Abstract
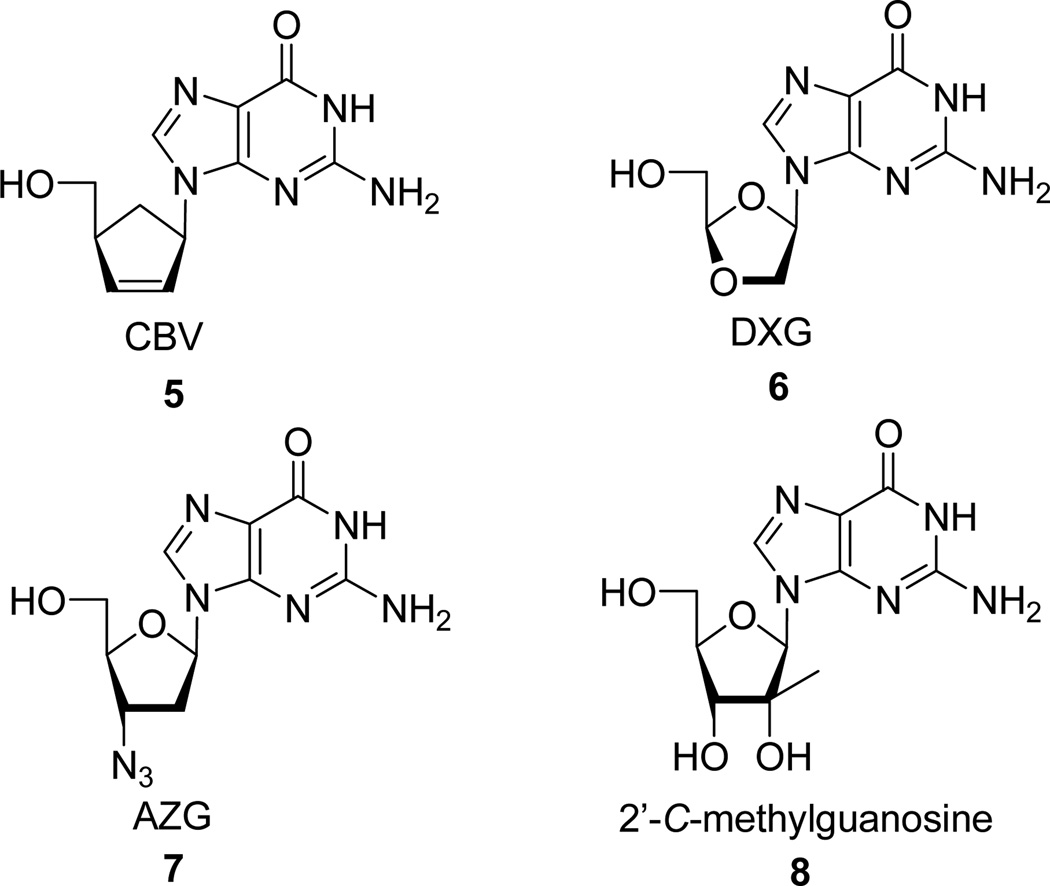
A series of 3,9-dihydro-9-oxo-5H-imidazo[1,2-A]purine nucleosides (tricylic nucleosides) were synthesized from 9-[4-α-(hydroxymethyl)cyclopent-2-ene-1-α-yl]guanine (CBV) 5, (−)-β-D-(2R,4R)-1,3-dioxolane-guanosine (DXG) 6, 3’-azido-3’-deoxy-guanosine (AZG) 7, and 2’-C-methylguanosine 8. Their in vitro activity against HIV and HCV were evaluated and correlated to their ability to degrade to their purine counterpart.
Keywords: Nucleosides, tricyclic, stability
1. Introduction
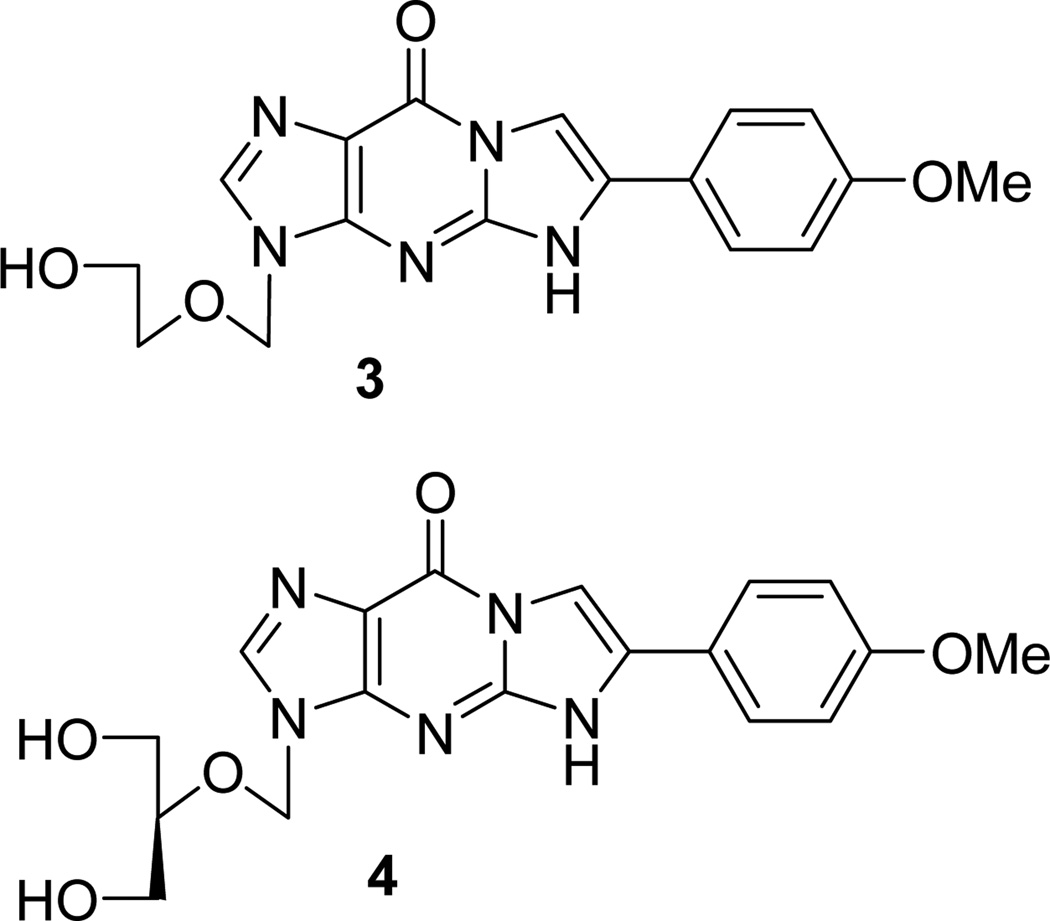
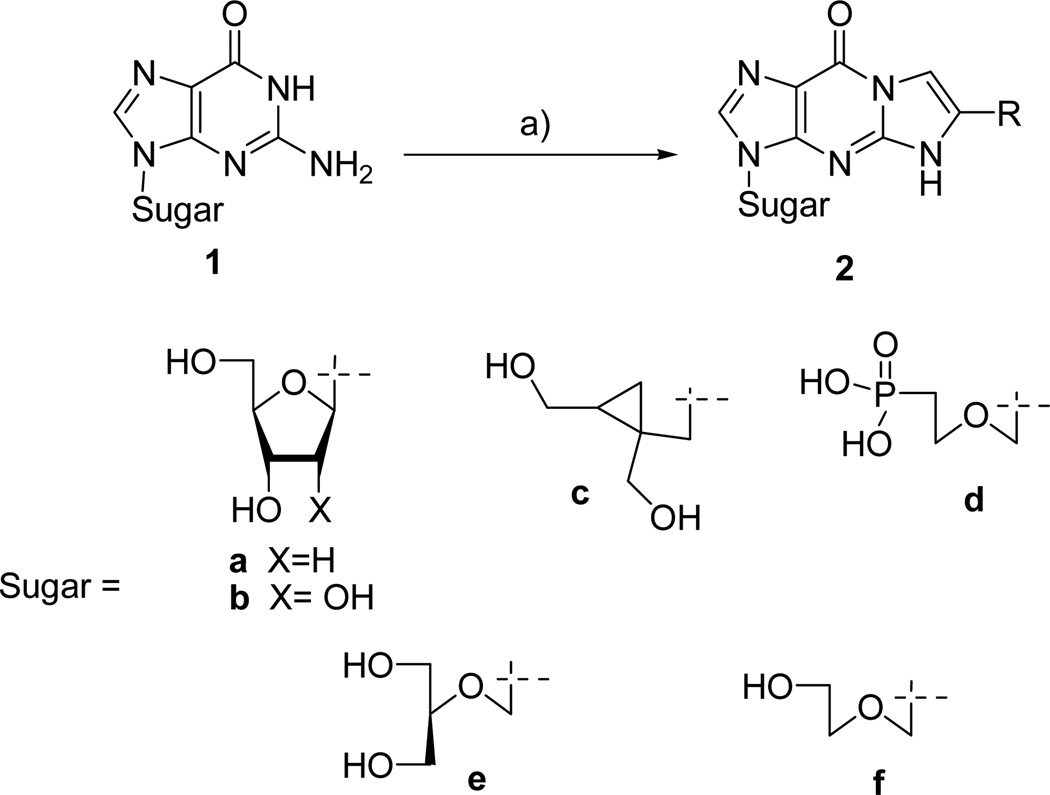
The alkylation-condensation of guanosine nucleosides 1 with phenylacyl bromide is known to produce the corresponding 3,9-dihydro-9-oxo-5H-imidazo[1,2-A]purine nucleoside 2 (or simply called tricyclic nucleoside). This reaction has been extensively studied using various substrates such as 2’-deoxyguanosine 1a [1] and guanosine 1b, the cyclopropyl-guanosine 1c, [2] 9-(2-phosphonyl–methoxyethyl)guanine (PMEG) 1d [3] and also the FDA approved ganciclovir (GCV) 1e and acyclovir (ACV) 1f. [4–13] The corresponding highly conjugated compounds 2 show some fluorescent properties and, surprisingly in some cases, exhibited potent and selective antiviral activity. Over the years, various studies have established the antiviral influence of the substitution on the tricylic moiety. The data obtained from more than 70 ACV and GCV analogs, indicated that compounds 3 and 4 were as active as their parent congeners against herpes simplex virus (HSV). [14
Because of these unexpected results for such highly modified nucleoside analogs, we investigated other guanosine derivatives that target viruses of major public health concern such as HIV and hepatitis C virus (HCV). HCV has infected an estimated 170 million individuals worldwide and is a major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma. Currently the only approved therapy for chronic HCV infection is pegylated interferon-α in combination with ribavirin, a modality often poorly tolerated and effective in only half of the genotype 1 population. [15–16] On the other hand an estimated 33 million people worldwide live with HIV. In 2007, it is estimated HIV killed an estimated 2.1 million people, including 330,000 children. Although treatments for HIV can slow the course of the disease, there is currently no vaccine or cure. For both HIV and HCV there is significant need for new and more effective therapies. Based on the literature [17–18] and our own antiviral expertise, we investigated the influence of this tricyclic modification on 9-[4-α-(hydroxymethyl)–cyclopent-2-ene-1-α-yl]guanine (CBV) 5, (−)-β-d-(2R,4R)-1,3-dioxolane-guanosine (DXG) 6, 3’-azido-3’-deoxyguanosine (AZG) 7, and 2’-C-methylguanosine 8 all well known for their anti-HIV [19] or anti-HCV activity. [20–23]
