This study evaluated the impact of age and pneumococcal vaccination on the density of pneumococcal nasopharyngeal carriage. Among colonized individuals, density decreased with increasing age. Time-trends analysis revealed that pneumococcal vaccination appeared to lower the density of nasopharyngeal carriage.
Abstract
Background. This study evaluated the impact of age and pneumococcal vaccination on the density of pneumococcal nasopharyngeal carriage.
Methods. A cluster-randomized trial was conducted in rural Gambia. In 11 villages (the vaccine group), all residents received 7-valent pneumococcal conjugate vaccine (PCV-7), while in another 10 villages (the control group), only children <30 months old or born during the study period received PCV-7. Cross-sectional surveys (CSSs) were conducted to collect nasopharyngeal swabs before vaccination (baseline CSS) and 4, 12, and 22 months after vaccination. Pneumococcal density was defined using a semiquantitative classification (range, 1–4) among colonized individuals. An age-trend analysis of density was conducted using data from the baseline CSS. Mean pneumococcal density was compared in CSSs conducted before and after vaccination.
Results. Mean bacterial density among colonized individuals in the baseline CSS was 2.57 for vaccine-type (VT) and non–vaccine-type (NVT) pneumococci; it decreased with age (P < .001 for VT and NVT). There was a decrease in the density of VT carriage following vaccination in individuals older than 5 years (from 2.44 to 1.88; P = .001) and in younger individuals (from 2.57 to 2.11; P = .070) in the vaccinated villages. Similar decreases in density were observed with NVT within vaccinated and control villages. No significant differences were found between vaccinated and control villages in the postvaccination comparisons for either VT or NVT.
Conclusions. A high density of carriage among young subjects might partly explain why children are more efficient than adults in pneumococcal transmission. PCV-7 vaccination lowered the density of VT and of NVT pneumococcal carriage in the before-after vaccination analysis.
Clinical Trials Registration. ISRCTN51695599.
Prevention of pneumococcal disease is a public health priority [1]. Nasopharyngeal pneumococcal carriage plays an important role in the pathogenesis of invasive pneumococcal disease (IPD) and pneumococcal transmission. The effectiveness of pneumococcal conjugate vaccines (PCVs) in preventing disease due to vaccine-serotype (VT) pneumococci is due in large measure to their impact on nasopharyngeal carriage in both vaccinees and their contacts [2–5]. Such a reduction is sometimes accompanied by an increase in carriage of non–vaccine-serotype (NVT) pneumococci [2–7].
How this increase in NVT pneumococcal carriage is brought about is still not fully understood. Elimination of VT pneumococci from the nasopharynx might open up an ecological niche that can be colonized by NVT pneumococci in the face of reduced competition from VT pneumococci [3, 5–7]. Alternatively, apparent replacement could be an artifact due to the “unmasking” of NVT serotypes as reduction in VT pneumococci makes it easier to detect NVT [6–9]. Because the commonly used serotyping methods require that only a few colonies are assessed, investigators are unlikely to detect cocolonization of serotypes present at low density.
Increasing attention is being paid to the nasopharyngeal bacterial load (or density) of pneumococcal colonization [10, 11]. Although data on the inverse association between the prevalence of pneumococcal nasopharyngeal carriage and age are widely available, we are unaware of any data on the association of pneumococcal nasopharyngeal density and age. PCVs may have an effect on carriage density among those who remain colonized, influencing their susceptibility to pneumonia and their ability to transmit the infection [6].
The aims of this study were to evaluate the impact of age—a surrogate for naturally acquired immunity—and 7-valent PCV (PCV-7) vaccination on the density of VT and NVT nasopharyngeal carriage recorded during a cluster-randomized trial (CRT) conducted in rural Gambia. All residents of 11 villages were vaccinated with PCV-7, whereas in 10 villages only young children received PCV-7 [12]. The prevalence of carriage with VT pneumococci fell after vaccination in all age groups and both study arms. There was little change in the prevalence of NVT carriage following vaccine introduction.
METHODS
Study Population
The study was conducted in 21 villages in the Sibanor area in the Western Region of Gambia. The combined population of the selected villages was 5441 in June 2006 [12], with 80–660 inhabitants per village. The prevalence of HIV infection among pregnant women attending the local hospital (in Sibanor) between 2000 and 2001 was 3% [13]. Other characteristics of the study population are described elsewhere [14].
The study was approved by the joint Medical Research Council (MRC)/Gambia Government Ethics Committee and by the ethics committee of the London School of Hygiene and Tropical Medicine. The conduct of the trial was guided by a data safety and monitoring board.
