Abstract
Aims: To establish a functional link between microRNA-107 (miR-107) and stem cell survival during ischemic preconditioning (IPC) of stem cells with multiple cycles of brief anoxia/re-oxygenation (10 or 30 min, one to three cycles) and show that the cytoprotective effects were independent of hypoxamir-210. Results: We demonstrated the induction of miR-107 in response to the IPC-induced activation of Akt/hypoxia inducible factor-1α (HIF-1α) in preconditioned mesenchymal stem cells (PCMSC), which showed improved survival during subsequent exposure to 6 h of lethal anoxia (p<0.05 vs. non-preconditioned MSC[non-PCMSC]). In silico analysis and luciferase activity assay confirmed programmed cell death-10 (PDCD10) as a putative target of miR-107 in PCMSC, which was significantly reduced during IPC and inversely related to stem cell survival under 6 h of lethal anoxia. Loss-of-function studies with miR-107 antagomir showed a significantly reduced survival of PCMSC. A comparison of miR-107 and miR-210 showed that both miRs participated independently via their respective putative target genes Pdcd10 and Casp8ap2. The simultaneous abrogation of Pdcd10 and Casp8ap2 had a stronger effect on PCMSC survival under lethal anoxia. The transplantation of PCMSC in an acute model of myocardial infarction showed a significantly improved survival of transplanted PCMSC with concomitantly enhanced miR-107 expression in PCMSC-transplanted animal hearts. Innovation: Cytoprotection afforded by IPC is regulated by miR-107 induction via Pdcd10 independent of miR-210/Casp8ap2 signaling, and the simultaneous abrogation miR-107/miR-210 has a stronger effect on the loss of PCMSC survival. Conclusion: IPC enhances stem cell survival via the combined participation of hypoxia responsive miRs miR-107 and miR-210 via their respective putative target genes Pdcd10 and Casp8ap2. Antioxid. Redox Signal. 00, 000–000.
Introduction
We have previously reported the effectiveness of ischemic preconditioning (IPC) by intermittent cyclical exposure to short anoxia/re-oxygenation (A/R) cycles to support stem cell survival under lethal anoxia and post-transplantation in the infarcted heart (9, 17).
Hypoxia significantly affects cell functionality, including metabolism, survival, proliferation, emigrational properties, and differentiation with a critical role for hypoxia inducible factor-1α (HIF-1α) signaling. Besides, hypoxia also alters a select group of microRNAs (miRs), designated as hypoxia responsive miRs (HRM) (20). These 19–22 nucleotides and long, single-stranded miRs post-transcriptionally regulate the expression of most genes, many of which are expressed in response to HIF-1α activity, the master regulator of oxygen-sensing mechanisms of cells, and their hypoxia responsiveness (29). HRM are being extensively studied for HIF-1α dependence and their association with anti-apoptotic signaling (19, 27). Although the functional significance of HRM remains an area of intense research, only a few studies have successfully established a link between stem cell preconditioning and the role of miRs (7, 17).
Innovation.
Hypoxia-regulated hypoxia inducible factor-1α (HIF-1α) dependent microRNAs (miRs) are critical regulators of pro-survival signaling in preconditioned mesenchymal stem cells (PCMSC). Our study is the first attempt which shows that HIF-1α-dependent miR-107 induced during the ischemic preconditioning of stem cells acted via Pdcd10 to impart cytoprotection; the effects of miR-107/Pdcd10 interaction were independent of miR-210/Casp8ap2 signaling, and the simultaneous abrogation of miR-107/miR-210 had a stronger combined effect on the loss of PCMSC survival.
Since the establishment of our pioneering strategy of cellular preconditioning to effectively prime stem cells by treatment with preconditioning mimetic diazoxide (26), various research groups have reported that the treatment of stem cells with hypoxia effectively supports their survival under lethal anoxia (2, 30). In our subsequent study, we hypothesized that IPC with repeated intermittent cycles of A/R primes the cells for improved survival during subsequent exposure to lethal anoxia (17). While elucidating the mechanism of cytoprotection, we implied the critical role of HIF-1α and miR-210 during IPC (17). We also defined Casp8ap2 as a downstream putative target of miR-210, which was abrogated in response to IPC (17). CASP8AP2 has a critical role in the apoptosis signaling complex to mediate Fas-induced apoptosis. It interacts with the death effector domain of caspase-8 or the Fas-associated death domain and facilitates its release from the apoptosis signaling complex to activate the downstream caspase cascade (13). Similar to miR-210, miR-107 is an important member of HRM (18, 20) and has been mostly studied for its role in tumor progression and proliferation (5, 8, 22). Nevertheless, little is known about its mechanistic participation in survival signaling during IPC. The present study was designed to determine the role of miR-107 and its putative target programmed cell death-10 (Pdcd10) in mesenchymal stem cells (MSC) survival in response to HIF-1α activation during IPC. Gain and loss-of-function studies showed that PDCD10 was induced in response to pro-apoptotic stimuli, and its pro-apoptotic role was mediated via the activation of caspase-3 (4). Given that miR-210 is a critical determinant of preconditioned MSC (PCMSC) survival under lethal anoxia, we sought its comparison with miR-107. Our results showed that miR-107 and miR-210 acted via their respective putative targets Pdcd10 and Casp8ap2. Moreover, the combined abrogation of miR-107/miR-210 or their putative targets genes Pdcd10/Casp8ap2 in PCMSC had a stronger combined effect in terms of cytoprotection.
