Abstract
Few mechanistic ideas from the pre-molecular era of biology have had as enduring an impact as the morphogen concept. In the classical view, cells in developing embryos obtain positional information by measuring morphogen concentrations and comparing them with fixed concentration thresholds; as a result, graded morphogen distributions map into discrete spatial arrangements of gene expression. Recent studies on Hedgehog and other morphogens suggest that establishing patterns of gene expression may be less a function of absolute morphogen concentrations, than of the dynamics of signal transduction, gene expression, and gradient formation. The data appoint away from any universal model of morphogen interpretation and suggest that organisms use multiple mechanisms for reading out developmental signals in order to accomplish specific patterning goals.
Introduction
A fundamental problem in developmental biology is how initially identical cells reliably achieve specific spatio-temporal patterns of differentiation. For the last half century, the most influential model has been that of the morphogen gradient. Classically, morphogens are molecules that form concentrations gradients in space, and to which cells respond in distinct ways depending on the morphogen dose they encounter. In recent years, the study of morphogen gradients has been a rich topic for research, with most studies focusing either on how such gradients form, or how they are interpreted by cells (reviewed in [1–5]). Here we take up the latter question — how graded signals are translated into discrete patterns of gene expression —and review literature suggesting that morphogen gradient interpretation cannot be entirely separated from the dynamics of signaling and morphogen gradient evolution over time.
Concentration landscapes and patterns: a one-to-one relationship?
Among modern biologists, the most widely accepted notion of what a morphogen is comes from experimental studies, such as those of Stumpf [6] and Lawrence [7] in insects, as interpreted and popularized in the context of Wolpert’s positional information theory [8]. Molecular identification of morphogens came much later, beginning with the maternal transcription factor Bicoid (Bcd), which acts as a cytoplasmic morphogen within the syncytial Drosophila embryo [9–11], and later the secreted TGF-β family member Decapentaplegic (Dpp), which patterns the Drosophila larval wing imaginal disc [12–14].
The subsequent molecular identification of additional families of signaling molecules— including other TGF-βs, FGFs, EGF, Hedgehogs, Wnts, and retinoids —as morphogens has depended upon experimental demonstrations that such molecules do act at a distance from their site of production and elicit distinct cellular responses in a concentration-dependent manner. The fact that cellular responses in a tissue correlate with morphogen concentration does not, however, imply that each cell simply “reads” the concentration it experiences, nor does it tell us how different concentrations are distinguished. Indeed, given the shallowness of many morphogen gradients, it has been suggested that, using only the cell-autonomous readout of a static gradient, it might be difficult to create the reliable, sharply-demarcated domains of gene expression that are commonly observed during patterning [4].
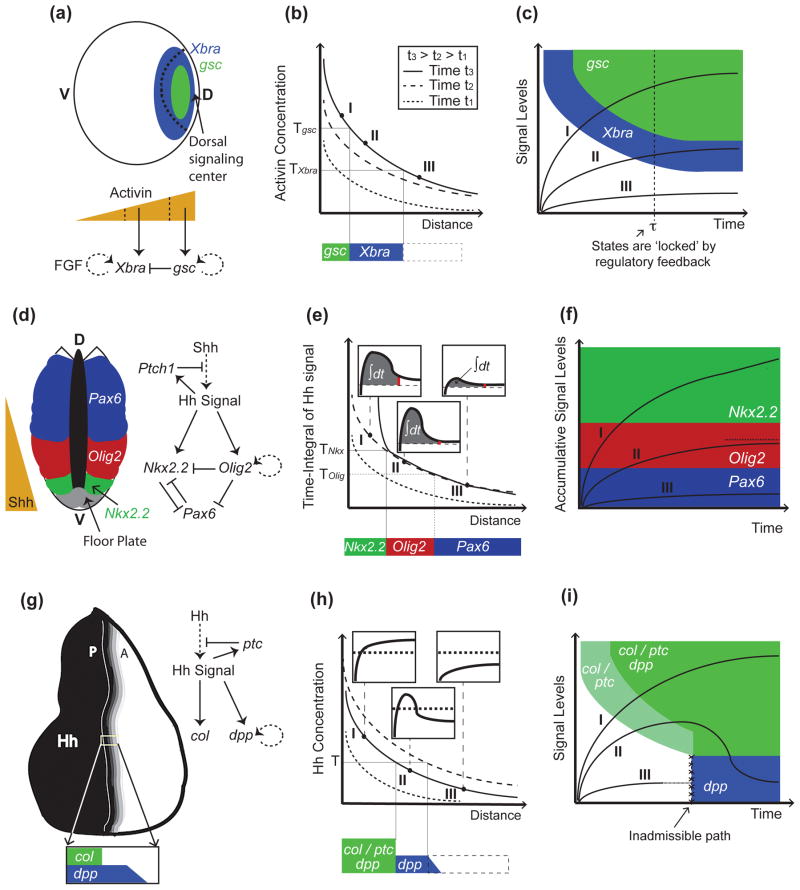