2. Results and discussion
2.1. Chemistry
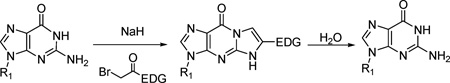
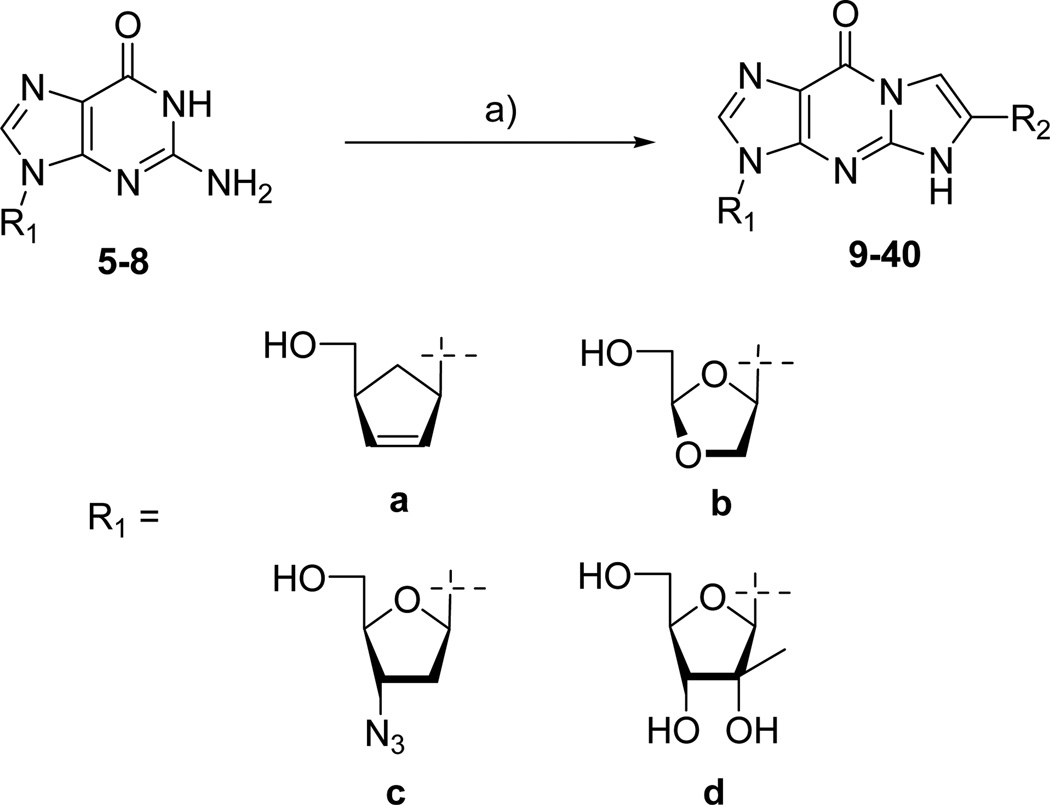
CBV 5, [24] DXG 6, AZG 7, [25] and 2’-C-methylguanosine 8, [26] were synthesized according to literature procedures. The corresponding tricyclic derivatives were prepared by reacting nucleoside analogs 5–8 with an appropriate bromoketone in the presence of sodium hydride following the previously reported method for this alkylation condensation reaction (Scheme 2). All the synthesized tricyclic nucleoside analogs, 9–40, have been characterized by 1H-NMR while their purity and nominal mass were checked by LC/MS before biological evaluation.
Scheme 2.
a) NaH, BrCH2(CO)R, DMF, rt, 10–50%
2.2. Antiviral evaluation and discussion
From our SAR effort (Table 1), several observations can be made. First, the antiviral selectivity seemed to be related to the structure of the sugar moiety. Indeed, the tricyclic compounds 36–39 with a 2’-C-methylribo sugar showed selective anti-HCV activity, like its parent nucleoside 8. On the other hand, compounds 9–34 synthesized from HIV active compound CBV 5, DXG 6 and AZG 7 showed selective anti-HIV activity. The second important point is that antiviral activities correlate with the electron richness of the annulated aryl group of the tricyclic derivative. Thus, compounds bearing an electron donor group such as 4-MeO-Ph (36), 4-NEt2-Ph (37), 4-NMe2-Ph (38), 2-thiophenyl (39) showed EC50 values against HCV below 10 µM. In the same manner, tricyclic compounds obtained from CBV, DXG, and AZG with a 2-thiophenyl (14, 27), 4-NEt2-Ph (12, 25, 33), 4-NMe2-Ph (13, 26, 34), 4-MeO-Ph (9, 18, 32) group possessed anti-HIV activity similar to their corresponding parent nucleoside analogues. However, other substitutions such as ethyl (16, 35), 4-CN-Ph (30) or 4-Cl-Ph (22) showed less or no activity in all cases. The influence of the electronic properties of the substitution observed in our case seems to be in accordance with the literature since electron withdrawing substituents such as 4-NO2-Ph, naphtalenyl, 3-MeO-Ph or 4-CF3-Ph are not active in the ACV/GCV series [14]. Finally, it is noteworthy that the most potent compounds in each series demonstrated a level of potency similar to the parent nucleoside.
