Abstract
Aims
Reliable detectors of worsening renal function (WRF) in Emergency Department (ED) patients with acute heart failure (AHF) are limited. We hypothesized that initial urinary neutrophil gelatinase-associated lipocalcin (NGAL) levels, and changes in urinary NGAL levels after initial ED AHF therapy, would be associated with WRF and adverse events.
Methods and results
Urinary NGAL upon ED presentation and 12–24 h after ED treatment was measured in a cohort of ED patients with AHF. NGAL was corrected for urinary creatinine (uCr). WRF was defined as RIFLE stages 1, 2, or 3, or a creatinine increase of ≥0.3 mg/dL. Patients were prospectively followed for 5- and 30-day adverse cardiovascular events. The 399 patients had a median age of 63 years, 50% were Caucasian, and 62% were male. Those with WRF at 72–96 h were more likely to have a higher initial NGAL value (71 vs. 32 ng NGAL/mg uCr) (P = 0.005), and a higher NGAL level at 12–24 h after ED therapy (107 vs. 25ng NGAL/mg uCr, P < 0.001). In a multivariable model, NGAL at 12–24 h remained a significant predictor of WRF (P = 0.012). Of all variables available 12–24 h after initial therapy, the only significant predictor of 30-day events was an elevated urinary NGAL level (P = 0.02).
Conclusions
Urinary NGAL levels determined 12–24 h after ED therapy are significantly associated with both WRF at 72–96 h and 30-day adverse events. This suggests that early management strategies may have an impact on subsequent WRF and outcomes. If confirmed, NGAL may have a role for guiding therapeutic decisions.
Keywords: Heart failure, NGAL, Outcomes, ED
Introduction
Treatment and disposition decision making in Emergency Department (ED) patients with acute heart failure (AHF) is problematic. AHF patients often have multiple co-morbidities that influence responses to therapy and post-treatment outcomes, complicating the decision of whether to discharge or admit. Amongst the most challenging of these co-morbidities is abnormal kidney function, which has been associated with increased risk of complications and in-hospital mortality.1–3 Moreover, patients with abnormal kidney function upon ED presentation can be challenging to treat effectively. It is often unclear whether elevated blood urea nitrogen (BUN) and creatinine are a result of intravascular volume depletion or poor kidney perfusion due to AHF. Changes in response to treatment can further complicate decision making; patients with creatinine increases of ≥0.3 mg/dL after initial therapy have an increased risk of subsequent adverse events,4 suggesting evolution of worsening renal function (WRF).
Unfortunately, early reliable markers of WRF in ED patients with AHF are lacking; an increase in BUN or creatinine that occurs 48–72 h after the renal insult may be the first indicator that WRF may have occurred. If a marker were available that provided earlier detection of WRF than BUN or creatinine, it could identify a group of patients at risk of WRF and afford physicians an opportunity to change their treatment strategies to avoid further kidney damage and reduce the risk of adverse events.
Neutrophil gelatinase-associated lipocalcin (NGAL) circulating in the serum is freely filtered by the glomerulus, then reabsorbed in the proximal tubule. When there is tubular injury, gene up-regulation leads directly to increased tubular secretion of NGAL, resulting in elevated urinary concentrations.5 Recently, NGAL has been found to be elevated after acute kidney injury (AKI) and is increased in both plasma and urine 48–72 h prior to an increase in creatinine.6,7 Thus, urinary NGAL is an indicator of tubular injury. The association of serum NGAL with renal function and adverse events has been explored in patients with chronic heart failure, and small cohorts of patients with AHF.8–12 These studies suggest an association between elevated serum NGAL levels and the development of both WRF and subsequent adverse events at 30–90 days after ED presentation. However, whether urinary NGAL is associated with WRF in patients with AHF is unknown, and whether urinary NGAL changes as a result of ED therapy are associated with WRF or adverse events has not been explored. Previous non-AHF studies suggest that urinary NGAL has a higher predictive value for WRF than serum NGAL.13
Because of its early detection in the urine, we hypothesized that baseline urinary NGAL, as well as changes in urinary NGAL after initial AHF therapy, would be associated with WRF. Based on previous data suggesting an increase in adverse events in those AHF patients with WRF, we further hypothesized that an elevated NGAL would also be associated with subsequent adverse events.
Methods
Subjects and setting
We included patients from an ongoing, prospective, observational cohort study of adults presenting to an ED with signs and symptoms consistent with AHF [the Decision Making in Acute Decompensated Heart Failure (DECIDE) study].14 The DECIDE study was designed to identify ED patients diagnosed with and treated for AHF. Its purpose was to derive a prediction rule to identify AHF patients who are at low risk for inpatient or outpatient death and serious in-hospital or out-of-hospital complications after a short (1 or 3 day) hospital stay.
Patients were screened for inclusion if they presented with dyspnoea, cough, or acute pulmonary oedema on chest radiography. Eligible patients were those that: (i) met modified Framingham criteria for AHF (Table 1); (ii) could be enrolled within 3 h of first physician contact; and (iii) received vasodilators or diuretics in the ED for treatment of AHF. The cohort study and this secondary analysis were approved by the Institutional Review Boards of each participating centre.
Table 1.
