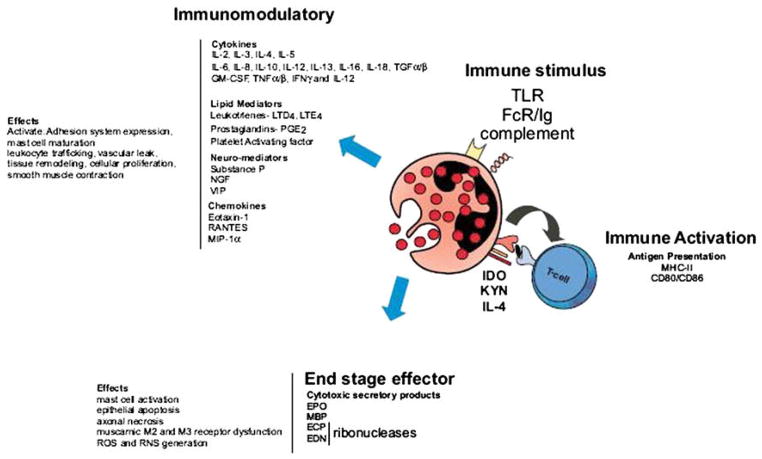
Eosinophil accumulation in the gastrointestinal (GI) tract is a common feature of numerous GI disorders including classic immunoglobulin (Ig)E-mediated food allergy,6 eosinophilic gastroenteritis (EGE),7 allergic colitis,8 eosinophilic esophagitis (EE),9,10 inflammatory bowel disease (IBD),11 and gastroesophageal reflux disease.12,13 The function of eosinophils in GI inflammation remains an enigma. Eosinophils can potentially initiate GI antigen-specific immune responses by acting as antigen-presenting cells (Fig. 1). Eosinophils express major histocompatibility complex class II molecules and relevant costimulatory molecules (CD40, CD28, CD86, B7.1, and B7.2) and secrete an array of cytokines (interleukin [IL]-2, IL-4, IL-12, and IL-10) capable of promoting lymphocyte proliferation, activation, and helper T cell type 1 or type 2 polarization. In addition, eosinophils can have proinflammatory effects including the up-regulation of GI adhesion systems and the modulation of leukocyte trafficking, tissue remodeling, and cellular activation states by releasing cytokines (IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-18, and transforming growth factor [TGF]-β), chemokines (RANTES [regulated on activation normal T-cell expressed and secreted] and eotaxin), and lipid mediators (platelet activating factor and leukotriene C4) (see Fig. 1). Finally, eosinophils can serve as major effector cells, inducing tissue damage and dysfunction by releasing toxic granule proteins (major basic protein [MBP], eosinophilic cationic protein [ECP], eosinophil peroxidase [EPO], and eosinophil-derived neurotoxin [EDN]) and lipid mediators.14 Consistent with multifunctional capabilities, there is accumulating evidence in various eosinophilic GI disorders (EGIDs) that eosinophils may have a dual function (ie, end-stage effector and immunoregulatory).1,14–18
Fig. 1.
Eosinophil function in GI inflammation. Eosinophils are bilobed granulocytes with eo-sinophilic staining of secondary granules. The secondary granules contain four primary cationic proteins: eosinophil peroxidase (EPO), major basic protein (MBP), eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN). All four proteins are cytotoxic molecules; in addition, ECP and EDN are ribonucleases. Eosinophils can be activated by immune stimulus by way of toll-like receptor (TLR), immunoglobulin, and complement. In addition to releasing their preformed cationic proteins, eosinophils can also release a variety of cyto-kines, chemokines, lipid mediators, and neuromodulators. Eosinophils activate T cells by serving as antigen-presenting cells. Eosinophils can also regulate T-cell polarization through synthesis of indoleamine 2,3-dioxygenase (IDO), an enzyme involved in oxidative metabolism of tryptophan, catalyzing the conversion of tryptophan to kynurenines (KYN), a regulator of T helper cell type 1 and 2 balance. Eosinophils generate an array of cytokines, chemokines, lipid mediators, and neuromodulators that regulate leukocyte trafficking, activation, and maturation; adhesion system expression; collagen synthesis; cellular proliferation; and mucus cell hypersecretion. Eosinophils can also act as an end-stage effector cell, secreting cationic proteins that can regulate mast cell function and generate reactive oxygen species (ROS), reactive nitrogen species (RNS), epithelial cell injury, and muscarnic receptor (M2 and M3) dysfunction. FcR, Fc receptor; GM-CSF, granulocyte-macrophage colony–stimulating factor; IFN, interferon; IL, interleukin; LT, leukotriene; MHC, major histo-compatibility complex; MIP, macrophage inflammatory protein; PG, prostaglandin; RANTES, regulated on activation normal T-cell expressed and secreted; TGF, transforming growth factor; TNF, tumor necrosis factor; VIP, vasoactive intestinal peptide. (Adapted from Rothen-berg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006;24:149; with permission.)
