Abstract
A two-step strategy for the synthesis of arrays of tricyclic tetrazolo-fused benzodiazepines and benzodiazepinones has been investigated. The protocol uses ortho-N-Boc phenylisocyanides and phenylglyoxaldehydes or ethyl glyoxylate in the 4-component Ugi-Azide reaction to afford MCR (Multi Component Reactions) derived adducts equipped with the desired diversity inputs. A subsequent acidic treatment (TFA/DCE) allows a simultaneous deprotection-cyclization leading to the final products.
Keywords: benzodiazepines, benzodiazepinones, tetrazoles, Ugi reaction, TMS-N3
1. Introduction
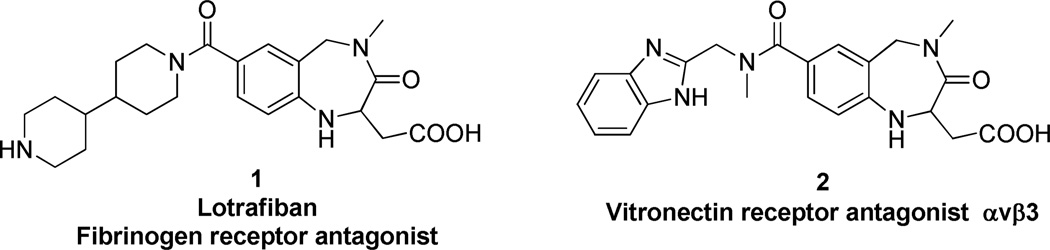
The benzodiazepine family represents one of the most prominent classes of privileged scaffolds in medicinal chemistry.1 Other than their well-known properties as psychoactive drugs, which function as sedative-hypnotics, anxiolytic, muscle relaxants, and anticonvulsants,2 they display a plethora of further activity patterns ranging from anticancer3 and anti-HIV properties4 to cholecystokinin receptors antagonism.5 Furthermore, this class possesses several examples of diverse biological activities of closely related structural analogs. One such example can be seen by comparing activities of compounds 1 and 2. Lotrafiban6 interacts highly selectively with the integrin GPIIbIIIa, whereas 2 binds highly preferentially to the integrin αvβ3 receptor.7 This change was merely initiated by simple modification of the ‘west-side’ amine group, thought to recognize the RGD motif.
Multicomponent reactions have recently met renewed interest due to their capability to generate high molecular diversity through conceptually and operationally simple chemistry, able to build complex molecules incorporating diversity reagents in an atom economic fashion.8 Indeed, since a widely exploited approach to elaborate MCR adducts consists of rigidifying them into nitrogen-containing heterocycles,9 a number of MCR based benzodiazepine syntheses have been reported.10
2. Results and Discussion
In continuation of our studies on the generation of potentially medicinally relevant chemotypes via the efficient post-condensation modifications of the Ugi reaction,11 we envisioned the possibility of fusing the multipurpose benzodiazepine core with the similarly interesting tetrazole moiety. Lately, we have explored the use of the Ugi-azide reaction to fruitfully embed the afore-mentioned scaffold in multicomponent derived backbones, which were subsequently submitted to secondary transformations resulting in drug-like entities.12 In fact, the 1,5-disubstituted tetrazole ring has been identified as a surrogate of the cis-amide peptide bond,13 opening new scenarios toward the preparation of peptidomimetics with enhanced in vivo stability.
