The benzoin reaction has been studied for more than a century, since its initial report by Wöhler and Liebig in 1832.[1,2] Nature has long utilized the concept of umpolung in the form of thiamine dependent enzymes that are required for life.[3] Despite this, a general method for the asymmetric cross-benzoin reaction has not yet been discovered.[ 4] The inherent problem with this transformation lies in the lack of chemoselectivity between aldehyde partners. A few elegant methodologies have addressed this problem making the synthesis of enantioenriched hetero-benzoin products accessible.[5,6]
Related to the cross-benzoin reaction of aldehydes is the cross aza-benzoin reaction of aldehydes and imines.[7] This concept has a variety of advantages. The difference in reactivity between an aldehyde and an imine is inherently greater but can also be tuned due to the trivalency of nitrogen. Furthermore, α-amido ketones represent an important class of medicinal agents and are a synthon for the ubiquitous 1,2-amino-alcohol motif.[8,9]
Murry, Frantz and coworkers reported the first example of an aldehyde-imine cross-benzoin reaction catalysed by thiazolylidene carbenes, using arylsulfonylamides as imine precursors.[10,11] Later, Miller and coworkers disclosed an asymmetric variant of this work implementing their peptide-derived thiazolium salt as a pre-catalyst to deliver aryl aldehyde derived α-amido ketones.[12] These workers further noted some epimerization of the newly formed stereocenter when using more activated coupling partners.
There has been speculation in the literature about the reversibility of active catalyst addition to an imine which has led to the dogma that slow generation of the imine in situ, or attenuation of its electrophilicity would be necessary for catalyst turnover.[13] We have recently disclosed a study of aza-Breslow intermediates derived from the interaction of one of our chiral triazolylidene carbenes with iminium salts.[14] This study revealed that addition of these carbene species to an iminium salt is facile, leading to a stable intermediate; however, in the presence of weak acid this process is highly reversible. We wondered if we could take advantage of this reversibility when electrophilic acyl-imines are used as substrates, negating the requirement for a slow addition protocol.
With the assumption that a less activated system would lead to increased stability of the newly formed stereocenter as well as a more synthetically valuable product, we began to evaluate the addition of butanal 1a to N-Boc imine 2a. In our study of aza-Breslow intermediates we found acetic acid to be a competent catalyst in regenerating the active carbene. Acetate salts have been used as bases in a variety of NHC-catalyzed processes and implementation of them in this system would generate catalytic amounts of the required acid in situ.[15] We were pleased to find that catalyst 3c in combination with cesium acetate provides the desired α-amido ketone 4a in good yield (89%) and excellent enantioselectivity (96%) (Table 1). Amine bases such as diisopropylethylamine are not effective, except in the case where catalytic amounts of acid are added to the reaction.[16] Addition of 4Å molecular sieves is necessary to suppress imine hydrolysis, likely due to the hygroscopic-nature of cesium acetate, while lowering the temperature led to a significant reduction in post reaction epimerization.
Table 1.
Reaction optimization.a
| ||||||
|---|---|---|---|---|---|---|
| Entry | Cat. | Temp [°C] | Base | Additive | Yield [%][b] | ee [%][c] |
| 1 | 3c | 0 | i-Pr2NEt | none | <5% | N/A |
| 2 | 3c | 0 | i-Pr2NEt | AcOH | 91 | 90 |
| 3 | 3c | 0 | NaOAc | none | 33 | 99 |
| 4 | 3c | −10 | CsOAc | 4Å MS | 83 | 90 |
| 5 | 3c | −20 | CsOAc | 4Å MS | 89 | 96 |
| 6 | 3c | −25 | CsOAc | 4Å MS | 68 | 97 |
| 7 | 3c | −30 | CsOAc | 4Å MS | 61 | 98 |
| 8 | 3a | −20 | CsOAc | 4Å MS | 38 | 25 |
| 9 | 3b | −20 | CsOAc | 4Å MS | 51 | 87 |
|
| ||||||
Reactions conducted with 1.5 equiv 1a and 1.0 equiv 2a.
