Abstract
Background and Aims
A significant number of species assigned to the Neotropical orchid sub-tribe Oncidiinae reward insect pollinators with oil produced in floral glands termed elaiophores. The latter may be glabrous (epithelial elaiophores) or hirsute (trichomal elaiophores). Although the detailed anatomy and ultrastructure of epithelial elaiophores have been studied for a number of genera, such as Oncidium Sw., Gomesa R. Br. and Trichocentrum Poepp. & Endl., hitherto, trichomal elaiophores have been investigated only for a single species of Oncidiinae, Ornithocephalus ciliatus Lindl. Furthermore, this is the only representative of the Ornithocephalus clade to be investigated to date. Here, an examination is made of the elaiophore anatomy and ultrastructure of a further four species currently assigned to this clade (Ornithocephalus gladiatus Hook., Phymatidium falcifolium Lindl., Zygostates grandiflora (Lindl.) Mansf. and Zygostates lunata Lindl.) and the results compared with those obtained for other Oncidiinae.
Methods
Elaiophore structure was examined for all species at three stages of flower development: closed bud, first day of anthesis and final stage of anthesis, using light microscopy, fluorescence microscopy, scanning electron microscopy, transmission electron microscopy and histochemistry.
Key Results
Elaiophores of O. gladiatus occur upon the lateral lobes of the labellum and display characters intermediate between those of typical epithelial and trichomal elaiophores, in that they are largely glabrous, consisting mainly of cuboidal epidermal cells, but bear short, unicellular hairs proximally. By contrast, the elaiophores of all the other species investigated occur on the callus and are of the trichomal type. In P. falcifolium, these unicellular hairs are capitate. In all species, oil secretion commenced at the closed floral bud stage. Ultrastructurally, the mainly trichomal elaiophores of the four representatives of the Ornithocephalus clade closely resembled the epithelial elaiophores of other Oncidiinae, in that their cells displayed an organelle complement typical of lipid-secreting cells. However, in some taxa, a number of noteworthy characters were present. For example, the elaiophore cuticle of O. gladiatus and P. falcifolium was bi-layered, the outer layer being lamellate, the inner reticulate. The cuticle of Z. grandiflora and Z. lunata was also lamellate, but here, a reticulate layer was absent. Accumulation of secreted oil resulted in the localized distension of the cuticle. Cuticular cracks and pores, however, were absent from all species. The walls of the secretory cells of Z. grandiflora were also atypical in that they had short protuberances or ingrowths, and contained cavities which are thought to be involved in the secretory process.
Conclusions
Of the species investigated, most displayed similar anatomical organization, their trichomal elaiophores occurring on the labellar callus. They, thus, differ from many other members of the Oncidiinae, where epithelial elaiophores are found either on the callus, or on the lateral lobes of the labellum. However, ultrastructurally, all elaiophores, whether those of representatives of the Ornithocephalus clade, or those of other oil-secreting Oncidiinae, possessed a similar complement of organelles, regardless of whether the elaiophores were trichomal or epithelial. In view of the latter, and the similar chemical composition of oils derived from all Oncidiinae investigated to date, it is probable that position and type of elaiophore, and possibly the structure of the overlying cuticle, play an important role in pollinator selection in these oil-secreting orchids.
Keywords: Anatomy, elaiophore, histochemistry, lipid secretion, micromorphology, oil glands, Oncidiinae, trichome, ultrastructure
INTRODUCTION
A significant proportion of species assigned to the Neotropical orchid sub-tribe Oncidiinae (sensu Chase et al., 2003) reward potential pollinators with floral oil (Chase et al., 2003; Chase, 2005). According to studies by Renner and Schaefer (2010), oil-mediated pollination is thought to have arisen, and the capacity to produce floral oil has been lost, on at least 28 and 36–40 occasions, respectively. In Orchidaceae, oil rewards appeared relatively recently, about five million years ago. Indeed, the production of floral oils in orchids is polyphyletic and is thought to have evolved at least five times in Oncidiinae alone (Silvera, 2002). In this sub-tribe, oil is produced by epidermal glands or glandular hairs, referred to as epithelial and trichomal elaiophores, respectively (Vogel, 1974), but their anatomy has been largely overlooked. However, recently, there has been a revived interest in the structure of orchid elaiophores (Davies and Stpiczyńska, 2008; Davies, 2009, and references therein), and in particular, those of Oncidiinae (Singer and Cocucci, 1999; Stpiczyńska et al., 2007; Pacek and Stpiczyńska, 2007; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Davies and Stpiczyńska, 2009). However, these studies were confined mainly to the epithelial elaiophores of species currently assigned to the Gomesa (formerly Oncidium) and Trichocentrum clades (sensu Williams et al., 2001), and hitherto, trichomal elaiophores have been investigated only for a single member of the Ornithocephalus clade (sensu Williams et al., 2001; Pacek and Stpiczyńska, 2007), namely, Ornithocephalus ciliatus Lindl. (as O. kruegeri Rchb.f.).
Elaiophores are common amongst members of the Ornithocephalus clade and both they, and the composition of the oil that they produce, are seemingly prone to parallelism (Reis et al., 2006; Singer et al., 2006). For example, Buchmann (1987) reported floral elaiophores in 50 species of Ornithocephalus Hook. and four species of Zygostates Lindl. According to Toscano de Brito (2001), those of Ornithocephalus are mainly of the trichomal type and usually occur on the labellum, although in some species they are found on the column or other parts of the flower. Similarly, the trichomal elaiophores of Zygostates occur on the labellar callus or petals. In Phymatidium delicatulum Lindl. and P. falcifolium Lindl. (as P. tillandsioides Barb Rodr.), they also occur on the labellum (Reis et al., 2006). In Hintonella mexicana Ames and Chytroglossa marileoniae Rchb.f., they occur at the base of the lip or upon the callus, respectively, whereas in Platyrhiza quadricolor Barb. Rodr., they are found at the base of the lip and upon the tabula infrastigmatica. Moreover, in some species, the individual hairs may be capitate, as in Phymatidium falcifolium. The pollinators of representatives of the Gomesa and Ornithocephalus clades differ. For example, Gomesa R. Br. (including those species formerly placed in Oncidium Sw. and Ornithophora Barb. Rodr.) and Oncidium Sw. (those species formerly placed in Sigmatostalix Rchb.f.) are pollinated by oil-gathering Tetrapedia and Centris bees (van der Pijl and Dodson, 1969; Buchmann, 1987; Dressler, 1990; Singer and Cocucci, 1999; van der Cingel, 2001), but the pollinators of Ornithocephalus are mainly species of Paratetrapedia, with P. testacea pollinating O. ciliatus Lindl (formerly O. avicula Rchb.f.) and O. cf. patentilobus