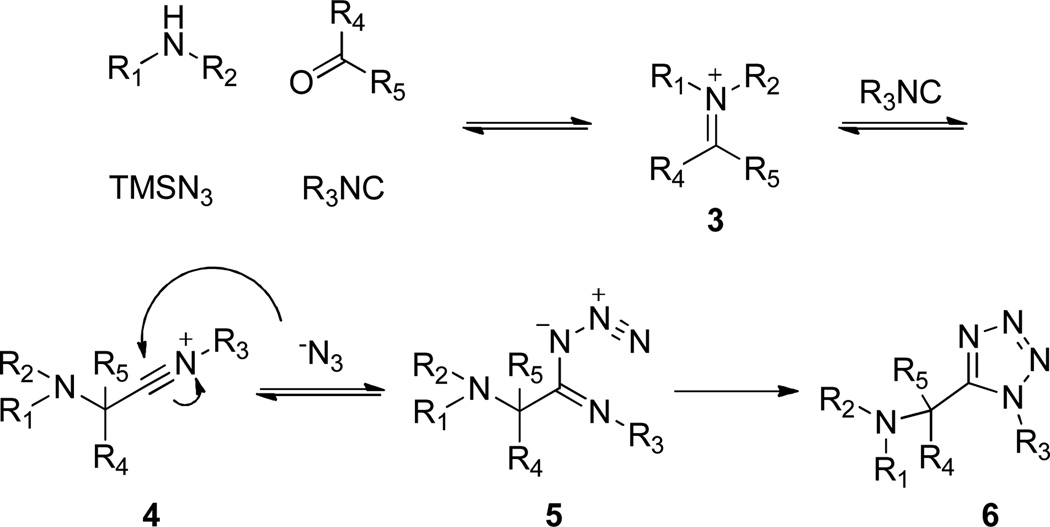
The well-established TMS-N3 variant of the Ugi 4-component condensation also constitutes the first and key step of this work. The process differs from the regular Ugi reaction with azidotrimethylsilane being used as a replacement for the carboxylic acid, while the other three inputs remain the same.
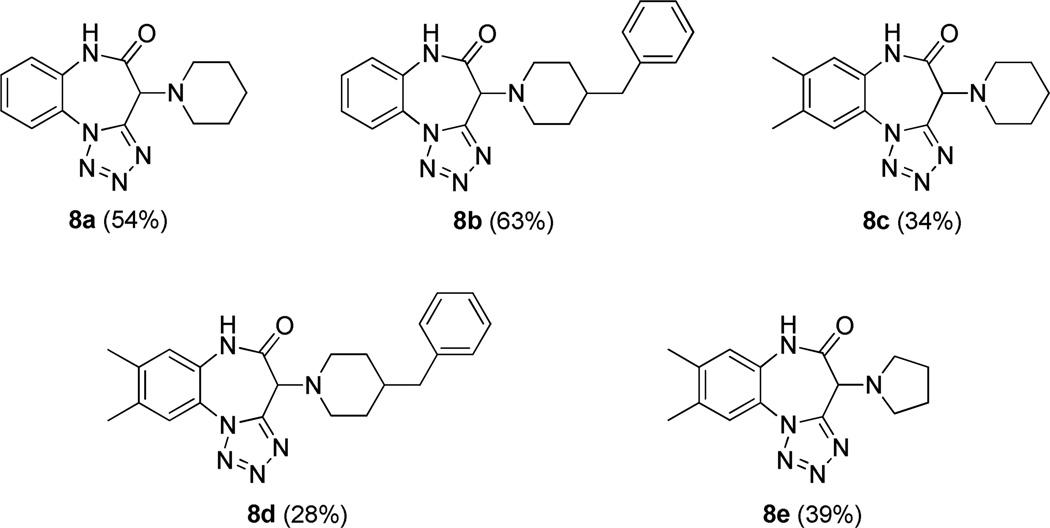
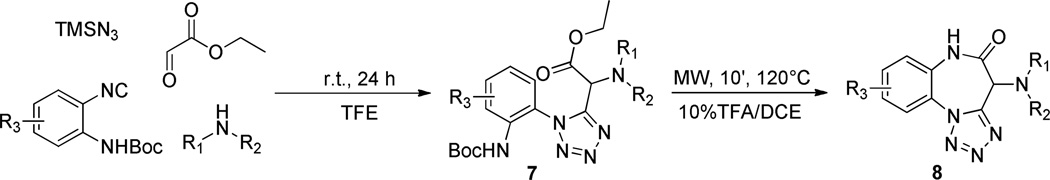
In this case, azide plays the role of the nucleophile instead of the carboxylate ion and traps the nitrilium intermediate species 4, resulting in the formation of the 1,5-disubstututed tetrazole 6 upon intramolecular cyclization.14 We initially decided to employ cyclic secondary amines and ethyl glyoxylate as the carbonyl input, along with the azide source and ortho-N-Boc phenylisocyanides, our plan being to endow the resulting products 7 with both an electrophilic ester functionality and a masked amino nucleophile. According to our experience, subsequent acidic treatment was most likely to cleave the Boc group and trigger cyclization rendering benzodiazepinones 8. The multicomponent step proceeded smoothly under mild conditions, especially when trifluoroethanol (TFE) was used as the solvent. Indeed, such a phenomenon is well-known and is the outcome of the low nucleophilicity of TFE, still a polar protic solvent suitable for the Ugi reaction, but unlike methanol unable to attack the electrophilic Schiff base intermediate.15 In order to keep the whole process simple and tailored for high-throughput applications, crude products were only quickly passed through flash chromatography and the semi-purified compounds 7 were directly submitted to the cyclization step. Predictably, the secondary transformation was readily promoted by dissolving 7 in a 10% TFA/DCE solution at elevated temperature (MW, 10 min, 120 °C). Hence, validation of the protocol was performed by preparing a small five member (8a–e) collection with good overall yields.