Trial Design
A comprehensive description of the trial design, randomization, and cross-sectional surveys (CSS) conducted before and after vaccination has been presented previously [12]. In brief, in one group of 11 villages, all individuals older than 30 months received 1 dose of PCV-7, whereas in 10 control villages, subjects older than 30 months received 1 dose of serogroup C meningocococal conjugate vaccine. In both study groups, all children younger than 30 months and infants born during the course of the trial received PCV-7 (3 doses at monthly intervals for infants and 1 dose for children >1 year of age at the time of vaccination). It was considered unethical not to give PCV-7 to all children <30 months of age because the efficacy of a 9-valent PCV of Gambian children was previously shown [15]. PCV-7 vaccination started in July 2006, and by September the first round of vaccination had been completed (Supplementary Figure 1).
CSSs were conducted before and 4–6 months (CCS-1), 12 months (CCS-2), and 22 months (CCS-3) after vaccination started, during which nasopharyngeal swabs were collected from approximately 1200 randomly selected, age-stratified subjects [12] (Supplementary Figure 1). In the last survey, only 446 samples were analyzed because many subjects in study villages had received a single dose of azithromycin during the course of the Gambian National Trachoma Elimination; all 446 samples were collected before the azithromycin campaign started in any of the study villages.
Nasopharygeal Samples
Sample collection and laboratory methods were similar during all surveys. Nasopharyngeal specimens were collected by application of a calcium alginate swab to the posterior wall of the nasopharynx, and swabs were inoculated into vials containing skim milk-tryptone-glucose-glycerol (STGG) transport medium. Inoculated vials were placed in a cold box and, within 8 hours, were stored at −70°C, in accordance with the World Health Organization protocol [16].
Laboratory Methods
A total of 10 µL of thawed, inoculated STGG medium was plated onto gentamicin blood agar (GBA) plates for selective isolation of Streptococcus pneumoniae and incubated for 18–24 hours at 35°C in 5% CO2. Pneumococcal identification was based on colony morphology and conventional methods of characterization (optochin susceptibility and bile solubility assays) [14]. Inoculated STGG medium was streaked onto the GBA plates to obtain decreasing inoculum sizes in 4 different quadrants of a Petri dish, from the first quadrant (where the diluted nasopharyngeal swabs was inoculated) to the fourth (the last to be covered by the loop) [17]. For samples from which at least 1 pneumococcal colony was isolated, density was semiquantitatively categorized as described previously [17]: category 4, >10 pneumococcal colonies counted in the fourth quadrant of the Petri dish; category 3, <10 pneumococcal colonies present in the fourth quadrant and >10 in the third quadrant; category 2, <10 pneumococcal colonies present in the third quadrant and >10 in the second quadrant; and category 1, <10 pneumococcal colonies counted in the second quadrant and any colony present in the first quadrant.
Serotyping was performed at MRC Fajara with capsular and factor typing sera (Statens Serum Institut, Copenhagen, Denmark), using a modified latex agglutination assay [18]. Equivocal results were confirmed by the Quellung reaction. Pneumococci were classified as follows: VT serotypes included those in PCV-7 (4, 6B, 9V, 14, 18C, 19F, and 23F) and serotype 6A; NVT comprised other pneumococcal serotypes not included in the above classification, as well as nontypeable pneumococci. All detected serotypes were included in the analysis for subjects carrying >1 serotype.
Analysis
The primary end points for the analysis were the density of VT and NVT pneumococci in nasopharyngeal swabs. Mean pneumococcal density among pneumococcal nasopharyngeal carriers was calculated before and after vaccination. Data from the postvaccination surveys (CSS-1–3) were combined to improve the statistical power and to reduce the number of comparisons, thereby limiting multiple testing. In addition, there was no consistent difference in pneumococcal density among the postvaccination surveys. Analyses were based on cluster-level summaries (ie, village means), since the number of clusters is small and because statistical models that allow for clustering (eg, random effects or generalized estimating equations) may be less robust under these circumstances [19]. The difference between mean density in control and vaccinated villages was adjusted for baseline density, using analysis of covariance. The comparison was performed separately for children ≤5 years of age and for individuals >5 years of age when the sample had been collected. We tested for interaction between age and trial arm, using the method of Cheung et al [20]. A paired t test was used to compare mean density in vaccinated villages following vaccination with baseline density, and a paired t test was also used to compare the differences (baseline vs postvaccination) between age groups (ie, to test for interaction between age and time).