Table 1.
In vitro anti-HCV activity, anti HIV activity and cellular toxicity of nucleoside analogs 9–40.
| Compound | R1 | R2 | Anti-HIV activity in human PBM cells (µM) |
Anti-HCV activity Huh-7 cells (µM) |
Cytotoxicity (CC50 in µM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 | EC90 | EC50 | EC90 | PBM | CEM | VERO | |||
| CBV, 5 | 0.043 | 0.29 | > 10 | > 10 | 7.0 | > 100 | > 100 | ||
| 9 | a | 4-MeO-Ph | 0.62 | 4.9 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 10 | a | 4-Me-Ph | 5.0 | 30.1 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 11 | a | 4-Br-Ph | 12.3 | 43.2 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 12 | a | 4-NEt2-Ph | 0.13 | 0.65 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 13 | a | 4-NMe2-Ph | 0.11 | 0.47 | > 10 | > 10 | 24.0 | > 100 | > 100 |
| 14 | a | 2-thiophenyl | 0.64 | 7.1 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 15 | a | 3-thiophenyl | 2.6 | 15.5 | > 10 | > 10 | > 100 | > 100 | > 100 |
| DXG, 6 | 0.51 | 2.4 | > 10 | > 10 | > 100 | > 100 | > 100 | ||
| 16 | b | Et | 31.5 | > 100 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 17 | b | Ph | 7.1 | 43.5 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 18 | b | 4-MeO-Ph | 3.45 | 23.9 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 19 | b | 3-MeO-Ph | 20.7 | 89.6 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 20 | b | 2-MeO-Ph | 8.5 | 26.8 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 21 | b | 4-Me-Ph | 4.9 | 23.9 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 22 | b | 4-Cl-Ph | > 100 | > 100 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 23 | b | 4-F-Ph | 43.5 | >100 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 24 | b | 2,4-F-Ph | 14.7 | 51.8 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 25 | b | 4-NEt2-Ph | 0.25 | 1.25 | > 10 | > 10 | > 100 | 80.8 | 63.0 |
| 26 | b | 4-NMe2-Ph | 0.61 | 1.5 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 27 | b | 2-thiophenyl | 3.0 | 16 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 28 | b | 3-thiophenyl | 0.91 | 6.6 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 29 | b | 4-N3-Ph | 2.6 | 11.4 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 30 | b | 4-CN-Ph | 69.3 | > 100 | > 10 | > 10 | > 100 | > 100 | > 100 |
| AZG, 7 | 0.18 | 1.98 | > 10 | > 10 | > 100 | > 100 | > 100 | ||
| 31 | c | Ph | 8.4 | 94.1 | > 10 | > 10 | 69.6 | 21.8 | > 100 |
| 32 | c | 4-MeO-Ph | 1.3 | 7.0 | > 10 | > 10 | 56.7 | 13.5 | > 100 |
| 33 | c | 4-NEt2-Ph | 0.04 | 0.34 | > 10 | > 10 | > 100 | 14.1 | 72.8 |
| 34 | c | 4-NMe2-Ph | 0.14 | 0.54 | > 10 | > 10 | > 100 | 74.6 | > 100 |
| 2’-C-MeG, 8 | > 100 | > 100 | 2.3 | 7.8 | > 100 | > 100 | > 100 | ||
| 35 | d | Et | > 100 | > 100 | > 10 | > 10 | > 100 | > 100 | > 100 |
| 36 | d | 4-MeO-Ph | > 100 | > 100 | ≥ 10 | > 10 | > 100 | > 100 | > 100 |
| 37 | d | 4-NEt2-Ph | > 100 | > 100 | ≤ 10 | nd | > 100 | > 100 | > 100 |
| 38 | d | 4-NMe2-Ph | > 100 | > 100 | 4.4 | 9.5 | > 100 | > 100 | > 100 |
| 39 | d | 2-thiophenyl | > 100 | > 100 | 6.9 | 25.6 | > 100 | > 100 | 44.3 |
| 40 | d | 3-thiophenyl | > 100 | > 100 | ≥ 10 | > 10 | > 100 | > 100 | > 100 |
nd: not determined
The similarities of antiviral profiles between parent nucleosides and corresponding tricyclic nucleoside, also observed with ACV and GCV derivatives, lead us to question the stability of the electron rich tricycles in cells or in solution. Indeed, several groups have suggested that this kind of tricycle might serve as a prodrug of the parent nucleoside [5] however; no data has ever been generated to support this hypothesis.
Since nucleosides are prodrugs that require conversion to the corresponding triphosphate anabolite via cellular kinases, the cellular level of phosphorylation for compound 38, which showed significant activity against HCV, was evaluated. However, after incubation of compound 38 at 50 µM in Huh-7 cells for 4 h no tricyclic-monophosphate (MP), -diphosphate (DP), -triphosphate (TP) or even simple compound 38 were detected by LC-MS/MS. On the other hand, significant levels of the parents compound 8 and its MP, DP and TP were detected intra and extracellular, indicating that the observed activity does not come from the triphosphate of 38, but from conversion to 8-TP. To confirm these results, the stability of various tricyclic compounds in solution was studied using LC-MS/MS for detection of potential products. Compound 33, one of the most potent compound against HIV, was unstable in water with a half life of 25 min. However, the same compound 33 was more stable in MeOH with only 1% conversion to 7 after 72 h. Compounds 32, 36 and 39 which displayed lower antiviral activity also reverted to parent nucleoside at slower rates (respectively 60% of 7, 30% of 8 and 60% of 8 after 3 weeks). These differences in the rate of decomposition could certainly explain the variations of activity in each series. A more stable tricyclic compound will more slowly convert to its active parent and conversely, a less stable tricyclic compound will generate more of its active nucleoside parent.
With these surprising stability results, we decided to turn our attention on to the tricyclic ACV analog 6-(4-MeOPh)-TACV 3, which, according to the literature, shows a similar potency and selectivity against HSV-1 and HSV-2 as ACV [14]. Compound 3 was synthesized following the published procedure and evaluated for activity against HSV-1. First of all, 3 showed some activity against HSV-1 (EC50 = 12.2 µM, EC90 = 23.7 µM) even though 3 appears less potent than ACV in our assay (EC50 = 0.075 µM, EC90 = 0.7 µM) and less potent than what has been reported. Using the method previously described, the stability of compound 3 in water was studied and we found that it slowly converted to ACV (4% after 4 days and 35% after 3 weeks).
3. Conclusions
Despite the abundance of literature concerning this type of tricyclic nucleosides, we are the first to report the instability of some 3,9-dihydro-9-oxo-5H-imidazo[1,2-A]purine nucleosides in aqueous media. These results indicate that the activity of the electron rich tricyclic nucleosides was derived from their ability to convert to the active parent nucleoside, thus acting as prodrugs. More studies will be needed in order to determine if the differences in solubility and polarity of these electron rich tricyclic nucleosides translates into any advantage for the in vivo delivery of therapeutically important nucleoside analogs.
