Abstract
Mechanical ventilation (MV) with O2-rich gas (MV-O2) offers life-saving treatment for newborn infants with respiratory failure, but it also can promote lung injury, which in neonates translates to defective alveolar formation and disordered lung elastin, a key determinant of lung growth and repair. Prior studies in preterm sheep and neonatal mice showed that MV-O2 stimulated lung elastase activity, causing degradation and remodeling of matrix elastin. These changes yielded an inflammatory response, with TGF-β activation, scattered elastic fibers, and increased apoptosis, culminating in defective alveolar septation and arrested lung growth. To see whether sustained inhibition of elastase activity would prevent these adverse pulmonary effects of MV-O2, we did studies comparing wild-type (WT) and mutant neonatal mice genetically modified to express in their vascular endothelium the human serine elastase inhibitor elafin (Eexp). Five-day-old WT and Eexp mice received MV with 40% O2 (MV-O2) for 24–36 h. WT and Eexp controls breathed 40% O2 without MV. MV-O2 increased lung elastase and MMP-9 activity, resulting in elastin degradation (urine desmosine doubled), TGF-β activation (pSmad-2 increased 6-fold), apoptosis (cleaved-caspase-3 increased 10-fold), and inflammation (NF-κB activation, influx of neutrophils and monocytes) in lungs of WT vs. unventilated controls. These changes were blocked or blunted during MV-O2 of Eexp mice. Scattered lung elastin and emphysematous alveoli observed in WT mice after 36 h of MV-O2 were attenuated in Eexp mice. Both WT and Eexp mice showed defective VEGF signaling (decreased lung VEGF-R2 protein) and loss of pulmonary microvessels after lengthy MV-O2, suggesting that elafin's beneficial effects during MV-O2 derived primarily from preserving matrix elastin and suppressing lung inflammation, thereby enabling alveolar formation during MV-O2. These results suggest that degradation and remodeling of lung elastin can contribute to defective lung growth in response to MV-O2 and might be targeted therapeutically to prevent ventilator-induced neonatal lung injury.
Keywords: elastin, alveolarization, neonatal chronic lung disease, bronchopulmonary dysplasia, ventilator-induced lung injury, elastase and matrix metalloproteinase-9, urine desmosine
mechanical ventilation (MV) with O2-rich gas (MV-O2) is a life-saving treatment for respiratory failure in newborn infants whose lungs are incompletely developed. Several studies have shown that prolonged exposure of developing lungs to cyclic stretch and hyperoxia adversely affect the formation of alveoli, resulting in lung growth arrest (1, 13, 14, 26, 27). Such failure of lung growth is typically associated with increased lung cell apoptosis (19, 23) and disordered matrix elastin (26, 37, 55), resulting in a condition that was first described as bronchopulmonary dysplasia (BPD) (42), herein called neonatal chronic lung disease (CLD).
Elastin plays a major role in mammalian lung development, serving as a formative framework of future alveoli and pulmonary blood vessels. Elastin-null mice die soon after birth from cardiorespiratory failure due to smooth muscle overgrowth in systemic and pulmonary arteries, defective airway branching, and failure of alveolar septation (35, 59). Numerous reports have linked excess, disordered lung elastin with impaired formation of alveoli and microvessels in lungs of premature infants who have died with CLD (26, 37, 55). A related study showed that urinary excretion of desmosine, a biomarker of elastin breakdown, increased in the first week of MV-O2 in newborns with evolving CLD (10). Fragmentation of elastic fibers in this disease has been attributed to inflammation linked to increased lung elastolytic activity (39, 44, 58), which may result from infection and/or hyperoxia, conditions that often complicate the course of extremely premature infants (9). Recent studies showed that mechanical forces can regulate elastase activity in the lung matrix, causing elastin binding sites to unfold and elastic fibers to fragment, which in turn can promote aberrant tissue remodeling and impaired lung function (31, 32). Consistent with these findings, authentic animal models of CLD induced by prolonged MV-O2 of premature primates and lambs exhibit lung inflammation and increased elastase activity, resulting in matrix remodeling and lung growth arrest (3, 8, 46, 61).
We previously showed that newborn mice treated with MV-O2 for up to 24 h displayed increased lung elastase activity, resulting in elastic fiber degradation and remodeling of the extracellular matrix (ECM) (6). These changes were linked to activation of transforming growth factor-β (TGF-β) and increased apoptosis, culminating in defective formation of alveoli and pulmonary microvessels, and widely dispersed elastic fibers in distal lung (6, 7, 40). In a subsequent study, we found that treatment of mechanically ventilated newborn mice with a single dose of recombinant human elafin, a serine elastase inhibitor, suppressed lung elastase activity, as well as matrix metalloproteinase (MMP)-9 activity, thereby preventing MV-O2-induced degradation and dispersion of lung elastin, influx of inflammatory cells, TGF-β activation, and apoptosis (25). Elafin treatment also attenuated the defective formation of alveoli seen in the lungs of vehicle-treated control mice after MV-O2 for 24 h. Earlier studies showed that mutant mice engineered to express human elafin in blood vessels were protected against several forms of cardiovascular and lung injury, including hypoxia-induced pulmonary hypertension (63), viral myocarditis (62), myocardial infarction (45), and models of vascular injury (16, 64).
We therefore tested the hypothesis that neonatal mice endowed with endogenous elafin would be protected against the adverse effects of prolonged MV-O2 on matrix elastin and lung growth. Because these mice were genetically modified to express elafin in their lung endothelium, we further hypothesized that elastase inhibition would be sustained beyond the 8-h duration observed after treatment with a single dose of elafin (25) and that targeting expression of elafin to endothelial cells might mitigate the adverse pulmonary vascular effects of MV-O2. We indeed discovered that elafin-expressing (Eexp) mice, contrary to wild-type (WT) pups, showed sustained inhibition of lung elastase and MMP-9 activity during MV-O2, resulting in suppressed elastin degradation, lung inflammation, TGF-β activation, and apoptosis. Scattered lung elastin and emphysematous alveoli observed in WT mice after 36 h of MV-O2 were attenuated in Eexp mice. These differences in lung structure between WT mice and Eexp mice occurred without apparent benefit of elafin with respect to MV-O2-induced disruption of vascular endothelial growth factor (VEGF) signaling or loss of lung microvessels. Thus inhibiting elastase activity in the developing lung exposed to mechanical stretch with O2-rich gas helps to preserve matrix elastin and alveolar formation, but without discernible protection against the adverse effects of MV-O2 on the pulmonary microcirculation.
METHODS
Transgenic mice.
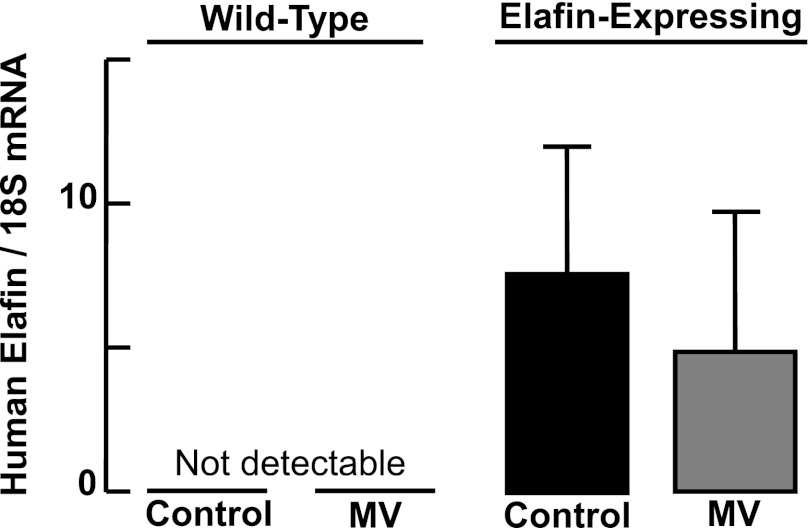
This study used transgenic mice generated to express the human serine elastase inhibitor elafin under regulation of the preproendothelin-1 promoter, assuring a high level of transgene expression in the pulmonary and systemic vasculature, with functional activity measured in both lung and heart tissue, as previously reported (62). These mice were bred into a CD-1 background, with breeding pairs confirmed as homozygous by Southern blot analysis. We used PCR to verify that all mutant mice used in this study expressed the elafin transgene, in contrast to WT pups that did not express the transgene. Preliminary studies showed that lung mRNA expression of human elafin was not significantly different in mechanically ventilated Eexp mice compared with unventilated Eexp controls (Fig. 1).
Fig. 1.
Quantitative RT-PCR for human elafin mRNA relative to 18S rRNA in lung confirms the presence of the transgene in the mutant mice, but not in wild-type pups. Mechanical ventilation (MV) with O2-rich gas (MV-O2) yielded no significant change in lung expression of elafin mRNA (P = 0.32). 18S rRNA was virtually identical between groups. Values are means and SD; n = 5–6/group.
Mechanical Ventilation Experiments
Experimental design.
The study was designed to test the hypothesis that neonatal mice genetically modified to express in their vascular endothelium the human serine elastase inhibitor elafin would be protected against lung injury induced by prolonged MV-O2. We used 5-day-old CD1 WT and Eexp mice that weighed 3–5 g (WT 3.9 ± 0.5 g; Eexp 3.7 ± 0.7 g).
