Abstract
Dementia is a frequent complication of Parkinson’s disease (PD). About half of PD dementia (PDD) is hypothesized to be due to progression of the underlying Lewy body pathology into limbic regions and the cerebral cortex while the other half is thought to be due to coexistent Alzheimer’s disease. Clinically, however, these are indistinguishable. The spread of amyloid plaques to the striatum has been reported to be a sensitive and specific indicator of dementia due to Alzheimer’s disease (AD). The purpose of the present study was to determine if the presence of striatal plaques might also be a useful indicator of the presence of diagnostic levels of AD pathology within PD subjects. We analyzed neuropathologically-confirmed cases of PD without dementia (PDND, N = 31), PDD without AD (PDD, N = 31) and PD with dementia meeting clinicopathological criteria for AD (PDAD, N =40). The minimum diagnostic criterion for AD was defined as including a clinical history of dementia, moderate or frequent CERAD cortical neuritic plaque density and Braak neurofibrillary stage III–VI. Striatal amyloid plaque densities were determined using Campbell-Switzer and Thioflavine S stains. Striatal plaque densities were significantly higher in PDAD compared to PDD (p<0.001). The presence of striatal plaques was approximately 80% sensitive and 80% specific for predicting AD. In comparison, the presence of cerebral cortex plaques alone was highly sensitive (100%) but had poor specificity (48% to 55%). The results suggest that striatal amyloid imaging may be clinically useful for making the distinction between PDD and PDAD.
Keywords: striatum, Lewy body, diagnosis, autopsy, neuropathology, biomarker
Introduction
When dementia presents at least one year after the onset of motor Parkinson’s disease (PD), patients are clinically diagnosed as Parkinson’s disease with dementia (PDD) [2,3]. However, if dementia precedes PD or the diagnosis is within 1 year of the onset of motor signs, patients are diagnosed as dementia with Lewy bodies (DLB) [3,4]. The cause of cognitive impairment and dementia in PDD is thought to be due primarily to either PD-related pathologic lesions (Lewy bodies and associated neuritic changes) or concomitant AD-type changes (amyloid plaques and neurofibrillary tangles) but other pathologies are also contributory, including vascular lesions, non-AD tauopathies, hippocampal sclerosis and progressive supranuclear palsy [2,5–10].
The presence of concomitant histopathology meeting clinicopathological criteria for AD within the setting of PDD has been reported to range from non-existent to 65% and varies depending on the specific neuropathological criteria used to diagnose AD [2,7,8,11–14]. A recent consensus paper has issued new guidelines for the neuropathological diagnosis of AD in an attempt to reconcile divergent opinions and assimilate new data [15]. The relative importance of AD and Lewy-type histopathology for dementia within PD has been debated but the majority of the research indicates both are significant contributors [13,16–20]. Currently, however, it is not possible to clinically separate PDD from PDAD as the characteristics of the dementia syndrome are very similar [14].
Striatal plaques in AD were first reported in 1984 [21]. It was soon realized that striatal plaques are a later disease development, being infrequent at preclinical stages and frequent when dementia is present [22–24]. The spread of amyloid plaques to the striatum and other brain regions beyond the cerebral cortex was later used as the basis of a staging scheme for AD by Thal and colleagues [22]. In this scheme, amyloid deposition first occurs in the neocortex and then progresses to allocortex, diencephalon and striatum, brainstem and finally cerebellum. We recently demonstrated, in an autopsy sample composed of AD and non-demented elderly control subjects, that striatal plaques have a high predictive value for the presence of clinicopathological AD [25].
Striatal plaques may have similar diagnostic value in the setting of PDD. Striatal plaques have been reported to be present in PDD and DLB [26–30] but their diagnostic value for AD in the context of PDD has not been specifically assessed. The purpose of this study was to determine if striatal plaques could be used to predict, in an autopsy sample of subjects with PDD, the presence of pathologically defined AD.
