Abstract
An efficient strategy for the oxidative carbonylation of aromatic amides via C-H/N-H activation to form phthalimides under Rh(III) catalyst has been developed. The reaction shows a preference for C–H bonds of electron-rich aromatic amides and tolerates a variety of functional groups.
The carbonylation of organic compounds has become one of the most important metal catalyzed C–C bond-forming processes.1 Carbon monoxide as a C1 feed stock has been employed in a variety of palladium catalyzed transformations,2,3 including the formation of esters from aryl halides,4 aryl tosylates, 5 alkyl and aryl indium reagents among many others.6 Furthermore, cyclopentanones have been formed via the Pauson-Khand reaction between an alkene, alkyne and CO.7 In these cases the organometallic intermediate inserts CO as a π-component to access molecules with increased complexity.
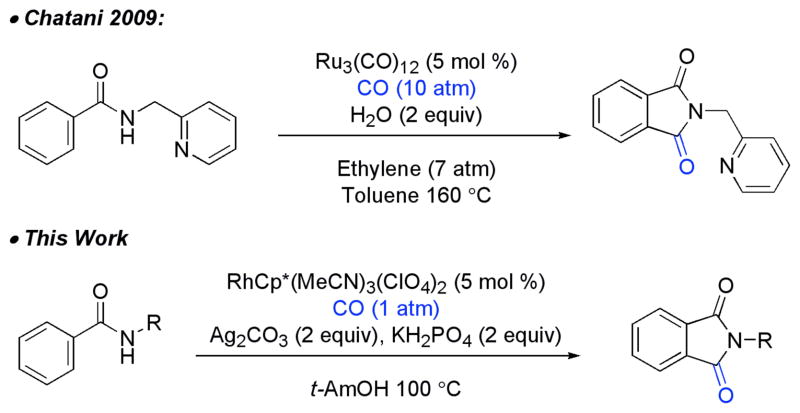
Previously, we8 and others9 have reported the coupling of benzamides and α,β-unsaturated amides with alkynes to access isoquinolones and pyridones utilizing Rh-(III) C–H activation. We imagined that if the alkyne was replaced with CO, we could access unique phthalimides via an analogous approach. This approach has been employed previously in work by Chatani and coworkers,10 who found that benzamides are able to direct C–H insertion in the presence of CO at high pressure utilizing ruthenium catalyst and pyridyl substituted amides (Scheme 1). Yu and coworkers have also demonstrated that palladium can facilitate the formation of succinamides via Pd (II) C–H activation from highly electron deficient amides under 1 atm of CO.11 We speculated that Rh(III) might allow for a more general approach to access phthalimides from a broad variety of amides with 1 atm of CO. Herein, we provide a complete description of our development of the methodology, mechanistic insights, and product derivatization.
Scheme 1.
Our studies began with conditions which were used with success in the formation of isoquinolones from benzamides and alkynes. When the alkyne is replaced with CO the desired product is obtained in 4% yield (Table 1, entry 1). A brief survey of oxidants did not result in appreciable increase in product (Table 1, entry 2 and 3). However, when the catalyst is replaced with its cationic counterpart in the presence of Ag2CO3,12 the desired phthalimide is obtained in 45% yield (Table 1, entry 6). A screen of solvents confirmed t-AmOH to be an ideal solvent (Table 1, entry 7 and 8), with increased concentration resulting in a greater yield (Table 1, entry 9). ClO4 was found to be a better anion partner for the catalyst because of increased solubility, and as a consequence higher yield is obtained (Table 1, entry 10). Different acid or base additives were examined to tune the pH, with KH2PO4 being ideal for the reaction and the desired product then is obtained in 94% yield (Table 1, entry 12).
Table 1.
Optimization

| ||||
|---|---|---|---|---|
| Entry | Catalystd | Oxidante | Solvent [M] | Yield(%) |
| 1a | [RhCp*Cl2]2 | Cu(OAc)2 | t-AmOH [0.3] | 4 |
| 2a | [RhCp*Cl2]2 | AgOAc | t-AmOH [0.3] | 7 |
| 3a | [RhCp*Cl2]2 | Ag2CO3 | t-AmOH [0.3] | 5 |
| 4 | A | Cu(OAc)2 | t-AmOH [0.3] | 2 |
| 5 | A | AgOAc | t-AmOH [0.3] | 18 |
| 6 | A | Ag2CO3 | t-AmOH [0.3] | 45 |
| 7 | A | Ag2CO3 | Dioxane [0.3] | 40 |
| 8 | A | Ag2CO3 | DCE [0.3] | 12 |
| 9 | A | Ag2CO3 | t-AmOH [0.6] | 56 |
| 10 | B | Ag2CO3 | t-AmOH [0.6] | 80 |
| 11b | B | Ag2CO3 | t-AmOH [0.6] | 85 |
| 12c | B | Ag2CO3 | t-AmOH [0.6] | 94 |
Catalyst Loading is 2.5 mol %.
