Abstract
The statins are a class of 3-hydroxy-3-methylglutaryl-coenzyme A-reductase inhibitors that are recognized to have pleiotropic properties. We previously reported the attenuation of LPS-induced murine acute lung injury (ALI) by simvastatin in vivo and identified relevant effects of simvastatin on endothelial cell (EC) signaling, activation, and barrier function in vitro. In particular, simvastatin induces the upregulation of integrin-β4, which in turn inhibits EC inflammatory responses via attenuation of MAPK signaling. The role of integrin-β4 in murine ALI protection by simvastatin, however, is unknown. We initially confirmed a time- and dose-dependent effect of simvastatin on increased integrin-β4 mRNA expression in human lung EC with peak protein expression evident at 16 h. Subsequently, reciprocal immunoprecipitation demonstrated an attenuation of LPS-induced integrin-β4 tyrosine phosphorylation by simvastatin (5 μM, 16 h). Increased expression of EC inflammatory cytokines [IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, regulated on activation normal T cell expressed and secreted (RANTES)] by LPS (500 ng/ml, 4 h) was also significantly attenuated by simvastatin pretreatment (5 μM, 16 h), but this effect was reversed by cotreatment with an integrin-β4-blocking antibody. Finally, although simvastatin (20 mg/kg) conferred significant protection in murine ALI as evidenced by decreased bronchoalveolar lavage fluid cell counts, protein, inflammatory cytokines (IL-6, IL-1β, MCP-1, RANTES), decreased Evans blue dye albumin extravasation in lung tissue, and changes on lung histology, these effects were reversed by the integrin-β4-blocking antibody (IV, 1 mg/kg, 2 h before LPS). These findings support integrin-β4 as an important mediator of ALI protection by simvastatin and implicate signaling by integrin-β4 as a novel therapeutic target in patients with ALI.
Keywords: statins, integrins, endothelial cells
the statins are a class of 3-hydroxy-3-methylglutaryl-coenzyme A-reductase inhibitors recognized to have potent anti-inflammatory and vascular-protective properties independent of their ability to lower serum cholesterol levels. In this regard, we previously reported the attenuation of murine acute lung injury (ALI) by pretreatment with simvastatin (12). The mechanisms underlying these effects, however, are complex and not fully characterized. For example, we have identified several distinct effects of statins on endothelial cell (EC) signaling and activation that are relevant to the pathophysiology of ALI including actin cytoskeletal rearrangement and EC barrier protection via differential effects on small GTPases RhoA and Rac1 (13) and inhibition of superoxide generation via NADPH oxidase inhibition (4). We have also reported the marked upregulation of integrin-β4 (13), a molecule that attenuates EC inflammatory responses via effects on MAPK signaling (3). This dramatic upregulation of EC integrin-β4 by statins has subsequently been corroborated (16); however, the role of integrin-β4 in murine ALI protection by simvastatin remains unclear.
Integrins, expressed in a variety of cell types and mediators of both inside-out and outside-in signaling, exist as transmembrane heterodimers consisting of a β-subunit required for activation and an α-subunit that serves a regulatory role. There are eight known β-subunits, all of which share significant structural homology except for integrin-β4, which is uniquely characterized by a long cytoplasmic tail (over 1,000 amino acids) that fails to show identity with the cytoplasmic domains of other β-subunits (11). Moreover, recent evidence suggests that integrin-β4 is associated with wholly distinct signaling properties. In particular, integrin-β4 expression and signaling is associated with an attenuation of EC inflammatory responses in vitro and ALI protection in vivo. This is in notable contrast to a variety of other integrin β-subunits that promote lung vascular permeability and inflammation. For example, integrins-β2, -β5, and -β6 have each been identified as independent mediators of increased lung vascular permeability associated with ALI (9, 22, 28).
The extracellular ligand for integrin-β4 is laminin. Integrin-β4 complexes only with integrin-α6 and is recognized as important for the formation of epithelial hemidesmosomes and is relevant to cancer biology. Increased integrin-β4 expression is linked to increased tumor invasiveness in squamous cell carcinomas (26) and papillary carcinomas of the thyroid (14) and portends a poor prognosis in both breast (24) and bladder cancer (10). Until recently, however, little was known about the role of integrin-β4 in normal EC physiology. We previously reported that integrin-β4 attenuates EC inflammatory responses via the negative regulation of MAPK signaling mediated by Src homology phosphatase-2 (SHP-2), a protein tyrosine phosphatase (3). In the current study, we investigated the functional contribution of these effects to simvastatin protection both in EC inflammatory responses in vitro and in vivo in a murine model of LPS-induced ALI.
MATERIALS AND METHODS
Materials and reagents.