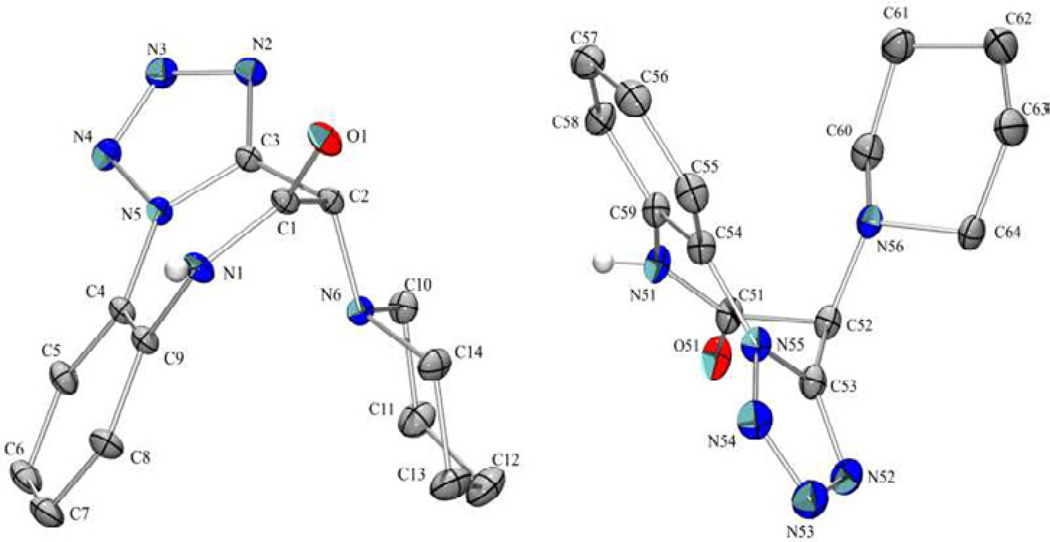
Whereas characterization of Ugi product 7 was skipped for the sake of procedural simplicity, unambiguous X-ray structural assignment was achieved for 8a (Figure 3).16
Figure 3.
Solved crystal structure of 8a showing two unique molecules in the asymmetric unit.
With a validated route in hand for five examples, we then proceeded with a parallel synthesis production campaign of 48 compounds (general structure 8) on a 0.2 millimolar scale. To our delight, after high-throughput purification by preparative HPLC-MS systems, all but one reaction was found to be successful, even if recovered amounts were extremely variable. From high-throughput perspective, extreme robustness of the strategy was demonstrated, at least for the amine input. In fact, while no additional isocyanides other than the two used in the validation phase were tried, 48 different secondary amines made up the diversity-enhancing pool.
