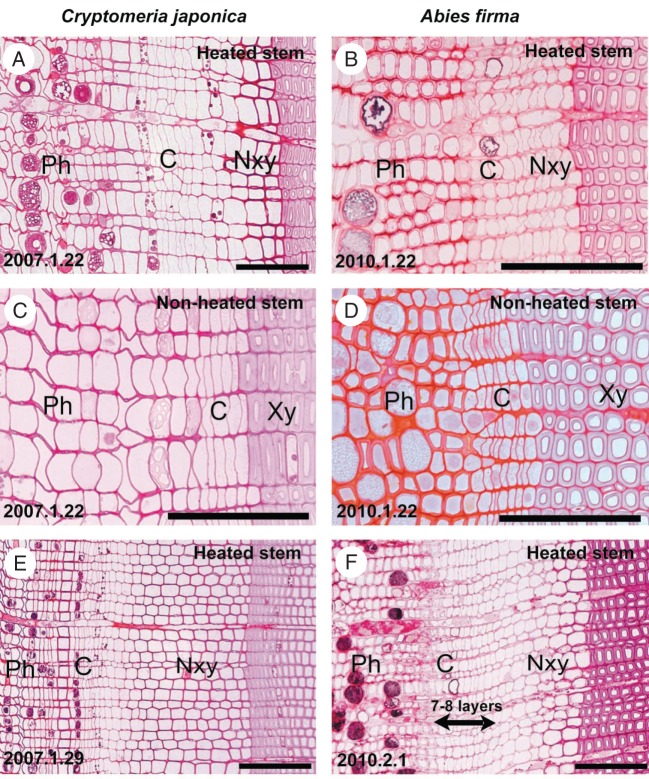
One week after cessation of heating, on 5 February 2007, there were no clear changes in the dimensions of the differentiating tracheids in the samples from adult C. japonica (Fig. 5A). In contrast, in the seedlings of A. firma, 1 week after cessation of heating, on 8 February 2010, tracheids with a smaller diameter were detected (Fig. 5B). After 2 weeks, on 13 February 2007 and 15 February 2010, obvious reductions in tracheid diameter were apparent in the samples from adult C. japonica and from seedlings of A. firma (Fig. 5C, D). After 3 weeks, on 19 February 2007 and 22 February 2010, tracheids with very clearly diminished diameters and thickened cell walls were observed in both C. japonica and A. firma (Fig. 5E, F). In addition, the numbers of radial layers of fusiform cambial cells were decreased from 7–8 to 5–6 in the cambial zone, indicating that cambial activity was lower on 22 February 2010 than on 1 February 2010 in stems of A. firma (Figs 4F and 5F). Scanning electron micrographs of these samples, 3 weeks after cessation of heating, revealed tracheids with diminished diameters in C. japonica and A. firma (Fig. 5G, H). The cells from both species were latewood tracheids (Fig. 5G, H).
Fig. 5.
Light micrographs showing transverse views of cambium and differentiating xylem after heating of Cryptomeria japonica and Abies firma stems was discontinued. (A, B) One week after cessation of heating, there were no obvious changes observed in the dimensions of the differentiating tracheids in C. japonica (A); and reductions in tracheid diameter (arrowheads) were first observed in A. firma (B). (C, D) Two weeks after cessation of heating, obvious reductions in tracheid diameter (arrowheads) were observed in C. japonica (C) and Abies firma (D). (E, F) After 3 weeks, in C. japonica (E) and in A. firma (F), more pronounced reductions in radial diameter and in the thickness of cell walls of tracheids (arrowheads) were observed. (G, H) Scanning electron micrographs indicate that reduced diameters of tracheids (asterisks) were associated with a decrease in temperature in C. japonica (G) and in A. firma (H) 3 weeks after discontinuation of heating. Abbreviations: C, cambium; Ph, phloem; Xy, xylem; Nxy, new xylem. Scale bars in light micrographs = 100 µm; scale bars in scanning electron micrographs = 20 µm.
There were differences in terms of the timing of reductions in the radial diameter of tracheids and increases in thickness of tracheid cell walls between C. japonica trees and A. firma seedlings (Table 1).
Table 1.