EOSINOPHIL-DERIVED CYTOKINES
Eosinophils can synthesize and secrete at least 35 important inflammatory and regulatory cytokines, chemokines, and growth factors. Those eosinophil-derived cytokines that have been quantified generally appear to be generated in relatively small amounts, suggesting an autocrine, paracrine, or juxtacrine role in regulating the function of the microenvironment. In some circumstances, however, eosinophils are the chief producers of cytokines such as TGF-β, which is linked with tissue remodeling in a variety of eosinophil-associated diseases such as asthma.19 Eosinophils store their cytokines intracellularly as preformed mediators in crystalloid granules and small secretory vesicles.20 This allows the immediate release of these mediators on eosinophil activation, instead of several hours or days required for other inflammatory cells. For example, the release of the chemokine RANTES was shown to occur within 60 to 120 minutes of eosinophil stimulation by interferon (IFN)-γ. This release of chemokines was related to rapid mobilization (within 10 minutes) of RANTES in small secretory vesicles that translocated this chemokine to the cell membrane before its release.20–22
Eosinophils contain a number of other granule-stored enzymes whose exact role in eosinophil function has not been defined.23 They include acid phosphatase (large amounts of which have been isolated from eosinophils), collagenase, arylsulphatase B, histaminase, phospholipase D, catalase, nonspecific esterases, and vitamin B12-binding protein. Eosinophils are also a source of matrix metalloproteinases (MMP), which have an important role in cell transmigration and inflammation,24–28 although lesser amounts are produced from eosinophils than from monocytes, macrophages, and neutrophils. The intracellular location of matrix MMP-9 has been localized to peri-nuclear regions and not the crystalloid granules.25
EOSINOPHIL-DERIVED GRANULE CATIONIC PROTEINS
Eosinophils secrete an array of cytotoxic granule cationic proteins (MBP, ECP, EPO, and EDN) that are capable of inducing tissue damage and dysfunction.17 Eosinophil granules contain a crystalloid core composed of MBP-1 (and MBP-2), and a matrix composed of ECP, EDN, and EPO.17 MBP, EPO, and ECP are toxic to a variety of tissues, including heart, brain, and bronchial epithelium.29–32 ECP and EDN are ribonucleases and have been shown to possess antiviral activity, and ECP causes voltage-insensitive, ion-selective toxic pores in the membranes of target cells, possibly facilitating the entry of other cytotoxic molecules.33–36 ECP also has a number of additional noncytotoxic activities including suppression of T-cell proliferative responses and immunoglobulin synthesis by B cells, mast cell degranulation, and stimulation of airway mucus secretion and glycosaminoglycan production by human fibroblasts.37 MBP has been shown to directly alter smooth muscle contraction responses by dysregulating vagal muscarinic M2 and M3 receptor function and to promote mast cell and basophil degranulation.38–40 MBP has been recently implicated in regulating peripheral nerve plasticity.41 EPO catalyzes the oxidation of pseudoha-lides (thyiocyanate), halides (chloride, bromide, and iodide), and nitric oxide (nitrite) to form highly reactive oxygen species (hypohalous acids), reactive nitrogen metabo-lites (nitric dioxide), and perioxynitrite-like oxidants, respectively. These molecules oxidize nucleophilic targets on proteins, promoting oxidative stress and subsequent cell death by apoptosis and necrosis.42–44
EOSINOPHIL DEGRANULATION
Eosinophils predominantly secrete their granule protein by regulated exocytosis and degranulation.45 In a process of piecemeal degranulation, eosinophils selectively release components of their specific granules.46 For example, activation of human eosinophils by IFN-γ promotes the mobilization of granule-derived RANTES to the cell periphery without inducing cationic protein release.47,48 Regulated exocytosis occurs by the formation of a docking complex composed of soluble N-ethylmaleimide–sensitive factor attachment protein (SNAP) receptors located on the vesicle and the target membrane. It is postulated that receptor-coupled activation of eosinophils leads to rapid mobilization of cytoplasmic vesicles to the plasma membrane, leading to the formation of a SNAP receptor complex (VAMP-2/SNAP-23/syntaxin-4) and to subsequent mediator release.45 The receptor-coupled activation of eosinophils may involve immunoglobulin, innate pattern recognition receptors (toll-like receptors [TLRs]), complement, or cytokine. Eosinophils express the Fc receptors (FcR) for IgA, IgD, IgG, and IgM.49 CD32 (FcγRII) is constitutively expressed on resting human eosinophils,50 and is up-regulated by IFN-γ.51 Eosinophils do not constitutively express the FcγRI (CD 64) or the low-affinity FcγRIII (CD16); however, expression can be up-regulated by cytokines IFN-γ, complement (C5a), and platelet activating factor.51 These receptors not only function as IgG receptors but also appear to have a role stimulating eosinophil survival, degranulation, and generation of leukotri-enes.52–55 Eosinophils express the IgA receptors (CD89).56 Ex vivo studies have demonstrated that eosinophil