Results
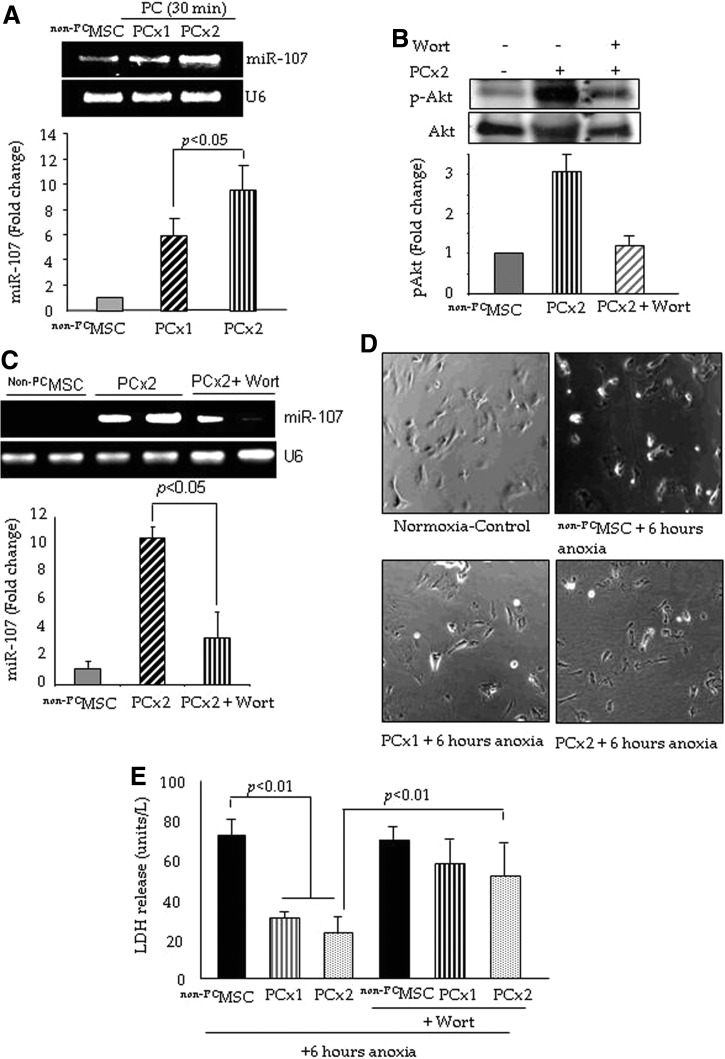
MiR-107 expression was significantly increased in PCMSC
MSC were successfully preconditioned using our optimized protocol by exposure to one cycle (PCx1) and two cycles (PCx2) of sub-lethal anoxia for 30-min with intermittent re-oxygenation. Since miR-107 is involved in anti-apoptotic gene regulation, we performed quantitative reverse transcription polymerase chain reaction (RT-PCR) to quantify its expression in PCMSC (Fig. 1A). miR-107 significantly increased in PCMSC after treatment with both PCx1 and PCx2 as compared with non-preconditioned MSC (non-PCMSC) (Fig. 1A); the induction of miR-107 was higher in PCx2 as compared with PCx1 (p<0.05; Fig. 1A). A time-course study for miR-107 induction showed insignificantly changed miR-107 induction between A/R phases of preconditioning cycles (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/ars). We did not observe any significant change in miR-103 expression in PCMSC (Supplementary Fig. S1B). PCMSC showed significantly higher activation of Akt, which was abrogated by the pretreatment of cells with 40 μM Wortmannin (Wort) for 45 min, whereas total Akt remained unchanged (Fig. 1B). Interestingly, the pretreatment of PCMSC with Wort also abrogated miR-107 (Fig. 1C). On subsequent exposure to 6 h of lethal anoxia, morphological integrity was better preserved in PCMSC as compared with non-PCMSC (Fig. 1D). The non-PCMSC under 6 h of lethal anoxia showed rounded and hyper-contracted morphology unlike PCMSC, which maintained their typical flat and elongated appearance (Fig. 1D). Lactate dehydrogenase (LDH) release was significantly reduced in PCMSC (both PCx1 and PCx2) as compared with non-PCMSC under 6 h of lethal anoxia (Fig. 1E). However, the preconditioning of MSC with PCx2 was more effective in reducing cellular injury, as indicated by reduced LDH leakage as compared with non-PCMSC. Furthermore, pretreatment with 40 μM Wort abolished the cytoprotective effects in PCMSC (both PCx1 and PCx2) (Fig. 1E). Consistent with LDH release assay, transferase-mediated dUTP nick-end labeling (TUNEL) positivity was significantly reduced in PCMSC (both PCx1 and PCx2) under 6 h of lethal anoxia as compared with non-PCMSC (Supplementary Fig. S1C). In another set of experiments, we treated both non-PCMSC and PCMSC with 100 μM H2O2 to determine their survival using respective cells without H2O2 treatment as the control (Supplementary Fig. S1D). Preconditioning by PCx2 did not change LDH release as compared with non-PCMSC, whereas LDH release was significantly reduced in PCMSC as compared with non-PCMSC on H2O2 treatment, thus implying that preconditioning was as effective in protecting the cells under H2O2 stress as it was against lethal anoxia (Supplementary Fig. S1D).
FIG. 1.
IPC induced miR-107 expression in MSC. (A) IPC significantly increased miR-107 expression in PCMSC as compared with native non-PCMSC. Cells treated with two cycles (PCx2) of 30 min A/R showed higher expression as compared with the cells treated with one cycle (PCx1). (B) Western blots showing significantly higher phosphorylation of Akt in PCMSC as compared with non-PCMSC, which was abrogated by pretreatment with 40 μM Wort for 45 min. (C) The induction of miR-107 in PCMSC was abrogated by pretreatment with Wort. Fold change for miR-107 in (A, C) was determined from densitometric ratio normalized to U6 expression and for pAkt in (B) from densitometric ratios between pAkt/total Akt in the respective samples. (D) Phase-contrast photomicrographs showed a better preserved morphology in the PCMSC treated with PCx2 as compared with PCx1 on subsequent exposure to 6 h of lethal anoxia. (E) Preconditioning attenuated cellular injury as examined by LDH assay subsequent to 6 h of lethal anoxia. Preconditioning by PCx2 was more effective in the protection of cells as compared with PCx1, using non-PCMSC as controls. Pretreatment with 40 μM Wort significantly abolished the effects of preconditioning. A/R, anoxia/re-oxygenation; IPC, ischemic preconditioning; LDH, lactate dehydrogenase; miR, microRNA; MSC, mesenchymal stem cells; PCMSC, preconditioned MSC; non-PCMSC, non-preconditioned MSC; Wort, Wortmannin.
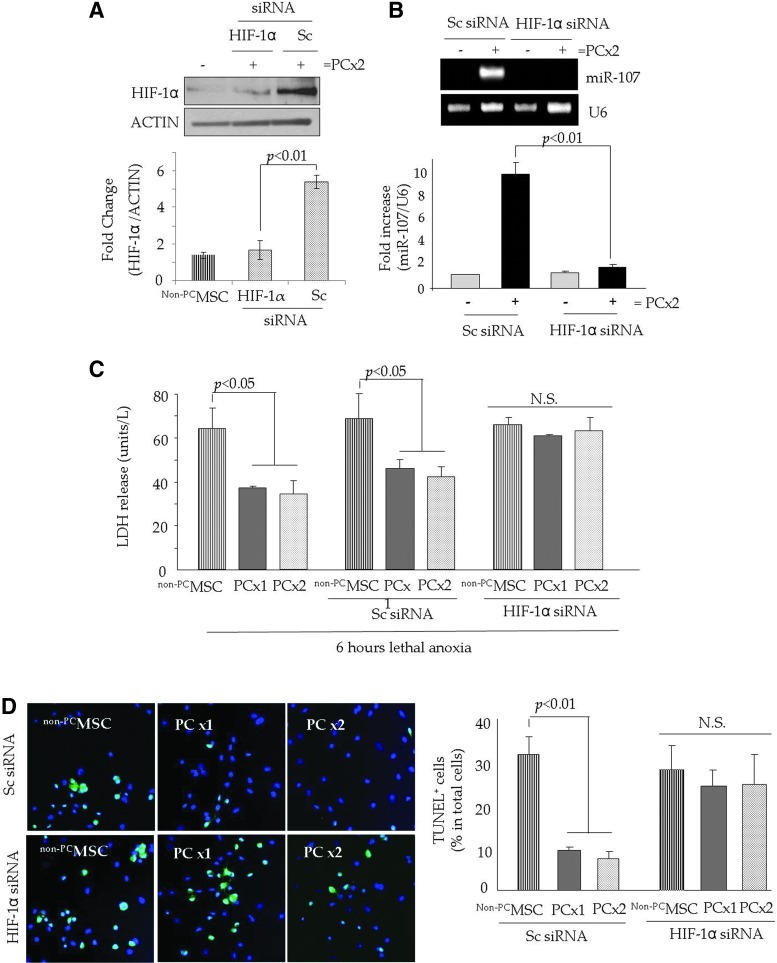
HIF-1α regulated miR-107 expression during the preconditioning of stem cells
To determine the regulatory role of HIF-1α in miR-107 expression during preconditioning, PCMSC were pretreated with HIF-1α siRNA using scramble (Sc) siRNA-transfected PCMSC as controls. We successfully abrogated HIF-1α in PCMSC by specific RNA interference as compared with Sc siRNA-transfected PCMSC (Fig. 2A). Western blot studies also showed that there was no activation of p53 in response to IPC in PCMSC as compared with non-PCMSC (Supplementary Fig. S2A). Abrogation of HIF-1α significantly abolished miR-107 in PCMSC as compared with non-PCMSC and Sc siRNA-transfected PCMSC (Fig. 2B). These molecular changes led to a loss of the cytoprotective effects of preconditioning, as was evident by significantly higher LDH leakage under 6 h of lethal anoxia in PCMSC (both PCx1 and PCx2) pretreated with HIF-1α siRNA as compared with Sc siRNA-treated controls (Fig. 2C). Consistent with LDH, TUNEL positivity was significantly increased in HIF-1α siRNA-transfected PCMSC as compared with Sc siRNA-transfected PCMSC (Fig. 2D). Non-PCMSC under 6 h of lethal anoxia were used as controls and showed significant LDH release and higher TUNEL positivity under 6 h of lethal anoxia (Fig. 2C, D).