4. Experimental procedure
4.1. Synthesis
Anhydrous solvents were purchased from Aldrich Chemical Company, Inc. Reagents were purchased from commercial sources. Unless noted otherwise, the materials used in the examples were obtained from readily available commercial suppliers or synthesized by standard methods known to one skilled in the art of chemical synthesis. 1H NMR spectra were taken on a Varian Unity Plus 400 spectrometer at room temperature and reported in ppm downfield from internal tetramethylsilane. Signal multiplicities are represented by s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quadruplet), br (broad), bs (broad singlet), m (multiplet). All J-values are in Hz. Mass spectral analyses were performed on a Micromass TOF instrument (Hewlett-Packard HPLC driven electrospray MS instrument). Analytical HPLC analyses were performed on a Hewlett-Packard HPLC with a Phenomenex Gemini-NX column (2 mm × 50 mm, 3 µm, C18, 110 Å). Mobile phase flow was 0.7 mL/min with a 3.5 min gradient from 96% aqueous media (0.05% formic acid) to 96% CH3CN (0.05% formic acid) with a 5.5 min total acquisition time and 190 to 360 nm PDA detection).
4.1.1. General procedure for the synthesis of compound 9–40
To a solution of 5–8 (1 equiv) in anhydrous DMF (20 mL/1 mmol of 5–8) was added sodium hydride as 60% suspension in oil (1.1 equiv). After being stirred for 2 h at room temperature, the resulting solution was treated with bromoketone (1.3 equiv) and stirred overnight. After methanolysis, the volatiles were evaporated and the residual solid was chromatographed on silica gel column using CH2Cl2-MeOH (9:1 or 8:2). If necessary, the crude material, after chromatography, was then washed with a minimum volume of MeOH to afford the desired compounds 9–40.
4.1.1.1. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(4-methoxyphenyl)-9-oxo-5Himidazo-[1,2-a]purine (9)
1H NMR (DMSO-d6) δ 1.58–1.64 (m, 1H), 2.58–2.66 (m, 1H), 2.83–2.87 (m, 1H), 3.39–3.43 (m, 2H), 3.76 (s, 3H, CH3), 4.73 (brs, 1H), 5.43–5.47 (m, 1H), 5.91–5.93 (m, 1H), 6.12–6.14 (m, 1H), 6.98 (d, 2H, J = 8.8 Hz), 7.71 (s, 1H), 7.80 (d, 2H, J = 8.8 Hz), 7.95 (s, 1H), 12.91 (brs, NH); 13C NMR (DMSO-d6) δ 34.8, 48.5, 55.9, 59.6, 64.7, 102.5, 115.1, 116.4, 121.1, 127.2, 129.6, 130.1, 137.3, 139.3, 146.9, 150.3, 152.0, 160.3; LC-MS Calcd for C20H18N5O3 377.4; observed (M+1) 378.3.
4.1.1.2. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(4-methylphenyl)-9-oxo-5Himidazo-[1,2-a]purine (10)
1H NMR (DMSO-d6) δ 1.58–1.65 (m, 1H), 2.30 (s, 3H, CH3), 2.58–2.66 (m, 1H), 2.83–2.87 (m, 1H), 3.39–3.43 (m, 2H), 4.72 (brs, 1H), 5.43–5.47 (m, 1H), 5.91–5.94 (m, 1H), 6.12–6.15 (m, 1H), 7.24 (d, 2H, J = 7.6 Hz), 7.74–7.78 (m, 3H), 8.09 (s, 1H), 12.90 (brs, NH). LC-MS Calcd for C20H19N5O3 361.4; observed (M+1) 362.5.
4.1.1.3. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(4-bromophenyl)-9-oxo-5Himidazo-[1,2-a]purine (11)
1H NMR (DMSO-d6) δ 1.58–1.65 (m, 1H), 2.59–2.67 (m, 1H), 2.83–2.87 (m, 1H), 3.39–3.43 (m, 2H), 4.69–4.73 (m, 1H), 5.43–5.47 (m, 1H), 5.92–5.94 (m, 1H), 6.13–6.15 (m, 1H), 7.65 (d, 2H, J = 8.8 Hz), 7.79–7.85 (m, 3H), 8.26 (s, 1H), 13.01 (brs, NH). LC-MS Calcd for C19H16BrN5O2 425.3/427.4; observed (M+1) 426.1/428.2.
4.1.1.4. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(4-diethylaminophenyl)-9-oxo-5Himidazo-[1,2-a]purine (12)
1H NMR (DMSO-d6) δ 1.03–1.07 (m, 6H, 2×CH3), 1.58–1.65 (m, 1H), 2.57–2.66 (m, 1H), 2.83–2.87 (m, 1H), 3.31–3.36 (m, 4H, 2×CH2), 3.39–3.43 (m, 2H, CH2), 4.72 (brs, 1H), 5.43–5.47 (m, 1H), 5.91–5.94 (m, 1H), 6.12–6.15 (m, 1H), 6.67 (d, 2H, J = 8.0 Hz), 7.63–7.68 (m, 3H), 7.85 (s, 1H), 12.82 (brs, NH). LCMS Calcd for C23H26N6O2 418.5; observed (M+1) 419.4.
4.1.1.5. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(4-dimethylaminophenyl)-9-oxo-5Himidazo-[1,2-a]purine (13)
1H NMR (DMSO-d6) δ 1.58–1.65 (m, 1H), 2.58–2.66 (m, 1H), 2.83–2.87 (m, 1H), 2.92 (s, 6H, 2×CH3), 3.39–3.43 (m, 2H), 4.71 (t, 1H, J = 5.6 Hz), 5.43–5.47 (m, 1H), 5.91–5.94 (m, 1H), 6.12–6.15 (m, 1H), 6.74 (d, 2H, J = 8.8 Hz), 7.67 (d, 2H, J = 8.8 Hz), 7.77 (s, 1H), 7.88 (s, 1H), 12.8 (brs, NH). LCMS Calcd for C21H22N6O2 390.4; observed (M+1) 391.4
4.1.1.6. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(2-thienyl)-9-oxo-5H-imidazo-[1,2-a]purine (14)
1H NMR (DMSO-d6) δ 1.59–1.65 (m, 1H), 2.58–2.66 (m, 1H), 2.83–2.87 (m, 1H), 3.39–3.43 (m, 2H, CH2), 4.71 (brs, 1H), 5.43–5.47 (m, 1H), 5.91–5.94 (m, 1H), 6.12–6.14 (m, 1H), 7.12–7.15 (m, 1H), 7.57–7.58 (m, 1H), 7.62 (d, 1H, J = 5.2 Hz), 7.79 (s, 1H), 7.88 (s, 1H), 13.19 (brs, NH). LCMS Calcd for C17H15N5O2S 353.4; observed (M+1) 354.5
4.1.1.7. 3-[4-(Hydroxymethyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(3-thienyl)-9-oxo-5H-imidazo-[1,2-a]purine (15)
1H NMR (DMSO-d6) δ 1.59–1.65 (m, 1H), 2.58–2.64 (m, 1H), 2.83–2.87 (m, 1H), 3.39–3.43 (m, 2H, CH2), 4.70–4.73 (m, 1H), 5.44–5.47 (m, 1H), 5.92–5.95 (m, 1H), 6.12–6.15 (m, 1H), 7.67–7.68 (m, 2H), 7.79 (s, 1H), 7.95–7.96 (m, 1H), 8.08 (s, 1H), 13.00 (brs, NH). LCMS Calcd for C17H15N5O2S 353.4; observed (M+1) 354.5.
4.1.1.8. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-ethyl-9-oxo-5H-imidazo-[1,2-a]purine (16)
1H NMR (DMSO-d6) δ 1.2 (t, 3H, J = 7.6 Hz, CH3), 2.55–2.61 (m, 2H, CH2), 3.41–3.29 (m, 2H, CH2), 4.16–4.21 (m, 1H), 4.45–4.48 (m, 1H), 5.01–5.02 (m, 1H), 5.12–5.15 (m, 1H), 6.26 (d, 1H, J = 5.2 Hz), 7.33 (s, 1H), 8.00 (s, 1H), 8.27 (s, 1H), 13.01 (brs, NH). LCMS Calcd for C17H15N5O2S 305.3; observed (M+1) 306.4.