So far, the most compelling support for the view that cells simply read morphogen concentrations comes from studies of activin, a member of the TGF-β family that patterns the dorsal mesoderm in the Xenopus embryo (reviewed in [15]; Fig. 1a). Activin controls the differential expression of the genes goosecoid (gsc) and Xenopus brachyury (Xbra) in a dose-dependent manner [16–19] (Fig. 1a), with cells responding autonomously as a function of absolute receptor occupancy (about 100 activin-bound receptors are sufficient to activate Xbra, while approximately 300 are required for gsc; [20]), relatively independent of signal duration (Fig. 1b). In vivo, the maintenance and sharpening of gene expression boundaries seems to be resolved downstream of activin signaling, at the level of target gene-interactions —for example Gsc-mediated repression of Xbra [21], and a positive feedback loop between Xbra and FGF signaling that amplifies expression of Xbra in its own domain [22,23] (Fig. 1a). Such network interactions are sufficient to ‘lock’ the regulatory state of cells, so that fates can be maintained when signaling levels drop below required thresholds, or after morphogen exposure is lost [19,24] (Fig. 1c).
Figure 1.
Models of morphogen gradient interpretation. (a-c), Mesoderm specification in Xenopus depends on activin concentrations. Activin is secreted from the dorsal signaling center, and forms a dorsal (D) to ventral (V) gradient that activates Xbra and gsc at successively increasing concentration thresholds (Tgsc>TXbra; (a)). In (b), (as well as in (e), and (h)), three gradients at successive time points are shown indicating the dynamics displayed by the gradients (legend only displayed in (b), but also applies to (e) and (h)). In the case of activin, genes are activated sequentially; cells exposed to high activin concentrations express first Xbra and then gsc (trajectory I in (c)). Then, Xbra is turned off by Gsc (a), resulting in mutually-exclusive domains (a). Once cells have acquired a stable pattern (e.g., after time τ in the trajectories in (c)), they no longer require activin signaling and are no longer under the control of the morphogen thresholds (dotted line in (c)). Cell states are now irreversible and maintained by positive feedback loops downstream of activin signaling ((a); dotted lines in the regulatory networks denote that the interaction may be not direct). (d-g), In the developing spinal cord, Shh is secreted in the floor plate in the ventral-most portion of the neural tube and establishes the expression of Nkx2.2, Olig2, and Pax6, among other neural markers, in specific domains of the neural tube (d). Not shown in (d-f), for the sake of simplicity, is that low levels of Pax6 persist in the Olig2 domain. As in the case of activin, the neural progenitor markers Olig2 and Nkx2.2 are also established sequentially (trajectory I in (f)), but in this case, the order of activation depends on the integration of Hh signaling over time (e). This is accomplished by a feed-forward loop in which activation of Nkx2.2 requires both sustained Shh exposure and Olig2-mediated repression of the Pax6 inhibitor (d). In (e), the time-integral (area under signal vs. time curves; see insets) is higher closer to the Shh source, because desensitization of cells is slower when exposed to higher Shh concentrations. In this model, cell fates are determined by thresholds in accumulated signal levels (f). In (f), it is assumed that the time-integral of the signal is bounded, at least in cells marked by Olig2 (dotted line in (f)), although it is unclear how this is accomplished. (g-i) In the Drosophila wing disc, Hh is expressed in the posterior (P) compartment and establishes a short-range gradient into the anterior (A) compartment (g). col (and ptc) and dpp become expressed in different domains as a consequence to a refinement in the Hh gradient (h). This ‘shift’ results from increased Hh sequestration and destruction by Ptc as ptc becomes upregulated by Hh signaling (g). Thus, unlike cells exposed to sustained signaling (I), or not receiving Hh at all (III), some cells in the middle of the gradient (II) receive Hh signaling only transiently (insets in (h)). This model of signal interpretation (represented by the state-space in (i)) only requires a single switching threshold, T. Unlike in the previous examples (panels (c) and (f)), col/ptc are turned on before dpp (i), despite the fact that cells are exposed to increasing concentrations. In (i), the white and blue domains are not connected (i.e., trajectory I is assumed to reach the white-blue boundary in infinite time).
