Abstract
Objective: The purpose of this was to evaluate the neuroprotective effects of near-infrared (NIR) light using an in-vivo rodent model of traumatic brain injury (TBI), controlled cortical impact (CCI), and to characterize changes at the behavioral and biochemical levels. Background data: NIR upregulates mitochondrial function, and decreases oxidative stress. Mitochondrial oxidative stress and apoptosis are important in TBI. NIR enhanced cell viability and mitochondrial function in previous in-vitro TBI models, supporting potential NIR in-vivo benefits. Methods: Sprague–Dawley rats were divided into three groups: severe TBI, sham surgery, and anesthetization only (behavioral response only). Cohorts in each group were administered either no NIR or NIR. They received two 670 nm LED treatments (5 min, 50 mW/cm2, 15 J/cm2) per day for 72 h (chemical analysis) or 10 days (behavioral). During the recovery period, animals were tested for locomotor and behavioral activities using a TruScan device. Frozen brain tissue was obtained at 72 h and evaluated for apoptotic markers and reduced glutathione (GSH) levels. Results: Significant differences were seen in the TBI plus and minus NIR (TBI+/−) and sham plus and minus NIR (S+/−) comparisons for some of the TruScan nose poke parameters. A statistically significant decrease was found in the Bax pro-apoptotic marker attributable to NIR exposure, along with lesser increases in Bcl-2 anti-apoptotic marker and GSH levels. Conclusions: These results show statistically significant, preclinical outcomes that support the use of NIR treatment after TBI in effecting changes at the behavioral, cellular, and chemical levels.
Introduction
Traumatic brain injury (TBI) is defined as damage to the brain resulting from external mechanical forces.1 Rapid acceleration or deceleration, impact, blast waves, or penetration by a projectile can lead to temporary or permanent brain dysfunction. TBI is classified based on severity, mechanism of injury, and pathological features. A variety of signs and symptoms are seen in brain injury patients, including headaches, nausea, vomiting, and cognitive and focal neurological deficit. Behavioral changes following TBI are characterized by lack of motivation, difficulty sequencing, inattention, and memory retrieval problems in humans. Neuropsychology testing in humans can delineate the abovementioned deficits. In rat TBI models, poor goal-seeking behavior, as studied through the use of TruScan behavior testing arenas, suggests the psychomotor effects of cortical contusion.
TBI is of major public health significance, affecting almost 1,600,000 people in the United States each year. TBI is also the hallmark injury among Iraq and Afghanistan war victims.2 Advancements in diagnosis and management of TBI patients include neuroimaging, intracranial pressure monitoring, early decompressive craniectomy, and neuro-critical care. In spite of improved clinical management, the morbidity and long- term disability following severe TBI is very high, hence warranting further research into new treatment modalities.
Novel therapies for management of TBI emphasizing recovery of injured brain tissue are on the horizon. Light in the far red to near-infrared regions (670–1000 nm) of the spectrum has been shown to promote cell survival and wound healing.3,4 Phototherapy has been applied clinically in the treatment of soft tissue injuries and to accelerate wound healing for >30 years.5,6 Photobiomodulation using cellular and animal models of neurological disease has shown benefit from exposure to 670 nm light.7 Recently, pulsed 808 nm laser has been shown to be of benefit to mice subjected to closed head injury,8 and case reports have been published describing improved cognitive function in two patients with chronic TBI after treatment with 633+870 nm light-emitting diodes (LED).9 Therefore, we proposed a study whose goal was to evaluate the neuroprotective effects of noninvasive low-intensity light treatments using light in the far red to near-infrared range and which is delivered using LED arrays, using an in-vivo rodent model of TBI, controlled cortical impact (CCI),10 also known as cortical contusion. This may lead, eventually, to clinically based noninvasive treatments that can be used in healthcare facilities and in the military field.
The CCI model, using an air-driven piston, has been well validated.11 Histological evaluations have observed widespread cortical damage and ablation of the gray matter, with lesser injury to the underlying white matter. Cell death from mitochondrial dysfunction is also seen.12
Materials and Methods
Sprague–Dawley rats were divided into two or three groups: severe TBI, sham surgery, and anesthetization only (control, behavioral response only), or TBI and sham surgery (for chemical assays.) CCI followed a modification of the method of Dixon.13 Anesthetization was induced with an isoflurane mixture. Rats were mounted in a stereotaxic frame, and a 6 mm craniotomy was made over the left parietal cortex centered at 3 mm posterior and 4 mm lateral to bregma. Moderate injury was delivered by an air driven 5 mm piston at 6 mm/sec, 3 mm deformation, and 50 msec dwell time.
Sham surgery consisted of the craniotomy without use of the cortical impactor. Cohorts in each group were administered either no NIR or NIR. They received two 670 nm LED treatments (5 min, 50 mW/cm2, 15 J/cm2) per day for 72 h (chemical analysis) or 10 days (behavioral). Irradiation was applied directly to the top of the head, from a distance of 0.5 cm.