FIG. 2.
HIF-1α-dependent induction of miR-107 improved PCMSC survival. (A) Western blot showing the successful abrogation of HIF-1α in the nuclear fraction of PCMSC with PCx2 and transfected with HIF-1α siRNA as compared with Sc siRNA-transfected PCMSC. Native non-PCMSC were used as controls. (B) RT-PCR showing the abrogation of miR-107 in PCMC (PCx2) in response to HIF-1α siRNA transfection as compared with Sc siRNA-transfected PCMSC. (C) The abrogation of HIF-1α led to the loss of cytoprotection in PCMSC as determined by LDH-release assay. PCMSC (both PCx1 and PCx2) with HIF-1α siRNA transfection had a significantly higher release of LDH under 6 h of lethal anoxia as compared with ScMSC. (D) Representative fluorescence images and quantitative analysis of TUNEL positivity (green) showed that the abrogation of HIF-1α significantly increased TUNEL-positive PCMSC as compared with Sc siRNA-transfected PCMSC. DAPI was used to visualize nuclei. HIF-1α, hypoxia inducible factor-1α; N.S., non-significant; RT-PCR, reverse transcription polymerase chain reaction; Sc, scramble; TUNEL, transferase-mediated dUTP nick-end labeling. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
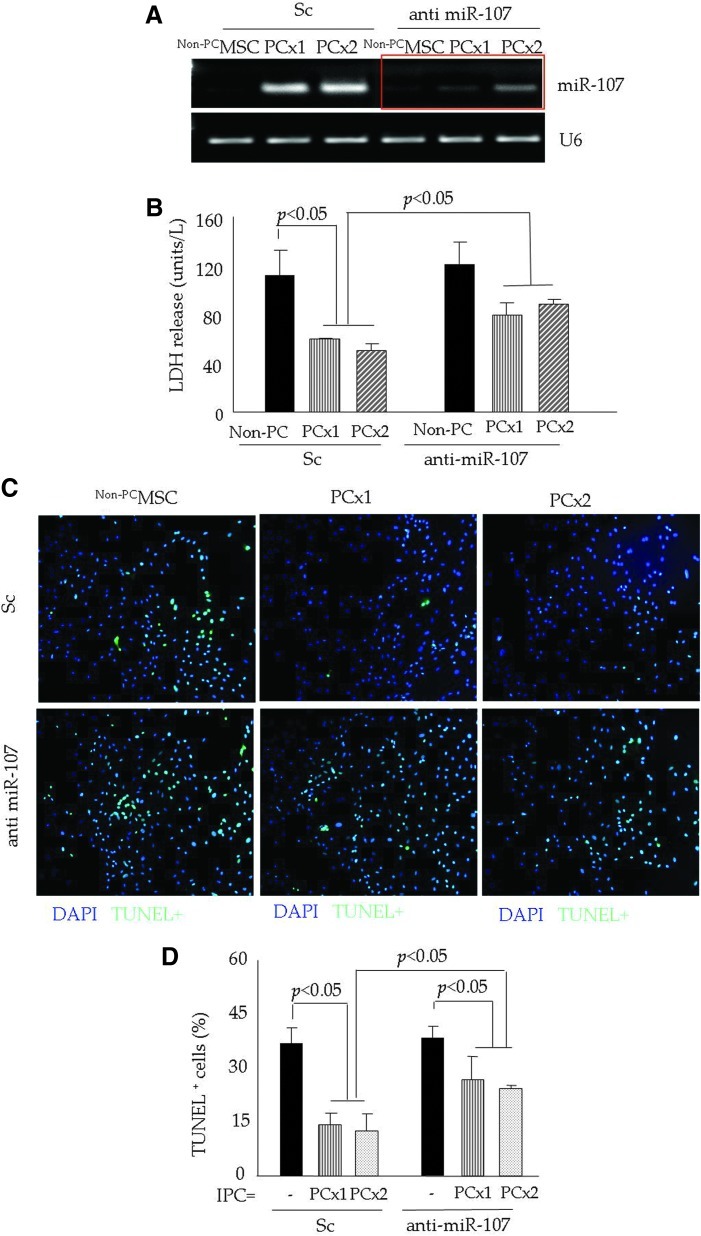
Abrogation of miR-107 and reduced PCMSC survival under lethal anoxia
We observed a significantly higher expression of miR-107 in PCMSC (both PCx1 and PCx2) pretreated with Sc as compared with non-PCMSC (Fig. 3A). To study the role of miR-107 in cytoprotection, PCMSC were pretreated with anti-miR-107 and successfully abrogated miR-107 induction in response to IPC (Fig. 3A). Non-PCMSC were used as a baseline control for both Sc siRNA and anti-miR-107 treatment. The effect of miR-107 abrogation was determined by exposing the cells to 6 h of lethal anoxia, which resulted in a significant loss of cytoprotection, as was evident from increased LDH leakage (Fig. 3B) and higher TUNEL positivity (Fig. 3C, D) in anti-miR-107 pretreated PCMSC in comparison to Sc siRNA-treated PCMSC.
FIG. 3.
Abrogation of miR-107 reduced PCMSC survival under lethal anoxia. (A) RT-PCR showing successful and specific knock-down of endogenous miR-107 in PCMSC by transfection with antisense molecules specific for miR-107. Sc siRNA-transfected MSC were used as controls and showed significant up-regulation of miR-107 after preconditioning (both PCx1 and PCx2). (B) LDH assay showing significantly higher LDH release from PCMSC pretreated with anti-miR-107 as compared with Sc siRNA-transfected MSC. (C, D) Representative fluorescence images from various treatment groups of cells after TUNEL. TUNEL positivity was significantly higher in PCMSC (both with PCx1 and PCx2) pretreated with anti-miR-107 as compared with Sc siRNA-transfected MSC. Non-PCMSC were used as controls. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
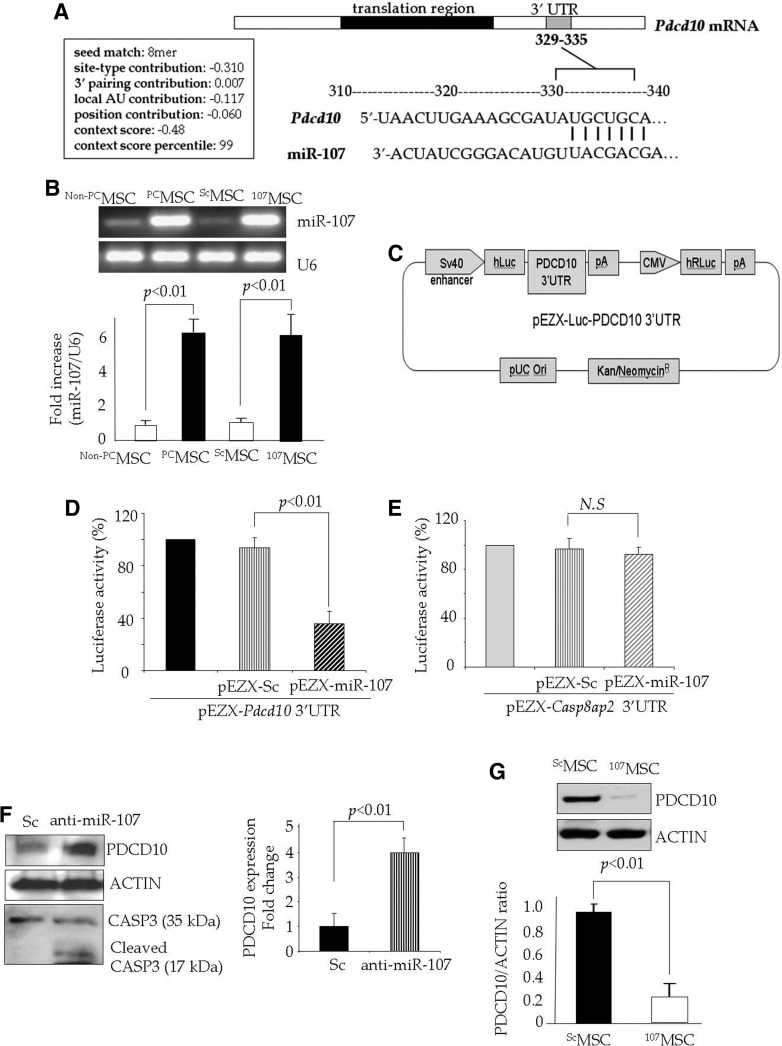
Cytoprotective effects of PDCD10/miR-107 in PCMSC
To further investigate the biological relevance of miR-107 induction during preconditioning and its participation in survival signaling in PCMSC, we employed three databases (miRANDA, Sanger MirBase, and Targetscan) for in silico search for the potential targets of miR-107. Our computational search found consensus putative target sites of miR-107 and miR-210 with high complementarity relevant to apoptosis in the 3′UTR region of Pdcd10 mRNA and Casp8ap2 (17), respectively (Fig. 4A). However, for miR-107 and miR-210, no complementarity was observed that was relevant to apoptosis in the 3′UTR region of Casp8ap2 and Pdcd10 mRNA, respectively. These data were confirmed by luciferase reporter assay. For luciferase reporter assay, we developed a precursor miR-107 expression vector (pEZX-miR-107), which could successfully transfect MSC to achieve a higher expression of miR-107 