Is activin’s function as a simple dose-dependent inducer of genes typical of all morphogens? Or is positional information sometimes encoded in other ways? It is inevitable that morphogen gradients change over time, initiating, spreading, and eventually being shut down. The cells that respond to morphogens often grow, move and differentiate, so that their relationships even with static morphogen gradients would be expected to change over time. Finally, the signals generated within cells by morphogens may themselves be dynamic, due, for example, to feedback or feed-forward effects. If cells normally experience morphogen gradients in such a spatiotemporally varying a manner, the question arises as to whether the information encoded in such dynamics is used in any meaningful way, and if so, to what end? Below we discuss some insights into these questions that have come out of the study of several morphogen systems, most notably the patterning of the vertebrate neural tube and the Drosophila wing disc by morphogens of the Hedgehog family.
Signal integration: the case for duration-encoding
Even cells that encounter similar morphogen levels at the time we observe them may have had very different histories of morphogen exposure. One measure of that history—the duration of exposure — can potentially be recorded by cells in the form of a signaling intermediate or gene product whose level accumulates during the period of morphogen exposure. That level, reflecting the time-integral of morphogen signaling, would normally be sensitive to both the amount and duration of morphogen exposure, but given equal levels of morphogen signaling, would directly reflect the duration.
There are several patterning systems in which it has been proposed that positional information is encoded in the duration of morphogen signaling. These include the patterning of rhombomeres in the zebrafish hindbrain by retinoic acid [25,26]; the specification of digits in the vertebrate limb in response to Sonic Hedgehog (Shh) signaling [27]; the specification of olfactory and lens placodal cells in the chick embryo in response to BMPs [28]; and the dorsoventral patterning of the chick neural tube by Shh [29].
The last of these examples is so far the best understood mechanistically, thanks to several recent studies [29–33]. In the developing vertebrate spinal cord, Shh is produced at the ventral midline of the neural tube and forms a ventral-to-dorsal concentration gradient responsible for assigning positional identities to neural progenitor cells (Fig. 1d). Although levels of Shh correlate with the establishment of neural progenitor domains, experimental evidence indicates that duration of exposure to Shh plays a critical role in establishing cell fates. For example, exposure to moderate Shh levels for about 6 hours causes cells to express the motor neuron marker, olig2, but longer exposures eventually lead them to downregulate olig2 and upregulate the V3 interneuron marker, Nkx2.2, that is normally restricted to the ventral-most region of the neural tube, where Shh levels are highest [29] (Fig. 1d). The gene regulatory mechanisms underlying this behavior have been partly worked out: Cells receiving and accumulating Shh signaling can immediately activate Olig2 expression, but Nkx2.2 activation cannot proceed because it is repressed by Pax6; once Olig2 accumulates sufficiently it represses Pax6 and Nkx2.2 expression proceeds. Thus, signal accumulation is recorded by a feed-forward gene-regulatory loop (Fig. 1d). Consistent with this model, patterns of Olig2 and Nkx2.2 are normally established sequentially, and on the time scale over which Pax6 becomes restricted to dorsal regions (Fig. 1e,f; [31]).
