Abstract
Background and Aims
There is still debate regarding the direction and strength of plant interactions under intermediate to high levels of stress. Furthermore, little is known on how disturbance may interact with physical stress in unproductive environments, although recent theory and models have shown that this interplay may induce a collapse of plant interactions and diversity. The few studies assessing such questions have considered the intensity of biotic interactions but not their importance, although this latter concept has been shown to be very useful for understanding the role of interactions in plant communities. The objective of this study was to assess the interplay between stress and disturbance for plant interactions in dry calcareous grasslands.
Methods
A field experiment was set up in the Dordogne, southern France, where the importance and intensity of biotic interactions undergone by four species were measured along a water stress gradient, and with and without mowing disturbance.
Key Results
The importance and intensity of interactions varied in a very similar way along treatments. Under undisturbed conditions, plant interactions switched from competition to neutral with increasing water stress for three of the four species, whereas the fourth species was not subject to any significant biotic interaction along the gradient. Responses to disturbance were more species-specific; for two species, competition disappeared with mowing in the wettest conditions, whereas for the two other species, competition switched to facilitation with mowing. Finally, there were no significant interactions for any species in the disturbed and driest conditions.
Conclusions
At very high levels of stress, plant performances become too weak to allow either competition or facilitation and disturbance may accelerate the collapse of interactions in dry conditions. The results suggest that the importance and direction of interactions are more likely to be positively related in stressful environments.
Keywords: Competition, facilitation, stress, disturbance, biotic interactions, unproductive environments, Teucrium chamaedrys, Koeleria vallesiana, Thymus serpyllum, Seseli montanum
INTRODUCTION
Plant interactions – either negative (Grime, 1973; Tilman, 1982) or positive (Hacker and Gaines, 1997; Bruno et al., 2003) – are known to be key factors structuring plant communities (for a review see Brooker et al., 2008). Although it is now widely recognized that competition dominates in benign environments (Grime, 1973; Huston, 1979), there is still debate regarding the role and direction of plant interactions in stressful conditions (Brooker et al., 2008). Grime (1973) stated that competition for light disappears under those conditions, whereas the ‘Stress Gradient Hypothesis’ (SGH) proposed that competition should switch to facilitation with increasing stress or disturbance (Bertness and Callaway, 1994; Brooker and Callaghan, 1998). Several empirical studies supported the SGH in terrestrial (e.g. Callaway et al., 2002; Liancourt et al., 2005), marine (Bulleri et al., 2011) and freshwater systems (Le Bagousse-Pinguet et al., 2012). However, others found an increase in competition in water-stressed environments (Tielbörger and Kadmon, 2000; Maestre and Cortina, 2004). Such inconsistencies may notably be due to the idiosyncrasy of plant interactions undergone by a species of a particular strategy, within a particular community limited by a particular resource (Liancourt et al., 2005; Michalet, 2007; Maestre et al., 2009). Additionally, facilitation has been proposed to wane at the extreme end of severity gradients (Michalet et al., 2006). This theoretical prediction has been supported by mathematical simulations (Travis et al., 2006; Xiao et al., 2009) and several experiments conducted along biotic disturbance gradients (Smit et al., 2007), and stress or physical disturbance gradients (Kitzberger et al., 2000; Veblen, 2008; Forey et al., 2010; de Bello et al., 2011). Michalet et al. (2006) argued that the collapse of facilitation in extremely severe environments may be due to the strong reduction in size and effects of the nurses impeding any environmental mitigation for other species. More recently, Malkinson and Tielbörger (2010) proposed that a collapse may also occur for competitive interactions when these appear in particular stressful conditions, such as arid ecosystems (e.g. Tielbörger and Kadmon, 2000). Data are still needed to assess the variation in biotic interactions from intermediate to high conditions of stress.
Most of these studies have focused on variation in either stress or disturbance along complex environmental gradients without controlling for variation in the other constraint. Few studies have so far disentangled the combinatory effects of these different direct gradients on biotic interactions, despite their frequent co-occurrence in natural systems (Graff et al., 2007; Kawai and Tokeshi, 2007; le Roux and McGeoch, 2010; Bulleri et al., 2011). Co-occurring environmental direct factors may have multiplicative rather than additive effects (Brooker and Callaghan, 1998; Freckleton et al., 2009; Malkinson and Tielbörger, 2010). Coupling high levels of stress and disturbance may accelerate the collapse of facilitation leading to rapid drops of diversity (Michalet et al., 2006; Forey et al., 2010) and extinctions of communities (Kefi et al., 2007). Recent studies showed in particular that biotic interactions depend strongly on the interaction between abiotic stress and consumer pressure in contrasted ecosystems and vary according to target species and season (Crain, 2008; Veblen, 2008; Smit et al., 2009; Brewer, 2011; Bulleri et al., 2011; Soliveres et al., 2011), although no common clear trend has so far been found. This might be linked to the difficulty of predicting the outcome of biotic interactions when considering selective disturbance processes (e.g. consumer pressure), which may induce complex indirect facilitation processes (e.g. associational defences against herbivores) varying along the abiotic stress gradient. We expect that non-selective disturbance processes (e.g. mowing) would theoretically lead to more convergent results (i.e. disturbance would affect the whole community – competitors, benefactors and beneficiaries – and potential biotic interactions would be direct across the whole stress gradient).