RESULTS
Samples
Analyses were based on 4792 samples (2094 obtained during the baseline CSS and 2698 collected during postvaccination CSSs). Overall, 136 samples were excluded from the analyses of the postvaccination surveys; 102 samples were from children <30 months of age, since children in this age group had received PCV-7 regardless of the trial arm, and 24 samples were from children sampled before vaccination.
The overall prevalence of pneumococcal carriage dropped from 71% in the baseline CSS to 44% in the postvaccination surveys [12]. Density data were available for >95% of the pneumococcal-colonized individuals (Table 1). The distribution of pneumococcal carriers is presented in Table 2.
Table 1.
Number of Samples Tested for Pneumococcal Carriage Density Data in Prevaccination and Postvaccination Cross-sectional Surveys
| Survey | Samples, No. | Pneumococcal Colonization, No. (%) | Density Available, No. (%) |
|---|---|---|---|
| Prevaccination | 2094 | 1488 (71) | 1390a (93) |
| Postvaccination | |||
| CSS-1 | 1109 | 511 (46) | 508 (>99) |
| CSS-2 | 1155 | 426 (37) | 425 (>99) |
| CSS-3 | 434 | 250 (58) | 239 (96) |
| Overall | 4792 | 2675 (48) | 2562 (96) |
Abbreviation: CSS, cross-sectional survey.
a Fifty samples were from children <30 months of age, an age group not included in the postvaccination surveys.
Table 2.
Age and Sex Distribution of Pneumococcal Carriers in the Different Cross-sectional Surveys
| Prevaccination |
Postvaccination |
|||
|---|---|---|---|---|
| Control Communities | Vaccinated Communities | Control Communities | Vaccinated Communities | |
| Male sex, % | 51.28 | 45.82 | 50.51 | 51.04 |