C. Schweinf. in Peru, whereas P. calcarata pollinates O. bicornis Lindl. and O. powellii Schltr. in Panama (van der Cingel, 2001; Toscano de Brito, 2001). Nevertheless, the composition of the oil that they secrete is, for the most part, similar. Thus, oil produced by members of the Gomesa clade, including Gomesa × amicta (Lindl.) M.W. Chase & N.H. Williams (formerly Oncidium × amictum Lindl.), G. cornigera (Lindl.) M.W. Chase & N.H. Williams (formerly O. cornigerum Lindl.), G. cuneata (Scheidw.) M.W. Chase & N.H. Williams (formerly O. truncatum Pabst), G. echinata (Barb. Rodr.) M.W. Chase & N.H. Williams (formerly Baptistonia echinata Barb. Rodr.), G. hookeri (Rolfe) M.W. Chase & N.H. Williams (formerly O. hookeri Rolfe), G. kautskyi (Pabst) M.W. Chase & N.H. Williams (formerly O. kautskyi Pabst), G. longicornu (Mutel) M.W. Chase & N.H. Williams (formerly Oncidium longicornu Mutel), G. pubes (Lindl.) M.W. Chase & N.H. Williams (formerly O. pubes Lindl.), G. radicans (Rchb.f.) M.W. Chase & N.H. Williams (formerly Ornithophora radicans (Rchb.f.) Garay & Pabst), G. venustum (Drapiez) M.W. Chase & N.H. Williams (formerly Oncidium trulliferum Lindl.) and G. welteri (Pabst) M.W. Chase & N.H. Williams (formerly O. welteri Pabst) is characterized by the presence of diacylglycerols, in which the acetyl group is invariably located at position 1 of the glycerol moiety, and the fatty acid at position 2. The long-chain fatty acid has either hydroxyl or acetoxy groups at positions 3 and 7. The major component of the floral oil of G. radicans is (2S,3'R,7'R)-1-acetyl-2-(3',7'-diacetoxy-eicosanoyl)-glycerol (Reis et al., 2003). Acylglycerols are thought also to occur in the floral oils of G. loefgrenii (Cogn.) M.W. Chase & N.H. Williams (formerly O. loefgrenii Cogn.) and Gomesa, as formerly circumscribed (R. B. Singer, pers. comm., 2006). Oils of certain other members of the Gomesa clade, such as G. praetexta (Rchb.f.) M.W. Chase & N.H. Williams (formerly O. enderianum auct.) and related taxa, also contain diacylglycerol derivatives. The composition of the oils of members of the Ornithocephalus clade, such as Phymatidium falcifolium (as P. tillandsioides) and Zygostates lunata Lindl., is similar to that of the Gomesa clade (Reis et al., 2000, 2003, 2006), and the floral oils of P. falcifolium (as P. tillandsioides) and P. delicatulum contain the same component as that of G. radicans, namely, 1-acetyl-2-(3',7'-diacetoxy-eicosanoyl)-glycerol. However, the floral oil of P. delicatulum Lindl. is unusual in that it is composed mainly of simple, linear hydrocarbons (Reis et al., 2006).
The ultrastructural investigation of the trichomal elaiophore of O. ciliatus (Pacek and Stpiczyńska, 2007) is the only detailed study to date of oil-secreting tissue in a representative of the Ornithocephalus clade. In this species, the elaiophore is represented by a tuft of unicellular, oil-secreting trichomes arising from the centre of the labellar callus. These hairs, which elongate rapidly during the course of anthesis, contain a centrally placed nucleus, plastids with small starch grains, and lipid droplets. Lipids can be detected even when the flowers are in bud stage, a week or so prior to anthesis. The outer wall of each hair has a thin cuticle, and this becomes detached at the base of the hair, as oil accumulates between it and the epidermal cell wall. Here, we extend our studies of the floral elaiophore to another four species of the Ornithocephalus clade, some displaying elaiophore characters intermediate to those of typical epithelial and trichomal elaiophores. Their elaiophore structure, in turn, is compared with that of other Oncidiinae.
MATERIALS AND METHODS
Plants for this study were grown at the Botanic Garden of the Maria Curie-Skłodowska University, Lublin, Poland. Four species were investigated, namely, Ornithocephalus gladiatus Hook. (accession number 104/07), Phymatidium falcifolium Lindl. (accession number 100/07), Zygostates grandiflora (Lindl.) Mansf. (accession number 99/07) and Z. lunata Lindl. (accession number 97/07). Abbreviations for authors of plant names follow Brummitt and Powell (1992) throughout.
The position of elaiophores in intact, fresh flowers of all investigated species was determined using an Olympus SZX12 stereo-microscope. The structure of elaiophores was examined using light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) at three stages of floral development: closed bud, first day of anthesis and the final stage of anthesis. Flowers at this last stage showed necrosis or abscission of floral parts. Lipid secretion was monitored throughout the life-span of the flower, from the closed bud stage (3–4 weeks prior to anthesis, when the bud is sufficiently large to dissect and already contains floral oil) until the end of anthesis.
Following macroscopic observations, the secretory tissue was dissected, and pieces of elaiophore tissue (approx 1 mm3) were fixed in 2·5 % glutaraldehyde/4 % formaldehyde in phosphate buffer (pH 7·4; 0·1 m) for 2 h at 4 °C, carefully washed three times in phosphate buffer and post-fixed in 1·5 % osmium tetroxide solution for 1·5 h. The fixed material was then dehydrated using a graded ethanol series, and infiltrated and embedded in LR White resin (LR White acrylic resin, medium grade, Sigma). Following polymerization at 60 °C, sections were cut at 70 nm for TEM using a Reichert Ultracut-S ultramicrotome and a glass knife, stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined using a Tesla BS-500 or FEI Tecnai Spirit G2 transmission electron microscope at an accelerating voltage of 25 kV.
Semi-thin sections (0·9–1·0 µm thick) were prepared for light and fluorescence microscopy. For general histology, they were stained with an aqueous solution of 1 % (w/v) methylene blue and 1 % (w/v) azure II (1 : 1) for 5–7 min. The sections were mounted in Euparal.
Histochemical tests were used to detect the presence of lipids and starch in elaiophore tissue. Lipids were selectively stained by treating hand-cut sections of fresh tissue, or semi-thin sections of fixed tissue with a saturated ethanolic solution of Sudan III or with auramine O (Jensen, 1962; Gahan, 1984). Depending on the stain employed, the tissue samples were examined using a Nikon E-200 or Nikon Eclipse 90i fluorescence microscope (FITC filter, excitation light 465–495 nm, barrier filter 515–555 nm). Insoluble polysaccharides were stained using the periodic acid–Schiff (PAS) reaction (Jensen, 1962). The presence of starch was detected by treating hand-cut sections with IKI followed by examination under a Nikon E-200 bright-field light microscope. Micrometry and photomicrography of elaiophores were accomplished using a Nikon Eclipse 90i light microscope with NIS-Elements AR or FEI Tecnai Spirit G2 (TEM Imaging & Analysis computer program) software.
For SEM, fixed pieces of labellum were dehydrated, subjected to critical-point drying using liquid CO2, sputter coated with gold and examined by means of a Tescan Vega II LS scanning electron microscope at an accelerating voltage of 90 kV.
RESULTS
Four representatives of the Ornithocephalus clade were examined: a single species of Ornithocephalus, another of Phymatidium, and two species of Zygostates.