1 equiv KH2PO4.
2 equiv KH2PO4.
A = RhCp*(MeCN)3(SbF6)2 B = RhCp*(MeCN)3(ClO4)2.
2 equiv.
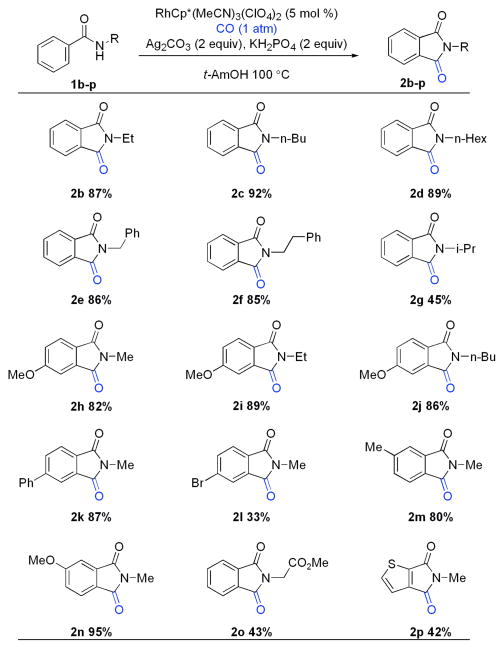
This reaction was examined with various amides under our optimized conditions (Chart 1). It is found that the reaction of amides (1b–1f) bearing alkyl groups at the nitrogen atom proceeds smoothly to deliver phthalimides in excellent yields. N-Isopropyl benzamide 1g is also tolerated but with slower reaction rate. On the other hand, N-cyclohexyl benzamide provides less than 10% desired product.
Chart 1.
Reaction Scope
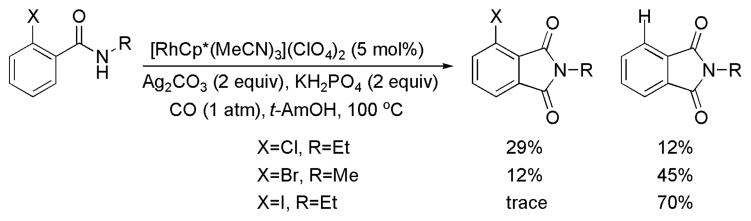
A variety of substitution patterns on the aromatic ring are permitted. Substrates bearing p-methoxy and p-phenyl substituents (1h–1k) are efficient. Amide bearing mildly electron-withdrawing group (1l) provides phthalimide in lower yield. Stronger electron-withdrawing substituents are not tolerated.13 Substitution at the meta position leads to 3-substituted phthalimides as single regioisomers (2m and 2n). Amides bearing ortho substituents such as methoxy, methyl, phenyl and fluoro afford phthalimides in minimal yields. Ortho Cl, Br, I amides mainly provide dehalogenated phthalimides (Scheme 2).
Scheme 2.
With respect to the aryl substituents on nitrogen, N-phenyl benzamide provides no phthalimide under our conditions. Amide derived from alanine methyl ester results in the desired product in moderate yield (2o). A thiophene derived amide is tolerated under our reaction conditions providing the derived imide in modest yield (2p).
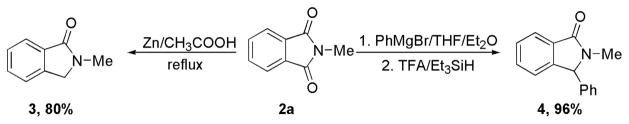
To demonstrate the synthetic utility of this reaction (Scheme 3), phthalimide 2a was subjected to Clemmensen reduction conditions. Treatment of 2a with Zn/CH3COOH under reflux14 provides reduction product 3 in 80% yield. Grignard Reaction of 2a and subsequently TFA/triethylsilane reduction15 affords lactam 4 in 96% yield.
Scheme 3.
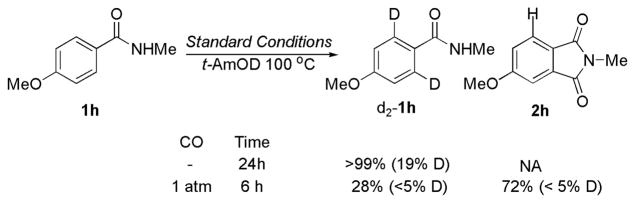
A series of experiments were designed to probe the mechanism of the reaction. When the reaction is conducted in t-AmOD in the absence of CO, 19% deuterium incorporation is observed at the ortho positions of amide 1h (Figure 1). If the same reaction is conducted in the presence of CO, no deuterium incorporation is observed in the unreacted starting material or in the product. These experiments suggest that, under the reaction conditions, the C–H insertion step is largely irreversible.