Materials and Methods
Case selection
The study took place at Banner Sun Health Research Institute (BSHRI), with autopsies performed on elderly subjects who had volunteered for the BSHRI Brain and Body Donation Program, a longitudinal clinicopathological study of normal aging, dementia and parkinsonism [31]. The study was approved by the Banner Health Institutional Review Board and all participants or next of kin gave informed consent. The database was queried for cases between the years of 1997–2010 that had clinicopathological diagnoses of PD [32] without dementia (PD, N=31), PDD [3] without pathological AD (PDD, N=31), and PDD with pathological AD (PDAD, N=40). For comparative purposes, subjects from a previously published study [25] with AD without PD (AD, N = 50) as well as non-demented normal control subjects without parkinsonism (NC, N = 62) were also included. For all subjects, those with concomitant clinicopathological diagnoses of other causes of parkinsonism such as corticobasal degeneration and progressive supranuclear palsy were excluded. Subjects (Table 1) were clinically characterized as previously described [31,33] through use of standardized periodic neurological and neuropsychological assessments and/or review of private medical records, self-report and telephone interviews with spouses and/or caregivers. All cases underwent autopsy and a standardized neuropathological assessment, resulting in the assignment of pathological diagnoses according to published recommendations. For clinicopathologic diagnoses, cases received a diagnosis of PD if they had two or more cardinal clinical signs as well as Lewy bodies and pigmented neuron loss in the substantia nigra. Cases received a diagnosis of AD if they had a clinical history of dementia and were classified as “intermediate” or “high” according to the 1997 NIA-Reagan criteria [34] as well as the revised criteria recently published [15]. Dementia with Lewy bodies was distinguished from PD with dementia according to consensus criteria published by the Dementia with Lewy Bodies Consortium [4]. Subjects with Lewy body-related histopathology were also classified according to the Unified Staging System for Lewy Body Disorders [13].
Table 1.
Demographics and pathologic descriptions of series. AD=Alzheimer’s disease, PDAD= Parkinson’s disease dementia with AD, PDD= Parkinson’s disease dementia without AD, PD= Parkinson’s disease, NC= non-demented normal control, NFT=neurofibrillary tangles, LB=Lewy bodies.
| AD | PDAD | PDD | PD | NC | P values | |
|---|---|---|---|---|---|---|
| N (M:F) | 50 (25:25) | 40 (30:10) | 31(22:9) | 31(18:13) | 62 (33:29) | 0.07 |
| age at death-mean (±stdev) | 81±10 | 81±5 | 77±6 | 80±7 | 87±6* | <0.001 |
| PD duration-mean (±stdev) | - | 10±6 | 15±8# | 14±8 | - | 0.01 |
| Number of cases | - | 37 | 31 | 25 | - | |
| Dementia duration-mean (±stdev) | 8±4* | 3±2 | 4±5 | - | - | <0.001 |
| Number of cases | 42 | 15 | 13 | - | - | |
| Braak NFT stage-median (range) | V (II–VI)* | IV (I–V)** | II (I–IV) | III (I–IV) | III (I–IV) | <0.001 |
| NIA Reagan-median | high** | intermediate** | not met | not met | not met | <0.001 |
| APOE4 allele, N (%) | 27 (54)** | 17 (43)** | 5 (16) | 6 (19) | 17 (27) | <0.001 |
| Unified LB stage, N (%) | ||||||
| lla. Brainstem Predominant | - | 3 (8) | 3 (10) | 8 (26) | - | 0.06 |
| llb. Limbic Predominant | - | 0 | 1 (3) | 3 (10) | - | 0.12 |
| lll. Brainstem/Limbic | - | 13 (33) | 19 (61)# | 16 (52)# | - | <0.001 |
| lV. Neocortical | - | 23 (59)┼ | 9 (29) | 4 (13) | - | <0.001 |
significantly greater than all other groups,
significantly greater than PDD, PD, and NC,
significantly greater than PDAD,
significantly greater than PDD and PD.