Four groups of mice, all born at term gestation, were studied (9–12 mice per group): WT controls that breathed 40% O2 without MV; WT pups that received MV-O2; Eexp controls that breathed 40% O2 without MV; and Eexp pups that received MV-O2. Mice randomly selected to receive MV-O2 underwent a tracheotomy after sedation with intramuscular ketamine [∼60 μg/g body weight (bw)] and xylazine (∼12 μg/g bw), followed by MV-O2 at 180 breaths/min from a customized small animal respirator (MicroVent 848; Harvard Apparatus, Holliston, MA) for either 24 or 36 h. Tidal volumes were similar between the MV-O2 groups (WT 8.5 ± 1.1 μl/g bw; Eexp 8.2 ± 1.0 μl/g bw). Spontaneously breathing WT and Eexp controls received sedation with ketamine and xylazine for sham surgery (superficial neck incision), after which they breathed 40% O2 without MV for 24 or 36 h.
Experiments lasting 24 h were performed to harvest lungs for quantitative measurement of elastase and MMP-9 activity, NF-κB activation and inflammation, and immunoblot analysis of specific proteins related to the ECM and to lung growth, including pulmonary microvessels. Experiments lasting 36 h were done to harvest lungs for quantitative assessment of lung structure by morphometry and to measure the abundance and distribution of relevant proteins by immunohistochemistry. The longer studies were intended to determine the impact of earlier differences in protease activity and inflammation on subsequent lung elastin deposition and alveolar formation.
Our assisted ventilation protocol was designed to avoid the severe lung injury that typically occurs in response to MV with very high inflation pressures and extreme hyperoxia. Hence, we used relatively modest tidal volumes (∼8 μl/g bw) and airway pressures (peak 13–20 cmH2O, mean 4–6 cmH2O) and limited the inspired O2 fraction to 40%, thereby simulating the MV strategy that has been used to treat infants with respiratory failure. In previous studies (7), similar ventilator settings yielded blood pH and Pco2 values within the physiological range (pH 7.30 ± 0.12, Pco2 37 ± 11 mmHg).
During MV, mice were maintained at a neutral thermal environment and fed via an orogastric tube every 2 h, initially with 10% glucose-0.25% saline solution and later with an elemental amino acid-based infant formula (AA Lipil, Mead-Johnson, Evansville, IN), yielding a daily fluid intake of ∼100–120 μl/g bw, as previously described (6). All pups received a single intramuscular dose of ampicillin (200 μg/g bw) and gentamicin (4 μg/g bw) at the start of each study to reduce the risk of infection. Sedation with ketamine and xylazine (10 and 2 μg/g bw, respectively) was repeated as needed to minimize spontaneous movement and assure comfort. Urine was obtained by suprapubic needle aspiration when the bladder became visibly distended, and 24-h urine collections were frozen for later measurement of desmosine and creatinine concentrations. At the end of each study, pups were euthanized with an intraperitoneal overdose of pentobarbital sodium, ∼150 μg/g bw, and the lungs were excised for various studies as described below.
All surgical and animal care procedures and experimental protocols were reviewed and approved by the Stanford University Institutional Animal Care and Use Committee.
Tissue Assays
Serine elastase activity.
Lung tissue from 24-h studies was snap frozen in liquid N2 and stored at −80°C for measurement of serine elastase activity by a modification of a previously described method (6, 8, 63) that utilizes DQ-elastin substrate (EnzChek Elastase Assay Kit, Invitrogen, Camarillo, CA, cat. no. E12056). Activity was measured in the presence and absence of the serine proteinase inhibitor N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone (0.01 mmol). The selective serine elastase inhibitor elafin (5 μg/ml) was added to some samples, in lieu of the above inhibitor, before DQ-elastin.
MMP-9 activity.
Frozen lung tissue from 24-h studies was homogenized in PBS and electrophoresed onto 10% Tris-glycine gel with 0.1% gelatin incorporated as a substrate (Invitrogen, cat. no. EC61752BOX). SDS was removed from the gels by shaking in 2.5% Triton X-100 for 1 h. The gels were equilibrated in Zymogram Developing Buffer (Invitrogen) to add the divalent metal cation required for enzymatic activity. Elastolytic bands were visualized by Coomassie stain. Densitometric quantification of the protease bands was performed using a Gel Documentation System (Bio-Rad, Hercules, CA), as previously described (25, 63).
Urinary desmosine.
Cumulative 24-h and 36-h urine specimens were frozen for subsequent radioimmunoassay measurements of desmosine and colorimetric assay for creatinine, as previously reported (52).
Protein extraction and immunoblots.
Lungs from 24-h studies (n = 4–6/group) were snap frozen in liquid N2 and stored at −80°C for later protein extraction and immunoblots, as previously described (7, 40). Immunoblot analysis was applied to measure the following proteins: cleaved caspase-3, using a 1:250 dilution of rabbit monoclonal antibody (catalog no. 9664, Cell Signaling Technology, Danvers, MA); pSMAD-2, by using a 1:1,000 dilution of mouse monoclonal antibody (catalog no. 3108, Cell Signaling); VEGF-R2, using a 1:200 dilution of rabbit polyclonal antibody (catalog no. sc-504, Santa Cruz Biotechnology, Santa Cruz, CA); p65-NF-κB, using a 1:700 dilution of rabbit polyclonal antibody (catalog no. sc-372, Santa Cruz); pIκBα, using a 1:1,000 dilution of mouse monoclonal antibody (catalog no. 9246, Cell Signaling); total IκBα, using a 1:1,000 dilution a mouse monoclonal antibody (catalog no. 4814, Cell Signaling); von Willebrand factor (vWF), using a 1:500 dilution of rabbit anti-human polyclonal antibody (catalog no. ab6994, Abcam, Cambridge, MA); endothelial nitric oxide synthase (eNOS), using a 1:1,000 dilution of mouse anti-human monoclonal antibody (catalog no. 610297, BD Transduction Laboratories, San Jose, CA); and CD-31, using a 1:1,000 dilution of rat anti-mouse monoclonal antibody (catalog no. DIA310, Dianova, Hamburg, Germany).
Membranes with applied antibodies were incubated overnight at 4°C, washed three times in a solution containing 0.1% Tween-20 and Tris-buffered saline buffer, and then incubated at room temperature for 1 h with a dilute (1:5,000) solution of a secondary antibody (goat anti-rabbit IgG-HRP; catalog no. sc-2301, Santa Cruz) conjugated to horseradish peroxidase, followed by three washes. The secondary antibody used for pIκBα and total IκBα was a 1:3,500 dilution of goat anti-mouse IgG-HRP (catalog no. 2302; Santa Cruz). Images were detected by chemiluminescence (ECL or ECL+ Detection Kit, Amersham Life Science, Piscataway, NJ) and quantified by densitometry using a Gel Documentation System (Bio-Rad). Membranes were stripped and reprobed with a 1:5,000 dilution of rabbit polyclonal anti-β-actin antibody (ab-8227, Abcam) and incubated for 1 h to provide an internal loading control.
Nuclear and cytoplasmic extracts for NF-κB-p65 and pIκBα proteins.
Lungs from 24-h studies (n = 3–5/group) were snap frozen in liquid N2 and stored at −80°C for later extraction of nuclear and cytoplasmic cell fractions and immunoblot analysis of p65 (nuclear) and pIκBα (cytoplasm) as indexes of NF-κB activation. Harvested lung tissue was pretreated with protease inhibitor (Pierce Biotechnology, Rockford, IL, cat. no. 78410) and homogenized in ice-cold collection buffer supplied in a nuclear protein extraction kit (NE-Per Kit, Pierce Biotechnology, cat. no. 78833). Nuclear and cytoplasmic extracts were obtained according to the manufacturer's instructions, and protein content was quantified by BCA assay. Optimized conditions for immunoblot assays of NF-κB-p65 and pIκBα proteins are reported in the preceding section.
Processing lungs for quantitative histology.
Lungs (n = 5–6/group) were fixed intratracheally with buffered 4% paraformaldehyde (PFA) overnight at 20 cmH2O, as previously described (6, 7). The fixed lungs were excised, their volume was measured by fluid displacement (50), and they were embedded in paraffin for isotropic uniform random sectioning, as previously described (40). Tissue sections (4 μm) were stained with hematoxylin and eosin for quantitative assessment of alveolar area by use of a Bioquant image analysis system (R & M Biometrics, Nashville, TN) (7); radial alveolar counts provided an index of alveolar number (20). The relative amount and distribution of insoluble cross-linked elastin was assessed by quantitative image analysis of Hart's-stained tissue sections, as previously described (6).
Immunohistochemical assessment of TGF-β activation and apoptosis.
To assess nuclear localization of pSmad-2, a marker of TGF-β activation, random tissue sections were pretreated for antigen retrieval (Target Retrieval Solution, Dako, Carpinteria, CA), followed by blocking serum and application of primary antibody (rabbit anti-pSmad-2, 1:500, Cell Signaling, Boston, MA), with overnight incubation at 4°C. Immune complexes were visualized with the relevant peroxidase-coupled secondary antibody by using the Vectastain Kit (Vector, Burlingame, CA). Apoptosis was detected by terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay using the ApopTag In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA) applied to PFA-fixed sections. The Bioquant image analysis system was used to quantify the ratio of stained nuclei to total nuclei in 10–15 ×400-magnification fields in two random sections per animal.
Immunohistochemical staining for monocytes/macrophages and neutrophils.