| Age, years, median (IQR) | 13.5 (7.5–34.0) | 13.5 (7.5–29.5) | 10.5 (6.5–18.5) | 11.2 (6.3–20.4) |
Baseline Pneumococcal Carriage Density
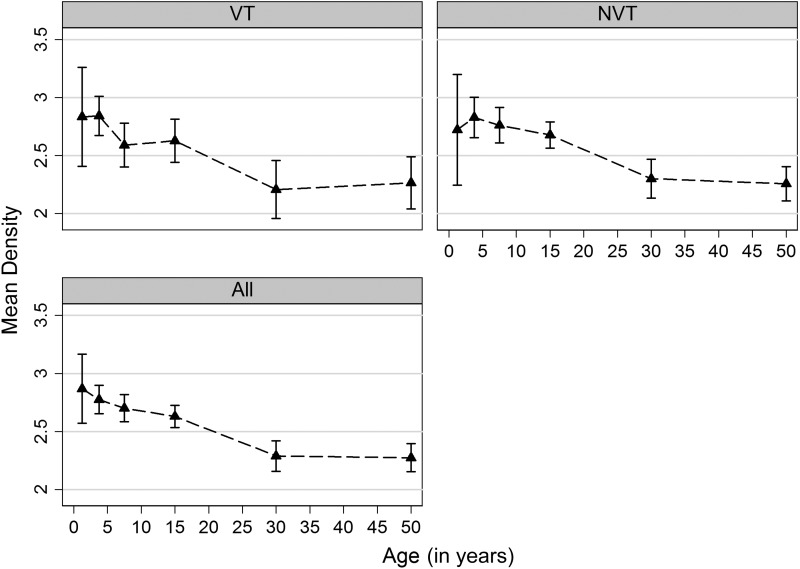
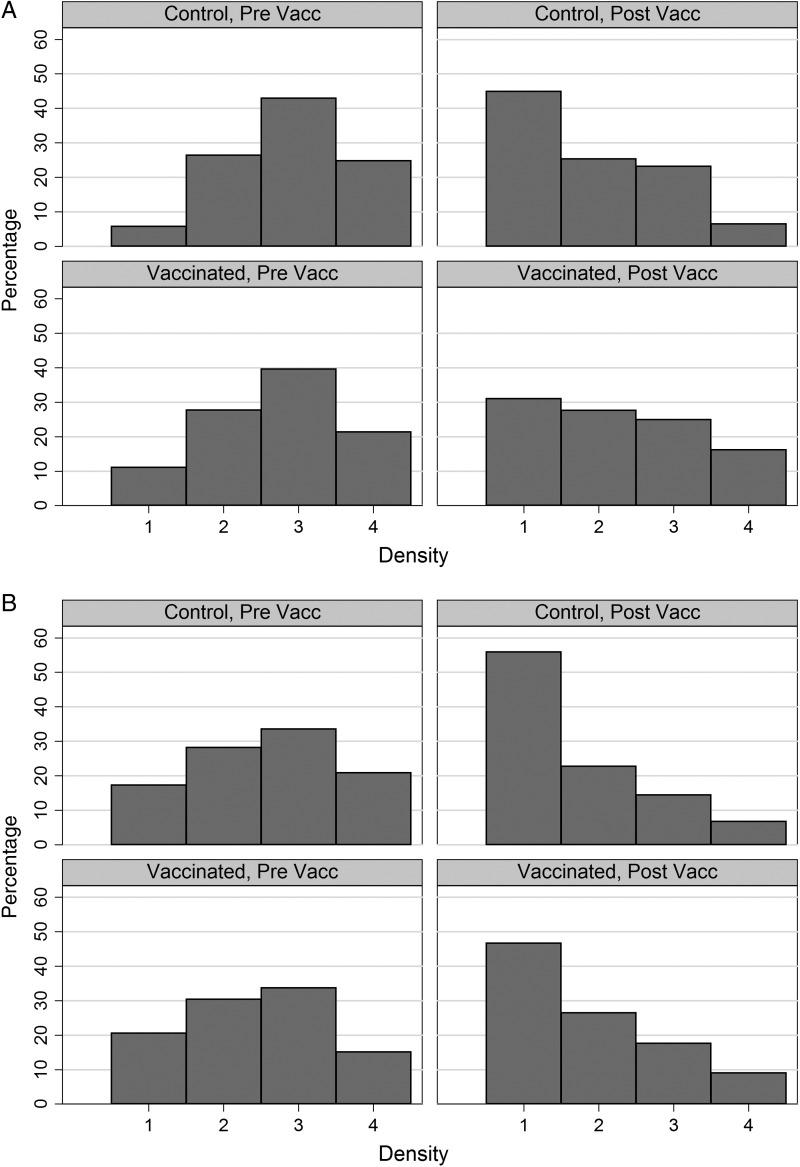
Mean density among colonized individuals in the baseline CSS decreased with age (P < .001 for VT, NVT, and any carriage) (Figure 1). Most pneumococcal carriers (83%) had a density of carriage of >1, and this was similar among VT (87%) and NVT (82%) carriers (Figure 2). There was no difference in overall density by sex (P = .560) for either VT (P = .231) or NVT carriage (P = .727).
Figure 1.
Mean density of pneumococcal carriage by age in the prevaccination cross-sectional survey. Age groups considered for the figure were 0–<2.5 years, 2.5–5 years, 6–9 years, 10–19 years, 20–39 years, and ≥40 years. Triangles represent mean density for each age group (plotted at the midpoint), and capped lines are 95% confidence intervals. Abbreviations: NVT, non–vaccine-type pneumococci; VT, vaccine-type pneumococci.
Figure 2.
Distribution of pneumococcal carriage density among infected individuals before (Pre Vacc) and after (Post Vacc) vaccination, in Vaccinated and Control groups. A, Children ≤5 years of age when the sample was collected. B, Individuals >5 years of age when the sample was collected.
Density varied by serotype (P < .001 in both age groups) (Supplementary Table 1). In the youngest age group, the density of VT serotypes ranged from 2.38 to 4.00, and the density of NVT ranged from 2.33 to 3.50. Among older subjects, densities were lower than those among young children for nearly all serotypes, ranging from 2.27 to 2.50 for VT and from 2.17 to 2.70 for NVT.