Interestingly, if one examines a direct readout of Shh signaling (a reporter for GLI transcription factor activity) within moderately short times after exposure of cultured neural plate explants, rather small differences are observed between the effects of high vs. low doses of the morphogen. Only later do large differences in signaling emerge, due to temporal adaptation [29]. This adaptation reflects two peculiarities of Hh signaling: The first is that the normal function of the Hh receptor Patched (Ptc) is to maintain signaling in an inactive (OFF) state, while binding of Hh to Ptc relieves this repression and activates signaling (in effect, signaling is a function of the total number of unoccupied receptors). The second feature is that ptc is nearly always a transcriptional target of Hh signaling, being strongly upregulated by members of the Hh family in both Drosophila and vertebrates (Fig. 1d,g). Together these phenomena help explain the slow ‘desensitization’ of Shh-treated cells in the neural tube [29].
One potential disadvantage of encoding positional information in the ‘time-integral’ of a morphogen signal is that the integral of a sustained signal grows continuously over time, making the positional value that a cell reads potentially very sensitive to the exact time it chooses to read it (Fig. 1e). For duration-encoded signals to provide robust patterning information, either morphogen signaling needs to be turned off, cells need to move away from the morphogen, or cell-intrinsic mechanisms need to “lock in” cell fates despite the presence of a continuously changing morphogen signal (Fig. 1f). One advantage offered by temporal adaptation of morphogen signals is that, by slowing down the rate of signal integration, patterning can be made less sensitive to the timing of these events.
The importance of morphogen gradient dynamics
A general consequence of temporal adaptation is that it creates a dynamic output even in response to a constant input. Because adaptation to Shh in the neural tube appears to vary inversely with Shh concentration [29] —a curious finding, which may relate to indirect effects of Shh target genes on Shh sensitivity [33] —sustained high-Shh exposure results in much longer-duration signaling than sustained low-Shh exposure [29,31]. Thus, cells in the neural tube might usefully employ duration-encoding as a way to translate small changes in static Shh levels into large changes in signaling.
However, there are good reasons to suspect that Shh levels are not static. Because Ptc appears to be the primary receptor for Hh clearance, in both vertebrates and invertebrates, the very same transcriptional feedback that causes cell-autonomous adaptation of Hh signals should also be expected to cause changes to the shapes of Hh gradients themselves. Although such gradient dynamics have been modeled in the vertebrate neural tube [34], they have been most extensively studied, and subjected to experimental verification, in the Drosophila wing disc [35,36].
In both systems, modeling led to the expectation that Hh gradients should undergo biphasic dynamics, first spreading out broadly (when Ptc levels are relatively low), and then retracting back toward the morphogen source (as Ptc is upregulated in response to signaling), ultimately producing a much shorter gradient with a much steeper slope. Experimental evidence in the wing disc supports the view that this refinement, or “spatial overshoot”, indeed takes place [36]. As a result of this overshoot, there is a domain of cells that is only transiently exposed to Hh signaling, sandwiched between cells that receive the signal continuously, and cells that never receive the signal [36] (Fig. 1h). In order for such a situation to produce nested patterns of gene expression, it was proposed, and experimentally verified, that some Hh targets, such as dpp, can be maintained by the memory of earlier Hh signaling (i.e. wing disc cells integrate and retain an earlier signal), while others (such as collier (col) and ptc itself) simply read out the current level of Hh signaling [36] (Fig. 1i). As in the chick neural tube, the end result is that cell fates that require continuous morphogen exposure lie closest to the morphogen source, and those that do not, lie further away. A major difference, however, is that—because of the remodeling of the morphogen gradient over time —cells nearest the Hh source in the wing disc display fates associated with responding to the current (not time-integrated) morphogen signal, whereas in the neural tube the cells closest to the Shh source display fates associated with long-time integration of the signal (Fig. 1f,i).