Integrin-β4 and integrin-β1 antibodies were purchased from Millipore (Billerica, MA). Mouse IgG was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other antibodies were purchased from Cell Signaling (Danvers, MA). The real-time RT-PCR kit was purchased from Qiagen (Valencia, CA), and the primers for integrin-β4 and GADPH were purchased from IDT (Coralville, IA). ELISA kits for IL-6 and IL-8 and Milliplex MAP mouse cytokine kit were purchased from Biolegend (San Diego, CA) and Millipore, respectively. All other reagents were purchased from Sigma (St. Louis, MO) unless otherwise specified.
Real-time PCR.
EC were grown in six-well plates to 80–95% confluence and were then treated with simvastatin for the times indicated. Cells were harvested, and total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized from total RNA (1 μg), and RT-PCR was performed for integrin-β4 using ABI Prism 7700 Sequence Detector System (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. GADPH was used as an endogenous control for normalization.
Immunoprecipitation and Western blotting.
Cells were treated as indicated and harvested with RIPA buffer containing proteinase inhibitors and phosphatase inhibitors per standard protocols. After sonication and centrifugation, the supernatant was collected. Equal amounts of protein were incubated with integrin-β4 or phosphorylated tyrosine antibodies for immunoprecipitation overnight. IgG agarose beads (20 μl) were added for 2 h, and the beads were centrifuged and washed with RIPA buffer ×4 followed by the addition of 30 μl of Laemmli sample buffer and boiling. Samples were then analyzed by SDS-PAGE gel. After transfer to a nitrocellulose membrane (Bio-Rad, Hercules, CA), Western blotting was performed using appropriate primary antibodies and horseradish peroxidase-conjugated secondary antibodies before visualization via chemiluminescence (Amersham Biosciences, Piscataway, NJ). Blot density was determined by Alpha Imager software (Alpha Innotech, San Leandro, CA).
Murine ALI model.
All experiments and animal care procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee. C57/Bl6 mice (Jackson Laboratory, Bar Harbor, ME), 8–12 wk old, were initially administered simvastatin (20 mg/kg) or vehicle via intraperitoneal injection overnight. The following day (24 h later), the mice were pretreated with an integrin-β4- or integrin-β1-blocking antibody (1 mg/g body wt) or a control antibody (mouse IgG, 1 mg/g body wt) intravenously 2 h before the repeat administration of simvastatin or vehicle and intratracheal LPS (1.25 mg/kg). On the third day, 24 h after LPS, the mice were killed and bronchoalveolar lavage (BAL) was performed in select animals as previously described (12). BAL fluid was assessed for cell counts, protein content, and cytokine levels. In separate animals, lungs were harvested for histological analysis.
Measurement of cytokines in EC media and in BAL fluid.
EC were grown to 75–80% confluence before treatment per the experimental conditions. The media was then briefly centrifuged and collected to measure cytokine concentration with commercially available ELISA kits according to the manufacturer's instruction. In the animal studies, BAL cytokines were measured with Millipore MAP mouse cytokine kit according to manufacturer's instruction.
Assessment of lung capillary leakage.
Evans blue dye albumin (EBA, 20 mg/kg) was injected into the internal jugular vein 1 h before the termination of the experiment to assess vascular leak as we have previously described (19). The lungs were then perfused with PBS and then excised before homogenization, incubation with 2 ml formamide, and centrifugation (12,000 g × 20 min). The optical density of the supernatant was then determined spectrophotometrically at 620 nm. The extravasated EBA concentration was calculated against a standard curve and expressed as μg EBA/ml.
Statistical analysis.
Shapiro-Wilk testing was used to confirm data were normally distributed. Student's t-test was used to compare the means of data from two experimental groups, whereas significant differences (P < 0.05) among multiple-group comparisons were confirmed by one-way ANOVA and post hoc multiple-comparisons testing. Results are expressed as means ± SE.
RESULTS
Regulation of integrin-β4 expression by simvastatin.