degranulation can be induced by IgA-coated particles, suggesting that IgA receptor interaction induces eosinophil degranulation.57 The expression or presence of the low-affinity IgE receptor (CD23) or the high-affinity IgE receptor on eosinophils remains controversial.58 Eosinophils express complement receptors (CRs), including CR1 (CD35), CR3 (CD11b/CD18), C3a, CR4 (CD11c), C5a, CD103, and receptors for C1q.49,59–61 CR1 is recognized by the complement fragments C3b, C4b, iC3b, and C1q. The expression of CR1 on eosinophils is regulated by certain stimuli including leukotriene B4, 15-hydroxyeicosatetraenoic acid, and 5- hydroperoxyeicosatetraenoic acid.62 CR3 has also been shown to be expressed on eosinophils; CR3 interacts with a number of ligands, including iC3b and ICAM1, promoting eosinophil priming and degranulation.63 Eosinophils have also been shown to express a number of TLRs, including TLR-1, TLR-2, TLR-4, TLR-5, TLR-6, TLR-7, TLR-8, TLR-9, and TLR-10.64–66 The level of TLR expression on the eosinophils is low relative to other granulocytes such as neutrophils, except for relatively elevated levels of TLR-7/8.64 Functional analysis using TLR-specific ligands revealed that TLR-7/8 ligands (R-848) induces eosinophil activation (superoxide production) and prolongs eosinophil survival. The expression of TLR-7/8 has been shown to be regulated by cytokines including IFN-γ.64
EOSINOPHILIC GASTROINTESTINAL DISORDERS
Eosinophil accumulation in the GI tract is a common feature of numerous GI disorders, including classic IgE-mediated food allergy,6 EGE,7 allergic colitis,8 EE,9,10 IBD,11 and gastroesophageal reflux disease.12,13
Esophageal Disorders
A number of disorders are accompanied by eosinophil infiltration into the esophagus, including recurrent vomiting, parasitic and fungal infections, IBD, hypereosinophilic syndrome, esophageal leiomyomatosis, myeloproliferative disorders, carcinomatosis, periarteritis, allergic vasculitis, scleroderma, and drug injury.67 Recently, there has been a significant amount of attention on the new and emerging eosinophil-associated esophageal disorder, EE. Patients who have primary EE commonly report symptoms that include vomiting, epigastric or chest pain, dysphagia, and respiratory obstructive problems.68,69 In EE, eosinophil levels are generally 20 to 24 eosinophils per high-power field, reaching 200 eosinophils per high-power field in some cases, and are predominantly localized to the proximal and distal esophagus. In addition, esophageal tissues from patients who have EE demonstrate thickened mucosa with basal layer hyperplasia and papillary lengthening. EE has been associated with esophageal dys-motility. The etiology of the motor disturbances is unclear; however, recent esopha-geal ultrasound studies have revealed the presence of a dysfunctional muscularis mucosa in patients who have EE, providing a possible explanation for the impaired esophageal dysmotility.70 Recently, investigators developed experimental models of EE to begin to examine the contribution of eosinophils in EE. These studies have elegantly delineated an important contribution for the eosinophil-sensitive molecules IL-5 and IL-13 in the recruitment of eosinophils into the esophagus during experimental EE. Although the direct contribution of eosinophils to specific aspects of disease remains unclear, these investigators demonstrated an association between eosinophil numbers in the esophagus and epithelial cell hyperplasia, suggesting a pathophysio-logic connection between eosinophils and the development of EE.
Small Bowel Disorders
EG and EGE present with a constellation of symptoms related to the degree and area of the GI tract affected; however, even patients who have isolated eosinophilic enteritis (eg, duodenitis) can have a range of GI symptoms. The mucosal form of EGE (the most common variant) is characterized by vomiting, abdominal pain (that can mimic acute appendicitis), diarrhea, blood loss in stools, iron-deficiency anemia, malabsorption, protein-losing enteropathy, and failure to thrive.71 The muscularis form is characterized by infiltration of eosinophils predominantly in the muscularis layer, leading to thickening of the bowel wall, which may result in GI obstructive symptoms mimicking pyloric stenosis or other causes of gastric outlet obstruction. The serosal form occurs in a minority of patients who have EGE and is characterized by exudative ascites with higher peripheral eosinophil counts compared with the other forms.72
Histologic analysis of the small bowel from patients who have EGE reveals extracel-lular deposition of eosinophil granule constituents, and indeed, extracellular MBP and ECP are immunohistochemically detectable at elevated levels.1 Further, Charcot-Leyden crystals, remnants of eosinophil degranulation, are commonly found on microscopic examination of stool samples. Electron microscopy studies have revealed ultrastructural changes in the secondary granules (indicative of eosinophil degranulation and mediator release) in duodenal samples from patients who have EGE. Patients who have EGE can have micronodules (with or without polyposis) noted on endoscopy, and these lesions often contain marked aggregates of lymphocytes and eosinophils.