Modified Framingham criteria
| Major | Minor |
|---|---|
| History of heart failure | Extremity oedema |
| Paroxysmal nocturnal dyspnoea | Night cough |
| Pulmonary or interstitial oedema (on chest X-ray) | Dyspnoea on exertion |
| Rales | Hepatomegaly |
| Cardiomegaly | Pleural effusion |
| S3 gallop | Tachycardia (≥130 b.p.m.) |
| Jugular venous distention | |
| Positive hepatojugular reflux |
Two major or one major and two minor criteria are required to establish a preliminary diagnosis of heart failure by the Framingham criteria.
Data collection
Trained study personnel approached qualified candidates who had been identified by ED physicians during their initial encounter. They explained the purpose of the study and obtained written informed consent from patients who indicated their willingness to participate. Socio-demographic and medical history data were collected using questionnaires administered to the patients, supplemented using medical records.
Clinical data were obtained to reflect baseline (within 3 h of being seen by the treating physician), and 12–24 h and 72–96 h after ED treatment. Because this was a convenience sample and treatment or disposition decisions were left to the discretion of the treating physicians, we expected the size of the cohort to decrease at each time point as a result of patients being discharged from the hospital. Study personnel collected vital signs and urinary output, which were typically obtained every 2–4 h in the ED and on the hospital floor, and hourly in the intensive care unit (ICU). Results of laboratory studies ordered by the treating physician as part of usual clinical care were also recorded. Study-specific blood and urine samples were obtained at the three time points to complete any missing clinical laboratory testing, and blood was also banked for future exploration. Blood and urine specimens were centrifuged and frozen at –70°C within 90 min of collection. Any tests performed for study purposes were not shared with the treating physician or inpatient team. Study personnel conducted follow-up interviews at both 5 and 30 days after enrolment to document adverse events, supplemented via medical records. When there was a conflict between patient-reported events and the medical record, the medical record was used as the source for adverse events. Patients who were still in the hospital at the 5-day follow-up were visited by study personnel.
Outcomes
The primary outcomes in this study were WRF and 5- and 30-day events. Previous studies have identified two definitions of interest of WRF in patients with AHF. A creatinine increase >0.3 mg/dL during hospitalization has been associated with adverse events and fulfils the Acute Kidney Injury Network (AKIN) criteria.1,15–17 Further, the RIFLE criteria have also been used to define AKI and WRF.18 For these reasons, patients were considered to have WRF if they fulfilled one of the following two criteria: (i) creatinine increase ≥0.3 mg/dL; or (ii) RIFLE criteria stages 1, 2, or 3. RIFLE stage 1 was defined as a creatinine increase of 1.5 from baseline or glomerular filtration rate (GFR) decrease of >25% from baseline. RIFLE stage 2 was defined as a creatinine increase of 2.0 from baseline or a GFR decrease of >50% from baseline. RIFLE stage 3 was defined as a creatinine increase of 3.0 from baseline or GFR decrease of >75% from baseline. The GFR was calculated using the MDRD (modification of diet in kidney disease) formula [GFR = 186 ×creatine −1.154 ×age−0.203 ×(0.742 if female) ×(1.210 if African American)].
The 5- and 30-day outcomes were defined as a composite endpoint: unscheduled ED visits for AHF, unscheduled hospital admissions for AHF, acute coronary syndrome, percutaneous coronary intervention, coronary artery bypass procedure, intubation, defibrillation, cardiopulmonary resuscitation, and death from any cause.
Neutrophil gelatinase-associated lipocalcin and creatinine assays
Urine NGAL was measured using the Abbott ARCHITECT ci4100 system (Abbott, Abbott Park, IL, USA) according to the manufacturer's instructions. The ARCHITECT urine NGAL assay is a magnetic microparticle-based chemiluminescent immunoassay. In our study, representative imprecision for the ARCHITECT urine NGAL assay was 4.2, 2.1, and 1.6% at 20.64, 107.1, and 1219.1 ng/mL NGAL, respectively. We also corrected for urinary dilution by using the following formula: NGAL/uCr/100, where uCr was urinary creatinine. The assay for creatinine used for research purposes (Abbott iSTAT) has a correlation of >99% with the laboratory-based assays used at all of the institutions throughout the study (Olympus 640, J & S Vitros 5600).
Analysis
There were four aims in this analysis: (i) to determine whether urinary NGAL levels measured at baseline were associated with WRF at 12–24 h and at 72–96 h; (ii) to determine whether urinary NGAL levels measured 12–24 h after baseline were associated with WRF at 72–96 h; (iii) to determine whether urinary NGAL levels at either baseline or at 12–24 h were associated with subsequent 5- or 30-day AHF-related events; and (iv) to determine whether absolute or relative changes in NGAL levels were associated with WRF at 72–96 h or subsequent 5- or 30-day AHF-related events.