Additionally, several authors have stressed the utility of considering the importance, rather than the intensity, of either competition or facilitation when assessing how species interactions may contribute to drive community composition and structure along environmental gradients (Brooker et al., 2005; Freckleton et al., 2009; Kikvidze and Brooker, 2010; Kikvidze et al., 2011; Rees et al., 2012). The importance of interactions has been defined as the change in performance of a target species due to neighbours as compared with the effect of other community drivers such as stress and disturbance (Brooker et al., 2005). Importance highlights better the role of biotic interactions compared with the role of other abiotic factors in community assembly and has been proposed to illustrate Grime's (1973) theories on competition (Brooker et al., 2005; Freckleton et al., 2009; Kikvidze and Brooker, 2010; Kikvidze et al., 2011; Rees et al., 2012). However, most empirical studies assessing variation in biotic interactions along severity gradients were based on measurements of the intensity of interactions; fewer were based on the importance (Ariza and Tielbörger, 2011; Kunstler et al., 2011) or on a comparison between the intensity and the importance of interactions (Sammul et al., 2000; Gaucherand et al., 2006; Zhang et al., 2008; le Roux and McGeoch, 2010). To our knowledge, only le Roux and McGeoch (2010) have analysed changes in the importance of interactions along two complex environmental gradients (altitude and wind exposure) and no study has assessed the crossed effects of direct stress and disturbance factors on the importance of interactions.
Our goal here is to assess variation in both the intensity and the importance of interactions along a water stress gradient, with and without mowing disturbance in dry calcareous grasslands. Calcareous grasslands are characterized by important water and nutrient stresses (Grime and Curtis, 1976) and a high species richness with considerable conservation value. Stress conditions in calcareous grasslands vary naturally, notably according to topography (e.g. exposure and steepness and shape of the slope), thus constituting a good system to measure the effects of a stress gradient on biotic interactions. Calcareous grasslands are currently subject to disturbance-based management strategies such as grazing or mowing (Kahmen et al., 2002), which may interact with the stress gradient and thus shape the community. We focused on variation in interactions with and without mowing disturbance in intermediately dry to extremely dry conditions, i.e. a position along the water stress gradient which has fuelled considerable debate in the literature. We used a combination of a natural water stress, choosing a range of communities from mesic to xeric soil and exposure conditions, and of an experimentally induced water stress, using a rainfall interception procedure, making use of the potential of studying an expanded gradient (Kawai and Tokeshi, 2007; Ariza and Tielbörger, 2011). Based on the most recent predictions (Michalet et al., 2006; Malkinson and Tielbörger, 2010), we expected that: (1) biotic interactions (positive or negative, intensity and importance) should collapse at the extreme end of the water stress gradient and (2) this collapse should occur earlier along the gradient if the system is subjected to disturbance (mowing).
MATERIALS AND METHODS
Study site
Sites were located close to Saint Sulpice de Mareuil (45°28′50″N, 0°30′22″E, 140 m a.s.l.) and La Rochebeaucourt (45°27′53″N, 0°23′33″E, 140 m a.s.l.) in the Périgord-Limousin Natural Park, south-western France. Mean winter temperature is 6·2 °C and mean summer temperature is 19·5 °C. Mean annual precipitation is 800 mm with the highest rainfall amounts in winter. The experiment was established during two average years (873 mm in 2009 and 773 mm in 2010, PreviMeteo SARL). The site is dominated by cretaceous limestones. In calcareous grasslands, vegetation is overall mainly limited by water and nutrient stresses (Grime and Curtis, 1976; Liancourt et al., 2005). However, calcareous grassland communities vary in cover, productivity and species composition, depending on soil depth, slope, exposure and management (Boullet, 1986; Royer, 1987). Like most calcareous grasslands across Europe, the selected sites were semi-natural. However, they were not subjected to any land use within the 30 years preceding the experiment, and thus human and domestic animal disturbances did not vary in our plots. However, because dynamics is so slow in these stressful environmental conditions, time since abandonment was not long enough to let our sites overtake the ‘grassland’ stage of secondary succession. We used a natural water stress gradient including eight semi-natural calcareous grassland sites ranging from Mesobromion (moist) to Xerobromion (dry) communities: soil moisture significantly decreased from the wettest Mesobromion (14·8 % of total soil weight in spring 2010) to the driest Xerobromion (2·2 % of total soil weight in spring 2010) sites. Above-ground vascular plant biomass significantly decreases along this gradient (maximum = 678 g m−2, minimum = 232 g m−2; F1,6 = 56·38, n = 8, r2 = 0·90, P < 0·001). Vegetation cover follows the same trend (maximum = 99 %, minimum = 50 %; F1,6 = 3·25, n = 8, r2 = 0·63, P < 0·05). Species nomenclature follows Tutin et al. (1964–1980). Species found are mostly slow-growing and stress-tolerant (sensu Grime 1974), in particular the dominant grass species Festuca ovina. Both the cover of this species and its vegetative height significantly decrease when passing from the wettest Mesobromion to the driest Xerobromion site (maximum cover = 52 %, minimum cover = 2 %; F1,6 = 7·67, n = 8, r2 = 0·56, P < 0·05; maximum height = 13·3 cm, minimum height = 5·5 cm; F1,6 = 11·45, n = 8, r2 = 0·66, P < 0·05). Other species are Anthyllis vulneraria, Coronilla minima, Fumana procumbens, Helichrysum stoechas, Potentilla verna and Carex humilis.
