Abstract
Recently, bitter taste receptors (TAS2Rs) were found in the lung and act to relax airway smooth muscle (ASM) via intracellular Ca2+ concentration signaling generated from restricted phospholipase C activation. As potential therapy, TAS2R agonists could be add-on treatment when patients fail to achieve adequate bronchodilation with chronic β-agonists. The β2-adrenergic receptor (β2AR) of ASM undergoes extensive functional desensitization. It remains unknown whether this desensitization affects TAS2R function, by cross talk at the receptors or distal common components in the relaxation machinery. We studied intracellular signaling and cell mechanics using isolated human ASM, mouse tracheal responses, and human bronchial responses to characterize TAS2R relaxation in the context of β2AR desensitization. In isolated human ASM, magnetic twisting cytometry revealed >90% loss of isoproterenol-promoted decrease in cell stiffness after 18-h exposure to albuterol. Under these same conditions of β2AR desensitization, the TAS2R agonist chloroquine relaxation response was unaffected. TAS2R-mediated stimulation of intracellular Ca2+ concentration in human ASM was unaltered by albuterol pretreatment, in contrast to cAMP signaling, which was desensitized by >90%. In mouse trachea, β2AR desensitization by β-agonist amounted to 92 ± 6.0% (P < 0.001), while, under these same conditions, TAS2R desensitization was not significant (11 ± 3.5%). In human lung slices, chronic β-agonist exposure culminated in 64 ± 5.7% (P < 0.001) desensitization of β2AR-mediated dilation of carbachol-constricted airways that was reversed by chloroquine. We conclude that there is no evidence for physiologically relevant cross-desensitization of TAS2R-mediated ASM relaxation from chronic β-agonist treatment. These findings portend a favorable therapeutic profile for TAS2R agonists for the treatment of bronchospasm in asthma or chronic obstructive lung disease.
Keywords: asthma, airway smooth muscle, β2-adrenergic receptor desensitization, chloroquine, bitter taste receptor
β-agonists are routinely utilized for the treatment of asthma, for acute therapy for bronchospasm, and for preventative or maintenance therapy when chronically administered. These agents relieve airflow obstruction by activating β2-adrenergic receptors (β2ARs), which are G protein-coupled receptors (GPCRs) expressed on airway epithelial and airway smooth muscle (ASM) cells (6, 44). β2ARs signal to Gαs, which activates adenylyl cyclase, thereby increasing intracellular cAMP and activating protein kinase A (PKA) (7, 16). PKA phosphorylation of myosin light chain kinase and related proteins results in ASM relaxation and bronchodilation (2, 24, 42). Human studies have shown that chronic administration of standard doses of β-agonist to subjects decreases β2AR expression and agonist-stimulated generation of cAMP in circulating mononuclear cells (9) and in airway epithelial cells and macrophages obtained by bronchoalveolar lavage (43). In the treatment of obstructive lung disease, chronic β-agonist use has been associated with tachyphylaxis (loss of clinical efficacy) (15, 29). In addition, increased bronchial hyperreactivity (11, 25) and adverse effects, including death (8, 20, 39, 40), have been observed in various clinical trials of chronic β-agonists. It should be noted, though, that none of these effects have been definitively linked to β-agonist-evoked regulation in ASM, although β2AR desensitization seems to be the most likely of these events to be mediated by such tachyphylaxis (19, 27).
For GPCRs, agonist-promoted desensitization is defined as a loss of function during persistent agonist exposure. When function is defined as coupling of β2AR to Gs with activation of adenylyl cyclase, β2AR desensitization is often quantified in terms of generation of the second-messenger cAMP. However, in a broader context relevant to bronchodilation, desensitization can be defined as a loss of physiological function, which is a failure to relax ASM. In this regard, when pathways from different classes of receptors ultimately converge on the same physiological function, desensitization can occur at points distant from the initial agonist-receptor coupling event. Furthermore, desensitization can occur via cross talk between two pharmacologically distinct GPCRs (28, 31). Such interactions can take place via a number of interactions, including second-messenger-promoted kinase activation, such as PKA and protein kinase C (27).
Using RNA microarrays, the complement of GPCRs on human ASM has been shown to be much greater than previously recognized (22) and has led to consideration of other receptors whose activation might lead to relaxation. One such class of receptors is the bitter taste receptors (TAS2Rs), some of which are expressed at levels greater than β2AR (18). TAS2Rs promote relaxation via specialized intracellular Ca2+ concentration ([Ca2+]i) signaling, leading to membrane hyperpolarization via the large-conductance, calcium-dependent K+ channel (BKCa) (18) and potentially other mechanisms (5). In isolated human bronchi, TAS2R agonist-mediated relaxation appears to be equal to or somewhat greater than that of the full β-agonist isoproterenol (17, 18). Given the large number of known bitter tastants (35), these findings have given rise to the notion that these agents might be utilized for the treatment of obstructive lung disease (18). Of particular interest might be bitter tastant use when β-agonist treatment has failed, such as under conditions of tachyphylaxis. β2AR and TAS2R relax ASM by different mechanisms, but, as introduced earlier, since they converge on a final common physiological function, there is the potential for the muscle that has become desensitized to the relaxation effects of β-agonists to be poorly responsive as well to TAS2R agonists. Here, we ascertain this potential using isolated human ASM mechanics and intracellular signaling in vitro, and intact human and mouse airways ex vivo, under conditions of β-agonist tachyphylaxis.
MATERIALS AND METHODS
Materials.