A second difference between the wing disc and neural tube models is that, in the wing disc, because of the gradient dynamics, there is no need for cells to encode any information about Hh concentration other than whether it is “high” or “low”, i.e. there need only be a single, binary signaling threshold. In contrast, the temporal adaptation model for the neural tube depends upon different signaling strengths producing different durations of signaling.
Looking ahead: anticipating a greater role for dynamics in patterning
The ability of the wing disc Hh gradient to specify multiple cell fates at distinct locations despite an absence of multiple response thresholds illustrates a general point: positional information can be encoded by morphogen gradients in multiple ways. Not only is there a reason to believe, as discussed above, that the time integrals of morphogen signals play a critical role in patterning, there is good reason to believe that cells can also measure the time derivatives of morphogen signals.
For example, it has been proposed that the relative rate of rise of Dpp concentration, rather than Dpp concentration itself, drives cell growth in the Drosophila wing disc [37]. In addition, recent studies indicate that signaling dynamics downstream of both Wnts and EGF (both can serve as morphogens) makes cellular responses to these molecules reflect fold changes in their concentrations (or the concentrations of their signaling intermediates), and not absolute levels [38,39]. Within the context of a dynamically-changing morphogen gradient, this implies that cells would tend to read out the relative time derivative of the gradient. For Wnts and EGFs, the mechanistic basis for such behavior is an adaptive circuit known as the incoherent feed-forward loop [40], in which a stimulus first activates and then, through a parallel pathway, later represses a response. In general, any adaptive circuit has the potential to make responses sensitive to the time-derivatives of stimuli. For example, in the Drosophila wing disc Hh gradient (Fig. 1g), in which the dynamics of gradient expansion and contraction are driven by an adaptive circuit (mediated by Hh-dependent ptc upregulation), the spatial extent of dpp expression will be a function of the rate at which Hh spreads relative to the rate at which ptc is upregulated.
Just as we are increasingly learning that cells can respond to the time-derivatives and time-integrals of morphogen signaling, evidence is also emerging that they may measure space-derivatives and space-integrals as well. For example, in the Dpp gradient of the Drosophila wing disc, there is good evidence that some cellular responses are driven by measurements that cells make of the difference between their own Dpp signaling and that of their neighbors, effectively a readout of spatial slope [41,42]. A measurement of the spatial integral of a morphogen gradient can be obtained whenever a morphogen induces (or represses) expression of something that diffuses rapidly throughout a morphogen field, such that its level reflects the total (integrated) amount of morphogen. This sort of mechanism has recently been suggested to underlie the scaling of morphogen gradients with tissue size, in cases in which the rapidly diffusing molecule feeds back upon the length scale of the morphogen gradient itself [43,44].
In coming years, it seems likely that many additional examples will emerge of dynamic morphogen gradients, in which cells measure and respond not just to morphogen levels, but to temporal and spatial derivatives and integrals. The challenge for the future will be not only to identify the mechanisms underlying such processes, but to understand how they may contribute to making patterning more precise, robust, or flexible.
