Abstract
Complex mixtures of polycyclic aromatic hydrocarbons (PAHs) are difficult to resolve because of the high degree of overlap in compound vapor pressures, boiling points and mass spectral fragmentation patterns. The objective of this research was to improve the separation of complex PAH mixtures (including 97 different parent, alkyl-, nitro-, oxy-, thio-, chloro-, bromo-, and high molecular weight PAHs) using GC×GC/ToF-MS by maximizing the orthogonality of different GC column combinations and improving the separation of PAHs from the sample matrix interferences, including unresolved complex mixtures (UCM). Four different combinations of non-polar, polar, liquid crystal and nano-stationary phase columns were tested. Each column combination was optimized and evaluated for orthogonality using a method based on conditional entropy that considers the quantitative peak distribution in the entire two-dimensional space. Finally, an atmospheric particulate matter with diameter < 2.5 µm (PM2.5) sample from Beijing, China, a soil sample from St. Maries Creosote Superfund Site, and a sediment sample from the Portland Harbor Superfund Site were analyzed for complex mixtures of PAHs. The highest chromatographic resolution, lowest synentropy, highest orthogonality and lowest interference from UCM were achieved using a 10 m × 0.15 mm × 0.10 µm LC-50 liquid crystal column in the first dimension and a 1.2 m × 0.10 mm × 0.10 µm NSP-35 nano-stationary phase column in the second dimension. In addition, the use of this column combination in GC×GC/ToF-MS resulted in significantly shorter analysis times (176 min) for complex PAH mixtures compared to one-dimensional GC/MS (257 min), as well as potentially reduced sample preparation time.
Introduction
Complex mixtures of polycyclic aromatic hydrocarbons (PAHs) are produced from the incomplete combustion of fossil fuels, biomass1,2, forest fires3, volcanic eruptions4, and hydrothermal processes5 and consist of a wide variety of different PAH compounds, including parent PAHs (PPAHs), alkyl-PAHs (MPAHs), nitro-PAHs (NPAHs), oxy-PAHs (OPAHs), thio-PAHs (SPAHs), high molecular weight PAHs (MW ≥ 300; HMW PAHs), bromo-PAHs (BrPAHs) and chloro-PAHs (ClPAHs). Some of these individual PAH, as well as complex mixtures of PAHs, are of concern because of their potential persistence, bioaccumulation, and toxicity, including carcinogenicity and mutagenicity6,7,8,9,10. In addition, the same sources that produce PAHs also produce other poorly characterized mixtures of organic compounds, commonly referred to as an unresolved complex mixture (UCM)11, that acts as matrix interferrances in the analysis. Therefore, the separation of complex mixtures of individual PAH compounds in environmental samples requires high chromatographic resolution.12
Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry13 (GC×GC/ToF-MS) is well suited for this task. GC×GC/ToF-MS has been used to identify more than 3,000 compounds in crude oil11 and more than 370 compounds in house dust14, as well as to characterize UCM in sediment15,16, biota17, and oil spills18. Multidimensional separation techniques, such as GC×GC/ToF-MS, generate a theoretical available peak capacity equivalent to the arithmetic product of the individual peak capacities of each dimension.19 However, this is rarely obtained because of the existing correlation between the two dimensions. The final information obtained in these analyses is the sum of the mean information of each individual dimension minus the cross information.20 A high degree of correlation between the different dimensions can reduce a multidimensional separation to a distribution of peaks along a diagonal.21 To minimize this correlation and approach theoretical peak capacity, orthogonality (the degree of independence between the dimensions of analysis) must be maximized and synentropy (the amount of information contributed equally from both dimensions22) must be minimized, so that the cross information across dimensions is close to zero.21
To date, a common column combination used for environmental sample analysis in GC×GC/ToF-MS includes a non-polar GC-column in the first dimension followed by a more polar column in the second dimension11, 14, 16,23,24,25. In a previous study, this column combination resulted in good chromatographic separation and good space occupation for GC×GC/ToF-MS analysis of a coal liquefaction middle distillate sample containing semi-volatile organic compounds (SOCs) with different physicochemical properties, such as paraffins (alkanes), naphthenes (cyclic alkenes), aromatics and phenols.23 However, this column combination may not be orthogonal enough to resolve complex mixtures of SOCs within a single class of compounds that share similar physicochemical properties and respond to the separation mechanisms in similar ways, such as complex mixtures of PAHs.
The objective of this research was to improve the separation of complex PAH mixtures using GC×GC/ToF-MS by maximizing the orthogonality of different column combinations and improving the separation of compounds from the sample matrix interferences. The orthogonality of four different GC column combinations was tested using a standard solution containing 97 PAHs, including PPAHs, MPAHs, NPAHs, OPAHs, SPAHs, ClPAHs, BrPAHs and HMW PAHs. GC-columns with different stationary phases and column dimensions were tested, including traditionally used non-polar (Rtx-5) and polar (Rxi-17) columns, liquid crystal (LC-50) and newly developed nano-stationary phase (NSP-35) columns. Each column combination was optimized and evaluated for orthogonality. The different column combinations were used to separate complex mixtures of PAHs in environmental samples, including an atmospheric particulate matter sample with diameter < 2.5 µm (PM2.5) collected from Beijing, China, a soil sample from St. Maries Creosote Superfund Site, and a sediment sample from the Portland Harbor Superfund Site. This paper serves as a model for optimizing the two-dimensional separation of other complex SOC mixtures from environmental matrices with significant matrix interferrances, including UCM.
