PREFACE
Podosomes and invadopodia are actin-based dynamic protrusions of the plasma membrane of metazoan cells that represent sites of attachment to, and degradation of, the extracellular matrix. Key proteins in these structures include the actin regulators cortactin and (N)-WASP, the adaptor proteins Tks4 and Tks5, and the metalloprotease MT1-MMP. Many cell types elaborate these structures, including invasive cancer cells, vascular smooth muscle and endothelial cells, and immune cells such as macrophages and dendritic cells. Recent progress has been made in our understanding of the regulatory and functional aspects of podosome and invadopodia biology and their role in human disease.
INTRODUCTION
In 1980, David-Pfeuty and Singer demonstrated that transformation of chicken embryo fibroblasts with Rous sarcoma virus (RSV), containing the oncogene src, caused relocalization of the cytoskeletal proteins vinculin and α-actinin away from the cell-extracellular matrix (ECM) contact points called focal adhesions and into circular clusters they called rosettes1. In 1985, Tarone, Marchisio and their colleagues demonstrated that these proteins were localized to protrusions of the ventral membrane that also contained actin and tyrosine phosphorylated proteins, and were sites of cell adhesion to the extracellular matrix2 (Box 1). They considered these structures cellular feet, and therefore called them podosomes. That same year, and also using RSV-transformed cells, Chen, Parsons and colleagues demonstrated that the tyrosine kinase Src was localized to sites of cell contact with the extracellular matrix3 (Box 1). Furthermore, they made the important observation that degradation of the ECM occurs at these contact sites3. In 1989, Chen demonstrated that these Src-enriched sites of degradation were in fact the podosomes4. To reflect the adhesive and degradative capacity of these structures, Chen coined the term invadopodia. Chen and colleagues subsequently extended these findings beyond RSV-transformed chicken and mouse fibroblasts to show that invadopodia could also be found in human cancer cell lines. In the meantime, Marchisio and colleagues demonstrated that podosomes could form in cultured osteoclasts5. They have now been described in other cell types, including macrophages and dendritic cells, endothelial cells and vascular smooth muscle cells6. The past twenty- five years has seen an expansion on research into podosomes and invadopodia, including the discovery of associated proteins, of stimuli required for their formation, and of in vivo relevance (Box 1 and Table 1). However, the control of their formation, and their function in vivo, remains somewhat enigmatic.
Box 1. Highlights from 25 years of Podosome/Invadopodia Research.
| 1985: | Actin- and phosphotyrosine-rich ventral protrusions are recognized as cell attachment points to the ECM, and called podosomes2. |
| 1985: | Src is localized to the sites of cell contact to ECM, and it is shown that ECM degradation occurs at these sites3. |
| 1988: | Podosomes are found in osteoclasts adhering to bone laminae5 |
| 1989: | The Src-enriched sites of degradation are shown to be identical to the actin-rich protrusions known as podosomes – the new name invadopodia is coined4. |
| 1990: | Bone resorption by osteoclasts is shown to require the podosome belt - the first physiologic process shown to require podosomes132. |
| 1994: | First description of invadopodia-dependent proteolytic activity in human cancer cells133. |
| 1997: | MT1-MMP is located in podosomes and invadopodia and is required for cancer cell invasion134,135. |
| 1999: | First demonstration of a podosome-associated disease - Wiskott-Aldrich syndrome49. |
| 2000: | Microtubules are required for podosome formation136. |
| 2005: | The adaptor protein Tks5 is shown to promote invadopodia formation, and be required for invasive behavior of human cancer cells31. |
| 2006–2008: | In vivo studies demonstrate that invadopodia-associated proteins are required for both tumor growth and metastasis51,53. |
| 2008: | ECM rigidity promotes invadopodia activity40. |
| 2009: | Reactive oxygen species are necessary for podosome and invadopodia formation28. |
| 2009–2010: | First demonstration that vascular pathophysiology involves podosome formation19,20, and that podosomes exist in vivo19. |
| 2010: | Detailed visualization of invadopodia elongation as cells traverse basement membrane41. |
| 2010: | First description of podosome structure and invasion in 3D, demonstrating that podosomes invade into the ECM using a mechanism similar to invadopodia13. |
| Description | Location | Protrusive Dimension |
Actin Rearrangement |
Lipids Involved | Microtubule Dependence |
Pericellular Proteolysis |
Duration of Structure |
|
|---|---|---|---|---|---|---|---|---|
| Lamellipodia137,138 | Surface-attached, sheet-like protrusions |
Leading edge of the cell |
Width: 0.1 – 0.2 µm |
Branched actin filaments |
Yes PI(4,5)P2139 |
Induces lamellipodia extension140 |
Minimal141 | Minutes142 |
| Filopodia137,138 | Finger-like projections |
Usually located in regions of lamellipodia |
Width: 0.1 – 0.3 µm Length: 3 –10 µm |
Parallel actin filament bundles |
Yes PI(4,5)P2, PI(3,4,5)P3 |
Regulates filopodia density in lamellipodia rich areas143 |
No | Minutes |
|
Focal Adhesions144,145 |
Large clusters of transmembrane receptors, integrins, and cytosolic proteins that connect ECM and actin cytoskeleton |
Leading edge of the cell |
Width: 2 – 6 µm | Predominately parallel actin filament bundles but branched actin at end146 |
Yes PI(4,5)P2 |
Inhibition of microtubules leads to increased focal adhesions147 |
Minimal | Hours – depending on rate of cell migration |
| Podosomes6 | Actin rich core that is surrounded by a ring of actin- associated and signaling proteins. Term applies to normal cells |
Ventral cell surface Often clustered behind the leading edge of the cell |
Width: 0.5 – 2 µm Length: > 2 µm |
Branched and unbranched actin39 |
Yes PI(3,4,5)P3148 |
Required for elongation and/or formation 13,136 |
Yes MT1-MMP, uPAR13,149 |
Minutes |
| Invadopodia6,11 | Actin rich core that is surrounded by a ring of actin- associated and signaling proteins. Term applies to normal cells |
Ventral cell surface Often situated under the nucleus |
Width: 0.5 – 2 µm Length: > 2 µm |
Branched actin at the cell surface. Unbranched actin through the tip of the protrusion41 |
Yes PI(3,4)P2, PI(3)P, lipid rafts (Cav-1)29,92 |
Required for elongation but not formation41 |
Yes MMP2, MMP9, MT1-MMP, seprase, uPAR ADAMs 12, 15, 19, |
Hours |
Given that the terms podosome and invadopodia were first applied to the same structure in the same cells — the ventral protrusions of Src-transformed fibroblasts — it is hardly surprising that confusion surrounds the nomenclature! Typically, structures of this type are referred to as podosomes when found in normal cells, and invadopodia when found in cancer cells. But what about the Src-transformed cells where they were originally discovered? Some of us have used podosomes and invadopodia interchangeably in this case, no doubt adding to the confusion. However, these cells are truly cancer cells, faithfully mimicking all the tumorigenic properties of the fibrosarcomas caused by RSV in its native host. Therefore, going forward, we advise using the term invadopodia to describe Src-transformed fibroblasts, but recommend using both search terms, and noting the cell type under study, when evaluating all older literature. A final comment on nomenclature: most recently a catch-all term — the invadosome — has been introduced to describe all adhesive structures involved in ECM degradation and invasion. This term can be convenient where no distinction is being made between podosomes and invadopodia. However, it would seem premature to replace the original terms until and unless it becomes clear that podosomes and invadopodia are identical. In this Review, we will use the ‘normal cells = podosomes’ and ‘cancer cells = invadopodia’ rule.
Our focus in this Review is on recent progress in understanding how podosome and invadopodia formation and function are regulated, and how these structures impact development and disease. We will discuss individual podosome and invadopodia components in this context.
THE CHARACTERISTICS OF PODOSOMES AND INVADOPODIA
Both podosomes and invadopodia are characteristically composed of an actin-rich core surrounded by adhesion and scaffolding proteins7. A chorus of actin nucleators, polymerization activators, actin binding and cross-linking proteins, kinases, small GTPases and scaffold proteins regulate the actin machinery within these dynamic structures6, with the half-life of actin turnover ranging from minutes to a few hours. Key players include the adaptor proteins Tks4 and Tks5, the actin regulators cortactin and (N)-WASP, the tyrosine kinase Src, and the transmembrane metalloprotease MT1-MMP. Some of these proteins are shown in Figure 1, but readers are referred to other recent reviews for comprehensive descriptions of all the molecular components of podosomes and invadopodia6,8–10.