4.1.1.9. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-phenyl-9-oxo-5H-imidazo-[1,2-a]purine (17)
1H NMR (DMSO-d6) δ 3.57–3.60 (m, 2H, CH2), 4.19–4.24 (m, 1H), 4.50–4.53 (m, 1H), 5.04 (t, 1H, J = 2.8 Hz), 5.14 (t, 1H, J = 6.0 Hz), 6.29 (d, 1H, J = 4.0 Hz), 7.34–7.38 (m, 1H), 7.43–7.47 (m, 2H), 7.88 (d, 2H, J = 7.2 Hz), 8.06 (s, 1H), 8.20 (s, 1H), 13.05 (brs, NH). LCMS Calcd for C17H15N5O5 353.3; observed (M+1) 354.4
4.1.1.10. 3-(β-D-1,3-Dioxolanyl)-2-cyclopenten-1-yl]-3,9-dihydro-6-(4-methoxyphenyl)-9-oxo-5Himidazo-[1,2-a]purine (18)
1H NMR (DMSO-d6) δ 3.56–3.60 (m, 2H, CH2), 3.77 (s, 3H, CH3), 4.19–4.24 (m, 1H), 4.49–4.53 (m, 1H), 5.02–5.05 (m, 1H), 5.12–5.15 (m, 1H), 6.29 (d, 1H, J = 4.8 Hz), 7.01 (d, 2H, J = 8.8 Hz), 7.81 (d, 2H, J = 8.8 Hz), 8.05 (s, 1H), 8.06 (s, 1H), 12.98 (brs, NH); 13C NMR (DMSO-d6) δ 56.0, 61.8, 71.2, 79.9, 102.6, 106.2, 115.1, 115.9, 120.9, 127.2, 129.8, 137.0, 146.3, 150.3, 151.9, 160.3; LCMS Calcd for C18H17N5O5 383.4; observed (M+1) 384.5.
4.1.1.11. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(3-methoxyphenyl)-9-oxo-5H-imidazo-[1,2-a]purine (19)
1H NMR (DMSO-d6) δ 3.56–3.60 (m, 2H, CH2), 3.80 (s, 3H, CH3), 4.19–4.24 (m, 1H), 4.49–4.53 (m, 1H), 5.03–5.05 (m, 1H), 5.12–5.15 (m, 1H), 6.29 (d, 1H, J = 4.8 Hz), 6.92 (d, 1H, J = 7.2 Hz), 7.32–7.37 (m, 1H), 7.45–7.48 (m, 2H), 8.05 (s, 1H), 8.27 (s, 1H), 13.05 (brs, NH). LCMS Calcd for C18H17N5O5 383.4; observed (M+1) 384.4.
4.1.1.12. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(2-methoxyphenyl)-9-oxo-5H-imidazo-[1,2-a]purine (20)
1H NMR (DMSO-d6) δ 3.56–3.60 (m, 2H, CH2), 3.95 (s, 3H, CH3), 4.19–4.23 (m, 1H), 4.49–4.51 (m, 1H), 5.03–5.05 (m, 1H), 5.10–5.13 (m, 1H), 6.29 (d, 1H, J = 3.6 Hz), 7.03–7.07 (m, 1H), 7.15–7.17 (m, 1H), 7.34–7.39 (m, 1H), 7.82–7.84 (m,1H), 7.92 (s, 1H), 8.04 (s, 1H), 13.05 (brs, NH). LCMS Calcd for C18H17N5O5 383.4; observed (M+1) 384.4.
4.1.1.13. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-methylphenyl)-9-oxo-5H-imidazo-[1,2-a]purine (21)
1H NMR (DMSO-d6) δ 2.30 (s, 3H, CH3), 3.56–3.60 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.47–4.50 (m, 1H), 5.03–5.04 (m, 1H), 5.14–5.17 (m, 1H), 6.29 (d, 1H, J = 4.4 Hz), 7.24 (d, 2H, J = 8.4 Hz), 7.75 (d, 2H, J = 8.4 Hz), 8.00 (s, 1H), 8.07 (s, 1H), 13.02 (brs, NH). LCMS Calcd for C18H17N5O4 367.4; observed (M+1) 368.5.
4.1.1.14. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-chlorophenyl)-9-oxo-5H-imidazo-[1,2-a]purine (22)
1H NMR (DMSO-d6) δ 3.55–3.60 (m, 2H, CH2), 4.19–4.22 (m, 1H), 4.47–4.49 (m, 1H), 5.03–5.04 (m, 1H), 5.14–5.19 (m, 1H), 6.29 (d, 1H, J = 4.4 Hz), 7.48 (d, 2H, J = 8.4 Hz), 7.89 (d, 2H, J = 8.4 Hz), 7.98 (s, 1H), 8.17 (s, 1H), 13.04 (brs, NH). LCMS Calcd for C17H14ClN5O4 387.8; observed (M+1) 388.1/390.2.
4.1.1.15. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-fluorophenyl)-9-oxo-5H-imidazo-[1,2-a]purine (23)
1H NMR (DMSO-d6) δ 3.57–3.60 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.51 (d, 1H, J = 9.2 Hz), 5.03–5.05 (m, 1H), 5.12 (t, 1H, J = 6.0 Hz), 6.29 (d, 1H, J = 4.4 Hz), 7.29–7.33 (m, 2H), 7.91–7.95 (m, 2H), 8.05 (s, 1H), 8.21 (s, 1H), 13.09 (brs, NH). LCMS Calcd for C17H14N5O4 371.3; observed (M+1) 372.2.
4.1.1.16. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(2,4-difluorophenyl)-9-oxo-5H-imidazo-[1,2-a]purine (24)
1H NMR (DMSO-d6) δ 3.56–3.59 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.50 (d, 1H, J = 9.2 Hz), 5.03–5.05 (m, 1H), 5.14 (brs, 1H), 6.29–6.31 (m, 1H), 7.24–7.29 (m, 1H), 7.43–7.49 (m, 1H), 7.81 (d, 1H, J = 3.2 Hz), 7.92–7.98 (m, 1H), 8.03 (s, 1H), 13.01 (brs, NH). LCMS Calcd for C17H13F2N5O4 389.3; observed (M+1) 390.3.
4.1.1.17. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-diethylaminophenyl)-9-oxo-5H-imidazo-[1,2-a]purine (25)
1H NMR (DMSO-d6) δ 1.07 (t, 6H, J = 6.8 Hz, 2×CH3), 3.27–3.37 (m, 4H, 2×CH2), 3.57–3.60 (m, 2H, CH2), 4.18–4.23 (m, 1H), 4.49–4.52 (m, 1H), 5.03–5.04 (m, 1H), 5.11 (t, 1H, J = 6.0 Hz), 6.28 (d, 1H, J = 4.8 Hz), 6.68 (d, 2H, J = 8.8 Hz), 7.64 (d, 2H, J = 8.8 Hz), 7.86 (s, 1H), 8.03 (s, 1H), 12.81 (brs, NH). LCMS Calcd for C21H24N6O4 424.5; observed (M+1) 425.6.
4.1.1.18. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-dimethylaminophenyl)-9-oxo-5H-imidazo-[1,2-a]purine (26)
1H NMR (DMSO-d6) δ 2.92 (s, 6H, 2×CH3), 3.57–3.60 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.49–4.52 (m, 1H), 5.03–5.04 (m, 1H), 5.12 (t, 1H, J = 6.0 Hz), 6.28 (d, 1H, J = 4.8 Hz), 6.74 (d, 2H, J = 8.8 Hz), 7.68 (d, 2H, J = 8.8 Hz), 7.90 (s, 1H), 8.02 (s, 1H), 12.84 (brs, NH). LCMS Calcd for C19H20N6O4 396.4; observed (M+1) 397.3.
4.1.1.19. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(2-thienyl)-9-oxo-5H-imidazo-[1,2-a]purine (27)
1H NMR (DMSO-d6) δ 3.56–3.60 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.49–4.51 (m, 1H), 5.02–5.04 (m, 1H), 5.12 (t, 1H, J = 6.4 Hz), 6.28 (d, 1H, J = 4.4 Hz), 7.13–7.15 (m, 1H), 7.58 (d, 1H, J = 3.2 Hz), 7.62–7.64 (m, 1H), 7.91 (s, 1H), 8.04 (s, 1H), 13.17 (brs, NH). LCMS Calcd for C15H13N5O4S 359.4; observed (M+1) 360.5.