Timing of reduction of tracheid diameters and increases in thickness of cell walls of tracheid after cessation of heating in Cryptomeria japonica and Abies firma stems
| Time after cessation of heating | Cryptomeria japonica | Abies firma |
|---|---|---|
| 1 week | No change in tracheid diameters | Start of reduction of tracheid diameters |
| 2 weeks | Start of reduction of tracheid diameters | Obvious reduction of tracheid diameters |
| 3 weeks | Clearly diminished diameters and thickened cell walls of tracheids | Clearly diminished diameters and thickened cell walls of tracheids |
Anatomical measurements of cell morphology
In stems of adult Cryptomeria japonica
There were no differences in terms of reductions in diameter of tracheids after cessation of heating between the two C. japonica trees. Typical images from C. japonica are shown in Fig. 6. Tracheids with larger diameters were located near the annual ring boundary (tracheids 2–6) and tracheids with significantly smaller diameters were located closer to the cambial zone (tracheids 7–14; P < 0·05; Fig. 6A, B). In addition, towards the cambial zone, the cell walls of tracheids (nos 6–12) were significantly thicker than those the near annual ring boundary (nos 1–5; P < 0·05; Fig. 6A, B). Near the annual ring boundary (tracheids 1–5 or 6), the percentage of cell wall area of tracheids was 36·55 % and the percentage of lumen area of tracheids was 63·45 % (Fig. 6C). In contrast, towards the cambial zone (tracheids 5 or 6–14), the percentage of cell wall area of tracheids was 63·50 % and the percentage of lumen area of tracheids was 36·50 % (Fig. 6C). The percentage of cell wall area of these tracheids was >50 % and, therefore, they were identified as latewood tracheids (Fig. 6C).
Fig. 6.
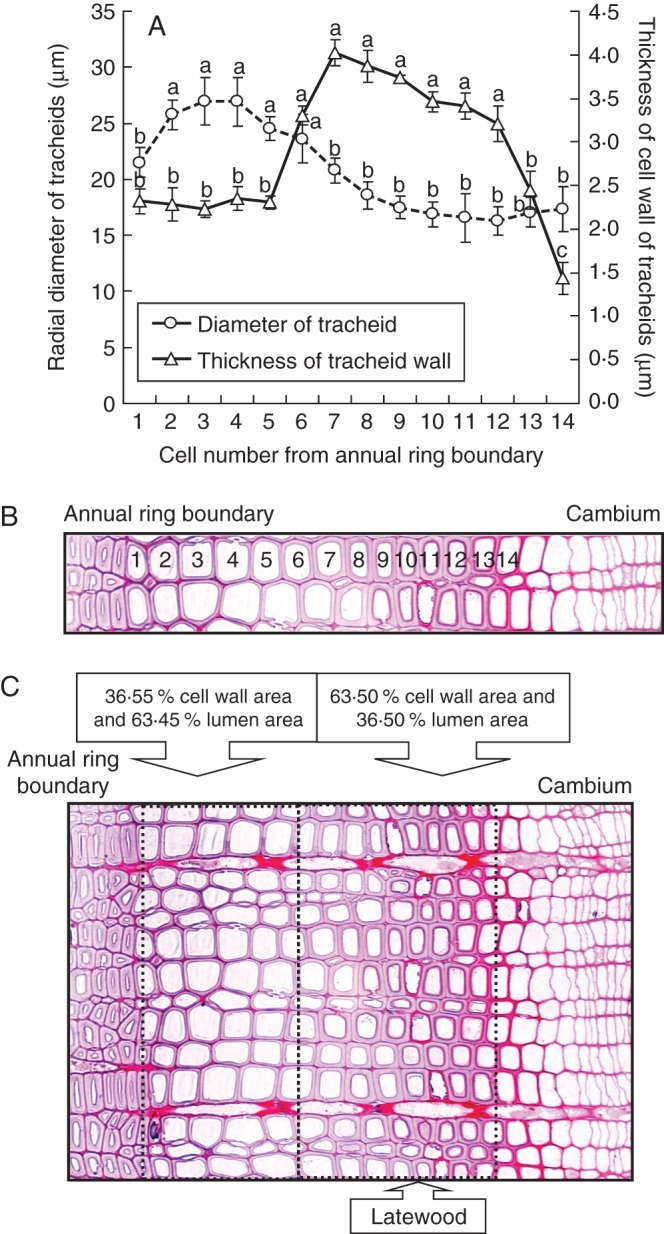
Light micrographs showing transverse views of cambium and xylem and measurements made 3 weeks after cessation of heating, on 19 February 2007, in stems of Cryptomeria japonica. (A) Cell diameters and cell wall thicknesses of differentiating tracheids in stems of adult C. japonica trees from the annual ring boundary towards the cambial zone after cessation of heating (n = 8). Columns and bars show mean values ± s.d. Means with the same letter are not significantly different at P < 0·05 (Fisher's LSD test). (B, C) Light micrographs showing 14 radial files of differentiating tracheids, including cambium (B), and the percentage of areas of the lumen and cell wall for each differentiating tracheid (C), as determined by image analysis of transverse sections. The latewood is indicated.