Ornithocephalus gladiatus
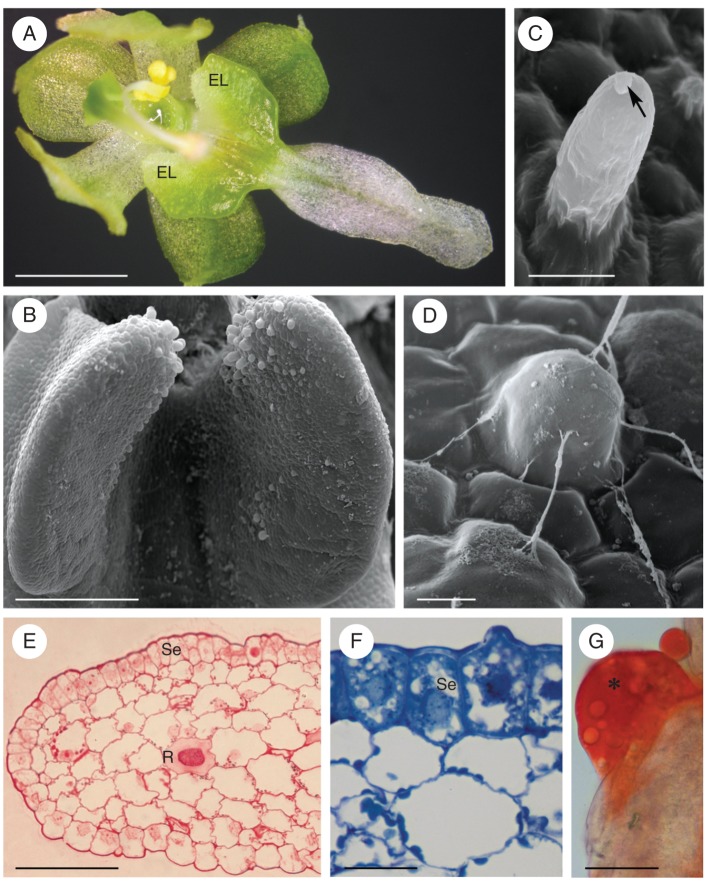
In O. gladiatus, light-green, ear-shaped elaiophores are located symmetrically on the lateral lobes of the labellum (Fig. 1A). Proximally, these glands are clothed with unicellular hairs (Fig. 1B–D) that increase in length with development of the flower (mean = 25·08 µm at bud stage; mean = 44·62 µm at the end of anthesis). An oily secretion was detected in flower buds 3 weeks prior to opening, and this accumulated throughout anthesis, mainly in a depression of the labellum, between the elaiophores (Fig. 1A).
Fig. 1.
Ornithocephalus gladiatus, SEM and LM. (A) Habit of flower with elaiophores located proximally on the labellum. (B) Detail of minutely hirsute elaiophore at bud stage. (C, D) Hairs at anthesis showing blistered cuticle (C, arrow) and strands of secretion (D). (E) Transverse section of elaiophore following the PAS reaction. Starch occurs predominantly in the subsecretory parenchyma. Idioblasts containing raphides occur in the ground parenchyma. (F) Secretory parenchyma cells stain intensely with methylene blue–azure II, in contrast to the weakly stained parenchyma cells. (G) Large lipid droplet (asterisk) stained with Sudan III on surface of elaiophore. Scale bars: (A) = 1 mm; (B) = 300 µm; (C, D) = 20 µm; (E) = 100 µm; (F, G) = 20 µm. Abbreviations (used in Figs 1–7): Bl, blistered cuticle; Ca, cavity in cell wall; Cl, lamellate layer of cuticle; Cr, reticulate layer of cuticle; Cw, cell wall; EL, elaiophore; Ep, elaioplast; ER, endoplasmic reticulum; G, dictyosome (Golgi apparatus); L, lipid droplet; m, mitochondrion; mf, myelin-like figure; N, nucleus; P, plastid; R, raphides; Se, secretory epidermis; st, starch; Sv, secretory vesicle; V, vacuole.
The elaiophore consists of a single-layered secretory epithelium of cuboidal or rectangular, epidermal cells and small subepithelial parenchyma cells (Fig. 1E, F) that stain intensely with methylene blue–azure II solution (Fig. 1F). Collateral vascular bundles occur in the underlying ground parenchyma. Raphides occur in the vacuoles of idioblastic parenchyma cells (Fig. 1E) and, during the bud stage, these cells contain numerous lipid droplets.
Lipid was detected, both within elaiophore tissue and on the surface of flower buds, following treatment with Sudan III (Fig. 1G), and the amount of lipids, both within epithelial cells and secretory hairs, had increased by the beginning of anthesis. Epithelial and parenchyma cells contained plastids that accumulated starch during the bud stage, but starch grains were larger and more abundant in ground parenchyma than in epidermal cells (Fig. 1E).
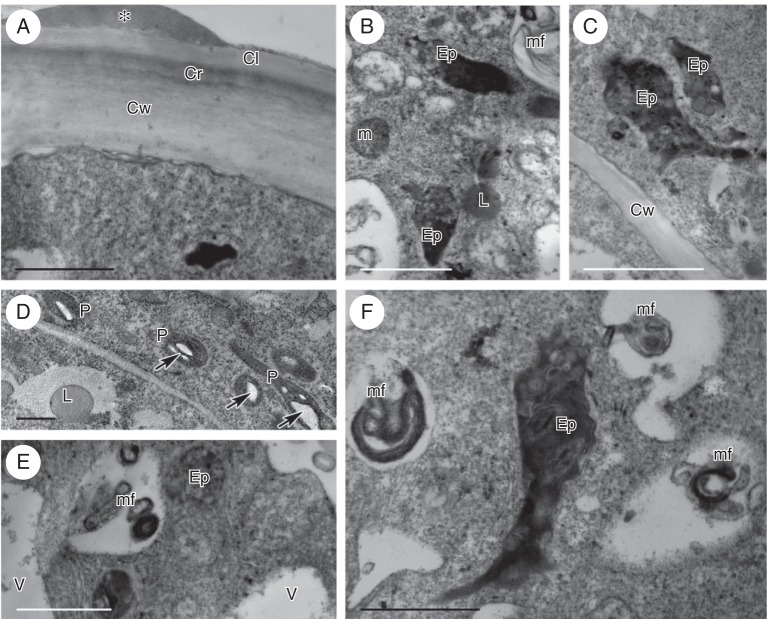
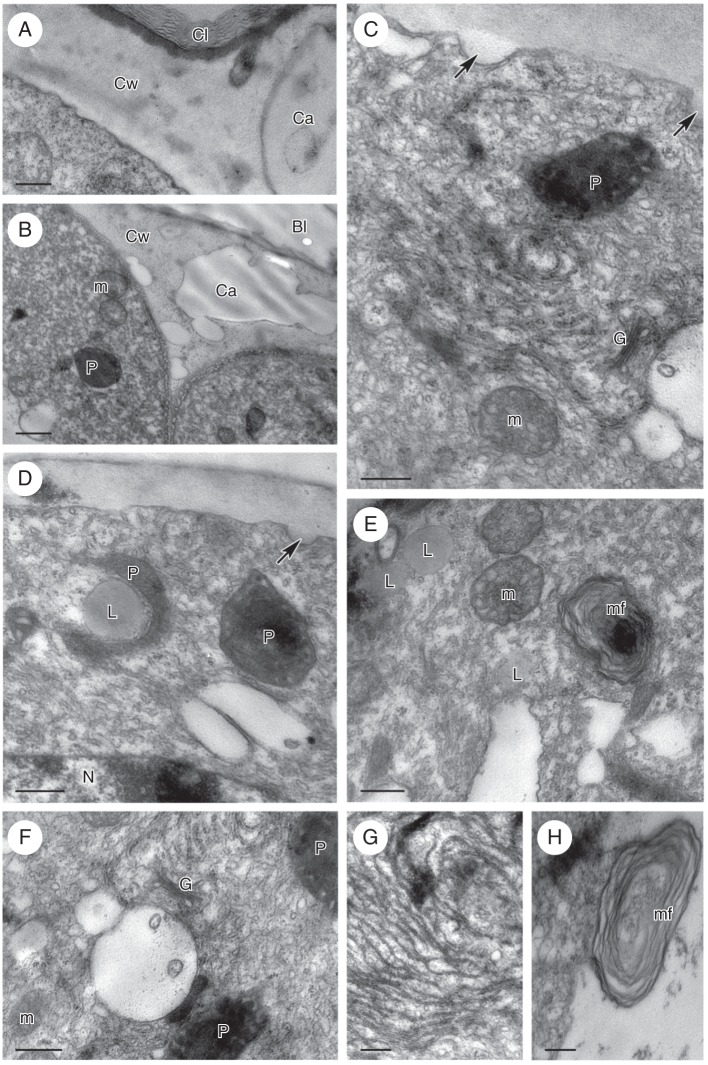
The cuticle present on the outer wall of epithelial cells comprised a lamellate layer overlying a reticulate cuticular layer (Fig. 2A). At the bud stage, the cuticle became slightly distended in response to the pressure exerted by the accumulation of secreted material, but by the final stage of anthesis, the cuticle had become wrinkled and had often separated from the cell wall (Fig. 1C). Lipid droplets were present both on the surface of the cell wall (Fig. 2A) and in the cytoplasm for all stages investigated (Fig. 2B). The cytoplasm of secretory cells was dense, granular, and contained abundant smooth and rough endoplasmic reticulum (SER and RER, respectively) profiles, as well as vacuoles of various sizes (Fig. 2A–F). During the bud stage, intravacuolar, myelin-like figures began to form, and these increased in size and abundance throughout the course of anthesis (Fig. 2B, E, F). Moreover, the leucoplasts contained one to several starch grains (Fig. 2D), but by the beginning of anthesis they had differentiated to form elaioplasts with numerous, large lipid droplets (Fig. 2B, C, E, F). At the final stage of anthesis, the cytoplasm began to show signs of autolysis and the cells became highly vacuolated, yet still contained large lipid droplets. Plasmodesmata were present throughout all investigated stages of secretion, mainly in the anticlinal walls of epidermal cells.