We selected the four species with the highest abundances among the pool of species present in all of the 32 study plots, Koeleria vallesiana, Seseli montanum, Teucrium chamaedrys and Thymus serpyllum, after removing from the pool the potential nurse species F. ovina. K. vallesiana is a short hexaploid bunch grass with thick leaves. S. montanum is an Apiaceae with a thick tap-root giving birth to subordinate stems. Te. chamaedrys and Th. serpyllum are two Lamiaceae with very high capacities of stoloniferous vegetative spreading. Th. serpyllum has woody stems but Te. Chamaedrys does not. The four species are common species of calcareous grasslands and either restricted to southern Europe (K. vallesiana and S. montanum) or found across the continent (Th. serpyllum and Te. chamaedrys, Royer 1987).
Experimental design
The experiment lasted from December 2008 to June 2010. We experimentally increased the length and the resolution of our natural water stress gradient by splitting each of the eight sites into two blocks: one was kept intact and the other was experimentally dried. We framed two plots (3·5 × 3·5 m each) within each block in order to apply a two-level mowing factor (control and mown). The design included a total of 32 plots (8 sites × 2 blocks × 2 levels of mowing).
The experimental drought was simulated through rainfall interception during two successive springs (from 10 April to 24 June 2009: 76 days and 270 intercepted millimetres, and from 19 April to 28 June 2010: 70 days and 119 intercepted millimetres). We chose spring because most of the growing season occurs in this period. We used roofs of transparent plastic allowing 90 % penetration of photosynthetically active radiation (CelloFlex 4TT, Prosyn Polyane, ETS Girard, Bruges, France). To avoid a temperature increase, roofs were tunnel-shaped. This allowed air circulation through 2-m-high gaps at both ends (mean air temperature from April to June 2010 in dried plots = 14·8 °C, s.d. = 0·25; in control plots = 14·6 °C, s.d. = 0·29). This kind of shelter has already been used in several studies investigating the effect of experimental droughts on grassland ecosystems (e.g. Kahmen et al., 2005). We used soil moisture measurements in control (unmown) plots to establish the stress gradient covariate. Eight measurements (in % of soil weight) per plot were made using a Theta probe device (ML2X, Delta-T Devices, Cambridge, UK) in spots where biomass had been removed. This process was repeated on three different dates during the 2010 growing season (28 April, 13 May and 27 May). We then averaged all measurements per plot and attributed a single value of soil moisture to each of the 16 blocks.
Induced mowing
We applied the mowing treatment by clipping 3 cm above ground and exporting mown biomass outside the plot three times during the experiment (November 2008, June 2009 and March 2010), thus simulating a more intensive management than usual in calcareous grasslands, to ensure significant changes in a short-term experiment.
We assessed the responses of the four selected target species to neighbours (biotic interactions) in all treatments using a neighbour removal procedure. We selected eight short and young individuals of each species (i.e. juveniles in the case of K. vallesiana and S. montanum and young ramets in the case of Te. chamaedrys and Th. serpyllum) within each plot in April 2009. Plant individuals are more likely to be facilitated when they are juveniles or suffer from competition for light when they are short. We regularly hand-weeded (completely removed) the above-ground biomass of neighbouring plants in a 10-cm-diameter circle around half of the targets and severed roots of neighbouring plants around each removal area. Thus, in each plot, we tagged individuals with and without neighbouring plants. There were a total of 1024 targets [8 communities × 2 blocks × 2 levels of mowing × 2 neighbouring levels × 4 species × 4 replicates].
Data collection
We used both survival and plant growth (represented by the standardized estimation of target net biomass differences between the end and the beginning of spring 2010) to evaluate target performance according to treatments. To estimate target initial biomass (27 April 2010), we measured non-destructive parameters [leaf number (L) and stretched plant vegetative height (H)] and transformed them in biomass values using allometric regressions. We established these regressions as follows: for each species, we measured L, H and dried biomass (W, 72 h, 60 °C) on 30 individuals growing outside the experimental plots and covering a wide range of sizes. We used these data to select the best allometric equations using a stepwise backward Akaike Information Criterion procedure. We saved the following equations: K. vallesiana: W = 0·0047 × H + 0·0036 × L + 0·00066 × H × L (r2 = 0·86); S. montanum: W = 0·043 × L (r2 = 0·77); Te. chamaedrys: W = 0·0022 × L (r2 = 0·87); Th. serpyllum: W = 0·0065 × L (r2 = 0·93). Surviving targets were sampled on 6 July 2010, dried (72 h, 60 °C) and weighed. We computed plant growth as follows: G = (Bf – Bi)/Bi, where Bi is the estimated initial biomass and Bf the weighed final biomass. Inside each plot, we averaged plant performance (survival or growth) per species in the presence (P+N) or in the absence (P−N) of neighbours before computing the indices described below.
We calculated the importance of interactions using Seifan et al.'s (2010) index ‘Iimp’: Iimp = Nimp/(|Nimp| + |Eimp|), where Nimp and Eimp are the neighbour and environmental contributions to plant performance, respectively: Nimp = P+N – P−N and Eimp = P−N – Pmax±N, where Pmax±N is the maximum value of species performance in all of the 32 plots.
We calculated the intensity of interactions using the Relative Interaction Index (‘RII’, Armas et al., 2004): RII = (P+N – P−N)/(P+N + P−N).
Iimp and RII are limited in their range [–1,1] and are symmetrical around 0. Negative values for Iimp and RII indicate competition whereas positive values indicate facilitation.