Reagents were obtained from Sigma (St. Louis, MO), with the exception of Dulbecco's modified Eagles's medium (DMEM)-Ham's F-12 (1:1), which was purchased from GIBCO (Grand Island, NY). The synthetic arginine-glycine-aspartic acid (RGD) containing peptide was purchased from American Peptide (Sunnyvale, CA). Albuterol, isoproterenol, salmeterol, acetylcholine, carbachol, and methacholine were reconstituted in either sterile distilled water or DMSO, frozen in aliquots, and diluted appropriately in serum-free media on the day of use.
ASM cell culture and characterization.
Human bronchi were obtained from lungs unsuitable for transplantation, in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings. Human ASM cells were prepared from these bronchi, as described previously (14), or were obtained from the fourth- and fifth-order bronchi of surgical lobectomies and pneumonectomies performed for malignancy. Cells were grown until confluence at 37°C in humidified air containing 5% CO2 and passaged with 0.25% trypsin-0.02% EDTA solution every 10–14 days. ASM cells in culture were elongated and spindle shaped, grew with the typical hill-and-valley appearance, and showed positive staining for smooth muscle-specific α-actin. In this study, we used cells in passages 3–7, as these cells retain native contractile protein expression (37). Unless otherwise specified, serum-deprived postconfluent cells were plated at 30,000 cells/cm2 on plastic wells (96-well Removawell, Immunlon II: Dynetech) previously coated with type I collagen (Vitrogen 100; Cohesion, Palo Alto, CA) at 500 ng/cm2. Cells were maintained in serum-free media for 24 h at 37°C in humidified air containing 5% CO2. These conditions have been optimized for seeding cultured cells on collagen matrix and for assessing their mechanical properties (3, 4, 18).
Magnetic twisting cytometry.
Dynamic changes in stiffness were measured as an indicator of contraction and relaxation of isolated human ASM cells, using magnetic twisting cytometry (MTC), as described by our laboratory in detail elsewhere (3, 18). In brief, RGD-coated ferrimagnetic microbeads (4.5 μm in diameter) bound to the surface of adherent human ASM cells were magnetized horizontally and then twisted in a vertically aligned homogenous magnetic field that was varying sinusoidally in time. This sinusoidal twisting magnetic field caused both a rotation and a pivoting displacement of the bead: as the bead moves, the cell develops internal stresses, which, in turn, resist bead motions (23). Lateral bead displacements in response to the resulting oscillatory torque were detected with a spatial resolution of ∼5 nm, and the ratio of specific torque to bead displacements was computed and expressed here as the cell stiffness in units of Pascal per nanometer (Pa/nm).
In this study, adherent human ASM cells were treated for 18 h, with or without 1 μM albuterol. In both albuterol-treated and -untreated ASM, methacholine increased cell stiffness in a dose-dependent manner, with a maximal response at 10 μM, which was not different between treated or untreated cells (data not shown). Unless otherwise stated, ASM cells were contracted with 10 μM methacholine, which was maintained during addition of multiple doses of isoproterenol or chloroquine. For each individual human ASM cell, changes in stiffness in response to isoproterenol or chloroquine were normalized to its respective methacholine-contracted stiffness.
[Ca2+]i and cAMP measurements.
For detecting changes in [Ca2+]i, adherent cells in 96-well plates were loaded with Fluo-4 AM (BD Biosciences) and probenecid for 1 h at 37°C. Receptor agonists were added by an automated pipetting system in triplicate, and the 525-nm signals were generated by excitation at 485 nm using a Flex Station II (Molecular Devices). Data were acquired every 1.5 s for 1 min. Unless otherwise stated, studies were performed in media containing 1.5 mM calcium. For cAMP measurements, human ASM cells were plated in 96-well plates and detected using a fluorescence-based assay (CatchPoint, Molecular Devices).
Ex vivo intact mouse airway physiology.
All mouse studies were approved by the Animal Care and Use Committee of the University of Maryland, School of Medicine. As described previously (18), we excised 5-mm sections of trachea from FVB/N mice (Taconic) and studied them in an isometric myograph system. In brief, tracheal rings fitted between a fixed wire and a transducer-coupled wire were maintained in Krebs buffer saturated with 95% O2/5% CO2 at 37°C. A passive tension of 5 mN was applied for each ring for a baseline. For relaxation studies, rings were contracted with acetylcholine (0.1 mM), which was maintained during addition of isoproterenol (10 μM) or chloroquine (1 mM). Then, rings were washed, removed from tension, and incubated with isoproterenol (10 μM) in Krebs buffer with 0.1 mM ascorbic acid for 18 h at 37°C in a 5% CO2/95% air atmosphere. Rings were then washed, contracted with acetylcholine, and relaxed again with isoproterenol or chloroquine, as indicated above. Desensitization was calculated as the percent loss of relaxation of the post-18-h isoproterenol-exposed rings compared with the preexposed rings (control), with each ring acting as its own control.
Human precision-cut lung slice preparation and airway function.
Healthy human lungs were received from the National Disease Research Interchange, and the human precision-cut lung slices (PCLSs) were prepared as previously described (12, 13). Human PCLSs (∼750 μm thick) were then maintained in 24-well plates with continuous agitation in 0.5-ml culture medium (DMEM/F12) supplemented with 15 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml of amphotericin B at 37°C in humidified air containing 5% CO2. After at least 48 h in culture, lung slices were washed and incubated for 18 h in the absence or presence of 50 nM salmeterol or 1 μM albuterol. Slices were then washed four times. After collecting a baseline quantitative microscopic image (under conditions of 0% contraction), human PCLSs were constricted with 1 μM carbachol and then treated sequentially with 1 μM isoproterenol and 50 μM chloroquine. For each treatment, airway images were collected after 15–20 min, and the lumenal area measured using a program written within Image J software. Bronchodilation was calculated as the percent reversal of the carbachol-induced reduction in airway cross-sectional area.