4.1.1.20. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(3-thienyl)-9-oxo-5H-imidazo-[1,2-a]purine (28)
1H NMR (DMSO-d6) δ 3.57–3.60 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.49–4.52 (m, 1H), 5.02–5.04 (m, 1H), 5.12–5.15 (m, 1H), 6.29 (d, 1H, J = 5.6 Hz), 7.67–7.68 (m,2H), 7.97 (s, 1H), 8.04 (s, 1H), 8.10 (s, 1H), 13.06 (brs, NH). LCMS Calcd for C15H18N5O4S 359.4; observed (M+1) 360.3.
4.1.1.21. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-azido-phenyl)-9-oxo-5H-imidazo-[1,2-a]purine (29)
1H NMR (DMSO-d6) δ 3.56–3.60 (m, 2H, CH2), 4.19–4.23 (m, 1H), 4.51 (d, 1H, J = 9.2 Hz), 5.00–5.03 (m, 1H), 5.12 (t, 1H, J = 6.0 Hz), 6.28 (d, 1H, J = 4.8 Hz), 7.19 (d, 2H, J = 8.4 Hz), 7.91 (d, 2H, J = 8.4 Hz), 8.04 (s, 1H), 8.20 (s, 1H), 13.07 (brs, NH). LCMS Calcd for C17H14N8O4 394.3; observed (M+1) 395.4.
4.1.1.22. 3-(β-D-1,3-Dioxolanyl)-3,9-dihydro-6-(4-cyano-phenyl)-9-oxo-5H-imidazo-[1,2-a]purine (29)
1H NMR (DMSO-d6) δ 3.57–3.60 (m, 2H, CH2), 4.19–4.24 (m, 1H), 4.50–4.53 (m, 1H), 5.03–5.04 (m, 1H), 5.11–5.15 (m, 1H), 6.29 (d, 1H, J = 4.4 Hz), 7.91–7.93 (m,2H), 8.05–8.08 (m, 3H), 8.47 (s, 1H), 13.25 (brs, NH). LCMS Calcd for C18H14N6O4 378.3; observed (M+1) 379.2.
4.1.1.23. 3-(3-Azido-2,3-dideoxy-β-D-erythro-pentofuranosyl)-3,9-dihydro-6-phenyl-9-oxo-5Himidazo-[1,2-a]purine (31)
1H NMR (DMSO-d6) δ 2.46–2.52 (m, 1H), 2.82–2.89 (m, 1H), 3.53–3.61 (m, 2H, CH2), 3.87–3.90 (m, 1H), 4.52–4.59 (m, 1H), 5.12–5.16 (m, 1H), 6.21 (t, 1H, J = 6.0 Hz), 7.35–7.39 (m, 1H), 7.43–7.47 (m, 2H), 7.87–7.89 (m, 2H), 8.16 (s, 1H), 8.20 (s, 1H), 13.02 (brs, NH). LCMS Calcd for C18H16N8O3 392.4; observed (M+1) 393.3.
4.1.1.24. 3-(3-Azido-2,3-dideoxy-β-D-erythro-pentofuranosyl)-3,9-dihydro-6-(4-methoxyphenyl)-9-oxo-5H-imidazo-[1,2-a]purine (32)
1H NMR (DMSO-d6) δ 2.46–2.52 (m, 1H), 2.83–2.90 (m, 1H), 3.53–3.61 (m, 2H, CH2), 3.77 (s, 3H, OCH3), 3.88–3.90 (m, 1H), 4.54–4.59 (m, 1H), 5.11–5.17 (m, 1H), 6.21 (t, 1H, J = 6.4 Hz), 7.01 (d, 2H, J = 8.6 Hz), 7.81 (d, 2H, J = 8.6 Hz), 8.06 (s, 1H), 8.15 (s, 1H), 12.93 (brs, NH). 13C NMR (DMSO-d6) δ 36.9, 55.9, 61.4, 61.8, 83.2, 85.2, 102.6, 115.1, 116.4, 121.0, 127.2, 129.8, 137.5, 146.8, 150.2, 151.9, 160.3; LCMS Calcd for C19H18N8O4 422.4; observed (M+1) 423.5.
4.1.1.25. 3-(3-Azido-2,3-dideoxy-β-D-erythro-pentofuranosyl)-3,9-dihydro-6-(4-diethylaminophenyl)-9-oxo-5H-imidazo-[1,2-a]purine (33)
1H NMR (CDCl3) δ 1.06–1.25 (m, 6H, 2×CH3), 2.30–2.35 (m, 1H), 3.10–3.15 (m, 1H), 3.22–3.29 (m, 2H, CH2), 3.77 (d, 1H, J = 12.4 Hz), 4.06 (d, 1H, J = 12.4 Hz), 4.19 (s, 1H), 4.44–4.46 (m, 1H), 6.10–6.14 (m, 1H), 6.58 (d, 2H, J = 8.6 Hz), 7.36 (d, 2H, J = 8.6 Hz), 7.55 (s, 1H), 7.65 (s, 1H). LCMS Calcd for C22H25N9O3 463.5; observed (M+1) 464.6
4.1.1.26. 3-(3-Azido-2,3-dideoxy-β-D-erythro-pentofuranosyl)-3,9-dihydro-6-(4-dimethylamino–phenyl)-9-oxo-5H-imidazo-[1,2-a]purine (34)
1H NMR (DMSO-d6) δ 2.81–3.02 (m, 8H, 2×CH3, CH2), 3.53–3.61 (m, 2H, CH2), 3.87–3.90 (m, 1H), 4.52–4.59 (m, 1H), 5.11–5.17 (m, 1H), 6.18–6.23 (m, 1H), 6.75 (d, 2H, J = 8.6 Hz), 7.68 (d, 2H, J = 8.6 Hz), 7.91 (s, 1H), 8.13 (s, 1H), 12.85 (brs, NH). LCMS Calcd for C20H21N9O3 435.5; observed (M+1) 436.5.
4.1.1.27. 3-(3-azido-2,3-dideoxy-β-D-erythro-pentofuranosyl)-3,9-dihydro-6-ethyl-9-oxo-5H-imidazo-[1,2-a]purine (35)
1H NMR (DMSO-d6) δ 0.79 (s, 3H, CH3), 1.19 (t, 3H, J = 7.6 Hz, CH3), 2.56–2.60 (m, 2H, CH2), 3.62–3.67 (m, 1H), 3.77–3.86 (m, 2H), 3.95–4.00 (m, 1H), 5.10 (s, OH), 5.15–5.17 (m, OH), 5.25 (d, J = 6.8 Hz, OH), 5.82 (s, 1H, H-1’), 7.31 (s, 1H), 8.21 (s, 1H), 12.91 (brs, NH). LCMS Calcd for C15H19N5O5 349.3; observed (M+1) 350.2.
4.1.1.28. 3-(β-D-2-C-Methyl-ribofuranosyl)-3,9-dihydro-6-(4-methoxy-phenyl)-9-oxo-5H-imidazo-[1,2-a]purine (36)
1H NMR (DMSO-d6) δ 0.80 (s, 3H, CH3), 3.62–3.70 (m, 1H), 3.77–3.88 (m, 4H), 3.96–4.00 (m, 1H), 4.06–4.10 (m, 1H), 5.10 (s, OH), 5.17–5.19 (m, OH), 5.29 (d, J = 6.4 Hz, OH), 5.86 (s, 1H, H-1’), 7.00 (d, 2H, J = 8.8 Hz), 7.81 (d, 2H, J = 8.8 Hz), 8.05 (s, 1H), 8.25 (s, 1H), 13.05 (brs, NH); 13C NMR (DMSO-d6) δ 20.7, 55.9, 60.0, 72.2, 79.3, 83.0, 91.2, 102.5, 115.2, 116.1, 121.0, 127.2, 129.8, 137.2, 146.9, 150.2, 151.9, 160.3; LCMS Calcd for C20H21N5O5 427.4; observed (M+1) 428.3.