Statistical analyses
We tested the interactive effects of the stress gradient (covariate), the mowing treatment (two-level factor) and species identity (four-level factor) on the Iimp and on the RII indices computed for both plant survival and growth using mixed analysis of covariance (ANCOVA) models. Blocks were included as a random variable in the models. The mowing treatment was nested within blocks. We used restricted maximum likelihood (REML) to estimate model parameters because this procedure allows us to deal with unbalanced data (Corbeil and Searle, 1976), which was the case of our growth data as there were a few subclasses (species × mowing) with no survival. We tested intercept significance at both ends of the gradient for each factor combination (species × mowing). We log-transformed soil moisture data to homogenize the gradient (i.e. to avoid aggregated point patterns) and to meet parametric model assumptions. Data analyses were performed using the R software for statistical computing (2·10·1).
RESULTS
Survival was high for all species (K. vallesiana: 0·92, s.d. = 0·15; S. montanum: 0·87, s.d. = 0·20; Te. chamaedrys: 0·71, s.d. = 0·33; Th. serpyllum: 0·67, s.d. = 0·33) and there were no significant effects of our treatments on the indices of both intensity (RII) and importance (Iimp) for survival. The only significant result for survival was the occurrence of globally positive RII for Te. chamaedrys (F3,42 = 2·95, P < 0·05), but with no significant changes in both indices (RII and Iimp) along treatments (data not shown). Below, ‘RII’ and ‘Iimp’ always refer to indices measured on growth.
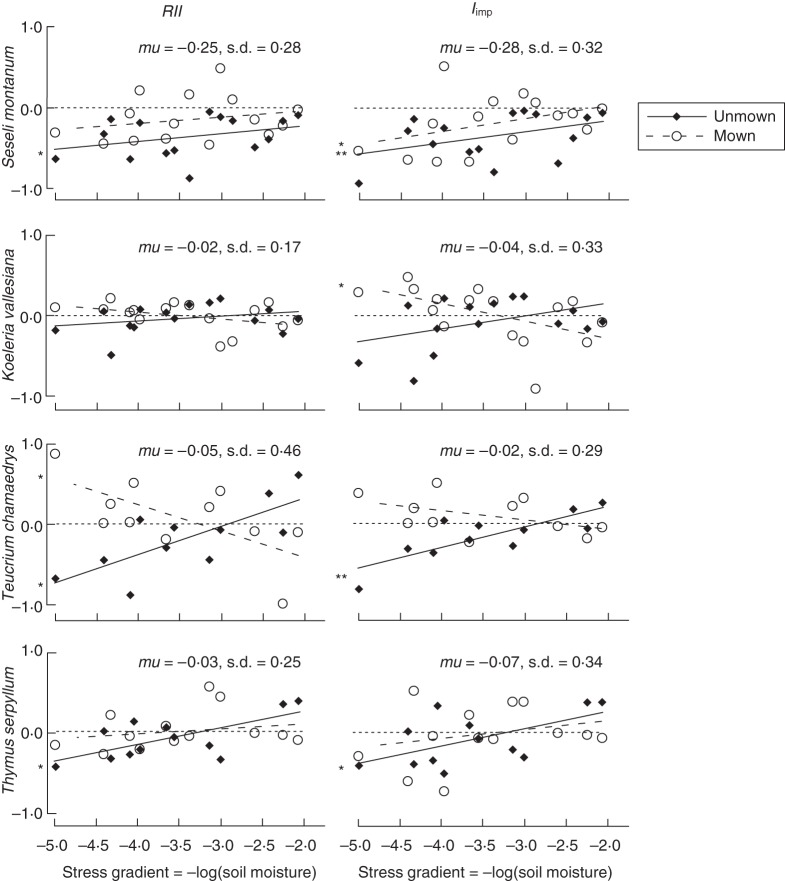
In summary, indices measured on growth show that interactions were strong in intensity and importance in the wettest part of the moisture gradient and mostly competitive in unmown plots and positive (or null) in mown plots, and collapsed in the driest plots whatever the mowing treatment or original interaction type (Fig. 1). The impact of plant interactions on growth was predominantly negative, as shown by the highly significant intercept effect for both RII and Iimp indices (Table 1, Fig. 1). Because results were quite similar for both indices, we comment on them simultaneously. There was a significant mowing effect, because the intensity and importance of competition globally decreased with mowing (Table 1, Fig. 1). In addition, there was a significant species effect because the general mean of RII and Iimp differed according to species, and S. montanum was particularly affected by competition as compared with the other species (Table 1, Fig. 1). However, there was a highly significant moisture × mowing interaction (in particular for RII), because the decrease in the intensity and importance of competition with mowing only occurred in the wettest part of the moisture gradient. Additionally, the significant three-way interaction (marginally significant for Iimp) between the moisture, mowing and species treatments indicates that this decrease in the intensity and importance of competition with mowing in the wettest part of the gradient was particularly strong for some species, and in particular Te. chamaedrys for RII and K. valesiana for Iimp (Table 1, Fig. 1). These two species were significantly facilitated, in intensity and importance, respectively, in the wettest plots but only when mown, as shown by the significantly positive intercepts of the corresponding curves at this side of the gradient (Fig. 1).
Fig. 1.
Variation in the intensity (Relative Interaction Index, RII) and the importance (Iimp index) of biotic interactions measured on growth along a water stress gradient (x-axis) for four target species and with and without mowing disturbance, as indicated in the key. Global mean (mu) and s.d. of each index and for each species are shown at the top right of each graph. Significant line intercepts at both gradient extremes are shown (*P < 0·05; **P < 0·01). Note that in all cases, there are no significant intercepts at the harsh extreme of the gradient.