Figure 1. Regulators of Podosome and Invadopodia Formation.

There are several components within the cell that are regulated to induce the formation and promote the function of podosomes and invadopodia. Some of the main components that are required for these structures are highlighted are, however this is not intended to be a totally comprehensive list.
Are podosomes and invadopodia in fact distinct structures? Here, opinion differs. Some take the view that they vary in both structure and function. Others consider that there is no precedent for cancer cells “inventing” new mechanisms (rather than co-opting and dysregulating normal cellular processes) to argue that they are, in essence, identical structures. Nevertheless, while podosomes and invadopodia are very similar in overall architecture and function, morphological and molecular distinctions have been noted (Table 1). For example, previous studies have suggested that invadopodia protrude further into the ECM and are stable for hours, when compared to the minimal protrusion and rapid turnover of podosomes, and that this accounts for the higher degradative ability of cancer cells11. More recently, total internal reflection fluorescence (TIRF) microscopy studies suggest that invadopodia are part of a superstructure found in areas of membrane ruffling that is composed of a core with filament-like invadopodia emanating from it12. In contrast, podosomes neither exhibit intense membrane ruffling nor form filament-like processes12. However, it has recently been demonstrated that under appropriate culture conditions, dendritic cells can elaborate long podosomes into ECM and degrade13. How many of the perceived differences will be explained by the culture conditions, or the cell type being examined, remains to be determined.
It is also important to distinguish podosomes and invadopodia from other cellular protrusions such as filopodia and lamellipodia, and from other adhesive structures such as focal adhesions. Each has distinct morphological characteristics (Table 1), and each form in a distinct spatial location in cells growing in 2D. Yet they share several common proteins, particularly those that orchestrate actin polymerization14. Therefore, it is necessary to consider protein and lipid composition, morphology and localization to distinguish between these structures (Table 1). In particular, the co-localization of ventral actin puncta with focal degradation of the ECM is a valuable means to distinguish podosomes and invadopodia from other membrane structures (Figure 2).
Figure 2. Structure and Function of Podosomes and Invadopodia.
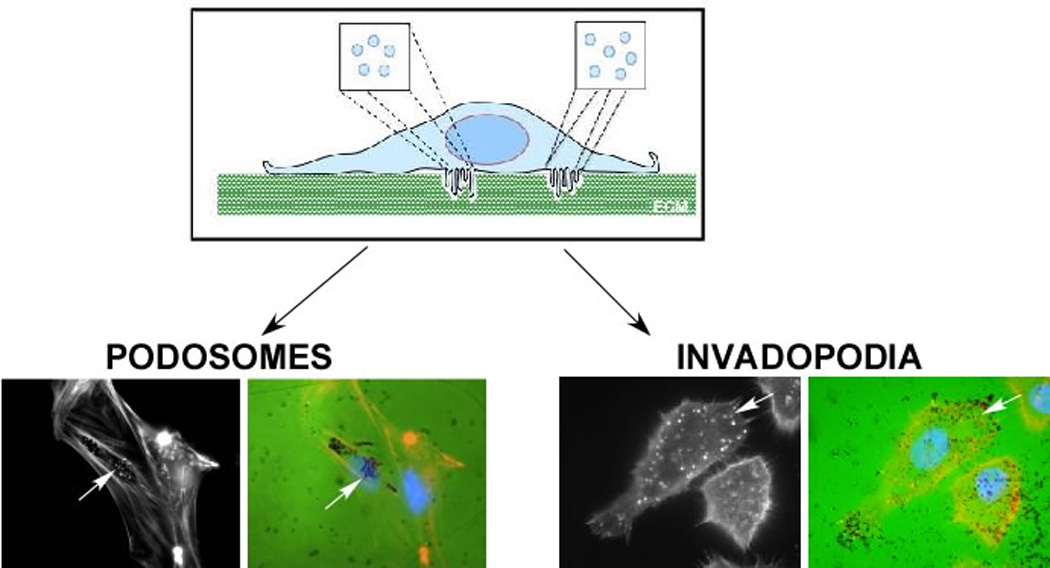
Podosomes and invadopodia are actin rich structures that are formed on the ventral membrane of the cell. These structures are often seen as individual puncta or rosettes that protrude into the extracellular matrix (ECM). Classically, presence of these structures is often confirmed by culturing cells on top of fluorescently-conjugated matrix (FITC-gelatin), staining cells for F-actin and examining co-localization between F-actin puncta and degradation of matrix (black regions). This is demonstrated in vascular smooth muscle cells (podosomes) and SCC61 head and neck squamous carcinoma cells (invadopodia) as indicated by arrows.
THE FUNCTION OF PODOSOMES AND INVADOPODIA
It is thought that podosomes or invadopodia allow a cell to coordinate ECM degradation with cell motility, to facilitate cell migration through tissue microenvironments. Cell migration is required during embryonic development and in adults in response to injury and infection. Abnormal cell migration can underlie developmental, vascular and immune diseases, as well as tumor metastasis. In keeping with this, podosomes are found in cell types involved in tissue remodeling and immune surveillance, and the presence of invadopodia is correlated with the ability of cancer cells to invade and metastasize
The maturation process for podosomes and invadopodia involves the recruitment and activation of multiple pericellular proteases, which facilitates ECM degradation15, and perhaps also cytokine release (Table 1). There are three main classes of protease present in these structures: zinc-regulated metalloproteases (MMP2, MMP9, MT1-MMP, the ADAM family of sheddases), the cathepsin cysteine proteases, and the serine proteases seprase and urokinase plasminogen activator16.
It is generally accepted that the invadopodia formed in human cancer cells and Src-transformed cells degrade ECM. However, there is some debate about the role of podosomes in ECM degradation. Earlier studies observed only shallow ECM degradation in podosome-containing cells16, and degradative ability was once thought to distinguish invadopodia from podosomes17. However, more recent studies in vascular smooth muscle cells, dendritic cells, and endothelial cells have demonstrated degradation of ECM13,18–21. From these and other studies, it appears that ECM degradation by podosomes is likely to be dependent on cell type and ECM substrate. Perhaps podosomes might also play a degradation-independent role in cell migration.
How might podosomes and invadopodia control cell motility? Planar cell migration is facilitated by the coordination of focal adhesion assembly, maturation and turnover with protrusion of the leading edge at lamellipodia22. Collective cell migration requires MT1-MMP degradation of collagen to generate ECM tracks23. Additionally, mechanical forces are recognized to play an important role, both for the formation of focal adhesions, and for the growth of adhesion-anchored stress fibers. Recently, podosomes and invadopodia were also shown to act as mechanosensors, as well as to exert traction on the underlying matrix, in a process requiring MT1-MMP24,25. More insight is likely to be achieved through investigating the dynamics of the actin comet-based structures of invadopodia, which consist of a stationary head that is localized to degradative patches and a tail that is motile26. Interestingly, these structures disappear during cell migration. A detailed understanding of the mechanism by which podosomes and invadopodia facilitate cell migration is not yet available, and will not be covered here.
VISUALIZING PODOSOMES AND INVADOPODIA
We have introduced the concept that podosomes and invadopodia can be distinguished from other protrusive and adhesive structures by a careful analysis of their morphology and protein and lipid composition. Here, we will review their morphological characteristics.
Podosomes and invadopodia in 2 dimensions
Both podosomes and invadopodia are usually visualized by co-staining cells with fluorescent phalloidin, which binds to F-actin, an obligate component of these structures and other associated-proteins such as the actin nucleator Arp2/3 or cortactin, which promotes the polymerization and rearrangement of the actin cytoskeleton. Since these proteins also co-localize with actin in other cellular structures, it is important to determine that the co-staining is on the ventral surface of the cell, using either confocal or TIRF microscopy. As an alternative, the presence of both F-actin and the adaptor protein Tks5 can be diagnostic, since Tks5 does not appear to localize to other actin-based structures such as focal adhesions but is a key component of both podosomes and invadopodia27–30. It is particularly telling that the expression of Tks5 promotes the formation of invadopodia in cells that normally do not have them, in a manner dependent on its PX domain31. Finally, the co-localization of F-actin, cortactin or Tks5 with focal ECM degradation is often used as an identifier for podosomes and invadopodia.