At days 1, 3, 5, and 10 of the recovery period, animals were tested for locomotor and behavioral activities using a TruScan device. Ten days was judged to be a sufficient amount of time in which to observe any differences in recovery among groups. Baseline values were also obtained for each animal pre-injury. Ten rats per injury group and five rats per sham or control group were used.
TruScan devices are Plexiglas cages that are used as open field arenas for animal activity measurements (Coulbourn Instruments). Activity is measured using patterns of infrared beam breaks that are analyzed by software to obtain parameters of locomotor activity. Two basic activity types are measured: movement and nose poke. Movement data include all types of horizontal, vertical, and stereotypic movements during a 30 min observation. Typical parameters include movement time, distance, velocity, turns, and jumps. In the nose poke experiment, a plate with baited holes is inserted into the floor of the arena, and the animal is observed as it seeks out food. Typical nose poke parameters include time to reach baited holes, number and type of hole entries, and repeat visits.
After 72 h, assay animals were killed and the brains were harvested, separated into four sections (left cortex upper [LCU], right cortex upper [RCU], left cortex lower [LCL], and left cortex lower [RCL]), and flash frozen. Seventy-two hours was the time frame chosen in order to maximize the possibility of observable differences, as further recovery time would tend to narrow these differences. Ten rats per injury group and five rats per sham group were used. No anesthetization-only controls were included in the assay groups. Reduced glutathione (GSH) levels were determined on flash frozen samples using a modified Chemicon GSH assay kit (Millipore). Western blot immunoassays were performed by loading 20 μg total protein (DC protein assay, Bio-Rad) per lane in an 18% polyacrylamide gel (sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE]) with transfer to a polyvinylidene fluoride (PVDF) membrane. Bax bands were visualized with 6A7 antibody (Santa Cruz Biotechnology), Bcl-2 with C-2 antibody (Santa Cruz) and β-actin with AC-74 antibody (Sigma-Aldrich). The total integrated density of the appropriate band was determined, after background correction, by use of the graphics program ImageJ. Initially, it was intended to correct these numbers using β-actin as a loading control. However, it was found that β-actin expression was not constant. Accordingly, as equal lane loading was used, the various band density values were used as is.
Statistical analysis was performed by two-sided unpaired t tests, with significance level set at p<0.05.