as compared with native non-PCMSC and ScMSC (107MSC vs. all groups; Fig. 4B). The expression of miR-107 in 107MSC was comparable with that of PCMSC (p>0.05 vs. PCMSC; Fig. 4B). A luciferase construct was designed as shown in Figure 4C. The participation of Pdcd10 as a putative target gene of miR-107 was confirmed by luciferase activity assay, which showed that the co-transfection of a precursor miR-107 expression vector (pEZX-miR-107) with the vector containing 3′UTR of the Pdcd10 gene significantly reduced luciferase activity in comparison to co-transfection with the miR- Sc vector (pEZX-miR-Sc) (Fig. 4D). These results provided clear evidence that exogenous forced the expression of miR-107 down-regulated Pdcd10 via targeting 3′UTR of this gene. We also performed luciferase activity assay using pEZX-miR-107 with a vector containing 3′UTR of the Casp8ap2 gene. However, luciferase activity was not significantly changed in comparison to the co-transfection with the miR-Sc vector (Fig. 4E). These results showed that miR-107 had its effects, which were independent of Casp8ap2, a putative target of miR-210 (17). The relationship between miR-107 and its putative target was confirmed by Western blotting, which showed that pretreatment of PCMSC with anti-miR-107 increased PDCD10 protein expression in comparison with Sc siRNA-pretreated PCMSC (Fig. 4F). Pretreatment with anti-miR-107 increased CASPASE-3 cleavage in PCMSC (Fig. 4F). Western blotting also showed that the induction of miR-107 in neonatal cardiomyocytes abrogated PDCD10 expression (Fig. 4G).
FIG. 4.
Pdcd10 is a putative target of miR-107 in PCMSC. (A) Computational studies showing a putative target site of miR-107 highly conserved in the Pdcd10 mRNA 3′UTR. (B) qRT-PCR showing the successful transfection of MSC for the transgenic expression of miR-107 using the pEZX-miR-107 plasmid. The expression of miR-107 was significantly higher in 107MSC as compared with the pEZX-miR-Sc plasmid-transfected ScMSC as control. The expression of miR-107 in 107MSC was comparable with PCMSC (p>0.05 107MSC vs. PCMSC). (C) Construction of luciferase construct (D, E). Luciferase activity assay performed by the co-transfection of MSC with pEZX-Luc vector containing (D) Pdcd10 3′UTR or (E) Casp8ap2 3′UTR along with a plasmid encoding miR-107. Significantly decreased luciferase activity was observed on co-transfection with the pEZX-Luc vector containing Pdcd10 3′UTR, whereas no significant decrease in luciferase activity was observed on co-transfection with Casp8ap2 3′UTR. The ratio of luciferase activity was calculated in either the presence or absence of miR-107. (F) Western blot showing significantly higher expression of PDCD10 in PCMSC pretreated with anti-miR-107 as compared with ScMSC. Fold change was determined in the PDCD10/actin ratio between the Sc siRNA and anti-miR-107-treated cells. (G) Western blot showing significant abrogation of PDCD10 in neonatal cardiomyocytes after the transfection of miR-107 as compared with the non-transfected cardiomyocytes. PDCD10, programmed cell death-10.
Combined effect of simultaneous induction of miR-107 and miR-210 on cytoprotection
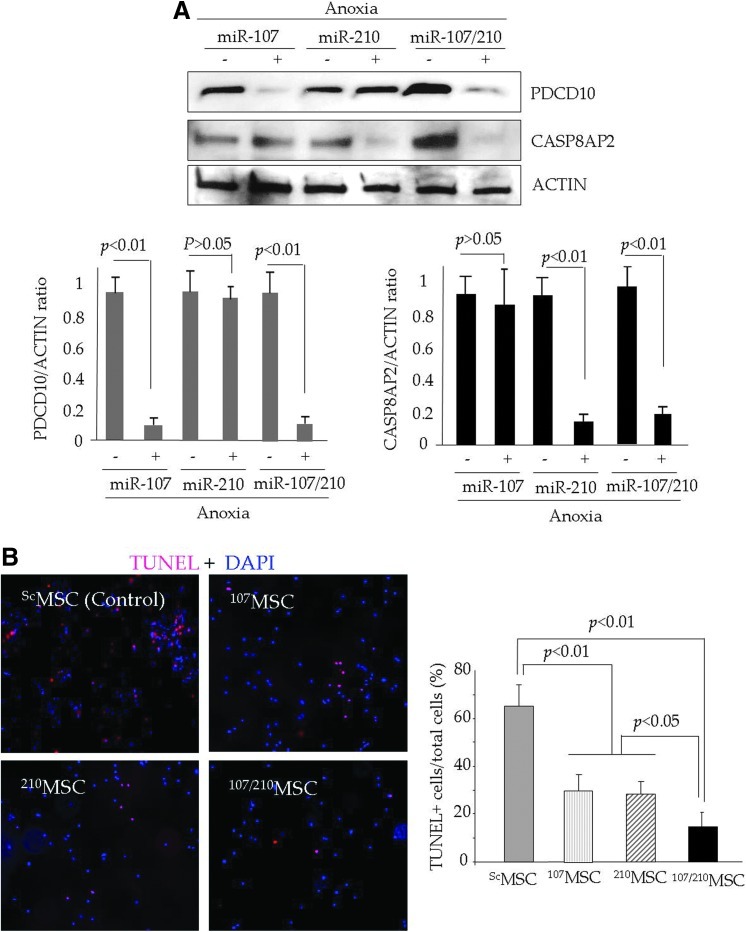
A gain-of-function study that determines cytoprotection after a combined overexpression of miR-107 and miR-210 in MSC was performed using 107MSC and 210MSC for individual miR-107 and miR-210 cytoprotective effects, whereas ScMSC were used as a baseline control. Single as well as combined overexpression of miR-107 and miR-210 led to individual and combined abrogation of PDCD10 and CASP8AP2 under lethal anoxia (Fig. 5A). TUNEL showed that combined overexpression miR-107 and miR-210 was more effective in protecting MSC as compared with individual overexpression of respective miR (Fig. 5B).
FIG. 5.