In 2D culture, the formation of both podosomes and invadopodia is restricted to the ventral surface of the cells. The structures often present as isolated puncta, often behind the leading edge of the cell in the case of podosomes, or under the nucleus in the case of invadopodia (Figure 3). In some Src-transformed fibroblasts, individual invadopodia cluster together into rosettes1 (Figure 3). Similar rosettes have also been observed in vascular smooth muscle cells, and occasionally in cancer cells. Rosette formation can be promoted by the expression of activated Src, activation of endogenous protein kinase C, Rho family GTPases, and certain integrins21,32–37. It is not known if there are functional differences between rosettes and individual podosomes or invadopodia. In osteoclasts cultured on glass, individual podosomes form transient circular rings that appear to fuse and form a podosome belt38. Under more physiological conditions, osteoclasts will form a similar F-actin rich structure composed of individual podosomes, called the sealing zone39. Perhaps clustering into rosettes and subsequent fusion is a maturation step that can occur in all cell types with the appropriate stimulus.
Figure 3. Podosomes and Invadopodia in 2-dimensions.
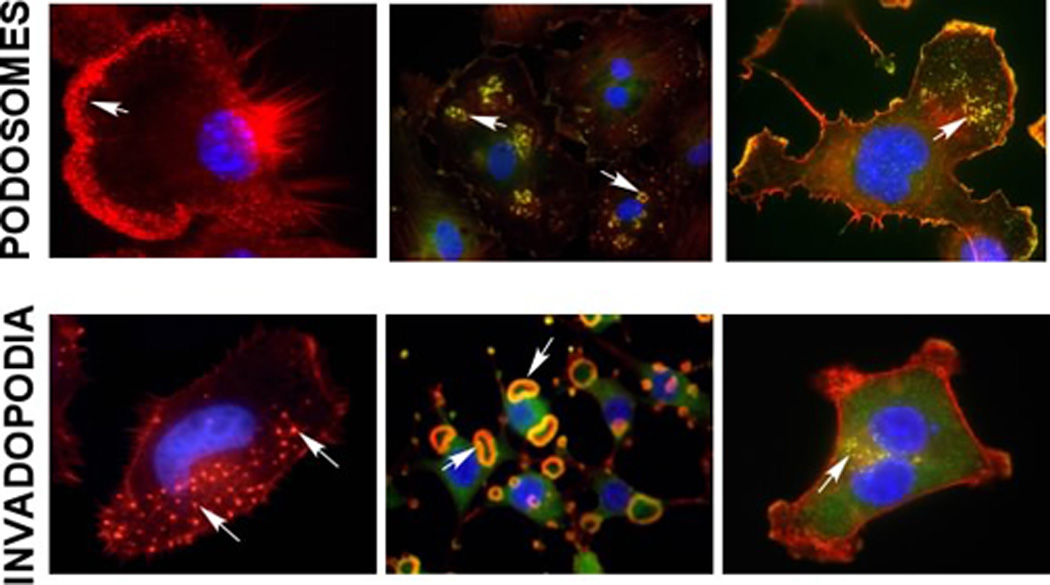
Formation of podosomes and invadopodia is frequently visualized by co-staining cells with F-actin (red) and the podosome and invadopodia associated protein cortactin (green). These structures can be seen in many cell types. Podosomes (top row): Macrophages (IC-21), vascular smooth muscle cells (VSMCs - A7r5 treated with 25nM PDGF) and neural crest stem cells (JOMA1.3, treated with 20nM PMA). Invadopodia (bottom row): head and neck squamous carcinoma cells (SCC61), Src-3T3 cells, and breast cancer cells (MDA-MB-231). Arrows denote podosomes and invadopodia.
In 2D, podosomes are relatively short-lived (2 – 20 min) whereas invadopodia can persist for several hours6. Morphological study of cells with podosomes usually involves plating cells directly onto glass coverslips, while to study invadopodia, the coverslips are frequently coated with a layer of defined ECM. A systematic analysis of how these culture conditions might affect podosome and invadopodia number, size, distribution and turnover has not been undertaken, although it is known that increasing ECM rigidity increases invadopodia formation40.
Podosomes and invadopodia in 3 dimensions
Cell culture in 3-dimensions (3D) is used to more faithfully mimic the in vivo environment and has been used to great effect by the research community to reveal key differences in the morphology, metabolism and survival of normal and cancer cells. Recent studies have used 3D systems to address podosome and invadopodia formation and function. In one of these, cancer cells were cultured on native basement membrane and the composition and function of invadopodia followed over time41. Passage of the cells through the basement membrane involved 3 stages that took place over 7 days: formation of invadopodia and perforation of the basement membrane at the sites of formation; extension and elongation of the invadopodia through and beyond the basement membrane; and invadopodia-led migration of the cells through the basement membrane41. Similar in depth studies of podosome formation in 3D have yet to be performed. However, when vascular smooth muscle cells and human monocyte derived macrophages are cultured in either collagen I or gelled collagen (fibrillar collagen with the architecture of Matrigel), respectively, they form long actin-rich protrusions that contain podosome-associated proteins42–44. The extension of long podosome-like structures is also associated with robust degradation activity in human macrophages, dendritic cells, and lymphocytes13,44,45. Similar MMP-dependent protrusive structures have been seen in 3D cultures of Src-transformed mouse sarcomas, and human melanoma, fibrosarcoma and breast cancer cells46–48. That these 3D structures may represent podosomes and invadopodia is supported by the presence of F-actin together with proteins such as talin, cortactin, FAK, MT1-MMP, N-WASP, paxillin, gelsolin, and β1-integrin, although many of these markers are also found in other adhesive structures. It will be important to rigorously establish the characteristics that determine the existence of podosomes and invadopodia in 3D.
Podosomes and invadopodia in vivo
There is much circumstantial evidence to suggest that podosomes and invadopodia are physiologically relevant structures. For example, loss of the obligatory podosome proteins WASP and Src results in defects in macrophage and osteoclast function, respectively, in vivo49,50. And key invadopodia-associated proteins are required for cancer progression in animal models51–53. Yet there is currently scant evidence for the existence of invadopodia and podosomes in vivo, although we did recently visualize Tks5- and cortactin-containing rosette-like structures in vascular smooth muscle cells in vivo19 (Figure 4). And our laboratory has also visualized Tks5-dependent protrusions in migrating embryonic cells (DAM and SAC unpublished) (Figure 4). With recent advances in the molecular characterization of the structures and enhanced microscopy techniques, we anticipate that the formation of podosomes and invadopodia will be a physiological event required for migration of many cell types in vivo.
Figure 4. Podosomes in vivo.
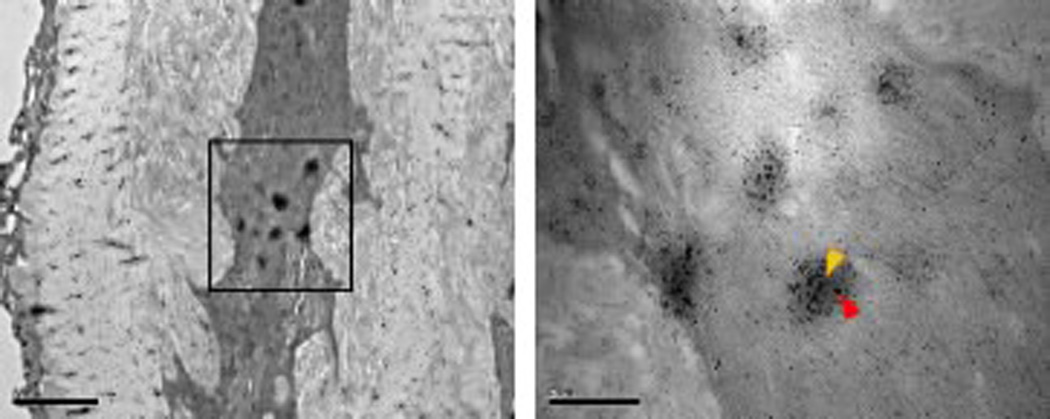
Podosome structures have recently been observed in vivo. (A) Immunoelectron microscopy for Tks5 (red arrowhead) and cortactin (yellow arrowhead) in murine VSMCs in vivo. Enlarged image of labeled aorta section (boxed area, right panel) (B) Protrusive structures visible in migratory trunk neural crest cells in (foxd3:GFP) zebrafish, Danio rerio.