In an effort to delineate the significance of eosinophil accumulation in eosinophil small bowel disorders, the author and colleagues2 developed an experimental-model oral antigen–induced eosinophilic GI inflammation that mimics eosinophil-associated small bowel disease—in particular, EGE. Oral administration of the antigen ovalbumin to ovalbumin-sensitized mice induced a pronounced eosinophilic inflammation of the small intestine (duodenum, jejunum, and ileum). Oral antigen challenge induced a prominent cellular infiltrate comprising predominantly eosinophils. Increased eosin-ophil numbers were observed in various segments of the GI tract, including the esophagus, stomach, small intestine, and Peyer’s patches. The oral antigen–challenged mice suffered from variable levels of reduced activity, increased respiratory rate, pilar erecti, and failure to thrive (cachexia). Postmortem GI examination of these mice revealed the presence of gastromegaly and evidence of gastric dysmotility.73 Employing an in vivo gastric retention assay, the author and colleagues demonstrated impaired gastric emptying in oral allergen–challenged mice. In addition, morphometric analysis revealed a significant decrease in the villus/crypt ratio in the small intestine of oral allergen–challenged mice compared with control-challenged animals. Similarly, patients who have a variety of inflammatory GI disorders also present histologically with reduction in the intestinal villus/crypt ratio.15 Employing eotaxin-1 (CCL11) deficient mice, the author and colleagues2 demonstrated that intestinal eosinophilic inflammation induced by oral antigen challenge is dependent on CCL11. Furthermore, they showed that the cachexia and gastric dysmotility is dependent on CCL11 and eosinophils, suggesting that eosinophils contribute to marked GI pathology including villus/crypt shortening, gastric dysmotility, gastromegaly, and failure to thrive. Electron microscopy analysis revealed that eosinophils in the jejunum of oral antigen–challenged mice are in close proximity to damaged enteric nerves.2 The enteric nerves contain swollen enlarged axonal chambers, with variable loss of internal organelles, including the dense core granules of Schwann cells. Of interest, these features, indicative of axonal necrosis, have been observed in patients with EGID.74 Notably, studies examining full-thickness intestinal biopsies from pediatric patients who have persistent obstructive symptoms have revealed eosinophil infiltration into the myenteric plexus.75
Colonic Disorders
Eosinophils accumulate in the colon of patients who have a variety of disorders including eosinophilic colitis, infections (including pinworms and dog hookworms), drug reactions, vasculitis (eg, Churg-Strauss syndrome), and IBD.3 IBD, Crohn’s disease, and ulcerative colitis (UC) are chronic, relapsing, remitting GI diseases and, in specific subtypes, are characterized by an eosinophilic inflammation of the intestine.76 Elevated levels of eosinophils have been observed in colonic biopsy samples from patients who have UC, and increased numbers of this cell and of eosinophil-derived granular proteins (MBP, ECP, EPO, and EDN) have been shown to correlate with morphologic changes to the GI tract, to disease severity, and to GI dysfunction.77,78 Immunohistochemistry analysis of inflamed colonic mucosa of patients who have UC has revealed evidence of eosinophil activation and degranulation.77 Eosinophils usually represent only a small percentage of the infiltrating leukocytes,11 but their level has been proposed to be a negative prognostic indicator.79
Forbes and colleagues80 previously employed a model of dextran sulfate sodium (DSS)-induced colitis to begin to examine the contribution of eosinophils in colonic epithelial injury. The model of DSS-induced colitis is a colonic epithelial injury model that is associated with a pronounced colonic eosinophilic inflammation. Electron microscopy studies revealed that the infiltrating eosinophils in the colon of DSS-treated mice appear to undergo cytolytic eosinophilic degranulation as evidenced by the presence of free eosinophilic granules in the extracellular spaces adjacent to these eosinophils.80 Consistent with this observation, Forbes and colleagues80 demonstrated elevated levels of colonic luminal EPO activity. Employing EPO-deficient mice and EPO antagonists, Forbes and colleagues80 showed that some of the pathologic features of DSS-induced colitis were significantly attenuated in the absence of EPO activity, indicating a role for eosinophils and EPO in the pathogenesis of DSS-induced colonic injury.
EPO catalyzes the oxidation of halides and pseudohalides (chloride, bromide, and thyiocyanate) with the products of respiratory burst (molecular oxygen and hydrogen peroxide [H2O2]) to generate cytotoxic oxidants (3-bromotyrosine, 3-chlorotyrosine, and hypothyiocynite). These cytotoxic oxidants induce tissue damage and cell death.81 EPO has also been shown to preferentially catalyze the oxidation of nitrite (NO2−), generating the highly toxic reactive nitrogen species (RNS) 3-nitrotyrosine and peroxynitrate.43,44 Eosinophils and EPO have been shown to play an important role in RNS-mediated oxidative stress–induced tissue injury in asthma.82,83 Clinical investigations have demonstrated elevated inducible nitric oxide synthase activity; nitric oxide, NO2−, and peroxynitrite (ONOO−) production; and protein nitration (3-ni-trotyrosine positive staining) in patients who have asthma compared with nonasth-matics.44,83,84 Immunohistochemical analysis of bronchial tissue revealed eosinophils colocalized with 3-nitrotyrosine positive staining suggesting that eosinophil-derived EPO directly contributes to the generation of ONOO− and NO2− and, thus, protein nitration in asthma.44 Notably, at physiologic levels of NO2− and in the presence of H2O2, eosinophils have been shown to promote protein nitration.44 UC has also been shown to be associated with increased inducible nitric oxide syn-thase activity and nitric oxide and RNS production.84 Furthermore, recent clinical studies have demonstrated an imbalance in secondary mucosal antioxidant pathways and in the production of reactive oxygen metabolites including H2O2, hypochlorous acid, and RNS in IBD.85 It is possible that the release of EPO in the lumen during experimental UC leads to the generation of RNS and reactive oxygen metabolites and the subsequent development of the pathophysiologic features of the disease.
