Abstract
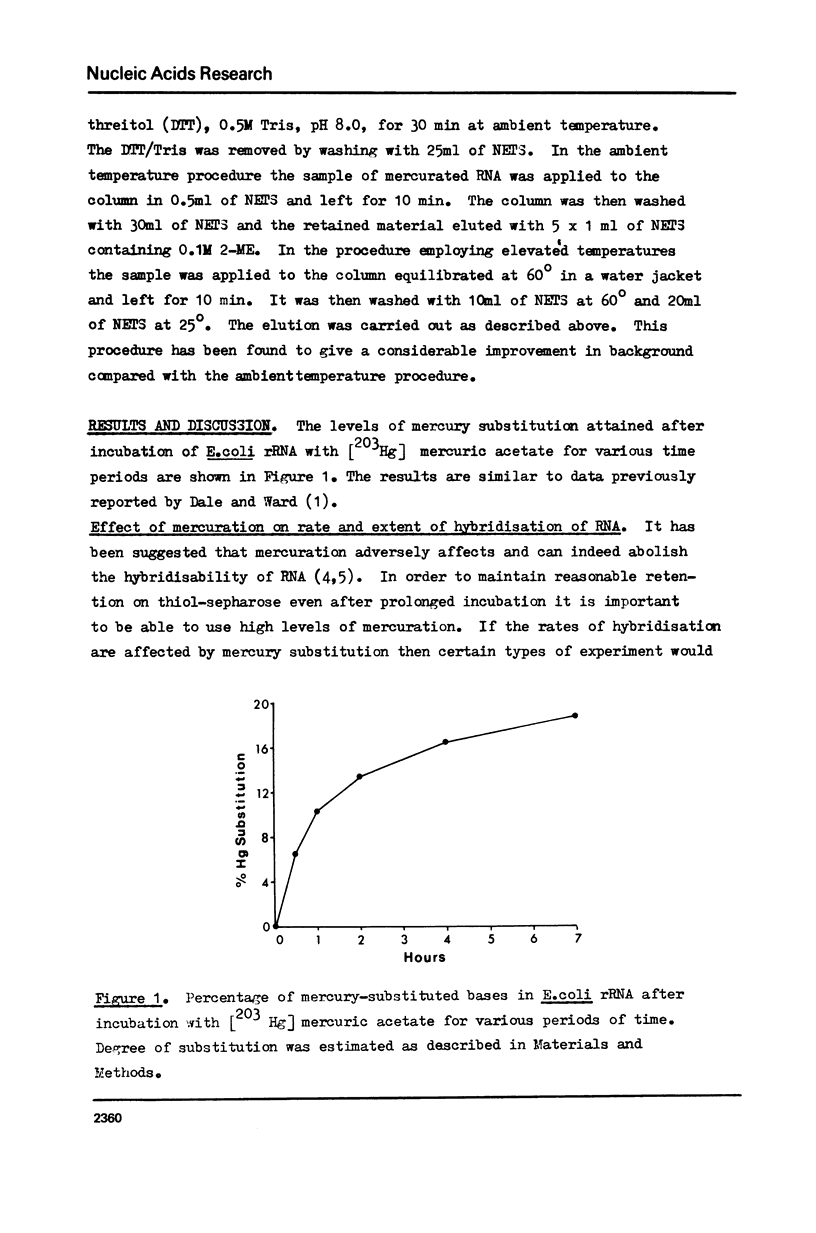
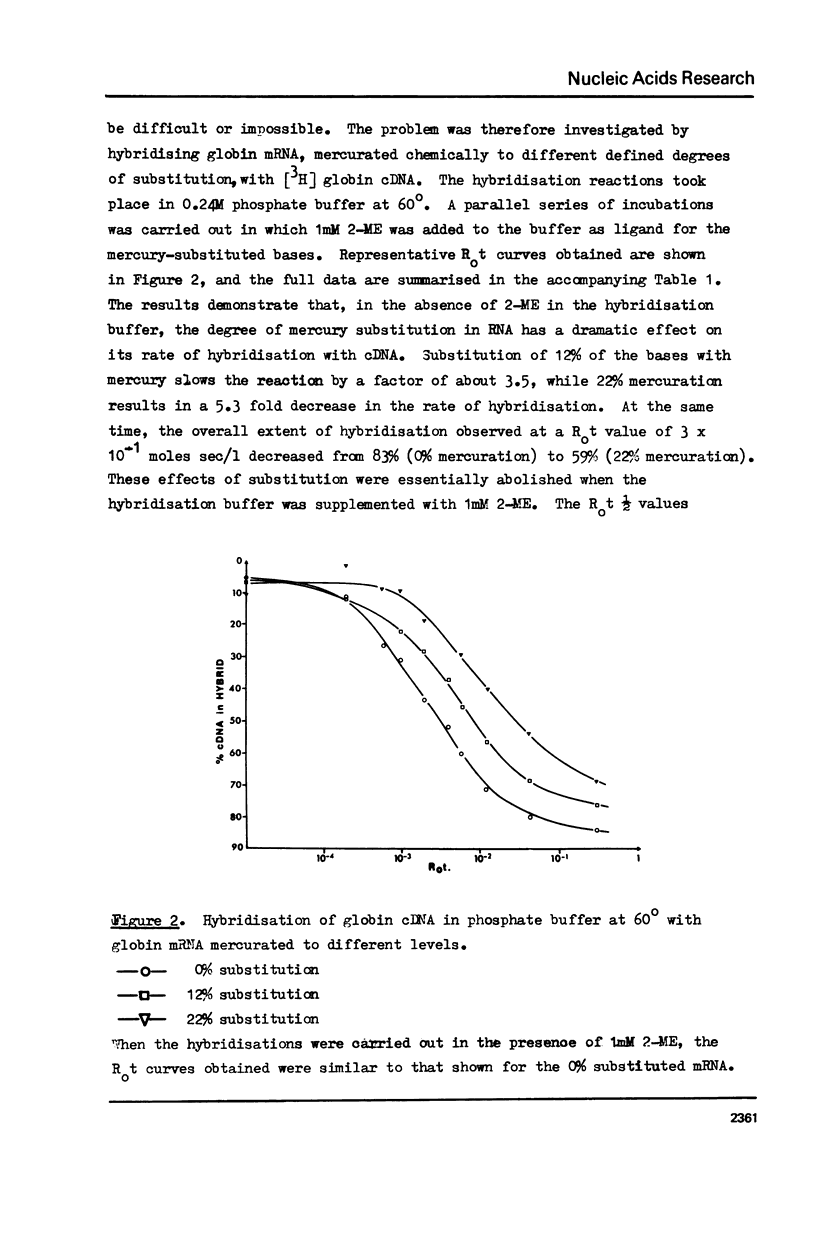
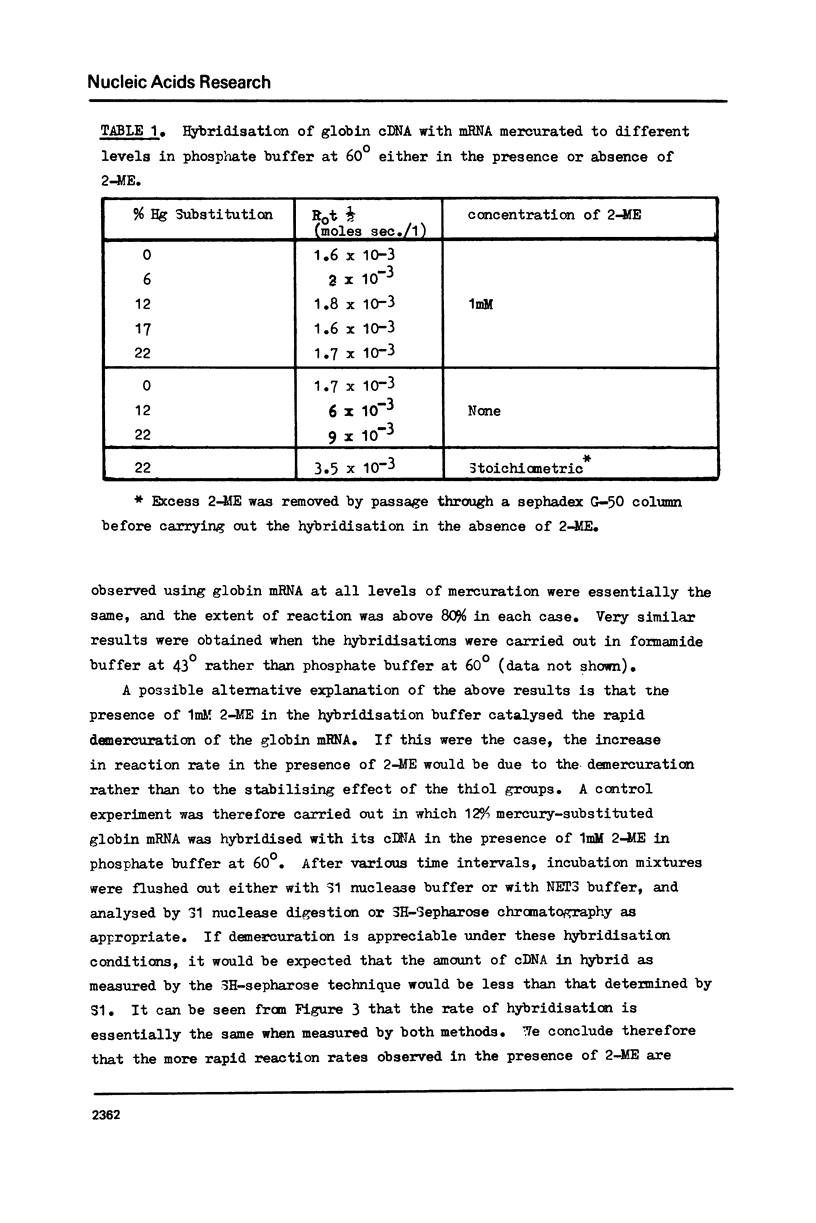
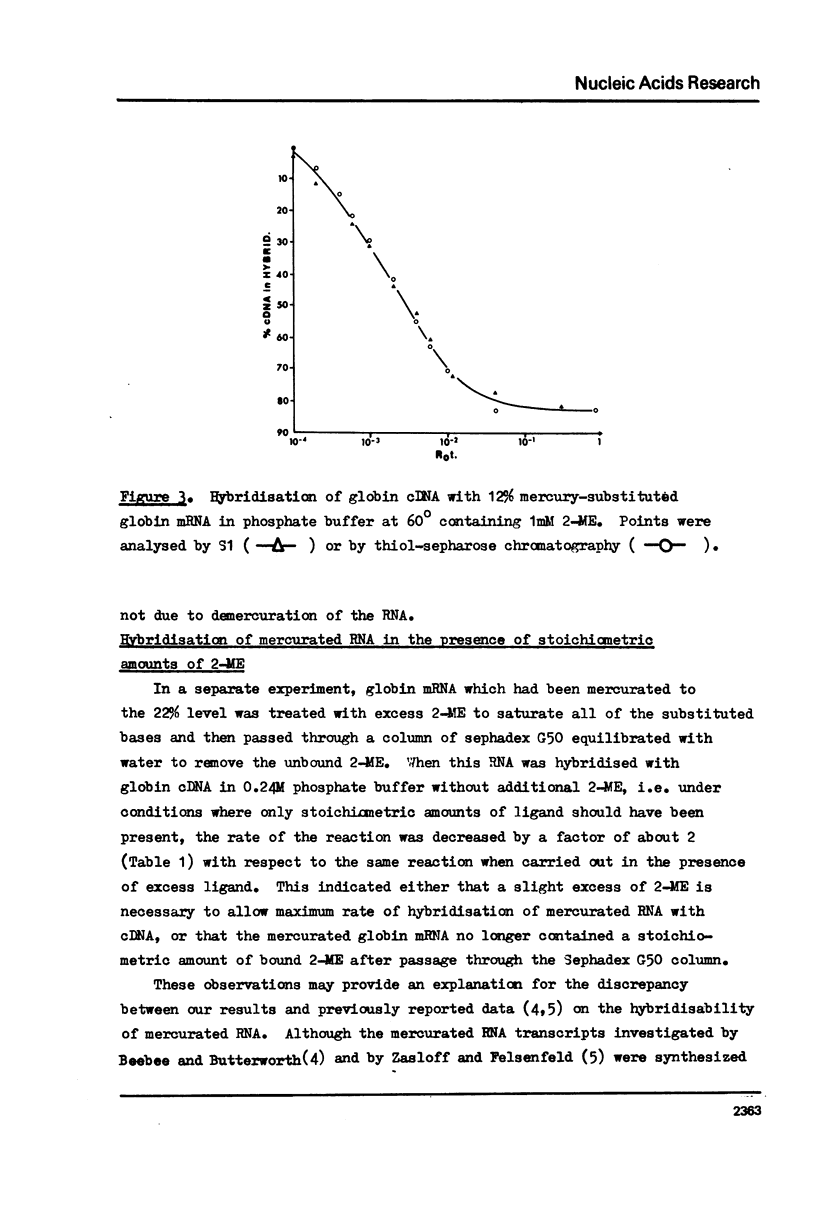
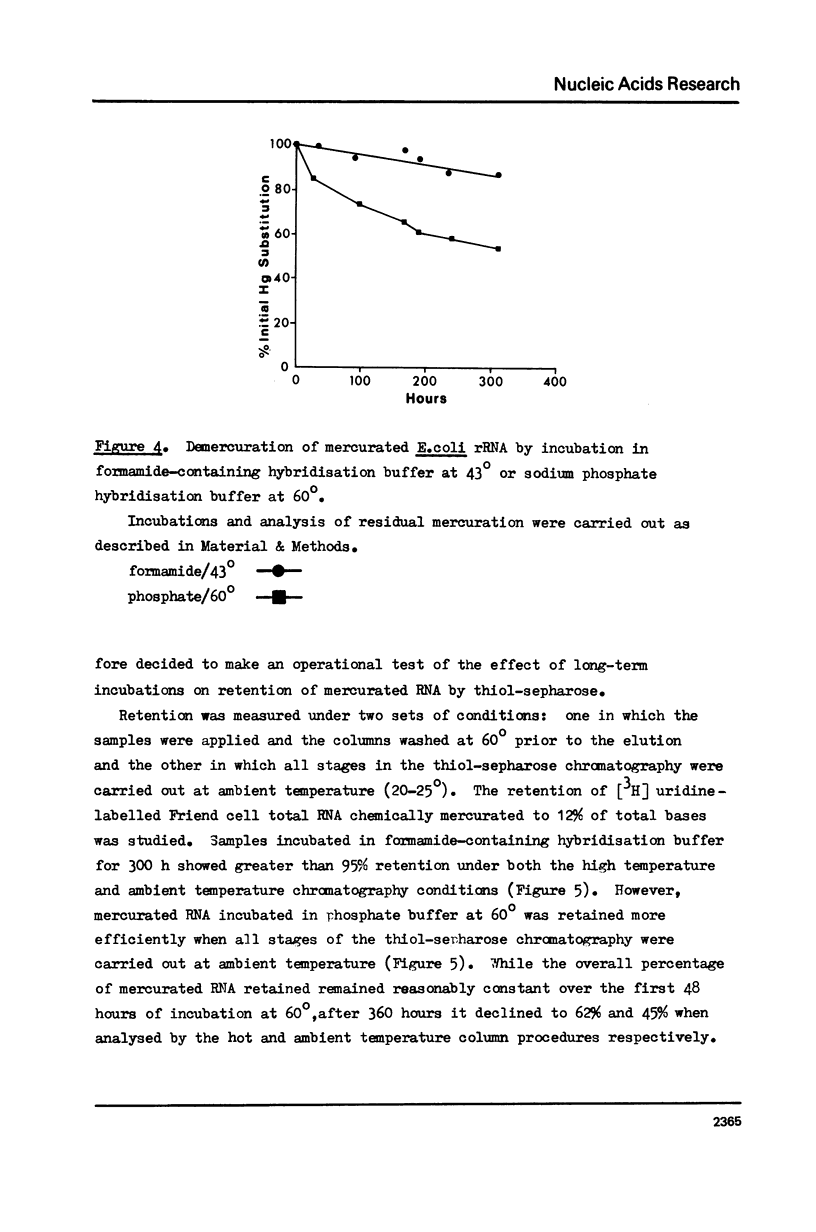
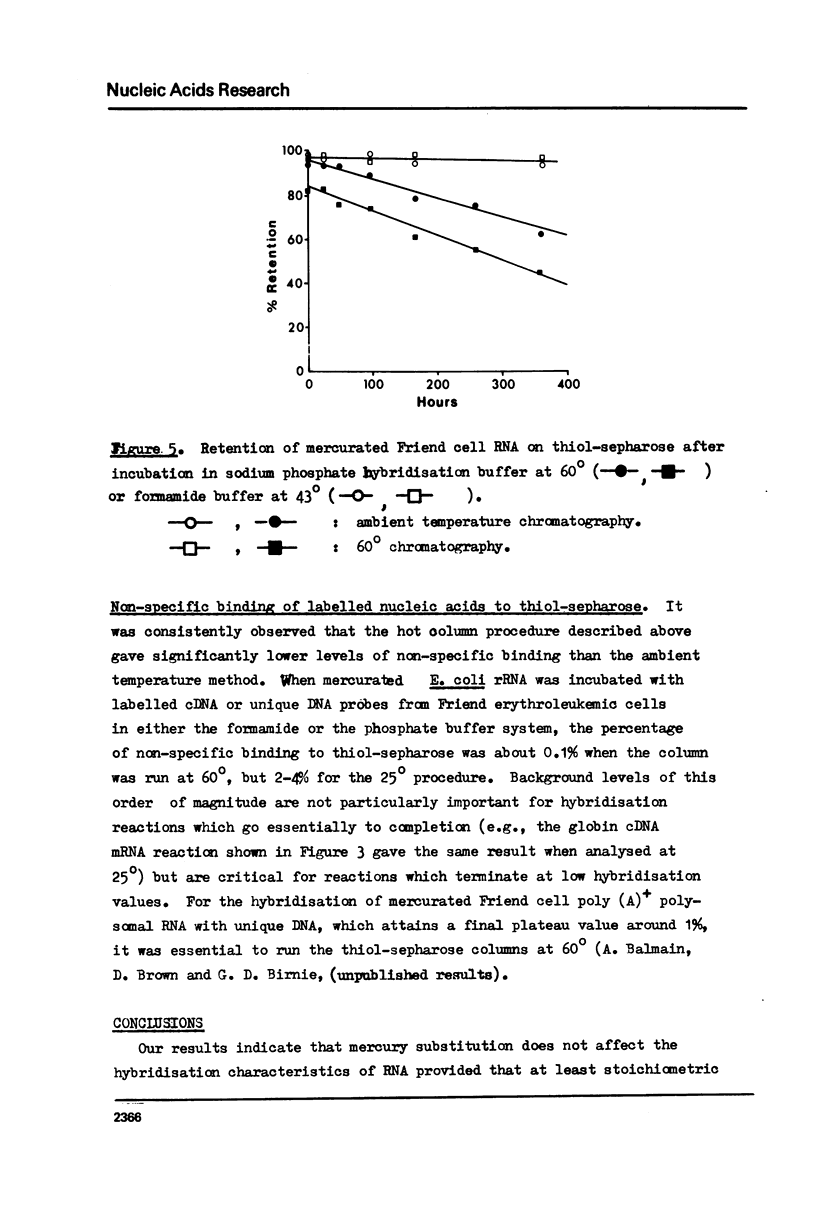
The effect of different levels of mercury substitution on the rate and extent of hybridisation of globin mRNA with a complementary DNA (cDNA) copy has been investigated. It was found that mercuration significantly reduces both the rate of hybridisation and the extent of the reaction, but that these effects are abolished when at least stoichiometric amounts of 2-mercaptoethanol are included