PODOSOME AND INVADOPODIA INITIATION
Many studies have demonstrated that stimulation with growth factors such as PDGF, TGFβ, and EGF will induce podosome or invadopodia formation in normal and cancer cells, respectively19,21,54,55. These stimuli will elicit the phosphorylation and/or activation of key podosome and invadopodia proteins discussed above through canonical signaling pathways, especially those involving Src and PKC. Novel mechanisms for podosome and invadopodia initiation that have come to light more recently include ROS signaling, integrin signaling, and microRNA control. These will be discussed here.
Integrin signaling
Integrin receptors directly interact with the ECM and transmit extracellular signals to the cytoplasm (“outside-in signaling”), as well as the status of the cell to the extracellular space (“inside-out signaling’). They are thus ideally placed to modulate invadopodia and podosome biology. Many studies have described the localization of integrins to podosomes and invadopodia. For example, the αvβ3 integrin is found in both osteoclast podosomes and the invadopodia of several cancer types56, and the β1 subunit is also found in both podosomes and invadopodia57,58. Few studies have directly addressed the role of the integrins in this structure, although podosome and invadopodia formation is modulated by the presence of ECM substrate13,59. Antibody-induced activation of β1 increases ECM degradation60, while interference with αvβ3 in osteoclasts results in defective podosome function61. More recently, the roles of ECM and integrins in the regulation of podosome and invadopodia formation were investigated in osteoclasts and Src-transformed fibroblasts36, which express both β1 and β3. Invadopodia still form in the absence of β3, but podosome and invadopodia formation is inhibited by loss of β1 (Figure 5)59. Furthermore, phosphorylation of the cytoplasmic tail of β1 promoted the formation of rosettes59. Interestingly, acute loss of β1 also promoted the formation of focal adhesions, reinforcing the idea of a reciprocal relationship between these two structures, as described later (Figure 5).
Figure 5. The stages of invadopodia formation.
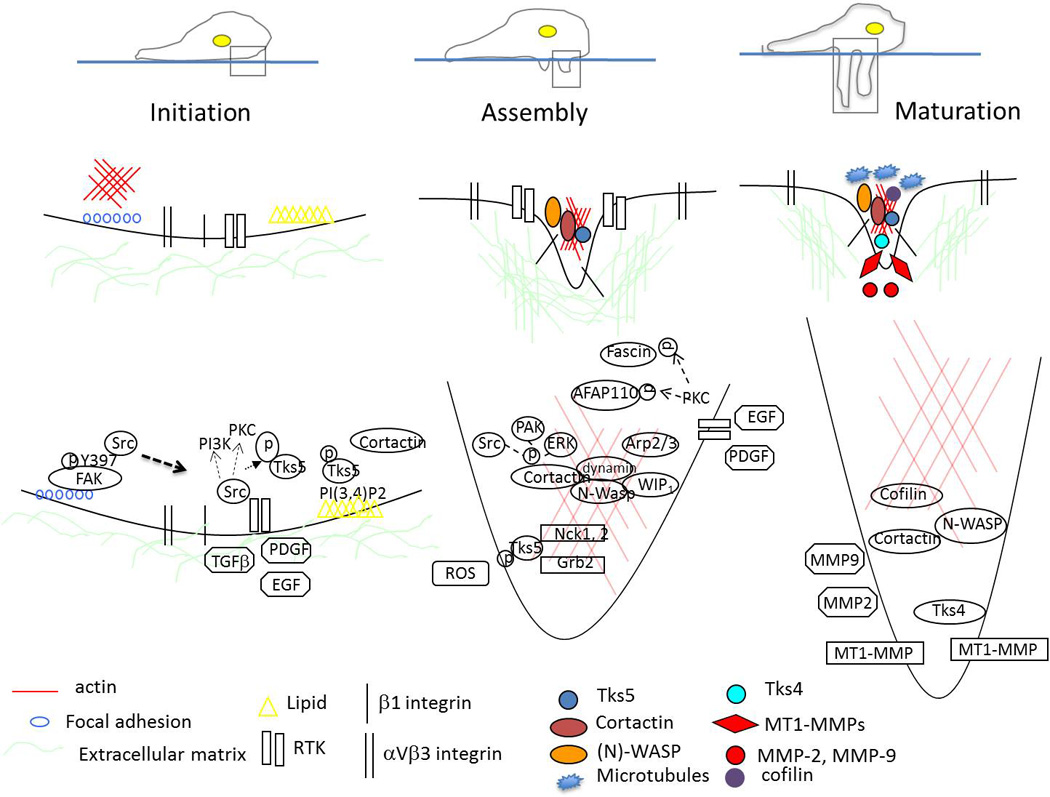
Initiation - Cells establish focal adhesions with the ECM through interaction of integrins, Src, and FAK (left side). In migrating cells, a switch occurs that will release Src to bind Tks5 and localize to regions containing PI(3,4)P2. These intracellular changes are initiated by factors such as EGF, PDGF, TGFβ (right side).
Assembly - Formation of invadopodia occurs through recruitment and activation of actin regulatory proteins (Arp2/3, WIP), phosphorylation of key invadopodia components (cortactin, Tks5, fascin, AFAP110), and production of ROS.
Maturation - Invadopodia promote degradation of ECM by coordinating secretion of MMP-2 and MMP-9, and enabling delivery (potentially through microtubules) and presentation of MT1-MMP to the tip of the protruding structure through the interaction of key invadopodia components (cortactin, Tks4, β1 integrin).
Src kinases and PKCs
Src family kinases play a pivotal role in the formation of both podosomes and invadopodia. The scaffold protein Tks5 was first identified as a Src substrate, as were other proteins present in these structures, including cortactin, the actin binding and cross-linking protein AFAP110 and the integrin effector and adaptor protein p130Cas (62,63). Given this, it is perhaps not surprising that activated Src promotes podosome or invadopodia formation, whereas inhibition of Src has the opposite effect. Src stimulates primary actin nucleation, rate of flux at the actin core and formation of the actin cloud and podosome belt within osteoclasts, suggesting that one role is in the initiation of podosome formation64. But the observation that the podosomes that do form in Src−/− osteoclasts have a fourfold longer life span with decreased actin flux suggests that it is also required for the disassembly of podosomes64. How is Src activated in normal and cancer cells? Few studies have addressed this question directly, although both PDGF and EGF, which promote podosome and invadopodia formation, activate Src family kinases65. Src also mediates integrin signals to the actin cytoskeleton66. Src and PKC act in concert to regulate podosome and invadopodia formation54,67. For example, experimental activation of PKC-α with phorbol esters or by membrane targeting induces AFAP110 to co-localize with and activate Src, via a mechanism that requires PI 3-kinase activity54. Interestingly, PKC-mediated phosphorylation of AFAP110 and fascin, an actin bundling protein, stabilizes podosomes and invadopodia46,68. Thus PKC–Src mediated pathways appear to have a predominate role in podosome and invadopodia formation. Recent studies have identified novel Src-dependent regulatory mechanisms in podosome and invadopodia formation that involve integrins, reactive oxygen species, and microRNA.
Reactive oxygen species
Most reactive oxygen species (ROS) in the cell are produced either as a by-product of oxidative phosphorylation in mitochondria, or by the action of members of the NADPH oxidase (Nox) family. Nox-catalyzed ROS production occurs to high levels in phagocytic cells as a host defense mechanism, and at lower levels in other cell types to facilitate mitogenesis and motility69. In cancer cells, ROS are produced at high levels because of metabolic stress, resulting in DNA damage and apoptosis70. More recently it has become apparent that low level ROS production in cancer cells, likely from Nox enzymes, facilitates invasion and metastasis70. Both podosome and invadopodia formation are dependent on ROS, and Nox components and ROS generation were detected in invadopodia28. Interestingly, the podosome and invadopodia proteins Tks4 and Tks5 share some architectural similarity with the phagocytic Nox organizer protein p47phox, which promotes catalysis by recruiting p67phox and Rac GTPases to the membrane components of Nox2, via association with p22phox. In a similar way, Tks proteins can also associate with both p22phox and p67phox orthologues via reciprocal SH3 domain-polyproline motif interactions28,71, which also promotes Nox catalysis. How do ROS promote invadopodia formation and function? Likely roles include increasing the expression of matrix metalloprotease (MMPs) and consequent remodeling of ECM, and transient modulation of the catalytic activity of key podosome and invadopodia regulators. For example, PKCs, and perhaps Src72, are activated by ROS73. Furthermore, ROS can transiently oxidize and inhibit protein tyrosine phosphatases and some lipid phosphatases74, which could also promote invadopodia formation. In keeping with this, knockdown of PTP-PEST increases invadopodia number28. In the future it will be important to catalog all phosphatases that are regulated by invadopodia-produced ROS, and determine their effect on invasiveness and migration.