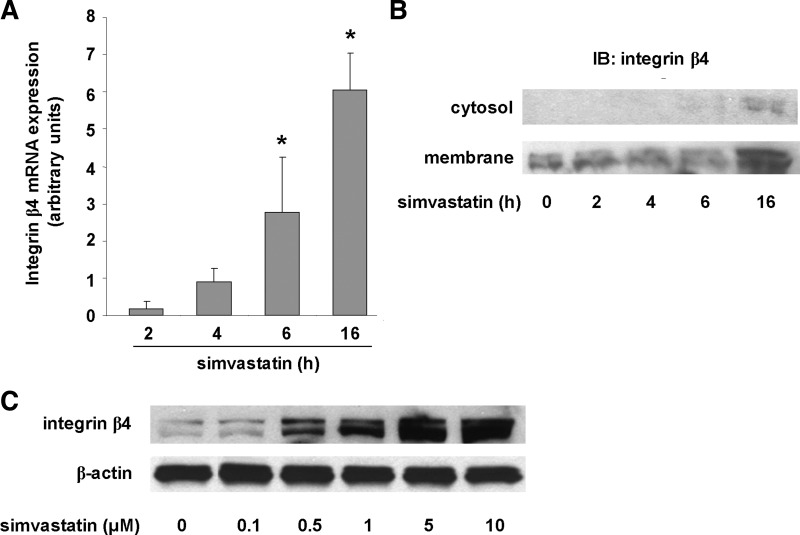
We previously reported increased expression of integrin-β4 in EC treated with simvastatin (5 μM, 24 h) detected by microarray analysis with increased protein levels confirmed by Western blotting (13, 29). To extend and further validate our earlier studies, we performed real-time PCR and characterized integrin-β4 mRNA changes over time in human pulmonary artery EC treated with simvastatin (5 μM). These studies confirmed time-dependent changes in integrin-β4 mRNA levels with a significant increase evident at 6 h, whereas levels were increased even further at 16 h (Fig. 1A). Consistent with these findings, there was no change in integrin-β4 protein levels by Western blotting of EC lysates during early time points (within 6 h), whereas a significant increase was evident at 16 h (Fig. 1B). Membrane fractionation confirmed localization of integrin-β4 at the cell membrane. In addition, we confirmed a dose-response effect with increased integrin-β4 expression evident in response to as little as 0.5 μM of simvastatin (16 h) and expression increasing proportionally with increasing concentrations of simvastatin, up to 10 μM (Fig. 1C).
Fig. 1.
Increased integrin-β4 gene and protein expression by simvastatin. A: qRT-PCR studies reveal significantly increased integrin-β4 expression in human pulmonary artery endothelial cell (EC) treated with simvastatin (5 μM). The time course of increased integrin-β4 mRNA levels is consistent with that of increased protein expression (B). Membrane fractionation confirms that protein expression is localized to the membrane but not the cytosol. (*P < 0.05 compared with 2 h). C: in addition, Western blotting confirmed a dose-response effect of simvastatin treatment (16 h) on EC integrin-β4 expression as shown. IB, immunoblotting.
Regulation of integrin-β4 tyrosine phosphorylation.
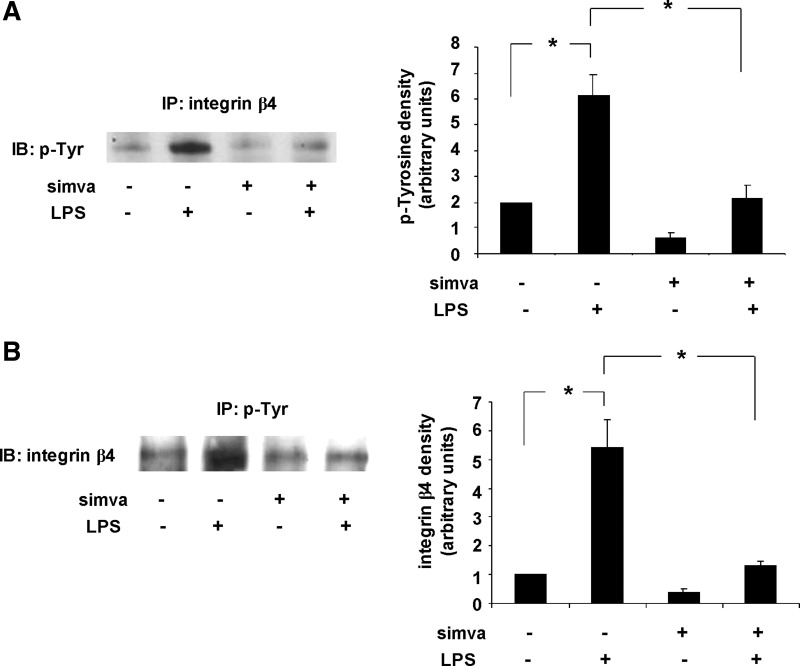
In an effort to link changes in integrin-β4 expression by simvastatin with changes in integrin-β4-mediated signaling, we performed immunoprecipitation experiments and assessed tyrosine phosphorylation of integrin-β4 in response to simvastatin. Human pulmonary artery EC were treated with simvastatin (5 μM, 16 h) or vehicle before treatment with LPS (500 ng/ml, 1 h) and the lysates subjected to immunoprecipitation with an integrin-β4 antibody followed by immunoblotting with a phospho-tyrosine antibody (Fig. 2A). Reciprocal experiments were also performed under the same conditions utilizing a phospho-tyrosine antibody for immunoprecipitation followed by immunoblotting with an integrin-β4 antibody (Fig. 2B). These studies confirmed increased tyrosine phosphorylation of integrin-β4 in response to LPS that was abrogated with simvastatin pretreatment. Notably, we repeated these studies using human lung microvascular EC and confirmed identical results (data not shown).