in the hybridisation medium. As a preliminary to using this technique to isolate specific groups of sequences after long-term hybridisations, we have investigated both the rate of demercuration of RNA and its retention on thiol-sepharose columns after extended incubation under commonly employed hybridisation conditions at 43 degrees or 60 degrees. Retention was essentially quantitative even after incubation times of 300 hours at 43 degrees, but decreased significantly after 48 hours at 60 degrees. It is concluded that thiol-sepharose chromatography offers considerable advantages over hydroxyapatite chromatography for the recovery of hybridised sequences, particularly with regard to the lower levels of non-specific binding obtained and its ability to distinguish directly between DNA-DNA and DNA-Hg RNA hybrids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebee T. J., Butterworth P. H. The use of mercurated nucleoside triphosphate as a probe in transcription studies in vitro. Eur J Biochem. 1976 Jul 15;66(3):543–550. doi: 10.1111/j.1432-1033.1976.tb10580.x. [DOI] [PubMed] [Google Scholar]
- Birnie G. D., MacPhail E., Young B. D., Getz M. J., Paul J. The diversity of the messenger RNA population in growing Friend cells. Cell Differ. 1974 Nov;3(4):221–232. doi: 10.1016/0045-6039(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Gilmour R. S., Affara N. A., Conkie D., Paul J. Globin messenger RNA synthesis and processing during haemoglobin induction in Friend cells. II. Evidence for post-transcriptional control in clone 707. Cell Differ. 1974 Jun;3(1):23–30. doi: 10.1016/0045-6039(74)90037-2. [DOI] [PubMed] [Google Scholar]
- Kleiman L., Birnie G. D., Young B. D., Paul J. Comparison of the base-sequence complexities of polysomal and nuclear RNAs in growing Friend erythroleukemia cells. Biochemistry. 1977 Mar 22;16(6):1218–1223. doi: 10.1021/bi00625a029. [DOI] [PubMed] [Google Scholar]
- Longacre S. S., Mach B. Purification of specific DNA sequences by sulfhydryl-Sepharose chromatography of mercurated polynucleotides. J Biol Chem. 1978 Oct 25;253(20):7500–7507. [PubMed] [Google Scholar]
- Young B. D., Birnie G. D. Complexity and specificity of polysomal poly(A+) RNA in mouse tissues. Biochemistry. 1976 Jun 29;15(13):2823–2829. doi: 10.1021/bi00658a019. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Analysis of in vitro transcription of duck reticulocyte chromatin using mercury-substituted ribonucleoside triphosphates. Biochemistry. 1977 Nov 15;16(23):5135–5145. doi: 10.1021/bi00642a029. [DOI] [PubMed] [Google Scholar]