Fig. 2.
Ornithocephalus gladiatus, TEM. (A) Elaiophore cell wall with bi-layered cuticle comprising an outer lamellate layer and an inner reticulate layer. A lipid droplet (asterisk) is present on the surface of the elaiophore and the parietal cytoplasm contains abundant ER. (B) The cytoplasm of the elaiophore cell contains elaioplasts, lipid droplets and mitochondria. (C) Elaioplasts of irregular shape contain lipid droplets. (D) Starch (arrows) is present in the plastids at bud stage. (E) Elaioplast and small, intravacuolar myelin-like figures. (F) Detail of elaioplast, with dark stroma and lipid droplets, intravacuolar, myelin-like figures, and cytoplasm with abundant ER. Scale bars = 1 µm. See Fig. 1 for abbreviations.
Phymatidium falcifolium Lindl
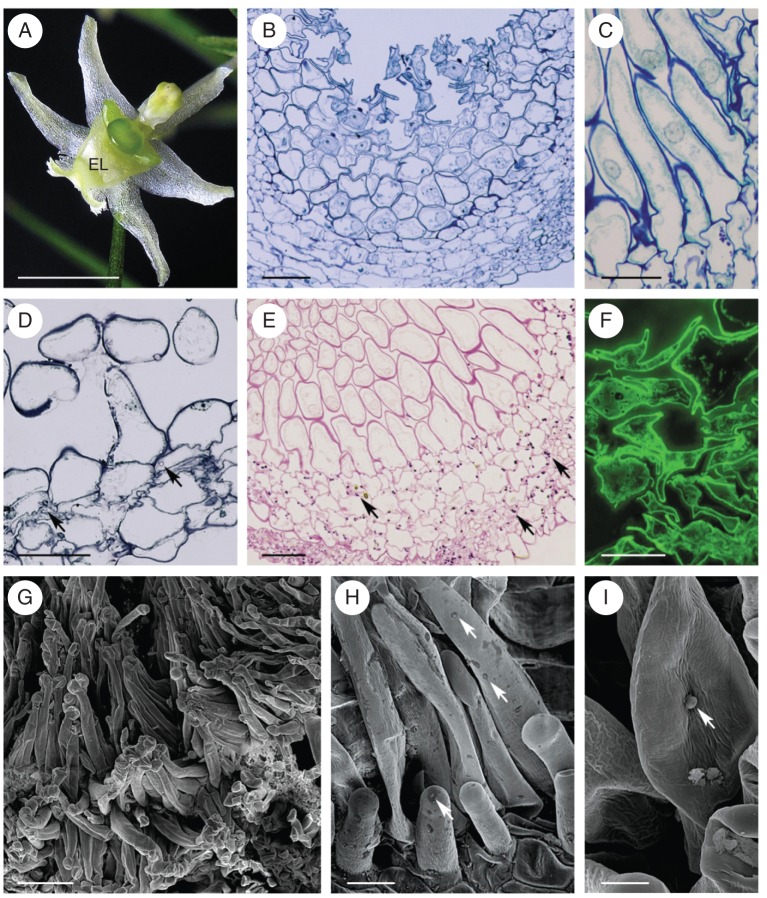
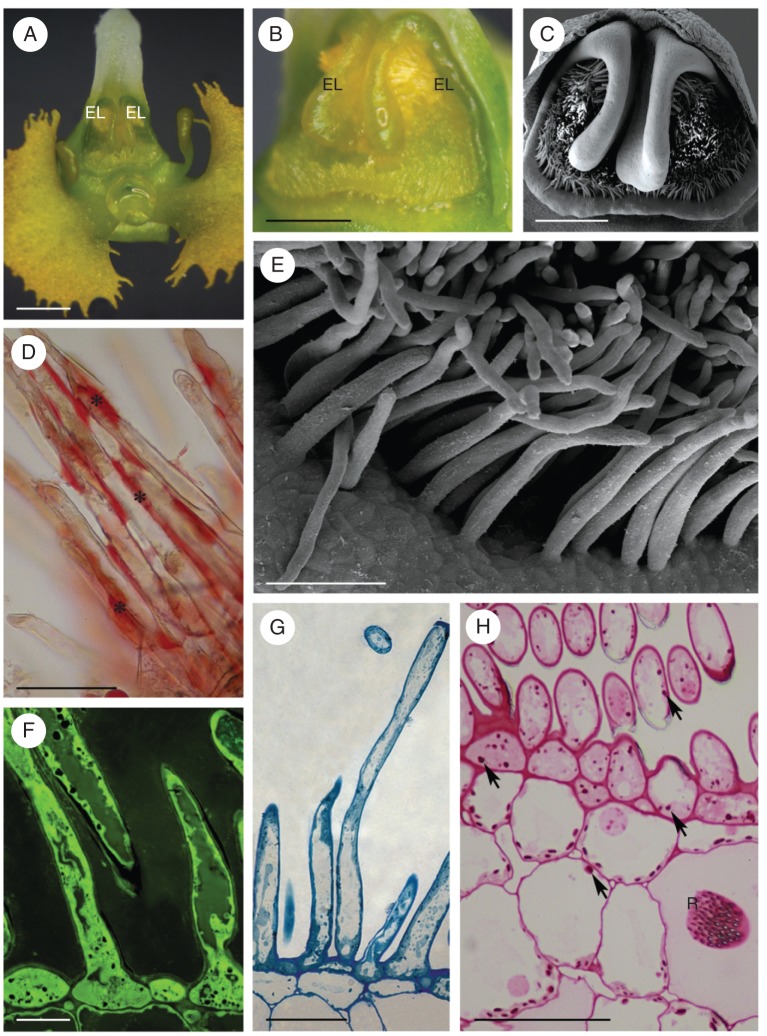
The sepals and petals of this species are greenish-white and the callus is centrally located on the labellum. The callus comprises two parts: proximally, a green globular structure, and distally, a white, more elongate region (Fig. 3A). The latter, which functions as an elaiophore, is densely clothed with unicellular, capitate hairs (Fig. 3B–I). In section, the elaiophore is seen to consist of a hirsute epidermis, a single layer of small, flattened subepidermal cells and underlying ground parenchyma with idioblasts containing raphides (Fig. 3B–E). The elaiophore hairs are unicellular and range in length from 29–68 µm, at the bud stage, to 113·5 µm, at the end of anthesis. They have a centrally positioned nucleus, parietal cytoplasm and a large vacuole (Fig. 3C). Their cell walls, which at the bud stage tended to be folded (Fig. 3B, F, G), became increasingly smooth at anthesis (Fig. 3C–E, H, I).