In stems of Abies firma seedlings
There were no obvious differences in terms of reductions in diameter of tracheids after cessation of heating among pairs of heated seedlings of A. firma. Typical images from A. firma are shown in Fig. 7. Wider tracheids were located near the annual ring boundary (tracheids 2–6) and significantly narrower tracheids were located closer to the cambial zone (tracheids 7–11; P < 0·05; Fig. 7A, B). In addition, towards the cambial zone, cell walls of tracheids were significantly thicker (nos 5–9) than those near the annual ring boundary (nos 1–4; P < 0·05; Fig. 7A, B). Near the annual ring boundary, the percentage of cell wall area of tracheids (nos 1–4 or 5) was 33·65 % and the percentage of lumen area of tracheids was 66·35 % (Fig. 8C). In contrast, towards the cambial zone, the percentage of cell wall area of tracheids (nos 4 or 5–10 or 11) was 66·25 % and the percentage of lumen area of tracheids was 33·75 % (Fig. 7C). Since the percentage of cell wall area of these tracheids was >50 %, they were identified as latewood tracheids (Fig. 7C).
Fig. 7.
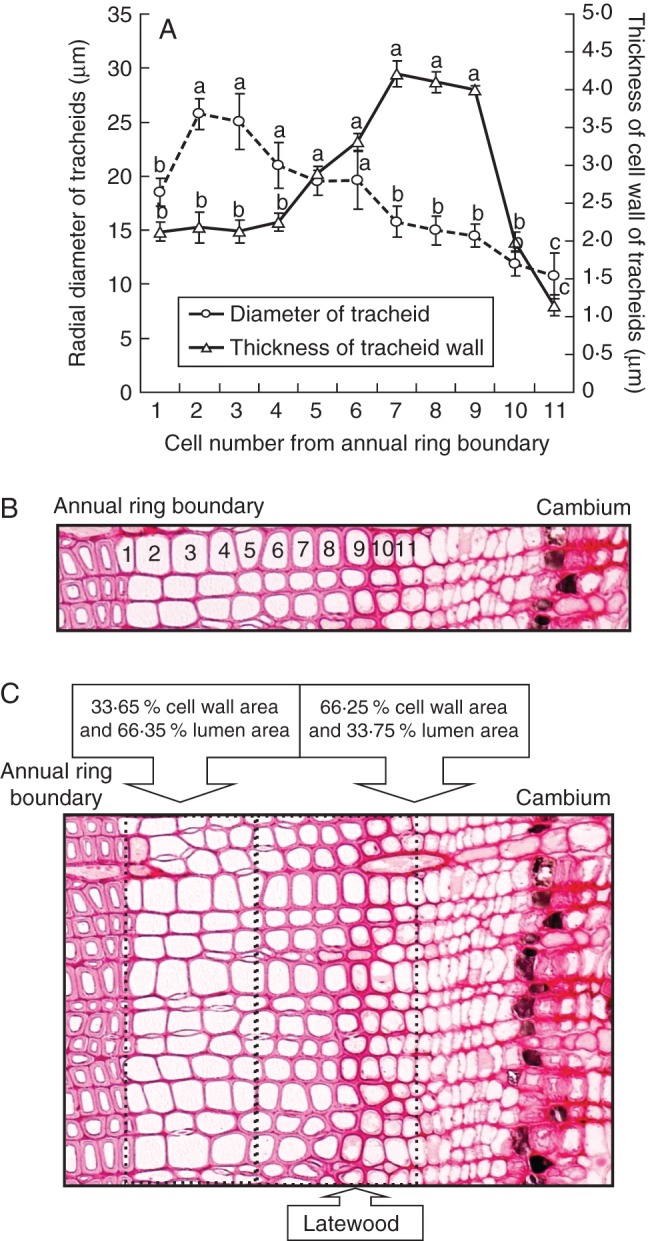
Light micrographs showing transverse views of cambium and xylem and measurements made 3 weeks after cessation of heating, on 22 February 2010, in stems of Abies firma. (A) Cell diameters and cell wall thicknesses of differentiating tracheids in stems of A. firma seedlings from the annual ring boundary towards the cambial zone after cessation of heating (n = 8). Columns and bars show mean values ± s.d. Means with the same letter are not significantly different at P < 0·05 (Fisher's LSD test). (B, C) Light micrographs showing 11 radial files of differentiating tracheids, including cambium (B), and the percentage of areas of the lumen and cell wall for each differentiating tracheid (C), as determined by image analysis of transverse sections. The latewood is indicated.