Acknowledgments
The authors acknowledge support from NIH grants GM067247 and GM076516.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413(6858):797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 2.Zhu AJ, Scott MP. Incredible journey: How do developmental signals travel through tissue? Genes Dev. 2004;18(24):2985–2997. doi: 10.1101/gad.1233104. [DOI] [PubMed] [Google Scholar]
- 3.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133(3):385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 4.Lander AD. Morpheus unbound: Reimagining the morphogen gradient. Cell. 2007;128(2):245–256. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Rogers KW, Schier AF. Morphogen gradients: From generation to interpretation. Annu Rev Cell Dev Biol. 2011 doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 6.Stumpf HF. Mechanism by which cells estimate their location within the body. Nature. 1966;212(5060):430–431. doi: 10.1038/212430a0. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence PA. Development and determination of hairs and bristles in the milkweed bug, Oncopeltus fasciatus (Lygaeidae, Hemiptera) J Cell Sci. 1966;1(4):475–498. doi: 10.1242/jcs.1.4.475. [DOI] [PubMed] [Google Scholar]
- 8.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 9.Driever W, Nusslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54(1):95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 10.Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54(1):83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 11.Struhl G, Struhl K, Macdonald PM. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell. 1989;57(7):1259–1273. doi: 10.1016/0092-8674(89)90062-7. [DOI] [PubMed] [Google Scholar]
- 12.Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121(8):2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 13.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a dpp morphogen gradient. Cell. 1996;85(3):357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 14.Lecuit T, Brook WJ, Ng M, Calleja M, Sun H, Cohen SM. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381(6581):387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- 15.Smith JC. Forming and interpreting gradients in the early Xenopus embryo. Cold Spring Harb Perspect Biol. 2009;1(1):a002477. doi: 10.1101/cshperspect.a002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347(6291):391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- 17.Gurdon JB, Dyson S, St Johnston D. Cells' perception of position in a concentration gradient. Cell. 1998;95(2):159–162. doi: 10.1016/s0092-8674(00)81747-x. [DOI] [PubMed] [Google Scholar]
- 18.Gurdon JB, Harger P, Mitchell A, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371(6497):487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- 19.Gurdon JB, Mitchell A, Mahony D. Direct and continuous assessment by cells of their position in a morphogen gradient. Nature. 1995;376(6540):520–521. doi: 10.1038/376520a0. [DOI] [PubMed] [Google Scholar]
- 20.Dyson S, Gurdon JB. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell. 1998;93(4):557–568. doi: 10.1016/s0092-8674(00)81185-x. [DOI] [PubMed] [Google Scholar]
- 21.Artinger M, Blitz I, Inoue K, Tran U, Cho KW. Interaction of goosecoid and brachyury in Xenopus mesoderm patterning. Mech Dev. 1997;65(1–2):187–196. doi: 10.1016/s0925-4773(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 22.Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J. 1994;13(19):4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte-Merker S, Smith JC. Mesoderm formation in response to brachyury requires FGF signalling. Curr Biol. 1995;5(1):62–67. doi: 10.1016/s0960-9822(95)00017-0. [DOI] [PubMed] [Google Scholar]
- 24.Jullien J, Gurdon J. Morphogen gradient interpretation by a regulated trafficking step during ligand-receptor transduction. Genes Dev. 2005;19(22):2682–2694. doi: 10.1101/gad.341605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavalas A. ArRAnging the hindbrain. Trends Neurosci. 2002;25(2):61–64. doi: 10.1016/s0166-2236(02)02067-2. [DOI] [PubMed] [Google Scholar]