Patient characteristics were described using median and range for continuous variables and counts and proportions for categorical variables. The Student's t-test, the Mann–Whitney U-test, χ2, or Fisher's exact tests were used for between-group comparisons as appropriate. Spearman's rho was used for correlations. Logistic regression was used to explore the association between NGAL and outcomes. To adjust for known covariates, we constructed multivariable models that included any covariates found to demonstrate a statistically significant univariable association with the outcome.19 Odds ratios (ORs) and 95% confidence intervals (CIs) are reported. All analyses were conducted using SPSS 18.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
There were 399 subjects with a baseline urinary NGAL. There were 200 patients still hospitalized who had urinary NGAL available at 12–24 h after ED therapy. Those 399 subjects with baseline NGAL measurements had a median age of 63 years, 50% were Caucasian, and 62% were male. Baseline median systolic blood pressure was 147 mmHg (70–262 mmHg), median BUN was 22 mg/dL (4–97 mg/dL), creatinine was 1.3 mg/dL (0.4–10.4 mg/dL), and brain natriuretic peptide (BNP) was 819 pg/mL (5–5000 pg/mL). The median NGAL level was 10.3 ng NGAL/mg uCr (0.2–2526.7 ng NGAL/mg uCr). Median GFR at ED presentation was 58.9 mL/min/1.73 m2 (6.5–171.3 mL/min/1.73 m2). Initial NGAL was modestly associated with initial GFR (ρ = –0.36, P = 0.01). Furosemide was administered in the ED in 284 (71.2%) of patients, topical nitroglycerin in 133 (73.5%), and intravenous nitroglycerin in 3 (1.7%).
Neutrophil gelatinase-associated lipocalcin and worsening renal function
Association of initial neutrophil gelatinase-associated lipocalcin values with worsening renal function at 12–24 and 72–96 h
At 12–24 h after ED evaluation, 32 (9%) of the 350 patients in whom WRF was calculable developed WRF; 23/32 had a creatinine increase of 0.3 mg/dL, and 20/32 were classified as RIFLE stages 1–3. Compared with those who did not develop WRF, those with WRF at 12–24 h were more likely to be younger (58 vs. 64 years), female (59% vs. 34%), have a higher initial BNP (median 1320 vs. 792 pg/mL), and a higher initial NGAL value (median 62 vs. 27 ng NGAL/mg uCr), all P < 0.05. The median dose of initial diuretic was 70 mg (range 10–160 mg) in those with WRF and 40 mg (range 40–160 mg) in those without WRF. In a multivariable model, age (OR = 0.96, 95% CI 0.93–0.98,P = 0.005) and BNP (OR = 1.0, 95% CI 1.00–1.00, P = 0.007) were significant predictors of WRF at 12–24h, while initial NGAL was not (OR = 1.00, 95% CI 0.99–1.02, P = 0.423).
At 72–96 h after ED evaluation, 46 (24%) of 192 patients in whom WRF was calculable developed WRF; 41/46 had a creatinine increase of ≥0.3 mg/dL, and 26/46 were classified as RIFLE stages 1–3. Compared with those who did not develop WRF by 72–96 h, those with WRF were significantly more likely to be female (32.8% vs. 17.9%), have a history of renal disease (42.6% vs. 16.8%), and a higher initial median systolic blood pressure (160 mmHg vs. 141 mmHg) and NGAL value (71.2 vs. 31.6 ng NGAL/mg uCr) (all P < 0.05) (Table 2). In a multivariable model using the significant univariable predictors of AKI, a history of renal disease (OR = 2.99, 95% CI 1.39–6.43, P = 0.005) was a significant predictor of WRF at 72–96 h, while baseline NGAL was not (OR = 1.00, 95% CI 1.00–1.001, P = 0.176).
Table 2.
Baseline patient characteristics by worsening renal function at 72–96 h
| Total | No WRF (n = 146) | WRF (n = 46) | Difference | 95% CI |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 64 | 25–94 | 65 | 25–94 | 62 | 32–92 | −3 | −7.55 | 1.55 | 0.202 |
| White | 103 | 54% | 82 | 56% | 21 | 48% | −8% | −0.25 | 0.08 | 0.325 |
| Male | 123 | 65% | 101 | 69% | 22 | 50% | −19% | −0.36 | −0.03 | 0.020 |
| History of heart failure | 134 | 71% | 103 | 71% | 31 | 71% | 0% | −0.16 | 0.15 | 0.990 |
| History of renal disease | 47 | 25% | 27 | 19% | 20 | 46% | 27% | 0.11 | 0.43 | <0.001 |
| Baseline vital signs, medication, and laboratory values | ||||||||||
| Systolic blood pressure (mmHg) | 144 | 92–262 | 141 | 92–247 | 160 | 96–262 | 19.5 | 0.66 | 38.34 | 0.016 |
| Heart rate (b.p.m.) | 92 | 49–164 | 93 | 49–164 | 92 | 53–140 | −0.5 | −7.48 | 6.48 | 0.523 |
| Respiratory rate (breath/min) | 20 | 12–40 | 20 | 15–40 | 20 | 12–34 | 0 | −2.22 | 2.22 | 0.805 |
| BNP (pg/mL) | 961 | 9–5000 | 892 | 86–4904 | 1159 | 9–5000 | 267 | −225.00 | 758.00 | 0.153 |
| Sodium (mmol/L) | 139 | 100–148 | 140 | 100–146 | 139 | 119–148 | −1 | −1.20 | 0.20 | 0.922 |
| BUN (mg/dL) | 23 | 5–97 | 23 | 5–97 | 23 | 6–90 | 0 | −10.55 | 10.55 | 0.806 |
| Tropinin (ng/mL) | 0 | 0.0–40.1 | 0 | 0.0–40.1 | 0 | 0.0–0.2 | 0 | −0.01 | 0.01 | 0.592 |
| Parenteral lasix dose (mg) | 40 | 10–160 | 40 | 10–160 | 40 | 20–100 | 0 | −20.96 | 20.96 | 0.824 |
| NGAL (ng NGAL/mg uCr) | 39 | 1–5041 | 32 | 2–4873 | 71 | 1–5041 | 40 | 47.50 | 126.70 | 0.005 |
| NGAL absolute change | 0 | −4520 to 5655 | 0 | −4520 to 541 | −10 | −1620 to 5655 | −10 | −19.90 | 39.13 | 0.476 |
| NGAL relative change | 2% | −5.3 | 0% | −5.3 | 13% | −2.6 | −13% | −25.22 | 50.22 | 0.834 |
| Five-day event | 11 | 6% | 8 | 6% | 3 | 7% | 2% | −0.07 | 0.10 | 0.712 |
| Thirty-day event | 40 | 21% | 30 | 21% | 10 | 23% | 2% | −0.12 | 0.16 | 0.835 |
BNP, b-type natriuretic peptide; BUN, blood urea nitrogen; NGAL, neutrophil gelatinase-associated lipocalcin; uCr, urinary creatinine; WRF, worsening renal function.