Fig. 3.
Phymatidium falcifolium, LM and SEM. (A) Habit of flower with elaiophore located on elongate part of labellar callus. (B) Section of the elaiophore at bud stage, with irregularly shaped hairs. (C) Hairs at anthesis, with centrally positioned nucleus and parietal cytoplasm. (D, E) Plastids at anthesis contain starch (arrows). Sections stained with methylene blue–azure II (D) and with PAS (E). (F) Secretory hairs at bud stage. Secretion stained yellow-green with auramine O. (G) Tuft of capitate hairs upon callus surface. (H) Detail of the hair surface with intact cuticle and traces of secretion (arrows). (I) Blistered cuticle (arrow) on surface of secretory hair. Scale bars: (A) = 2 mm; (B, D, E) = 50 µm; (C, F, H) = 20 µm; (G) = 100 µm; (I) = 10 µm. See Fig. 1 for abbreviations.
Secretory activity of the elaiophore commences at the early bud stage and lasts until the end of anthesis. One week prior to opening, lipids were present in the floral bud, mainly in secretory hair cells and the subepidermal parenchyma. However, at anthesis, the surface of the elaiophore had also become coated with surface secretion. Cuticular pores or cracks were not observed for secretory cells. The cuticle consisted of an outer lamellate and an inner reticulate layer. The surface secretion stained with Sudan III and with auramine O (Fig. 3F). Plastids in parenchyma cells contained starch, both at the bud stage and at the beginning of anthesis, but fewer starch grains were present in secretory hairs (Fig. 3D, E). Moreover, at the final stage of anthesis, there was a reduction in the amount of starch. During this stage, starch was found exclusively in cells of the ground parenchyma.
Zygostates grandiflora
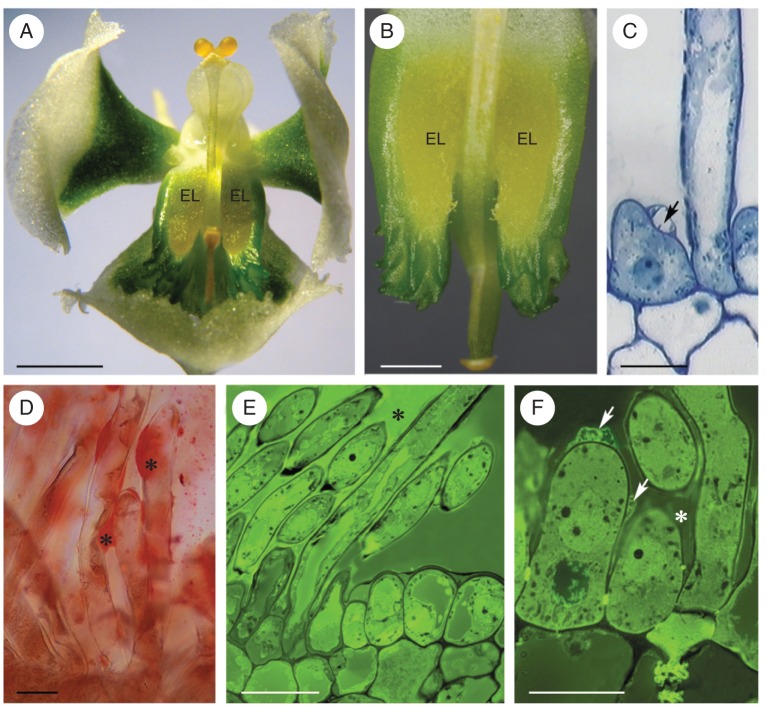
This species has whitish-green flowers, and the elaiophore is located in a shallow depression of the labellar callus (Fig. 4A, B). At the bud stage, the elaiophore was green, but at anthesis it became yellow and, thus, contrasted markedly with the green background. The entire surface of the elaiophore was clothed with unicellular hairs (Fig. 4C–F) that varied in length from 60–95 µm at the bud stage to 283 µm by the end of anthesis. Beneath the hirsute epidermis occurred a single layer of small parenchymatous cells, overlying ground parenchyma that contained vascular bundles. Raphides were frequently observed in idoblasts scattered throughout the ground parenchyma.
Fig. 4.
Zygostates grandiflora LM. (A) Habit of flower with elaiophores symmetrically located upon labellum. (B) Tuft of yellow elaiophore hairs. (C) Secretory hair and epidermal cell with blistered cuticle (arrow). (D) Lipid coating secretory hairs (asterisks) stained with Sudan III. (E, F) Secretory hairs with secretion (asterisks) stained green-yellow with auramine O. In (F) the blistered cuticle is indicated by arrows. Scale bars: (A) = 5 mm; (B) = 3 mm; (C, D, F) = 20 µm; (E) = 50 µm. See Fig. 1 for abbreviations.