Clinical investigations have demonstrated increased levels of a number of other eosinophil granular proteins, including MBP, ECP, and EDN, in biopsy samples from patients who have colonic injury, strengthening a causal link to this granulocyte.77,78 Furuta and colleagues,86 employing the oxazalone model of experimental colitis, demonstrated a role for MBP in disease pathogenesis. Moreover, MBP-deficient mice were less susceptible to oxazolone-induced colitis compared with wild-type mice. In vitro analysis demonstrated that MBP promoted increased intestinal epithelial cell permeability. Notably, the increase in intestinal epithelial cell permeability was associated with the down-regulation of tight junction protein occludin-1 on colonic epithelial cells.86 Clinical and experimental analysis has provided evidence of a casual link between increased intestinal permeability and susceptibility to IBD.87 Further experimental analysis is required to fully delineate the contribution of eosinophil granule proteins in the pathogenesis of IBD.
Previous clinical investigations have also demonstrated collagen deposition in the intestinal biopsy samples from patients who have IBD.88 The collagen deposition is thought to be primarily associated with cellular inflammation and TGF-β and insulin-like growth factor-I expression.88 Furthermore, eosinophils have been linked to fibroblast activation and fibrosis and stricture formation in Crohn’s disease.89,90 There is evidence to suggest that eosinophils may be involved in remodeling and tissue repair through fibroblast stimulation by release of ECP and TGF-β.91 Notably, clinical studies have previously demonstrated that the level of eosinophil activation is elevated in the quiescent phase of UC compared with the active phase.92 Experimental DSS-induced colitis is characterized by extensive deposition of collagen in the colonic submucosa.80 Notably, eosinophils are interspersed throughout the fibrotic layer, suggesting that eosinophils may contribute to collagen deposition. The mechanism causing collagen deposition is currently unknown; however, it is tempting to speculate that eosinophil-derived TGF-β contributes, at least in part, to colonic remodeling in IBD. Recent investigations have demonstrated that eosinophils produce TGF-β during chronic inflammation.93,94 Further analysis is required to define the contribution of eo-sinophils to colonic remodeling.
SUMMARY
EGIDs are becoming more prevalent in the Western world. EGIDs are associated with a variety of nonspecific common GI symptoms and laboratory findings, making their diagnosis completely dependent on microscopic examination of GI biopsy samples, generally obtained during endoscopic evaluation. A variety of clinical and experimental models have revealed that eosinophils promote potent proinflammatory effects mediated by their ability to release their cytotoxic secondary granule constituents and a variety of lipid mediators and cytokines. Although much progress has been made, there is still a paucity of knowledge concerning the individual role of eosinophil-derived granule proteins and inflammatory mediators in EGIDs. It is hoped that further clinical and experimental investigation will unravel the individual role of eo-sinophil-derived mediators in the pathogenesis of EGIDs.
Acknowledgments
The author would like to thank the numerous colleagues who contributed to the body of information presented in this review, including Drs. Elizabeth Forbes, Luqman Seidu, Marc Rothenberg, and Paul Foster. The author is also grateful to Lisa Roberts and Courtney Wilkens for their assistance with the manuscript preparation.
This work was supported in part by the Crohn’s and Colitis Foundation of America.
Footnotes
References
- 1.Rothenberg ME, Mishra A, Brandt EB, et al. Gastrointestinal eosinophils in health and disease. Adv Immunol. 2001;78:291–328. doi: 10.1016/s0065-2776(01)78007-8. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rothenberg ME, Forbes E, et al. Chemokines in eosinophil-associated gastrointestinal disorders. Curr Allergy Asthma Rep. 2004;4(1):74–82. doi: 10.1007/s11882-004-0047-8. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113(1):11–28. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 4.Hogan SP, Foster PS, Rothenberg ME. Experimental analysis of eosinophil-associated gastrointestinal diseases. Curr Opin Allergy Clin Immunol. 2002;2:239–48. doi: 10.1097/00130832-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg ME, Mishra A, Brandt EB, et al. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–55. doi: 10.1034/j.1600-065x.2001.790114.x. [DOI] [PubMed] [Google Scholar]
- 6.Moon A, Kleinman RE. Allergic gastroenteropathy in children. Ann Allergy Asthma Immunol. 1995;74(1):5–12. [PubMed] [Google Scholar]
- 7.Keshavarzian A, Saverymuttu SH, Tai PC, et al. Activated eosinophils in familial eosinophilic gastroenteritis. Gastroenterology. 1985;88(4):1041–9. doi: 10.1016/s0016-5085(85)80026-3. [DOI] [PubMed] [Google Scholar]
- 8.Sherman MP, Cox KL. Neonatal eosinophilic colitis. J Pediatr. 1982;100(4):587–9. doi: 10.1016/s0022-3476(82)80760-9. [DOI] [PubMed] [Google Scholar]