12–24 h neutrophil gelatinase-associated lipocalcin to predict worsening renal function at 72–96 h
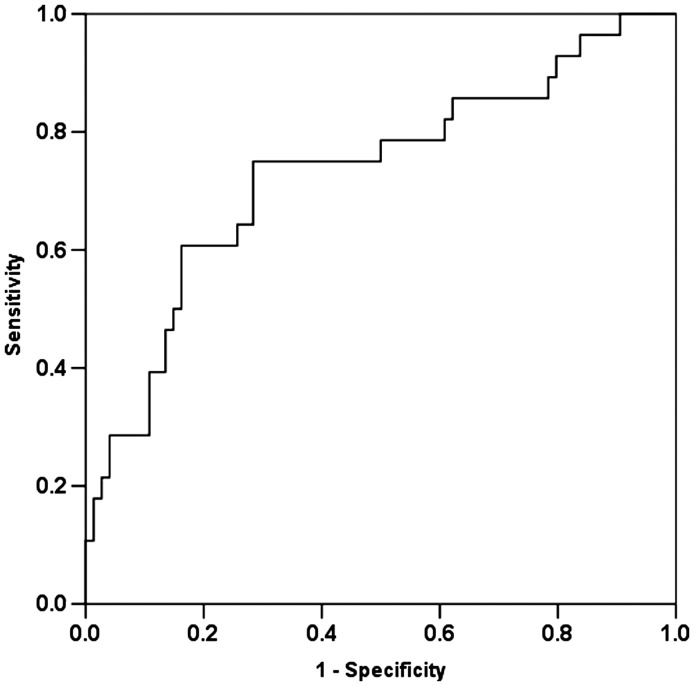
A subset of 102 patients had urine available for NGAL measurement at 12–24 h after ED therapy and had WRF status determined at 72–96 h. Twenty-eight of these patients developed WRF; 23/28 had a creatinine increase ≥0.3 mg/dL, and 14/28 were categorized as RIFLE stages 1–3. Compared with those who did not develop WRF by 72–96h, those patients who developed WRF were more likely to have a history of renal disease (50% vs. 20%), and exhibited both higher creatinine (median 2.3 vs. 1.3 mg/dL) and NGAL values at 12–24 h (median 107 vs. 25 ng NGAL/mg uCr), all P < 0.05) (Table 3 and Figure 1). In a multivariable model including 12–24 h creatinine and 12–24 h NGAL, NGAL remained a significant predictor of WRF (OR = 1.02, 95% CI 1.01–1.04, P = 0.012).
Table 3.