Statistical analysis.
For MTC, cAMP, and mouse trachea studies, we used Student's t-test, the ANOVA, with adjusting for multiple comparisons by applying the Bonferroni methods, or nested design analysis to control any random effect caused by repeated measurements of multiple cells (MTC) in the same subject. To satisfy the normal distribution assumptions associated with ANOVA, cell stiffness data were converted to log scale before analyses. For PCLS, analysis was by ANOVA using the Holm-Sidak method for pairwise multiple comparisons. All analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC), and the two-sided P values < 0.05 were considered significant.
RESULTS
Chloroquine is highly efficacious in decreasing the stiffness of human ASM cells.
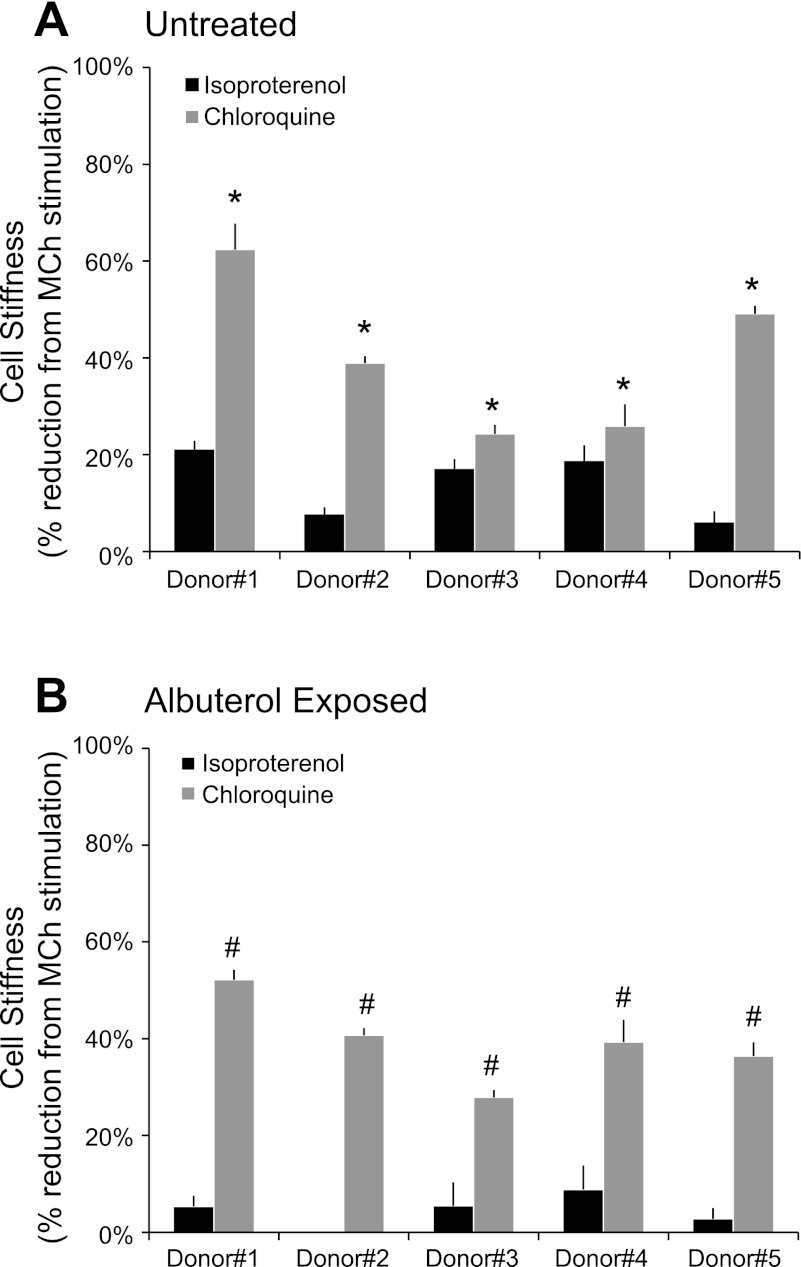
We established primary human ASM cell cultures from five separate lungs and, using MTC, measured their mechanical responses to the β2AR agonist isoproterenol and a selective TAS2R agonist chloroquine. For these studies, ASM cells were first contracted for 5 min with methacholine (10 μM) and then relaxed with increasing doses of either isoproterenol or chloroquine. In methacholine-contracted ASM cells, maximal decreases in stiffness occurred with 10 μM isoproterenol and 1 mM chloroquine, respectively, consistent with our laboratory's previously reported responses under other conditions (18). Human ASM cells isolated from the lung donors showed some degree of between-cell and -donor variation in isoproterenol- and chloroquine-induced stiffness responses (Fig. 1, A and B). Nevertheless, the maximal reduction in cell stiffness induced by chloroquine was greater in magnitude than isoproterenol, with differences ranging from ∼7 to 44% among individual lung donors (Fig. 2A). Applying nested design analysis, we found statistical differences between the effects of chloroquine and isoproterenol on cell stiffness as early as 6 s (P < 0.0001 vs. isoproterenol) upon and throughout drug stimulation, with the maximal differences at time 87 s (P < 0.00005 vs. isoproterenol). In addition, there were significant differences (P < 0.00001) in the rate of stiffness decreases between isoproterenol (−0.00018/s) and chloroquine (−0.00251/s). These findings demonstrate that TAS2R activation by chloroquine has a faster onset of action and greater efficacy than the full β2-agonist isoproterenol in relaxing methacholine-contracted human ASM.