Temperature after cessation of heating
During localized heating, stems of C. japonica and A. firma were exposed to a constant temperature of 20–22 °C. When heating was discontinued, the temperature fell rapidly in the heated portions of the stems of both species (Fig. 2). Therefore, the cambium of the two species was exposed to a rapid decrease in temperature from approx. 22 °C to between 0·3 and 3·8 °C during the formation of earlywood. The anatomical measurements showed that significantly narrowed tracheids with thickened cell walls were produced after discontinuation of heating when the minimum atmospheric temperature ranged between 0·3 and 3·8 °C (Figs 2, 6 and 7).
DISCUSSION
In the present study, it was observed that a rapid decrease in temperature induced the formation of narrower tracheids with thickened cell walls after cambial cells of conifers had been reactivated by localized heating. Reductions in tracheid diameter were observed first in stems of A. firma seedlings 1 week after heating was discontinued. More pronounced reductions in tracheid diameter and thicker cell walls were observed within 2 or 3 weeks after cessation of heating of A. firma stems. In stems of C. japonica, reductions in tracheid diameter were first detected 2 weeks after heating was stopped and obvious thickening of cell walls was evident after 3 weeks. The present observations suggest that decreases in the radial diameter of tracheids begin before increases in the thickness of cell walls of tracheids upon a rapid decrease in temperature.
Previous researchers proposed that latewood formation in conifers is controlled by both internal and external factors (Larson, 1964; Denne and Dodd, 1981; Timell, 1986; Heide and Prestrud, 2005). Certain environmental conditions, such as severe drought conditions, can induce the formation of latewood in conifers (Shepherd, 1964; Zahner et al., 1964; Larson, 1969b). In addition, Jenkins and Shepherd (1972) suggested that the formation of latewood in Pinus radiata in late summer might be a result of elevated summer temperatures. In contrast, in Picea abies, latewood formation occurred under low-temperature conditions in late summer (Gindl and Grabner, 2000). In the present study, it was found that, upon cessation of localized heating of stems of C. japonica and A. firma, the rapid decrease in temperature from approx. 22 °C to between 0 and 4 °C induced changes in the morphology of tracheids that had been reactivated by the localized heating. The present results with these two model systems suggest that a rapid decrease in the temperature of the stem might be critical for latewood formation in conifers. Thus, rapid changes in natural environmental conditions might induce changes in morphology of tracheids.
Environmental factors such as temperature are known to have a profound effect on the dimensions of tracheids, and variations in these dimensions are evident across individual tree rings (Richardson and Dinwoodie, 1960; Denne and Dodd, 1981). A long photoperiod was associated with larger diameter tracheids in Picea sitchensis (Larson, 1964, 1969a), while transfer of Pinus radiata seedlings to a lower temperature resulted in decreases in tracheid diameter (Jenkins, 1975). Elevation by approx. 10 °C of the temperature of stems of P. sitchensis seedlings, by use of a heating coil, promoted the radial expansion of tracheids (Richardson, 1966), and tracheid diameters were found to increase in P. sitchensis and Abies balsamea with higher ambient temperatures (Krause et al., 2010), with night-time temperatures having a greater effect on tracheid expansion than daytime temperatures in Pseudotsuga menziesii, Sequoia sempervirens, Larix sibirica and Podocarpus latifolius (Richardson and Dinwoodie, 1960; Richardson, 1964; Antonova and Stasova, 1997; Dünisch, 2010). Moreover, low night-time temperatures were associated with reduced expansion of tracheids in Podocarpus latifolius seedlings (Dünisch, 2010), indicating that low temperatures might have a major effect on xylem differentiation. The present results demonstrate clearly that the radial diameter of tracheids, produced after localized heating, was large and that a rapid decrease in temperature, due to cessation of heating, induced a conspicuous decrease in tracheid diameter. Therefore, the results support the hypothesis that reductions in tracheid diameter might be related to rapid decreases in temperature.