Lungs from 24-h studies (4–5/group) were instilled intratracheally with IHC Zinc Fixative (BD Pharmingen, San Jose, CA, cat. no. 51-7438KZ) at an inflation pressure of 20 cmH2O overnight at room temperature and were processed as described above for PFA-fixed lungs. Zinc-fixed lung sections were incubated with peroxidase blocking reagent (Dako North America, Carpinteria, CA), then incubated with normal goat serum, and next incubated with antibodies against specific antigens: rat anti-F4/80 (1:400, Abcam), rat anti-Ly-6G (Gr-1) (1:200, eBioscience, San Diego, CA). The slides then were incubated with biotinylated goat anti-rat secondary antibody (1:200, Santa Cruz Biotechnology), washed in PBS and incubated with streptavidin-HRP and diaminobenzidine (+) (Dako North America). Slides were lightly counterstained with hematoxylin I (Richard-Allan Scientific, Kalamazoo, MI). Two tissue sections per animal stained with F4/80 for monocytes/macrophages and with Ly-6G for neutrophils were analyzed by counting the number of positively stained cells in 20 fields at ×400 magnification.
Quantification of microvessels (20–100 μm) in distal lung.
Lungs from 24-h studies (4–5/group) were instilled with IHC Zinc Fixative (BD Pharmingen, cat. no. 51-7438KZ) at an inflation pressure of 20 cmH2O overnight at room temperature, and processed as described above. Localization of the endothelial cell marker, CD-31, was done by incubating 4-μm zinc-fixed tissue sections with the primary antibody, rat anti-CD-31 (1:100, BD Biosciences, San Jose, CA). Immune complexes were visualized with the relevant peroxidase-coupled secondary antibody, provided in the Vectastain Kit (Vector Laboratories). Quantitative image analysis was used to measure the surface area of CD-31 relative to the surface area of distal lung tissue by use of the Bioquant image analysis system. We then applied a modification of a previously described immunohistochemical and morphometric approach (5) for assessing the number of 20-to 100-μm diameter blood vessels in 10 high-power (×400 magnification) fields of distal lung per animal.
RNA extraction and quantitative real-time PCR.
Lungs from 8-h studies (6/group) were excised, weighed, snap frozen in liquid N2, and stored at −80°C for subsequent two-step mRNA extraction using TRIzol reagent (Invitrogen, Carlsbad, CA) and purification with RNeasy Mini Kit columns (Qiagen, Valencia, CA), as previously described (25). Quantitative real-time PCR was applied to measure lung mRNA expression of human elafin and interleukin (IL)-1β using proprietary primer-probe sets [TaqMan Gene Expression Assays, Applied Biosystems, Foster City, CA; human peptidase-3 (elafin), cat. no. Hs00160066-m1, and mouse IL-1β, cat. no. Mm00434228-m1].
Statistical Analysis
Data in the text and figures are reported as means ± SD. Two-way analysis of variance and the Bonferroni post hoc test were performed to compare the two groups of controls and two groups of mechanically ventilated WT and Eexp mice. To compare datasets from two groups of mice (immunoblot analysis), we used Student's unpaired t-test or the nonparametric Mann-Whitney test (for data sets with a skewed distribution). Statistical analysis was done by use of Prism 5 software package (GraphPad, San Diego, CA). Differences were considered statistically significant when the P value was <0.05.
RESULTS
Suppressed Proteolytic Activity and Elastin Breakdown During MV-O2 in Eexp Mice
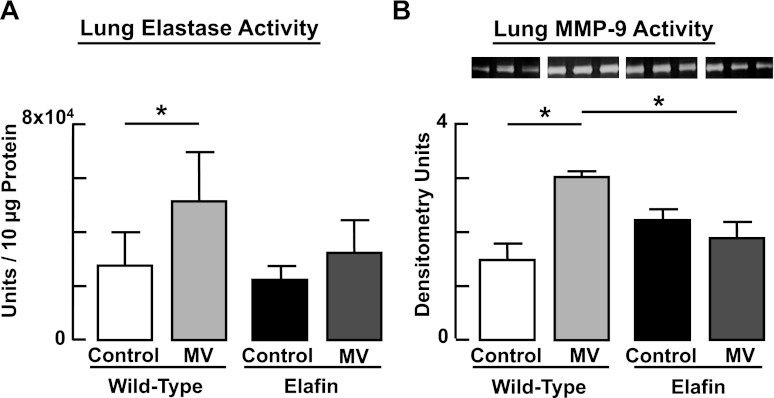
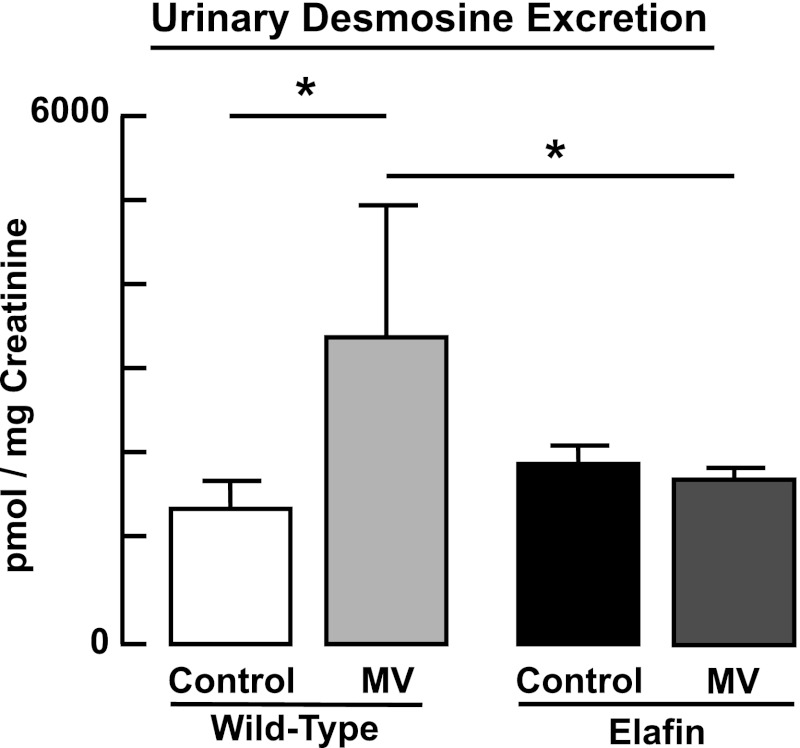
MV-O2 for 24 h caused a twofold increase of both serine elastase activity and MMP-9 activity in lungs of WT mice (Fig. 2). These MV-O2-evoked changes in elastase and MMP-9 activity were suppressed in Eexp mice. Though elafin does not directly inhibit MMPs, by suppressing elastase activity it can inhibit activation of the proform of MMPs and also prevent inactivation of tissue inhibitors of MMPs (30). Urinary excretion of desmosine, a biomarker of elastin breakdown, increased significantly after 24 h of MV-O2 in WT mice, but not in Eexp mice (Fig. 3).
Fig. 2.
Increased lung proteolytic activity elicited by MV-O2 is suppressed in elafin-expressing mice. Elastase activity (A) and matrix metalloproteinase (MMP)-9 (B) activity measured in the lungs of 6-day-old wild-type mice after MV-O2 for 24 h is increased when compared with unventilated controls that breathed 40% O2 for 24 h. In contrast, neither lung elastase activity nor MMP-9 activity increased in response to MV-O2 in lungs of elafin-expressing mice. *Significant difference between groups, P < 0.05. Values are means and SD; n = 4–5/group for elastase activity, n = 3/group for MMP-9 activity.
Fig. 3.
Increased degradation of lung elastin during MV-O2 is suppressed in elafin-expressing mice. Cumulative 24-h urinary excretion of desmosine, normalized to creatinine excretion, is increased in 6-day-old wild-type mice after MV-O2 for 24 h, compared with unventilated controls that breathed 40% O2 for 24 h. In contrast, urinary desmosine excretion was unchanged in response to MV-O2 in elafin-expressing mice when compared with unventilated controls. *Significant difference between groups, P < 0.05. Values are means and SD; n = 4–12/group.
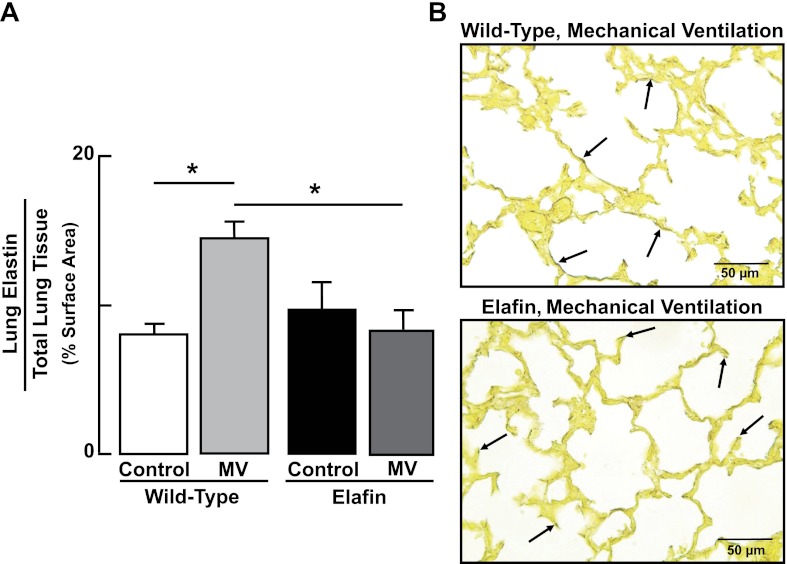
To determine whether inhibition of elastin degradation preserved the normal distribution of elastic fibers at the tips of secondary septa in lungs of Eexp mice exposed to lengthy MV-O2, we used quantitative image analysis to assess the amount and distribution of elastin in lung tissue sections treated with Hart's elastin stain. In WT mice, MV-O2 resulted in elastic fibers being strewn throughout the walls of distal air spaces (Fig. 4). In contrast, lungs of Eexp mice displayed a normal distribution of elastin at the septal tips, with considerably less dispersion of elastic fibers in alveolar walls after MV-O2.