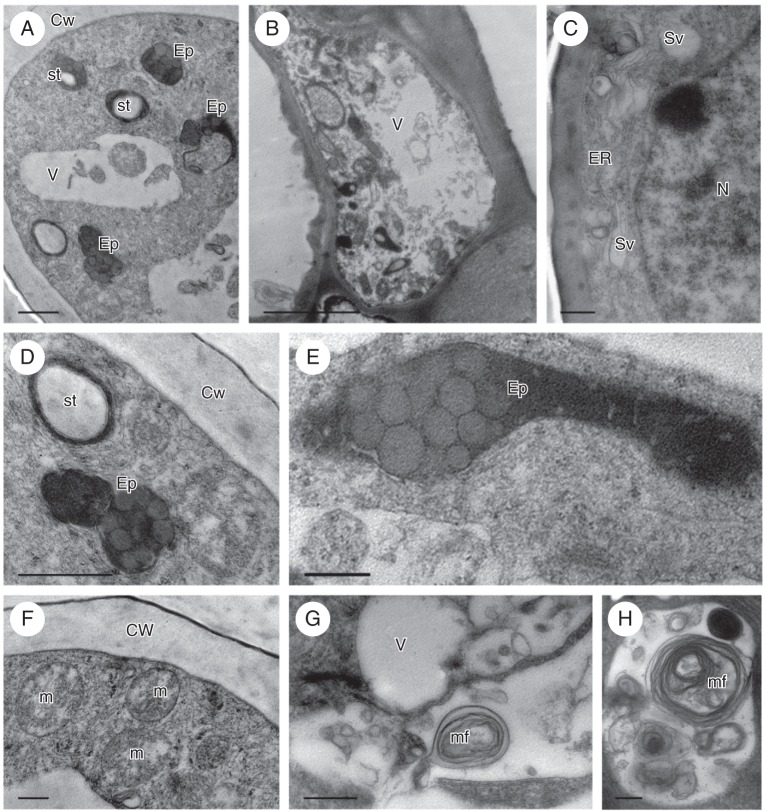
Although secretion was absent 3 weeks before the flower opened, secretory activity was recorded in floral buds 1 week prior to anthesis. Copious secretion was present both on the surface and within secretory and subepidermal cells (Fig. 4D–F). The volume of secretion increased throughout the course of anthesis. Frequently, the cuticle of the secretory hairs became distended and formed blisters on the surface of the cell wall (Fig. 4C, F). The outer cell wall of hairs and glabrous epidermal cells possessed a lamellate cuticle, beneath which frequently occurred a strongly osmiophilic layer. This was present at all stages of secretory activity (Fig. 5A). Cavities were present in the cell walls, and these became particularly pronounced at anthesis (Fig. 5A, B). Secretion accumulated both in these cavities and beneath the cuticle, eventually resulting in the distension and rupture of the cuticle (Figs 4C, F and 5B). Very short ingrowths of the cell wall were often present (Fig. 5C, D), especially at the bud stage. The parietal cytoplasm of elaiophore hairs stained intensely with methylene blue–azure II solution (Fig. 4C). The nucleus was centrally located, and vacuoles of various sizes occurred at the base and apex of each hair, often with small vacuoles encircling a larger one. SER (Fig. 5A–G) and circular profiles of partly rough ER with strongly osmiophilic contents were present (Fig. 5C, G). Often, particularly at the final stage of anthesis, SER cisternae became dilated and irregular in outline, as they became laden with lipids (Fig. 5B). Free, spherical lipid droplets were also present, scattered throughout the cytoplasm (Fig. 5D, E). Membranes of endoplasmic reticulum (ER) were often closely associated with the plasmalemma (Fig. 5B–D). At anthesis, ER membranes frequently became modified, resulting in the formation of myelin-like figures (Fig. 5E, G) that often projected into the vacuole (Fig. 5H), as the tonoplast became increasingly invaginated. The elaioplasts of Z. grandiflora have a highly osmiophilic stroma, with few internal membranes, but numerous, small plastoglobuli and lipid droplets. Compared with the other three species investigated, they had a more regular, oval outline (Fig. 5B–D). Trichome plastids contained a single starch grain. Larger starch grains were often present during the bud stage, in parenchyma cells situated close to vascular bundles, but these gradually disappeared at anthesis. Mitochondria, dictyosomes, (Golgi apparatus) and numerous secretory vesicles were commonly observed in hair cells (Fig. 5B, E, F).
Fig. 5.
Zygostates grandiflora, TEM. (A) Cell wall with lamellate cuticle, beneath which occur an osmiophilic layer and a large wall cavity. (B) Cell wall cavities and blistered cuticle. The parietal cytoplasm contains abundant ER profiles, plastids and mitochondria. (C) Small protuberances or ingrowths of cell wall (arrows). The cytoplasm contains plastids, dictyosomes (Golgi apparatus), mitochondria and ER that is in close contact with the plasmalemma. (D) Lipid droplet associated with plastid, whereas the ER is continuous with the plasmalemma. Arrow indicates protuberance or ingrowth of cell wall. (E) Profiles of ER, mitochondria, lipid bodies and cytoplasmic, myelin-like figure. (F) Plastids, dictyosomes (Golgi apparatus), small vacuoles and predominantly smooth ER with dilated cisternae. (G) Detail of myelin-like figure formed in the cytoplasm from ER membranes. (H) Myelin-like figure extruding into vacuole. Scale bars: (A, C, E–H) = 0·5 µm; (B, D) = 1 µm. See Fig. 1 for abbreviations.
Zygostates lunata Lindl
As in the two last species, the elaiophore of Z. lunata is of the trichomal type, and is located upon the callus. It is yellow-green in colour and, thus, contrasts with the white labellum (Fig. 6A, B). It bears a tuft of unicellular hairs (Fig. 6C–H) whose surfaces glisten with a coating of oil. The hairs measure 110 µm at the bud stage and increase to 242 µm at the final stage of anthesis. In general, the elaiophore anatomy of Z. lunata is very similar to that of the other three species investigated.
Fig. 6.
Zygostates lunata, SEM and LM. (A) Habit of flower with yellow elaiophores symmetrically located upon labellar callus. (B) Tuft of yellow elaiophore hairs coated with secretion. (C) Elaiophore hairs, at bud stage, located on the callus. (D) Lipids on the surface of elaiophore hairs (asterisks), stained with Sudan III. (E) Elaiophore hairs with secretory residues on the surface of cell walls. (F) Yellow-green secretion both within and on the surface of secretory hairs, following staining with auramine O. (G) Grey lipid droplets in secretory hairs stained with methylene blue–azure II. (H) Staining with PAS reveals the presence of starch in the plastids of both secretory hairs and parenchyma cells (arrows). Scale bars: (A) = 1 mm; (B) = 0·5 mm; (C) = 0·4 mm; (D, G, H) = 50 µm; (E) = 90 µm; (F) = 20 µm. See Fig. 1 for abbreviations.
Secretion was present in closed buds some 4 weeks before opening, and the amount of lipid present increased throughout the course of anthesis. The secretion stained intensely with Sudan III and auramine O (Fig. 6D, F), and relatively large, irregularly shaped lipid droplets stained grey with methylene blue–azure II (Fig. 6G). Starch grains were present in the plastids of both hair and parenchyma cells (Fig. 6H) and were particular abundant near vascular bundles at the bud stage. At anthesis, the amount of starch present diminished significantly. TEM revealed that the plastids were predominantly elaioplasts. They were irregularly shaped and contained several large lipid droplets and a single, variously sized starch grain (Fig. 7A, B, D, E). Elaioplasts were present in cells during all the secretory stages investigated. The cytoplasm contained abundant SER, RER and mitochondria (Fig. 7A, C, D, F). Membranes of the ER were particularly abundant in the parietal cytoplasm and were often closely associated with the plasmalemma (Fig. 7C, D). Secretory vesicles also occurred close to the plasmalemma (Fig. 7C). Vacuoles increased in size from bud to final stage of anthesis and, during all stages of secretory activity, they enclosed myelin-like figures. These too increased in size as anthesis progressed (Fig. 7G, H). Cell wall cavities were not observed, but the cuticle of the secretory epidermis and hairs comprised a thin lamellate layer. Although the cuticle became locally distended, neither cuticular cracks nor pores were observed (Figs 6E and 7B).
Fig. 7.