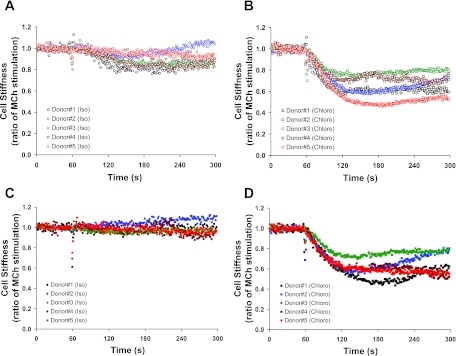
Fig. 1.
Dynamic changes of cell stiffness in response to a full β2-adrenergic receptor (β2AR) agonist isoproterenol (Iso) and a selective bitter taste receptor (TAS2R) agonist chloroquine (Chloro) in human airway smooth muscle (ASM), as assessed by Magnetic Twisting Cytometry. Primary human ASM cell cultures were established from 5 separate lung donors. Isolated human ASM cells were untreated (A and B) or treated for 18 h with 1 μM albuterol (C and D). Cells were contracted with 10 μM methacholine (MCh) and then relaxed with 10 μM Iso (A and C) or 1 mM Chloro (B and D). For each cell, changes in stiffness in response to Iso or Chloro were normalized to its respective MCh-contracted stiffness. Values are presented as median (A: n = 53–266; B: n = 62–281; C: n = 58–248; D: n = 70–233 individual cell measurements).
Fig. 2.
Maximum stiffness reduction of MCh-contracted human ASM induced by Iso and Chloro in untreated (A) and albuterol-exposed (B) cells. Values are means ± SE (A: n = 53–281; B: n = 58–248 individual cell measurements). *P < 0.01; #P < 0.00005.
Prolonged exposure to albuterol induces β2AR desensitization in isolated human ASM.
To induce β2AR desensitization (13), we treated isolated human ASM cells for 18 h with 1 μM albuterol. Subsequently, cells were washed, the response to methacholine ascertained, and then their mechanical responses to the β2AR agonist isoproterenol and a selective TAS2R agonist chloroquine were measured. Albuterol-treated and -untreated cells exhibited differential cellular responses to isoproterenol (Fig. 1, A and C). In albuterol-untreated cells, isoproterenol (10 μM) caused stiffness decreases that ranged from ∼8 to 21% reduction from the methacholine-contracted state (Fig. 2A). Ten micromoles of this full β2-agonist isoproterenol had little effect on the stiffness of albuterol-treated cells contracted with methacholine, even at higher doses of isoproterenol (data not shown). These findings are consistent with previous findings in human lung slices (13) and demonstrate a direct effect, independent of the epithelium, of long-term β-agonist exposure on receptor desensitization in isolated human ASM cells. As shown in Fig. 1, this extensive β2AR desensitization by β-agonist was observed with cells from each of the five donors.
β2AR desensitized in human ASM cells are fully responsive to chloroquine.
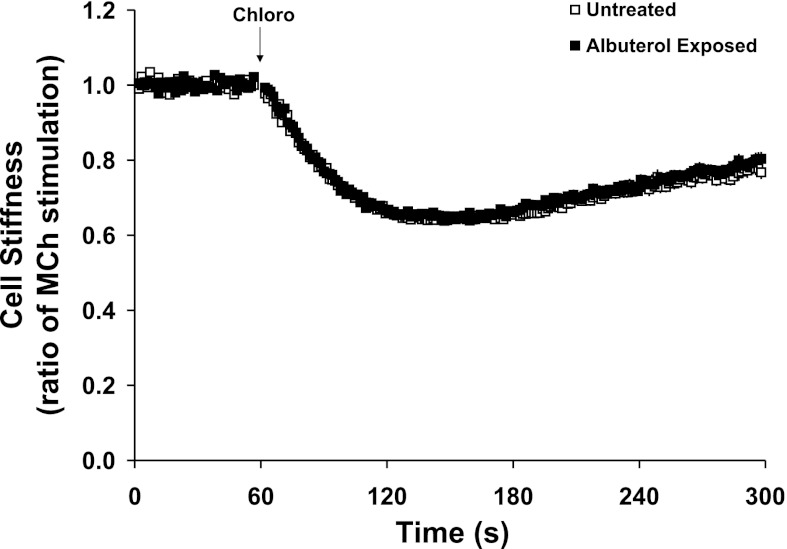
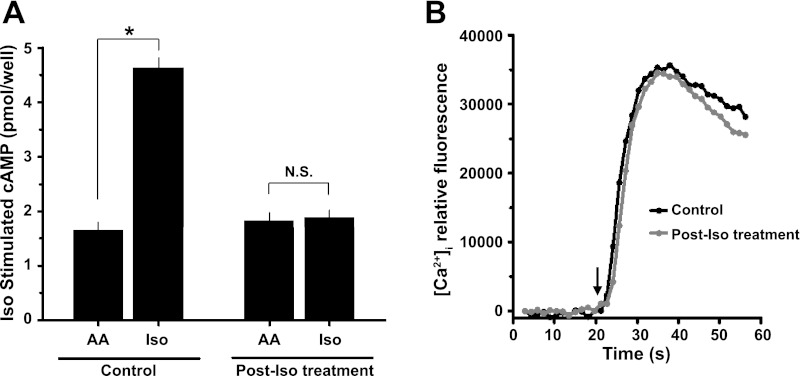
Under these same conditions that essentially eliminated the effectiveness of a full β2AR agonist isoproterenol, we found that a selective TAS2R agonist chloroquine (1 mM) substantially decreased stiffness of albuterol-treated cells from each donor (Fig. 1, B and D, and Fig. 2). In fact, chloroquine evoked nearly identical degrees of reduction in stiffness of both albuterol-treated and -untreated human ASM cells (Fig. 3), amounting to 39 ± 3.9 and 40 ± 7.2% maximal reduction from their respective methacholine-contracted state (P = nonsignificant). These results indicate a lack of physiologically relevant cross-desensitization between the β2AR pathway and the TAS2R pathway. To further confirm this notion, human ASM in culture were exposed to carrier (ascorbic acid) or β-agonist for 18 h and washed, and cAMP (the β-agonist response) or [Ca2+]i (the TAS2R response) was measured with exposure to isoproterenol or chloroquine, respectively. As shown in Fig. 4A, long-term β-agonist exposure markedly desensitized (>90%) the isoproterenol-stimulated cAMP response, but did not alter chloroquine-mediated [Ca2+]i release (Fig. 4B).