The 12–24 h patient characteristics by worsening renal function at 72–96 h
| Total | No WRF (n = 74) | WRF (n = 28) | Difference | 95% CI |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 62 | 30–90 | 62 | 30–90 | 63 | 32–84 | 1 | −3.90 | 4.90 | 0.828 |
| White | 53 | 52% | 42 | 57% | 11 | 39% | −18% | −0.39 | 0.04 | 0.115 |
| Male | 67 | 66% | 51 | 69% | 16 | 57% | −12% | −0.33 | 0.09 | 0.264 |
| History of heart failure | 75 | 74% | 55 | 74% | 20 | 71% | −3% | −0.22 | 0.17 | 0.804 |
| History of renal disease | 29 | 28% | 15 | 20% | 14 | 50% | 30% | 0.09 | 0.50 | 0.003 |
| 12–24 h vital signs, medication, and laboratory values | ||||||||||
| Systolic blood pressure (mmHg) | 124 | 81–227 | 124 | 81–227 | 124 | 92–184 | 1 | 18.87 | −17.87 | 0.402 |
| Heart rate (b.p.m.) | 83 | 47–130 | 83 | 47–130 | 81 | 58–128 | −2 | −12.61 | 8.61 | 0.832 |
| Respiratory rate (breaths/min) | 19 | 12–32 | 19 | 12–32 | 20 | 16–29 | 1 | −0.32 | 2.32 | 0.466 |
| BNP (pg/mL) | 1215 | 78–6730 | 1119 | 78–6730 | 1505 | 129–6730 | 387 | −572.94 | 1345.94 | 0.191 |
| Sodium (mmol/L) | 140 | 131–148 | 140 | 131–148 | 139 | 134–144 | −1 | −2.35 | 0.35 | 0.113 |
| BUN (mg/dL) | 22 | 71–85 | 21 | 9–78 | 26 | 7–85 | 5 | −9.53 | 19.53 | 0.280 |
| Tropinin (ng/mL) | 0 | 0.0–8.9 | 0 | 0.0–8.9 | 0.1 | 0.0–3.0 | 0 | −0.02 | 0.04 | 0.481 |
| Creatinine (mg/dL) | 1 | 0.4–93 | 1.27 | 0.4–93 | 2 | 0.7–11 | 1 | 0.14 | 1.82 | 0.016 |
| NGAL (ng NGAL/mg uCr ) | 30 | 3–10 697 | 25 | 3–12 166 | 107 | 8–10 697 | 82 | −55.60 | 220.10 | <0.001 |
| Five-day event | 7 | 7% | 4 | 5% | 3 | 12% | 6% | −0.07 | 0.20 | 0.372 |
| Thirty-day event | 25 | 25% | 16 | 22% | 9 | 32% | 10% | −0.10 | 0.30 | 0.315 |
BNP, b-type natriuretic peptide; BUN, blood urea nitrogen; NGAL, neutrophil gelatinase-associated lipocalcin; uCr, urinary creatinine; WRF, worsening renal function.
Figure 1.
Receiver operating characteristic curve for 12–24 h neutrophil gelatinase-associated lipocalcin and acute kidney injury at 72–96 h (area under the receiver operating characteristic curve 0.717, 95% confidence interval 0.601–0.833).
Neutrophil gelatinase-associated lipocalcin and adverse events
Initial neutrophil gelatinase-associated lipocalcin values and their association with 5- and 30-day adverse events
Of the 399 patients enrolled, 396 (99%) were contacted for follow-up at 5 days, and 393 (98%) were contacted at 30 days. There were 23 (6%) subjects who had 5-day adverse events and 83 (21%) who had 30-day events. Those patients with a 5-day adverse event had a higher median initial respiratory rate (24 vs. 20 breaths/min) (P = 0.01). Patients with 5-day events had initial NGAL levels that were similar to those of patients without events (median 43.7 vs. 28.0 ng NGAL/mg uCr) (P = 0.31). The proportion of patients with WRF at 12–24 h who experienced adverse events was similar to the proportion of patients with WRF at 12–24 h who did not experience adverse events (10% vs. 9%, P = 0.71).
Patients with 30-day events had initial NGAL levels that were similar to those of patients without events (36.7 vs. 27.3 ng NGAL/mg uCr) (P = 0.123). The proportion of patients with WRF at 12–24 h who experienced 30-day adverse events was similar to the proportion of patients without WRF at 12–24 h who did not experience 30-day adverse events (10% vs. 7%, P = 0.47).
12–24 h neutrophil gelatinase-associated lipocalcin values and their association with 5- and 30-day adverse events
Of the patients with NGAL measured at 12–24 h, 13/198 (6.6%) had a 5-day event and 48/197 (24.4%) had a 30-day event. Compared with patients without 5-day events, those with 5-day events had a significantly higher median serum sodium at 12–24 h(143 vs. 140 mEq/L, P = 0.05) and a trend toward a significantly higher 12–24 h median NGAL level (46 vs. 26 ng NGAL/mg uCr, respectively, P = 0.06). In a multivariable model, neither NGAL nor serum sodium remained significant predictors of 5-day events (P > 0.05). The only significant predictor of 30-day events was an elevated 12–24 h NGAL level (median 42 vs. 25 ng NGAL/mg uCr, respectively, P = 0.04) (Table 4).
Table 4.