Fig. 2.
Simvastatin (simva) inhibits LPS-induced tyrosine phosphorylation of integrin-β4. A: human pulmonary artery EC were treated with vehicle or simvastatin (5 μM, 16 h) before stimulation with LPS (500 ng/ml, 1 h). Cells were lysed and integrin-β4 immunoprecipitated (IP) with a specific antibody before IB with an antibody specific for phosphorylated tyrosine. B: in reciprocal experiments, under identical conditions, cells were subjected to IP with an antibody specific for phosphorylated tyrosine before IB for integrin-β4 (n = 3/experimental condition, *P < 0.05).
Role of integrin-β4 in the attenuation of LPS-induced EC inflammatory responses by simvastatin.
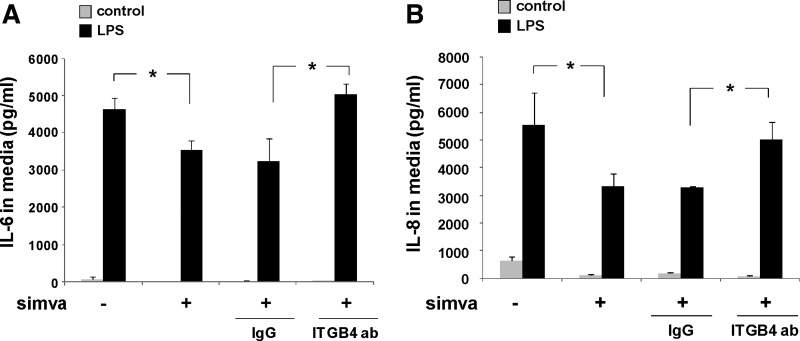
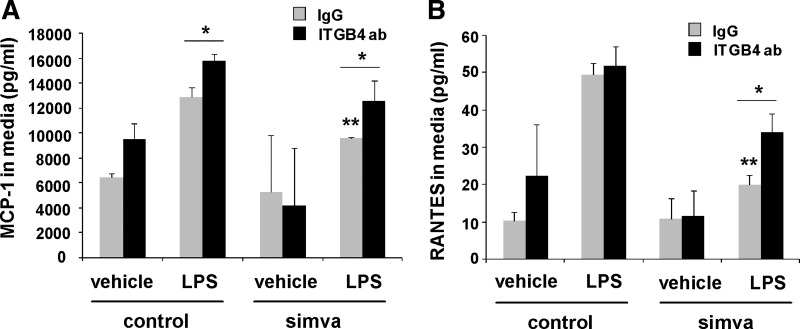
Because we have previously reported an anti-inflammatory role for integrin-β4 in EC responses to LPS (3), we investigated these effects in the context of simvastatin treatment. In our initial experiments, human pulmonary artery EC were treated with simvastatin (5 μM, 16 h) or vehicle either alone or with subsequent treatment with either an integrin-β4-blocking antibody (20 μg/ml, 2 h) or a control antibody (IgG) before LPS stimulation (500 ng/ml, 4 h). Media were then collected and IL-6 and IL-8 levels measured (Fig. 3). These studies confirmed a significant attenuation of LPS-induced IL-6 and IL-8 expression by simvastatin alone that was abrogated by treatment with the integrin-β4-blocking antibody. Additional studies to assess other inflammatory cytokines revealed a significant attenuation of both monocyte chemoattractant protein (MCP)-1 and regulated on activation normal T cell expressed and secreted (RANTES) expression in LPS-stimulated EC pretreated with simvastatin (5 μM, 16 h) that was significantly reversed by treatment with the integrin-β4-blocking antibody (Fig. 4). Interestingly, the integrin-β4-blocking antibody also affected an increase in MCP-1 expression in these studies when administered by itself. Of note, separate studies were conducted using siRNA specific for integrin-β4, but these experiments confirmed an overriding effect of simvastatin resulting in increased-β4 expression despite the use of siRNA that effectively inhibited integrin-β4 expression when used alone.
Fig. 3.
The attenuation of LPS-induced EC IL-6 and IL-8 expression by simvastatin is mediated by integrin-β4. EC were grown to confluence and treated with simvastatin (5 μM, 16 h) or vehicle before LPS (500 ng/ml, 4 h), and levels of IL-6 (A) and IL-8 (B) were measured in the media. Compared with controls, simvastatin affected a significant decrease in LPS-induced IL-6 and IL-8 expression. Moreover, pretreatment with an integrin-β4-blocking antibody (ITGB4 ab, 20 μg/ml, 2 h before LPS) reversed the effects of simvastatin, whereas use of a control antibody (IgG, 20 μg/ml) had no effect (n = 3/condition, *P < 0.05).
Fig. 4.