Fig. 3.
Dynamic changes of cell stiffness in response to 1 mM Chloro in untreated and albuterol-exposed human ASM. To control random effects due to multiple cell measurements from the same donor, the nested regression was used for group comparisons. Values are means ± SE (n = 775–859 individual cell measurements from 5 lung donors).
Fig. 4.
β2AR desensitization and intracellular signaling in isolated human ASM cells. A: cultured primary human ASM cells were incubated for 18 h with ascorbic acid (AA) or β-agonist (Iso), and cAMP was measured by CatchPoint assay (Molecular Devices). Iso-mediated accumulation of cAMP (15 min) is abrogated in human ASM cells that were pretreated for 18 h with β-agonist, but not in untreated cells (means ± SE, n = 4 experiments). NS, nonsignificant. *P < 0.01. B: human ASM cells were loaded with Fluo-4 AM, and intracellular Ca2+ concentration ([Ca2+]i) release evoked by TAS2R activation was measured. Arrow indicates the time of Chloro addition. [Ca2+]i transient curves to 1 mM Chloro is unabated in human ASM cells that were pretreated for 18 h with β-agonist. Results shown are from a single representative experiment of 3 performed.
Chloroquine responsiveness during β2AR desensitization in intact airways.
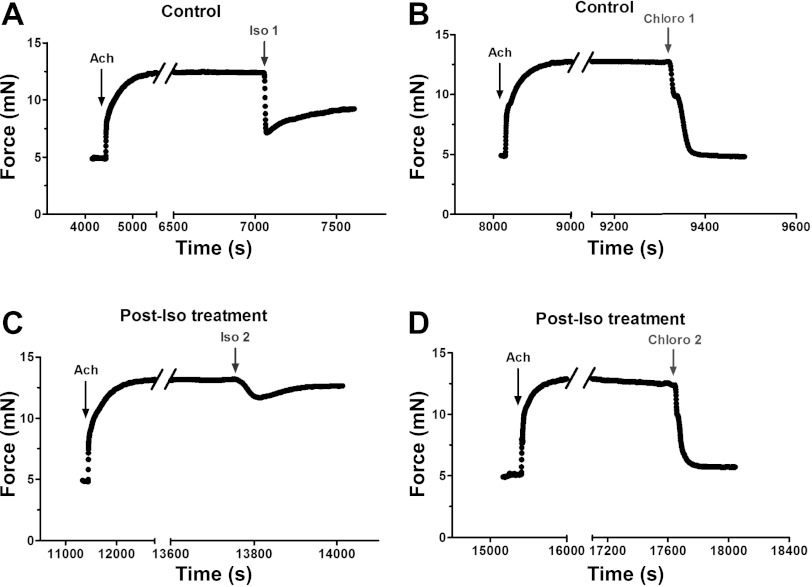
We next examined the efficacy of chloroquine in relaxing ASM of intact mouse airways, under conditions of β2AR desensitization. For each control tissue precontracted with a submaximal dose of acetylcholine, both isoproterenol and chloroquine decreased the force-generating capacity of mouse trachealis (Fig. 5, A and B). Consistent with our laboratory's previous report (18), however, chloroquine-promoted reduction in ASM tension was greater in magnitude than that of isoproterenol in tissues that had not been pretreated with β-agonist (Fig. 6). In tissues that were pretreated for 18 h with isoproterenol, and then washed, acetylcholine-induced tension development was similar in magnitude as in time-matched, untreated tissues (data not shown). Subsequent isoproterenol treatment, however, showed a significant decrease in relaxation (from 59 ± 5.2 to 5.3 ± 4.3%, P < 0.001), representing a 92 ± 6.0% desensitization of the response (Fig. 5, A and C). In contrast, as with isolated ASM measurements with MTC, chloroquine markedly decreased ASM tension in tissues that had been pretreated with β-agonist, with desensitization amounting to 11 ± 3.5%, which was not statistically significant (Fig. 5D). Indeed, the reduction in ASM tension with chloroquine was indistinguishable from that of time-matched, non-β-agonist-treated airways (Fig. 6).
Fig. 5.
TAS2R activation evokes ASM relaxation under conditions of β2AR desensitization. Intact mouse trachealis were studied in the absence (Control; A and B) or after 18 h of exposure to the β-agonist Iso (Post-Iso treatment; C and D). Rings were contracted with 0.1 mM acetylcholine (ACh) and relaxed with 10 μM Iso [Iso1 (A), Iso2 (C)]. After each Iso treatment, the ring was washed and then rechallenged with the same dose of ACh, followed by 1 mM Chloro addition [Chloro1 (B), Chloro2 (D)]. ACh was maintained in the bath when Iso and Chloro were added. Results shown are from a single representative experiment of at least 4 performed.