The 12–24 h patient characteristics by 30 day event
| Total | No 30-day event (n = 149) | 30-day event (n = 48) | Difference | 95% CI |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 64 | 30–90 | 63 | 31–89 | 64 | 30–90 | 1 | −2.92 | 4.92 | 0.636 |
| White | 104 | 53% | 78 | 52% | 26 | 54% | 2% | −0.14 | 0.18 | 0.826 |
| Male | 131 | 66% | 98 | 66% | 33 | 69% | 3% | −0.12 | 0.18 | 0.704 |
| History of heart failure | 150 | 76% | 115 | 77% | 35 | 73% | −4% | −0.19 | 0.10 | 0.547 |
| History of renal disease | 52 | 26% | 40 | 27% | 12 | 25% | −2% | −0.16 | 0.12 | 0.801 |
| 12–24 h vital signs, medication, and laboratory values | ||||||||||
| Systolic blood pressure (mmHg) | 125 | 81–227 | 127 | 81–227 | 125 | 84–184 | −4 | −12.40 | 4.40 | 0.796 |
| Heart rate (b.p.m.) | 78 | 11–130 | 78 | 47–130 | 78 | 11–123 | 1 | −2.92 | 4.92 | 0.920 |
| Respiratory rate (breaths/min) | 18 | 12–32 | 18 | 12–32 | 18 | 16–30 | −1 | −6.93 | 5.93 | 0.689 |
| BNP (pg/mL) | 976 | 30–6730 | 976 | 30–5001 | 998 | 195–6730 | 22 | −567.92 | 610.92 | 0.383 |
| Sodium (mmol/L) | 140 | 125–148 | 140 | 131–148 | 140 | 125–147 | 0 | −1.51 | 1.51 | 0.064 |
| BUN (mg/dL) | 21 | 6–96 | 21 | 6–90 | 22 | 71–96 | 1 | −5.61 | 6.61 | 0.681 |
| Tropinin (ng/mL) | 0 | 0.0–8.9 | 0.03 | 0.0–8.9 | 0.03 | 0.0–4.9 | 0 | −0.03 | 0.03 | 0.325 |
| Creatinine (mg/dL) | 1 | 0.4–93 | 1 | 1–93 | 2 | 0.4–11 | 0 | −0.33 | 0.69 | 0.330 |
| NGAL (ng NGAL/mg uCr) | 28 | 36–10 697 | 25.1 | 3–1658 | 42 | 4–10 697 | 17 | 1.62 | 32.70 | 0.035 |
| WRF | 28 | 28% | 19 | 25% | 9 | 36% | 11% | −0.11 | 0.32 | 0.315 |
BNP, b-type natriuretic peptide; BUN, blood urea nitrogen; NGAL, neutrophil gelatinase-associated lipocalcin; uCr, urinary creatinine; WRF, worsening renal function.
Absolute and relative changes in neutrophil gelatinase-associated lipocalcin and the association with worsening renal function and adverse events
Of the 200 patients who had serial NGAL measurements obtained, 97 (48.5%) had values that increased from their baseline value. The median absolute change was –0.3 ng NGAL/mg uCr and the median relative change was –1.1%. Neither the absolute nor the relative changes in NGAL were significantly associated with WRF at 72–96 h or 5- and 30-day events.
Discussion
Our study, the first to evaluate urinary NGAL levels obtained before and after ED therapy for AHF, and the largest overall NGAL investigation in AHF, suggests that NGAL obtained 12–24 h after initial therapy was not only significantly associated with WRF at 72–96 h, but also was the sole predictor of 30-day adverse events. This finding, not previously reported in a cohort of ED patients with AHF, suggests that initial response to therapy may be as important as patient characteristics at ED presentation in determining risk for WRF and subsequent adverse events. Our results align with previous work indicating that WRF is associated with adverse events10,20 and suggest that NGAL is an upstream marker that could identify this high-risk cohort prior to WRF development. If confirmed in a prospective analysis, our findings could impact how ED and hospitalized patients with AHF are risk stratified and how subsequent therapeutic and disposition decisions are made.
NGAL levels rise quickly after acute ischaemic or nephrotoxic injury.6,7,21 NGAL has been found to be elevated in the plasma and urine in adults and children who develop WRF following cardiopulmonary bypass surgery.22,23 Further, urinary NGAL at 2 h after bypass surgery had better test characteristics for prediction of WRF than serum NGAL, and in a multivariable analysis was the most powerful independent predictor of AKI. It has also been elevated in plasma and urine in adult patients in intensive care who develop WRF from a number of conditions including sepsis and ischaemia.24 NGAL has also been associated with WRF in patients with chronic heart failure.8,9 Previous studies have evaluated the diagnostic and prognostic utility of serum and plasma NGAL in ED patients with AHF, and have found results similar to ours. In a cohort of 194 patients, initial plasma NGAL levels were significantly different in those with and without 30-day events.10 Further, hospital discharge NGAL values were significantly higher in those patients with 30-day events. In a smaller study of 91 patients with AHF, serum NGAL was also associated with WRF at 5 days.11
These results have a pattern consistent with our findings. However, there are several features of our study which distinguish it from the above: (i) we measured urinary NGAL, rather than plasma or serum NGAL; (ii) we measured NGAL values at the time of ED presentation in 399 patients and after ED therapy in 200 patients; and (iii) our results suggest that NGAL values 12–24 h after initial therapy are useful for looking at both WRF and adverse events. Taken in total, our results, combined with others, suggest that urinary NGAL, especially 12–24 h after AHF therapy, may be a better predictor of WRF and adverse events than serum NGAL and other readily available laboratory data 12–24 h after therapy.