The attenuation of LPS-induced EC monocyte chemoattractant protein (MCP)-1 and regulated on activation normal T cell expressed and secreted (RANTES) expression by simvastatin is mediated by integrin-β4. EC were grown to confluence and treated with simvastatin or vehicle (5 μM, 16 h), followed by an integrin-β4-blocking antibody (ITGB4 ab, 20 μg/ml) or a control antibody (IgG) 2 h before LPS (500 ng/ml, 4 h). Media were collected and levels of MCP-1 (A) and RANTES (B) measured. Whereas pretreatment with the control antibody had no effect, pretreatment with an integrin-β4-blocking antibody reversed the attenuation of LPS-induced MCP-1 and RANTES expression by simvastatin (n = 3/condition, *P < 0.05, **P < 0.05 compared with LPS alone).
Role of integrin-β4 in murine ALI protection by simvastatin.
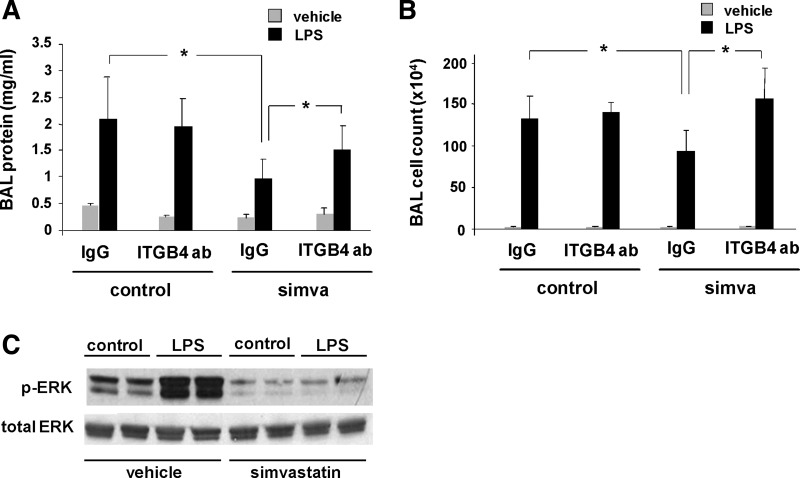
We sought to extend our in vitro findings to our previously established murine model of LPS-induced ALI. Consistent with our previous report (12), mice were pretreated with simvastatin (20 mg/kg) or vehicle by intraperitoneal injection 20 h before treatment with an integrin-β4-blocking antibody (1 mg/kg iv) or either an integrin-β1-blocking antibody or mouse IgG as controls. This was followed 4 h later by concomitant treatment with a second dose of simvastatin (20 mg/kg ip) and intratracheal LPS (1.25 mg/kg). BAL fluid was then collected 24 h later and assessed for protein content and cell counts (Fig. 5, A and B). Increased BAL fluid protein levels and total cell counts (predominantly neutrophils) induced by LPS were significantly reduced in animals treated with both simvastatin and the control antibody (IgG). In contrast, animals treated with the integrin-β4-blocking antibody exhibited a significant reversal in these effects of simvastatin. Notably, we observed no effects with the administration of the integrin-β1-blocking antibody in these experiments (data not shown). We next sought to confirm an attenuation of integrin-β4-mediated signaling by simvastatin in vivo. Because we have previously reported an inhibitory role for integrin-β4 in EC inflammatory responses associated with attenuated MAPK signaling (3), whole lung homogenates from mice pretreated with vehicle or simvastatin and then administered LPS (as above) were subjected to Western blotting and probed for Erk phosphorylation (Fig. 5C). These experiments confirmed a dramatic increase in Erk activation in response to LPS that was abrogated by simvastatin.
Fig. 5.
Protection by simvastatin in murine acute lung injury (ALI) is attenuated by inhibition of integrin-β4 as assessed by bronchoalveolar lavage (BAL) protein and cell counts. Mice were administered simvastatin (20 mg/kg, 24 h) or vehicle intraperitoneally followed by intravenous integrin-β4 blocking antibody (ITGB4, 1 mg/kg) or a control antibody (IgG, 1 mg/kg) 4 h before concomitant treatment with a second dose of simvastatin and LPS (1.25 mg/kg intratracheally). BAL fluid was collected at 24 h and assessed for protein content (A) and cell counts (B) (n ≥ 3 animals/group, *P < 0.05). C: in separate experiments, Western blots of whole lung homogenates confirmed an attenuation of integrin-β4-mediated signaling (ERK phosphorylation) by simvastatin in LPS-treated mice (representative blots shown from duplicate experiments).