Zygostates lunata, TEM. (A) Transverse section of apical part of secretory hair showing elaioplasts, plastids containing starch, vacuoles and granular cytoplasm. (B) Basal part of secretory hair with parietal cytoplasm containing numerous plastids and abundant ER profiles. (C) Parietal cytoplasm of hair with ER, secretory vesicles and nucleus. (D) Detail of (A) showing two kinds of plastid: the first almost completely occupied by a starch grain, the other, a typical elaioplast containing numerous lipid droplets. (E) Detail of elongate elaioplast containing numerous lipid droplets. (F) ER membranes and mitochondria in parietal cytoplasm of secretory hair. (G) Vacuoles containing membranes, small vesicles and myelin-like figures. (H) Vacuole enclosing numerous myelin-like figures. Scale bars: (A, D, G, H) = 1 µm; (B) = 50 µm; (C, E, F) = 0·50 µm. See Fig. 1 for abbreviations.
DISCUSSION
The position of elaiophores differs in the four species investigated. In O. gladiatus, which has an intermediate type of elaiophore, they are located symmetrically on the lateral lobes of the labellum, whereas in P. falcifolium, Z. grandiflora and Z. lunata, which have trichomal elaiophores, they occur upon the callus. Elaiophores of other Oncidiinae also occur on the lateral lobes of the labellum [e.g. Gomesa paranensoides M. W. Chase & N. H. Williams (as Oncidium paranaense Kraenzl.), Gomesa venusta (Drapiez) M. W. Chase & N. H. Williams (as Oncidium trulliferum Lindl.) and Trichocentrum cavendishianum (Bateman) M. W. Chase & N. H. Williams] or on the callus [e.g. Gomesa bifolia (Sims) M. W. Chase & N. H. Williams, Gomesa loefgrenii (as Oncidium loefgrenii) and Gomesa radicans (as Ornithophora radicans); Singer and Cocucci 1999; Stpiczyńska et al. 2007; Stpiczyńska and Davies 2008; Aliscioni et al. 2009]. Each of these species, with the exception of G. bifolia, has epithelial elaiophores.
The secretory tissue of O. gladiatus is composed of cuboidal epidermal cells and small epidermal hairs. A similar intermediate type of elaiophore was also recorded for G. bifolia (Aliscioni et al., 2009). By contrast, in Phymatidium falcifolium, Zygostates grandiflora and Zygostates lunata, oil is secreted by unicellular epidermal hairs. However, whereas those of P. falcifolium are capitate, those of the remaining species investigated here are not. Unicellular trichomes have also been recorded for O. ciliatus (as O. kruegerii; Pacek and Stpiczyńska, 2007). Elaiophore trichomes vary greatly in length. For example, in Z. grandiflora, the longest elaiophore hairs measured approx. 283 µm.
Regardless of whether the elaiophore is of the epithelial or trichomal type, a single layer of small, compactly arranged subsecretory parenchyma is present, and this probably supports the secretory process, since its cells frequently contain starch and/or lipid droplets. An identical layer has also been recorded for the elaiophores of other Oncidiinae (Pacek and Stpiczynska, 2007, Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008).
The elaiophore epidermal cells of the four species investigated are typical of secretory cells, in that they have strongly osmiophilic cytoplasm and relatively large, centrally located nuclei that are surrounded by numerous plastids. These plastids frequently contain starch, at least in the bud stage, as do those of other Oncidiinae (Stpiczyńska et al., 2007, Pacek and Stpiczyńska, 2007; Stpiczyńska and Davies, 2008; Davies and Stpiczyńska, 2009; Aliscioni et al., 2009). Plastids of the four species studied here accumulate numerous lipid droplets and, usually after starch depletion, they differentiate to form elaioplasts. The elaioplasts of Z. grandiflora were more or less spherical in shape and resembled those of Gomesa radicans and G. recurva R. Br. (Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008), in contrast to other species where they were somewhat elongate and less regular in shape. As well as lipid droplets, the elaioplasts contained osmiophilic plastoglobuli. These were frequently seen in Z. lunata, and may be involved in the synthesis of volatiles, since flowers of this species, unlike those of the other taxa investigated here, are highly fragrant. Both lipid droplets and numerous plastoglobuli have also been reported for other fragrant Oncidiinae, such as Gomesa crispa (Lindl.) Klotzsch ex Rchb.f., G. loefgrenii, G. radicans, G. recurva, O. cheirophorum Rchb.f., O. ornithorhynchum Kunth and T. cavendishianum (Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008; Davies and Stpiczyńska, 2008).
The presence of both fragrance and oil-reward in the same orchid flower may significantly increase the range of insect species that visit it, since for some potential pollinators, oil is the reward, for others, the fragrance, yet both groups use olfactory cues to locate the flower.
SER was the most abundant organelle in secretory cells of all four species investigated, but RER was also present, regardless of developmental stage of the flower, and both plastids and SER were involved in the synthesis of secreted lipids. The latter are transported, either directly along the ER or by secretory vesicles that originate in the ER, to the plasmalemma. Both organelles were present in the parietal cytoplasm of all four species, the ER and secretory vesicles being closely associated with the plasmalemma. Similar transport of secretion from ER to plasmalemma was proposed for nectar by Kronestedt-Robards and Robards (1991). The presence of secretory vesicles in the parietal cytoplasm has also been recorded for elaiophores of O. ornithorhynchum and T. cavendishianum (Stpiczyńska et al, 2007; Davies and Stpiczyńska, 2009) and, at present, a granulocrine mode of secretion by elaiophore cells cannot be ruled out.
In all the species investigated here, a lamellate cuticle was present on the outer cell wall of elaiophore secretory cells and, in the case of O. gladiatus and P. falcifolium, this was supported by a reticulate layer. A lamellate or stratified cuticle may facilitate cuticular blistering in response to the pressure exerted by the sub-cuticular accumulation of secreted material. In this way, the secreted material remains protected from the environment, but the thin layer of cuticle that is left provides purchase for alighting, oil-gathering insects. A lamellate cuticle also occurs in Gomesa bifolia, G. loefgrenii and G. venusta (Stpiczyńska et al., 2007; Davies and Stpiczyńska, 2009; Aliscioni et al., 2009). Variation in cuticle texture may provide space for secreted material (Jeffree, 1996). According to Buchmann (1987), the secretion produced by trichomal elaiophores is not covered by a protective cuticle. However, the present study does not support this assertion, in that cuticular blisters were observed for all species investigated. An osmiophilic layer was frequently observed just beneath the cuticle and, in section, this appeared to consist of globular elements that probably represent droplets of secreted material similar to those found in the elaiophores of Malpighiaceous Galphimia brasiliensis (L.) Adr. Juss. (Castro et al., 2001). The outer cell wall of the secretory cells of Z. grandiflora contained cavities that probably facilitated the accumulation of secretion and/or its transport across the wall. Elaiophore cell-wall cavities are also known to occur in other Oncidiinae, such as G. bifolia, G. loefgreni, G. venusta and T. cavendishianum, where they play a similar role (Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009).