Fig. 4.
MV-O2-induced maldistribution of lung elastin is attenuated in elafin-expressing mice. A: summary data (means and SD) showing increased dispersion of lung elastin, assessed by quantitative image analysis of Hart's-stained tissue sections, in 6-day-old wild-type mice after MV-O2 for 36 h, compared with unventilated controls that breathed 40% O2 for 36 h. Lung elastin distribution was virtually unchanged in response to MV-O2 in elafin-expressing mice when compared with unventilated controls. Surface density of lung elastin, expressed relative to total lung tissue area, was assessed by quantitative image analysis of Hart's-stained tissue sections (12 ×400 fields/animal). *Significant difference between groups, P < 0.05. Values are means and SD; n = 4–6/group. B: representative images of lung tissue sections (×400) showing elastic fiber stain (arrows, darkened areas against yellow counterstained tissue) in distal lung of wild-type mouse (top) compared with elafin-expressing mouse (bottom) after MV-O2 for 36 h.
Suppressed Lung Inflammation During MV-O2 in Eexp Mice
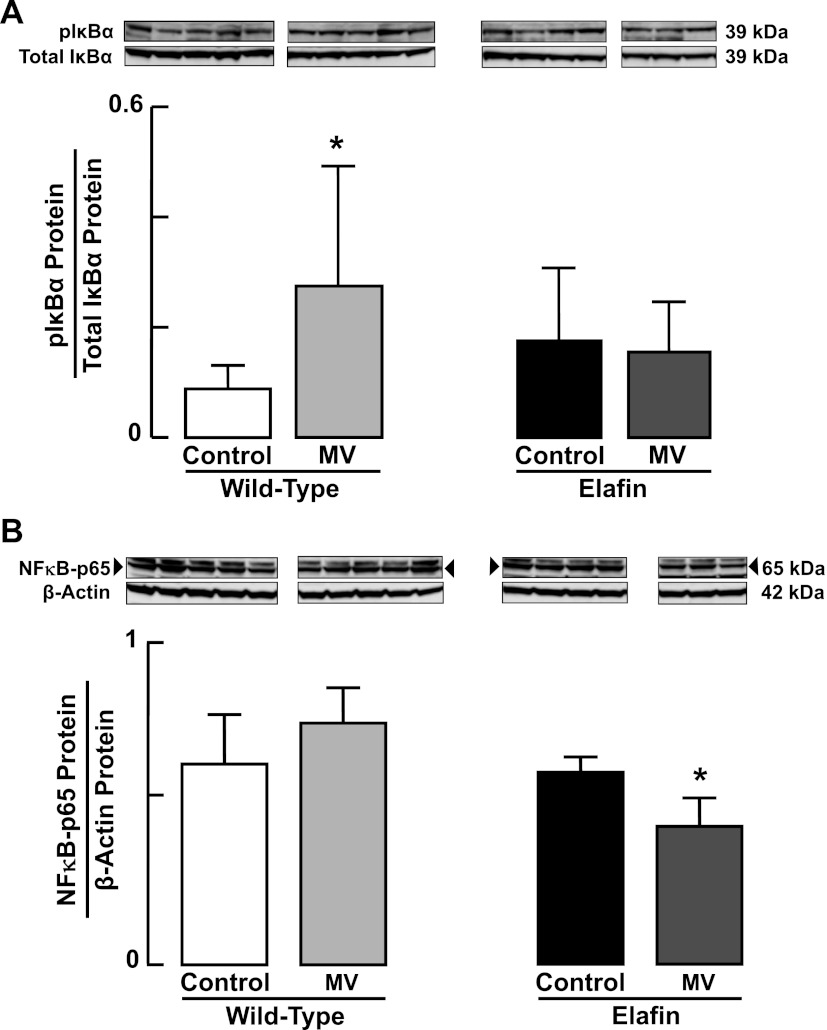
Besides inhibiting elastolytic activity and degradation of elastic fibers, elafin has been reported to suppress activation of the transcription factor NF-κB, which in turn can stimulate an inflammatory response in endothelial cells, monocytes, and macrophages exposed to endotoxin and other atherogenic stimuli (12, 24). We therefore assessed NF-κB activation in lungs of WT and Eexp mice by measuring pIκBα, a key positive regulator of NF-κB transcriptional activity, and p65 protein, a subunit of the NF-κB complex that mediates inflammatory and immune responses. MV-O2 for 24 h led to a threefold increase in lung content of pIκBα protein (Fig. 5A) in WT mice, but without a significant increase in lung content of p65 nuclear protein (Fig. 5B). In contrast, lungs of Eexp mice showed unchanged abundance of pIκBα protein (Fig. 5A), and significantly reduced p65 nuclear protein content after 24 h of MV-O2 (Fig. 5B). Since NF-κB is known to regulate expression of several proinflammatory cytokines, including IL-1β, we assessed lung expression of IL-1β in Eexp mice and found that it was significantly reduced in response to MV-O2 compared with its expression in lungs of unventilated Eexp controls (data not shown).
Fig. 5.
Increased pIκBα-NF-κB signaling during MV-O2 is suppressed in elafin-expressing mice. A: immunoblots showing increased pIκBα protein relative to total IκBα protein in cytoplasmic extracts of lungs obtained from 6-day-old wild-type mice after MV-O2 for 24 h, compared with unventilated controls that breathed 40% O2 for 24 h (left). pIκBα protein was unchanged in response to MV-O2 in cytoplasmic extracts of lungs obtained from elafin-expressing mice compared with unventilated controls (right). B: immunoblots showing suppression of NF-κB-p65 protein relative to β-actin protein in nuclear extracts of lungs obtained from 6-day-old elafin-expressing mice after MV-O2 for 24 h, compared with unventilated controls that breathed 40% O2 for 24 h (right). NF-κB-p65 protein did not change significantly in nuclear extracts of lungs obtained from wild-type mice after MV-O2 for 24 h (left). *Significant difference compared with Control, P < 0.05. Values are means and SD; n = 3–5/group.
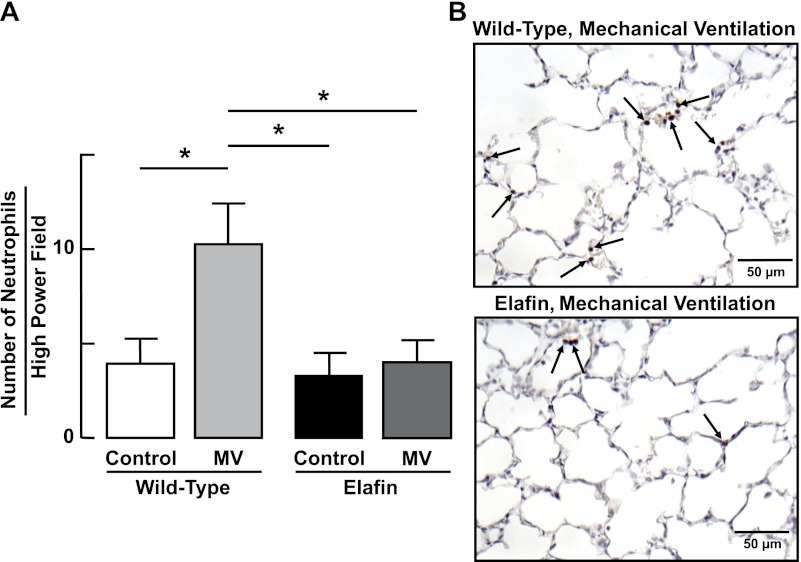
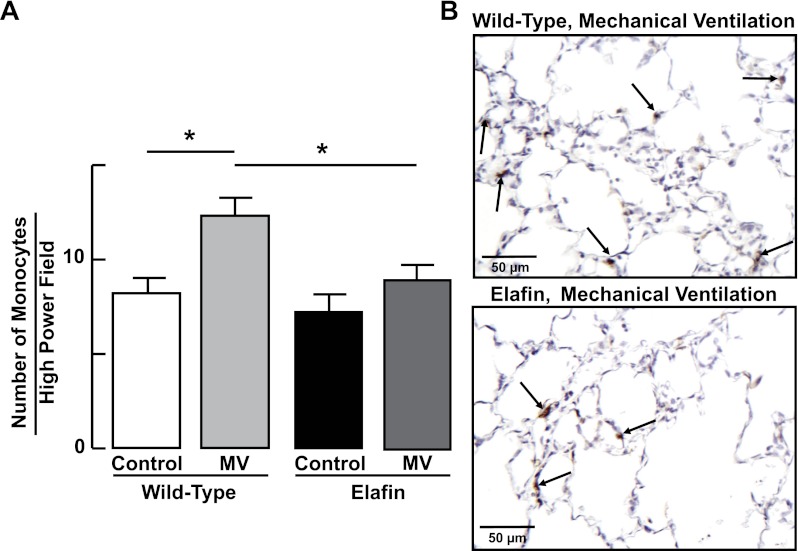
Consistent with these findings, MV-O2 for 24 h evoked an inflammatory response in WT mice, as evidenced by a threefold increase in neutrophils (Fig. 6), and a lesser but significant increase in monocytes (Fig. 7) in distal lung. The inflammatory response to MV-O2 was suppressed in Eexp mice, whose lungs exhibited no significant increase of neutrophils (Fig. 6) or monocytes (Fig. 7) after 24 h of MV-O2.