1. Experimental section
Materials and Reagents
Standards of 19 PPAHs, 10 MPAHs, 19 NPAHs, 5 OPAHs, 2 SPAHs and 15 HMW PAHs were purchased from AccuStandard (New Haven, CT, USA). Standards of 17 ClPAHs and 10 BrPAHs were synthesized by Dr. Takeshi Ohura at the University of Shizouka in Shizouka, Japan26,27. The list of the 97 PAHs measured can be found in the Table S1. Atmospheric particulate matter with diameter < 2.5 µm (PM2.5) was collected at Peking University in Beijing, China during the 2008 Beijing Olympic Games and its sample preparation, that included pressurized liquid extraction with dichloromethane and solid phase extraction, has been previously described28. A soil sample was collected from St. Maries Creosote Superfund Site in St. Maries, Idaho and a sediment sample was collected from Portland Harbor Superfund Site in Portland, Oregon. Samples were prepared following the procedures described elsewhere.29,30
GC×GC/ToF-MS Analysis
The standards and environmental samples were analyzed using a GC×GC/ToF-MS Pegasus 4D instrument (Leco, St Joseph, MI, USA) with four different GC column combinations. The instrument consisted of an Agilent 6890 gas chromatograph (Palo Alto, CA, USA) with a secondary oven, a split/splitless injector, and a non-moving quad-jet dual stage modulator. The two GC columns were connected using an Agilent CPM union (part no. 188–5361) for 0.1–0.25 mm I.D. columns. The GC oven temperature, modulation period, and gas flow rate were optimized for each column combination, with other parameters, including injection method and temperature, transfer line temperature and source temperature, fixed. The optimization was performed using the standard solution of 97 PAHs in ethyl acetate with a concentration of 1 ng/uL.
Column combination “A1” consisted of a 35 m × 0.25 mm × 0.10 µm Rtx-5ms column with 5 m guard column (Restek, Bellefonte, PA, USA) in the first dimension and a 1.2 m × 0.10 mm × 0.10 µm Rxi-17 column (Restek, Bellefonte, PA, USA) in the second dimension. A previously optimized GC temperature program for this column combination was used31, with an increase in the final hold time from 9 to 19 min in order to ensure complete elution of HMW PAHs. Three modulation periods were tested (3, 5 and 7 s), with three flow rates (0.8, 1, 1.2 mL/min). The hot pulse in the modulation period was, on average, 30% lower than the cold pulse, ensuring maximum peak height ratios in the second dimension.32 Better chromatographic resolution was obtained with shorter modulation periods (Figure S1). However, the signal-to-noise ratios were reduced because of the larger number of sub-peaks generated by the modulating process. In addition, some sub-peaks did not elute within a single modulation period and produced “wraparounds” in the next chromatographic run. After optimization, 1 µL of the 1 ng/µL standard solution in ethyl acetate was injected and analyzed using the conditions shown in Table 1. The total run time for column combination “A1” was 64 min.
Table 1.
GC×GC/ToF-MS optimized operation parameters for the analysis of PAHs with four different column combinations
| Combination "A1" | Combination "A2" | Combination "B" | Combination "C" | |
|---|---|---|---|---|
| 1D column | Rtx-5ms (35 m × 0.25 mm × 0.25 µm) |
Rxi-5ms (10 m × 0.10 mm × 0.10 µm) |
LC-50 (10 m × 0.15 mm × 0.10 µm) |
LC-50 (10 m × 0.15 mm × 0.10 µm) |
| Max Temperature | 320 °C | 350 °C | 270 °C | 270 °C |
| 2D column | Rxi-17 (1.2 m × 0.10 mm × 0.10 µm) |
Rxi-17 (1.2 m × 0.10 mm × 0.10 µm) |
Rxi-17 (1.2 m × 0.10 mm × 0.10 µm) |
NSP-35 (1.2 m × 0.10 mm × 0.10 µm) |
| Max Temperature | 360 °C | 360 °C | 360 °C | 360 °C |
| Injection Volume | 1 µL | 1 µL | 1 µL | 1 µL |
| Inlet Temperature | 300 °C | 300 °C | 250 °C | 250 °C |
| Carrier Gas | He | He | He | He |
| Carrier Gas Flow | 1.20 mL/min (constant) | 0.80 mL/min (constant) | 0.80 mL/min (constant) | 0.80 mL/min (constant) |
| 1D Oven Program | 60 °C (1 min), 6 °C/min to 300 °C (3 min), 20 °C/min to 320 °C (19 min) |
60 °C (3 min), 16 °C/min to 320 °C (6 min) |
90 °C (2 min), 20 °C/min to 170 °C, 2 °C/min to 270 °C (120 min) |
90 °C (2 min), 20 °C/min to 170 °C, 2 °C/min to 270 °C (120 min) |
| 2D Oven Program | 80 °C (1 min), 6 °C/min to 320 °C (3 min), 20 °C/min to 340 °C (19 min) |
80 °C (3 min), 16 °C/min to 340 °C (6 min) |
120 °C (2 min), 20 °C/min to 200 °C, 2.5 °C/min to 325 °C (120 min) |
120 °C (2 min), 20 °C/min to 200 °C, 2.5 °C/min to 325 °C (120 min) |
| Modulator Temp. Offset | 35 °C | 35 °C | 45 °C | 45 °C |
| Modulation Time | 5 s | 7 s | 5 s | 5 s |
| Hot Pulse Time | 1 s | 1.5 s | 1 s | 1 s |
| Cold Time | 1.5 s | 2 s | 1.5 s | 1.5 s |
| Transfer Line Temp. | 285 °C | 285 °C | 285 °C | 285 °C |
| Ion Source Temperature | 250 °C | 250 °C | 250 °C | 250 °C |
| Scan Speed | 151.5 spectra/second | 151.5 spectra/second | 151.5 spectra/second | 151.5 spectra/second |
| Total run time | 64 min | 25.25 min | 176 min | 176 min |
Column combination “A2” consisted of a 10 m × 0.10 mm × 0.10 µm Rxi-5ms column (Restek, Bellefonte, PA, USA) in the first dimension, followed by a 1.2 m × 0.10 mm × 0.10 µm Rxi-17 column (Restek, Bellefonte, PA, USA) in the second dimension. This column combination was included in the experiments in order to compare the orthogonality of different column combinations with similar column lengths and inner diameters, as well as gas flow rate. Rxi-5ms is a low polarity, high inertness stationary phase similar to Rtx-5ms used in column combination “A1”33. A single GC temperature ramp was used due to the relatively short column length in the first dimension and four temperature ramps were tested (6, 12, 16, 20 °C/min), with three modulation periods (3, 5, 7 s). After optimization, 1 µL of the 1 ng/µL standard solution in ethyl acetate was injected and analyzed using the conditions shown in Table 1. The total run time for column combination “A2” was 25.25 min.