Fig. 6.
TAS2R activation is highly efficacious in relaxing ASM under conditions of β2AR desensitization. As described above in Fig. 4, intact mouse trachealis were studied before (Control) or after treatment with the β-agonist Iso (Post-Iso treatment). Subsequent Iso and Chloro responses were measured in rings contracted with ACh. Values are means ± SE (n = 4 experiments). *P < 0.01.
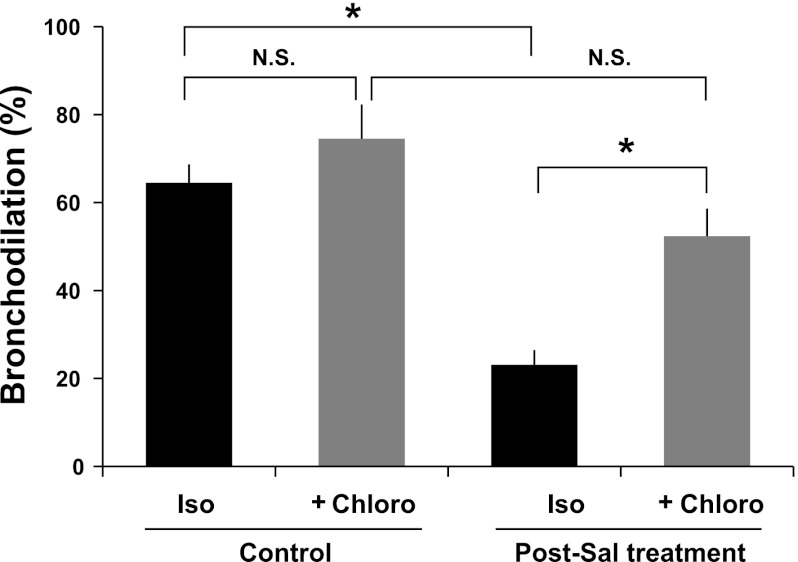
Based on our established intact human airways model, demonstrating decreased sensitivity to isoproterenol-mediated bronchodilation, following chronic exposure to β-agonists (12, 13) and compelling evidence for increased bronchial hyperresponsiveness with regular use of long-acting β-agonist salmeterol (11, 12, 45, 46), we incubated human PCLSs for 18 h in the absence (control) or presence of 50 nM salmeterol. This dosage of salmeterol is as equi-effective in bronchodilating PCLSs and in desensitizing β2AR when chronically administered, as 1 μM albuterol (12). For each control PCLS precontracted with a submaximal dose of carbachol, both isoproterenol and subsequent chloroquine increased the luminal area of these airways, amounting to 65 ± 4.2 and 75 ± 7.8% bronchodilation (P = nonsignificant), respectively, at doses that were 1/10th and 1/20th of those used for mouse tracheal rings (Fig. 7). In human PCLSs that were pretreated for 18 h with salmeterol, however, isoproterenol treatment showed 64 ± 5.7% desensitization of airway lumen dilation (P < 0.001 vs. control isoproterenol), and subsequent chloroquine treatment markedly bronchodilated these carbachol-constricted airways (Fig. 7). Chloroquine also effectively rescued desensitization in carbachol-constricted airways (73 ± 6.7% bronchodilation; mean ± SE, n = 4) that were chronically exposed to albuterol. Taken together, our findings in intact mouse and human airway physiology ex vivo and isolated human ASM mechanics and intracellular signaling in vitro demonstrate a consistent β2AR desensitization with chronic β-agonist exposure in ASM, regardless of the model. Despite such β2AR desensitization, however, we show no physiologically relevant cross-desensitization between the β2AR pathway and the TAS2R pathway, and that TAS2R activation is highly efficacious in relaxing ASM and bronchodilating the airways under conditions of β-agonist tachyphylaxis.
Fig. 7.
TAS2R activation dilates intact human small airways under conditions of β2AR desensitization. Human precision-cut lung slices (PCLSs) were incubated for 18 h in the absence (Control) or presence of long-acting β-agonist salmeterol (Post-Sal treatment), washed, and constricted with carbachol. Carbachol-constricted airways were treated sequentially with 1 μM Iso and 50 μM Chloro. Values are means ± SE (obtained using 102 PCLSs derived from 4 donors). *P < 0.005.
DISCUSSION
We have contrasted signaling and mechanical responses of primary human ASM cells and airway relaxation responses in human and mouse bronchi to a selective TAS2R agonist, chloroquine, in the context of the β-agonist-desensitized state. These studies were prompted by the recent finding that TAS2Rs are expressed on ASM and act to markedly relax ASM and bronchodilate the airways. These studies have brought to the forefront the potential for a new class of direct bronchodilators acting at TAS2Rs. These agents might be used in patients with asthma or chronic obstructive lung disease who have not achieved adequate control on chronic β-agonists, “indirect” bronchodilators (such as M3-receptor antagonists), and other agents. As introduced earlier, chronic β-agonist activation of β2AR is associated with clinical deterioration, which may be due to β2AR desensitization. Such desensitization, particularly when defined by the end-organ response, can act across multiple nodal points, including at the receptor, G protein, the “initial” effector (such as adenylyl cyclase), and more distal effectors. Cross talk has been shown among multiple GPCRs, even those with disparate structures and ligands (26, 30–33, 36). We thus felt it necessary to explore the potential for chronic β-agonist activation of β2AR on ASM to cross-desensitize TAS2R responses. Studies were carried out measuring intracellular signaling, isolated ASM mechanics, and intact mouse and human airways.