MicroRNAs
MicroRNAs (miRs) are highly conserved small RNAs that inhibit gene expression, either by inhibiting translation or inducing degradation of target mRNAs, and in so doing control pathways involved in development, tumor growth and metastasis75,76. In vascular smooth muscle cells, miRs-143 and -145 regulate the switch between differentiated (contractile) and de-differentiated (synthetic or migratory) phenotypes, which occurs in response to vascular stress and injury77,78, by controlling podosome formation both in vitro and in vivo78,79. The miRs normally repress podosome formation by inhibiting the expression of key podosome regulators PDGF receptor-α and PKCε (in the case of miR-143) and fascin (in the case of miR-145)19. Downregulation of the miRs, and subsequent podosome formation, is initiated by a pathway involving PDGF (which is released in response to vascular stress), Src and p53 (19). Currently it is not known whether select miRs also regulate invadopodia formation in cancer cells. However, there appears to be an overall down-regulation of miRs during transformation, metastasis, and metastatic relapse75,80, suggesting that this is a possibility worth exploring.
ASSEMBLY AND TURNOVER
Podosomes and invadopodia are dynamic structures whose formation and turnover are tightly controlled. Here we will discuss what is known about the assembly, maturation and turnover of these structures.
Initiation of podosome and invadopodia formation
The shift from a quiescent to a migratory phenotype is often characterized by the dissolution of focal adhesions and the formation of podosomes or invadopodia14,81 at sites of ECM contact (Figure 5). Podosomes and invadopodia have many of the same components as focal adhesions, structures associated with stable attachment to ECM, and whose dissolution is required for cell motility. One study has suggested that there is a reciprocal relationship between focal adhesions and invadopodia, with focal adhesion kinase (FAK) — a scaffold and tyrosine kinase that, together with Src, promotes the turnover of focal adhesions — functioning as a negative regulator of invadopodia through the spatial control of Src81. In this view, phosphorylation of FAK at tyrosine 397 promotes its association with Src, and the phosphorylation of Src substrates at focal adhesions. Depletion of FAK releases active Src to promote the phosphorylation of invadopodia proteins and increase invadopodia formation. Other studies have confirmed that inhibition of FAK promotes invadopodia formation82,83. However, there are also reports that FAK is present in invadopodia, and that increased FAK expression promotes invadopodia formation40,84. Furthermore, the FAK-related kinase Pyk2 is an obligate component of osteoclast podosomes85–88. In Src-transformed fibroblasts, the formation of invadopodia is initiated in the vicinity of focal adhesions in response to the focal generation of PI3,4-P2 (29). This lipid recruits Tks5, via association with its PX domain27. Mueller and co-workers proposed that aggregation of cortactin at sites of ECM attachment was a key early step in invadopodia formation15. Condeelis and co-workers showed that Tks5 co-localized with cortactin to invadopodia precursors, and suggested that Tks5 may be the scaffold that recruits cortactin89. In this model, the focal generation of PI3,4-P2 is the key initiating step in podosome and invadopodia formation, yet we have little idea how this lipid is generated at, or localized to, the membrane near focal adhesions (Figure 5).
How might Tks5 recruit cortactin, and/or orchestrate actin polymerization? Tks5 can bind, directly or indirectly, to the key actin regulators Nck1, Nck2, (N)-WASP and Grb2 (29,30). Cortactin also associates with several actin regulatory proteins, including (N)-WASP and Arp2/3, which forms an active complex through cdc4290, WIP and dynamin, each of which are required for podosome and invadopodia formation6. It seems likely that one or more of these proteins act as a bridge between cortactin and Tks5 and that this is dependent on the phosphorylation status of cortactin. Cortactin is phosphorylated by several kinases, including those of the Src, PAK and ERK families, which regulates its interaction with other proteins53. For example, tyrosine phosphorylation of cortactin in response to EGF stimulation causes its dissociation from cofilin, leading to the generation of barbed ends from severed actin filaments, and initiating actin polymerization and invadopodia formation89 (Figure 5). Subsequent cortactin dephosphorylation blocks the severing ability of cofilin, to enable invadopodia stabilization and degradative capacity89,90.
Maturation of podosomes and invadopodia
Both cortactin and Tks4 have been shown to regulate secretion and localization of MMPs at invadopodia respectively10,91,92, and appear to play a role in the maturation of podosomes and invadopodia. In keeping with a key role for cortactin, its recruitment to future sites of ECM degradation immediately precedes the trafficking of proteases to these sites15. In contrast, knockdown of Tks5 inhibits invadopodia formation without affecting the secretion of metalloproteases31. Loss of the Tks5-related scaffold protein Tks4 has an intermediate phenotype: immature invadopodia (which contain Tks5 and cortactin) are formed and MMPs are secreted, but MT1-MMP fails to localize to the invadopodia, and ECM degradation does not take place92. Together, these data suggest a model in which Tks5 and cortactin act to generate invadopodia and secrete metalloproteases, respectively (Figure 5). Tks4 subsequently localizes and/or stabilizes MT1-MMP in the structure to allow focal activation of MMPs and subsequent ECM degradation (Figure 5).
We have already described that the localization of MT1-MMP to podosomes and invadopodia is a key maturation step. A brief description of how MT1-MMP localization is controlled by intracellular trafficking and ECM binding will serve to illustrate the complexity of its regulation. MT1-MMP is delivered to invadopodia in a number of ways. First, a significant fraction derives from endocytic recycling, in a similar manner to integrins. MT1-MMP is efficiently internalized by both clathrin- and caveolae-mediated endocytosis, with trafficking to endosomal and lysosomal compartments for either recycling or degradation. The 20 amino acid cytoplasmic domain of MT1-MMP plays a key role in regulating its endocytosis93. Currently attention focuses on Src phosphorylation of MT1-MMP within the AP2-clathrin adaptor binding sequence, which is predicted to impede endocytosis94. Src also phosphorylates endophilin A2, reducing its affinity for the GTPase dynamin, thus inhibiting clathrin-mediated endocytosis94. Additionally, clustering of MT1-MMP with β1 integrin as a consequence of type-I collagen binding slows endocytosis95. Second, MT1-MMP can be mobilized from intracellular stores by a Rab8-dependent secretory pathway96. Third, there is an important role for the coordinated actions of the actin cytoskeleton and the exocytic machinery. In this regard, activated Cdc42 and RhoA promote association of the polarity regulator IQGAP1 (which links microtubules with the actin cytoskeleton) and the exocyst complex, which localizes to invadopodia and is required for MT1-MMP localization97,98. Since MT1-MMP is not localized at invadopodia in the absence of Tks4 it will be important to determine how Tks4 interfaces with the complex control of MT1-MMP turnover. These data suggest that the MT1-MMP is accumulated at invadopodia through a balance of endocytosis and exocytosis, providing many intervention points to control the cell surface expression of this key protease.
The microtubule network and the actin cytoskeleton are closely linked and cooperate during planar cell migration99. Similar cooperation between microtubules and the actin cytoskeleton appears to promote podosome and invadopodia formation and function. For example, microtubules are required for podosome formation and turnover in macrophages and osteoclasts6 and a recent study showed that the elongation of invadopodia into basement membranes is dependent on a microtubule network that forms at the base of the structure, suggesting delivery of components to the protruding tip41 (Figure 5). Furthermore kinesins, motor proteins which transport cargo along microtubules, are required for delivery of MT1-MMP to podosomes100. How do the actin and microtubule networks cooperate? Myosin, which helps transport organelles and vesicles along actin, also transports microtubule ends along actin tracks to foci of cortical actin during cell migration101. In dendritic cells, myosin inhibition by blebbistatin prevents podosome elongation and invasion13. These data suggest that the trafficking of podosome and invadopodia proteins regulates their elongation and invasive capabilities, although it remains to be determined which key proteins are trafficked in this way.