Changes in Pneumococcal Carriage Density Following Vaccination
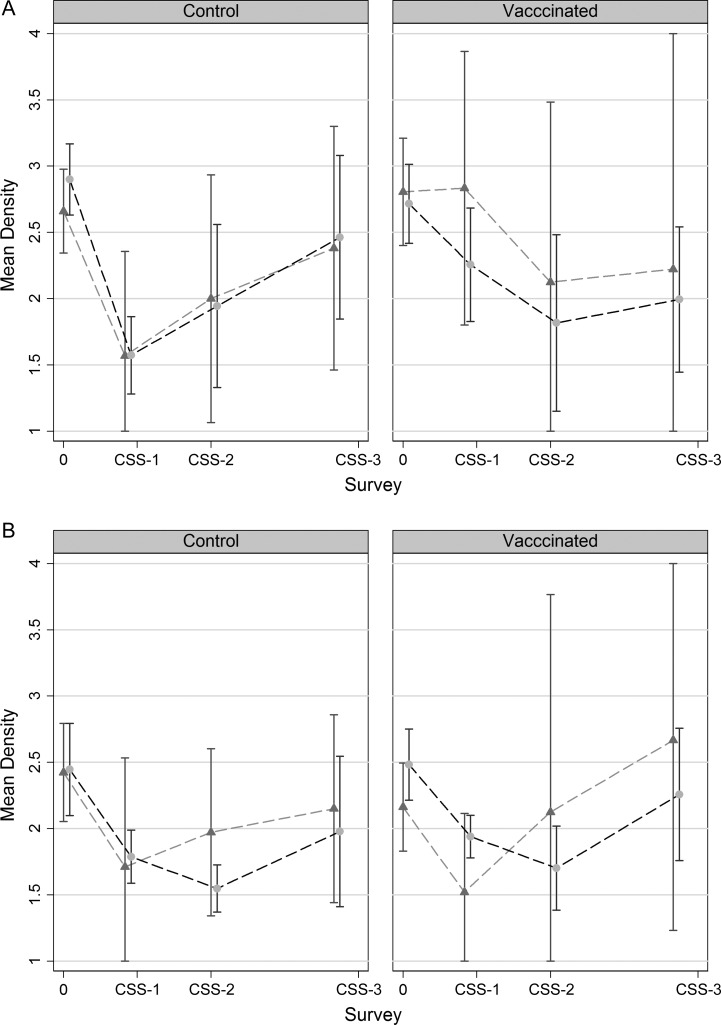
The highest mean density was observed in the prevaccination survey, except for VT in vaccinated villages, where density was highest in CSS-3 (Figure 3). As confidence intervals were very wide, further analysis was performed on pooled results from the 3 postvaccination surveys. A comparison of the mean densities of VT and NVT carriage between all postvaccination CSSs and the baseline CSS is shown in Table 3. In the vaccinated villages, there was a significant decrease in the density of carriage with any pneumococcus following vaccination in individuals older than 5 years (from 2.44 to 1.88; difference = −0.56; P = .001) and a decrease of borderline significance among the younger age group (from 2.57 to 2.11; difference = −0.46; P = .07) (Table 3A). In the control villages, there was also a decrease in density following vaccination, both among the older individuals (from 2.44 to 1.75; difference = −0.69; P = .001), as well as among the younger individuals (from 2.76 to 1.99; difference = −0.78; P = .002) (Table 3B). Similar decreases in density following vaccination were observed for colonization with both VT and NVT pneumococci (Tables 3A and B). Approximately 50% and 40% of pneumococcal carriers had a density of carriage of >1 in the postvaccination surveys (Figure 2).
Figure 3.
Mean density of vaccine-type (triangles) and non–vaccine-type (circles) pneumococcal carriage at prevaccination (0) and at 3 postvaccination surveys (CSS-1 to CSS-3). Capped lines represent 95% confidence intervals. A, Children ≤5 years of age when the sample was collected. B, Individuals >5 years of age when the sample was collected. Abbreviation: CSS, cross-sectional survey.
Table 3.
Mean Density of Pneumococcal Carriage in Infected Individuals During the Prevaccination and Postvaccination Cross-Sectional Surveys in Vaccinated Villages and Control Villages, by Age at Sample Collection
| Age ≤ 5 Years |
Age > 5 Years |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Arm, Serotype Group | Prevaccination | Postvaccinationa | Difference (95% CI) | P | Prevaccination | Postvaccinationa | Difference (95% CI) | P | Pintb |
| Vaccinated villages | |||||||||
| VT | 2.87 | 2.48 | −0.39 (−0.82 to 0.04) | .071 | 2.14 | 1.78 | −0.36 (−0.64 to −0.07) | .021 | .902 |
| NVT | 2.72 | 2.08 | −0.64 (−1.28 to 0.01) | .052 | 2.48 | 1.91 | −0.57 (−0.90 to −0.25) | .003 | .582 |
| All | 2.57 | 2.11 | −0.46 (−0.97 to 0.04) | .067 | 2.44 | 1.88 | −0.56 (−0.83 to −0.29) | .001 | .702 |
| Control villages | |||||||||
| VT | 2.70 | 1.94 | −0.76 (−1.49 to −0.04) | .042 | 2.42 | 1.78 | −0.64 (−1.00 to −0.28) | .003 | .685 |
| NVT | 2.90 | 2.03 | −0.87 (−1.46 to −0.28) | .010 | 2.45 | 1.74 | −0.71 (−1.06 to −0.36) | .001 | .895 |
| All | 2.76 | 1.99 | −0.78 (−1.19 to −0.36) | .002 | 2.44 | 1.75 | −0.69 (−1.01 to −0.37) | .001 | .696 |
Data are mean densities, expressed in arbitrary units, as defined in the Methods section.