Tissue processing and histological methods
Tissue processing methods have been previously described [25,31]. Briefly, the cerebrum was cut in the coronal plane at the time of brain removal into 1 cm thick slices and then divided into left and right halves. The slices from the right half were frozen between slabs of dry ice while the slices from the left half were fixed by immersion in neutral-buffered 4% formaldehyde for 48 hours at 4 degrees C. Following cryoprotection in ethylene glycol and glycerol with 0.1 M pH 7.4 phosphate buffer, selected 3 × 4 cm blocks were sectioned at 40 or 80 μm thickness on a sliding freezing microtome. Sections were stained with hematoxylin and eosin (H&E), thioflavine S and enhanced silver methods for amyloid plaques and neurofibrillary tangles, using the Campbell-Switzer and Gallyas methods respectively. The Campbell-Switzer stain has been reported by several groups to be as sensitive as Aβ immunohistochemistry for the detection of all types of senile plaques including diffuse plaques [22]. Thioflavine S is one of the methods recommended and validated for neuritic plaque density grading by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [1] while the Braak neurofibrillary tangle staging protocol was originally described using the Gallyas stain [35]. The validity and accuracy of this combination of stains for estimating the density of Aβ deposits has also been established in our laboratory through strong correlations with autoradiographic binding of an imaging ligand, Florbetapir, in postmortem human brain sections (R = 0.95) from AD subjects [36], and with ELISA biochemical measures of Aβ (R = 0.89) in cerebral cortex extracts [37].
Histopathological scoring was performed blinded to clinical and neuropathological diagnosis. Amyloid plaque densities were graded using the CERAD templates, and neurofibrillary tangles were assessed using the original Braak and Braak protocol with the Gallyas stain [35]. All subjects were genotyped for apolipoprotein E (ApoE) using a modification of a standard method [38,39]. In a subset of 41 cases (NC= 20; AD = 3; ADPD = 7, PDD = 5; PDND = 6) striatal tyrosine hydroxylase (TH) concentrations were assessed in the posterior putamen using an ELISA method previously published [40].
Plaque density measures
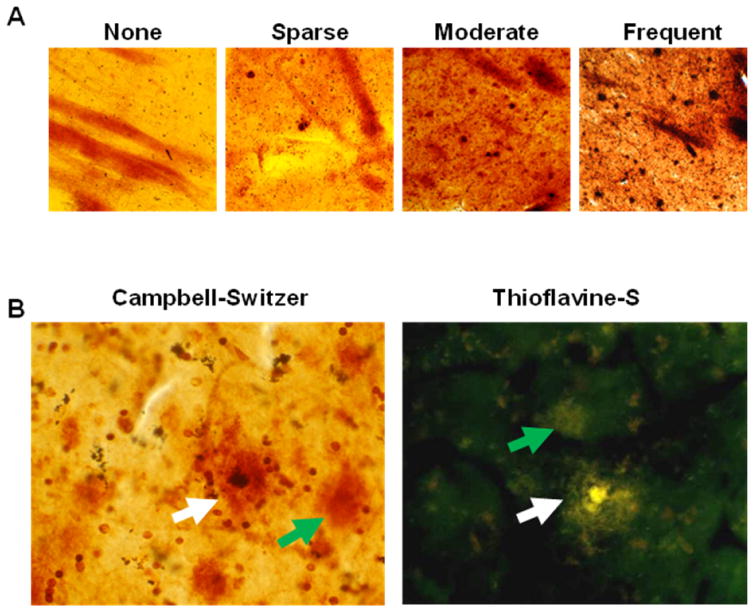
Amyloid plaque density was graded and staged at standard sites in frontal, temporal and parietal cortex, based on the aggregate impression from the thick sections stained with thioflavine S, Campbell-Switzer and Gallyas methods. Plaque density scores were obtained by assigning values of none (0), sparse (1), moderate (2) and frequent (3), according to the published CERAD templates [1]; representative images are located in Figure 1. Scores for plaque density were derived by considering all types of plaques together (cored + neuritic + diffuse = total plaques) as well as separately for cored and neuritic plaques (NP) considered together, without diffuse plaques (Table 2).
Figure 1.
A) Representative images taken at 10× magnification of plaque density scores in the putamen of none (0), sparse (1), moderate (2) and frequent (3), according to the published CERAD criteria [1]. B) Representative images taken at 40× magnification of neuritic (white arrow) and diffuse plaques (green arrow) with Thioflavine-S and Campbell-Switzer staining.
Table 2.