Column combination “B” consisted of a 10 m × 0.15 mm × 0.10 µm LC-50 liquid crystal column (J&K Scientific, Edwardsville, Nova Scotia, Canada) in the first dimension, followed by a 1.2 m × 0.10 mm × 0.10 µm Rxi-17 (Restek, Bellefonte, PA, USA) in the second dimension. The liquid crystal column was used in the first dimension, rather than the second dimension, to avoid excessive wraparound of later eluting PAHs due to the relatively low temperature limit of this GC column (270 °C) and strong retention of these PAHs25. Combination “C” consisted of a 10 m × 0.15 mm × 0.10 µm LC-50 liquid crystal column (J&K Scientific, Edwardsville, Nova Scotia, Canada) in the first dimension, followed by a 1.2 m × 0.10 mm × 0.10 µm NSP-35 nano-stationary phase column (J&K Scientific, Edwardsville, Nova Scotia, Canada) in the second dimension. Five primary oven temperature programs were tested to optimize combinations “B” and “C”: 1) 90 °C for 2 min, 20 °C/min to 170 °C, 2 °C/min to 270 °C and held for 28 min; 2) 90 °C for 2 min, 2 °C/min to 170 °C, held for 8 min, 10 °C/min to 270 °C and held for 20 min; 3) 90 °C for 1 min, 20 °C/min to 270 °C and held for 50 min; 4) 90 °C for 5 min, 20 °C/min to 170 °C, held for 25 min, 20 °C/min to 270 °C and held for 20 min; and 5) 90 °C for 5 min, 9 °C/min to 270 °C, held for 35 min. Three modulation periods (3, 5, 7 s) were tested. Using the optimized conditions shown in Table 1 for column combinations “B” and “C”, 1 µL of the 1 ng/µL standard solution in ethyl acetate was injected via a splitless injector at 250 °C using He as the carrier gas and a constant column flow rate of 0.80 mL/min. The total run time for column combinations “B and “C” was 176 min.
For all four column combinations, the temperature of the transfer line was held at 285 °C. An acquisition rate of 151.5 spectra/second was used with the ion source at 250 °C. The data processing for all four GC column combinations was performed using Leco ChromaTOF version 4.33 (Leco, St Joseph, MI, USA). The optimized conditions and PAH retention times for the four column combinations tested are given in Table 1 and Table S1, respectively.
2. Results and Discussion
Chromatographic Separation of Complex PAH Mixtures
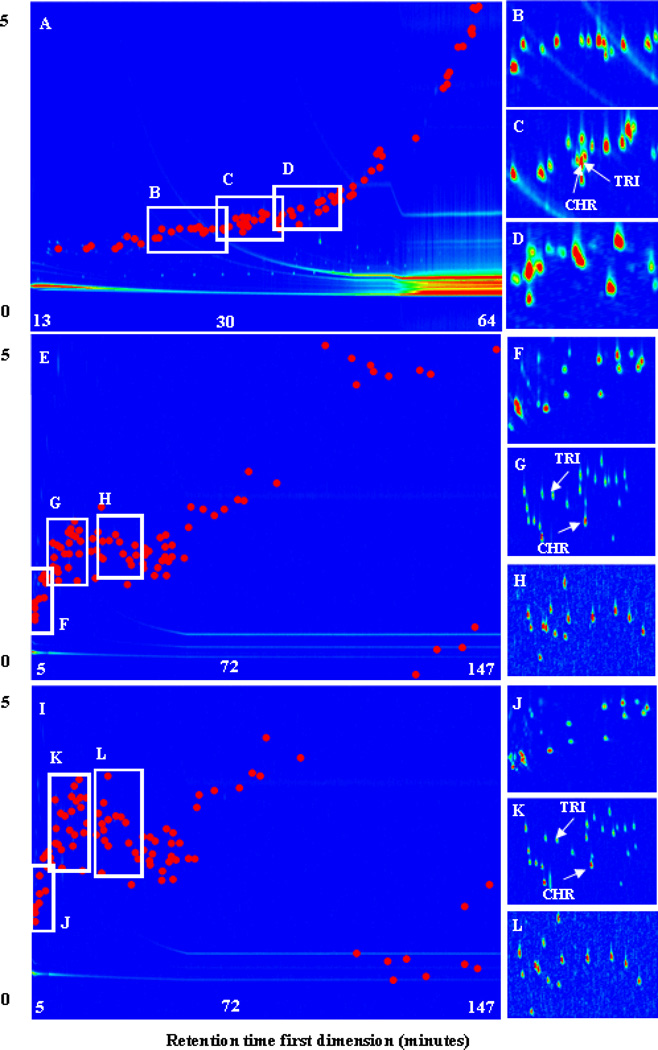
Versions of column combination “A1” (Rtx-5ms×Rxi-17) are commonly used in GC×GC/ToF-MS.11, 14, 16, 24, 34 The total ion chromatogram (TIC) of the standard solution containing 97 PAHs analyzed with combination “A1” is shown in Figure 1A (Figure S2 for black and white version). The PAH elution pattern showed a strong correlation between the retention times in the two dimensions and the majority of the two-dimensional space in the chromatogram was not occupied. Ideally, the chromatographic peaks (points in Figure 1A) would fill the entire two-dimensional space in a more random pattern. Of the 97 PAHs detected, 37 co-eluted completely or partially, with 3 of these 37 being the HMW PAHs with m/z 302. Table S2 shows the retention times in each dimension for PAH isomers showing complete co-elution in column combination “A1”, including chrysene (CHR) and triphenylene (TRI) and benzo[b]fluoranthene (BbF) and benzo[k]fluoranthene (BkF). Some of these co-elutions are shown in Figures 1B, 1C and 1D. These PAH isomer pairs were not resolved because there is significant overlap in their mass spectra. The column bleed for column combination “A1” eluted in approximately 1 s in the second dimension and throughout the first dimension. The column bleed intensity was highest during the final minutes of the run.