Simultaneous overexpression of miR-107 and miR-210 has a stronger pro-survival effect on PCMSC survival. (A) Western blot showing the abrogation of PDCD10 and CASP8AP2 in MSC after individual or combined overexpression of miR-107 and miR-210 under lethal anoxia. As compared with ScMSC, 107MSC and 210MSC significantly abrogated PDCD10 and CASP8AP2, respectively, under lethal anoxia. On the same note, 107/210MSC showed the successful simultaneous abrogation of both PDCD10 and CASP8AP2. (B) The TUNEL assay was performed to assess the cytoprotective effects of these molecular events. Although 107MSC and 210MSC were effectively protected against lethal anoxia, 107/210MSC showed significantly higher survival as compared with 107MSC and 210MSC. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars).
Combined effect of simultaneous abrogation of miR-107 and miR-210 on cytoprotection
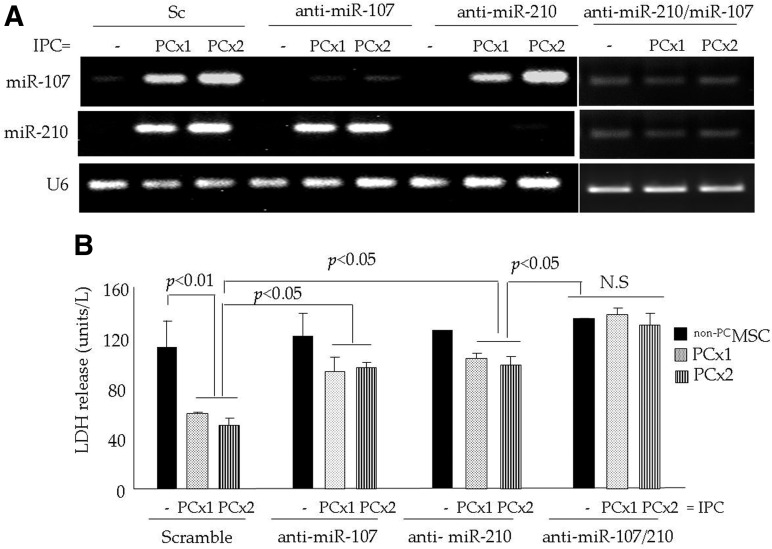
We successfully abrogated miR-107, miR-210, and both miR-107/miR-210 by the pretreatment of PCMSC with anti-miR-107, anti-miR-210, and both anti-miR-107/miR-210, respectively (Fig. 6A). Sc siRNA-treated PCMSC and non-PCMSC were used as controls. Subsequent exposure to 6 h of lethal anoxia showed a significant loss of cytoprotection in all three antisense treatment groups of cells in comparison to the Sc siRNA pretreated PCMSC as determined by LDH (Fig. 6B) and TUNEL assays (Supplementary Fig. S2B, C). TUNEL positivity in Sc siRNA-transfected PCMSC (PCx2) was (8.8±1.2), which was significantly lower as compared with the PCMSC (PCx2) transfected with anti-miR-107 (17.8±2.3) and anti-miR-210 (25.8±1.7). Although TUNEL-positive PCMSC pretreated with anti-miR-107 (17.8±2.3) and anti-miR-210 (25.8±1.7) were statistically insignificant from each other, the abrogation of miR-210 led to higher TUNEL positivity. On the contrary, PCMSC with the simultaneous abrogation of miR-107/miR-210 had higher TUNEL positivity (37.4±2.2). This was similar to the non-PCMSC with simultaneous miR-107/miR-210 abrogation (p>0.05) but significantly higher as compared with the PCMSC pretreated with either anti-miR-107 or anti-miR-210 (Supplementary Fig. S2B, C). A similar trend was also observed in PCMSC (with PCx1) (Supplementary Fig. S2B, C).
FIG. 6.
Simultaneous abrogation of miR-107 and miR-210 has a stronger combined effect on PCMSC survival. (A) qRT-PCR showing the successful abrogation of miR-107, miR-210 and the simultaneous abrogation of miR-107/miR-210 in PCMSC (both PCx1 and PCx2) after the pretreatment of cells with anti-miR107, anti-miR-210, and anti-miR107/miR-210, respectively. Pretreatment with Sc siRNA did not change miR-107 and miR-210 in PCMSC (both PCx1 and PCx2). Non-PCMSC were used as a baseline control. (B). LDH release as an indicator of cellular injury was significantly increased in PCMSC (both PCx1 and PCx2) pretreated with anti-miR-107, anti-miR-210, and anti-miR-107/miR-210 as compared with Sc siRNA-transfected PCMSC subsequent to 6 h of lethal anoxia.
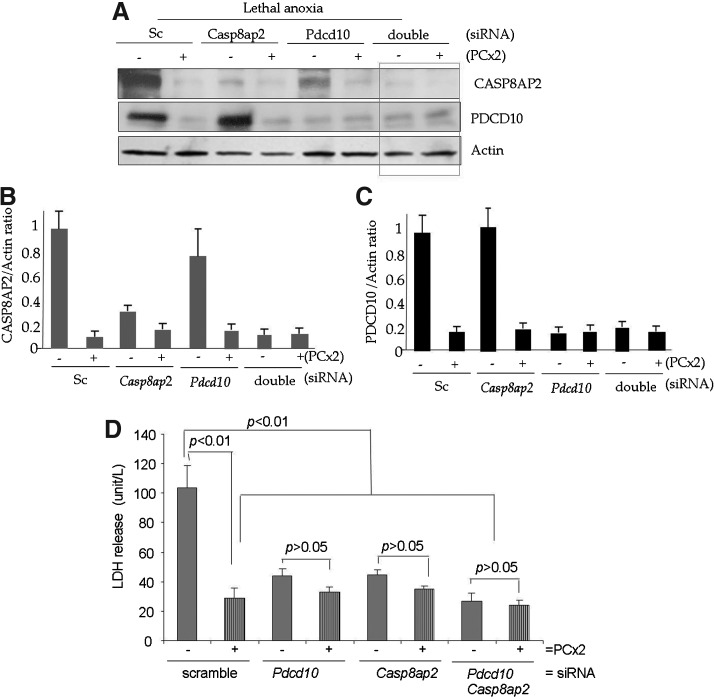
In a parallel set of experiments, we abrogated the putative targets of miR-107 and miR-210, Pdcd10 and Casp8ap2, respectively, in PCMSC either individually or simultaneously by the pretreatment of PCMSC with siRNA specific for Pdcd10, Casp8ap2, and both Pdcd10 siRNA/Casp8ap2, respectively (Fig. 7). Sc siRNA-transfected PCMSC and non-PCMSC were used as controls. Western blots showed the successful and specific abrogation of PDCD10, CASP8AP2, and PDCD10/CASP8AP2 in the respective siRNA treatment cell groups (Fig. 7A–C). In accordance with data from anti-miR treatment (Fig. 6), subsequent exposure to 6 h of lethal anoxia resulted in significantly improved survival in all cell treatment groups with respective siRNA treatments as was evident from reduced LDH release (Fig. 7D) and TUNEL positivity (Supplementary Fig. S3). PCMSC with Sc siRNA treatment control showed significantly low LDH release (Fig. 7D) as well as TUNEL positivity (Supplementary Fig. S3). On the contrary, non-PCMSC with Sc siRNA had higher LDH release and TUNEL positivity. Although TUNEL positivity in non-PCMSC pretreated with individual Casp8ap2 siRNA (29±5.7) or Pdcd10 siRNA (27.4±6.1) was statistically insignificant (p>0.05 Casp8ap2 siRNA vs. Pdcd10 siRNA pretreatment), Casp8ap2 siRNA pretreatment tended to be more effective. On the contrary, the simultaneous abrogation of Casp8ap2/Pdcd10 in non-PCMSC was more effective and showed a stronger combined effect in terms of reduced TUNEL positivity (19.2±3.1) as compared with cells with single siRNA treatment (Supplementary Fig. S3). Similarly, the simultaneous abrogation of Casp8ap2/Pdcd10 in PCMSC showed reduced TUNEL positivity (13.1±4.2) as compared with the abrogation of either Casp8ap2 (19.2±3.1) or Pdcd10 (18.4±4.3) with respective siRNA treatment (Supplementary Fig. S3).