If our findings can be prospectively confirmed, the next step would be to determine whether urinary NGAL levels, especially those 12–24 h after initial therapy, could direct a therapeutic strategy that would reduce the risk of developing WRF. While renal protective agents have been universally disappointing, strategies aimed at minimizing ongoing injury, rather than attempting to protect the kidney while therapeutic insult continues, may result in improved outcomes.25,26 One alternative would be to consider changing treatment approaches, such as minimizing diuretic use and utilizing intravenous vasodilators earlier in the course of treatment. Another alternative would be exploring substitutes for traditional therapy. Treatment approaches utilizing ultrafiltration and vasopressin or aldosterone antagonists would be a potential future aim in this pathway of research. Changing a patient's early (WRF) and intermediate (5- and 30-day events) trajectory by implementing tailored therapy in the first 12–24 h of care would be a paradigm shift to the current approach to AHF therapy. This therapeutic approach has remained essentially unchanged over the last two decades, consisting largely of loop diuretics, often as the sole therapy.27,28 To date, none of the novel AHF therapeutic agents which have been studied has been able to improve both symptoms and outcomes.29–32 Exploring the earlier use of alternative therapeutics in selected patients with early predictors of renal dysfunction, prior to the development of WRF, may prove beneficial.
A marker such as urinary NGAL, which is associated with subsequent development of WRF, also has the potential to improve risk stratification. NGAL offers a unique opportunity to identify those at risk of subsequent WRF and adverse events, prior to developing WRF as defined by traditional markers of renal injury. This could have an impact in terms of targeting at-risk patients for more intensive monitoring and tailoring therapy to improve outcomes. Finally, incorporating NGAL into predictive instruments could also identify a cohort at low risk (i.e. normal NGAL levels) of subsequent adverse events, thereby potentially improving utilization of resources.
Limitations
While our findings suggest that urinary NGAL levels measured 12–24 h after initiating therapy are associated with both subsequent development of WRF and adverse outcomes, there are some noteworthy limitations. First, the number of patients with WRF at 12–24 h was limited. This is not surprising considering that WRF may not develop until 48–72 h after initial injury. The small number of patients also limits the number of predictors that can be analysed in a multivariable model. Secondly, not all subjects had blood and urine available at each time point. Many of the patients were already discharged from the ED or hospital prior to the time point of data collection. Given our close follow-up and a lack of adverse events in this group, it is unlikely they developed clinically significant WRF. Thirdly, our definition of WRF was purposely broad. The RIFLE criteria use relatively large changes in GFR and creatinine to define WRF. However, based on previous data suggesting that clinically important WRF may occur at increases in serum creatinine as low as 0.3 mg/dL, and the AKIN definition, we also included subjects who met criteria for WRF by this definition. Importantly, previous research from a select cohort of patients suggests that while WRF may be the final common pathway of several distinct pathophysiological processes, their clinical impact may be dramatically different.33
Previous studies suggest that urinary or serum NGAL values are most accurate for predicting WRF in a homogeneous cohort of patients who have an acute and readily identifiable nephrotoxic insult, such as cardiopulmonary bypass or contrast administration. Patients with AHF are heterogeneous and may have chronic, ongoing nephrotoxic insults, resulting in NGAL being a less accurate predictor of WRF. While diuretic use has been associated with subsequent WRF, diuretic dose or initiation of diuretic therapy has not been correlated with NGAL levels. This suggests that a select group of patients who respond adversely to aggressive diuretic administration may be able to be identified by NGAL elevation prior to development of subsequent WRF.34,35
While the ED diagnosis of AHF may not always agree with that obtained by a cardiology panel review of the medical record, previous data suggest that the rate of disagreement is ≤15%.28,36 Our aim was to evaluate NGAL in a cohort of patients felt to have AHF by the ED physician, and not those felt to have AHF at hospital discharge. Because this was an observational study, diuretic dosing was not controlled and not equal across patients. Therapy was left to the discretion of the treating clinician, which may confound the results (i.e. those patients who received a higher dose of diuretic may have required this amount in order to achieve a clinical response). Moreover, transient changes in renal function during hospitalization may not be as important as permanent changes post-hospitalization.4 We did not follow patient's renal function after hospitalization to determine whether these changes were transient or permanent. Finally, those patients still hospitalized at day 5 may have been protected from some of the adverse events, especially early ED and hospital readmission.
Conclusion
Our results suggest that urinary NGAL levels determined 12–24 h after ED therapy for AHF are significantly associated with both WRF at 72–96 h and 30-day adverse events. This important finding suggests that early management strategies may have a significant impact on subsequent WRF and outcomes. Future studies should consider a role for NGAL in guiding therapeutic or disposition decisions that could alter the course of WRF and improve outcomes.
Funding
Abbott Point of Care, the National Heart, Lung and Blood Institute (grant K23HL085387); an institutional Clinical and Translational Science Award NIH/NCRR (grant no. 1UL1RR026314-01). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of interest: S.P.C, prior and current grant/research support from Biosignetics, Inovise Medical Inc., Abbott Point-of-Care, NIH/NHLBI, Corthera, and BRAHMS; prior and current consulting for Abbot Point-of-Care, PDL BioPharma, Astellas, Otsuka Pharmaceuticals, Bayer, Trevena, Novartis, The Medicines Company, and Corthera. K.H., research support from Abbott POC. C.J.L., K.M., S.R., and M.S. research support from Abbott POC. G.J.F., grant support from Dyax, Corthera, and The Medicines Company; Advisory Board for Quest Diagnostics/Hemocue and Daiichi Sankyo. N.W. and D.S. have no conflicts of interest to declare. A.B.S., current grant support from Abbott Diagnostics, NIH/NHLBI (R01HL088459-02), NIH/NHLBI (K23HL085387-01A2), NIH/NHLBI (K12HL1090-01), Centers for Disease Control, Corthera, Roche Diagnostics, Abbott Diagnostics, and Thermo Fisher; current consultant for Roche Diagnostics.