Figure 1.
Chromatograms showing the separation of 97 PAHs by GC×GC/ToF-MS using 3 different column combinations: (A) TIC for Combination “A” showing co-elution of PAHs at (1D ‘minutes’, 2D ‘seconds’): (B) 23.57, 1.19 to 30.24, 1.79; (C) 28.41, 1.30 to 34.24, 1.80; (D) 33.90, 1.37 to 37.23, 1.97. (E) TIC for Combination “B”, with improved separation at (F) 5, 0.75 to 10.83, 1.65; (G) 10.58, 1.19 to 23.91, 2.69; (H) 22.99, 1.17 to 36.32, 2.67. (I) TIC for combination “C” with a further improvement in separation at: (J) 5, 1.00 to 11.67, 2.50; (K) 11.40, 1.68 to 24.75, 3.68; (L) 25.40, 1.89 to 38.73, 3.39. Chrysene (CHR) and Triphenylene (TRI) are labeled for each column combination
Column combination “A2” (Rxi-5ms×Rxi-17) was tested in order to compare to column combinations “B” and “C” for columns with similar lengths, inner diameters, and film thicknesses, but different stationary phases. The TIC of the standard solution containing 97 PAHs analyzed with combination “A2” is shown in Figure S3. Eighty-two of the 97 PAHs were detected with column combination “A2”. While the PAH elution order in column combination “A2” was similar to column combination “A1”, 49 PAHs co-eluted completely or partially with column combination “A2”, compared to 37 in column combination “A1”, due to the relatively short column length in the first dimension. Some examples of complete co-elutions are shown in Table S2. The column bleed for column combination “A2” eluted in approximately 1 s in the second dimension and throughout the first dimension.
Column combination “B” (LC-50×DB-17) was tested in an effort to reduce the correlation between the first and second dimensions by using stationary phases with different separation mechanisms. The liquid crystalline stationary phase column (LC-50) used in the first dimension was more selective for the separation of isomers35, such chrysene (CHR) and triphenylene (TRI), because the separation mechanism depends on molecular shape2,36,37,38. The TIC of the standard solution of 97 PAHs analyzed with column combination “B” is shown in Figure 1E. Compared to column combination “A1” (Figure 1A), the PAHs occupied a wider range of the two-dimensional chromatographic space with column combination “B”. Figures 1F, 1G, and 1H show better separation of individual PAHs and, of the 91 individual PAHs detected, only 13 PAHs partially co-eluted. In addition, the resolution of PAH isomers with complete co-elution with column combination “A1” and “A2” was improved in column combination “B” (Table S2). The column bleed for column combination “B” eluted in approximately 0.5 s in the second dimension and throughout the first dimension.
Column combination “C” (LC50×NSP-35) was tested in an effort to further improve the chromatographic separation of complex PAH mixtures over column combination “B” by using a nano-stationary phase capillary column (NSP-35) in the second dimension. Nano-stationary phases are different from conventional stationary phases because they are lower molecular weight, smaller molecular size, and have shorter methylphenylsiloxane chain length39. Like conventional stationary phases, nano-stationary phases range from non-polar to polar. The specific orientation of the molecules making up the nano-stationary phase provide higher selectivity and shorter column length compared to conventional stationary phases39. Compared to column combinations “A1” (Figure 1A), “A2” (Figure 1E), and “B” (Figure 1E), the PAHs occupied a wider range of the two-dimensional chromatographic space with column combination “C” (Figure 1I). Figures 1J, 1K, and 1L show that there are no complete or partial co-elutions of the 89 PAHs detected. The resolution of some PAH isomers was improved in column combination “C” as compared to column combinations “A1”, “A2”, and “B” (Table S2). The column bleed for column combination “C” eluted in approximately 0.5 seconds in the second dimension and throughout the first dimension.
Full 2D color TIC for the four column combinations can be found in Figures S4, S5, S6 and S7. The total run time for column combinations “B” and “C” was 176 min because of the temperature limitations of the LC-50 column and the low vapor pressures (high boiling points) of the HMW PAHs. If the HMW PAHs were not included in the method, the analysis times for combinations “B” and “C” would be substantially reduced to less than 100 min. The corresponding analysis in one-dimensional GC/MS would require four different instrument runs, and result in lower chromatographic resolution, for a total run time of 257.5 min: NPAHs and OPAHs method (45.7 min)28, PPAH and MPAHs method (46 min)28, HMW PAH method (115.9 min)28, and Cl and Br-PAH method (49.9 min)40.
Orthogonality of Column Combinations
The correlation between the retention times in the first and second dimensions was used as an initial evaluation of the degree of association between the different column combinations tested. A high degree of correlation would suggest similar separation mechanisms between the first and second dimensions21. Figure S8 shows the results of these correlations for the four different column combinations. The HMW PAHs were not included in these correlations because they wrapped around the chromatogram in combinations “B” (Figure 1E) and “C” (Figure 1I) due to the relatively low temperature limit of the liquid crystal column used. The wrap-around can be solved by using higher oven temperatures, faster gas flow rates or increasing the modulation time during the period of HMW PAH elution. Wrap around becomes a problem only when the compounds co-elute with column bleed or other compounds present in the mixture.