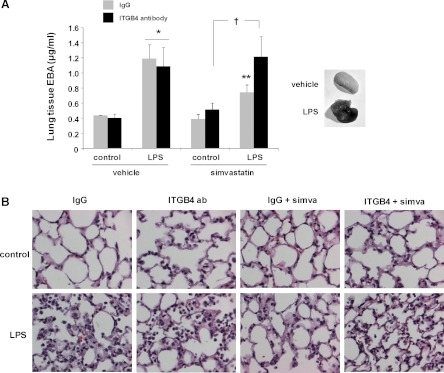
In separate studies with the same experimental conditions, we measured changes in EBA extravasation to assess lung capillary leakage. These studies confirmed increased EBA tissue content in response to LPS that was abrogated by simvastatin, whereas administration of the integrin-β4-blocking antibody fully reversed these effects of simvastatin (Fig. 6A). In addition, lungs were also collected and used for histological evaluation, in which a significant increase in lung tissue edema and inflammatory cell infiltration in LPS-treated animals compared with controls that was markedly attenuated by simvastatin pretreatment was observed. In contrast, lungs from simvastatin-treated animals that also received the integrin-β- blocking antibody demonstrated significant injury and were essentially indistinguishable from those of animals that received LPS alone (Fig. 6B).
Fig. 6.
Protection by simvastatin in murine ALI is attenuated by inhibition of integrin-β4 as assessed by Evans blue dye albumin (EBA) extravasation and lung histology. Mice were administered simvastatin (20 mg/kg, 24 h) or vehicle intraperitoneally followed by intravenous integrin-β4-blocking antibody (ITGB4, 1 mg/kg) or a control antibody (IgG, 1 mg/kg) 4 h before concomitant treatment with a second dose of simvastatin and LPS (1.25 mg/kg, intratracheally). A: in select animals, EBA was injected and lung tissue extravasation was measured (n = 3 animals/group, *P < 0.05 compared with controls, **P < 0.05 compared with LPS alone, †P < 0.05). B: in separate experiments, lungs were collected for histology (representative images shown).
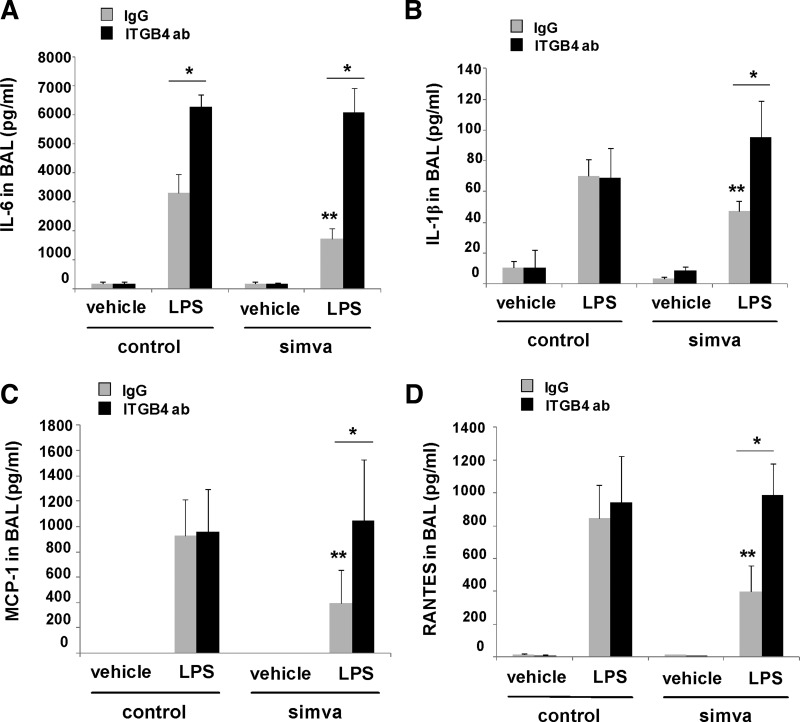
Finally, the animal studies were repeated using the integrin-β4-blocking antibody, and BAL fluid was collected to assess changes in inflammatory cytokine levels. These experiments identified several cytokines that were markedly increased in the BAL fluid in response to LPS alone but significantly reduced by pretreatment with simvastatin including IL-6, IL-1β, MCP-1, and RANTES (Fig. 7). Furthermore, reductions in each of these by simvastatin were found to be significantly attenuated by use of the integrin-β4-blocking antibody. Of note, although BAL fluid KC levels (a murine IL-8 homolog) were reduced in mice treated with simvastatin and LPS compared with LPS alone, we did not detect a significant reversal in BAL KC levels in LPS and simvastatin-treated animals that also received the blocking antibody. Similarly, although IL-1β levels were significantly altered in the BAL fluid, we did not detect a significant effect of either simvastatin or the blocking antibody on IL-1β levels in our in vitro studies.
Fig. 7.