Striatal and cortical plaque scores for all groups. All values are the median followed by the range in parentheses. The AD and PDAD groups had significantly higher plaque scores (both for total plaques and neuritic/cored plaques) in all areas analyzed. AD=Alzheimer’s disease, CERAD= consortium to establish a registry for Alzheimer’s disease, PDAD= Parkinson’s disease dementia with AD, PDD= Parkinson’s disease dementia without AD, PD= Parkinson’s disease, NC= non-demented normal control, NP= neuritic/cored plaques.
| AD | PDAD | PDD | PDND | NC | P values | |
|---|---|---|---|---|---|---|
| CERAD NP cerebral cortex score | 3 (2–3)** | 2 (2–3)** | 0 (0–3) | 0 (0–2) | 0 (0–1) | <0.001 |
|
| ||||||
| Total striatal plaque density | 2.75 (0–3)** | 1.5 (0–3)** | 0 (0–2.5) | 0 (0–2.5) | 0 (0–3) | <0.001 |
| NP striatal density | 0.5 (0–2)* | 0.25 (0–2)** | 0 (0–0.5) | 0 (0–0.5) | 0 (0–1) | <0.001 |
|
| ||||||
| Total cortical plaque density | 3 (2–3)* | 2.67 (1–3)** | 0 (0–2.5) | 0.5 (0–3) | 1.5 (0–3) | <0.001 |
| NP cortical density | 3 (2–3)** | 2.5 (1–3)** | 1 (0–3) | 1 (0–3) | 1 (0–3) | <0.001 |
significantly greater than all other groups,
significantly greater than PDD, PD, and NC
The striatum was assessed within the posterior putamen at the level of the full development of the lentiform nucleus. The caudate nucleus was not assessed. Semi-quantitative grading of striatal plaque density in PD cases was performed by 4 raters (BND, GES, TGB, LIS) blinded to case diagnostic status. In a subset of 29 cases graded by all raters, Spearman correlations between all raters were found to be strong and statistically significant (Spearman rho ranged between 0.85–0.96; all p values <0.001). After all grading was complete, data were un-blinded for analysis.
Statistical analysis
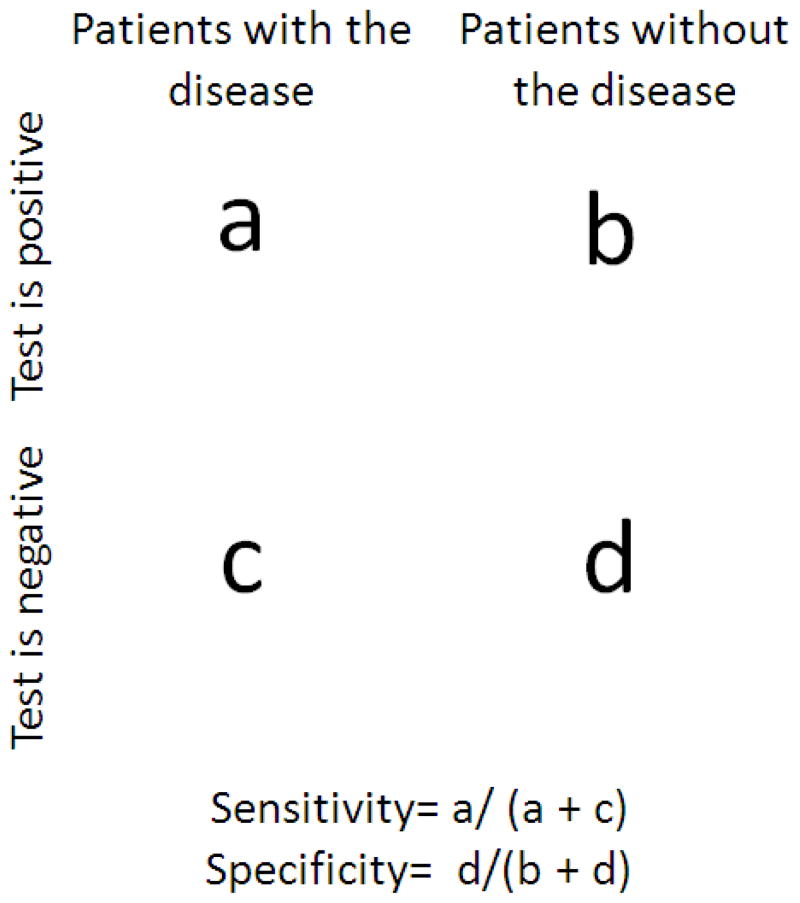
Kruskal-Wallis non-parametric analysis of variance and chi-square tests were used to analyze clinical and pathologic differences between groups. Dunn’s post hoc pairwise analysis was used as appropriate. All statistical analyses were performed with Sigma Plot 12.0 (Systat Software, Inc., San Jose, CA, USA). The significance level for all tests was set at p < 0.05. Sensitivity and specificity calculations were performed separately using all three PD groups (PDND, PDD and PDAD) or only the PDD and PDAD groups, in order to simulate possible clinical settings. A diagram of how sensitivity and specificity were calculated is located in Figure 2.