- 9.Furuta GT. Eosinophils in the esophagus: acid is not the only cause. J Pediatr Gastroenterol Nutr. 1998;26(4):468–71. doi: 10.1097/00005176-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Fox VL, Nurko S, Furuta GT. Eosinophilic esophagitis. Gastrointest Endosc. 2002;56:260–70. doi: 10.1016/s0016-5107(02)70188-0. [DOI] [PubMed] [Google Scholar]
- 11.Walsh RE, Gaginella TS. The eosinophil in inflammatory bowel disease. Scand J Gastroenterol. 1991;26:1217–24. doi: 10.3109/00365529108998617. [DOI] [PubMed] [Google Scholar]
- 12.Winter HS, Madara JL, Stafford RJ, et al. Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis. Gastroenterology. 1982;83(4):818–23. [PubMed] [Google Scholar]
- 13.Brown LF, Goldman H, Antonioli DA. Intraepithelial eosinophils in endoscopic biopsies of adults with reflux esophagitis. Am J Surg Pathol. 1984;8(12):899–905. doi: 10.1097/00000478-198412000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338(22):1592–600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 15.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103(5 Pt 1):717–28. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 16.Furuta GT, Ackerman SJ, Wershil BK. The role of the eosinophil in gastrointestinal diseases. Curr Opin Gastroenterol. 1995;11:541–7. [Google Scholar]
- 17.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39(177):177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 18.Weller PF. The immunobiology of eosinophils. N Engl J Med. 1991;324:1110–8. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- 19.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25(9):477–82. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–55. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 21.Lacy P, Moqbel R. Eokines: synthesis, storage and release from human eosinophils. Mem Inst Oswaldo Cruz. 1997;92(Suppl):H125–33. doi: 10.1590/s0074-02761997000800017. [DOI] [PubMed] [Google Scholar]
- 22.Moqbel R, Lacy P. Eosinophil cytokines. In: Busse WW, Holgate ST, editors. Inflammatory mechanisms in asthma. Vol. 117. New York: Marcel Dekker, Inc; 1998. pp. 227–46. [Google Scholar]
- 23.Spry CJF. A comprehensive review and guide to the scientific and medical literature. Oxford (UK): Oxford Medical Publications; 1988. Eosinophils. [Google Scholar]
- 24.Gauthier MC, Racine C, Ferland C, et al. Expression of membrane type-4 matrix metalloproteinase (metalloproteinase-17) by human eosinophils. Int J Biochem Cell Biol. 2003;35(12):1667–73. doi: 10.1016/s1357-2725(03)00136-5. [DOI] [PubMed] [Google Scholar]
- 25.Ohno I, Ohtani H, Nitta Y, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997;16(3):212–9. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- 26.Okada S, Kita H, George TJ, et al. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997;17(4):519–28. doi: 10.1165/ajrcmb.17.4.2877. [DOI] [PubMed] [Google Scholar]
- 27.Schwingshackl A, Duszyk M, Brown N, et al. Human eosinophils release matrix metal-loproteinase-9 on stimulation with TNF-β. J Allergy Clin Immunol. 1999;104(5):983–9. doi: 10.1016/s0091-6749(99)70079-5. [DOI] [PubMed] [Google Scholar]
- 28.Wiehler S, Cuvelier SL, Chakrabarti S, et al. p38 MAP kinase regulates rapid matrix metalloproteinase-9 release from eosinophils. Biochem Biophys Res Commun. 2004;315(2):463–70. doi: 10.1016/j.bbrc.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 29.Tai P-C, hayes DJ, Clark JB, et al. Toxic effects of eosinophil secretion products on isolated rat heart cells in vitro. Biochem J. 1982;204:75–80. doi: 10.1042/bj2040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venge P, Dahl R, Hallgren R, et al. Cationic proteins of human eosinophils and their role in the inflammatory reaction. In: Mahmoud AAF, Austin KF, editors. The eosinophil in health and disease. New York: Grune and Stratton; 1980. pp. 1131–42. [Google Scholar]
- 31.Frigas E, Loegering DA, Gleich GJ. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980;42(1):35–43. [PubMed] [Google Scholar]
- 32.Gleich GJ, Frigas E, Loegering DA, et al. The cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925. [PubMed] [Google Scholar]
- 33.Young JD, Peterson CG, Venge P, et al. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321(6070):613–6. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 34.Slifman NR, Loegering DA, McKean DJ, et al. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986;137(9):2913–7. [PubMed] [Google Scholar]
- 35.Gleich GJ, Loegering DA, Bell MP, et al. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci USA. 1986;83(10):3146–50. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70(5):691–8. [PubMed] [Google Scholar]
- 37.Venge P, Bystrom J, Carlson M, et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999;29:1172–86. doi: 10.1046/j.1365-2222.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 38.Zheutlin LM, Ackerman SJ, Gleich GJ, et al. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. J Immunol. 1984;133(4):2180–5. [PubMed] [Google Scholar]
- 39.Piliponsky AM, Pickholtz D, Gleich GJ, et al. Human eosinophils induce histamine release from antigen-activated rat peritoneal mast cells: a possible role for mast cells in late-phase allergic reactions. J Allergy Clin Immunol. 2001;107(6):993–1000. doi: 10.1067/mai.2001.114656. [DOI] [PubMed] [Google Scholar]