Figure 1.
Reversible C–H activation
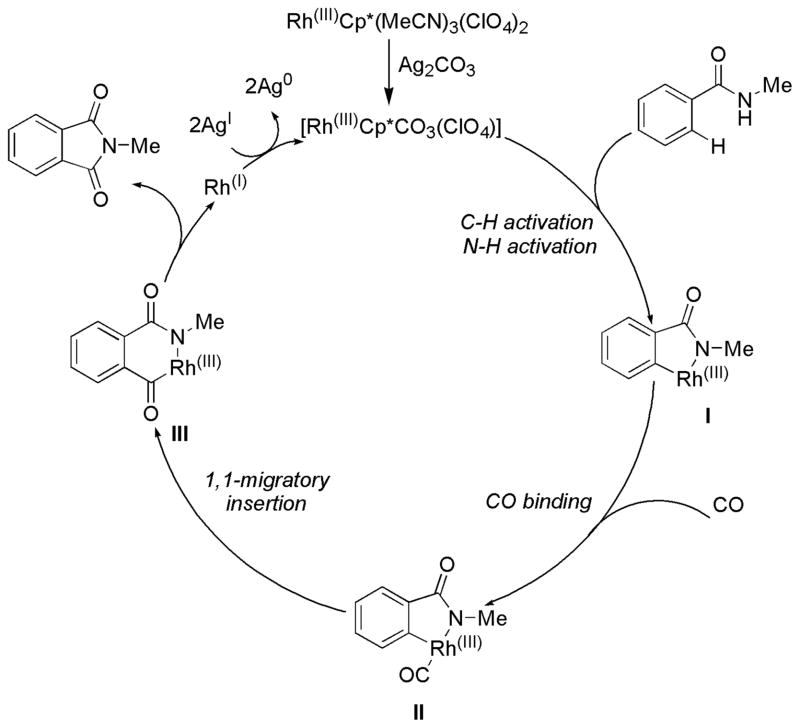
On the basis of the experiment results, a plausible mechanism for the RhCp*(MeCN)3(ClO4)2-catalyzed oxidative carbonylation of benzamides with CO is proposed (Scheme 4). The pre-catalyst decomposes to incorporate a carbonate ligand. 16 Coordination of an equivalent of amide directs C–H activation event to generate the 5-membered rhodacycle I. A molecule of CO can coordinate to the putative rhodacycle to form species II. Migratory insertion of CO into the Rh-C bond then takes place to forge the new C-C bond, generating 6-membered rhodacycle III. At this point reductive elimination can occur to afford the desired phthalimide and a Rhodium (I) species. This species can undergo two single electron oxidations via Ag2CO3 to render the catalytically active rhodium complex.
Scheme 4.
In conclusion, we have developed a Rh(III) catalyzed oxidative carbonylation phthalimide synthesis via C-H/N-H activation. The reaction utilizes Rh(III) catalyst in the presence of Ag(I) oxidant, and is proposed to proceed by N-H metalation of the amide followed by ortho C-H activation. The resultant rhodacycle undergoes CO insertion to form phthalimides in good to excellent yields. Importantly, in this reaction, the amide substrates and the coupling partner CO are both fully incorporated into the desired products.
Supplementary Material
Acknowledgments
We thank the NIGMS for generous support of this research (GM80442). T.R. thanks Amgen and Roche for unrestricted support. We thank Johnson Matthey for a generous loan of Rh salts.
Footnotes
This article is part of ChemComm ‘Advances in catalytic C-C bond formation via late transition metals’web issue.
Electronic Supplementary Information (ESI) available: Experiment procedures for the preparation of catalyst and coupling products and characterization of catalyst and prouducts; copies of 1H and 13CNMR spectra of all compounds. See DOI: 10.1039/b000000x/
Notes and references
- 1.Kollár L. Modern Carbonylation Methods. Wiley-VCH; Weinheim: 2008. [Google Scholar]
- 2.(a) Schoenberg A, Bartoleti I, Heck RF. J Org Chem. 1974;39:3318. [Google Scholar]; (b) Schoenberg A, Heck RF. J Org Chem. 1974;39:3327. [Google Scholar]; (c) Giri R, Yu JQ. J Am Chem Soc. 2008;130:14082. doi: 10.1021/ja8063827. [DOI] [PubMed] [Google Scholar]; (d) Giri R, Lam JK, Yu JQ. J Am Chem Soc. 2010;132:686. doi: 10.1021/ja9077705. [DOI] [PubMed] [Google Scholar]; (e) Haffemayer B, Gaunt MJ. Chem Sci. 2011;2:312. [Google Scholar]; (f) Lu Y, Leow D, Wang X, Engle KM, Yu JQ. Chem Sci. 2011;2:967. [Google Scholar]
- 3.During the preparation of this manuscript, a related Pd(II) catalyzed carbonylation of N-alkoxybenzamides was reported. See: Wrigglesworth JW, Cox B, Lloyd-Jones GC, Booker-Milburn KI. Org Lett. 2011;13 doi: 10.10211/ol202187h.