Table 1.
Mixed ANCOVA effects of soil moisture (covariate), mowing (factor) and species (factor) on the Relative Interaction Index (RII) and the Iimp index computed on the growth data
| d.f. | den d.f. |
RII |
Iimp |
|||
|---|---|---|---|---|---|---|
| F | P | F | P | |||
| Intercept | 1 | 58 | 14·40 | *** | 15·30 | *** |
| Moisture | 1 | 13 | 2·42 | n.s. | 5·25 | * |
| Mowing | 1 | 13 | 9·14 | ** | 6·14 | * |
| Species | 3 | 58 | 5·81 | ** | 5·09 | ** |
| Moisture ×mowing | 1 | 13 | 15·55 | ** | 9·25 | ** |
| Moisture × species | 3 | 58 | 1·45 | n.s. | 1·91 | n.s. |
| Mowing × species | 3 | 58 | 1·37 | n.s. | 0·76 | n.s. |
| Moisture × mowing × species | 3 | 58 | 6·17 | *** | 2·28 | † |
d.f., numerator degrees of freedom; den d.f., denominator degrees of freedom; n.s., not significant; †P < 0·1; *P < 0·05; **P < 0·01; ***P < 0·001.
DISCUSSION
Consistent with our first hypothesis, our results clearly showed that plant interactions – whether positive or negative – were globally more intense and more important at the benign end of the soil moisture gradient, and collapsed when stress conditions became too severe. The responses to mowing were more complex and species-specific. For two species, mowing decreased the intensity and importance of competition at the benign end of the soil moisture gradient, inducing an early collapse of interactions along the stress gradient, consistent with our second hypothesis. However, for the two other species, competition switched to facilitation (either in intensity for one species or in importance for the other one) with mowing at the benign end of the stress gradient, but these new interactions did not collapse more rapidly along the stress gradient, thus contradicting our second hypothesis. Overall, results were very similar using either the index of intensity or importance of interactions.
Variation in plant interactions along the stress gradient in the absence of disturbance
In unmown plots at the wettest end of our stress gradient, plant–plant interactions measured on target biomass production as either intensity or importance were significantly negative for three of the four investigated species (S. montanum, Te. chamaedrys and Th. serpyllum). There was a similar tendency for the fourth species (K. vallesiana), but this pattern was marginally significant and only for the importance of interactions. Competition intensity and importance both linearly decreased with increasing water stress to become null at the severe end of the gradient.
Michalet et al. (2006) and Malkinson and Tielbörger (2010) proposed that biotic interactions should collapse at the extreme end of stress or disturbance gradients because the effects of neighbours should vanish with decreasing performance and size, as originally proposed by Grime in his triangular strategy model (Grime, 1974). This theoretical prediction has been supported by mathematical simulations (Travis et al., 2006; Xiao et al., 2009) and several experiments conducted along biotic or physical disturbance gradients (Brooker et al., 2005; Smit et al., 2007; Forey et al., 2010). Fewer experiments have confirmed this hypothesis along stress gradients (Kitzberger et al., 2000; de Bello et al., 2011). Thus, our study including four target species brings additional support to the theoretical prediction that biotic interactions are not important for community structure under extreme stress conditions. At our sites, the decrease in the intensity and importance of net biotic interactions may be explained by the structure of Xerobromion communities located in the dry part of our water stress gradient, and in particular by the morphology and functional strategy of the dominant species. These communities have a very low biomass and appear more like a mat dominated by several short-sized forbs than a grassland dominated by tall competitors or bunch grass benefactors. Another explanation for the decrease of – mainly negative – biotic interactions along the stress gradient is that competition might have been progressively balanced by an increase in facilitation with increasing stress. The metrics we used estimate the net effect of biotic interactions, which is no more than the algebraic sum of facilitation and competition (Callaway and Walker, 1997; Brooker and Callaghan, 1998). With decreasing soil moisture, potential positive effects of the plant canopy on soil water availability might have balanced its negative effects on light availability, as proposed by the model of Holmgren et al. (1997).
Our results are more consistent with Malkinson and Tielbörger (2010), predicting that all interactions should vanish under extreme stress conditions, than Michalet et al. (2006) who rather proposed that competition should first switch to facilitation at an intermediate position along an environmental severity gradient [consistent with Bertness and Callaway's (1994) model], before collapsing at the end of the gradient. Indeed, in the absence of mowing we never found any significant facilitation for growth, even in intermediately water-stressed conditions. The decrease in competition from the wettest end to an intermediate position along the water stress gradient is consistent with previous experimental studies conducted in calcareous grasslands (Corcket et al., 2003; Liancourt et al., 2005), but Liancourt et al. (2005) also detected facilitation in semi-dry conditions. We propose two explanations for the observed absence of facilitation. First, our results were observed for growth, and it is well known that in the same system, a target species may be facilitated for survival while suffering from competition for growth (Goldberg et al., 2001; Liancourt et al., 2005). In our study, survival was high and, although one species was facilitated for survival (Te. chamaedrys), neither its intensity nor its importance significantly varied along the stress gradient. Second, the high survival of our target species and subsequent insensitivity to potential facilitative effects of neighbours might also be linked to their drought-tolerant strategy. Several authors have shown that stress-tolerant species are not good candidates for facilitation, because of their low shade-tolerance and low sensitivity to stress mitigation provided by neighbours as compared with drought-intolerant species (Liancourt et al., 2005; Forey et al., 2010).