Fig. 6.
Lung influx of neutrophils during MV-O2 is suppressed in elafin-expressing mice. A: summary data (means and SD) showing increased numbers of neutrophils (counted in 20 ×400 fields) in lungs of 6-day-old wild-type mice after MV-O2 for 24 h, compared with unventilated controls that breathed 40% O2 for 24 h. Neutrophil counts were unchanged in response to MV-O2 in elafin-expressing mice compared with unventilated controls. *Significant difference between groups, P < 0.05. Values are means and SD; n = 4–6/group. B: representative images of lung tissue sections (×400) showing neutrophils (arrows, brown stain of Ly-6G neutrophil antibody) in lungs of wild-type (top) and elafin-expressing (bottom) mice after MV-O2 for 24 h.
Fig. 7.
Lung influx of monocytes during MV-O2 is suppressed in elafin-expressing mice. A: summary data (means and SD) showing increased numbers of monocytes (counted in 20 ×400 fields) in lungs of 6-day-old wild-type mice after MV-O2 for 24 h, compared with unventilated controls that breathed 40% O2 for 24 h. Monocyte counts did not increase significantly in response to MV-O2 in elafin-expressing mice compared with unventilated controls. *Significant difference between groups, P < 0.05. Values are means and SD; n = 4–6/group. B: representative images of lung tissue sections (×400) showing monocytes (arrows, brown stain of F4/80 monocyte antibody) in lungs of wild-type (top) and elafin-expressing (bottom) mice after MV-O2 for 24 h.
Suppressed MV-O2 Induced TGF-β Activation and Apoptosis in Eexp Mice
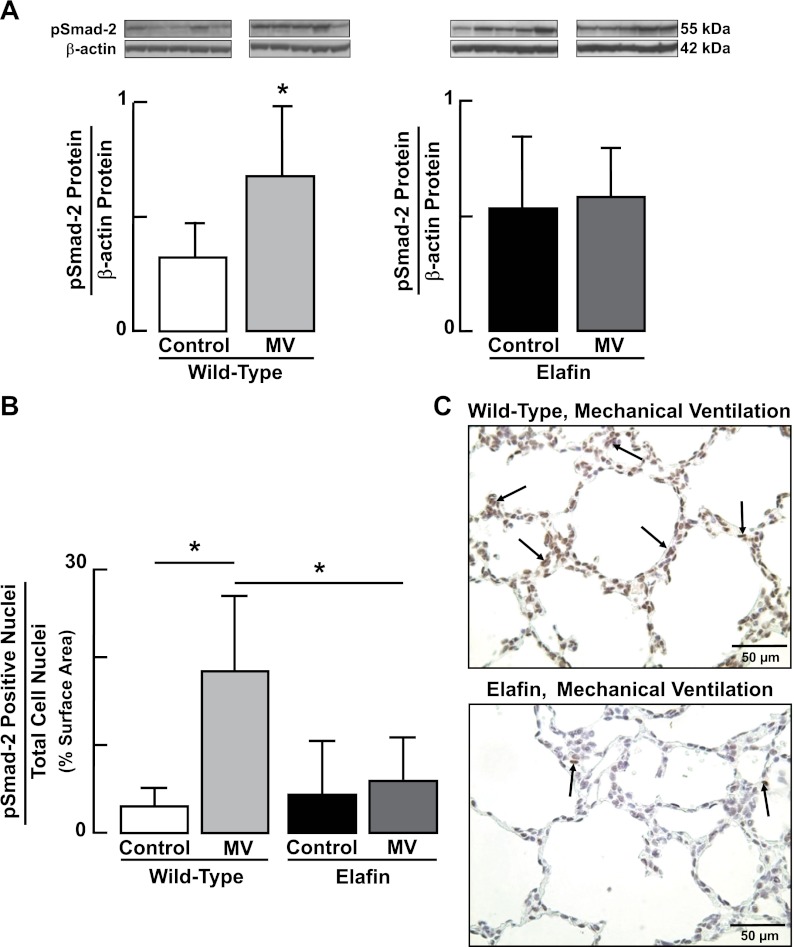
We previously reported increased TGF-β signaling in lungs of newborn mice exposed to MV for 24 h, with or without associated hyperoxia (25, 40). In this study, lungs of WT mice showed a twofold increase of pSmad-2 protein after MV-O2 for 24 h (Fig. 8A) and a sixfold increase in nuclear expression of pSmad-2 protein in distal lung tissue after 36 h of MV-O2 (Fig. 8B). These changes, which are indicative of TGF-β activation, were abrogated in Eexp mice exposed to MV-O2 for 24–36 h (Fig. 8).
Fig. 8.
Increased activation of TGF-β in the lungs of newborn mice during MV-O2 is suppressed in elafin-expressing mice. A: immunoblots showing increased pSmad-2 protein relative to β-actin protein, indicative of TGF-β activation, in lungs of 6-day-old wild-type mice after MV-O2 for 24 h, compared with unventilated controls that breathed 40% O2 for 24 h (left). pSmad-2 protein was unchanged in response to MV-O2 in lungs of elafin-expressing mice compared with unventilated controls (right). B: summary data showing increased nuclear staining for pSmad-2 (assessed in 12 random ×400 fields/animal) in lungs of 6-day-old wild-type mice after MV-O2 for 36 h, compared with unventilated controls that breathed 40% O2 for 36 h. pSmad-2 nuclear staining was unchanged in lungs of elafin-expressing mice compared with unventilated controls. *Significant difference compared with Control, P < 0.05. Values are means and SD; n = 5/group. C: representative images of lung tissue sections (×400) showing pSmad-2 protein expression (arrows, brown stain) in lungs of 6-day-old wild-type (top) and elafin-expressing (bottom) mice after MV-O2 for 36 h.
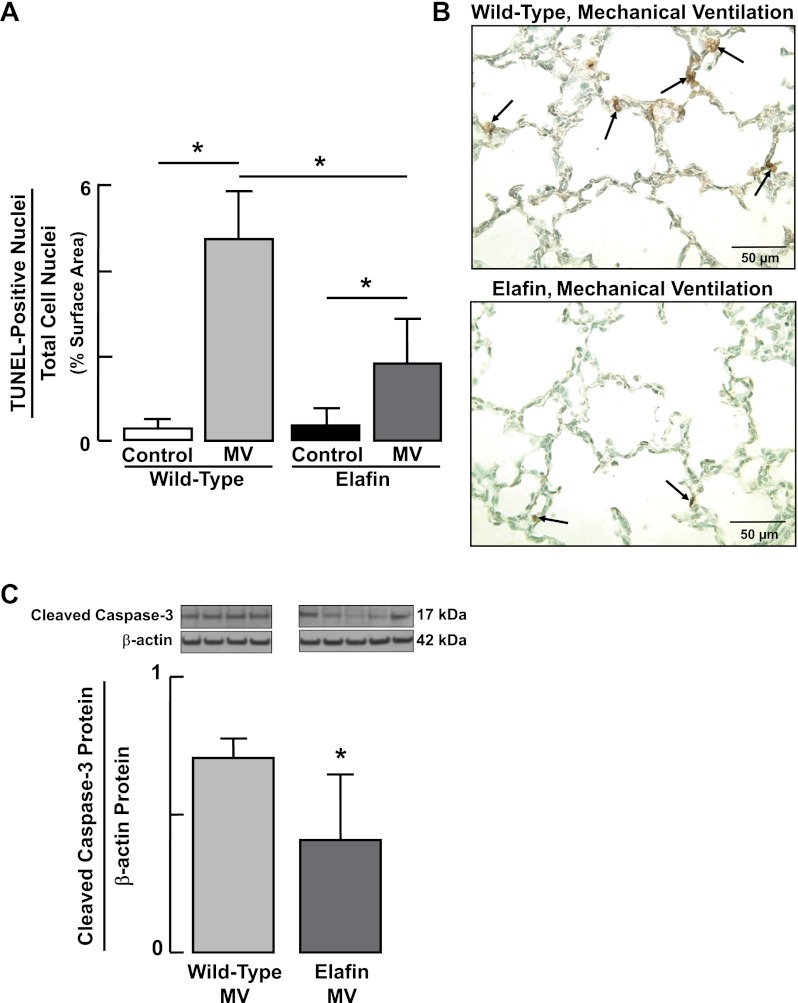
As TGF-β activation is known to induce apoptosis of both alveolar epithelial cells (2) and microvascular endothelial cells (36), we assessed apoptosis in lungs of WT and Eexp mice by immunoblot measurement of cleaved caspase-3 after 24 h of MV-O2, and by quantitative immunohistochemistry using the TUNEL assay, after 36 h of MV-O2. MV-O2 for 36 h caused a 16-fold increase in apoptosis in lungs of WT mice; this was significantly greater than the increase in lung cell apoptosis noted in Eexp mice (Fig. 9, A and B). Likewise, the increased lung abundance of cleaved caspase-3 that occurred in WT mice after 24 h of MV-O2 was suppressed in Eexp mice (Fig. 9C).
Fig. 9.