Figure 2.
Diagram of how sensitivity and specificity were determined. Sensitivities were calculated by dividing the number of cases containing a positive test (i.e. specified plaque measure) and patients with the disease (i.e. AD or dementia) - a in the figure above) by the total number of cases having the disease diagnosis regardless of test outcome (a + c). Specificities were calculated by dividing the number of cases having a negative test (i.e. lacking the specified plaque measure) and without the disease (i.e. no AD or dementia) - d in the figure above and by the total number of cases lacking the disease regardless of test outcome (b + d).
Results
The basic characteristics of the study subjects are shown in Table 1. Seven of the AD cases also met clinicopathological criteria for vascular dementia (VaD) and 3 had the additional diagnosis of hippocampal sclerosis (HS). Subjects with AD alone did not differ significantly in any demographic or AD-related neuropathological measures from those with AD/VaD or AD/HS and therefore all were grouped together for further analysis. There were no differences amongst groups with respect to gender ratios. The normal control group was significantly older than all other groups at the time of death. With respect to Parkinson’s disease duration, cases having PDD had significantly longer disease duration when compared to PDAD. With respect to dementia duration PDAD and PDD did not differ but AD cases had a significantly longer duration when compared to either. As expected, Braak neurofibrillary stage and NIA Reagan criteria were significantly greater in any case diagnosed with AD (AD, PDAD) as compared to other groups. The percentage of cases carrying at least one APOE4 allele was greater in the AD and PDAD groups than in PDD, PDND, and NC. With respect to Lewy-type histopathology, cases of PDD and PD were more frequently classified as Unified Stage III (Brainstem and Limbic) while PDAD cases had a higher proportion of stage IV (Neocortical) cases. There were no other significant differences amongst groups with respect to demographics and basic neuropathology.
Table 2 shows scores for plaque densities. As expected, the AD and PDAD cases had significantly higher cerebral cortex total and neuritic plaque density scores when compared to PDD, PDND and NC. Striatal plaques (total, all types) were present in 48/50 AD subjects, 32/40 PDAD, 6/31 PDD, 7/31 PDND and 22/62 NC subjects (Table 3) while striatal neuritic/cored plaques were present in 43/50 AD, 19/40 PDAD, 1/31 PDD, 4/31 PDND, and 14/62 NC subjects. Whether considering only the three PD groups, or only the two PD groups with dementia, thereby simulating possibly relevant clinical settings, the presence of striatal plaques had sensitivities and specificities of approximately 80% (plus or minus 1%) in both group settings for the presence of clinicopathological AD. In comparison, the presence of cerebral cortex plaques was 100% sensitive but only 48% specific (when PDND, PDAD and PDD groups were all included) or 55% specific (when only the PDAD and PDD groups were included). When striatal neuritic and/or cored plaques were used as the diagnostic marker (rather than total plaques as above), the sensitivities were much lower (results not shown). With respect to predicting the presence of dementia in all subjects (PD, PDD, PD/AD, AD and normals), the presence of any striatal amyloid plaques had a sensitivity of 71% and specificity of 69%, while cortical plaques had a sensitivity of 86% and specificity of 35%.
Table 3.
Percentage of all cases in specified group having any type of amyloid plaque (total) and cored/neuritic plaques (NP). AD=Alzheimer’s disease, PDAD= Parkinson’s disease dementia with AD, PDD= Parkinson’s disease dementia without AD, PD= Parkinson’s disease, NC= non-demented normal control.
| AD | PDAD | PDD | PDND | NC | |
|---|---|---|---|---|---|
| Striatal Plaques present | |||||
| total | 96% | 80% | 19% | 23% | 35% |
| NP | 86% | 48% | 3% | 13% | 22% |
|
| |||||
| Cortical Plaques present | |||||
| total | 100% | 100% | 45% | 58% | 68% |
| NP | 100% | 100% | 39% | 55% | 65% |
Analysis of the subset of cases with available putamen TH concentrations showed no significant differences based on the presence (N= 19) or absence (N=22) of striatal plaques. However, when comparing only those with PD (ADPD, PDND, or PDD), cases with striatal plaques (N=9) had a higher mean TH concentration than those without (N=9) (mean TH concentration 18.25 vs. 2.9 ng/mg; P = 0.042).