Interactive effects of the water stress gradient and the mowing factor on biotic interactions
At the benign end of our moisture gradient, mowing increased both the RII and Iimp indices and for all species, but the magnitude of this increase and subsequent changes in direction of interactions with mowing were species-specific. Plant interactions switched from competition to neutral (intensity) and from competition to weaker competition (importance) for S. montanum. Competition switched to neutral, both in intensity and in importance, for Th. serpyllum. These results are consistent with classic ecological theory (Grime, 1973; Huston, 1979) linking disturbance to a release from competition (Grubb, 1977). In mesic calcareous grasslands, vascular plants have been shown to suffer from competition by bryophytes (Otsus and Zobel, 2004; Ingerpuu and Kupper, 2007; Jeschke and Kiehl, 2008). In our wettest plots, the bryophytes layer was thicker and was damaged by the mowing treatment. This might explain why disturbance induced a collapse of competition earlier along the gradient for three species, consistent with our second hypothesis and with other studies showing the high dependency of biotic interactions on the interaction between physical stress and disturbance (Crain, 2008; Veblen, 2008; Bulleri et al., 2011; Soliveres et al., 2011).
For the two other species, mowing rather changed the direction of interactions, at least for either the intensity or importance. K. vallesiana had neutral interaction intensity all along the gradient with and without mowing treatment, but the importance of interactions switched from neutral to facilitation with mowing in the wettest conditions. In contrast, Th. chamaedrys switched from competition to facilitation with mowing when considering interaction intensity and from competition to neutral when considering importance. The occurrence of facilitation with disturbance is a process widely described along biotic disturbance gradients [i.e. associational defences, which are a case of indirect facilitation; Smit et al. (2007), Vandenberghe et al. (2009) and Anthelme and Michalet (2009)], consistent with the model of Bertness and Callaway (1994). To our knowledge no studies have found this result along a mowing disturbance gradient, where it is very unlikely that neighbours protect potential beneficiaries from the mechanical constraint induced by the mower, which is the case in the associational defences' interactions. However, we suggest that mown targets may suffer more from drought and thus may be more demanding of stress mitigation by neighbours than unmown targets, as shown in physically disturbed communities (Choler et al., 2001; Kawai and Tokeshi, 2007).
Intensity versus importance of interactions
Results were not very different using either the intensity or the importance index, at least in unmown plots. In the mown plots there were subtle differences in interaction with the species effects but the collapse of plant interactions with increasing stress was also observed with the two indices. This weak difference between those indices is not in accordance with Welden and Slauson's (1986) predictions that competition intensity should be stable along productivity gradients, while competition importance should increase with decreasing stress. However, Brooker et al. (2005) showed that comparisons between interactions importance and intensity along productivity gradients are system-dependent. They re-analysed two datasets, one from a productive environment (Reader et al., 1994) and one from a semi-arid system (Pugnaire and Luque, 2001). They validated Welden and Slauson's predictions in the productive environment, but found results similar to ours (decreasing importance and intensity along a water stress gradient) in the semi-arid system. Thus, our results provide new evidence suggesting that the importance and direction of interactions are more likely to be positively related in stressful environments.
Our study strongly supports models predicting a reduction of the magnitude of net plant–plant interactions, whether positive or negative, on an intermediate-to-high stress gradient because of decreases in the potential of plants to be benefactors or competitors. It also shows that this reduction is likely to be accelerated by disturbance. Intensity and importance seem to be correlated along stress gradients in unproductive environments. Further studies questioning the combinatory effects of stress and disturbance on plant–plant interactions should consider other factors potentially influencing them (e.g. life-history stages, Goldberg et al., 2001; performance proxy, Malkinson and Tielbörger, 2010; seasons effect, Soliveres et al., 2011). It would also be interesting to investigate potential differences between the effects on biotic interactions of natural and experimental stress gradients combined with disturbance.
ACKNOWLEDGEMENTS
This work was funded by the University of Bordeaux 1 and French ANR 09 – STRA – 09O2LA. We are grateful to the Périgord-Limousin Natural Park, the municipality of La Rochebeaucourt and Mr C. Allard for their support. We acknowledge all students who provided help in the field.