Although the correlations were statistically significant (p<0.001) for all of the different column combinations tested, the correlation was reduced by 52.25% and the r2 coefficient was reduced by 77.31% using combination “C” (LC-50×NSP-35) as compared to combination “A1” (Rtx-5ms×Rxi-17) (Figure S4). The same parameters were reduced by 53.71% (correlation coefficient) and 78.69% (r2 coefficient) using combination “C” (LC-50×NSP-35) as compared to combination “A2” (Rxi-5ms×Rxi-17). Figures S8E, S8F, S8G, and S8H show the residuals from the linear regressions shown in Figures S8A, S8B, S8C and S8D, respectively. The residuals for column combination “A1” (Rtx-5×Rxi-17) (Figure S8E) and column combination “A2” (Rxi-5ms×Rxi-17) (Figure S8F) confirm that there was a strong correlation between the retention times in both dimensions. The residuals for column combinations “B” and “C” are shown in Figures S8G and S8H, respectively, and are more normally distributed as compared to column combinations “A1” and “A2”. This behavior showed that, although analyte vapor pressures play a major role in all GC separations (including GC×GC and independent of the stationary phase used),41 when using column combination “C”, this linear correlation was minimized.
An approach based on conditional entropy42 was used to evaluate the orthogonality of the different column combinations tested. The entropy for a discrete single random variable and a pair of discrete random variables is defined by equations 1 and 2:
| (1) |
| (2) |
where p(x) and p(x,y) are the probability of a peak appearing at a particular retention time in one and two-dimensional separations, respectively.42 However, because two-dimensional chromatography deals with dimensions that are in some way correlated, a term describing this conditionality is necessary. Equation 3 describes the conditional entropy for a variable Y, given X, which can be obtained from the chain rule for conditional entropy (equation 4)42:
| (3) |
| (4) |
The orthogonality (Φ) of a two-dimensional system is defined as the percentage of the ratio of the conditional entropy of Y, given X, and the conditional entropy of Y (equation 5)42:
| (5) |
where H(Y|X) is the entropy of variable Y (retention time in the second dimension) conditioned on the variable X (retention time in the first dimension), H(Y) is the entropy of variable Y (retention time in the second dimension) and Φ% is the percent orthogonality of the two-dimensional system.
In order to calculate this, the retention time of the individual PAHs must be normalized to the minimum and maximum retention time in each dimension according to equation 6:
| (6) |
where (tr)min is the retention time of the earliest eluting PAH in the run, (tr)max the retention time of the latest eluting PAH in the run, (tr)i the PAH of interest and (tr)norm the normalized retention time for the PAH of interest. The two-dimensional chromatograms were then divided into an 8 × 8 matrix according to the optimized number of bins for a histogram containing 97 data points42. All of the PAHs in each chromatogram were placed in one of the 64 boxes, according to their retention times. Figure S9 shows how the different PAH were distributed in the normalized two-dimensional space for each column combination. The conditional entropy H(Y|X) and orthogonality (Φ) of the four different column combinations were calculated using equations 1–5 and are given in Figure S9. The greatest orthogonality (70.85%) was obtained using column combination “C” (LC-50×NSP-35). Column combination “C” used the greatest amount of two-dimensional space available for the separation of PAHs and was approximately two times the space used by column combinations “A1” and “A2”.
Analysis of Environmental Samples
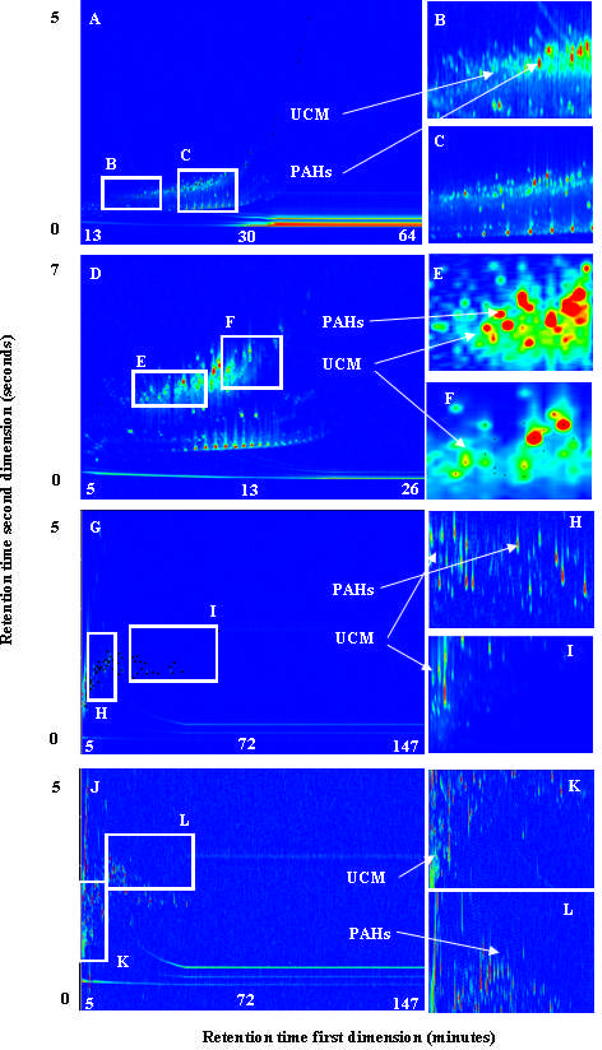
A PM2.5 sample was collected from Beijing, China during the 2008 Olympic Games28 and the extract analyzed for complex PAH mixtures in order to compare the different column combinations using an environmental matrix containing UCM. For reference, the one-dimensional chromatogram obtained using a GC/MS equipped with a 30 m × 0.25 mm × 0.25 µm DB-5ms is shown in Figure S10. Figures 2A and 2D (Figure S11 for black and white version) show the TIC obtained with column combinations “A1” and “A2”, respectively. These figures show that the UCM in the PM2.5 extract was distributed throughout most of the first dimension. A total of 51 of the 97 PAHs analyzed for were identified in the Beijing PM2.5 using column combination “A1” and combination “A2”.