Conifer seedlings grown at lower temperatures produce tracheids with thicker cell walls than those grown at higher temperatures (Richardson, 1964; Larson, 1967; Denne, 1971). In Picea glehnii, the thickness of cell walls of the final three arrays of tracheids was positively correlated with summer temperatures (Yasue et al., 1997, 2000). Under natural conditions, increased cell wall thickness of tracheids occurred due to greater availability of photosynthate in the cambial region (Larson, 1964). In the present study, the production of earlywood tracheids was observed during continuous heating. When heating was discontinued, the cambium was exposed to a rapid decrease in temperature, which induced the formation of reduced diameters of tracheids, but cell wall thickening of tracheids was still continued. Therefore, it can be hypothesized that, after receiving a lower temperature, reactivated cambium might be responsible for terminating the differentiation of tracheids that needs a considerable amount of photosynthate, which can be obtained from stored carbohydrate around the cambium. However, we cannot exclude the possibility that interruption to the seasonal cycle of the cambium might have some effects on the formation of cambial derivatives.
It has already been confirmed that temperature is a limiting factor in the onset of cambial reactivation and xylem differentiation in quiescent dormant conifers (Savidge and Wareing, 1981; Barnett and Miller, 1994; Oribe and Kubo, 1997; Oribe et al., 2001, 2003; Gričar et al., 2006; Begum et al., 2010a, b) and in a hardwood (Begum et al., 2007, 2008). Increases in temperature or higher than normal late winter to early spring temperatures induce earlier cambial reactivation and xylem differentiation in trees (Oribe and Kubo, 1997; Oribe et al., 2001, 2003; Gričar et al., 2006; Begum et al., 2007, 2008, 2010a, b), and continuous heating induces continuous earlywood formation (Begum et al., 2010a, b). Similarly, in the present study, earlier cambial reactivation and earlywood formation were induced by localized heating during winter dormancy in February. In addition, the accumulation of temperatures above a threshold temperature of 5 °C, expressed in terms of degree-days, could be used to predict the effect of temperature on the date of onset of cambial activity in Pinus sylvestris (Seo et al., 2008; Swidrak et al., 2011). In a previous study, it was observed that cambial reactivation and earlywood formation occurred above a certain threshold temperature, namely 10 or 11 °C, in C. japonica (Begum et al., 2010a). It was also reported that continuation of both cambial activity and earlywood formation requires a constant elevated threshold temperature (Begum et al., 2010a).
It has been reported that bud burst and the development of new leaves are related to cambial activity and xylem differentiation (Aloni, 1991; Suzuki et al., 1996). Larson (1964) found that, in Pinus resinosa, the transition from earlywood to latewood was associated with cessation of needle growth. Larson (1969b) also found that when needle growth is suppressed by drought, latewood tracheids are formed. Therefore, needle elongation appears to be correlated with latewood formation. However, in the present study, the localized rapid decrease in temperature during earlywood formation induced changes in the diameter and thickness of tracheids, indicating that cambium and its derivatives in stems of conifers can respond to changes in environmental conditions independently of any physiological changes in leaves or needles. Under natural conditions, latewood formation is associated with a decrease in the total amount of endogenous auxin in cambial regions in Abies balsamea and Pinus densiflora (Sundberg et al., 1987; Funada et al., 2001). No obvious changes in levels of auxin in cambial regions were detected during winter dormancy in a previous study of P. densiflora and Larix kaempferi (Funada et al., 2001, 2002), but we cannot exclude the possibility that rapid changes in the temperature of stems might induce changes in endogenous levels of auxin and/or the transport of auxin in cambial regions.
It can be concluded that a rapid decrease in temperature might be expected to have a direct effect on expansion and wall thickening of tracheids of C. japonica and A. firma stems. After cessation of heating, cambium and differentiating tracheids exposed to a rapid decrease in temperature from approx. 22 °C to between 0 and 4 °C that induced reductions of the radial diameter and increases in the thickness of cell walls in differentiating tracheids. The results indicate that cambium and its derivatives can respond directly to changes in temperature and that a rapid decrease in temperature in locally heated stems might be critical for the induction of latewood formation in conifers. Moreover, rapid changes in environmental conditions might have some possibility to change endogenous balances that control formation of latewood in conifers.
ACKNOWLEDGEMENTS
This work was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (nos. 20120009, 20·5659, 21380107, 22·00104, 23380105 and 24380090).