LITERATURE CITED
- Anthelme F, Michalet R. Grass-to-tree facilitation in an arid grazed environment (Air Mountains, Sahara) Basic and Applied Ecology. 2009;10:437–446. [Google Scholar]
- Ariza C, Tielbörger K. An evolutionary approach to studying the relative importance of plant–plant interactions along environmental gradients. Functional Ecology. 2011;25:932–942. [Google Scholar]
- Armas C, Ordiales R, Pugnaire FI. Measuring plant interactions: a new comparative index. Ecology. 2004;85:2682–2686. [Google Scholar]
- de Bello F, Dolezal J, Dvorsky M, et al. Cushions of Thylacospermum caespitosum (Caryophyllaceae) do not facilitate other plants under extreme altitude and dry conditions in the north-west Himalayas. Annals of Botany. 2011;108:567–573. doi: 10.1093/aob/mcr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertness MD, Callaway R. Positive interactions in communities. Trends in Ecology & Evolution. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Boullet V. Les pelouses calcicoles (Festuco-Brometea) du domaine atlantique français et ses abords au nord de la Gironde et du Lot. Essai de synthèse phyto sociologique. Université des Sciences et Techniques de Lille; 1986. [Google Scholar]
- Brewer JS. Disturbance-mediated competition between perennial plants along a resource supply gradient. Journal of Ecology. 2011;99:1219–1228. [Google Scholar]
- Brooker RW, Callaghan TV. The balance between positive and negative plant interactions and its relationship to environmental gradients: a model. Oikos. 1998;81:196–207. [Google Scholar]
- Brooker R, Kikvidze Z, Pugnaire FI, et al. The importance of importance. Oikos. 2005;109:63–70. [Google Scholar]
- Brooker RW, Maestre FT, Callaway RM, et al. Facilitation in plant communities: the past, the present, and the future. Journal of Ecology. 2008;96:18–34. [Google Scholar]
- Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution. 2003;18:119–125. [Google Scholar]
- Bulleri F, Cristaudo C, Alestra T, Benedetti-Cecchi L. Crossing gradients of consumer pressure and physical stress on shallow rocky reefs: a test of the stress-gradient hypothesis. Journal of Ecology. 2011;99:335–344. [Google Scholar]
- Callaway RM, Walker LR. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- Callaway RM, Brooker RW, Choler P, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Choler P, Michalet R, Callaway RM. Facilitation and competition on gradients in alpine plant communities. Ecology. 2001;82:3295–3308. [Google Scholar]
- Corbeil RR, Searle SR. Restricted maximum likelihood (REML) estimation of variance components in mixed model. Technometrics. 1976;18:31–38. [Google Scholar]
- Corcket E, Liancourt P, Callaway RM, Michalet R. The relative importance of competition for two dominant grass species as affected by environmental manipulations in the field. Ecoscience. 2003;10:186–194. [Google Scholar]
- Crain CM. Interactions between marsh plant species vary in direction and strength depending on environmental and consumer context. Journal of Ecology. 2008;96:166–173. [Google Scholar]
- Forey E, Touzard B, Michalet R. Does disturbance drive the collapse of biotic interactions at the severe end of a diversity-biomass gradient? Plant Ecology. 2010;206:287–295. [Google Scholar]
- Freckleton RP, Watkinson AR, Rees M. Measuring the importance of competition in plant communities. Journal of Ecology. 2009;97:379–384. [Google Scholar]
- Gaucherand S, Liancourt P, Lavorel S. Importance and intensity of competition along a fertility gradient and across species. Journal of Vegetation Science. 2006;17:455–464. [Google Scholar]
- Goldberg DE, Turkington R, Olsvig-Whittaker L, Dyer AR. Density dependence in an annual plant community: variation among life history stages. Ecological Monographs. 2001;71:423–446. [Google Scholar]
- Graff P, Aguiar MR, Chaneton EJ. Shifts in positive and negative plant interactions along a grazing intensity gradient. Ecology. 2007;88:188–199. doi: 10.1890/0012-9658(2007)88[188:sipanp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Grime JP. Competitive exclusion in herbaceous vegetation. Nature. 1973;242:344–347. [Google Scholar]
- Grime JP. Vegetation classification by reference to strategies. Nature. 1974;250:26–31. [Google Scholar]
- Grime JP, Curtis AV. Interaction of drought and mineral nutrient stress in calcareous grassland. Journal of Ecology. 1976;64:975–988. [Google Scholar]
- Grubb PJ. Maintenance of species-richness in plant communities – importance of regeneration niche. Biological Reviews of the Cambridge Philosophical Society. 1977;52:107–145. [Google Scholar]
- Hacker SD, Gaines SD. Some implications of direct positive interactions for community species diversity. Ecology. 1997;78:1990–2003. [Google Scholar]
- Holmgren M, Scheffer M, Huston MA. The interplay of facilitation and competition in plant communities. Ecology. 1997;78:1966–1975. [Google Scholar]
- Huston M. General hypothesis of species-diversity. American Naturalist. 1979;113:81–101. [Google Scholar]
- Ingerpuu N, Kupper T. Response of calcareous grassland vegetation to mowing and fluctuating weather conditions. Journal of Vegetation Science. 2007;18:141–146. [Google Scholar]
- Jeschke M, Kiehl K. Effects of a dense moss layer on germination and establishment of vascular plants in newly created calcareous grasslands. Flora. 2008;203:557–566. [Google Scholar]
- Kahmen A, Perner J, Buchmann N. Diversity-dependent productivity in semi-natural grasslands following climate perturbations. Functional Ecology. 2005;19:594–601. [Google Scholar]
- Kahmen S, Poschlod P, Schreiber KF. Conservation management of calcareous grasslands. Changes in plant species composition and response of functional traits during 25 years. Biological Conservation. 2002;104:319–328. [Google Scholar]
- Kawai T, Tokeshi M. Testing the facilitation-competition paradigm under the stress-gradient hypothesis: decoupling multiple stress factors. Proceedings of the Royal Society B-Biological Sciences. 2007;274:2503–2508. doi: 10.1098/rspb.2007.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefi S, Rietkerk M, Alados CL, et al. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature. 2007;449:213–U5. doi: 10.1038/nature06111. [DOI] [PubMed] [Google Scholar]