Figure 2.
TIC for Beijing atmospheric PM2.5 extract analyzed using the four different column combinations: (A) Combination “A1” (D) Combination “A2”, (G) Combination “B”, (J) Combination “C”. The inner boxes show the UCM distributed in different places in the four chromatograms
When the Beijing PM2.5 extract was analyzed using column combination “B” and “C” (Figures 2G and 2J and Figure S11 for black and white version), the UCM eluted within the first 10 min and was distributed more throughout the second dimension than the first dimension compared to column combinations “A1” and “A2”. Although the UCM co-eluted with some of the early eluting PAHs, such as acenaphthene, acenaphthylene, 1-nitronaphthalene, 1,3- and 2,6-dimethylnaphthalenes; the majority of the chromatogram (retention times > 15 min) had no UCM present and the majority of PAHs did not co-elute with the UCM. A total of 53 PAHs were detected in a 176 min run using column combinations “B” and “C”.
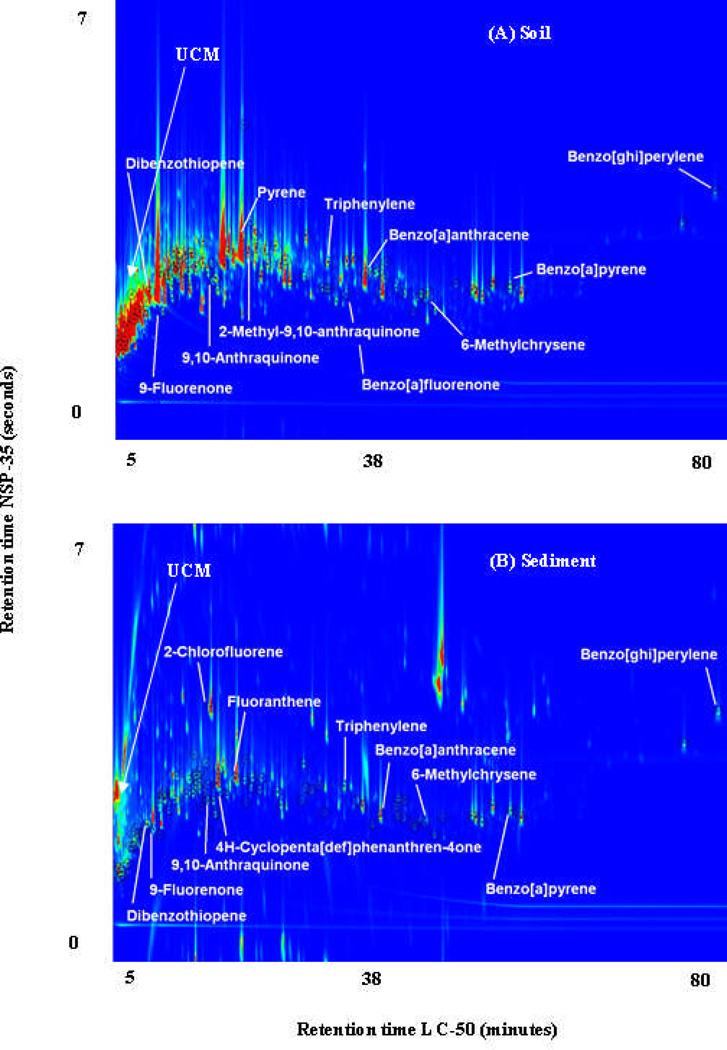
In addition, column combination “C” was used to analyze a soil sample extract from St. Maries Creosote Superfund Site and a sediment sample extract from the Portland Harbor Superfund Site for complex PAH mixtures. Figure 3 (Figure S12 for black and white version) shows the TIC for both. As with the Beijing PM2.5, the UCM from these environmental matrices eluted quickly in the first dimension and was more distributed in the second dimension. The majority of the PAHs detected eluted after the UCM. A total of 93 PAHs were identified in the St. Maries Creosote soil sample extract and 91 PAHs were identified in the Portland Harbor sediment sample extract, including PPAH, MPAH, OPAH, and ClPAH.
Figure 3.
TIC of environmental samples analyzed using column combination “C”. (A) Soil sample extract from St. Maries Creosote Superfund Site, and (B) Sediment sample extract from Portland Harbor Superfund Site. Some of the PAHs identified have been labeled.
Column combination “C” resulted in greater orthogonality for the separation of complex PAH mixtures, both for standard solutions containing 97 different PAHs with similar physicochemical properties and for environmental matrices containing UCM and other matrix interferrances. In addition, the use of column combination “C” in GC×GC/ToF-MS results in significantly shorter analysis times for complex PAH mixtures compared to one-dimensional GC/MS, as well as potentially reduced sample preparation. Future work will include the development of a method for quantification of complex PAH mixtures using column combination “C” and GC×GC/ToF-MS.
Supplementary Material
Acknowledgements
Funding for this research was provided by the U.S. National Science Foundation (ATM-0841165). This publication was made possible in part by National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), grants P30 ES00210 and P42 ES016465 and the National Children’s Study Formative Research Project (HHSN267200700021C, National Institute of Child Health and Human Development). The authors thank the analytical chemistry core of OSU’s Superfund Research Program for supplying standards and Prof. Takeshi Ohura at the University of Shizouka in Shizouka, Japan for supplying ClPAH and BrPAH standards. Its contents are solely responsibility of the authors and do not necessarily represent the official view of NIEHS, NIH, National Institute of Child Health and Human Development. We dedicate this manuscript to the memory of Dr. Rebecca Dickhut of Virginia Institute of Marine Sciences.