In studies of isolated ASM mechanics with MTC, chloroquine had a more rapid onset and greater efficacy than isoproterenol in decreasing stiffness of an individual human ASM cell from each lung donor. As previously shown in human lung slices (13), here we have also demonstrated β2AR desensitization in isolated human ASM cells with long-term exposure to β-agonists. Whereas this exposure inhibited generation of cAMP and completely abrogated the ability of an individual human ASM cell to decrease stiffness in response to isoproterenol, such receptor desensitization did not affect the efficacy of chloroquine. Chloroquine evoked intracellular calcium release and decreased stiffness of isolated human ASM cells, even with β-agonist-promoted β2AR desensitization. Scaling up to the level of intact airways ex vivo, chloroquine markedly inhibited force-generating capacity of mouse trachealis, as well as effectively bronchodilated human airways that exhibited tolerance to β-agonists. These findings further confirm that TAS2R activation utilizes a novel GPCR pathway that is distinct from Gq/Gs-mediated activation of downstream targets and suggest limited deleterious cross talk with β-agonists in promoting ASM relaxation.
Desensitization of GPCR signaling is frequently encountered in both in vitro and in vivo systems. Some receptors display little or no detectable desensitization (21), while others, such as the β2AR, undergo a marked decrease in function, as exhibited by measurements of the second-messenger cAMP (Fig. 4A) or the end-organ response (Figs. 2, 6, and 7). Our laboratory has previously shown only a modest degree of desensitization (20–30%) of TAS2R function with bitter tastant exposure in human ASM and monkey bronchus (38). At issue for the present studies is whether the markedly desensitized relaxation response evoked by prolonged β-agonist treatment affects the relaxation response to TAS2R activation. As discussed earlier, ample evidence suggests that interaction can occur between different GPCRs at multiple points within the signaling cascade, from initial receptor activation and coupling to G protein, to further downstream components. We show here no evidence for such interaction between chronic β-agonist evoked β2AR desensitization and TAS2R function in ASM, as determined by intracellular signaling measurements, single cell mechanics, and mouse and human airway relaxation responses. Importantly, our studies, derived from tissues retaining much of the native architecture of small human airways, provide further support for the idea that parallel pathways for bronchodilation exist that could be used therapeutically.
TAS2Rs belong to a family of GPCRs that is composed of 25 members (1, 10, 34), and, in human ASM, three dominant TAS2Rs (subtypes 10, 14, and 31) are detected at the mRNA and functional levels (18). TAS2Rs function in airways is mediated by [Ca2+]i release, but, nevertheless, leads to ASM relaxation and bronchodilation. This is based on a concordance of relaxation potency and efficacy with measured [Ca2+]i release, loss of TAS2R-mediated relaxation when [Ca2+]i release was blocked, and stimulation of spatially and temporally distinct [Ca2+]i events evoked by TAS2R agonists in intact ASM (18). In that study, we considered that the BKCa channels were activated and thus played some role in the relaxation response, based on a sensitivity of TAS2R-mediated relaxation of mouse airways, and individual ASM cells, to the BKCa-channel inhibitor iberiotoxin. This remarkable degree of ASM relaxation and its unique mechanism of action has led to the consideration of developing TAS2R agonists for treating obstructive lung disease. TAS2Rs are also expressed on airway epithelial cells and, upon activation, increase ciliary beat frequency (41), which may also have a therapeutic effect by increasing mucous clearance. Our laboratory has previously shown, using submaximal doses, that the acute relaxation effects of β-agonist and TAS2R agonist are additive (18). Thus concomitant use of both agents can be envisioned, providing two direct bronchodilators, which act via different mechanisms. We now know that, even under conditions of marked β2AR desensitization of the relaxation response, TAS2R agonists maintain full efficacy, which is a favorable property in consideration of taking forward TAS2R agonists as novel treatment options.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL107361 (to S. S. An), HL104119 (to W. C. H. Wang), HL097796 (to R. A. Panettieri, Jr.), and HL045967 and HL071609 (to S. B. Liggett). S. S. An was also supported by American Asthma Foundation (Sandler: 108183) grant, and R. A. Panettieri, Jr. by National Institute of Environmental Health Sciences Grant ES013508,. R. C. Kurten was supported by Division of Research Resources UL1RR029884. Human tissue used for this research project was provided by the National Disease Research Interchange through the generous gift of donor families.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.S.A., W.C.W., R.A.P., and S.B.L. conception and design of research; S.S.A., W.C.W., C.J.K.-W., D.Y.L., and R.C.K. performed experiments; S.S.A., W.C.W., C.J.K.-W., K.A., D.Y.L., R.C.K., and R.A.P. analyzed data; S.S.A., W.C.W., C.J.K.-W., K.A., R.C.K., R.A.P., and S.B.L. interpreted results of experiments; S.S.A., W.C.W., and R.C.K. prepared figures; S.S.A. and S.B.L. drafted manuscript; S.S.A., W.C.W., R.C.K., R.A.P., and S.B.L. edited and revised manuscript; S.S.A., W.C.W., C.J.K.-W., K.A., D.Y.L., R.C.K., R.A.P., and S.B.L. approved final version of manuscript.