Discussion
We sought to determine if the presence, density or type of striatal plaques was predictive of the presence of the clinicopathological diagnosis of AD in subjects with PD and dementia. We defined clinicopathological AD as requiring dementia as well as NIA-Reagan or NIA-Alzheimer’s Association “intermediate” or “high” probability ratings. The NIA-Alzheimer’s Associations guidelines are a recently published revision of the NIA-Reagan criteria [15]. The presence of any type (diffuse, neuritic or cored) of striatal plaques predicted the clinicopathological diagnosis of AD with 80% sensitivity and 80% specificity. Requiring the presence of neuritic or cored striatal plaques or requiring higher densities of striatal plaques resulted in less diagnostic accuracy. The higher striatal TH concentrations in subjects having striatal plaques, as compared to subjects without, are somewhat surprising but may indicate that PDAD has similarities with DLB/AD, as the latter group has been repeatedly shown to have less severe degeneration of the nigrostriatal dopaminergic system than what is seen in PD [13]. Additional studies with larger numbers of cases will be needed to confirm this finding.
The results presented here suggest that, with the use of amyloid imaging, the presence of striatal plaques could clinically distinguish PDD from PDAD. Previously, imaging reports of striatal amyloid plaques have been primarily concerned with their presence in subjects with early-onset, autosomal dominant inheritance of AD [41–44]. Few studies have documented striatal amyloid in late-onset sporadic AD [45–47]; one of these found the striatal amyloid signal in late-onset subjects to be equivalent to that in early-onset disease [47]. Most imaging studies in PD have been restricted to estimates of cortical amyloid load [48–51]. Cortical amyloid, however, is a relatively non-specific finding due to the presence of cortical amyloid plaques in a large proportion of neurologically normal elderly subjects [24,52–60], and this is further supported by the low specificity found in our study for cortical plaques.
There has been some debate as to the specificity of amyloid imaging ligands for Aβ amyloid. It is probable that, like their parent histological staining compounds, these ligands will bind with any β-pleated sheet structures. However, amyloid ligands may not appreciably bind to in-situ Lewy bodies. Previous literature has shown that one amyloid ligand (Pittsburgh compound B or PiB) does not produce a significant autoradiographic signal within sections of PD amygdala with frequent Lewy bodies [61] and a case study of pathologically confirmed DLB showed no correlation between Lewy body densities and PiB retention [62]. This agrees with histological knowledge that Lewy bodies are only weakly fluorescent with amyloid stains such as thioflavine S. Previous studies have found that PiB does produce an autoradiographic signal co-localized with neurofibrillary tangles [61,63] but the signal was so weak as to be unlikely to affect the much stronger signal associated with amyloid plaques. It also appears likely, based on postmortem autoradiography studies with antemortem amyloid imaging, that all types of Aβ amyloid, including diffuse, cored and neuritic plaques as well as amyloid angiopathy, will elicit strong amyloid imaging signals [61,63,64]. Additionally one autopsy case study showed clear correspondence between the striatal amyloid imaging signal and striatal diffuse plaques [64]. The largest amyloid imaging study with postmortem correlation to date did not analyze the striatum but confirmed that amyloid imaging is a sensitive and valid marker of postmortem Aβ amyloid deposits [36]. One study found the degree of uptake of PiB in putamen was closely associated with cognitive performance in AD [65]. Large antemortem-postmortem correlative studies are needed to determine whether a positive striatal amyloid imaging signal would be a sensitive and specific marker of concurrent PD and AD and their clinical severities. As approximately 48 to 78% of PD cases will develop dementia sometime during the course of their illness [11,14], the ability to determine the cause of this is a crucial objective if effective treatments are to be developed.
Acknowledgments
The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. Other members of the Arizona Parkinson’s Disease Consortium include Christine Belden, PhD, Erika Driver-Dunckley, MD, Virgilio Evidente, MD, and Sandra Jacobson, MD.