FIG. 7.
Simultaneous abrogation of Casp8ap2 and Pdcd10 has a stronger cytoprotection in PCMSC. (A–C) Western blot and densitometry analysis showing the successful abrogation of CASP8AP2, PDCD10 and the simultaneous abrogation of CASP8AP2/PDCD10 in PCMSC after the pretreatment of cells with siRNA specific for Casp8ap2 and Pdcd10. The pretreatment with Sc siRNA did not change the Casp8ap2, Pdcd10 in PCMSC (PCx2). Non-PCMSC were used as a baseline control. (D) LDH release was significantly reduced in PCMSC (two cycles 30 min A/R; PCx2) pretreated with Casp8ap2, Pdcd10, and Casp8ap2/Pdcd10 siRNA as compared with the Sc siRNA-transfected PCMSC after 6 h of anoxia. Non-PCMSC were used as a baseline control.
PCMSC with higher miR-107 showed improved survival in the infarcted heart
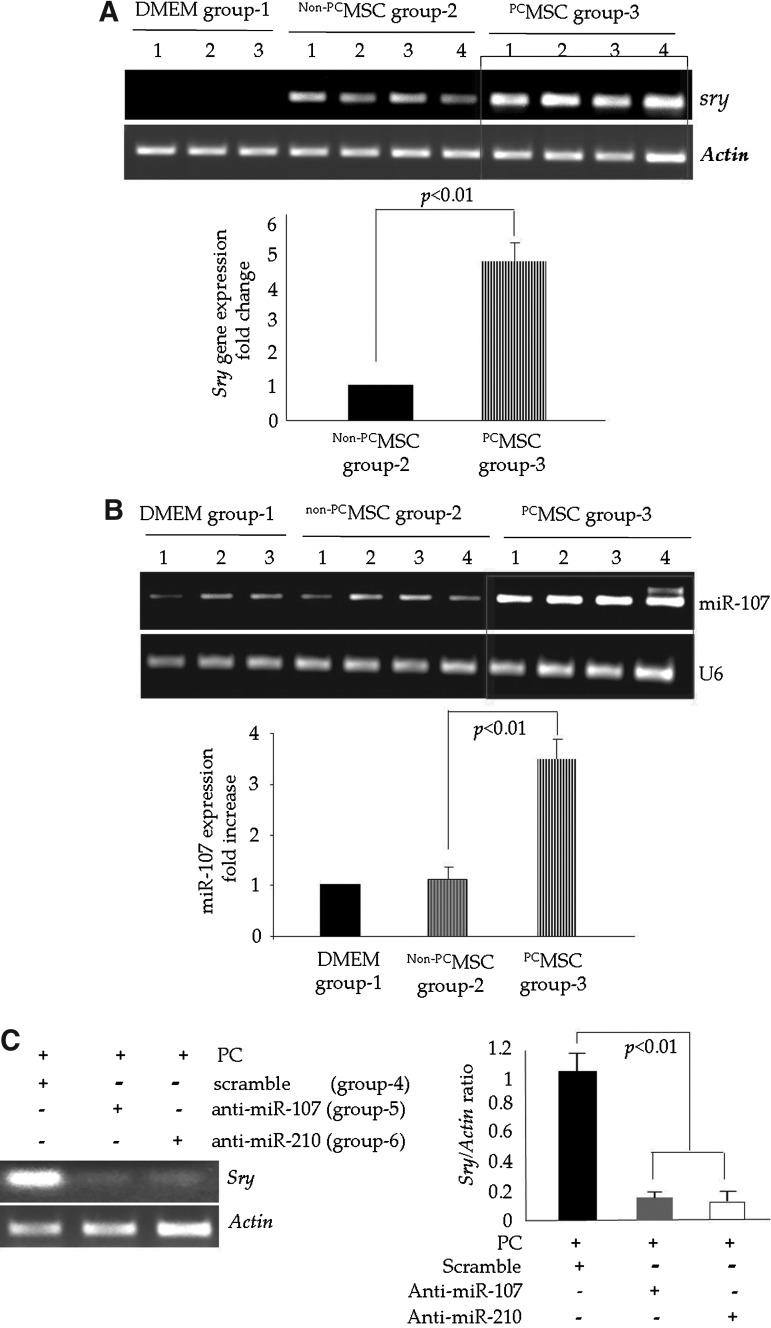
During the first phase of in vivo studies, three animal groups were prepared for in vivo studies: Dulbecco's Modified Eagle Medium (DMEM)-injected group-1 control, non-PCMSC-transplanted group-2, and PCMSC-transplanted group-3 (two-cycles of 30 min A/R, PCx2). The animal hearts (n=4) were harvested 4 days after their respective treatment for molecular studies to assess the survival of the transplanted cell graft. Sry-gene studies by qRT-PCR in female recipient hearts showed significantly higher survival (approximately fivefold higher) of male PCMSC in group-3 as compared with non-PCMSC group-2 (p<0.05; Fig. 8A). The DMEM-injected group-1 served as a negative control, as no sry-gene was detected (Fig. 8A). qRT-PCR for miR-107 showed significantly increased miR-107 expression (∼3.5-fold higher) in the PCMSC group-3 as compared with the non-PCMSC group-2 (Fig. 8B).
FIG. 8.
PCMSC had improved survival in the infarcted rat heart. (A) PCR for sry-gene expression in the female animal hearts from different treatment groups on day 4 after their respective treatment. Sry-gene expression was significantly higher in the PCMSC-transplanted animal hearts of group-3 as compared with the Non-PCMSC-transplanted animal hearts of group-2. The DMEM-treated animal hearts of group-1 did not show the sry-gene and served as a negative control. (B) qRT-PCR showing a higher level of expression of miR-107 in the infarcted rat heart on day 4 post-transplantation of PCMSC as compared with Non-PCMSC and DMEM-treated animal hearts. (C) PCR for the sry-gene analysis of animal hearts transplanted with PCMSC trnasfected with anti-miR-107 and anti-miR-210 using Sc-transfected PCMSC as a control. Transfection with anti-miR-107 and anti-miR-210 significantly abolished the cytoprotective effects of preconditioning. PCMSC with anti-miR-107 (animal group-5) and anti-miR-210 (animal group-6) transfection showed significantly lower survival as compared with the Sc-transfected PCMSC (animal group-4). DMEM, Dulbecco's Modified Eagle Medium.
We also performed loss-of-function studies by the transfection of PCMSC with anti-miR-107 (group-5) and anti-miR-210 (group-6) before preconditioning and later injected these cells into the infarcted hearts. The animals treated with ScMSC (group-4) served as controls (Fig. 8C). PCMSC with their respective anti-miR-107 and anti-miR-210 transfections showed significantly reduced survival post-transplantation (Fig. 8C). These data clearly demonstrated the importance of miR-107 and miR-210 as critical determinants of cytoprotection during preconditioning.
Discussion
The important findings of our study include (i) miR-107, one of the known HRM, which is up-regulated in the PCMSC in response to Akt/HIF-1α activation; (ii) miR-107 plays a mechanistic role in the PCMSC survival via its putative target Pdcd10; (iii) the Pro-survival effect of miR-107/PDCD10 interaction is independent of miR-210 and its known target Casp8ap2 during IPC of MSC (17); and (iv) miR-107 and miR-210 have a stronger combined pro-survival role in PCMSC via their respective putative targets Pdcd10 and Casp8ap2.