Turnover of podosomes and invadopodia
Podosomes and invadopodia are dynamic structures, with half-lives of actin turnover ranging from minutes to hours. Yet almost nothing is known about how their turnover is regulated. Nor is it clear that the entire structure disassembles. Most studies follow only the turnover of actin, fluorescently-tagged cortactin and AFAP-110 have also been visualized68,102. It remains possible that a core structure remaining in the plasma membrane becomes uncoupled from actin polymerization to effect turnover. Alternatively, parts of the structures may turnover rapidly. For example, in invadopodia actin comets the actin head structure turns over rapidly, while the filamentous tail region persists for hours26. Protein phosphorylation is important for podosome or invadopodia formation and/or turnover. The tyrosine phosphorylation of Tks5 is critical for invadopodia formation30, whereas both the tyrosine phosphorylation of cortactin and the serine phosphorylation of AFAP-110 promotes invadopodia turnover68,89. Phosphatases likely play key roles in controlling podosome and invadopodia formation and turnover, but few have been characterized to date. PTPε plays a positive role in osteoclasts via dephosphorylation of tyrosine 527 and subsequent activation of Src103, and PTP1B is required for invadopodia formation104. In contrast, the tyrosine phosphatase PTP-PEST negatively regulates invadopodia formation28. The 5’ inositol phosphatase synaptojanin is localized to invadopodia, and is required for their formation105, whereas the 3’ inositol phosphatase and tumor suppressor PTEN represses invadopodia formation106. Finally, the serine protease calpain promotes dendritic cell podosome turnover by cleaving the podosome proteins talin, Pyk2 and WASP107. It is important to extend these studies to determine the effect of lipid and protein phosphatases on turnover, as well as isolate other classes of regulators.
PODOSOMES AND INVADOPODIA IN BIOLOGY
Several studies have suggested that podosome proteins might have a role in embryonic development. Furthermore, several human diseases that are a consequence of abnormal cell migration or invasion have been linked to de-regulation of podosome or invadopodia components. These include the genetic diseases Wiskott-Aldrich syndrome and Frank-ter-Haar syndrome, as well as atherosclerosis, tumor progression and metastasis.
Podosomes in development
During development, cells migrate from a centralized location to distal sites in the developing embryo. Cell migration begins in gastrulation and later becomes restricted to certain cell types, particularly those such as neural crest derivatives that have undergone epithelial mesenchymal transitions (EMT). Genes involved in the control of EMT during embryonic development, for example the transcription factors Twist, Snail and Slug, also regulate tumor formation and cancer progression108–111,112. Furthermore, invadopodia are frequently found in cancer cells that have undergone EMT, and Twist can promote invadopodia formation113. Yet few studies have investigated whether podosomes and/or podosome-associated proteins are required for embryonic development. Recently, a mutation in the gene encoding the podosome and invadopodia associated protein Tks4 was shown to cause the autosomal recessive disorder Frank-ter-Haar syndrome (FTHS)114, characterized by skeletal, cardiac, metabolic, and ocular defects, which are replicated in a mouse model of Tks4 loss114. These phenotypes are consistent with defects in neural crest-derived cells. To date, it is unclear whether loss of Tks4 results in abnormal podosome formation in FTHS patients or what mechanistic role it plays in this disease. However, we have recently used a zebrafish model to implicate the related protein Tks5 in neural crest cell migration, and detected podosomes in neural crest stem cells in culture (DAM and SAC unpublished). Interestingly, Src family kinases, MT1-MMP, ADAM19, and collagen, all of which are podosome-associated proteins, appear to play a role in cell movement during gastrulation115–121. Podosome-associated proteins are also involved in genetic diseases characterized by craniofacial disorders122. While it is possible that these defects could be attributed to increased apoptosis or decreased proliferation, it seems more likely that podosome-associated proteins might be required for cell migration during development.
Podosome and invadopodia proteins in disease
Wiskott-Aldrich Syndrome (WAS) is an X-linked recessive disease in which patients present with eczema, thrombocytopenia, and severe immune deficiencies123. It is caused by mutations in the WASP gene, encoding the actin binding protein WASP, which is predominately expressed in hematopoietic cells123. The observation that macrophages and dendritic cells from WAS patients are defective in podosome formation provided the first link between podosomes and a human disease49. It has recently been proposed that chemotactic factors cause the recruitment of WASP to focal adhesions, where it acts as a scaffold between integrins and the actin filaments forming in the podosome core124.
Atherosclerosis is the accumulation of vascular smooth muscle cells in response to injury and vascular stresses such as ischemia, and is attributed to both increased cell proliferation and cell migration. As noted earlier, miRs-143 and -145 control the switch of vascular smooth muscle cells from the differentiated state to the synthetic, motile state125. Furthermore, reduced expression of these miRs was noted in aortic aneurysms77. Deletion of the gene encoding miR-143 and -145 results in arterial thickening and a blunted response to vasopressive stimuli that is correlated with increased podosome formation and cell migration in primary aortic smooth muscle cells in vivo and in vitro19,77. Interestingly, TGF-β, which is activated by disruption of blood flow and ischemia, induces podosome rosettes in arterial endothelial cells cultured ex vivo20. Together these data suggest that podosome formation plays a role in vascular homeostasis.
There is a growing body of literature describing the role of invadopodia proteins in invasive cell behavior in tissue culture systems. And several invadopodia proteins are known to be expressed in human cancer tissue. For example, cortactin levels correlate with aggressiveness in squamous cell carcinoma of the head and neck52. Tks5 has been detected in several human tumor samples31. And sites of invadopodia formation are capable of degrading ECM in human lobular breast carcinoma tissue examined ex vivo126. But few studies have critically examined the role of invadopodia in tumorigenesis in vivo. One of the earliest studies examined AMAP1, an ArfGAP that acts as a bridge between paxillin and cortactin, and co-localizes with them at invadopodia127. Expression in breast cancer cells of mutant AMAP1 proteins unable to mediate trimeric complex formation with cortactin and paxillin had only a minor effect on primary tumor growth in mammary fat pads, but a more pronounced effect on subsequent metastasis to the lung. Such a phenotype is consistent with studies demonstrating that invadopodia proteins are typically not required for tumor cell growth in vitro. However, an interesting complexity has emerged from the in vivo study of other invadopodia proteins. For example, inhibition of MMPs prevents primary tumor growth in a variety of mouse models128. Over-expression of MT1-MMP in cancer cells promotes tumorigenesis, and conversely, inhibition of the enzyme reduces tumor growth as well as invasion129. Knockdown of cortactin impairs tumor growth in a model of head-and-neck cancer32, and knockdown of Tks5 also impairs primary tumor growth51.
Why the discrepancy between the cell based and animal models? Consideration of data derived from 3-dimensional (3D) culture systems perhaps offers a way to rationalize the data. Tumor cell growth in 3D matrices of type I collagen requires MT1-MMP and metalloprotease activity, whereas growth in 2D on top of type I collagen does not48,129. Likewise, cortactin promotes growth in the 3D culture environments of agarose and matrigel52. The serine/threonine kinase LIMK, and the adaptors Tks4 and Tks5 are also required for efficient growth in, but not on top of, 3D collagen (130 and Barbara Blouw, Matthew Buschman, Begona Diaz and SAC, unpublished). The mechanism(s) by which invadopodia are required for 3D growth has not yet been fully explored, but might involve the induction of proteolysis. In the case of cortactin knockdown, growth can be rescued by co-culture with cortactin-expressing cells, suggesting that invadopodia-directed proteolysis may release autocrine growth factors from the cell surface52. Focal ECM degradation might also promote ECM–integrin signaling, and be necessary for expansion of the tumor into the microenvironment. In vivo, invadopodia-mediated regulation of pericellular proteolysis could also be responsible for the regulated production of pro-angiogenic factors such as VEGF, which might explain the reduced angiogenesis observed in Tks5 knockdown tumors131. Finally, many of the stromal cells present in the tumor microenvironment, including macrophages, fibroblasts, and endothelial cells, can form podosomes. It will be interesting to determine what role podosomes might play in tumor progression.