Increased lung cell apoptosis induced during MV-O2 is suppressed in elafin-expressing mice. A: summary data (means and SD) showing increased terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL)-positive nuclear staining, an index of apoptosis, in lungs of 6-day-old wild-type mice after MV-O2 for 36 h, compared with unventilated controls that breathed 40% O2 for 36 h. Lungs of elafin-expressing mice also exhibited increased TUNEL stain after MV-O2 for 36 h compared with unventilated controls, but the magnitude of the increase was significantly less than it was in wild-type mice. TUNEL stain was assessed by quantitative image analysis of 12 ×400 fields/animal. B: representative images of lung tissue sections (×400) showing TUNEL-stained nuclei (arrows, brown stain) in distal lungs of wild-type (top) and elafin-expressing (bottom) mice after MV-O2 for 36 h. C: immunoblots for cleaved caspase-3, showing suppression of apoptosis in elafin-expressing mice, compared with wild-type mice, after MV-O2 for 24 h. *Significant difference between groups, P < 0.05. Values are means and SD; n = 4–6/group for (A), n = 4–5/group for (C).
Attenuated Lung Structural Changes After Prolonged MV-O2 in Eexp Mice
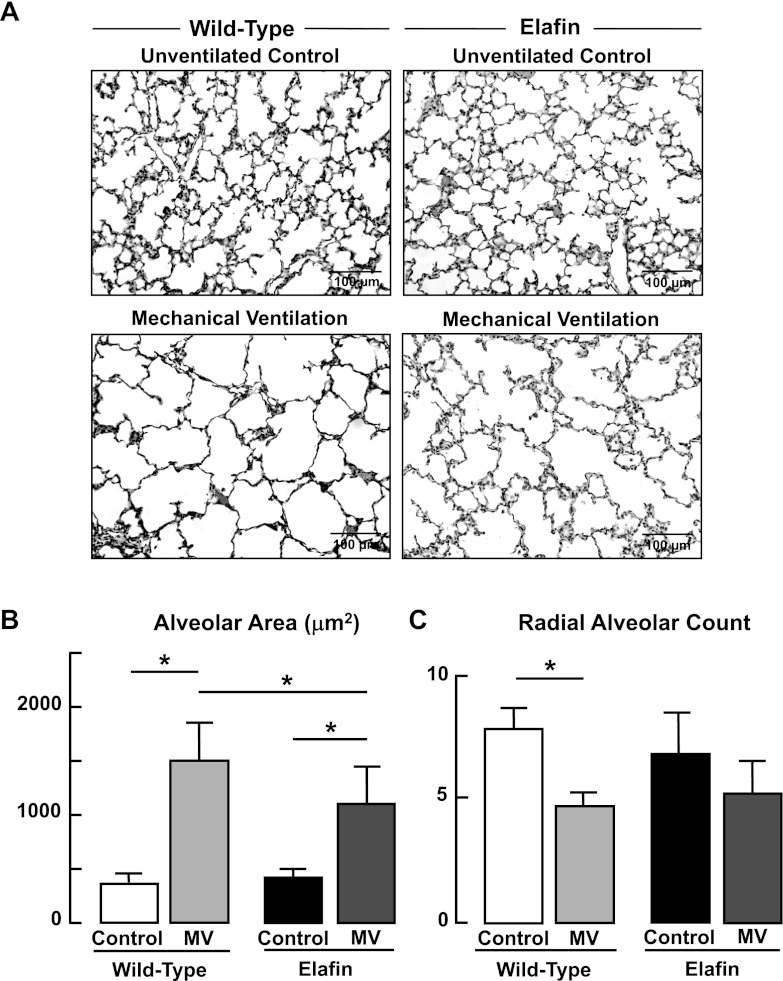
Previous studies showed that MV-O2 for 24 h inhibits alveolar septation and thereby impairs lung growth in newborn mice (6, 7). A subsequent study showed that intratracheal treatment of neonatal mice with recombinant human elafin mitigated this MV-O2-evoked effect on lung structure (25). In this study, WT pups received MV-O2 for a longer period of time (36 h), after which morphometric assessment of lung structure showed a fivefold increase in alveolar size and a 40% reduction in alveolar number as assessed by radial alveolar counts (Fig. 10). These changes in lung structure were attenuated in Eexp pups that were exposed to MV-O2 for 36 h. Lung volumes did not increase with MV-O2 in either WT (Control 77 ± 3 μl/g bw; MV-O2 68 ± 2 μl/g bw) or Eexp (Control 75 ± 8 μl/g bw; MV-O2 67 ± 12 μl/g bw) pups. These results indicate that structural changes caused by MV-O2 in WT mice reflect reduced alveolar septation that was attenuated in Eexp mice subjected to MV-O2 for 36 h.
Fig. 10.
Impaired alveolar formation elicited by MV-O2 is attenuated in elafin-expressing mice. A: representative lung tissue sections (×200) from 6-day-old wild-type and elafin-expressing mice after MV-O2 for 36 h, showing increased air space size in both groups when compared with unventilated controls that breathed 40% O2 for 36 h. B: summary data (means and SD) for alveolar area, assessed by quantitative image analysis of lung tissue sections from wild-type and elafin-expressing mice after MV-O2 for 36 h, compared with unventilated controls. C: summary data (means and SD) for radial alveolar counts, an index of alveolar number, of lung tissue sections from wild-type and elafin-expressing mice after MV-O2 for 36 h, compared with unventilated controls that breathed 40% O2 for 36 h. Radial alveolar counts decreased significantly after MV-O2 of wild-type mice, whereas there was not a significant reduction seen in the lungs of elafin-expressing mice. *Significant difference between groups, P < 0.05; n = 4–5/group.
Elafin Does Not Prevent the Adverse Effects of MV-O2 on Lung Microvessels
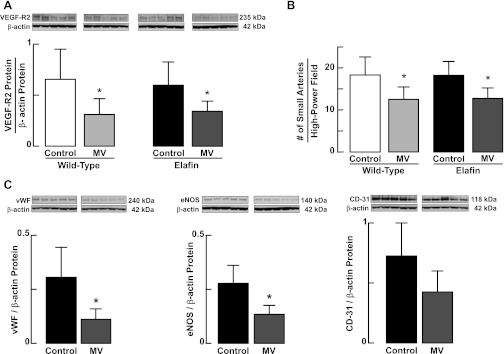
As MV-O2 can disrupt VEGF signaling, resulting in reduced expression of VEGF-R2 (7, 8) and decreased abundance of microvessels in lung (6), we reasoned that the adverse effects of MV-O2 on the pulmonary circulation also might be suppressed by endogenous elafin. We found, however, that MV-O2 for 24 h yielded similar decreases in VEGF-R2 protein (Fig. 11A) and the number of microvessels (Fig. 11B) in lungs of both WT and Eexp pups. Immunoblot analysis of three other endothelial cell markers, vWF, eNOS, and CD-31, confirmed that endothelial expression of elafin did not mitigate the adverse effects of MV-O2 on the pulmonary vasculature. MV-O2 for 24 h reduced lung abundance of vWF and eNOS proteins, and suppressed lung content of CD-31 when compared with unventilated Eexp controls, although the latter difference was not statistically significant (Fig. 11C). Thus elafin targeted to the pulmonary endothelium did not prevent MV-O2-induced loss of lung microvessels but did help to preserve alveolar structure.
Fig. 11.
Endogenous elafin expression does not protect against the adverse effects of MV-O2 on the pulmonary circulation. A: immunoblots for VEGF-R2 protein relative to β-actin in lungs of 6-day-old wild-type and elafin-expressing mice after breathing 40% O2 for 24 h either without mechanical ventilation (Control) or with MV, showing that lung content of VEGF-R2 protein decreased to a similar degree in both wild-type and elafin-expressing pups. B: MV with 40% O2 for 24 h reduced the number of small arteries (20–100 μm in diameter) in lungs of both wild-type and elafin-expressing neonatal mice. Small arteries were quantified in zinc-fixed lung tissue sections that were immunostained for the endothelial marker CD-31. Bars show average obtained from imaging 10–15 ×400 fields in 2 tissue sections/animal. C: immunoblots for 3 endothelial cell markers, von Willebrand factor (vWf), endothelial nitric oxide synthase (eNOS), and CD-31 [also known as platelet endothelial cell adhesion molecule (PECAM)-1] relative to β-actin in lungs of elafin-expressing mice after breathing 40% O2 for 24 h either without MV (Control) or with MV, showing that lung content of vWf and eNOS proteins decreased significantly in response to MV-O2. The pattern of suppression of CD-31 was similar, but the difference between Control and MV groups was not statistically significant (P = 0.12). Values are means and SD; n = 5/group. *Significant difference vs. Control, P < 0.05.
DISCUSSION
We recently reported (25) that intratracheal treatment of newborn mice with a single dose of recombinant human elafin at the start of MV-O2 suppressed lung elastase and MMP-9 activity for 8 h, thus preventing elastic fiber fragmentation and resultant inflammation, TGF-β activation, and apoptosis, and enabling lung growth during MV-O2 for 24 h. In this study we set out to determine whether endogenous expression of elafin in the lungs of newborn mice would yield more sustained suppression of proteolytic activity and protection against the adverse pulmonary effects of MV-O2 for up to 36 h. We discovered that neonatal mice genetically modified to express elafin in lung endothelium, in response to MV-O2, exhibited suppressed lung elastolytic activity, elastin breakdown, inflammation, TGF-β activation and apoptosis, and attenuated lung structural abnormalities seen in control mice after MV-O2 for 36 h. Taken together, these findings provide new insights regarding the pathogenesis and possible prevention of lung growth arrest caused by lengthy exposure to MV-O2 during early postnatal life. It is noteworthy, however, that endogenous expression of elafin in the lung endothelium did not protect against the adverse effects of MV-O2 on the pulmonary vasculature, reflected in reduced abundance of endothelial cell markers and loss of lung microvessels observed in both WT and Eexp mice after 24 h of MV-O2.