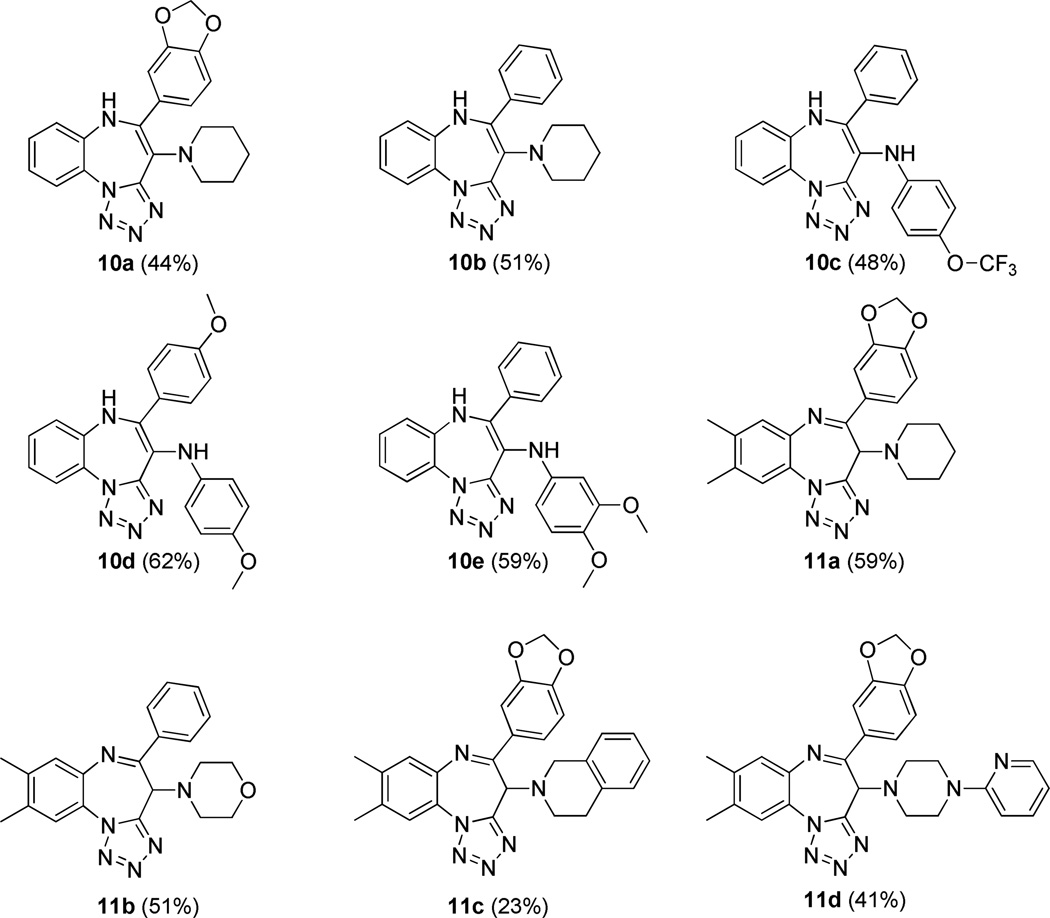
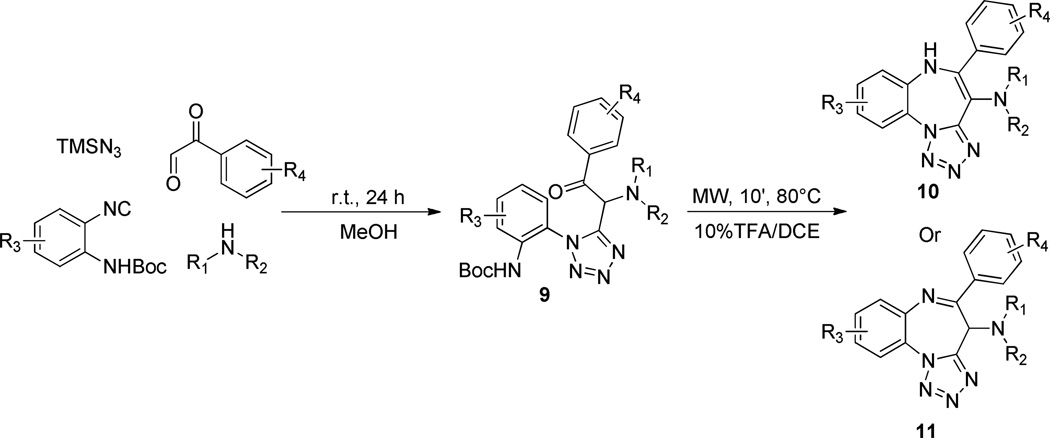
Having developed an operationally friendly fast entry into an interesting fused heterocyclic scaffold containing a benzodiazepine and a tetrazole,17 our next goal was to investigate replacement of ethyl glyoxylate with arylglyoxaldehydes to pave the way toward other chemotypes (10 and 11), and also to increase the number of diversity reagents by one over the first methodology. Arylglyoxaldehydes are indeed commercially available, and the additional carbonyl group they contain is ideal for reaction with unmasked internal amino nucleophiles. In this case, use of trifluoroethanol proved to be unnecessary, as methanol was capable to provide comparable yields under the same conditions, and cyclization step turned out to be even easier to accomplish, (MW, 10 min, 80 °C). Although good overall yields were obtained and the scope was extended to both primary and secondary amines, two distinct sets of compounds were isolated. Indeed, while adducts 9 derived from primary amines constantly led to enamine-type structures 10, their analogues stemming from the incorporation of a secondary amine input furnished either adducts 10 or 11. Over and above this issue, both 10 and 11 showed marked tendency to instability and after long term storage as dried solids at room temperature or short term exposure to silica gel or other acidic media, conversion into a plethora of degradation products was observed, probably via both hydrolysis and oxidation. Consequently, progression to parallel production of benzotetrazolodiazepines 10 and 11 was not carried out. Interconversion of structures 10 and 11 under acidic, basic or thermal conditions was never observed.