Abbreviations: CI, confidence interval; NVT, non–vaccine-type pneumococci; VT, vaccine-type pneumococci.
a Data are for all 3 postvaccination cross-sectional surveys combined.
b P value for interaction between group (control or vaccinated) and age category.
Although serotype distribution within VT and NVT varied between prevaccination and postvaccination surveys, this variation would account for <5% of the difference in mean density observed in the comparisons (data not shown).
Comparison of Pneumococcal Carriage Density Between Vaccinated and Control Groups in the Postvaccination Surveys
Mean densities in the postvaccination surveys among children ≤5 years of age were 1.99 and 2.11 in the control and vaccinated groups, respectively, and among individuals aged >5 years were 1.75 and 1.88, respectively. Differences between the vaccinated and control groups were not statistically significant for any of the comparisons (Table 4).
Table 4.
Comparison of Mean Density of Pneumococcal Carriage in Infected Individuals During the 3 Postvaccination Cross-Sectional Surveys in Vaccinated and Control Villages, by Age at Sample Collection
| Age ≤ 5 Years |
Age > 5 Years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotype Group | Control Villages | Vaccinated Villages | Difference | Differenceadja (95% CI) | P | Control Villages | Vaccinated Villages | Difference | Differenceadja (95% CI) | P | Pintb |
| VT | 1.94 | 2.43 | 0.49 | 0.53 (−0.03 to 1.08) | .060 | 1.78 | 1.78 | 0.00 | 0.15 (−0.24 to 0.54) | .435 | .102 |
| NVT | 2.04 | 2.07 | 0.03 | −0.09 (−0.61 to 0.44) | .738 | 1.74 | 1.91 | 0.17 | 0.17 (−0.01 to 0.35) | .062 | .582 |
| All | 1.99 | 2.11 | 0.12 | 0.13 (−0.34 to 0.60) | .579 | 1.75 | 1.88 | 0.13 | 0.13 (−0.04 to 0.30) | .135 | .981 |
Data are mean densities, expressed in arbitrary units, as defined in the Methods section.
Abbreviations: CI, confidence interval; NVT, non–vaccine-type pneumococci; VT, vaccine-type pneumococci.
a Adjusted for baseline density.
b P value for interaction between group (control or vaccinated) and age category.
DISCUSSION
To the best of our knowledge, this is the first extensive community study to measure the density of pneumococcal nasopharyngeal carriage. The data were collected as part of a CRT conducted in rural Gambia, an area with a high prevalence of pneumococcal nasopharyngeal carriage [14]. We evaluated the density of pneumococcal carriage in different age groups before and after PCV-7 vaccination. Our main finding was that the density of pneumococcal carriage decreased with increasing age. We also showed in a before-after analysis that the density of pneumococcal carriage was lower after vaccination.
We have shown a strong inverse correlation between pneumococcal nasopharyngeal density and age, which is in agreement with the fact that the overall prevalence of pneumococcal carriage falls with increasing age [14, 21–23]. Younger individuals have a higher prevalence of nasopharyngeal pneumococcal carriage and, at the same time, a higher density of pneumococci in the nasopharynx. This may be due in part to less close contact between adults than between young children, but it is also likely to be due to the progressive acquisition of immunity as a result of prior pneumococcal carriage. Immunity acquired by prior pneumococcal exposure may prevent colonization and also bring a quick decrease in density of infection that, in turn, leads to accelerated clearance, as observed in mouse models [24]. Several mechanisms might be responsible for the naturally acquired immune response that protects against carriage and reduces carriage density. Some studies have shown a negative association between the presence of antibodies to individual pneumococcal polysaccharides and the risk of subsequent acquisition of pneumococci of that serotype [25, 26]. In our study, we were not able to match antibody concentrations to the prevalence of carriage at the individual level. However, antibody concentrations reached a plateau in the study villages at approximately 10 years of age (Ota et al, unpublished data), while the decrease in the prevalence of pneumococcal carriage [12] and the pneumococcal density continued beyond this age, suggesting that other non–serotype-specific mechanisms, such as acquisition of humoral or cellular immune responses (ie, interleukin 17 release [17]) to conserved antigens, are involved in this process [27–29]. The occurrence of the highest density of carriage in young children might explain, at least in part, why child carriers transmit pneumococcal infection more efficiently than adults, as we have shown in our study community [30] and as has been detected elsewhere [4, 31, 32]. Reduction in high-density carriage among young children by vaccination may contribute to the vaccine's herd effect.