Isolated yield after chromatography.
Enantiomeric excess determined by HPLC analysis on a chiral stationary phase.
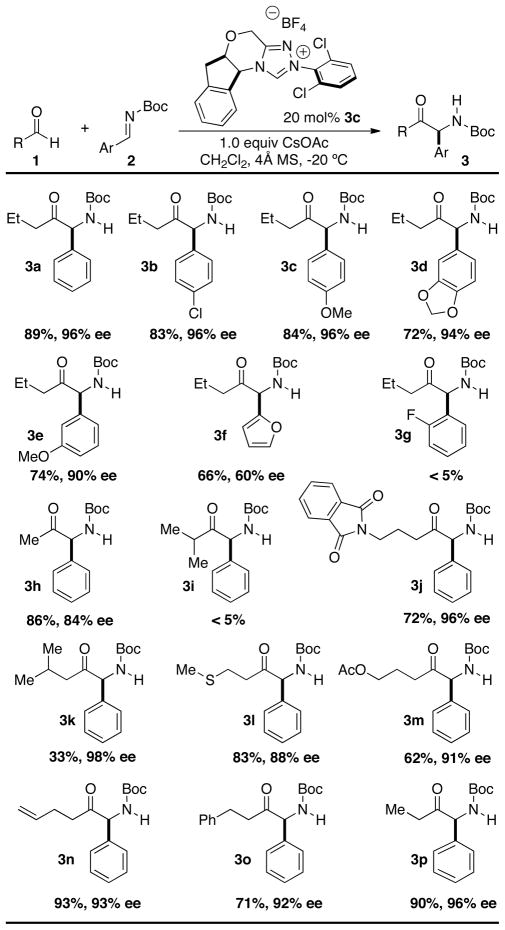
With an efficient catalyst system in hand we evaluated the scope of this transformation (Table 2). A variety of aliphatic aldehydes were examined containing a diverse range of functionality. Straight-chain aldehydes produce the desired products in high yield (71–93%) and excellent enantioselectivity (84–93% ee), while β-branched aldehydes participate in excellent enantioselectivity (98% ee) albeit lower yields (33%).[17] Heteroatoms can be incorporated into the tether without deleterious impact on yield or enantioselectivity, allowing for the incorporation of functionality such as thioethers, imides, and esters. Currently, α-branched aldehydes do not participate. The scope of the imine partner was assessed using N-Boc imines of varying electronic nature. Both electron rich and electron poor aryl derivatives work with equal utility (72–74% yield) despite some small variation in enantioselectivity (90–94% ee). The common methylenedioxyphenyl motif can be incorporated with high selectivity (94% ee) as well as heterocycles such as furan, albeit in lower selectivity (60% ee). Ortho-substituted aryl derivatives lead to an unexpected loss of reactivity.
Table 2.
Reaction Scopea
|
Reactions conducted with 1.5 equiv 1 and 1.0 equiv 2.
Isolated yield after chromatography.
Enantiomeric excess determined by HPLC analysis on a chiral stationary phase.
Absolute stereochemistry assigned by analogy; see supporting information.
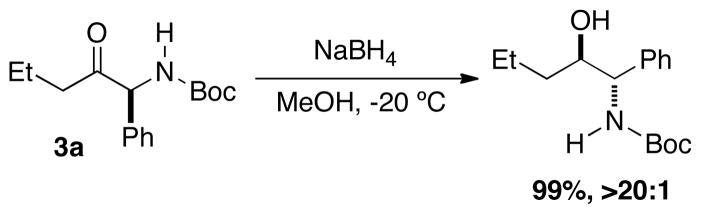
The α-amido ketone products are useful intermediates in the synthesis of 1,2-aminoalcohols. Reduction of 3a provides quantitative yield of the anti-1,2-aminoalcohol as the sole diastereomer (Scheme 1).[18]
Scheme 1.