Secretory activity in all species investigated here began at the bud stage, in some cases up to 4 weeks prior to anthesis. Elaiophore secretory activity at bud stage also occurs in O. ornithorhynchum (Davies and Stpiczyńska, 2009). The secretion of oil may also cause parts of the flower to reflect light and thereby attract potential pollinators. Moreover, its reflective and hydrophobic properties may protect the bud from scorch and desiccation at this vulnerable stage of its development. Unlike the nectar of many orchid and non-orchidaceous taxa (Nepi, 2007; Nepi and Stpiczyńska, 2008, and references therein), preliminary macroscopic investigations indicate that unused lipid secretions are not re-absorbed (either to conserve material and energy, or as a measure to reduce theft by non-pollinator insects). However, this requires further investigation. What is certain is that the trichomal elaiophores of representatives of the Ornithocephalus clade investigated here share many ultrastructural features with the epithelial elaiophores of other Oncidiinae. In view of this, coupled with the observation that the floral oils of all Oncidiinae investigated to date have a similar chemical composition, it is probable that the position and type of elaiophore, and possibly the structure of the overlying cuticle, all play an important role in pollinator selection in these oil-secreting orchids. For example, the type of elaiophore may be related to the size of the pollinator, those orchids with epithelial elaiophores being pollinated by relatively large insects that grasp the tabula infrastigmatica with their mandibles, straddle the flower and use their first or second pair of legs to brush the paired, lateral elaiophores. Conversely, those orchid species with delicate, centrally located, trichomal elaiophores may be visited by smaller bees whose brushing behaviour is gentler. Unfortunately, in the absence of adequate field data, this remains merely a hypothesis. Moreover, some taxa display atypical characters, such as elaiophore cell walls with ingrowths or cavities. This diversity raises questions about the origin of the elaiophore. Thus, it is proposed that future work should focus on other oil-secreting and nectariferous Oncidiinae, and that the anatomy and ultrastructure of their elaiophores and nectaries be compared.
ACKNOWLEDGEMENTS
This project was supported financially by the Ministry of Science and Higher Education, Republic of Poland/National Science Center via grant N N303 602938 to A.P. and M.S.
LITERATURE CITED
- Aliscioni SS, Torretta JP, Bello ME, Galati BG. Elaiophores in Gomesa bifolia (Sims) M.W. Chase & N.H. Williams (Oncidiinae: Cymbidieae: Orchidaceae): structure and oil secretion. Annals of Botany. 2009;104:1141–1149. doi: 10.1093/aob/mcp199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–396. [Google Scholar]
- Brummitt RK, Powell CE. Authors of plant names. Kew: Royal Botanic Gardens; 1992. [Google Scholar]
- Castro MA, Vega AS, Mulgura ME. Structure and ultrastructure of leaf and calyx glands in Galphimia brasiliensis (Malpighiaceae) American Journal of Botany. 2001;88:1935–1944. [PubMed] [Google Scholar]
- Chase MW. Classification of Orchidaceae in the age of DNA data. Curtis's Botanical Magazine. 2005;22:2–7. [Google Scholar]
- Chase MW, Barret RL, Cameron KN, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Sabah, Malaysia: Natural History Publications (Borneo); 2003. pp. 69–89. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination: America, Africa, Asia and Australia. Rotterdam, The Netherlands: A.A. Balkema Publishers; 2001. [Google Scholar]
- Davies KL. Food-hair form and diversification in orchids. In: Kull T, Arditti J, Sek Man Wong, editors. Orchid Biology – reviews and perspectives. Dordrecht: Springer Science+Business Media BV; 2009. pp. 159–184. Vol. X. [Google Scholar]
- Davies KL, Stpiczyńska M. The anatomical basis of floral, food-reward production in Orchidaceae. In: Teixeira da Silva JA, editor. Floriculture, ornamental and plant biotechnology: advances and topical issues. 1st edn. Vol. 5. Isleworth, UK: Global Science Books; 2008. pp. 392–407. [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative histology of floral elaiophores in the orchids Rudolfiella picta (Schltr.) Hoehne (Maxillariinae sensu lato) and Oncidium ornithorhynchum H.B.K. (Oncidiinae sensu lato) Annals of Botany. 2009;104:221–234. doi: 10.1093/aob/mcp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Harvard University Press; 1990. [Google Scholar]
- Gahan PB. Plant histochemistry and cytochemistry: an introduction. London: Academic Press; 1984. [Google Scholar]
- Jeffree CE. Structure and ontogeny of plant cuticles. In: Kerstiens G, editor. Plant cuticles: an integrated functional approach. Oxford: BIOS Scientific Publishers; 1996. pp. 33–82. [Google Scholar]
- Jensen WA. Botanical histochemistry: principle and practice. San Francisco, CA: W.H. Freeman; 1962. [Google Scholar]
- Kronestedt-Robards E, Robards AW. Exocytosis in gland cells. In: Hawes CR, Coleman JOD, Evans DE, editors. Endocytosis, exocytosis and vesicle traffic in plants. Cambridge: Cambridge University Press; 1991. pp. 199–232. [Google Scholar]
- Nepi M. Nectary structure and ultrastructure. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and nectar. Dordrecht: Springer; 2007. pp. 129–166. [Google Scholar]
- Nepi M, Stpiczyńska M. The complexity of nectar: secretion and resorption dynamically regulate nectar feature. Naturwissenshaften. 2008;95:117–184. doi: 10.1007/s00114-007-0307-2. [DOI] [PubMed] [Google Scholar]
- Pacek A, Stpiczyńska M. The structure of elaiophores in Oncidium cheirophorum Rchb.f and Ornithocephalus kruegeri Rchb.f. (Orchidaceae) Acta Agrobotanica. 2007;60:9–14. [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1969. [Google Scholar]
- Reis MG, de Faria AD, Bittrich V, Amaral MCE, Marsaioli AJ. The chemistry of flower rewards: Oncidium (Orchidaceae) Journal of the Brazilian Chemical Society. 2000;11:600–608. [Google Scholar]
- Reis MG, de Faria AD, Amaral MCE, Marsaioli AJ. Oncidinol – a novel diacylglycerol from Ornithophora radicans Barb. Rodr. (Orchidaceae) floral oil. Tetrahedron Letters. 2003;44:8519–8523. [Google Scholar]
- Reis MG, Singer RB, Gonçalves R, Marsaioli AJ. The chemical composition of Phymatidium delicatulum and P. tillandsioides (Orchidaceae) floral oils. Natural Product Communications. 2006;1:757–761. [Google Scholar]
- Renner SS, Schaefer H. The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philosophical Transactions of the Royal Society: Biological Sciences. 2010;365:423–435. doi: 10.1098/rstb.2009.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera KI. 2002 Adaptive radiation of oil-rewarding compounds among Neotropical orchid species (Oncidiinae). M.Sc. Thesis, University of Florida, USA. [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana. 1999;14:47–56. [Google Scholar]
- Singer RB, Marsaioli AJ, Flach A, Reis MG. The ecology and chemistry of pollination in Brazilian orchids: recent advances. In. In: Teixeira da Silva JA, editor. Floriculture, ornamental and plant biotechnology: advances and topical issues. London: Global Science Books; 2006. pp. 569–582. Vol. IV. [Google Scholar]
- Stpiczyńska M, Davies KL. Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst (Oncidiinae: Orchidaceae) Annals of Botany. 2008;101:375–384. doi: 10.1093/aob/mcm297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Elaiophore diversity in three contrasting members of Oncidiinae Benth. (Orchidaceae) Botanical Journal of the Linnean Society. 2007;155:135–148. [Google Scholar]
- Toscano de Brito AVL. Systematic review of the Ornithocephalus group (Oncidiinae: Orchidaceae) with comments on Hofmeisterella. Lindleyana. 2001;16:157–217. [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen. Abhandlungen Akademie Wissenschaften Mathematisch-Naturwissenschaften Klasse Tropische und Subtropische Pflanzenwelt. 1974;7:1–267. [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae) Lindleyana. 2001;16:113–139. [Google Scholar]