References
- 1.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson’s disease: a prospective, community-based study. Ann Neurol. 2005;58:773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 3.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 5.Kalaitzakis ME, Pearce RK. The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 2009;118:587–598. doi: 10.1007/s00401-009-0597-x. [DOI] [PubMed] [Google Scholar]
- 6.Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C, Williams DR, de Silva R, Lees AJ, Revesz T. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papapetropoulos S, Lieberman A, Gonzalez J, Mash DC. Can Alzheimer’s type pathology influence the clinical phenotype of Parkinson’s disease? Acta Neurol Scand. 2005;111:353–359. doi: 10.1111/j.1600-0404.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm. 2002;109:329–339. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- 9.Adler CH, Caviness JN, Sabbagh MN, Shill HA, Connor DJ, Sue L, Evidente VG, Driver-Dunckley E, Beach TG. Heterogeneous neuropathological findings in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 2010;120:827–828. doi: 10.1007/s00401-010-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SA, Evidente VG, Caviness JN, Shill HA, Sabbagh MN, Connor DJ, Hentz JG, Adler CH, Beach TG. Are there differences in cerebral white matter lesion burdens between Parkinson’s disease patients with or without dementia? Acta Neuropathol. 2010;119:147–149. doi: 10.1007/s00401-009-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papapetropoulos S, Gonzalez J, Lieberman A, Villar JM, Mash DC. Dementia in Parkinson’s disease: a post-mortem study in a population of brain donors. Int J Geriatr Psychiatry. 2005;20:418–422. doi: 10.1002/gps.1297. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 13.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, Caviness JN, Shill HA, Sue LI, Ziabreva I, Perry E, Ballard CG, Aarsland D, Walker DG, Beach TG. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J, Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson’s disease with concomitant dementia. Folia Neuropathol. 2004;42:141–150. [PubMed] [Google Scholar]
- 17.Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64:1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 18.Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 19.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol. 2003;106:83–88. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 20.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000;100:285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 21.Rudelli RD, Ambler MW, Wisniewski HM. Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol. 1984;64:273–281. doi: 10.1007/BF00690393. [DOI] [PubMed] [Google Scholar]
- 22.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 23.Selden N, Mesulam MM, Geula C. Human striatum: the distribution of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1994;648:327–331. doi: 10.1016/0006-8993(94)91136-3. [DOI] [PubMed] [Google Scholar]
- 24.Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13:226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Beach TG, Sue LI, Walker DG, Sabbagh MN, Serrano G, Dugger B, Mariner M, Yantos K, Henry-Watson J, Chiarolanza G, Hidalgo J, Souders L. Striatal Amyloid Plaque Density Predicts Braak Neurofibrillary Stage and Clinicopathological Alzheimer’s Disease: Implications for Amyloid Imaging. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. Striatal beta-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol. 2008;67:155–161. doi: 10.1097/NEN.0b013e31816362aa. [DOI] [PubMed] [Google Scholar]
- 27.Kalaitzakis ME, Walls AJ, Pearce RK, Gentleman SM. Striatal Abeta peptide deposition mirrors dementia and differentiates DLB and PDD from other Parkinsonian syndromes. Neurobiol Dis. 2011;41:377–384. doi: 10.1016/j.nbd.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi Y, Uchikado H, Dickson DW. Neuropathology of Parkinson’s disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 2007;13(Suppl 3):S221–224. doi: 10.1016/S1353-8020(08)70005-1. [DOI] [PubMed] [Google Scholar]
- 29.Halliday GM, Song YJ, Harding AJ. Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm. 2011;118:713–719. doi: 10.1007/s00702-011-0641-6. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112:253–260. doi: 10.1007/s00401-006-0088-2. [DOI] [PubMed] [Google Scholar]
- 31.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes AJ. Clinicopathological aspects of Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):13–20. doi: 10.1159/000113471. [DOI] [PubMed] [Google Scholar]