The study animals were maintained at the Zablocki Veterans Affairs Medical Center, Milwaukee, WI. All procedures were performed under protocols approved by the local Institutional Animal Care and Use Committee (IACUC). A total of 104 animals were used, with a mortality of 10.
Results
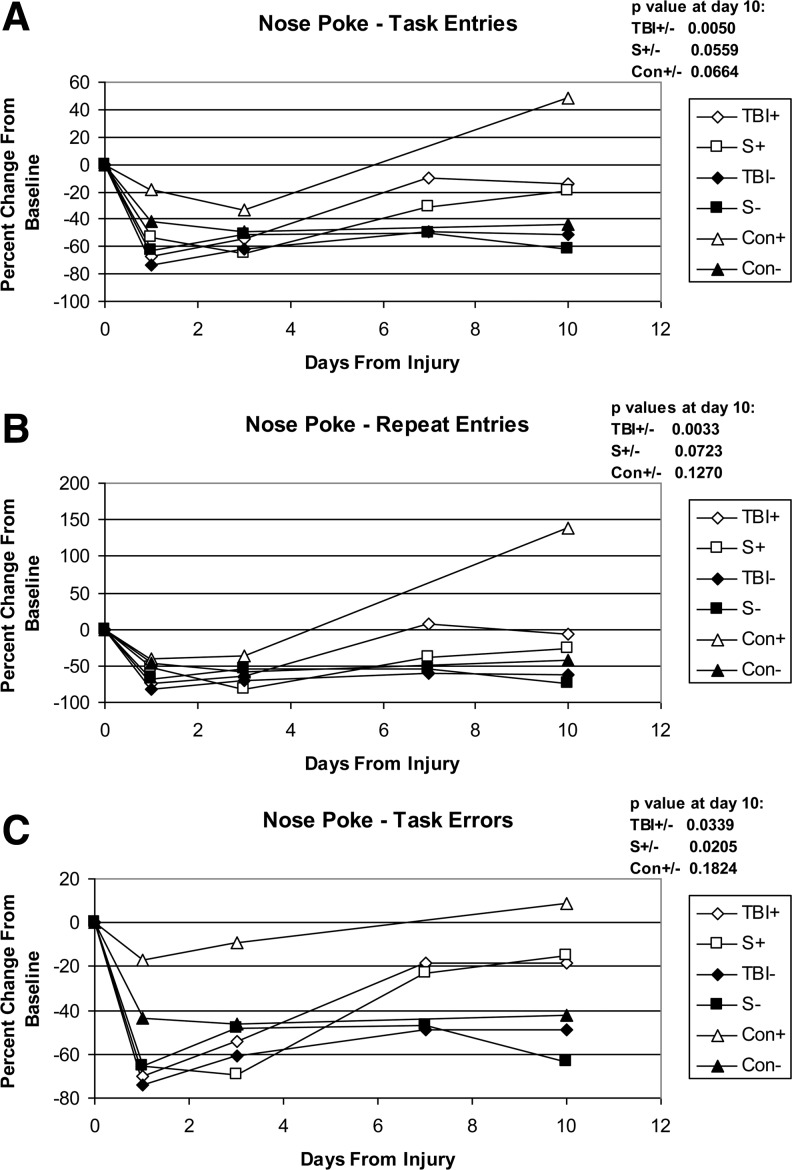
General movement data showed no statistically significant differences among groups. Significance was seen when analyzing nose poke data (Fig. 1). These data showed significant differences in the TBI plus and minus NIR (TBI+/−) and sham plus and minus NIR (S+/−) comparisons for some of the nose poke parameters. NIR improved the values in the TBI+ group compared with the TBI- group from −51.3±9.3 to −14.0±7.4 (standard error of the mean) for task entries, from −62.5±11.2 to −6.2±12.4 for repeat entries, and from −48.6±9.0 to −18.1±9.8 for task errors. The values are expressed as the average of the change from baseline for each individual animal. The corresponding changes for the S+ and S- groups were −61.4±30.3 to −19.9±12.8, −74.9±14.8 to −26.2±11.2, and −63.6±11.5 to −15.1±12.3. The TBI versus sham comparisons, with or without NIR, were not significant; in fact, the sham results were usually slightly lower than the corresponding TBI results. The Con+/− comparisons also show effects from the NIR, although not statistically significant.
FIG. 1.
TruScan nose poke data. Data are expressed as percent change from baseline; calculated individually per animal and averaged. Errors bars are not included in the graphs to allow for better clarity. The p value for each comparison pair at day 10 is indicated on each graph. A p value of 0.05 is considered significant. (A) Task entries: the total number of entries from the beginning of the run to the Nth novel entry (all baited holes entered at least once). (B) Repeat entries: repeat entries in holes previously entered, including successive entries in the current hole. (C) Task errors: the total number of entries in unbaited holes from the beginning to task completion.
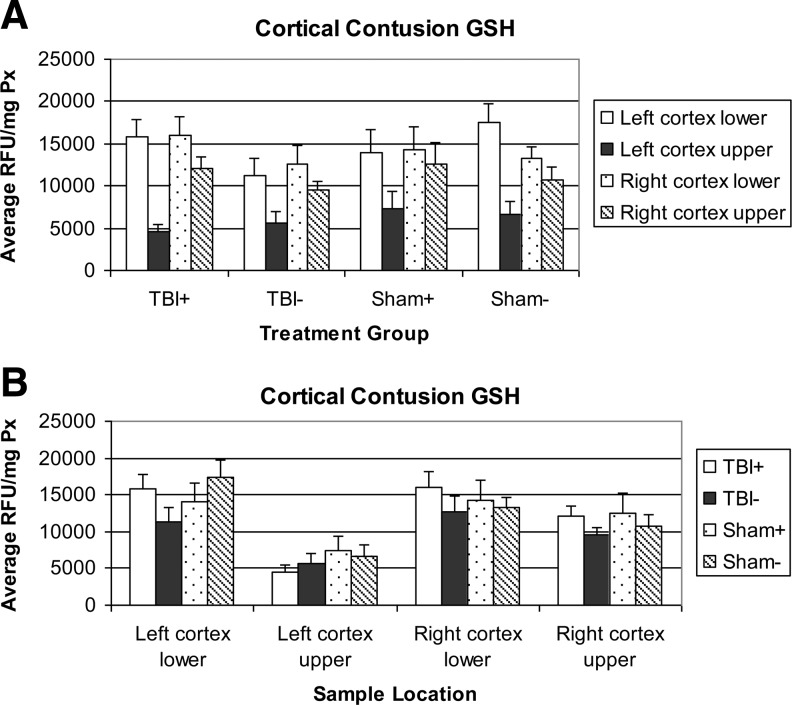
GSH levels are presented in Fig. 2, sorted by group or by sample location. The most striking feature of these graphs is the clear reduction in GSH levels in the upper left cortex (LCU), the location of the injury site. This reduction is common to both the TBI and sham groups and does not respond to the application of NIR. The other three regions show small increases, although not significant in the TBI comparisons due to NIR application.
FIG. 2.
Reduced glutathione (GSH) levels in tissue extracts. Levels are expressed in relative fluorescence units per milligram of protein content (RFU/mg Px). Errors bars represent the standard error of the mean. (A) Sorted by treatment group. (B) Sorted by sample location.