- 40.Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol. 2001;107(2):211–8. doi: 10.1067/mai.2001.112940. [DOI] [PubMed] [Google Scholar]
- 41.Morgan RK, Costello RW, Durcan N, et al. Diverse effects of eosinophil cationic granule proteins on IMR-32 nerve cell signaling and survival. Am J Respir Cell Mol Biol. 2005;28:28. doi: 10.1165/rcmb.2005-0056OC. [DOI] [PubMed] [Google Scholar]
- 42.Agosti JM, Altman LC, Ayars GH, et al. The injurious effect of eosinophil peroxi-dase, hydrogen peroxide, and halides on pneumocytes in vitro. J Allergy Clin Immunol. 1987;79(3):496–504. doi: 10.1016/0091-6749(87)90368-x. [DOI] [PubMed] [Google Scholar]
- 43.Wu W, Chen Y, Hazen SL. Eosinophil peroxidase, nitrates, protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosino-philic inflammatory disorders. J Biol Chem. 1999;274(36):25933–44. doi: 10.1074/jbc.274.36.25933. [DOI] [PubMed] [Google Scholar]
- 44.MacPherson JC, Comhair SA, Erzurum SC, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–72. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 45.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol. 2003;111(5):923–32. [PubMed] [Google Scholar]
- 46.Dvorak AM, Furitsu T, Letourneau L, et al. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991;138(1):69–82. [PMC free article] [PubMed] [Google Scholar]
- 47.Lacy P, Mahmudi-Azer S, Bablitz B, et al. Rapid mobilization of intracellularly stored RANTES in response to interferon-γ in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- 48.Bandeira-Melo C, Gillard G, Ghiran I, et al. EliCell: a gel-phase dual antibody capture and detection assay to measure cytokine release from eosinophils. J Immunol Methods. 2000;244(1–2):105–15. doi: 10.1016/s0022-1759(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 49.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51(2):213–340. [PubMed] [Google Scholar]
- 50.Hartnell A, Moqbel R, Walsh GM, et al. Fc gamma and CD1 1/CD 1 8 receptor expression on normal density and low density human eosinophils. Immunology. 1990;69(2):264–70. [PMC free article] [PubMed] [Google Scholar]
- 51.Hartnell A, Kay AB, Wardlaw AJ. IFN-γ induces expression of Fc γ RIII (CD16) on human eosinophils. J Immunol. 1992;148(5):1471–8. [PubMed] [Google Scholar]
- 52.Kim JT, Schimming AW, Kita H. Ligation of Fc gamma RII (CD32) pivotally regulates survival of human eosinophils. J Immunol. 1999;162(7):4253–9. [PubMed] [Google Scholar]
- 53.Kita H, Abu-Ghazaleh RI, Gleich GJ, et al. Regulation of Ig-induced eosinophil degranulation by adenosine 3′,5′-cyclic monophosphate. J Immunol. 1991;146(8):2712–8. [PubMed] [Google Scholar]
- 54.Cromwell O, Moqbel R, Fitzharris P, et al. Leukotriene C4 generation from human eosinophils stimulated with IgG–Aspergillus fumigatus antigen immune complexes. 1988;82(4):535–43. doi: 10.1016/0091-6749(88)90962-1. [DOI] [PubMed] [Google Scholar]
- 55.Cromwell O, Wardlaw AJ, Champion A, et al. IgG-dependent generation of platelet-activating factor by normal and low density human eosinophils. 1990;145(11):3862–8. [PubMed] [Google Scholar]
- 56.Monteiro RC, Hostoffer RW, Cooper MD, et al. Definition of immunoglobulin-A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993;92:1681–5. doi: 10.1172/JCI116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abu Ghazaleh R, Fujisawa T, Mestecky J, et al. IgA-induced eosinophil degranulation. J Immunol. 1989;142(7):2393–400. [PubMed] [Google Scholar]
- 58.Kita H, Gleich GJ. Eosinophils and IgE receptors: a continuing controversy. Blood. 1997;89(10):3497–501. [PubMed] [Google Scholar]
- 59.Walsh GM, Hartnell A, Moqbel R, et al. Receptor expression and functional status of cultured human eosinophils derived from umbilical cord blood mononuclear cells. Blood. 1990;76(1):105–11. [PubMed] [Google Scholar]
- 60.DiScipio RG, Daffern PJ, Jagels MA, et al. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transen-dothelial migration in vitro and in vivo. J Immunol. 1999;162(2):1127–36. [PubMed] [Google Scholar]
- 61.Daffern PJ, Pfiefer PH, Ember JA, et al. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–27. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischer E, Capron M, Prin L, et al. Human eosinophils express CR1 and CR3 complement receptors for cleavage fragments of C3. 1986;97(2):297–306. doi: 10.1016/0008-8749(86)90400-4. [DOI] [PubMed] [Google Scholar]
- 63.Koenderman L, Kuijpers TW, Blom M, et al. Characteristics of CR3-mediated aggregation in human eosinophils: effect of priming by platelet-activating factor. J Allergy Clin Immunol. 1991;87(5):947–54. doi: 10.1016/0091-6749(91)90416-l. [DOI] [PubMed] [Google Scholar]
- 64.Nagase H, Okugawa S, Ota Y, et al. Expression and function of toll-like receptors in eosinophils: activation by toll-like receptor 7 ligand. J Immunol. 2003;171(8):3977–82. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 65.Plotz SG, Lentschat A, Behrendt H, et al. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood. 2001;97(1):235–41. doi: 10.1182/blood.v97.1.235. [DOI] [PubMed] [Google Scholar]