Elafin Targeted to the Lung Endothelium
A low-molecular-weight (∼6 kDa) inhibitor of human neutrophil elastase and proteinase-3, elafin is expressed in several human tissues, including lung (41), where it is secreted by epithelial cells and detectable in airway secretions (48, 49). Elafin is secreted as a proform, referred to as “preelafin” (also known as “Trappin-2”), which bears a transglutaminase domain that facilitates its adherence to ECM proteins and helps preserve its antiproteolytic action (22). Elafin does not appear to be expressed in mice (47), but mutant mice that were engineered to express elafin in lung were protected against injury caused by intratracheal injection of Pseudomonas aeruginosa (51). In our study, elafin was targeted to the lung endothelium by virtue of its linkage to a preproendothelin-1 promoter, which allows for expansive elafin expression throughout the lung parenchyma. Previous studies with this transgenic mouse model showed high lung expression of the elafin transgene, which mitigated the pulmonary hypertensive response to chronic hypoxia (63).
Suppression of Lung Elastolytic Activity Prevents Elastic Fiber Fragmentation and Associated Inflammation
In an earlier study (6) we found that MV-O2 for 8 h caused a fourfold increase of serine elastase activity in lungs of 4-day-old Balb/c mice, resulting in increased tropoelastin production and scattering of elastic fibers throughout the walls of terminal air spaces after 24 h of MV-O2. These changes evolved without apparent inflammation, prompting speculation that increased elastase activity derived from lung parenchymal cells, perhaps from smooth muscle cells or fibroblasts, both of which exhibit inducible elastase activity (43, 56, 60).
In this study, exposure of 5-day-old, WT CD-1 mice to MV-O2 for 24 h caused a twofold increase of serine elastase activity and MMP-9 activity in lung, and a corresponding increase in urinary excretion of desmosine, a biomarker of elastin degradation. These changes were inhibited in Eexp mice after 24 h of MV-O2. Likewise, the associated inflammatory response seen in WT mice, as reflected by NF-κB activation and alveolar influx of neutrophils and monocytes, was fully suppressed in Eexp mice. Elastase in Eexp mice was associated with decreased dispersion of elastic fibers and reduced apoptosis than was seen in the lungs of WT mice after 24 h of MV-O2. Elastin fragments are known to induce pulmonary inflammation and apoptosis and thereby contribute to emphysematous changes in lung structure (4, 27). Inhibition of elastin degradation and associated inflammation likely helped to reduce the lung structural abnormalities seen in Eexp mice, compared with WT mice, after MV-O2 for 36 h.
Suppression of TGF-β Activation by Elafin
We previously reported that MV-O2 for 24 h increased TGF-β in lungs of preterm lambs, leading to dysregulated elastin production and defective alveolar septation (8). Similar findings were noted in the lungs of mechanically ventilated newborn mice that showed increased pulmonary expression of pSmad-2, a marker of TGF-β activation (40). A study of adult mice showed that increased elastase activity can trigger the release of growth factors from the lung, including TGF-β, which can boost tropoelastin production in lung fibroblasts (11). Another study showed enhanced release of TGF-β in cultured human airway smooth muscle cells exposed to neutrophil elastase by a mechanism involving NF-κB activation (34). TGF-β has been shown to increase production of tropoelastin mRNA and protein in cultured neonatal lung fibroblasts (38) and to induce apoptosis of pulmonary epithelial and microvascular endothelial cells (2, 36). Excessive TGF-β signaling has been linked to impaired elastogenesis, apoptosis, and defective alveolar septation in the lungs of neonatal mice devoid of latent TGF-β binding proteins 3 and 4 (15, 17, 18). Other reports indicate that overexpression of TGF-β in lungs of newborn rodents results in abnormal formation of alveoli and microvessels, similar to the pathology seen in CLD (21, 57).
In this study, inhibition of increased TGF-β signaling in response to MV-O2 in Eexp mice likely contributed to preserving lung elastin at the tips of secondary septa and reducing the apoptosis seen in lungs of WT mice after MV-O2. Thus elafin suppression of TGF-β activation may account, at least in part, for stabilizing lung elastin, mitigating apoptosis, and enabling lung growth in Eexp mice during MV-O2 for 36 h. Our finding that endogenous elafin did not prevent loss of lung microvessels associated with prolonged MV-O2 suggests that elafin's suppression of TGF-β signaling had little or no impact on endothelial cell survival during exposure to cyclic stretch with O2-rich gas. This finding also may help to explain why lung growth was not fully preserved during MV-O2 for 36 h, since VEGF signaling and angiogenesis are critical determinants of normal alveolar formation and lung growth (54).
Working Model Revisited
In a recent report describing the lung-protective effects of intratracheal delivery of elafin in mechanically ventilated newborn mice, we proposed a working model of lung growth arrest caused by MV-O2 and the impact of elafin treatment on this form of lung injury (25). Our thesis was that prolonged cyclic stretch with O2-rich gas evokes increased serine elastase activity in lung, which leads to degradation and dispersion of matrix elastin. Fragmented elastic fibers, coupled with NF-κB activation, yield an inflammatory response that further stimulates elastase and MMP-9 activity. This increased proteolytic activity causes TGF-β activation, which results in increased lung cell apoptosis and matrix remodeling, culminating in defective alveolar septation and lung growth arrest. The impact of elafin treatment was to suppress serine elastase activity induced by MV-O2, thereby abrogating fragmentation and dispersion of elastin and suppressing the inflammatory response, notably activation of NF-κB and MMP-9, and lung influx of neutrophils and monocytes. Although elastase inhibition is not known to suppress MMPs directly, inhibiting elastase activity has been shown to block activation of the proform of these enzymes and to prevent inactivation of tissue inhibitors of MMPs (30). Elastase inhibition also may block activation and release of TGF-β from the extracellular matrix (11), which in turn may protect against apoptosis and loss of alveolar septa (2, 36), thus enabling lung growth during MV-O2. Our results, using a second experimental approach, namely neonatal mice genetically modified to express elafin in the lung endothelium during MV-O2 for up to 36 h, are consistent with this hypothetical model of ventilator-induced neonatal lung growth arrest. The apparent failure of endogenous elafin to prevent impaired VEGF signaling and loss of lung microvessels during MV-O2 may help explain the incomplete maintenance of alveolar growth in Eexp mice exposed to MV-O2 for 36 h.
Why elafin targeted to the endothelium did not protect against MV-O2-induced vascular injury is unclear. Lung expression of human elafin was not reduced to a significant degree by lengthy MV-O2 of Eexp pups. Although there was less apoptosis noted in lungs of Eexp mice than in WT mice after MV-O2 for 24 h, we cannot exclude the possibility that lung endothelial cells constituted a greater proportion of the apoptotic cells in Eexp mice than in WT mice after lengthy MV-O2. Our finding that elafin expression is linked to NF-κB inhibition during MV-O2 could account for the apparent failure of elafin to preserve the survival of lung endothelial cells exposed to MV-O2, as a recent report showed that inhibiting NF-κB in neonatal mice disrupts postnatal pulmonary angiogenesis and alveolarization, thereby causing lung growth arrest (29). This study found that blocking constitutive NF-κB activity in lung endothelial cells of newborn mice lessened survival and proliferation of these cells, reduced their expression of VEGF-R2, and impaired in vitro angiogenesis. Thus, by inhibiting NF-κB activation during MV-O2, elafin may have undermined pulmonary endothelial cell survival while suppressing the inflammatory response to MV-O2 and preserving matrix elastin.
Our results are consistent with the notion that lung growth is dependent on no fewer than two distinct molecular pathways that regulate the formation of alveoli and microvessels in the developing lung. One such regulatory mechanism appears to encompass the extracellular matrix, more specifically the components that dictate the elastic properties of the lung, namely tropoelastin and the various genes that govern elastin assembly, of which TGF-β is a key modulator. A second regulatory mechanism features a proangiogenic molecular pathway that includes the hypoxia inducible factors, HIF-1α and HIF-1β, VEGF and its receptors, and several downstream targets including nitric oxide and apelin. On the basis of this study's results, elafin, by inhibiting elastase activity and associated inflammation, helps to preserve the integrity of elastic fibers and suppress TGF-β signaling during MV-O2, thereby limiting apoptosis and alveolar disruption. However, elafin afforded no apparent protection against the adverse effects of MV-O2 on the proangiogenic regulatory pathway, perhaps related to its inhibition of NF-κB, such that VEGF signaling was impaired and pulmonary microvessels were lost.