The usefulness of measurement of the density of pneumococcal carriage in the nasopharynx as a tool for the diagnosis of pneumococcal pneumonia is of current interest [6]. Bacterial densities are increased among pneumonic individuals, compared with healthy controls [6, 11]. Our findings indicate that if the ratio of carriage density between a case of suspected pneumonia and a healthy asymptomatic carrier is to be used as a diagnostic test, the impact of age on carriage density will have to be taken into account in setting “normal” values.
We have shown previously that vaccination with PCV-7 lowered the prevalence of nasopharyngeal VT carriage in both fully vaccinated communities and in those in which only children younger than 30 months received PCV-7, suggesting that young children are the main drivers of carriage in the community as a whole [12]. The results of the current study, which showed reductions in the density of VT carriage in vaccinated and control communities (in which only part of the population was vaccinated), are in keeping with this hypothesis and in agreement with the findings from a CRT conducted in a Navajo population, in which the density of pneumococcal carriage (also measured as a semiquantitative variable) among vaccinated children was almost 40% lower than that among unvaccinated children [33]. Reduction in density is also in agreement with the hypothesis stated above, which asserted that accelerated clearance (that would lead to a shift to a lower density of carriage) rather than inhibition of colonization may be an important mechanism of protection against pneumococcal carriage acquired either through natural exposure or through vaccination [24]. If reduction in the density of carriage results in less effective transmission, as seem likely, this could be an important mechanism through which PCVs exert an indirect protective herd effect. We did not find any significant difference in carriage density between subjects from full or partially vaccinated communities, contrasting with the findings in relation to the prevalence of VT carriage, in which differences between study arms were small but statistically significant in some of the comparisons [12].
An anomalous finding was the detection of a reduction in the density of NVT carriage following vaccination. Because we do not expect any direct effect of PCV-7 vaccination on serotypes not included in the vaccine, alternative explanations are needed. One possibility is that the decrease in density of NVT was a consequence of the unmasking of low-density NVT after the removal VT as a result of vaccination. Some investigators have suggested that the observed increase of NVT nasopharyngeal carriage after vaccine introduction is an artifact of unmasking, in which the reduction in the prevalence of VT makes it easier to detect NVT present in the population that were undetected in the absence of vaccination [6, 9]. Because the commonly used serotyping methods evaluate only a limited number of colonies, investigators may fail to detect cocolonization with serotypes present at low density. In the CRT discussed above conducted in a Navajo population [33], investigators found a decrease in the prevalence and density of VT but not of NVT in the vaccinated arm. In that study, the serotyping method was more sensitive than ours, making unmasking less likely. Another possible explanation for our findings of a reduction in the density of NVT carriage after vaccination is that there were some secular trends unrelated to vaccination. However, it is difficult to envisage what changes could have occurred in the study villages that could have affected the density of carriage during the conduct of the study, because there were no significant climatic or social variations during the study period or changes in medical practice, apart from the administration of azithromycin at the time of the last CSS, an event that has been addressed in the analysis. The methods by which samples were collected, stored, and tested were identical in all CSSs, and quality controls were in place through the study. Any secular change affecting density should probably have had the same impact on the density of NVT and VT.
The analysis presented here has additional limitations. Density was measured as a semiquantitative and subjective variable, but technicians were blind as to which study group a sample belonged. Newer methods (eg, real-time polymerase chain reaction) are able to provide fully quantitative measurements and are likely to make measurement of bacterial density a more feasible end point for vaccine trials than has been the case in the past. Because of the heterogeneity of the results of the different postvaccination surveys, we averaged the results for the main analysis. In addition, analysis was oversimplified by grouping serotypes in 2 groups (VT and NVT). Density differs by serotype and serotype distribution within VT and NVT varied in the pre vs the postvaccination CSSs. However, such variation has not significantly altered the mean densities in the comparisons (data not shown).
In summary, our analysis has given insights into why children are more efficient than adults in pneumococcal transmission when they are nasopharyngeal carriers. Our time-trends analysis also shows that vaccination may lower the density of pneumococcal carriage in wholly and partially vaccinated communities. Further studies to support or disprove our findings of an effect of vaccination on NVT are needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the individuals who participated in our study. We are especially grateful to the study field team (lead by M. A. Kinteh), the laboratory team, and the data management team, as well as the Clinical Trials Support Unit. Our thanks extend also to staff at the Expanded Program on Immunization unit located in Sibanor and to Dr J. Erskine, director of the Sibanor WEC Mission Hospital, for their support during the course of the study.