Footnotes
Associated Content
Supporting Information: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lima ALC, Farrington JW, Reddy CM. Combustion-Derived Polycyclic Aromatic Hydrocarbons in the Environment—A Review. Environ. Forensics. 2005;6(2):109–131. [Google Scholar]
- 2.Hutzinger O. The Handbook of Environmental Chemistry. Springer; New York: 1998. [Google Scholar]
- 3.Okuda T, Kumata H, Zakaria MP, Naraoka H, Ishiwatari R, Takada H. Source identification of Malaysian atmospheric polycyclic aromatic hydrocarbons nearby forest fires using molecular and isotopic compositions. Atmospheric Environment. 2002;36(4):611–618. [Google Scholar]
- 4.Zolotov MY, Shock EL. A thermodynamic assessment of the potential synthesis of condensed hydrocarbons during cooling and dilution of volcanic gases. J. Geophys. Res. 2000;105:539–559. doi: 10.1029/1999jb900369. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka T, Mizota C, Murae T, Hashimoto J. A currently forming petroleum associated with hydrothermal mineralization in a submarine caldera, Kagoshima Bay, Japan. Geochem. J. 1999;33:355–367. [Google Scholar]
- 6.Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. A review of atmospheric Polycyclic Aromatic Hidrocarbons: sources fate and behavior. Water Air Soil Pollut. 1991;60:279–300. [Google Scholar]
- 7.Miet K, Budzinski H, Villenave E. Heterogeneous Reactions of Oh Radicals with Particulate-Pyrene and 1-Nitropyrene of Atmospheric Interest. Polycyclic Aromat. Compd. 2009;29(5):267–281. [Google Scholar]
- 8.Lee J, Lane DA. Formation of oxidized products from the reaction of gaseous phenanthrene with the OH radical in a reaction chamber. Atmos. Environ. 2010;44(20):2469–2477. [Google Scholar]
- 9.Durant JL, Busby WF, Lafleur AL, Penman BW, Crespi CL. Human Cell Mutagenicity of Oxygenated, Nitrated and Unsubstituted Polycyclic Aromatic Hydrocarbons Associated with Urban Aerosols. Mutat. Res. 1996;371:123–157. doi: 10.1016/s0165-1218(96)90103-2. [DOI] [PubMed] [Google Scholar]
- 10.Dipple A. Polycyclic Hydrocarbons and Carcinogenesis. Vol. 283. American Chemical Society; 1985. Polycyclic Aromatic Hydrocarbon Carcinogenesis; pp. 1–17. [Google Scholar]
- 11.Melbye AG, Brakstad OG, Hokstad JN, Gregersen IK, Hansen BH, Booth AM, Rowland SJ, Tollefsen KE. Chemical and toxicological characterization of an unresolved complex mixture-rich biodegraded crude oil. Environ. Toxicol. Chem. 2009;28(9):1815–1824. doi: 10.1897/08-545.1. [DOI] [PubMed] [Google Scholar]
- 12.Poster DL, Schantz MM, Sander LC, Wise SA. Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: a critical review of gas chromatographic (GC) methods. Anal. Bioanal. Chem. 2006;386(4):859–881. doi: 10.1007/s00216-006-0771-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Phillips JB. Comprehensive Two-Dimensional Gas Chromatography using an On-Column Thermal Modulator Interface. J. Chromatogr. Sci. 1991;29(6):227–231. [Google Scholar]
- 14.Hilton DC, Jones RS, Sjödin A. A method for rapid, non-targeted screening for environmental contaminants in household dust. J. Chromatogr., A. 2010;1217(44):6851–6856. doi: 10.1016/j.chroma.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Frysinger GS, Gaines RB, Xu L, Reddy CM. Resolving the Unresolved Complex Mixture in Petroleum-Contaminated Sediments. Environ. Sci. Technol. 2003;37(8):1653–1662. doi: 10.1021/es020742n. [DOI] [PubMed] [Google Scholar]
- 16.Reddy CM, Eglinton TI, Hounshell A, White HK, Xu L, Gaines RB, Frysinger GS. The West Falmouth Oil Spill after Thirty Years: The Persistence of Petroleum Hydrocarbons in Marsh Sediments. Environ. Sci. Technol. 2002;36(22):4754–4760. doi: 10.1021/es020656n. [DOI] [PubMed] [Google Scholar]
- 17.Booth AM, Sutton PA, Lewis CA, Lewis AC, Scarlett A, Chau W, Widdows J, Rowland SJ. Unresolved Complex Mixtures of Aromatic Hydrocarbons: Thousands of Overlooked Persistent, Bioaccumulative, and Toxic Contaminants in Mussels. Environ. Sci. Technol. 2006;41(2):457–464. [PubMed] [Google Scholar]
- 18.Nelson RK, Kile BM, Plata D, Sylva SP, Li X, Reddy CM, Gaines RB, Frysinger GS, Reichenbach SE. Tracking the Weathering of an Oil Spill with Comprehensive Two-Dimensional Gas Chromatography. Environ. Forensics. 2006;7(1):33–44. [Google Scholar]
- 19.Cortez HJ. Multidimensional Chromatography: Techniques and Applications. New York: 1990. [Google Scholar]
- 20.Erni F, Frei RW. Two-dimensional column liquid chromatographic technique for resolution of complex mixtures. J. Chromatogr., A. 1978;149(0):561–569. [Google Scholar]
- 21.Venkatramani CJ, Xu J, Phillips JB. Separation Orthogonality in Temperature-Programmed Comprehensive Two-Dimensional Gas Chromatography. Anal. Chem. 1996;68(9):1486–1492. doi: 10.1021/ac951048b. [DOI] [PubMed] [Google Scholar]
- 22.Slonecker PJ, Li X, Ridgway TH, Dorsey JG. Informational Orthogonality of Two-Dimensional Chromatographic Separations. Analytical Chemistry. 1996;68(4):682–689. doi: 10.1021/ac950852v. [DOI] [PubMed] [Google Scholar]