Several markers were examined using Western blot immunoassays of tissue samples. These consisted of Bax, a pro-apoptotic protein, Bcl-2, an anti-apoptotic marker, and β-actin, a structural protein.
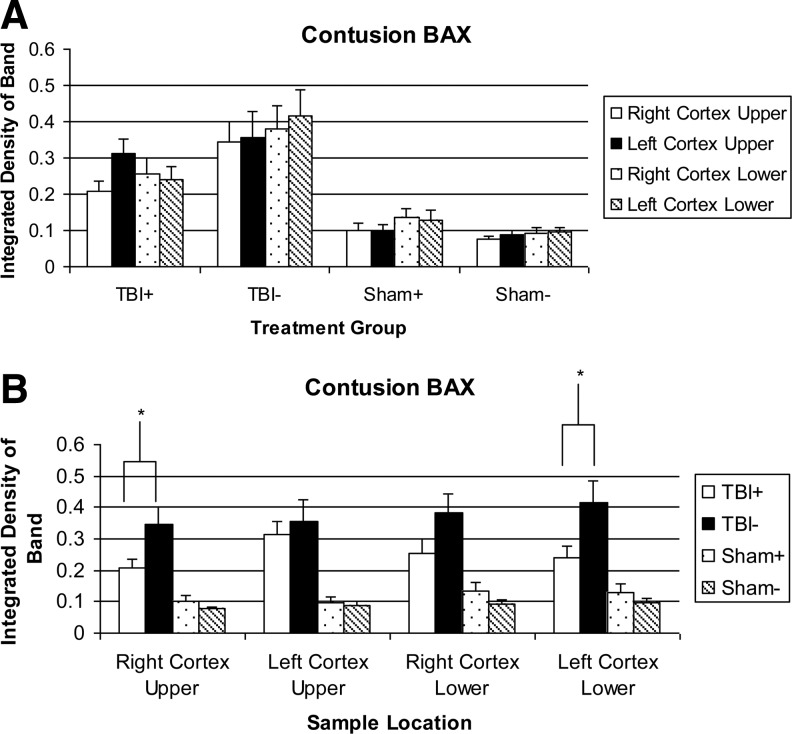
A typical example of a Western blot probed for Bax is shown in Fig. 3. Band density measurements are shown in Fig. 4. The sham groups overall have a lower Bax expression than the TBI groups. The S+ levels show no significant difference from the S- levels. The TBI+ samples in each region showed less Bax than the corresponding TBI- samples. This difference was significant in the RCU (0.207±0.028 vs. 0.345±0.055) and LCL (0.242±0.035 vs. 0.416±0.071) regions, and close to significant in the RCL (0.255±0.043 vs. 0.381±0.062) region. The LCU region showed no significant reduction.
FIG. 3.
An example of a Western blot probed for Bax pro-apoptotic indicator. Lanes 1–4: TBI-, 5–8: TBI-, 9–12: TBI+13–16: TBI+. Within each group of four lanes, the sample order is right cortex upper, left cortex upper, right cortex lower, left cortex lower. Molecular weight markers seen (left edge of figure) are, from top, 30 and 20 kDa.
FIG. 4.
Bax levels in tissue extracts. * - indicates a significant treatment effect (p<0.05). (A) Sorted by treatment group. (B) Sorted by sample location.
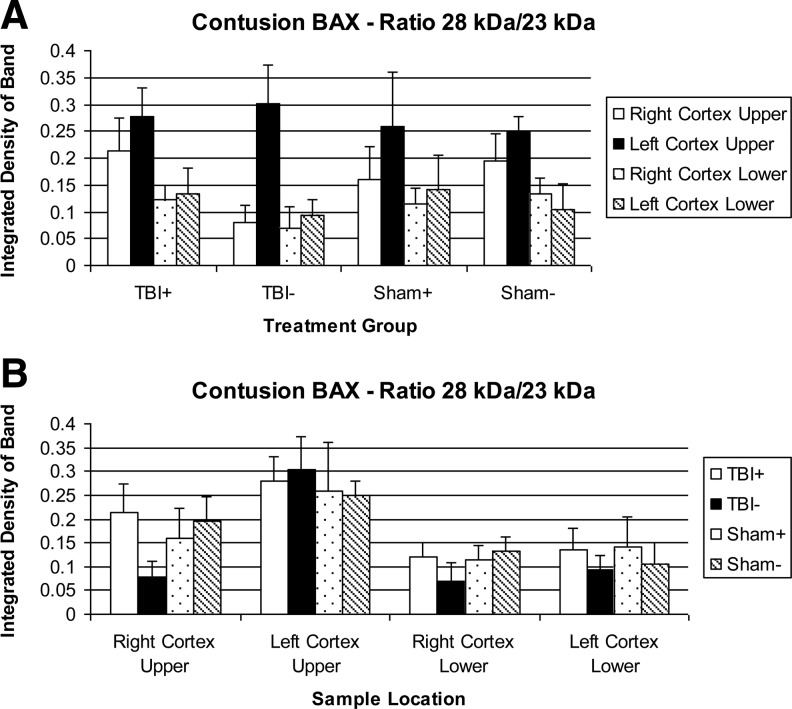
A novel phenomenon in the Bax Western blots was the appearance of a new band at ∼28 kDa, and a decrease in the intensity of the typical 23 kDa band (Fig. 3). This new band appears most often in the LCU region. The ratio of the 28 kDa/23 kDa bands was calculated (Fig. 5) and clearly shows an increase in the LCU, with also a small increase in the adjacent RCU area. The TBI groups show an increase over the sham groups, but the extra band is sometimes seen in sham animals. NIR treatment does not have a significant effect on this phenomenon.