4.1.1.29. 3-(β-D-2-C-Methyl-ribofuranosyl)-3,9-dihydro-6-(4-diethylaminophenyl)-9-oxo-5Himidazo-[1,2-a]purine (37)
1H NMR (DMSO-d6) δ 0.79 (s, 3H, CH3), 1.00–1.13 (m, 6H, 2×CH3), 3.33–3.40 (m, 4H, 2×CH2), 3.63–3.68 (m, 1H), 3.78–3.87 (m, 2H), 3.95–4.00 (m, 1H), 5.07 (s, OH), 5.17–5.19 (m, OH), 5.28 (d, J = 6.8 Hz, OH), 5.86 (s, 1H, H-1’), 6.67 (d, 2H, J = 8.8 Hz), 7.63 (d, 2H, J = 8.8 Hz), 7.84 (s, 1H), 8.22 (s, 1H), 12.85 (brs, NH). LCMS Calcd for C23H28N6O5 468.5; observed (M+1) 469.6.
4.1.1.30. 3-(β-D-2-C-methyl-ribofuranosyl)-3,9-dihydro-6-(4-dimethylaminophenyl)-9-oxo-5Himidazo-[1,2-a]purine (38)
1H NMR (DMSO-d6) δ 0.79 (s, 3H, CH3), 2.92 (s, 6H, 2×CH3), 3.63–3.68 (m, 1H), 3.79–3.97 (m, 2H), 3.96–4.01 (m, 1H), 5.08 (s, OH), 5.17–5.19 (m, OH), 5.28 (d, J = 6.8 Hz, OH), 5.86 (s, 1H, H-1’), 6.74 (d, 2H, J = 8.8 Hz), 7.67 (d, 2H, J = 8.8 Hz), 7.90 (s, 1H), 8.25 (s, 1H), 12.89 (brs, NH). LCMS Calcd for C21H24N6O5 440.4; observed (M+1) 441.5.
4.1.1.31. 3-(β-D-2-C-Methyl-ribofuranosyl)-3,9-dihydro-6-(2-thienyl)-9-oxo-5H-imidazo-[1,2-a]purine (39)
1H NMR (DMSO-d6) δ 0.80 (s, 3H, CH3), 3.64–3.68 (m, 1H), 3.79–3.87 (m, 2H), 3.96–4.00 (m, 1H), 5.09 (s, OH), 5.17–5.19 (m, OH), 5.29 (d, J = 6.8 Hz, OH), 5.85 (s, 1H, H-1’), 7.12–7.15 (m, 1H), 7.56 (d, 1H, J = 2.4 Hz), 7.62 (d, 1H, J = 5.2 Hz), 7.89 (s, 1H), 8.25 (s, 1H), 13.23 (brs, NH). LCMS Calcd for C17H17N5O5S 403.4; observed (M+1) 404.5.
4.1.1.32. 3-(β-D-2-C-Methyl-ribofuranosyl)-3,9-dihydro-6-(3-thienyl)-9-oxo-5H-imidazo-[1,2-a]purine (40)
1H NMR (DMSO-d6) δ 0.79 (s, 3H, CH3), 3.64–3.68 (m, 1H), 3.78–3.85 (m, 2H), 3.97–4.02 (m, 1H), 5.09 (s, OH), 5.17–5.19 (m, OH), 5.28 (d, J = 6.8 Hz, OH), 5.85 (s, 1H, H-1’), 7.67–7.68 (m, 2H), 7.95–7.96 (m, 1H), 7.95 (s, 1H), 8.26 (s, 1H), 13.01 (brs, NH). LCMS Calcd for C17H17N5O5S 403.4; observed (M+1) 404.4.
4.2. LC/MS/MS method for stability studies
The HPLC system was a Dionex Packing Ultimate 3000 modular LC system consisting of a quaternary pump, vacuum degasser, thermostated autosampler, and thermostated column compartment (Dionex, CA). A TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Electron Corp.) was used for detection. Thermo Xcalibur software version 1.3 was used to control the HPLC, the mass spectrometer and to perform data analysis. The separation was performed on a Hypersil GOLD-C18 column (50 × 1 mm, 3 µm particle size) (Thermo Scientific, Waltham, MA, USA). The mobile phase A consisted of 10 mM ammonium formate buffer, pH 3 adjusted using formic acid. An alternative mobile phase A consisted of water only. The mobile phase B consisted of methanol. The initial conditions were 95% A and 5% B at 50 µl min−1. From 1 min to 2 min B was increased from 5% to 95% and decreased to 5% after 4 min. The total run time was 15 min (including time for column regeneration). The column temperature was kept constant at 30°C. Analytes were protonated by electrospray ionization (ESI) in positive mode. Selected Reaction Monitoring (SRM) mode was used for the acquisition. The intensity of selected product ion in the MS/MS spectrum of each compound was optimized using direct infusion of the analytes in the corresponding mobile phase (at concentration 100 µM) separately into the instrument using a syringe pump at 5 µl min−1. The sheath, ion sweep and auxiliary gas (nitrogen) were set at 50, 0.5 and 1 arbitrary units (au) respectively. The collision gas (argon) pressure was set at 1.5 mTorr. The spray voltage was 4000 V. The capillary was heated at 300°C. 0.1 second scan time was used for all transitions. The collision-induced dissociation (CID) was used at −6 V. Tube lens was 107 V and collision energy was 20 V. The following m/z transitions were used for 38 m/z 441 → 295, 32 m/z 423 → 282, 33 m/z 464 → 323, 7: m/z 293 → 152, 36 m/z 428 → m/z 282, 39 m/z 404 → 258, 8: m/z 298 → 152, 3 356 → 282 and ACV 226 → 152. 3TC was used as internal standard, with the following SRM: m/z 230 → 112.
4.2.1 Detection of nucleoside MP, DP and TP intracellularly
4.2.1.1 Cell culture and drug extraction
The 2’-C-methyl analogues were incubated at 50 µM in Huh-7 cells at 37°C for 4 hr. The cells were washed twice with 1 X ice cold PBS and lysed using 60% methanol. After centrifugation to remove cellular debris, the supernatant was evaporated to dryness under a stream of air. Dried extracts were maintained at −80 °C until analysis.
4.2.1.2 Sample preparation
To separate the nucleoside mono-, di- and triphosphates and unchanged nucleosides, half of the samples were reconstituted in 200 µL water containing AZT, AZT-MP and AZT-TP 50 nM and subjected to an anion exchange Solid Phase Extraction. QMA cartridges were conditioned by rinsing with 750 µl of distilled dionized H2O (ddH2O). The samples were loaded onto the cartridges followed by a rinse with 300 µl ddH2O. Unchanged nucleosides were collected first with 750 µl ddH2O. The cartridge was conditioned with 750 µl of ddH2O and 300 µl 20 mM KCl. Nucleoside mono-, di- and triphosphates were removed by sequential washes with 750 µl of 100 mM KCl, 120 mM KCl and 400 mM KCl, respectively. The cartridge was rinsed with 150 µl of the corresponding buffer between each. The fractions were collected for de-phosphorylation.
The internal standard (3TC, 10 µl of a solution at 1 µM) was added to the fractions at this point. The pH was lowered to 4.5 by the addition of 400 mM ammonium acetate buffer, and phosphate groups were removed by addition of 2 unit of type XA sweet potato acid phosphatase per ml eluent and incubation at 37 °C for one hour. To remove salt from the resulting fractions, the samples were loaded onto Waters OASIS HLB cartridges pre-conditioned with 2 ml MeOH and 2 ml ddH2O, and washed with 3 ml of ddH2O. Nucleoside analogs of interest were eluted with 1 ml of methanol and dried under a stream of air. The residue was reconstituted with 100 µl. The other half of the samples was directly reconstituted with 100 µl of water containing 100 nM 3TC, were filtered by ultrafiltration (COSTAR filters 0.2 µm). Fractions from both SPE and direct reconstitution were injected onto the LC-MS/MS system for intracellular nucleoside detection. The method described previously (4.2) was used.
4.2.2 Conditions for stability studies
A known concentration of the analogs was diluted in two separate fractions, water and methanol, directly from a stock solution synthesized the same day and injected onto the LC/MS/MS using the method previously described 4.2. Injections were performed every 15 min for the first two hr, then every hr, every day and finally every week. Half of the sample was kept at room temperature and the other half was kept in the autosampler at 4°C. Standard calibration of the nucleosides 7, 8 and ACV ranging from 10 to 1000 nM were used in order to calculate the percentage of G analog formation.