- 66.Sabroe I, Jones EC, Usher LR, et al. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopoly-saccharide responses. J Immunol. 2002;168(9):4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 67.Ahmad M, Soetikno RM, Ahmed A. The differential diagnosis of eosinophilic esophagitis. J Clin Gastroenterol. 2000;30(3):242–4. doi: 10.1097/00004836-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Orenstein SR, Shalaby TM, Di Lorenzo C, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–30. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 69.Walsh SV, Antonioli DA, Goldman H, et al. Allergic esophagitis in children: a clin-icopathological entity. Am J Surg Pathol. 1999;23(4):390–6. doi: 10.1097/00000478-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Fox VL, Nurko S, Teitelbaum JE, et al. High-resolution EUS in children with eosin-ophilic ‘‘allergic’’ esophagitis. Gastrointest Endosc. 2003;57(1):30–6. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 71.Kelly KJ. Eosinophilic gastroenteritis. J Pediatr Gastroenterol Nutr. 2000;30(Suppl):S28–35. doi: 10.1097/00005176-200001001-00005. [DOI] [PubMed] [Google Scholar]
- 72.Talley NJ, Shorter RG, Phillips SF, et al. Eosinophilic gastroenteritis: a clinicopath-ological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31(1):54–8. doi: 10.1136/gut.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2(4):353–60. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 74.Dvorak AM, Onderdonk AB, McLeod RS, et al. Ultrastructural identification of exocytosis of granules from human gut eosinophils in vivo. Int Arch Allergy Immunol. 1993;102(1):33–45. doi: 10.1159/000236548. [DOI] [PubMed] [Google Scholar]
- 75.Schappi MG, Smith VV, Milla PJ, et al. Eosinophilic myenteric ganglionitis is associated with functional intestinal obstruction. Gut. 2003;52:752–5. doi: 10.1136/gut.52.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15(1):79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carvalho AT, Elia CC, de Souza HS, et al. Immunohistochemical study of intestinal eosinophils in inflammatory bowel disease. J Clin Gastroenterol. 2003;36(2):120–5. doi: 10.1097/00004836-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Jeziorska M, Haboubi N, Schofield P, et al. Distribution and activation of eosino-phils in inflammatory bowel disease using an improved immunohistochemical technique. J Pathol. 2001;194(4):484–92. doi: 10.1002/path.904. [DOI] [PubMed] [Google Scholar]
- 79.Desreumaux P, Nutten S, Colombel JF. Activated eosinophils in inflammatory bowel disease: do they matter? Am J Gastroenterol. 1999;94(12):3396–8. doi: 10.1111/j.1572-0241.1999.01657.x. [DOI] [PubMed] [Google Scholar]
- 80.Forbes E, Murase T, Yang M, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664–75. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 81.Kruidenier L, Verspaget HW. Oxidative stress as a pathogenic factor in inflammatory bowel disease—radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 82.Saleh D, Ernst P, Lim S, et al. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. Faseb J. 1998;12(11):929–37. [PubMed] [Google Scholar]
- 83.Massaro AF, Mehta S, Lilly CM, et al. Elevated nitric oxide concentrations in isolated lower airway gas of asthmatic subjects. Am J Respir Crit Care Med. 1996;153:1510–4. doi: 10.1164/ajrccm.153.5.8630594. [DOI] [PubMed] [Google Scholar]
- 84.Kimura H, Hokari R, Miura S, et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42(2):180–7. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kruidenier L, Kuiper I, van Duijn W, et al. Imbalanced secondary mucosal antiox-idant response in inflammatory bowel disease. J Pathol. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- 86.Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G890–7. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 87.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):401–7. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lawrance IC. Inflammation location, but not type, determines the increase in TGF-B1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16–26. doi: 10.1097/00054725-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Gelbmann CM, Mestermann S, Gross V, et al. Strictures in Crohn’s disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut. 1999;45(2):210–7. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu X, Rivkind A, Pikarsky A, et al. Mast cells and eosinophils have a potential profibrogenic role in Crohn disease. Scand J Gastroenterol. 2004;39(5):440–7. doi: 10.1080/00365520310008566. [DOI] [PubMed] [Google Scholar]
- 91.Levi-Schaffer F, Garbuzenko E, Rubin A, et al. Human eosinophils regulate human lung-and skin-derived fibroblast properties in vitro: a role for transforming growth factor β (TGF-β) 1999;96:9660–5. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lampinen M, Ronnblom A, Amin K, et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54(12):1714–20. doi: 10.1136/gut.2005.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL–13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–7. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 94.Ohkawara Y, Tamura G, Iwasaki T, et al. Activation and transforming growth factor-β production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol. 2000;23:444–51. doi: 10.1165/ajrcmb.23.4.3875. [DOI] [PubMed] [Google Scholar]