FIG. 5.
Ratio of 28 kDa to 23 kDa Bax levels in tissue extracts. (A) Sorted by treatment group. (B) Sorted by sample location.
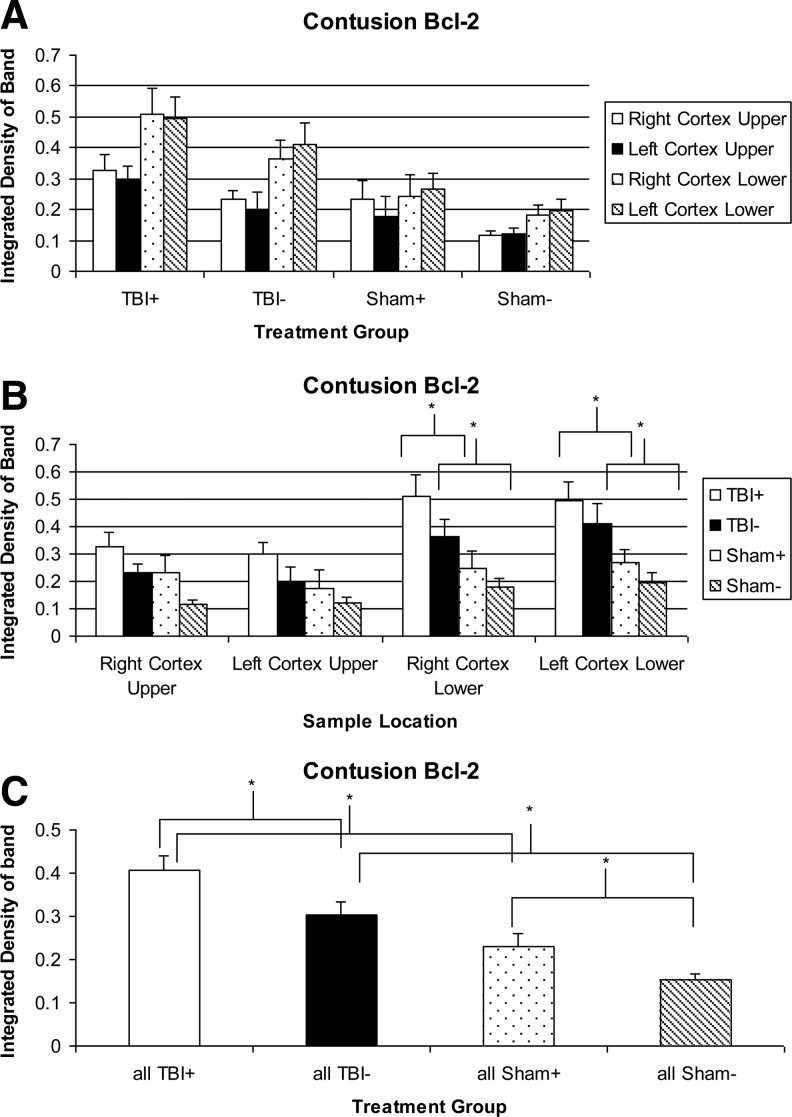
A typical Western blot for the Bcl-2 is shown in Fig. 6; densities in Fig. 7. TBI seems to increase Bcl-2 levels in all brain regions, with significant increases in the two lower regions, and a significant increase overall if all four regions are combined, in both NIR treated and untreated groups. Bcl-2 levels are always higher in the lower regions in any particular group. NIR increases Bcl-2 levels, although not significantly unless all regions are combined (0.407±0.034 vs. 0.302±0.032).
FIG. 6.
An example of a Western blot probed for Bcl-2 anti-apoptotic indicator. Lanes 1–4: TBI+, 5–8: TBI+, 9–12: TBI+13–16: TBI+. Within each group of four lanes, the sample order is right cortex upper, left cortex upper, right cortex lower, left cortex lower. Markers not visible because of stripping.