Inhibition of integrin-β4 reverses the attenuation of cytokine expression by simvastatin in murine ALI. Mice were administered simvastatin (20 mg/kg, 24 h) or vehicle intraperitoneally followed by intravenous integrin-β4 blocking antibody (ITGB4, 1 mg/kg) or a control antibody (IgG, 1 mg/kg) 4 h before concomitant treatment with a second dose of simvastatin and intratracheal LPS (1.25 mg/kg). BAL fluid was collected at 24 h and cytokines measured including IL-6 (A), IL-1β (B), MCP-1 (C), and RANTES (D) (n ≥ 3 animals/group, *P < 0.05, **P < 0.05 compared with LPS alone).
DISCUSSION
The potential role for statins in the treatment of ALI is an area of ongoing investigation and is supported by abundant in vitro data (4, 13, 27), findings in relevant animal models (12, 17, 18, 23), as well as numerous observational reports involving relevant patient populations (1, 7, 15, 20) and data from recent human studies (5, 21). These drugs are recognized to have pleiotropic properties, including direct effects on EC inflammatory responses and EC barrier function via differential Rho GTPase activation resulting in both dynamic cytoskeletal rearrangement and the inhibition of NADPH oxidase activity (4, 13). The relative contributions of these complex effects to the therapeutic potential of statins in ALI, however, are unknown. We have previously identified the dramatic upregulation of integrin-β4 in EC treated with simvastatin and, subsequently, have reported a functional role for integrin-β4 in attenuating EC inflammatory responses (3, 12). Our findings now indicate that integrin-β4 is a critical mediator of the protective properties of statins in murine ALI.
We found that EC integrin-β4 mRNA and protein levels increase in a time-dependent manner in response to simvastatin. These effects are associated with an overall decrease in LPS-induced tyrosine phosphorylation of integrin-β4. We previously reported that integrin-β4 serves as a “brake” on LPS-induced EC inflammatory responses via the negative regulation of the tyrosine phosphatase, SHP-2, which mediates MAPK signaling and the downstream expression of inflammatory cytokines, including IL-6 and IL-8 (3). Notably, tyrosine phosphorylation of integrin-β4 (on Tyr 1526) has been found to be required for Erk activation in human umbilical vein EC (6). Consistent with this, we found that integrin-β4 tyrosine phosphorylation was significantly increased in response to LPS stimulation, which in turn drives MAPK signaling, including Erk, and cytokine expression in human pulmonary artery EC. We speculate that these signaling events are attenuated by the overexpression of unphosphorylated integrin-β4 in response to simvastatin and accounts to a significant degree for the anti-inflammatory properties of statins in this context. The posttranslational changes that result in decreased tyrosine phosphorylation of integrin-β4 despite the increased protein expression induced by statins, however, are unknown and are a focus of our ongoing research.
A potential limitation of our study is our reliance solely on a blocking antibody to inhibit integrin-β4. Although additional studies are planned to explore these effects in more detail, the use of the blocking antibody is supported by successful use of this strategy by other investigators (8). Moreover, our in vitro findings using the blocking antibody corroborate our previous report that relied on the use of siRNA specific for integrin-β4, which identified a role for integrin-β4 in the elaboration of EC inflammatory responses (3). Finally, the use of the blocking antibody in our animal model, administered intravenously, was based on a protocol established by other investigators to investigate the role of integrin-β5 in rodent models of ALI (22).
The clinical implications of our findings are significant because statins are currently being investigated for their therapeutic potential in ALI. Although the appeal of statin therapy in this context is strong given their availability, affordability, and their generally favorable safety profile, their pleiotropic properties may lead to complex and potentially suboptimal clinical effects in patients with ALI. As our data suggest that integrin-β4 upregulation is a critical mediator of the statin-protective effects in murine ALI, it may be that a more targeted approach, focused on integrin-β4-mediated EC signaling, could affect more significant clinical benefits.
Finally, our results should help to inform the interpretation of clinical trials of statins in ALI. Specifically, there is evidence that the pleiotropic properties of individual statins may be highly variable, including their effects on endothelial function (2, 25). This may be a function of their relative lipophilicity because the more lipophilic statins (e.g., simvastatin) have increased extrahepatic tissue penetration relative to the more hydrophilic statins (e.g., rosuvastatin), which are more hepato-selective. Thus the lipophilic statins may have more prominent direct endothelial effects, including effects on integrin-β4 expression and signaling, relevant to attenuating ALI.
In summary, we explored the role of integrin-β4 on the attenuation of EC inflammatory signaling in vitro by simvastatin as well as protection by simvastatin in vivo in a murine model of ALI. We confirmed increased expression of EC integrin-β4 in response to simvastatin treatment and observed a significant reduction in simvastatin anti-inflammatory effects, both in vitro and in vivo, associated with inhibition of integrin-β4. Our findings support a novel regulatory role for integrin-β4 in ALI and implicate integrin-β4 signaling as a potentially important focus of strategies aimed at attenuating ALI in patients.