Conclusions/Perspectives
Tremendous strides have been made in our understanding of podosomes and invadopodia in recent years, yet many important questions remain. Are there molecular differences between the two structures? What role do they play in the mechanics of cell migration? How many cell types elaborate podosomes during development? What are the master switches that control their formation and function? What role does the ECM play in formation of these structures? How is pericellular proteolysis controlled by podosomes and invadopodia? Which diseases might be caused by alterations in podosome biology? How do invadopodia promote tumor growth and progression?
Even as we address these questions, there is already strong circumstantial evidence to suggest that modulation of podosomes and invadopodia might represent a viable therapeutic strategy to alter the course of several diseases. For example, podosome and invadopodia inhibitors might have value in the treatment of atherosclerosis and cancer. Transient activators of podosome formation might also ameliorate the progressive skeletal anomalies of FTHS infants and perhaps other craniofacial syndromes, as well as immunodeficiency. Many of the known components of podosomes and invadopodia are scaffolding or adaptor proteins with no catalytic activity, and are therefore unlikely drug targets. In the future, it will be important to identify key enzymatic regulators, with an eye to the generation of novel targeted therapies.
SUMMARY.
Podosomes and invadopodia are actin-based dynamic protrusions of the plasma membrane. They act as sites of attachment to, and degradation of, the extracellular matrix.
These structures contain actin regulators such as cortactin and (N)-WASP, adaptor proteins such as Tks4 and Tks5, and several pericellular proteases.
Podosomes are found in vascular smooth muscle and endothelial cells, as well as cells derived from monocyte lineages. Their presence correlates with migratory ability.
Invadopodia are found in invasive human cancer cells. In 2D culture, their presence correlates with invasive behavior. However, in 3D culture and in vivo, invadopodia proteins are required for cell growth.
Podosome proteins have been implicated in human developmental and immune disorders and dysregulation of podosome formation is associated with atherosclerosis.
Small molecule regulation of podosomes and invadopodia might represent a novel therapeutic strategy to treat several diseases.
ACKNOWLEDGEMENTS
We are grateful to Begoña Diaz, Matt Buschman and Christine Gould for reading the manuscript, Jeff Tsai for help in figure preparation, and Barbara Blouw, Begoña Diaz, Leonardo Elia and Manuela Quintavalle for the images of podosomes and invadopodia. The Courtneidge laboratory is supported by grants from the National Cancer Institute and the Mathers Foundation. Danielle Murphy is supported by a postdoctoral fellowship from the American Cancer Society.
GLOSSARY
Epithelial mesenchymal transitions;
- PX domain
a protein domain of approximately 120 amino acids that associates with phosphatidylinositol lipids
- barbed end
the end of an F-actin polymer to which a G-actin monomer is attached
- lamellipodia
actin based projections of the leading edge of a migrating cell
- actin cloud
a concentration of actin that surrounds podosome cores in osteoclasts
- podosome belt
a fusion of podosomes at the periphery of osteoclasts cultured in 2D
- outside-in signalling
the transmission of extracellular signals to the cytoplasm by the engagement of integrin receptors with the ECM
- inside-out signaling
the transmission of the status of the cell to the extracellular space via integrin receptors
REFERENCES
- 1.David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77:6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen WT, Chen JM, Parsons SJ, Parsons JT. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985;316:156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- 4.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 5.Zambonin-Zallone A, Teti A, Carano A, Marchisio PC. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res. 1988;3:517–523. doi: 10.1002/jbmr.5650030507. [DOI] [PubMed] [Google Scholar]
- 6.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Linder S, Kopp P. Podosomes at a glance. J Cell Sci. 2005;118:2079–2082. doi: 10.1242/jcs.02390. [DOI] [PubMed] [Google Scholar]
- 8.Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–149. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 9.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–590. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver AM. Invadopodia. Curr Biol. 2008;18:R362–R364. doi: 10.1016/j.cub.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Artym VV, Matsumoto K, Mueller SC, Yamada KM. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur J Cell Biol. 2011;90:172–180. doi: 10.1016/j.ejcb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gawden-Bone C, et al. Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J Cell Sci. 2010;123:1427–1437. doi: 10.1242/jcs.056515. This is the first description of highly protrusive podosomes that have the ability to extensively degrade ECM, in a similar fashion to invadopodia.
- 14.Block MR, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 16.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Ayala I, Baldassarre M, Caldieri G, Buccione R. Invadopodia: a guided tour. Eur J Cell Biol. 2006;85:159–164. doi: 10.1016/j.ejcb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Burgstaller G, Gimona M. Podosome-mediated matrix resorption and cell motility in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;288:H3001–H3005. doi: 10.1152/ajpheart.01002.2004. [DOI] [PubMed] [Google Scholar]
- 19. Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. This is the first demonstration that microRNAs can control podosome formation, and the first time that podosomes have been visualized in vivo.
- 20. Rottiers P, et al. TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J Cell Sci. 2009;122:4311–4318. doi: 10.1242/jcs.057448. Podosomes are seen in ex vivo cultures of endothelial cells for the first time.
- 21.Varon C, et al. Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26:3582–3594. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf K, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 24.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collin O, et al. Self-organized podosomes are dynamic mechanosensors. Curr Biol. 2008;18:1288–1294. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldassarre M, et al. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Abram CL, et al. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278:16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- 28. Diaz B, et al. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. This paper provides the first demonstration that reactive oxygen species control the formation of invadopodia and podosomes.
- 29. Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. This paper describes how invadopodia form near focal adhesions at membrane sites containing PI 3,4P2.
- 30.Stylli SS, et al. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J Cell Sci. 2009;122:2727–2740. doi: 10.1242/jcs.046680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seals DF, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. This paper describes that Tks5 is essential to invadopodia formation and invasion, and that its expression can promote invadopodia formation in noninvasive cancer cells.
- 32.Billottet C, et al. Regulatory signals for endothelial podosome formation. Eur J Cell Biol. 2008;87:543–554. doi: 10.1016/j.ejcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Cougoule C, et al. Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis. Blood. 2010;115:1444–1452. doi: 10.1182/blood-2009-04-218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau V, Tatin F, Varon C, Genot E. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol. 2003;23:6809–6822. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ory S, Brazier H, Pawlak G, Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87:469–477. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 36. Destaing O, et al. {beta}1A Integrin is a Master Regulator of Invadosome Organization and Function. Mol Biol Cell. 2010;21:4108–4119. doi: 10.1091/mbc.E10-07-0580. A mechanistic analysis of how integrins regulate invadopodia and podosome formation.
- 37.Berdeaux RL, Diaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–323. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luxenburg C, et al. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander NR, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. A fascinating microscopic analysis of the temporal elements controlling invadopodia formation and movement through the basement membrane.
- 42.Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Cote GP. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res. 2007;100:1328–1336. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- 43.Van Goethem E, et al. Macrophage podosomes go 3D. Eur J Cell Biol. 2011;90:224–236. doi: 10.1016/j.ejcb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol. 2010;184:1049–1061. doi: 10.4049/jimmunol.0902223. [DOI] [PubMed] [Google Scholar]
- 45.Carman CV, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li A, et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tolde O, Rosel D, Vesely P, Folk P, Brabek J. The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010;89:674–680. doi: 10.1016/j.ejcb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 48. Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. The first demonstration that MT1-MMP is required for 3D, but not 2D growth of cancer cells.
- 49. Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. This is the first time that a podosome protein was implicated in a human disease.