FIG. 7.
Bcl-2 levels in tissue extracts. * - indicates a significant treatment effect (p<0.05). (A) Sorted by treatment group. (B) Sorted by sample location. (C) All sample regions combined.
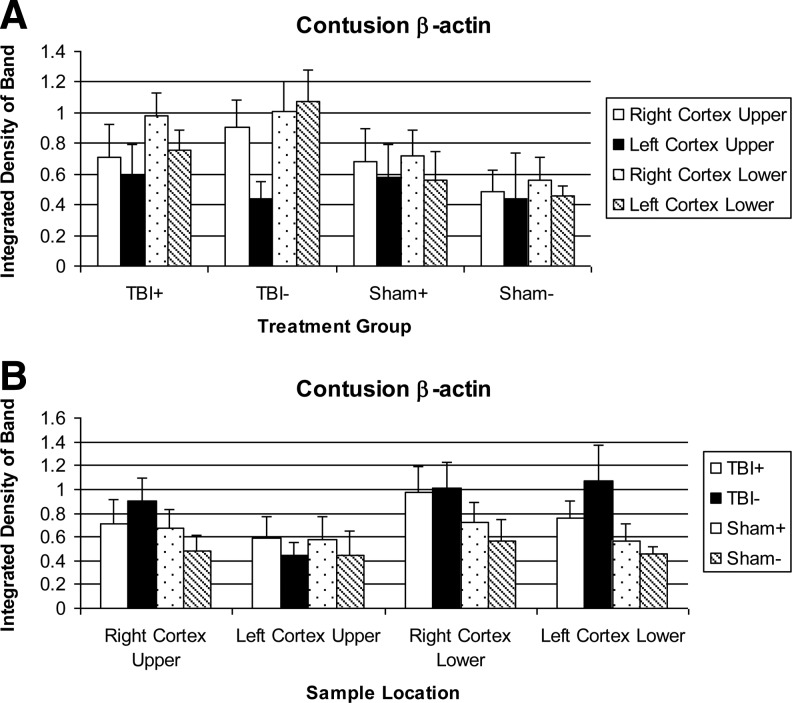
A typical Western blot for β-actin is shown in Fig. 8. β-Actin expression is not a constant, as the loading in each lane was adjusted to give equal protein amounts. β-Actin levels are shown in Fig. 9. None of the TBI+/−, S+/−, TBI+/S+, and TBI-/S- comparisons showed significance.
FIG. 8.
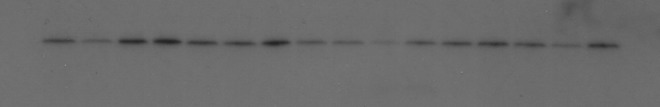
An example of a Western blot probed for β-actin cytoskeletal protein. Lanes 1–4: S-, 5–8: S-, 9–12: S- 13–16: S-. Within each group of four lanes, the sample order is right cortex upper, left cortex upper, right cortex lower, left cortex lower. Markers not visible because of stripping.
FIG. 9.
β-Actin levels in tissue extracts. (A) Sorted by treatment group. (B) Sorted by sample location.
The β-actin level is generally higher in the TBI groups than in the sham groups, except in the LCU. The β-actin level is almost always lower in the LCU, sometimes dramatically. In some cases, the β-actin is also lower in regions lateral or inferior to the injury area. β-Actin levels are not sensitive to NIR.
Discussion
The sham rats underwent the same craniotomy as the TBI rats, but were not subjected to contusion. These sham rats appeared to have sustained as much injury as the TBI rats, and recovered no better. In addition to the craniotomy and contusions, all rats also underwent anesthetization. The control rats did not undergo extensive surgery or injury and received no insult to the brain itself. These control rats also showed some impairment at 24 h, confirming that anesthetization and handling do have a deleterious effect on behavioral measures, but generally it is not as large as the effects in the sham and TBI groups. In all groups, the use of NIR light exposure caused a noticeable improvement in nose poke behavior by the 10 day mark. It appears that the rats suffered from varying degrees and combinations of impairment resulting from anesthetization, surgery, and contusion. In particular, the amount of damage from the craniotomy was quite variable, with some sham rats showing visible cortical tissue damage. Whether NIR treatment affects any particular injury, or a combination of these injuries, is not apparent at this time.
Despite these uncertainties in the causes of the behavioral impairments seen, there is evidence of a definite beneficial effect of exposure to NIR light during recovery. Significant, or near-significant, effects of NIR were seen in the TBI+/− and S+/- comparisons using the outcome parameters of task entries, repeat entries, and task errors. In addition, the Con+/- comparisons showed a definite effect from the NIR. Taken all together, these observations represent a picture of a more active and goal-seeking animal after exposure to NIR.