LITERATURE CITED
- Aloni R. Wood formation in deciduous hardwood trees. In: Raghavendra AS, editor. Physiology of trees. New York: Wiley and Sons; 1991. pp. 175–197. [Google Scholar]
- Antonova GF, Stasova VV. Effects of environmental factors on wood formation in larch (Larix sibirica Ldb.) stems. Trees. 1997;11:462–468. [Google Scholar]
- Barnett JR, Miller H. The effect of applied heat on graft union formation in dormant Picea sitchensis (Bong.) Carr. Journal of Experimental Botany. 1994;45:135–143. [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata) Annals of Botany. 2007;100:439–447. doi: 10.1093/aob/mcm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R. Responses to ambient temperature of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiology. 2008;28:1813–1819. doi: 10.1093/treephys/28.12.1813. [DOI] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Cambial sensitivity to rising temperatures from late winter to early spring in the evergreen conifer. Cryptomeria japonica. Trees. 2010a;24:43–52. [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Annals of Botany. 2010b;106:885–895. doi: 10.1093/aob/mcq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denne MP. Temperature and tracheid development in Pinus sylvestris seedlings. Journal of Experimental Botany. 1971;22:362–370. [Google Scholar]
- Denne MP, Dodd RS. The environmental control of xylem differentiation. In: Barnett JR, editor. Xylem cell development. Tunbridge Wells, UK: Castle House; 1981. pp. 236–255. [Google Scholar]
- Dünisch O. Low night temperatures cause reduced tracheid expansion in Podocarpus latifolius. IAWA Journal. 2010;31:245–255. [Google Scholar]
- Funada R, Kubo T, Fushitani M. Relationship between patterns of latewood formation and levels of endogenous auxin in the trunk of sugi (Cryptomeria japonica) Mokuzai Gakkaishi. 1987;33:253–260. [Google Scholar]
- Funada R, Kubo T, Fushitani M. Early and latewood formation in Pinus densiflora trees with different amounts of crown. IAWA Bulletin, New Series. 1990;11:281–288. [Google Scholar]
- Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M. Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora stems in relation to earlywood–latewood transition and cessation of tracheid production. Holzforschung. 2001;55:128–134. [Google Scholar]
- Funada R, Kubo T, Sugiyama T, Fushitani M. Changes in levels of endogenous plant hormones in cambial regions of stems of Larix kaempferi at the onset of cambial activity in springtime. Journal of Wood Science. 2002;48:75–80. [Google Scholar]
- Gindl W, Grabner M. Characteristics of spruce (Picea abies L. Karst) latewood formed under abnormal low temperatures. Holzforschung. 2000;54:9–11. [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Koch G, Schmitt U, Oven P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies) Annals of Botany. 2006;97:943–951. doi: 10.1093/aob/mcl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide OM, Prestrud AK. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiology. 2005;25:109–114. doi: 10.1093/treephys/25.1.109. [DOI] [PubMed] [Google Scholar]
- Jenkins PA. Influence of temperature change on wood formation in Pinus radiata grown in controlled environments. New Zealand Journal of Botany. 1975;13:579–592. [Google Scholar]
- Jenkins PA, Shepherd KR. Influence of temperature on cambial activity and cell diameter in Pinus radiata D. Don. Journal of the Institute of Wood Science. 1972;6:36–39. [Google Scholar]
- Krause C, Rossi S, Thibeault-Martel M, Plourde PY. Relationship of climates and cell features in stems and roots of black spruce and balsam fir. Annals of Forrest Science. 2010;67:402–408. [Google Scholar]
- Larson PR. Some indirect effects of environment on wood formation. In: Zimmermann MH, editor. The formation of wood in forest tress. New York: Academic Press; 1964. pp. 345–365. [Google Scholar]
- Larson PR. Effects of temperature on the growth and wood formation of ten Pinus resinosa sources. Silvae Genetica. 1967;16:58–65. [Google Scholar]
- Larson PR. Incorporation of 14C into the developing walls of Pinus resinosa tracheids (earlywood and latewood) Holzforschung. 1969a;23:17–26. [Google Scholar]
- Larson PR. Wood formation and the concept of wood quality. Yale University: School of Forestry Bulletin. 1969b;74:24–53. [Google Scholar]