REFERENCES
- 1. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell 100: 693– 702, 2000 [DOI] [PubMed] [Google Scholar]
- 2. An SS, Askovich PS, Zarembinski TI, Ahn K, Peltier JM, von Rechenberg M, Sahasrabudhe S, Fredberg JJ. A novel small molecule target in human airway smooth muscle for potential treatment of obstructive lung diseases: a staged high-throughput biophysical screening. Respir Res 12: 8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol 35: 55– 64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol 283: C792– C801, 2002 [DOI] [PubMed] [Google Scholar]
- 5. An SS, Robinett KS, Deshpande DA, Wang WC, Liggett SB. Reply to: Activation of BK channels may not be required for bitter tastant-induced bronchodilation. Nat Med 18: 650– 651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnes PJ. β-Adrenoceptors on smooth muscle, nerves and inflammatory cells. Life Sci 52: 2101– 2109, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Barnes PJ. New drugs for asthma. Nat Rev Drug Discov 3: 831– 844, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Beasley R, Pearce N, Crane J, Burgess C. β-Agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol 103: S18– S30, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Brodde OE, Brinkmann M, Schemuth R, O'Hara N, Daul A. Terbutaline-induced desensitization of human lymphocyte β2-adrenoceptors. Accelerated restoration of β-adrenoceptor responsiveness by prednisone and ketotifen. J Clin Invest 76: 1096– 1101, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJP. T2Rs function as bitter taste receptors. Cell 100: 703– 711, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting β2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med 327: 1198– 1203, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Cooper PR, Kurten RC, Zhang J, Nicholls DJ, Dainty IA, Panettieri RA. Formoterol and salmeterol induce a similar degree of β2-adrenoceptor tolerance in human small airways but via different mechanisms. Br J Pharmacol 163: 521– 532, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper PR, Panettieri RA. Steroids completely reverse albuterol-induced β2-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol 122: 734– 740, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol 158: 1429– 1441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies AO, Lefkowitz RJ. Regulation of β-adrenergic receptors by steroid hormones. Annu Rev Physiol 46: 119– 130, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal 18: 2105– 2120, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Deshpande DA, Robinett KS, Wang WCH, Sham JSK, An SS, Liggett SB. Bronchodilator activity of bitter tastants in human tissue correspondence. Nat Med 17: 776– 778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deshpande DA, Wang WCH, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JSK, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299– 1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dohlman HG. Diminishing returns. Nature 418: 591, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull 56: 1054– 1070, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Eason MG, Liggett SB. Subtype-selective desensitization of α2-adrenergic receptors. Different mechanisms control short and long term agonist-promoted desensitization of α2C10, α2C4, and α2C2. J Biol Chem 267: 25473– 25479, 1992 [PubMed] [Google Scholar]
- 22. Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA 105: 5230– 5235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Komalavilas P, Penn RB, Flynn CR, Thresher J, Lopes LB, Furnish EJ, Guo M, Pallero MA, Murphy-Ullrich JE, Brophy CM. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol 294: L69– L78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraan J, Koeter GH, vd Mark TW, Sluiter HJ, de Vries K. Changes in bronchial hyperreactivity induced by 4 weeks of treatment with antiasthmatic drugs in patients with allergic asthma: a comparison between budesonide and terbutaline. J Allergy Clin Immunol 76: 628– 636, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Liang M, Eason MG, Jewell-Motz EA, Williams MA, Theiss CT, Dorn GW, 2nd, Liggett SB. Phosphorylation and functional desensitization of the alpha2A-adrenergic receptor by protein kinase C. Mol Pharmacol 54: 44– 49, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Liggett SB. Desensitization of the beta-adrenergic receptor: distinct molecular determinants of phosphorylation by specific kinases. Pharmacol Res 24, Suppl 1: 29– 41, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Liggett SB. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci Signaling 4: pe36, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists. Is there cause for concern? Drug Saf 16: 295– 308, 1997 [DOI] [PubMed] [Google Scholar]
- 30. McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by β-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway beta-agonist paradox. J Clin Invest 112: 619– 626, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGraw DW, Elwing JM, Fogel KM, Wang WCH, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest 117: 1391– 1398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGraw DW, Fogel KM, Kong S, Litonjua AA, Kranias EG, Aronow BJ, Liggett SB. Transcriptional response to persistent β2-adrenergic receptor signaling reveals regulation of phospholamban, which alters airway contractility. Physiol Genomics 27: 171– 177, 2006 [DOI] [PubMed] [Google Scholar]
- 33. McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, Liggett SB. Airway smooth muscle prostaglandin-EP1 receptors directly modulate β2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest 116: 1400– 1409, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol 154: 37– 72, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35: 157– 170, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA, Jr, Emala CW. Gi-coupled γ-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 45: 1232– 1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 256: C329– C335, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol 45: 1069– 1074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med 123: 322– 328, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Sears MR, Taylor DR. The beta 2-agonist controversy. Observations, explanations and relationship to asthma epidemiology. Drug Saf 11: 259– 283, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131– 1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231– 236, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Turki J, Green SA, Newman KB, Meyers MA, Liggett SB. Human lung cell β2-adrenergic receptors desensitize in response to in vivo administered β-agonist. Am J Physiol Lung Cell Mol Physiol 269: L709– L714, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Wang WCH, Mihlbachler KA, Brunnett AC, Liggett SB. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway β2-adrenergic receptor physiologic signaling. Proc Natl Acad Sci USA 106: 15007– 15012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long-acting inhaled β2-agonist. Am J Respir Crit Care Med 154: 1603– 1607, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Yates DH, Worsdell M, Barnes PJ. Effect of regular salmeterol treatment on albuterol-induced bronchoprotection in mild asthma. Am J Respir Crit Care Med 156: 988– 991, 1997 [DOI] [PubMed] [Google Scholar]