- 26.Maden M. Retinoid signalling in the development of the central nervous system. Nat Rev Neurosci. 2002;3(11):843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- 27.Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308(2):343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjodal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell. 2007;13(1):141–149. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450(7170):717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 30.Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135(6):1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- *31.Dessaud E, Ribes V, Balaskas N, Yang LL, Pierani A, Kicheva A, Novitch BG, Briscoe J, Sasai N. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010;8(6):e1000382. doi: 10.1371/journal.pbio.1000382. This work suggests that patterning by Shh signaling in the vertebrate neural tube results from integrating the morphogen signal over time. The time-integral is calculated using a feed forward loop in the gene regulatory network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribes V, Balaskas N, Sasai N, Cruz C, Dessaud E, Cayuso J, Tozer S, Yang LL, Novitch B, Marti E, Briscoe J. Distinct sonic hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010;24(11):1186–1200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Lek M, Dias JM, Marklund U, Uhde CW, Kurdija S, Lei Q, Sussel L, Rubenstein JL, Matise MP, Arnold HH, Jessell TM, et al. A homeodomain feedback circuit underlies step-function interpretation of a Shh morphogen gradient during ventral neural patterning. Development. 2010;137(23):4051–4060. doi: 10.1242/dev.054288. This study shows that Shh targets provide for delayed feed-forward amplification, or attenuation, of Shh signals, resulting in a sharp, non-graded pattern of Shh activity in the neural tube. The authors also propose that a temporal switch, but not different Shh concentrations, drives differentiation between neural and non-neuronal (floorplate) cells. [DOI] [PubMed] [Google Scholar]
- 34.Saha K, Schaffer DV. Signal dynamics in sonic hedgehog tissue patterning. Development. 2006;133(5):889–900. doi: 10.1242/dev.02254. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing hedgehog. Cell. 1996;87(3):553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- *36.Nahmad M, Stathopoulos A. Dynamic interpretation of hedgehog signaling in the Drosophila wing disc. PLoS Biol. 2009;7(9):e1000202. doi: 10.1371/journal.pbio.1000202. This study introduces a model of Hh signal interpretation in which a signal-dependent refinement of the Hh gradient controls the duration of Hh exposure in receiving cells. The authors show that this spatial shift of the gradient is required to establish different patterns of expression in the Drosophila wing disc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Julicher F, Gonzalez-Gaitan M. Dynamics of Dpp signaling and proliferation control. Science. 2011;331(6021):1154–1159. doi: 10.1126/science.1200037. [DOI] [PubMed] [Google Scholar]
- *38.Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell. 2009;36(5):872–884. doi: 10.1016/j.molcel.2009.11.017. Using Xenopus embryos as a model system, this study provides evidence that Wnt signaling is interpreted as a function of relative changes in signal levels, rather than absolute levels. This interpretation mechanism depends on the gene regulatory properties of an incoherent feed forward loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Cohen-Saidon C, Cohen AA, Sigal A, Liron Y, Alon U. Dynamics and variability of ERK2 response to EGF in individual living cells. Mol Cell. 2009;36(5):885–893. doi: 10.1016/j.molcel.2009.11.025. Using cultured cells, the authors provide evidence that EGF signaling, like Wnt signaling in ref 38, is interpreted as a function of relative changes in signal levels, not absolute levels. [DOI] [PubMed] [Google Scholar]
- 40.Goentoro L, Shoval O, Kirschner MW, Alon U. The incoherent feedforward loop can provide fold-change detection in gene regulation. Mol Cell. 2009;36(5):894–899. doi: 10.1016/j.molcel.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the fat signaling pathway. Dev Cell. 2008;15(2):309–321. doi: 10.1016/j.devcel.2008.06.003. This study elucidates the mechanism by which cells in a morphogen gradient may respond to differences between their own morphogen signaling and that of their neighbors, thus calculating a measure of local gradient slope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogulja D, Irvine KD. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123(3):449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Zvi D, Pyrowolakis G, Barkai N, Shilo BZ. Expansion-repression mechanism for scaling the Dpp activation gradient in Drosophila wing imaginal discs. Curr Biol. 2011;21(16):1391–1396. doi: 10.1016/j.cub.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Zvi D, Barkai N. Scaling of morphogen gradients by an expansion-repression integral feedback control. Proc Natl Acad Sci U S A. 2010;107(15):6924–6929. doi: 10.1073/pnas.0912734107. [DOI] [PMC free article] [PubMed] [Google Scholar]