- Mork E. Die qualität des fichtenholzes unter besonderer rücksichtnahme auf schleifund papierholz. Der Papier Fabrikant. 1928;26:741–747. [Google Scholar]
- Murakami Y, Funada R, Sano Y, Ohtani J. The differentiation of contact cells and isolation cells in the xylem ray parenchyma of Populus maximowiczii. Annals of Botany. 1999;84:429–435. [Google Scholar]
- Nakaba S, Sano Y, Kubo T, Funada R. The positional distribution of cell death of ray parenchyma in a conifer. Abies sachalinensis. Plant Cell Reports. 2006;25:1143–1148. doi: 10.1007/s00299-006-0194-6. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Kubo T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiology. 1997;17:81–87. doi: 10.1093/treephys/17.2.81. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees. 2003;17:185–192. [Google Scholar]
- Richardson SD. The external environment and tracheid size in conifers. In: Zimmermann MH, editor. The formation of wood in forests trees. New York: Academic Press; 1964. pp. 367–388. [Google Scholar]
- Richardson SD. Studies on the physiology of xylem development. III. Effects of temperature, defoliation and stem girdling on tracheid size in conifer seedlings. Journal of the Institute of Wood Science. 1966;12:3–12. [Google Scholar]
- Richardson SD, Dinwoodie JM. Studies on the physiology of xylem development. I. The effect of night temperature on tracheid size and wood density in conifers. Journal of the Institute of Wood Science. 1960;6:3–13. [Google Scholar]
- Sano Y, Kawakami Y, Ohtani J. Variation in the structure of inter-tracheary pit membranes in Abies sachalinensis, as observed by field-emission scanning electron microscopy. IAWA Journal. 1999;20:375–388. [Google Scholar]
- Savidge RA, Wareing PF. Plant growth regulators and the differentiation of vascular elements. In: Barnett JR, editor. Xylem cell development. Tunbridge Wells, UK: Castle House; 1981. pp. 192–235. [Google Scholar]
- Schweingruber FH. Tree rings: basics and applications of dendrochronology. Dordrecht, The Netherlands: D. Reidel Publishing Company. 1988. pp. 95–141. [Google Scholar]
- Shepherd KR. Some observations on the effect of drought on the growth of Pinus radiata D. Don. Australian Forestry Journal. 1964;28:7–22. [Google Scholar]
- Seo JW, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. Estimating the onset of cambial activity in scots pine in northern Finland by means of the heat-sum approach. Tree Physiology. 2008;28:105–112. doi: 10.1093/treephys/28.1.105. [DOI] [PubMed] [Google Scholar]
- Sundberg B, Little CHA, Riding RT, Sandberg G. Levels of endogenous indole-3-acetic acid in the vascular cambium region of Abies balsamea trees during the activity–rest–quiescence transition. Physiologia Plantarum. 1987;71:163–170. [Google Scholar]
- Suzuki M, Yoda K, Suzuki H. Phenological comparison of the onset of vessel formation between ring-porous and diffuse-porous deciduous trees in a Japanese temperate forest. IAWA Journal. 1996;17:431–444. [Google Scholar]
- Swidrak I, Gruber A, Kofler W, Oberhuber Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiology. 2011;31:483–493. doi: 10.1093/treephys/tpr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timell TE. Compression wood in gymnosperms 2. Berlin: Springer-Verlag; 1986. Physiology of compression wood formation; pp. 1207–1218. [Google Scholar]
- Uggla C, Magel E, Moritz T, Sundberg B. Functions and dynamics of auxin and carbohydrates during earlywood/latewood transition in scots pine. Plant Physiology. 2001;125:2029–2039. doi: 10.1104/pp.125.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzicki TJ. Mechanisms of xylem differentiation in Pinus sylvestris. L. Journal of Experimental Botany. 1971;22:670–687. [Google Scholar]
- Yasue K, Funada R, Fukazawa K, Ohtani J. Tree-ring width and maximum density of Picea glehnii as indicators of climatic changes in northern Hokkaido, Japan. Canadian Journal of Forest Research. 1997;27:1962–1970. [Google Scholar]
- Yasue K, Funada R, Kobayashi O, Ohtani J. The effects of tracheid dimensions on variations in maximum density of Picea glehnii and relationships to climatic factors. Trees. 2000;14:223–229. [Google Scholar]
- Zahner R, Lotan JE, Baughman WD. Earlywood–latewood features of red pine grown under simulated drought and irrigation. Forest Science. 1964;10:361–370. [Google Scholar]