Clinical Relevance
In newborn infants whose lungs are incompletely developed, a lengthy period of MV-O2 often leads to a chronic form of respiratory distress that has been designated as “the new BPD” (33), herein described as CLD. Most infants who are afflicted with this disease have been born very prematurely and are at risk of acquiring respiratory distress syndrome. Such infants are typically treated with surfactant, which facilitates effective respiratory gas exchange in response to gentle MV applied with relatively small tidal volumes and modest amounts of extra O2. Development of CLD has been linked to increased lung inflammation and elastase activity, resulting in breakdown of lung elastin, documented by increased urinary excretion of desmosine (10, 39). These observations provided rationale for a clinical trial of α1-proteinase inhibitor (α1-PI) treatment designed to prevent or reduce the severity of CLD in high-risk, extremely premature infants (53). Although there was no statistically significant difference in the incidence of CLD in infants treated with α1-PI compared with infants that received placebo, there was a trend toward less CLD in the α1-PI-treated group (P = 0.06), which also had a lower incidence of pulmonary hemorrhage. Despite this intriguing outcome, there have been no subsequent clinical trials testing the potential benefit of either α1-PI treatment or other more potent, specific elastase inhibitors, such as elafin, in protecting high-risk newborns from the deleterious effects of MV-O2. Coupled with our recent report, which showed the feasibility and efficacy of elafin treatment to protect against ventilator-induced lung injury in newborn mice (25), the results of this study provide further rationale for conducting such a trial.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL086631 (R. D. Bland), HL108797, project 2 (R. D. Bland), HL086216 (K. Parai), and HL082662 (M. R. Nicolls); by Deutsche Forschungsgemeinschaft Grant HI1315/3-1 (A. Hilgendorff); and from funds provided by the Vera Moulton Wall Center for Pulmonary Vascular Disease at Stanford University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Albertine KH, Kim BI, Kullama LK, Starcher BC, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med 159: 945–958, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med 173: 318–326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 28: 555–562, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bland RD, Albertine KH, Carlton DP, Kullama LK, Davis PL, Cho SC, Kim B, Dahl M, Tabatabaei N. Chronic lung injury in preterm lambs: abnormalities of the pulmonary circulation and lung fluid balance. Pediatr Res 48: 64–74, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol 294: L3–L14, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol 293: L1099–L1110, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KSK. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 292: L1370–L1384, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF, Wedig KE. Risk factors for the degradation of lung elastic fibers in the ventilated neonate. Am Rev Respir Dis 146: 204–212, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Bruce MC, Wedig KE, Jentoft N, Martin RJ, Cheng PW, Boat TF, Fanaroff AA. Altered urinary excretion of elastin cross-links in premature infants who develop bronchopulmonary dysplasia. Am Rev Respir Dis 131: 568–572, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Buczek-Thomas JA, Lucey EC, Stone PJ, Chu CL, Rich CB, Carreras I, Goldstein RH, Foster JA, Nugent MA. Elastase mediates the release of growth factors from lung in vivo. Am J Respir Cell Mol Biol 31: 344–350, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Butler MW, Robertson I, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG. Elafin prevents lipopolysaccharide-induced AP-1 and NF-κB activation via an effect on the ubiquitin-proteasome pathway. J Biol Chem 281: 34730–34735, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Coalson JJ. Pathology of chronic lung disease of early infancy. In: Chronic Lung Disease in Early Infancy, edited by Bland RD, Coalson JJ. New York: Dekker, 2000, p. 85–124 [Google Scholar]
- 14. Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 160: 1333–1346, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol 167: 419–428, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cowan B, Baron O, Crack J, Coulber C, Wilson GJ, Rabinovitch M. Elafin, a serine elastase inhibitor, attenuates post-cardiac transplant coronary arteriopathy and reduces myocardial necrosis in rabbits afer heterotopic cardiac transplantation. J Clin Invest 97: 2452–2468, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dabovic B, Chen Y, Choi J, Davis EC, Sakai LY, Todorovic V, Vassallo M, Zilberberg L, Singh A, Rifkin DB. Control of lung development by latent TGF-β binding proteins. J Cell Physiol 226: 1499–1509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-β activity. J Cell Physiol 219: 14–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das KC, Ravi D, Holland W. Increased apoptosis and expression of p21 and p53 in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal 6: 109–116, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child 35: 544–547, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyot N, Zani ML, Maurel MC, Dallet-Choisy S, Moreau T. Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase. Biochemistry 44: 15610–15618, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hargitai B, Szabo V, Hajdu J, Harmath A, Pataki M, Farid P, Papp Z, Szende B. Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr Res 50: 110–114, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Henriksen PA, Hitt M, Xing Z, Wang J, Haslett C, Riemersma RA, Webb DJ, Kotelevtsev YV, Sallenave JM. Adenoviral gene delivery of elafin and secretory leukocyte protease inhibitor attenuates NF-κB-dependent inflammatory responses of human endothelial cells and macrophages to atherogenic stimuli. J Immunol 172: 4535–4544, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hilgendorff A, Parai K, Ertsey R, Jain N, Navarro EF, Peterson JL, Tamosiuniene R, Nicolls MR, Starcher BC, Rabinovitch M, Bland RD. Inhibiting lung elastase activity enables lung growth in mechanically ventilated newborn mice. Am J Respir Crit Care Med 184: 537–546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hislop AA, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev 15: 147–164, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 116: 753–759, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710–717, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Iosef C, Alastalo TP, Hou Y, Chen C, Adams ES, Lyu SC, Cornfield DN, Alvira CM. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol 302: L1023–L1036, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Itoh Y, Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem 270: 16518–16521, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Jesudason R, Black L, Majumdar A, Stone P, Suki B. Differential effects of static and cyclic stretching during elastase digestion on the mechanical properties of extracellular matrices. J Appl Physiol 103: 803–811, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Jesudason R, Sato S, Parameswaran H, Araujo AD, Majumdar A, Allen PG, Bartolak-Suki E, Suki B. Mechanical forces regulate elastase activity and binding site availability in lung elastin. Biophys J 99: 3076–3083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, Wang CH, Chung KF, Kuo HP. Neutrophil-derived elastase induces TGF-β1 secretion in human airway smooth muscle via NF-κB pathway. Am J Respir Cell Mol Biol 35: 407–414, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature 393: 276–280, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Lu Q, Patel B, Harrington EO, Rounds S. Transforming growth factor-β1 causes pulmonary microvascular endothelial cell apoptosis via ALK5. Am J Physiol Lung Cell Mol Physiol 296: L825–L838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Margraf LR, Tomashefski JF, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis 143: 391–400, 1991 [DOI] [PubMed] [Google Scholar]
- 38. McGowan SE. Influences of endogenous and exogenous TGF-β on elastin in rat lung fibroblasts and aortic smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 263: L257–L263, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DKI, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. J Clin Invest 72: 656–666, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol 298: L23–L35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nara K, Ito S, Ito T, Suzuki Y, Ghoneim MA, Tachibana S, Hirose S. Elastase inhibitor elafin is a new type of proteinase inhibitor which has a transglutaminase-mediated anchoring sequence termed “cementoin.” J Biochem 115: 441–448, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: bronchopulmonary dysplasia. N Engl J Med 276: 357–368, 1967 [DOI] [PubMed] [Google Scholar]
- 43. Numanami H, Koyama S, Sato E, Haniuda M, Nelson DK, Hoyt JC, Freels JL, Habib MP, Robbins RA. Serine protease inhibitors modulate chemotactic cytokine production by human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol 284: L882–L890, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis 130: 817–821, 1984 [DOI] [PubMed] [Google Scholar]
- 45. Ohta K, Nakajima T, Cheah AY, Zaidi SH, Kaviani N, Dawood F, You XM, Liu P, Husain M, Rabinovitch M. Elafin-overexpressing mice have improved cardiac function after myocardial infarction. Am J Physiol Heart Circ Physiol 287: H286–H292, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol 272: L452–L460, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Sallenave JM, Cunningham GA, James RM, McLachlan G, Haslett C. Regulation of pulmonary and systemic bacterial lipopolysaccharide responses in transgenic mice expressing human elafin. Infect Immun 71: 3766–3774, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sallenave JM, Silva A. Characterization and gene sequence of the precursor of elafin, an elastase-specific inhibitor in bronchial secretions. Am J Respir Cell Mol Biol 8: 439–445, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Sallenave JM, Silva A, Marsden ME, Ryle AP. Secretion of mucus proteinase inhibitor and elafin by Clara cell and type II pneumocyte cell lines. Am J Respir Cell Mol Biol 8: 126–133, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26: 57–60, 1970 [PubMed] [Google Scholar]
- 51. Simpson AJ, Wallace WA, Marsden ME, Govan JR, Porteous DJ, Haslett C, Sallenave JM. Adenoviral augmentation of elafin protects the lung against acute injury mediated by activated neutrophils and bacterial infection. J Immunol 167: 1778–1786, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Starcher B, Green M, Scott M. Measurement of urinary desmosine as an indicator of acute pulmonary disease. Respiration 62: 252–257, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Stiskal JA, Dunn MS, Shennan AT, O'Brien KK, Kelly EN, Koppel RI, Cox DW, Ito S, SLC, Rabinovitch M. α1-proteinase inhibitor therapy for the prevention of chronic lung disease of prematurity: a randomized, controlled trial. Pediatrics 101: 89–94, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 175: 978–985, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 106: 1452–1459, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Thompson K, Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol 166: 495–505, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Watterberg KL, Carmichael DF, Gerdes JS, Werner S, Backstrom C, Murphy S. Secretory leukocyte protease inhibitor and lung inflammation in developing bronchopulmonary dysplasia. J Pediatr 125: 264–269, 1994 [DOI] [PubMed] [Google Scholar]
- 59. Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Wigle DA, Thompson KE, Yablonsky S, Zaidi SH, Coulber C, Jones PL, Rabinovitch M. AML1-like transcription factor induces serine elastase activity in ovine pulmonary artery smooth muscle cells. Circ Res 83: 252–263, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'Donnell E, Cataltepe S. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 291: L619–L627, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Zaidi SH, Hui CC, Cheah AY, You XM, Husain M, Rabinovitch M. Targeted overexpression of elafin protects mice against cardiac dysfunction and mortality following viral myocarditis. J Clin Invest 103: 1211–1219, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105: 516–521, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Zaidi SH, You XM, Ciura S, O'Blenes S, Husain M, Rabinovitch M. Suppressed smooth muscle proliferation and inflammatory cell invasion after arterial injury in elafin-overexpressing mice. J Clin Invest 105: 1687–1695, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]