GRANTS
This work was supported by the NHLBI (HL 77134, HL 58064).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.C., J.G.G., and J.R.J. conception and design of research; W.C., S.S., S.M., and S.F.M. performed experiments; W.C. and J.R.J. analyzed data; W.C., J.G.G., and J.R.J. interpreted results of experiments; W.C., S.F.M., and J.R.J. prepared figures; W.C. and J.R.J. drafted manuscript; J.G.G. and J.R.J. edited and revised manuscript; J.G.G. and J.R.J. approved final version of manuscript.
REFERENCES
- 1. Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 110: 880– 885, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bellia A, Rizza S, Galli A, Fabiano R, Donadel G, Lombardo MF, Cardillo C, Sbraccia P, Tesauro M, Lauro D. Early vascular and metabolic effects of rosuvastatin compared with simvastatin in patients with type 2 diabetes. Atherosclerosis 210: 199– 201 [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Garcia JG, Jacobson JR. Integrin beta4 attenuates SHP-2 and MAPK signaling and reduces human lung endothelial inflammatory responses. J Cell Biochem 110: 718– 724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol 295: L575– L583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 183: 620– 626 [DOI] [PubMed] [Google Scholar]
- 6. Dans M, Gagnoux-Palacios L, Blaikie P, Klein S, Mariotti A, Giancotti FG. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J Biol Chem 276: 1494– 1502, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Falagas ME, Makris GC, Matthaiou DK, Rafailidis PI. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother 61: 774– 785, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Feng C, Ye C, Liu X, Ma H, Li M. Beta4 integrin is involved in statin-induced endothelial cell death. Biochem Biophys Res Commun 323: 858– 864, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, Matthay MA, Pittet JF. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res 102: 804– 812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grossman HB, Lee C, Bromberg J, Liebert M. Expression of the alpha6beta4 integrin provides prognostic information in bladder cancer. Oncol Rep 7: 13– 16, 2000 [PubMed] [Google Scholar]
- 11. Hogervorst F, Kuikman I, von dem Borne AE, Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J 9: 765– 770, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 288: L1026– L1032, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol 30: 662– 670, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kitajiri S, Hosaka N, Hiraumi H, Hirose T, Ikehara S. Increased expression of integrin beta-4 in papillary thyroid carcinoma with gross lymph node metastasis. Pathol Int 52: 438– 441, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 32: 75– 79, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Morikawa S, Takabe W, Mataki C, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. Global analysis of RNA expression profile in human vascular cells treated with statins. J Atheroscler Thromb 11: 62– 72, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Muller HC, Hellwig K, Rosseau S, Tschernig T, Schmiedl A, Gutbier B, Schmeck B, Hippenstiel S, Peters H, Morawietz L, Suttorp N, Witzenrath M. Simvastatin attenuates ventilator-induced lung injury in mice. Crit Care 14: R143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naidu BV, Woolley SM, Farivar AS, Thomas R, Fraga C, Mulligan MS. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg 126: 482– 489, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245– 1251, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Schmidt H, Hennen R, Keller A, Russ M, Muller-Werdan U, Werdan K, Buerke M. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med 32: 1248– 1251, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Shyamsundar M, McKeown ST, O'Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS, McAuley DF. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med 179: 1107– 1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK, Frank JA, Matthay MA, Sheppard D, Pittet JF. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol 36: 377– 386, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suda K, Eom J, Jaw JE, Mui T, Bai N, Or C, Ngan D, Li Y, Man SP, Van Eeden S, Sin DD. Endotoxin-induced cardiovascular dysfunction in mice: effect of simvastatin. J Appl Physiol 111: 1118– 1124, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res 4: 407– 410, 1998 [PubMed] [Google Scholar]
- 25. Turner NA, Midgley L, O'Regan DJ, Porter KE. Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion. J Cardiovasc Pharmacol 50: 458– 461, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Van Waes C, Kozarsky KF, Warren AB, Kidd L, Paugh D, Liebert M, Carey TE. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res 51: 2395– 2402, 1991 [PubMed] [Google Scholar]
- 27. Wu X, Lin D, Li G, Zuo Z. Statin post-treatment provides protection against simulated ischemia in bovine pulmonary arterial endothelial cells. Eur J Pharmacol 636: 114– 120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu J, Gao XP, Ramchandran R, Zhao YY, Vogel SM, Malik AB. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins. Nat Immunol 9: 880– 886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang LQ, Ma SF, Grigoryev D, Lavoie TL, Xiao HQ, Setterquist R, Li H, Jacobson J, Garcia JG, Ye SQ. Temporal gene expression analysis of human coronary artery endothelial cells treated with Simvastatin. Gene Expr 14: 229– 239, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]