Reduced GSH is an important reducing agent for reactive oxygen species, and plays a role in neuroprotection and neurotoxicity.14 The reduction in GSH levels in the samples from the LCU in the TBI and sham groups is indicative of an injury common to both, most likely a necrosis caused by the craniotomy. This reduction does not respond to NIR. The small increases seen in the GSH levels of the other three regions in the TBI groups may represent a diffuse injury, of much less magnitude, which does respond to NIR. This injury may represent the effects of the shearing forces imparted to the entire brain by the blow of the piston, and may correspond to diffuse axonal injury (DAI) seen in humans.15 The injury may be more of an apoptotic nature.
Bax is a pro-apoptotic protein that is typically present in elevated levels during apoptotic cell death.16 Reduction in Bax levels can be attributed to a reduction in cell death. Bcl-2 is an anti-apoptotic marker from the same gene family.17 The relative amounts of Bax and Bcl-2 have an effect on whether a cell will undergo apoptosis. Bcl-2 protects against apoptosis by heterodimerizing with Bax, preventing the homodimerization of Bax needed for it to become active. An increase in Bcl-2 expression could represent some degree of protection afforded by the treatment.
The overall lower Bax levels in the sham groups compared with the TBI groups indicates the efficacy of the cortical contusion model in inducing measurable injury. The lack of significant difference between the S+ and S- levels shows that NIR had no effect on Bax levels in animals that did not receive impact injury. The lower Bax levels in the TBI+ samples in each region compared to the corresponding TBI- strongly suggests that the NIR treatments may be reducing BAX expression. The lack of a significant reduction in the LCU, however, is possibly indicative of the predominance of a non-NIR responsive necrotic cell death mechanism in this area.
Bax levels were not, in general, sensitive to the brain region within a group, but were uniformly elevated in the injured groups versus the sham groups. This seems to indicate that the apoptotic cell death mechanism related to Bax expression may be the result of a diffuse type of injury, rather than a direct injury localized to the impact site.
The appearance of a new band at ∼28 kDa in the contusion Bax Western blots was surprising. The identity of this protein is unknown, but it does react with the Bax antibody, and may be a conjugate of Bax with some small protein. A similar phenomenon has been seen before.18
It is possible that the appearance of this 28 kDa band may be an indication of some sort of local injury. The increased expression of the new protein in the LCU, taken together with the increase in the TBI groups compared with the sham groups, may point to this phenomenon being associated with local injury in, or near, the impact area, that does not respond to NIR. This might be some sort of necrotic, rather than apoptotic, mechanism.
Injury seems to increase Bcl-2 levels in all brain regions, in both treated and untreated groups. These increases may represent an upregulation of Bcl-2 expression as a healing response to a diffuse injury caused by the shearing forces. Increases in Bcl-2 expression have been seen following TBI in rats19 and humans.20
The observed increase in Bcl-2 in the lower two regions, compared with the upper regions in the same group, may be the result of a necrotic injury in the upper levels, caused by the impactor, or injury from the craniotomy common to all groups. This injury, resulting in necrotic cell death, should result in decreased levels of all markers in those areas. NIR seems to increase Bcl-2 levels, although the increase is not significant unless all regions are combined. This increase in Bcl-2 may indicate a further healing response, attributable to NIR.
β-Actin is a structural protein associated with the cellular cytoskeleton. This protein is often used as an internal loading control in Western blot analysis, as its expression is thought to be fairly even in all cells. In our tests, however, variations were seen in the β-actin levels that could not be correlated to independent protein loading measurements. In addition, β-actin has come under criticism as an appropriate loading control21 and has been implicated in the apoptotic model of cell death.22 Accordingly, we chose to consider β-actin as an independent measurement that could possibly reveal information concerning cell death and treatment efficacy.
Although not significant, there are a few trends visible in the contusion β-actin Western blots. The generally higher levels seen in the TBI groups compared with the sham groups raises the question as to whether β-actin is involved in apoptosis. The usually lower β-actin in the LCU area, along with occasional decreases in adjacent areas, may be caused by a necrotic cell death, common to both TBI and sham groups. Damage outside the LCU target area could be caused by hemorrhage adjacent to the initial damage. The unresponsiveness to NIR treatment further points to a necrotic type of cell death.
Conclusions
The design of this study appears to have caused two distinct types of injury. The first, common to both the TBI and sham groups, appears to be a localized, necrotic type of cell death. This injury was caused by the craniotomy itself, although the piston impact most likely added to the magnitude. Hemorrhage may have played a role in this injury; especially as this type of damage was visible in some actual tissue samples.
This local damage is characterized by decreases in the Bax, Bcl-2, and β-actin markers, a reduction in the GSH levels, and a deterioration of movement and activity parameters. There is also the appearance of a new form of marker that reacts with Bax antibody. This damage does not appear to respond to treatment by NIR.