- 50.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 51.Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark ES, et al. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene. 2009;28:431–444. doi: 10.1038/onc.2008.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver AM. Cortactin in tumor invasiveness. Cancer Lett. 2008;265:157–166. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC. Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol. 2004;24:7578–7597. doi: 10.1128/MCB.24.17.7578-7597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zambonin-Zallone A, et al. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a beta 3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp Cell Res. 1989;182:645–652. doi: 10.1016/0014-4827(89)90266-8. [DOI] [PubMed] [Google Scholar]
- 57.Helfrich MH, et al. Beta 1 integrins and osteoclast function: involvement in collagen recognition and bone resorption. Bone. 1996;19:317–328. doi: 10.1016/s8756-3282(96)00223-2. [DOI] [PubMed] [Google Scholar]
- 58.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99(Pt 2):213–225. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 59.Destaing O, et al. beta1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–4119. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakahara H, et al. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura I, et al. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci. 1999;112(Pt 22):3985–3993. doi: 10.1242/jcs.112.22.3985. [DOI] [PubMed] [Google Scholar]
- 62.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 63.Lock P, Abram CL, Gibson T, Courtneidge SA. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Destaing O, et al. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 66.Obergfell A, et al. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 68.Dorfleutner A, et al. Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J Cell Sci. 2008;121:2394–2405. doi: 10.1242/jcs.026187. [DOI] [PubMed] [Google Scholar]
- 69.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grek CL, Tew KD. Redox metabolism and malignancy. Curr Opin Pharmacol. 2010;10:362–368. doi: 10.1016/j.coph.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gianni D, et al. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchoragedependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu WS, et al. Reactive oxygen species mediated sustained activation of protein kinase C alpha and extracellular signal-regulated kinase for migration of human hepatoma cell Hepg2. Mol Cancer Res. 2006;4:747–758. doi: 10.1158/1541-7786.MCR-06-0096. [DOI] [PubMed] [Google Scholar]
- 74.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 75.Baranwal S, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126:1283–1290. doi: 10.1002/ijc.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 77.Elia L, et al. The knockout of miR-143 and-145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xin M, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cordes KR, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 81.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S, Yamashita H, Weidow B, Weaver AM, Quaranta V. Laminin-332-beta1 integrin interactions negatively regulate invadopodia. J Cell Physiol. 2010;223:134–142. doi: 10.1002/jcp.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vitale S, Avizienyte E, Brunton VG, Frame MC. Focal adhesion kinase is not required for Src-induced formation of invadopodia in KM12C colon cancer cells and can interfere with their assembly. Eur J Cell Biol. 2008;87:569–579. doi: 10.1016/j.ejcb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J Biol Chem. 2002;277:12487–12490. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- 85.Bruzzaniti A, et al. Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclasts. Mol Cell Biol. 2009;29:3644–3656. doi: 10.1128/MCB.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duong LT, et al. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gil-Henn H, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/ −) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duong le T. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J Biol Chem. 2003;278:11502–11512. doi: 10.1074/jbc.M206579200. [DOI] [PubMed] [Google Scholar]
- 89. Oser M, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. This paper provides a mechanism by which cortactin phosphorylation promotes invadopodia assembly.
- 90.Yamaguchi H, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. This is the first demonstration of the regulated secretion of MMPs at invadopodia.
- 92.Buschman MD, et al. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. A comprehensive review of the regulation of MT1-MMP trafficking.
- 94.Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell. 2005;9:185–196. doi: 10.1016/j.devcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 95.Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bravo-Cordero JJ, et al. MT1-MMP proinvasive activity is regulated by a novel Rab8- dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 98.Sakurai-Yageta M, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 100.Wiesner C, Faix J, Himmel M, Bentzien F, Linder S. KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages. Blood. 116:1559–1569. doi: 10.1182/blood-2009-12-257089. [DOI] [PubMed] [Google Scholar]
- 101.Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 102.Badowski C, et al. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol Biol Cell. 2008;19:633–645. doi: 10.1091/mbc.E06-01-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Granot-Attas S, Luxenburg C, Finkelshtein E, Elson A. Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol Biol Cell. 2009;20:4324–4334. doi: 10.1091/mbc.E08-11-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cortesio CL, et al. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chuang YY, et al. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004;64:8271–8275. doi: 10.1158/0008-5472.CAN-04-2097. [DOI] [PubMed] [Google Scholar]
- 106.Mukhopadhyay UK, et al. Doubles game: Src-Stat3 versus p53-PTEN in cellular migration and invasion. Mol Cell Biol. 2010;30:4980–4995. doi: 10.1128/MCB.00004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–2385. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- 108.Blanco MJ, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 109.Come C, et al. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 110.Hartwell KA, et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A. 2006;103:18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eckert MA, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iqbal Z, et al. Disruption of the podosome adaptor protein TKS4 (SH3PXD2B) causes the skeletal dysplasia eye, cardiac abnormalities of Frank-Ter Haar Syndrome. Am J Hum Genet. 2010;86:254–261. doi: 10.1016/j.ajhg.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baas D, Malbouyres M, Haftek-Terreau Z, Le Guellec D, Ruggiero F. Craniofacial cartilage morphogenesis requires zebrafish col11a1 activity. Matrix Biol. 2009 doi: 10.1016/j.matbio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 116.Coyle RC, Latimer A, Jessen JR. Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp Cell Res. 2008;314:2150–2162. doi: 10.1016/j.yexcr.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 117.Jopling C, den Hertog J. Fyn/Yes and non-canonical Wnt signalling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 2005;6:426–431. doi: 10.1038/sj.embor.7400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neuner R, Cousin H, McCusker C, Coyne M, Alfandari D. Xenopus ADAM19 is involved in neural, neural crest and muscle development. Mech Dev. 2009;126:240–255. doi: 10.1016/j.mod.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharma D, Holets L, Zhang X, Kinsey WH. Role of Fyn kinase in signaling associated with epiboly during zebrafish development. Dev Biol. 2005;285:462–476. doi: 10.1016/j.ydbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 121.Takatsuka A, et al. Ablation of Csk in neural crest lineages causes corneal anomaly by deregulating collagen fibril organization and cell motility. Dev Biol. 2008;315:474–488. doi: 10.1016/j.ydbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 122.Cejudo-Martin P, Courtneidge SA. Podosomal proteins as causes of human syndromes: A role in craniofacial development? Genesis. 2011 doi: 10.1002/dvg.20732. [DOI] [PubMed] [Google Scholar]
- 123.Kirchhausen T. Wiskott-Aldrich syndrome: a gene, a multifunctional protein and the beginnings of an explanation. Mol Med Today. 1998;4:300–304. doi: 10.1016/s1357-4310(98)01268-4. [DOI] [PubMed] [Google Scholar]
- 124.Monypenny J, et al. Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur J Cell Biol. 2010 doi: 10.1016/j.ejcb.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Curr Drug Targets. 11:943–949. doi: 10.2174/138945010791591313. [DOI] [PubMed] [Google Scholar]
- 126.Busco G, et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010 doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 127.Onodera Y, et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hotary KB, et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 130.Scott RW, et al. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol. 2010;191:169–185. doi: 10.1083/jcb.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deryugina EI, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 132.Miyauchi A, et al. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J Cell Biol. 1990;111:2543–2552. doi: 10.1083/jcb.111.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monsky WL, et al. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–5710. [PubMed] [Google Scholar]
- 134.Nakahara H, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1- matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci U S A. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sato T, et al. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J Cell Sci. 1997;110:589–596. doi: 10.1242/jcs.110.5.589. [DOI] [PubMed] [Google Scholar]
- 136.Linder S, Hufner K, Wintergerst U, Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J Cell Sci. 2000;113:4165–4176. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- 137.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 138.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 139.Raucher D, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 140.Nachmias VT, Kavaler J, Jacubowitz S. Reversible association of myosin with the platelet cytoskeleton. Nature. 1985;313:70–72. doi: 10.1038/313070a0. [DOI] [PubMed] [Google Scholar]
- 141.Dikovsky D, Bianco-Peled H, Seliktar D. Defining the role of matrix compliance and proteolysis in three-dimensional cell spreading and remodeling. Biophys J. 2008;94:2914–2925. doi: 10.1529/biophysj.107.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schober JM, Komarova YA, Chaga OY, Akhmanova A, Borisy GG. Microtubule-targeting-dependent reorganization of filopodia. J Cell Sci. 2007;120:1235–1244. doi: 10.1242/jcs.003913. [DOI] [PubMed] [Google Scholar]
- 144.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 145.Wolfenson H, Henis YI, Geiger B, Bershadsky AD. The heel and toe of the cell's foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil Cytoskeleton. 2009;66:1017–1029. doi: 10.1002/cm.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Franz CM, Muller DJ. Analyzing focal adhesion structure by atomic force microscopy. J Cell Sci. 2005;118:5315–5323. doi: 10.1242/jcs.02653. [DOI] [PubMed] [Google Scholar]
- 147.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- 148.Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA. Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2- containing signaling proteins with gelsolin. J Biol Chem. 2001;276:47434–47444. doi: 10.1074/jbc.M107494200. [DOI] [PubMed] [Google Scholar]
- 149.Furlan F, et al. Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. J Bone Miner Res. 2007;22:1387–1396. doi: 10.1359/jbmr.070516. [DOI] [PubMed] [Google Scholar]