- 23.Omais B, Courtiade M, Charon N, Ponthus J, Thiébaut D. Considerations on Orthogonality Duality in Comprehensive Two-Dimensional Gas Chromatography. Anal. Chem. 2011;83(19):7550–7554. doi: 10.1021/ac201103e. [DOI] [PubMed] [Google Scholar]
- 24.Skoczyńska E, Korytár P, Boer Jd. Maximizing Chromatographic Information from Environmental Extracts by GCxGC-ToF-MS. Environ. Sci. Technol. 2008;42(17):6611–6618. doi: 10.1021/es703229t. [DOI] [PubMed] [Google Scholar]
- 25.Ieda T, Ochiai N, Miyawaki T, Ohura T, Horii Y. Environmental analysis of chlorinated and brominated polycyclic aromatic hydrocarbons by comprehensive two-dimensional gas chromatography coupled to high-resolution time-of-flight mass spectrometry. Journal of Chromatography A. 2011;1218(21):3224–3232. doi: 10.1016/j.chroma.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Ohura T, Kitazawa A, Amagai T, Makino M. Ocurrence, Profiles and Photoestabilities of Chlorinated Polycyclic Aromatic Hydrocarbons Associated with Particulates in Urban Air. Environ. Sci. Technol. 2005;39:85–91. [PubMed] [Google Scholar]
- 27.Kitazawa A, Amagai T, Ohura T. Temproal Trends and Relationships of Particulate Chlorinated Polycyclic Aromatic Hydrocarbons and Their Parent Compounds in Urban Air. Environ. Sci. Technol. 2006;40:4592–4598. doi: 10.1021/es0602703. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu T-W, Dashwood RH, Zhang W, Wang X, Simonich SLM. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011;45(16):6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genualdi SA, Killin RK, Woods J, Wilson G, Schmedding D, Simonich SLM. Trans-Pacific and Regional Atmospheric Transport of Polycyclic Aromatic Hydrocarbons and Pesticides in Biomass Burning Emissions to Western North America. Environmental Science & Technology. 2009;43(4):1061–1066. doi: 10.1021/es802163c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primbs T, Genualdi S, Simonich SM. Solvent Selection for Pressurized Lquid Extraction of Polymeric Solvents in Air Sampling. Environ. Toxicol. Chem. 2008;27:1267–1272. doi: 10.1897/07-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usenko S, Hageman KJ, Schmedding DW, Wilson GR, Simonich SL. Trace Analysis of Semivolatile Organic Compounds in Large Volume Samples of Snow, Lake Water, and Groundwater. Environmental Science & Technology. 2005;39(16):6006–6015. doi: 10.1021/es0506511. [DOI] [PubMed] [Google Scholar]
- 32.Hoh E, Mastovska K, Lehotay SJ. Optimization of separation and detection conditions for comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry analysis of polychlorinated dibenzo-p-dioxins and dibenzofurans. Journal of Chromatography A. 2007;1145(1–2):210–221. doi: 10.1016/j.chroma.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 33.Restek-Corporation Rxi. 3-in-1 Technology. http://www.restek.com/Landing-Pages/Content/gen_B003
- 34.Hoh E, Lehotay S, Mastovska K, Ngo HL, Pangallo KC, Reddy CM. Capabilities of Direct Sample Introduction- Comprehensive Two-Dimensional Gas Chromatography - Time-of-Flight Mass Spectrometry to Analyze Organic Chemicals of Interest in Fish Oils. Environ. Sci. Technol. 2009;43:3240–3247. doi: 10.1021/es803486x. [DOI] [PubMed] [Google Scholar]
- 35.Kelker H, Winterscheidt H. The behaviour of crystalline liquids as solvents in gas-liquid-chromatography. Fresenius' J. Anal. Chem. 1966;220(1):1–8. [Google Scholar]
- 36.Janini GM, Johnston K, Zielinski WL. Use of a nematic liquid crystal for gas-liquid chromatographic separation of polyaromatic hydrocarbons. Anal. Chem. 1975;47(4):670–674. doi: 10.1021/ac60354a016. [DOI] [PubMed] [Google Scholar]
- 37.Janini GM, Muschik GM, Schroer JA, Zielinsk WL. Gas-liquid chromatographic evaluation and gas-chromatography/mass spectrometric application of new high-temperature liquid crystal stationary phases for polycyclic aromatic hydrocarbon separations. Anal. Chem. 1976;48(13):1879–1883. doi: 10.1021/ac50007a017. [DOI] [PubMed] [Google Scholar]
- 38.Wise S, Sander L, Chang H, Markides K, Lee M. Shape selectivity in liquid and gas chromatography: Polymeric octadecylsilane (C18) and liquid crystalline stationary phases. Chromatographia. 1988;25(6):473–479. [Google Scholar]
- 39.Britten AJ, Naikwadi KP. New nano stationary phase GC capillary columns for fast analysis of PAH by GC and GC/MS. Int. J. Environ. Anal. Chem. 2009;89(15):1113–1123. [Google Scholar]
- 40.Gonzales L. Trace analysis of halogenated polycyclic aromatic hydrocarbons from an electronic waste recycling area in Guangzhou, China. Oregon State University; Corvallis, OR: 2011. [Google Scholar]
- 41.Mostafa A, Górecki T, Tranchida PQ, Mondello L. Comprehensive Chromatography in Combination with Mass Spectrometry. John Wiley & Sons, Inc.; 2011. History, Evolution, and Optimization Aspects of Comprehensive Two-Dimensional Gas Chromatography; pp. 93–144. [Google Scholar]
- 42.Pourhaghighi MR, Karzand M, Girault HH. Orthogonality of Two-Dimensional Separations Based on Conditional Entropy. Anal. Chem. 2011;83(20):7676–7681. doi: 10.1021/ac2017772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.