The second type of damage is of a diffuse type that can be seen across all four brain regions, and is apparent at the biochemical level in the TBI groups. The damage may be related to diffuse axonal injury, caused by the shearing forces imparted on the brain as a whole by the cortical impactor piston. This damage increases the Bax, Bcl-2, β-actin, and GSH levels, and depresses motor activity. NIR decreases Bax, increases Bcl-2 and GSH levels, and improves some of the more active, goal-seeking behavior measures.
In this study, we have found evidence for the utility of NIR light exposure in improving the recovery process after injury in a rat model of TBI. These results show a statistically significant, preclinical effect in rats that received CCI. More work is needed to clarify the clinical significance of this effect.
We have also found a statistically significant decrease in the Bax pro-apoptotic marker attributable to NIR, along with lesser increases in Bcl-2 anti-apoptotic marker and reduced GSH levels. This further supports the utility of NIR treatment at the cellular and chemical level.
Acknowledgments
This work was supported by the Defense Advanced Research Projects Agency (DARPA) Grant # W911NF-09-0117, Project # 56482LSDRP09267 (to Dr. Whelan), and the Bleser Endowed Chair in Neurology (to Dr. Whelan), as well as the Baumann Research Endowment (to Dr. Whelan). We gratefully acknowledge Brandi Jones and Joeylynn Nawrocki for technical assistance as well as Debbie Dye for administrative support in preparation of the manuscript.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Maas A.I. Stocchetti N. Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 2.Ling G. Bandak F. Armonda R. Grant G. Ecklund J. Explosive Blast Neurotrauma. J. Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- 3.Whelan H.T. Smits R.L. Buchmann E.V., et al. Effects of NASA light-emitting diode irradiation on wound healing. J. Clin. Laser Med. Surg. 2001;19:305–314. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- 4.Whelan H.T. Buchmann E.V. Dhokalia A., et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J. Clin. Laser Med. Surg. 2003;21:67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- 5.Karu T. Primary and secondary mechanisms of action of visibile to near-IR radiation on cells. J. Photochem. Photobiol. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 6.Karu T. Low power laser therapy, biomedical photonics handbook. Boca Raton FL: CRC Press, LLC; 2003. [Google Scholar]
- 7.Quirk B.J. DeSmet K.D. Henry M. Buchmann E. Wong–Riley M. Eells J.T. Whelan H.T. Therapeutic effect of near infrared (NIR) light on Parkinson's disease models. Front. Biosci. (Elite ed.) 2012;4:818–823. doi: 10.2741/E421. [DOI] [PubMed] [Google Scholar]
- 8.Oron A. Oron U. Streeter J., et al. Near infrared transcranial laser therapy applied at various modes to mice following traumatic brain injury significantly reduces long-term neurological deficits. J.Neurotrama. 2012;29:401–407. doi: 10.1089/neu.2011.2062. [DOI] [PubMed] [Google Scholar]
- 9.Naeser M.A. Saltmarche A. Krengel M.H. Hamblin M.R. Knight J.A. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed. Laser Surg. 2011;29:351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lighthall J.W. Controlled cortical impact: a new experimental brain injury model. J.Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 11.Manvelyan H. Contemporary experimental models of traumatic brain injury. Georgian Med. News. 2006;140:1306. [PubMed] [Google Scholar]
- 12.Robertson C. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J. Bioenerg. Biomembr. 2004;36:363–368. doi: 10.1023/B:JOBB.0000041769.06954.e4. [DOI] [PubMed] [Google Scholar]
- 13.Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 14.Monks T.J. Ghersi–Egea J.F. Philbert M. Cooper A.J. Lock E.A. Symposium overview: the role of glutathione in neuroprotection and neurotoxicity. Toxicol. Sci. 1999;51:161–177. doi: 10.1093/toxsci/51.2.161. [DOI] [PubMed] [Google Scholar]
- 15.Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 16.Gross A. Jockel J. Wei M.C. Korsmeyer S.J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikhailov V. Mikhalova M. Pulkrabek D.J. Dong Z. Venkatachalam M.A. Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 2001;276:18,361–18,374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 18.Antonsson B. Montessuit S. Sanchez B. Martinou J. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11,615–11,623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 19.Wennersten A. Holmin S. Mathiesen T. Characterization of Bax and Bcl-2 in apoptosis after experimental traumatic brain injury in the rat. Acta Neuropathol. 2003;105:281–288. doi: 10.1007/s00401-002-0649-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X. Chen Y. Jenkins L.W. Kochanek P.M. Clark R.S.B. Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Crit. Care. 2005;9:66–75. doi: 10.1186/cc2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittmer A. Dittmer J. B-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27:2844–2845. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- 22.Tang H.L. Le A.P. Lung H.L. The increase in mitochondrial association with actin precedes Bax translocation in apoptosis. Biochem. J. 2006;396:1–5. doi: 10.1042/BJ20060241. [DOI] [PMC free article] [PubMed] [Google Scholar]