Diastereoselective Reduct ion of Amido-ketones.
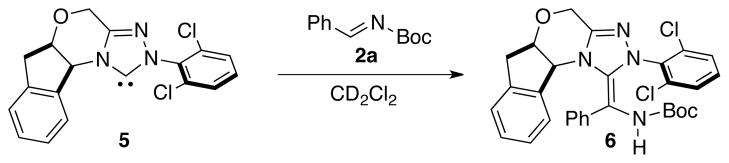
Upon devoloping an efficient catalyst system, we were interestested in probing the interaction between N-Boc imines and our active carbene to gain further evidence for the reversible formation of an aza-Breslow intermediate in the catalytic cycle. Subjection of pre-catalyst 3c to excess KOtBu shows clean formation of carbene 5 by 1H NMR. Addition of 1.0 equivalent of N-Boc imine 2a immediately produces a bright yellow solution and reveals the formation of a new species assigned by 1H NMR and HRMS to be aza-Breslow intermediate 6 (Scheme 2).[19] To test the reversibility of this process under the reaction conditions, 6 was treated with excess AcOH, regenerating a colorless solution. 1H NMR analysis confirms the disappearance of intermediate 6 but neither carbene 5 or imine 2a could be identified, suggesting that the carbene may not be the resting state under these conditions.[20]
Scheme 2.
NMR Studies of Catalyst/Substrate Interaction
In conclusion, we have developed a highly enantioselective cross aza-benzoin reaction of aliphatic aldehydes and N-Boc imines. This transformation provides access to useful 1,2-amino alcohol synthons in a single step. Crucial insight pertaining to the reversible formation of aza-Breslow intermediates in the catalytic cycle has enabled the direct implementation of highly reactive imines as substrates, negating the requirement for a slow-addition protocol.
Experimental Section
To a dry 4 mL vial with a magnetic stir bar, was added triazolium salt pre-catalyst 3a (26 mg, 0.058 mmol, 0.2 equiv), cesium acetate (55 mg, 0.29 mmol, 1.0 equiv), activated 4Å molecular sieves (8–12 mesh) (5–10 beads) and dichloromethane (1.5 mL). The vial was then placed in a −20 °C cooling bath with constant stirring and purged with argon. After 5 min the aldehyde (0.43 mmol, 1.5 equiv) was added via syringe followed by a solution of the imine (0.28 mmol, 1.0 equiv) in dichloromethane (0.5 mL). The reaction was stirred at −20 °C for 24 h at which point acetic acid (50 μL) was added and the whole mixture placed directly on a silica gel column and eluted with a hexanes:EtOAc mixture (typically 20:1). After chromatography the desired amido-ketone was obtained as a white amorphous solid.
Supplementary Material
Acknowledgments
We thank NGIMS for generous support of this research (GM 72586), and Donald Gauthier (Merck) for a gift of aminoindanol. TR thanks Amgen and Roche for support.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author
References
- 1.Liebig J, Wöhler F. Ann Pharm. 1832;3:249–282. [Google Scholar]
- 2.For reviews of NHC catalysis including the benzoin reaction see: Moore J, Rovis T. Top Curr Chem. 2009;291:77–144. doi: 10.1007/978-3-642-02815-1_18.Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z.
- 3.Nilsson U, Meshalkina L, Lindqvist Y, Schneider G. J Biol Chem. 1997;272:1864. doi: 10.1074/jbc.272.3.1864. [DOI] [PubMed] [Google Scholar]
- 4.For seminal work on the cross-benzoin reaction see: Stetter H, Dämbkes G. Synthesis. 1977:403–404.For select recent results, see: Jin MY, Kim SM, Han H, Ryu DH, Yang JW. Org Lett. 2011;13:880–883. doi: 10.1021/ol102937w.Rose CA, Gundala S, Fagan CL, Franz JF, Connon SJ, Zeitler K. Chem Sci. 2012;3:735–740.