The response of a cell to oxygen changes in its microenvironment is imperative for the maintenance of cellular oxygen homeostasis. The oxygen-sensing mechanism of cells revolves around HIF-1 signaling, which regulates hundreds of genes, either directly or indirectly, to help the cell acclimatize to subtle oxygen changes (28). Gene profiling studies showed that a vast array of genes influenced by HIF-1α activity was cell-type dependent (16). With an emerging role of miRs as key regulators of cellular function (23), a select group of miRs including miR-107 and miR-210 have been identified as HRM (18). These HRM participate in the cellular response to oxygen changes either by facilitating HIF-1α stability or by getting regulated by HIF-1α to fine tune expression of the genes required by the cells for survival under hypoxia. On the same note, HRM may also shut down the post-transcriptional activity of the genes that are not required for the maintenance of cellular homeostasis under hypoxia. Hypoxamir-210, one of the miRs with hypoxia signature, has been extensively studied for its relationship with HIF-1 signaling. Loss-and-gain-of-function studies provided mounting evidence that miR-210 is an important determinant of cellular functions (3, 7, 15, 24). We have reported that miR-210 was up-regulated during the IPC of stem cells in an Akt/HIF-1α-dependent manner and improved PCMSC survival under lethal anoxia and post-transplantation in an infarcted heart via its putative target gene Casp8ap2 (17). Our results provided mounting evidence that Akt activation was stabilized by HIF-1α, whereas the activity and stabilization of HIF-1α was associated with an altered expression of both miR-107 and miR-210. HIF-1α-specific RNA interference led to the abrogation of miR-107 and miR-210, thus showing that the transcription factor HIF-1α was a critical determinant of both the miRs during the preconditioning of stem cells. Given the significance of miR-210 in Akt/HIF-1α-mediated cell survival, vector-based and ex-vivo stem-cell-based delivery of miR-210 have been performed for myocardial repair with encouraging results (7, 9, 12).
Besides miR-210, we observed that miR-107 had a significantly altered expression during the IPC of MSC. Although little is known about miR-107 for its function in stem cell functions in general and during the preconditioning of stem cells in particular, miR-107 has been mostly studied for its association with the pathogenesis of human cancers (6, 8, 22); miR-107 targets CDK6 expression to induce cell-cycle arrest and the inhibition of tumor cell invasion (8). Similarly, p53-induced miR-107 inhibits apoptosis via the down-regulation of HIF-1β and suppresses tumor growth and tumor angiogenesis in colon cancer cells (31). It was also shown that p53 activated the pantothenate kinase 1 (PANK1) gene, and its intron miR-107 down-regulated two important cell cycle genes, Cdk6 and p130 (RBL2), to suppress tumorigenesis (1). Toll-like receptors down-regulate miR-107 to enhance CDK6 levels and increase the adhesion of macrophages (11). In this study, we provide the first evidence of the preconditioning induced regulation of miR-107 and its cytoprotective effects on PCMSC via its putative target gene (Pdcd10). Similar to miR-210, we observed Akt/HIF-1α dependent induction of miR-107, which acted via Pdcd10, as predicted by in silico analysis and validated by luciferase activity assay. Pdcd10 (also called cerebral cavernous malformation-3 or CCM3) is typically associated with apoptosis either directly (4) or via cell-cycle modulation (21). Loss-of-function studies with PDCD10 specific siRNA demonstrated a significant reduction in apoptosis during oxygen/serum deprivation (4). On the contrary, constitutive expression of Pdcd10 was observed in peripheral blood T-cells in patients suffering from cutaneous T-cell lymphoma and was associated with phosphatase-A2, which supported the mitogenesis and survival of cells (21). A combined regulatory participation of miR-107 and miR-103 has been reported in the migrational activity of neuroblastoma cells by the transcriptional regulation of a common putative target CDK5R1/p35 (25). We observed that during the IPC of MSC, the induction of miR-107 had an anti-apoptotic influence on the PCMSC cultured under lethal anoxia, which were independent of miR-103 involvement. Post-transplantation survival of PCMSC was significantly higher as compared with non-PCMSC. The pretreatment of PCMSC with anti-miR-107 and anti-miR-210 significantly abolished the cytoprotective effects of preconditioning. An interesting aspect of our data was the stronger combined effect of miR-107 and miR-210 induction during preconditioning. While demonstrating the pro-survival effects of miR-107 and miR-210 in PCMSC, we observed that the knock-down of each miR with respective antagomir reduced stem cell survival, both in vitro as well as post-transplantation in the infarcted heart. However, the simultaneous knock-down of miR-107/miR-210 had a stronger combined pro-apoptotic effect on PCMSC, which was higher as compared with the knock-down of individual miR. Similarly, the simultaneous abrogation of Pdcd10 and Casp8ap2 was more effective in simulating the pro-survival effects of IPC as compared with the pretreatment of PCMSC with either Pdcd10 or Casp8ap2 alone.
Despite interesting and novel findings, our study has limitations. Although we had studied the in vivo survival of PCMSC in the infarcted myocardium, we did not determine the cardiacprotective effects and functional impact of the transplanted cells in the heart, especially in response to miR-107 signaling. Future studies would be required to investigate how miR-107 and miR-210 co-ordinate for better prognosis after the transplantation of stem cells with the transgenic overexpression of the two miRs in comparison to the preconditioned stem cells.
In conclusion, we demonstrated a functional coordination between two members of the select group of HRM in alleviating the pro-apoptotic effects of lethal anoxia. Although miR-107 and miR-210 were induced in response to IPC, which activated Akt/HIF-1α signaling in PCMSC, the two miRs independently exerted their pro-survival effects through their respective putative target genes. Moreover, their independent signaling had stronger combined anti-apoptotic effects on preconditioned stem cells to support their survival under ischemic stress in vitro and post-transplantation. Our study results supported the existing dogma regarding the multifaceted nature of signaling involved in IPC (10).
Materials and Methods
Our study conformed to the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised-1996) and protocol approved by the Institutional Animal Care and Use Committee, University of Cincinnati. All surgical manipulations were performed under general anesthesia.
Purification and preconditioning of MSC
MSC were purified from young male Fischer-344 rat bone marrow and preconditioned as described in Supplementary Data (14, 17).
MiR isolation and detection
MiRs were isolated and detected by using mirVana™ miRNA Isolation and qRT-PCR miRNA Detection Kits with specific miR primers respectively provided by Ambion Inc., as per the manufacturer's instructions.
siRNA transfection
Predesigned siRNA for HIF-1α and Sc siRNA (Santa Cruz Biotechnology) were used to knock down gene expression (17). The transient transfection of siRNA was performed with Lipofectamine-2000™ (Invitrogen) as per the manufacturer's protocols.
Transfection with miR inhibitors and for overexpression of miR-107/miR-210
To knock down miR-107 and miR-210, the siPORT™ NeoFx™ transfection agent was used for the transfection of respective anti-miR (Ambion, Inc.), as detailed in Supplementary data.
Luciferase reporter assays and Western blotting
For detailed methods, please see Supplementary Data.
Rat heart model of acute myocardial infarction and cell transplantation
The survival of cells with their respective treatment was determined in vivo using a rat heart model of acute coronary artery ligation (17). Female young (6–8 weeks) Fisher-344 rats weighing ∼200 g were anesthetized by an intra-peritoneal injection of pentobarbital (30 mg/kg body weight). Sex-mismatched transplantation of male donor cells was performed during the acute phase after permanent LAD coronary artery ligation. Cell transplantation (1×106 cells in 70 μl basal DMEM/heart) was carried out at multiple sites (3–4 sites/heart) intramyocardially in the free wall of the left ventricle. After the injection, the chests of the animals were closed, and the animals were allowed to recover. The animals were kept on 0.1 mg/kg bis in die Buprinex during the first 24 h after surgery for pain management. The animals were euthanized on day 4 after their respective treatment for collection of the heart tissue samples for molecular studies.