Acknowledgements
The authors would like to acknowledge Dr Eoin Cotter and his colleagues at University College Dublin, Clinical Research Centre, Dublin, Ireland for their assistance performing NGAL testing.
References
- 1.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, O'Connor CM, Rich MW, Stevenson LW, Young J, Krumholz HM. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 3.Collins SP, Lindsell CJ, Naftilan AJ, Peacock WF, Diercks D, Hiestand B, Maisel A, Storrow AB. Low-risk acute heart failure patients: external validation of the Society of Chest Pain Center's recommendations. Crit Pathw Cardiol. 2009;8:99–103. doi: 10.1097/HPC.0b013e3181b5a534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–547. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury—where do we stand today? Nephrol Dial Transplant. 2011;26:762–764. doi: 10.1093/ndt/gfr006. [DOI] [PubMed] [Google Scholar]
- 6.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 8.Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10:997–1000. doi: 10.1016/j.ejheart.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, Clopton P, van Veldhuisen DJ. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–851. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail. 2010;16:49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvelos M, Lourenco P, Dias C, Amorim M, Rema J, Leite AB, Guimarães JT, Almeida P, Bettencourt P. Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.07.080. in press. [DOI] [PubMed] [Google Scholar]
- 13.Shemin D, Dworkin LD. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for early acute kidney injury. Crit Care Clin. 2011;27:379–389. doi: 10.1016/j.ccc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Storrow AB, Collins S, Lindsell CJ, Disalvo T, Han J, Weintraub NL. Improving Heart Failure Risk Stratification in the ED: Stratify 1R01HL088459-01; Treatment Endpoints in Acute Decompensated Heart Failure 1K23HL085387-01A2. 2007 Vanderbilt University and University of Cincinnati: NHLBI. [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair JE, Khan S, Konstam MA, Swedberg K, Zannad F, Burnett JC, Jr, Grinfeld L, Maggioni AP, Udelson JE, Zimmer CA, Ouyang J, Chen CF, Gheorghiade M EVEREST Investigators. Weight changes after hospitalization for worsening heart failure and subsequent re-hospitalization and mortality in the EVEREST trial. Eur Heart J. 2009;30:1666–1673. doi: 10.1093/eurheartj/ehp144. [DOI] [PubMed] [Google Scholar]
- 17.Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G, Teerlink JR, Greenberg BH, Filippatos G, Teichman SL, Metra M Pre-RELAX-AHF study group. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre-RELAX-AHF. Eur J Heart Fail. 2011;13:961–967. doi: 10.1093/eurjhf/hfr060. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 20.Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 23.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagshaw SM. Acute kidney injury: diagnosis and classification of AKI: AKIN or RIFLE? Nat Rev Nephrol. 2010;6:71–73. doi: 10.1038/nrneph.2009.225. [DOI] [PubMed] [Google Scholar]
- 25.Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, Cotter G, Weatherley BD, Ponikowski P, Teerlink JR, Cleland JG, O'Connor CM, Givertz MM. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) J Amn Coll Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Pang PS, Mehra M, Maggioni AP, Filippatos G, Middlebrooks J, Turlapaty P, Kazei D, Gheorghiade M RENO-DEFEND Investigators. Rationale, design, and results from RENO-DEFEND 1: a randomized, dose-finding study of the selective A1 adenosine antagonist SLV320 in patients hospitalized with acute heart failure. Am Heart J. 2011;161 doi: 10.1016/j.ahj.2011.03.004. 1012–1023 e3. [DOI] [PubMed] [Google Scholar]
- 27.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Collins SP, Peacock WF, Lindsell CJ, Clopton P, Diercks DB, Hiestand B, Hogan C, Kontos MC, Mueller C, Nowak R, Chen WJ, Huang CH, Abraham WT, Amsterdam E, Breidthardt T, Daniels L, Hasan A, Hudson M, McCord J, Naz T, Wagoner LE, Maisel A. S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Ann Emerg Med. 2009;53:748–757. doi: 10.1016/j.annemergmed.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 29.VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–40. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 30.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghiade M, Gattis WA, O'Connor CM, Adams KF, Jr, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 32.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M SURVIVE Investigators. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 33.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13:877–884. doi: 10.1093/eurjhf/hfr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, von Bary C, Endemann D, Banas B, Mack M, Böger CA, Riegger G, Luchner A. Kidney injury molecule-1 and N-acetyl-β-d-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail. 2011;13:1104–1110. doi: 10.1093/eurjhf/hfr102. [DOI] [PubMed] [Google Scholar]
- 35.Damman K, Ng Kam Chuen MJ, MacFadyen RJ, Lip GY, Gaze D, Collinson PO, Hillege HL, van Oeveren W, Voors AA, van Veldhuisen DJ. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57:2233–2241. doi: 10.1016/j.jacc.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 36.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC McCullough PA; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]