- Kikvidze Z, Brooker R. Towards a more exact definition of the importance of competition – a reply to Freckleton et al. (2009) Journal of Ecology. 2010;98:719–724. [Google Scholar]
- Kikvidze Z, Suzuki M, Brooker R. Importance versus intensity of ecological effects: why context matters. Trends in Ecology & Evolution. 2011;26:383–388. doi: 10.1016/j.tree.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Kitzberger T, Steinaker DF, Veblen TT. Effects of climatic variability on facilitation of tree establishment in northern Patagonia. Ecology. 2000;81:1914–1924. [Google Scholar]
- Kunstler G, Albert CH, Courbaud B, et al. Effects of competition on tree radial-growth vary in importance but not in intensity along climatic gradients. Journal of Ecology. 2011;99:300–312. [Google Scholar]
- Le Bagousse-Pinguet Y, Gross EM, Straile D. Release from competition and protection determine the outcome of plant interactions along a grazing gradient. Oikos. 2012;121:95–101. [Google Scholar]
- Liancourt P, Callaway RM, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. [Google Scholar]
- Maestre FT, Cortina J. Do positive interactions increase with abiotic stress? – A test from a semi-arid steppe. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:S331–S333. doi: 10.1098/rsbl.2004.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology. 2009;97:199–205. [Google Scholar]
- Malkinson D, Tielbörger K. What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos. 2010;119:1546–1552. [Google Scholar]
- Michalet R. Highlighting the multiple drivers of change in interactions along stress gradients. New Phytologist. 2007;173:3–6. doi: 10.1111/j.1469-8137.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- Michalet R, Brooker RW, Cavieres LA, et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecology Letters. 2006;9:767–773. doi: 10.1111/j.1461-0248.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- Otsus M, Zobel M. Moisture conditions and the presence of bryophytes determine fescue species abundance in a dry calcareous grassland. Oecologia. 2004;138:293–299. doi: 10.1007/s00442-003-1428-8. [DOI] [PubMed] [Google Scholar]
- Pugnaire FI, Luque MT. Changes in plant interactions along a gradient of environmental stress. Oikos. 2001;93:42–49. [Google Scholar]
- Reader RJ, Wilson SD, Belcher JW, et al. Plant competition in relation to neighbor biomass – an intercontinental study with Poa pratensis. Ecology. 1994;75:1753–1760. [Google Scholar]
- Rees M, Childs DZ, Freckleton RP. Assessing the role of competition and stress: a critique of importance indices and the development of a new approach. Journal of Ecology. 2012;100:577–585. [Google Scholar]
- le Roux PC, McGeoch MA. Interaction intensity and importance along two stress gradients: adding shape to the stress-gradient hypothesis. Oecologia. 2010;162:733–745. doi: 10.1007/s00442-009-1484-9. [DOI] [PubMed] [Google Scholar]
- Royer JM. Les pelouses des Festuco-Brometea. D'un exemple régional à une vision eurosibérienne: étude phytosociologique et phytogéographique. France: Thèse d'état, Université de Franche-Comté, Besançon; 1987. [Google Scholar]
- Sammul M, Kull K, Oksanen L, Veromann P. Competition intensity and its importance: results of field experiments with Anthoxanthum odoratum. Oecologia. 2000;125:18–25. doi: 10.1007/PL00008887. [DOI] [PubMed] [Google Scholar]
- Seifan M, Seifan T, Ariza C, Tielborger K. Facilitating an importance index. Journal of Ecology. 2010;98:356–361. [Google Scholar]
- Smit C, Vandenberghe C, den Ouden J, Muller-Scharer H. Nurse plants, tree saplings and grazing pressure: changes in facilitation along a biotic environmental gradient. Oecologia. 2007;152:265–273. doi: 10.1007/s00442-006-0650-6. [DOI] [PubMed] [Google Scholar]
- Smit C, Rietkerk M, Wassen MJ. Inclusion of biotic stress (consumer pressure) alters predictions from the stress gradient hypothesis. Journal of Ecology. 2009;97:1215–1219. [Google Scholar]
- Soliveres S, Garcia-Palacios P, Castillo-Monroy AP, Maestre FT, Escudero A, Valladares F. Temporal dynamics of herbivory and water availability interactively modulate the outcome of a grass-shrub interaction in a semi-arid ecosystem. Oikos. 2011;120:710–719. [Google Scholar]
- Tielbörger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton Monographs in Population Biology 17. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Travis JMJ, Brooker RW, Clark EJ, Dytham C. The distribution of positive and negative species interactions across environmental gradients on a dual-lattice model. Journal of Theoretical Biology. 2006;241:896–902. doi: 10.1016/j.jtbi.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, et al. Flora Europaea. vols 1–5. Cambridge: Cambridge University Press; 1964–1980. [Google Scholar]
- Vandenberghe C, Smit C, Pohl M, Buttler A, Frelechoux F. Does the strength of facilitation by nurse shrubs depend on grazing resistance of tree saplings? Basic and Applied Ecology. 2009;10:427–436. [Google Scholar]
- Veblen KE. Season- and herbivore-dependent competition and facilitation in a semiarid savanna. Ecology. 2008;89:1532–1540. doi: 10.1890/07-0973.1. [DOI] [PubMed] [Google Scholar]
- Welden CW, Slauson WL. The intensity of competition versus its importance – an overlooked distinction and some implications. Quarterly Review of Biology. 1986;61:23–44. doi: 10.1086/414724. [DOI] [PubMed] [Google Scholar]
- Xiao S, Michalet R, Wang G, Chen SY. The interplay between species' positive and negative interactions shapes the community biomass-species richness relationship. Oikos. 2009;118:1343–1348. [Google Scholar]
- Zhang JY, Cheng GW, Yu FH, Krauchi N, Li MH. Intensity and importance of competition for a grass (Festuca rubra) and a legume (Trifolium pratense) vary with environmental changes. Journal of Integrative Plant Biology. 2008;50:1570–1579. doi: 10.1111/j.1744-7909.2008.00699.x. [DOI] [PubMed] [Google Scholar]