The myocardial tissue samples from various treatment groups of female animals were used for PCR to determine male transplanted cells' survival. Tissue samples were snap frozen in liquid nitrogen and powdered. DNA purification was performed using the Genomic DNA Isolation kit (Qiagen), and the concentration of the purified DNA was determined by spectrophotometry. The primer sequences for sry-gene and actin were as follows: sry-gene, forward=5′-gaggcacaagtt ggctcaaca-3′ and reverse=5′-ctcctgcaaaaagggccttt-3′; actin, forward=5′-agccatgta cgtagccatcc-3′ and reverse=5′-ctctcagctgtggtggtgaa-3′. qPCR for Pdcd10 was performed using primers purchased from SA Biosciences as per manufacturer's instructions.
Statistical analyses
All values were expressed as mean±standard error. A comparison between two mean values was evaluated by an unpaired Student two-tailed t-test, and between three or more groups was evaluated by one-way analysis of variance followed by Bonferroni post-hoc analysis. Statistical significance was accepted at p<0.05.
Supplementary Material
Abbreviations Used
- A/R
anoxia/re-oxygenation
- HIF-1α
hypoxia inducible factor-1α
- HRM
hypoxia responsive miRs
- IPC
ischemic preconditioning
- LDH
lactate dehydrogenase
- miR
microRNA
- MSC
mesenchymal stem cells
- Non PCMSC
non-preconditioned MSC
- PCMSC
preconditioned MSC
- PCx1
preconditioning with A/R 30 min 1 cycle
- PCx2
preconditioning with A/R 30 min 2 cycles
- Sc
scramble
- TUNEL
transferase-mediated dUTP nick-end labeling
- Wort
Wortmannin
Acknowledgments
This work was supported by NIH (USA) grants R37HL074272; HL-080686; HL087246(M.A); HL-087288; HL-089535; and HL106190-01(Kh.H.H).
Author Disclosure Statement
The authors declare that they have nothing to disclose.
References
- 1.Bohlig L. Friedrich M. Engeland K. p53 activates the PANK1/miRNA-107 gene leading to downregulation of CDK6 and p130 cell cycle proteins. Nucleic Acids Res. 2011;39:440–453. doi: 10.1093/nar/gkq796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chacko SM. Ahmed S. Selvendiran K. Kuppusamy ML. Khan M. Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2011;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan YC. Banerjee J. Choi SY. Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L. Tanriover G. Yano H. Friedlander R. Louvi A. Gunel M. Apoptotic functions of PDCD10/CCM3, the gene mutated in cerebral cavernous malformation 3. Stroke. 2009;40:1474–1481. doi: 10.1161/STROKEAHA.108.527135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen PS. Su JL. Cha ST. Tarn WY. Wang MY. Hsu HC. Lin MT. Chu CY. Hua KT. Chen CN. Kuo TC. Chang KJ. Hsiao M. Chang YW. Chen JS. Yang PC. Kuo ML. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2011;121:3442–3455. doi: 10.1172/JCI45390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta J. Smith A. Lang JC. Islam M. Dutt D. Teknos TN. Pan Q. microRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cvarepsilon. Oncogene. 2011 doi: 10.1038/onc.2011.565. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasanaro P. Greco S. Lorenzi M. Pescatori M. Brioschi M. Kulshreshtha R. Banfi C. Stubbs A. Calin GA. Ivan M. Capogrossi MC. Martelli F. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem. 2009;284:35134–35143. doi: 10.1074/jbc.M109.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng L. Xie Y. Zhang H. Wu Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol. 2012;29:856–863. doi: 10.1007/s12032-011-9823-1. [DOI] [PubMed] [Google Scholar]
- 9.Ha Won Kim MA. Shujia Jiang. Haider Khawaja H. Direct transfer of miR-210 from preconditioned stem cells to the host cardiomyocytes via gap junctions promote functional recovery of the ischemic myocardium. Circulation. 2011;124(21 Supplement: S Meeting Abstract: A131) [Google Scholar]
- 10.Haider H. Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010;3:89–102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy EJ. Sheedy FJ. Santamaria D. Barbacid M. O'Neill LA. Toll-like receptor-4 (TLR4) down-regulates microRNA-107, increasing macrophage adhesion via cyclin-dependent kinase 6. J Biol Chem. 2011;286:25531–25539. doi: 10.1074/jbc.M111.256206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S. Huang M. Li Z. Jia F. Ghosh Z. Lijkwan MA. Fasanaro P. Sun N. Wang X. Martelli F. Robbins RC. Wu JC. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai Y. Kimura T. Murakami A. Yajima N. Sakamaki K. Yonehara S. The CED-4-homologous protein FLASH is involved in Fas-mediated activation of caspase-8 during apoptosis. Nature. 1999;398:777–785. doi: 10.1038/19709. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S. Haider H. Idris NM. Salim A. Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 15.Kane NM. Meloni M. Spencer HL. Craig MA. Strehl R. Milligan G. Houslay MD. Mountford JC. Emanueli C. Baker AH. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2011;30:1389–1397. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 16.Kelly BD. Hackett SF. Hirota K. Oshima Y. Cai Z. Berg-Dixon S. Rowan A. Yan Z. Campochiaro PA. Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 17.Kim HW. Haider HK. Jiang S. Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulshreshtha R. Davuluri RV. Calin GA. Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- 19.Kulshreshtha R. Ferracin M. Negrini M. Calin GA. Davuluri RV. Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle. 2007;6:1426–1431. [PubMed] [Google Scholar]
- 20.Kulshreshtha R. Ferracin M. Wojcik SE. Garzon R. Alder H. Agosto-Perez FJ. Davuluri R. Liu CG. Croce CM. Negrini M. Calin GA. Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauenborg B. Kopp K. Krejsgaard T. Eriksen KW. Geisler C. Dabelsteen S. Gniadecki R. Zhang Q. Wasik MA. Woetmann A. Odum N. Programmed cell death-10 enhances proliferation and protects malignant T cells from apoptosis. Apmis. 2010;118:719–728. doi: 10.1111/j.1600-0463.2010.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X. Zhang Y. Shi Y. Dong G. Liang J. Han Y. Wang X. Zhao Q. Ding J. Wu K. Fan D. MicroRNA-107, an oncogene microRNA that regulates tumour invasion and metastasis by targeting DICER1 in gastric cancer. J Cell Mol Med. 2011;15:1887–1895. doi: 10.1111/j.1582-4934.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z. Sall A. Yang D. MicroRNA: an emerging therapeutic target and intervention tool. Int J Mol Sci. 2008;9:978–999. doi: 10.3390/ijms9060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu ZH. Yang G. Zhao T. Cao GJ. Xiong L. Xia W. Huang X. Wu LY. Wu K. Fan M. Shao NS. Zhu LL. Small ncRNA expression and regulation under hypoxia in neural progenitor cells. Cell Mol Neurobiol. 2011;31:1–5. doi: 10.1007/s10571-010-9556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncini S. Salvi A. Zuccotti P. Viero G. Quattrone A. Barlati S. De Petro G. Venturin M. Riva P. The role of miR-103 and miR-107 in regulation of CDK5R1 expression and in cellular migration. PLoS One. 2011;6:e20038. doi: 10.1371/journal.pone.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niagara MI. Haider H. Jiang S. Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 27.Nie Y. Han BM. Liu XB. Yang JJ. Wang F. Cong XF. Chen X. Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int J Biol Sci. 2011;7:762–768. doi: 10.7150/ijbs.7.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 30.Stubbs SL. Hsiao ST. Peshavariya H. Lim SY. Dusting GJ. Dilley RJ. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012 doi: 10.1089/scd.2011.0289. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Yamakuchi M. Lotterman CD. Bao C. Hruban RH. Karim B. Mendell JT. Huso D. Lowenstein CJ. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A. 2011;107:6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.