- 4.Mägerlein W, Indolese AF, Beller M. Angew Chem, Int Ed. 2001;40:2856. doi: 10.1002/1521-3773(20010803)40:15<2856::AID-ANIE2856>3.0.CO;2-1. and references cited in. [DOI] [PubMed] [Google Scholar]
- 5.Munday RH, Martinelli JR, Buchwald SL. J Am Chem Soc. 2008;130:2754. doi: 10.1021/ja711449e. and references cited in. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Jin L, Li P, Lei A. J Am Chem Soc. 2008;130:9429. doi: 10.1021/ja801116s. [DOI] [PubMed] [Google Scholar]
- 7.(a) Pauson PL. Tetrahedron. 1985;41:5855. [Google Scholar]; (b) Schore NE. Org React. 1991;40:1. [Google Scholar]; (c) Ingate ST, Marco-Contelles J. Org Prep Proced Int. 1998;30:121. [Google Scholar]; (d) Geis O, Schmalz HG. Angew Chem Int Ed. 1998;37:911. doi: 10.1002/(SICI)1521-3773(19980420)37:7<911::AID-ANIE911>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]; (e) Chung YK. Coord Chem Rev. 1999;188:297. [Google Scholar]; (f) Brummond KM, Kent JL. Tetrahedron. 2000;56:3263. [Google Scholar]; (g) Wender PA, Deschamps NM, Gamber GG. Angew Chem, Int Ed. 2003;42:1853. doi: 10.1002/anie.200350949. [DOI] [PubMed] [Google Scholar]; (h) Wender PA, Croatt MP, Deschamps NM. J Am Chem Soc. 2004;126:5948. doi: 10.1021/ja0489487. [DOI] [PubMed] [Google Scholar]; (i) Ni Y, Montgomery J. J Am Chem Soc. 2006;128:2609. doi: 10.1021/ja057741q. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Hyster TK, Rovis T. J Am Chem Soc. 2010;132:10565. doi: 10.1021/ja103776u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hyster TK, Rovis T. Chem Sci. 2011;2:1606. doi: 10.1039/C1SC00235J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Guimond N, Gouliaras C, Fagnou K. J Am Chem Soc. 2010;132:6908. doi: 10.1021/ja102571b. [DOI] [PubMed] [Google Scholar]; (b) Mochida S, Umeda N, Hirano K, Satoh T, Miura M. Chem Lett. 2010;39:744. [Google Scholar]; (c) Song G, Chen D, Pan CL, Crabtree RH, Li X. J Org Chem. 2010;75:7487. doi: 10.1021/jo101596d. [DOI] [PubMed] [Google Scholar]; (d) Guimond N, Gorelsky SL, Fagnou K. J Am Chem Soc. 2011;133:6449. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]
- 10.(a) Inoue S, Shiota H, Fukumoto Y, Chatani N. J Am Chem Soc. 2009;131:6898. doi: 10.1021/ja900046z. [DOI] [PubMed] [Google Scholar]; (b) Hasegawa N, Charra V, Inoue S, Fukumoto Y, Chatani N. J Am Chem Soc. 2011;133:8070. doi: 10.1021/ja2001709. [DOI] [PubMed] [Google Scholar]
- 11.Yoo EJ, Wasa M, Yu J-Q. J Am Chem Soc. 2010;132:17378. doi: 10.1021/ja108754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White C, Thompson SJ, Maitlis PM. J Chem Soc, Dalton Trans. 1977:1654. [Google Scholar]
- 13.For example, p-CHO benzamide affords 11% desired product.
- 14.Brewster JH, Fusco AM. J Org Chem. 1963;28:501. [Google Scholar]
- 15.Bousquet T, Fleury JF, Daïch A, Netchitaïlo P. Tetrahedron. 2006;62:706. [Google Scholar]
- 16.The use of AgOTf as oxidant affords no desired product. In the presence of AgOTf and Li2CO3, the desired product is obtained in 23% yield.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.