Disclaimer. Wyeth Lederle Vaccines (Pfizer) did not participate in designing the study, writing the manuscript, or deciding to submit the manuscript for publication.
Financial support. This work was supported by the United Kingdom Medical Research Council. Study vaccines were donated by Wyeth Lederle Vaccines (Pfizer).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–36. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 3.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millar EV, Watt JP, Bronsdon MA, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47:989–96. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 5.Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–2. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 6.Klugman KP. Contribution of vaccines to our understanding of pneumococcal disease. Philos Trans R Soc Lond B Biol Sci. 2011;366:2790–8. doi: 10.1098/rstb.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipsitch M. Interpreting results from trials of pneumococcal conjugate vaccines: a statistical test for detecting vaccine-induced increases in carriage of nonvaccine serotypes. Am J Epidemiol. 2001;154:85–92. doi: 10.1093/aje/154.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Frazao N, Sa-Leao R, de LH. Impact of a single dose of the 7-valent pneumococcal conjugate vaccine on colonization. Vaccine. 2010;28:3445–52. doi: 10.1016/j.vaccine.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrich WC, Madhi SA, Adrian PV, et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis. 2012;54:601–9. doi: 10.1093/cid/cir859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu HT, Yoshida LM, Suzuki M, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30:11–8. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]
- 12.Roca A, Hill PC, Townend J, et al. Effects of community-wide vaccination with pcv-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med. 2011;8:e1001107. doi: 10.1371/journal.pmed.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schim van der Loeff MF, Sarge-Njie R, Ceesay S, et al. Regional differences in HIV trends in the Gambia: results from sentinel surveillance among pregnant women. AIDS. 2003;17:1841–6. doi: 10.1097/01.aids.0000076303.76477.49. [DOI] [PubMed] [Google Scholar]
- 14.Hill PC, Akisanya A, Sankareh K, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis. 2006;43:673–9. doi: 10.1086/506941. [DOI] [PubMed] [Google Scholar]
- 15.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien KL, Nohynek H. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 17.Turner P, Turner C, Kaewcharernnet N, Mon NY, Goldblatt D, Nosten F. A prospective study of urinary pneumococcal antigen detection in healthy Karen mothers with high rates of pneumococcal nasopharyngeal carriage. BMC Infect Dis. 2011;11:108. doi: 10.1186/1471-2334-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3:e168. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes RJ, Moulton LH. Cluster randomized trials. Boca Raton, FL: Champman and Hall/CRC Press; 2009. [Google Scholar]
- 20.Cheung YB, Zaman SM, Nsekpong ED, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediatr Infect Dis J. 2009;28:990–5. doi: 10.1097/INF.0b013e3181a78185. [DOI] [PubMed] [Google Scholar]
- 21.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27:59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain M, Melegaro A, Pebody RG, et al. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect. 2005;133:891–8. doi: 10.1017/S0950268805004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. 2010;10:304. doi: 10.1186/1471-2334-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldblatt D, Hussain M, Andrews N, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192:387–93. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 26.Weinberger DM, Dagan R, Givon-Lavi N, Regev-Yochay G, Malley R, Lipsitch M. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J Infect Dis. 2008;197:1511–8. doi: 10.1086/587941. [DOI] [PubMed] [Google Scholar]
- 27.Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun. 2004;72:2689–97. doi: 10.1128/IAI.72.5.2689-2697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun. 2004;72:5807–13. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Bernatoniene J, Bagrade L, et al. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur J Immunol. 2006;36:46–57. doi: 10.1002/eji.200535101. [DOI] [PubMed] [Google Scholar]
- 30.Hill PC, Townend J, Antonio M, et al. Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis. 2010;50:1468–76. doi: 10.1086/652443. [DOI] [PubMed] [Google Scholar]
- 31.Feikin DR, Klugman KP, Facklam RR, Zell ER, Schuchat A, Whitney CG. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin Infect Dis. 2005;41:481–7. doi: 10.1086/432015. [DOI] [PubMed] [Google Scholar]
- 32.Walter ND, Taylor TH, Jr., Dowell SF, Mathis S, Moore MR. Holiday spikes in pneumococcal disease among older adults. N Engl J Med. 2009;361:2584–5. doi: 10.1056/NEJMc0904844. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.