- 5.For the first example of an asymmetric cross-benzoin reaction using enzyme catalysis see: Dünkelmann P, Kolter-Jung D, Nitsche A, Demir AS, Siegert P, Lingen B, Baumann M, Pohl M, Müller M. J Am Chem Soc. 2002;124:12084–12085. doi: 10.1021/ja0271476.for the asymmetric cross-silyl-benzoin reaction catalysed by metallophosphites see: Linghu X, Potnick JR, Johnson JS. J Am Chem Soc. 2004;126:3070–3071. doi: 10.1021/ja0496468.
- 6.For an asymmetric cross benzoin reaction of aldehydes with trifluoromethylketones see: Enders D, Grossmann A, Fronert J, Raabe G. Chem Commun. 2010;46:6282–6284. doi: 10.1039/c0cc02013c.
- 7.For a homo-aza-benzoin reaction, see: Reich BJE, Justice AK, Beckstead BT, Reibenspies JH, Miller SA. J Org Chem. 2004;69:1357–1359. doi: 10.1021/jo035245j.
- 8.Lee A, Huang L, Ellman J. J Am Chem Soc. 1999;121:9907–9914. [Google Scholar]
- 9.Ager DJ, Prakash I, Schaad DR. Chem Rev. 1996;96:835–876. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]
- 10.Murry JA, Frantz DE, Soheili A, Tillyer R, Grabowski EJJ, Reider PJ. J Am Chem Soc. 2001;123:9696–9697. doi: 10.1021/ja0165943. [DOI] [PubMed] [Google Scholar]
- 11.For other examples using imines or iminium ions as acceptors, see: Castells J, López-Calahorra F, Bassedas M, Urrios P. Synthesis. 1988:314–315.Li GQ, Dai LX, You SL. Chem Commun. 2007:852–854. doi: 10.1039/b611646a.Enders D, Henseler A, Lowins S. Synthesis. 2009;24:4125–4128.
- 12.Mennen SM, Gipson JD, Kim YR, Miller SJ. J Am Chem Soc. 2005;127:1654–1655. doi: 10.1021/ja042650z. [DOI] [PubMed] [Google Scholar]
- 13.He M, Bode JW. Org Lett. 2005;7:3131–3134. doi: 10.1021/ol051234w. [DOI] [PubMed] [Google Scholar]
- 14.DiRocco DA, Oberg KM, Rovis T. J Am Chem Soc. 2012;134 doi: 10.1021/ja302031v.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Lathrop SP, Rovis T. J Am Chem Soc. 2009;131:13628–13630. doi: 10.1021/ja905342e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ozboya KE, Rovis T. Chem Sci. 2011;2:1835–1838. doi: 10.1039/C1SC00175B. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao X, DiRocco DA, Rovis T. J Am Chem Soc. 2011;133:12466–12469. doi: 10.1021/ja205714g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu G, Wilkerson PD, Toth CA, Xu H. Org Lett. 2012;14:858–861. doi: 10.1021/ol203375y. [DOI] [PubMed] [Google Scholar]; (e) DiRocco DA, Noey EL, Houk KN, Rovis T. Angew Chem Int Ed. 2012;51:2391–2394. doi: 10.1002/anie.201107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.We have observed increases in reactivity if the aldehyde is not distilled prior to use, which we attribute to the presence of carboxylic acid impurities.
- 17.Aromatic aldehydes do not provide adduct under these conditions.
- 18.Ghorai MK, Kumar A, Tiwari DP. J Org Chem. 2010;75:137–151. doi: 10.1021/jo902244y. [DOI] [PubMed] [Google Scholar]
- 19.See supporting information for details.
- 20.In parallel work, we have found that the coupling of aldehydes with in situ generated iminium electrophiles leads to generation of aza-Breslow intermediates in situ, the formation of which is reversible under the reaction conditions; DiRocco DA, Rovis T. manuscript submitted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.