4.3. Antiviral activity evaluation
The antiviral activity of compounds 9–40 was evaluated against HIV [27] in activated primary human PBM cells and in Huh-7 cells with a HCV subgenomic RNA replicon system. [28] Cytotoxicity was evaluated in the HCV replicon and normal PBM cells, along with CEM and Vero cells [29]. Compound 3 was evaluated for activity against HSV-1 (strain F) by plaque reduction assay in Vero cells using methodologies described previously [30].
Figure 1.
Structure of tricyclic derivatives of ACV and GCV
Figure 2.
Structure of CBV, DXG, AZG and 2’-C-methylguanosine
Scheme 1.
a) NaH, BrCH2(CO)R, DMF, rt
Acknowledgments
This work was supported in part by NIH grant 5R01-AI-071846, 5P30-AI-50409 (CFAR), 5R37-AI-041980 and by the Department of Veterans Affairs. We would like also to thanks Dr Ethel Garnier for helpful discussions and critical reading of the manuscript. Dr. Schinazi is the founder and a shareholder of RFS Pharma, LLC. Emory University received no funding from RFS Pharma, LLC to perform this work and vice versa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hofman T, Zweig K, Engels JW. Synthesis. 2005:1797–1800. [Google Scholar]
- 2.Ostrowski T, Golankiewicz B, De Clercq E, Balzarini J. Bioorg. Med. Chem. 2006;14:3535–3542. doi: 10.1016/j.bmc.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Horejsi K, Andrei G, De Clercq E, Snoeck R, Pohl R, Holy A. Bioorg. Med. Chem. 2006;14:8057–8065. doi: 10.1016/j.bmc.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski T, Golankiewicz B, De Clercq E, Balzarini J. Bioorg. Med. Chem. 2005;13:2089–2096. doi: 10.1016/j.bmc.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Ostrowski T, Golankiewicz B, De Clercq E, Balzarini J. J. Med. Chem. 2002;45:5052–5057. doi: 10.1021/jm020827z. [DOI] [PubMed] [Google Scholar]
- 6.Balzarini J, Ostrowski T, Golinski T, De Clercq E, Golankiewicz B. Gene Therapy. 2002;9:1173–1182. doi: 10.1038/sj.gt.3301779. [DOI] [PubMed] [Google Scholar]
- 7.Golankiewicz B, Ostrowski T, Andrei G, Snoeck R, De Clercq E. J. Med. Chem. 1994;37:3187–3190. doi: 10.1021/jm00045a025. [DOI] [PubMed] [Google Scholar]
- 8.Zielenkiewicz A, Golankiewicz B, Zielenkiewicz W. J. Sol. Chem. 2001;30:575. [Google Scholar]; f) Golinski T, Januszczyk P, Wenska G, Golankiewicz B, De Clercq E, Balzarini J. Nucleosides, Nucleotides Nucleic Acids. 2005:571–575. doi: 10.1081/ncn-200061817. [DOI] [PubMed] [Google Scholar]
- 9.Golankiewicz B, Ostrowski T, Golinski T, Januszczyk P, Zeidler J, Baranowski D, De Clercq E. J. Med. Chem. 2001;44:4284–4287. doi: 10.1021/jm010922s. [DOI] [PubMed] [Google Scholar]
- 10.Boryski J, Golankiewicz B, De Clercq E. J. Med. Chem. 1991;34:2380–2383. doi: 10.1021/jm00112a010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Lin J, Pan D. Bioorg. Med. Chem. Lett. 2007;17:741–744. doi: 10.1016/j.bmcl.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 12.Golinski T, Wenska G, Golankiewicz B, Balzarini J, De Clercq E. Nucleosides, Nucleotides Nucleic Acids. 2003:911–914. doi: 10.1081/NCN-120022684. [DOI] [PubMed] [Google Scholar]
- 13.Suwinska K, Golankiewicz B, Zielenkiewicz W. Acta Cryst. 2001:767–769. doi: 10.1107/s0108270101005364. [DOI] [PubMed] [Google Scholar]
- 14.See mini-review: Golankiewicz B, Ostrowski T. Antiviral Res. 2006;71:134–140. doi: 10.1016/j.antiviral.2006.05.004.
- 15.Management of Hepatitis C. National Institutes of Health Consensus Conference Statement; June 10–12, 2002.2002. [Google Scholar]
- 16.Davis GL. Gastroenterology. 2000;118:S104. doi: 10.1016/s0016-5085(00)70009-6. [DOI] [PubMed] [Google Scholar]
- 17.For a recent review on the anti-HCV compounds in development including 2’-C-nucleosides analogs see: Liu-Young G, Kozal MJ. AIDS Patient Care STDS. 2008;22:449–457. doi: 10.1089/apc.2007.0199.
- 18.For a review on HIV-1 reverse transcriptase inhibitors see: Jochmans D. Virus Res. 2008;134:157–170. doi: 10.1016/j.virusres.2008.01.003.
- 19.Parikh UM, Koontz DL, Chu CK, Schinazi RF, Mellors JW. Antimicrob. Agents Chemother. 2005;49:1139–1144. doi: 10.1128/AAC.49.3.1139-1144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eldrup AB, Allerson CR, Bennet CF, Bera S, Bhat B, Bosserman M, Brooks J, Burlein C, Carrol SS, Cook PD, Getty KL, MacCoss M, McMasters DR, Plseon DB, Prakash TP, Prhavc M, Song Q, Tomassini JE, Xia JJ. J. Med. Chem. 2004;47:2283–2295. doi: 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- 21.Sommadossi JP, Lacolla P. WO 01/92282. P. Intl. Patent Appl. 2001
- 22.Clark JL, Hollecker L, Mason JC, Stuyver LJ, Tharnish PM, Lostia S, McBrayer T, Schinazi RF, Watanabe KA, Otto MJ, Furman PA, Stec WJ, Patterson SE, Pankiewicz KW. J. Med. Chem. 2005;48:5504–5508. doi: 10.1021/jm0502788. [DOI] [PubMed] [Google Scholar]
- 23.Murakami E, Bao H, Ramesh M, McBrayer TR, Whitaker T, Steur HMM, Schinazi RF, Stuyver LJ, Obikhod A, Otto MJ, Furman PA. Antimicrob. Agents Chemother. 2007;51:503–509. doi: 10.1128/AAC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daluge SM, Martin MT, Sickles BR, Livingston DA. Nucleosides Nucleotides Nucleic Acids. 2000;19:297–327. doi: 10.1080/15257770008033011. [DOI] [PubMed] [Google Scholar]
- 25.Herdewijn P, Balzarini J, Baba M, Pauwels R, Van Aerschot A, Janssen G, De Clercq E. J. Med. Chem. 1988;31:2040–2048. doi: 10.1021/jm00118a033. [DOI] [PubMed] [Google Scholar]
- 26.Li NS, Piccirilli JA. J. Org. Chem. 2006;71:4018–4020. doi: 10.1021/jo0602165. [DOI] [PubMed] [Google Scholar]
- 27.Schinazi RF, Sommadossi JP, Saalman V, Cannon DL, Xie MW, Hart GC, Hahn EF. Antimicrob. Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuyver LJ, Whitaker T, McBrayer TR, Hernandez-Santiago BI, Lostia S, Tharnish PM, Ramesh M, Chu CK, Jordan R, Shi J, Rachakonda S, Watanabe KA, Otto MJ, Schinazi RF. Antimicrob. Agents Chemother. 2003;47:244–254. doi: 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuyver LJ, Lostia S, Adams M, Mathew J, Pai BS, Grier J, Tharnish P, Choi Y, Chong Y, Choo H, Chu CK, Otto MJ, Schinazi RF. Antimicrob. Agents Chemother. 2002;46:3854–3860. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schinazi RF, Peters J, Williams CC, Chance D, Nahmias A. Antimicrob. Agents Chemother. 1982;22:499–507. doi: 10.1128/aac.22.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]