- 33.Adler CH, Connor DJ, Hentz JG, Sabbagh MN, Caviness JN, Shill HA, Noble B, Beach TG. Incidental Lewy body disease: clinical comparison to a control cohort. Mov Disord. 2010;25:642–646. doi: 10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 35.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 36.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Zehntner SP, Skovronsky DM. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maarouf CL, Daugs ID, Kokjohn TA, Walker DG, Hunter JM, Kruchowsky JC, Woltjer R, Kaye J, Castano EM, Sabbagh MN, Beach TG, Roher AE. Alzheimer’s disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One. 2011;6:e27291. doi: 10.1371/journal.pone.0027291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 39.Beach TG, Sue L, Scott S, Layne K, Newell A, Walker D, Baker M, Sahara N, Yen SH, Hutton M, Caselli R, Adler C, Connor D, Sabbagh M. Hippocampal sclerosis dementia with tauopathy. Brain Pathol. 2003;13:263–278. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115:445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villemagne VL, Ataka S, Mizuno T, Brooks WS, Wada Y, Kondo M, Jones G, Watanabe Y, Mulligan R, Nakagawa M, Miki T, Shimada H, O’Keefe GJ, Masters CL, Mori H, Rowe CC. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009;66:1537–1544. doi: 10.1001/archneurol.2009.285. [DOI] [PubMed] [Google Scholar]
- 42.Ringman JM, Gylys KH, Medina LD, Fox M, Kepe V, Flores DL, Apostolova LG, Barrio JR, Small G, Silverman DH, Siu E, Cederbaum S, Hecimovic S, Malnar M, Chakraverty S, Goate AM, Bird TD, Leverenz JB. Biochemical, neuropathological, and neuroimaging characteristics of early-onset Alzheimer’s disease due to a novel PSEN1 mutation. Neurosci Lett. 2011;487:287–292. doi: 10.1016/j.neulet.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koivunen J, Verkkoniemi A, Aalto S, Paetau A, Ahonen JP, Viitanen M, Nagren K, Rokka J, Haaparanta M, Kalimo H, Rinne JO. PET amyloid ligand [11C]PIB uptake shows predominantly striatal increase in variant Alzheimer’s disease. Brain. 2008;131:1845–1853. doi: 10.1093/brain/awn107. [DOI] [PubMed] [Google Scholar]
- 44.Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, Hoge JA, Cohen AD, Ikonomovic MD, Saxton JA, Snitz BE, Pollen DA, Moonis M, Lippa CF, Swearer JM, Johnson KA, Rentz DM, Fischman AJ, Aizenstein HJ, DeKosky ST. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raji CA, Becker JT, Tsopelas ND, Price JC, Mathis CA, Saxton JA, Lopresti BJ, Hoge JA, Ziolko SK, DeKosky ST, Klunk WE. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer’s disease with Pittsburgh Compound B. J Neurosci Methods. 2008;172:277–282. doi: 10.1016/j.jneumeth.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 47.Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O’Neil JP, Janabi M, Baker SL, Agarwal N, Bonasera SJ, Mormino EC, Weiner MW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain. 2010;133:512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Nagren K, Chaudhury KR, Masters CL, Brooks DJ. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 49.Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, Hershey T, Perlmutter JS. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25:2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74:77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brooks DJ. Imaging amyloid in Parkinson’s disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord. 2009;24(Suppl 2):S742–747. doi: 10.1002/mds.22581. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 53.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 55.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 56.Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, Beyreuther K, Masters CL. A4 amyloid protein deposition and the diagnosis of Alzheimer’s disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988;38:1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- 57.Crystal HA, Dickson DW, Sliwinski MJ, Lipton RB, Grober E, Marks-Nelson H, Antis P. Pathological markers associated with normal aging and dementia in the elderly. Ann Neurol. 1993;34:566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- 58.Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett. 2010;473:168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994;4:138–150. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- 60.Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O’Keefe G, Masters CL, Rowe CC. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Ye L, Velasco A, Fraser G, Beach TG, Sue L, Osredkar T, Libri V, Spillantini MG, Goedert M, Lockhart A. In vitro high affinity alpha-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J Neurochem. 2008;105:1428–1437. doi: 10.1111/j.1471-4159.2008.05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kantarci K, Yang C, Schneider JA, Senjem ML, Reyes DA, Lowe VJ, Barnes LL, Aggarwal NT, Bennett DA, Smith GE, Petersen RC, Jack CR, Jr, Boeve BF. Ante mortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 64.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grimmer T, Henriksen G, Wester HJ, Forstl H, Klunk WE, Mathis CA, Kurz A, Drzezga A. Clinical severity of Alzheimer’s disease is associated with PIB uptake in PET. Neurobiol Aging. 2009;30:1902–1909. doi: 10.1016/j.neurobiolaging.2008.01.016. [DOI] [PubMed] [Google Scholar]