3. Conclusions
In summary, we have described herein an efficient and straightforward preparation of tetrazolo-fused benzodiazepines and benzodiazepinones involving an Ugi-azide multicomponent reaction for initial diversity generation followed by an acid-promoted post-condensation cyclization step. The procedure is perfectly amenable for high-throughput applications and library production for the benzodiazepinones 8 and, notwithstanding the previously discussed limitations, it represents a useful tool to gain access to richly substituted benzodiazepines 10 and 11 which are relatively stable when stored at low temperature.
4. Experimental Section
Solvents were purchased from commercial providers and used without further purification. Other reagents were used as obtained from commercial providers except when otherwise noted. Analytical thin layer chromatography (TLC) was performed on pre-coated silica gel plates. Visualization was accomplished with UV light or by staining with basic KMnO4 solution. Column chromatography was performed using automated chromatographic systems. Melting points were determined in an open glass capillary and are uncorrected. NMR spectra were recorded in CDCl3 at 400 MHz (1H NMR) and 100 MHz (13C NMR). Low and high resolution mass spectra were obtained using ESI methods.
General procedure for the preparation of benzotetrazolodiazepinones 8
Ethyl glyoxylate (1 mmol, 102 mg), amine (1 mmol), azidotrimethylsilane (1 mmol, 115 mg) and isocyanide (1 mmol) were dissolved in trifluoroethanol (0.5 mL) in a 10 mL MW vial. The reaction was allowed to run at room temperature for 24 h. Crude mixture was concentrated in vacuo and purified by flash chromatography (Hexane/EtOAc). Ugi adduct was then dissolved in a 10% TFA/DCE solution and heated at 120 °C for 10 min by means of microwave irradiation. Reaction mixture was diluted with EtOAc (15 mL) and washed with NaHCO3 sat. solution (15 mL). The aqueous phase was then re-extracted with EtOAc (15 mL), combined organics were dried over MgSO4 and crude mixture was purified by flash chromatography (Hexane/EtOAc) to afford expected benzotetrazolodiazepinones.
4-(piperidin-1-yl)-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-5(6H)-one (8a): white solid, 54% yield; product purified using 5 to 50% EtOAc in Hexane gradient; m.p. 225 – 227 °C; 1H NMR (400 MHz, CDCl3) δ ppm 9.23 (s, 1H), 7.95 (dd, J = 8.1, 1.4 Hz, 1H), 7.53 (td, J = 7.8, 1.5 Hz, 1H), 7.40 (td, J = 7.8, 1.3 Hz, 1H), 7.25 (dd, J = 8.0, 1.3 Hz, 1H), 4.78 (s, 1H), 2.59 – 2.21 (m, 2H), 2.20 – 1.91 (m, 2H), 1.34 – 1.01 (m, 6H).; 13C NMR (100 MHz, CDCl3) δ ppm 168.1, 150.8, 130.7, 129.6, 126.3, 122.8, 121.3, 66.4, 52.2, 25.8, 24.1; [M+H]+ = 285.2; HRMS (ESI): m/z calcd for C14H17N6O [M+H]+ : 285.1459, found: 285.1459.
4-(4-benzylpiperidin-1-yl)-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-5(6H)-one (8b): white solid, 63% yield; product purified using 5 to 40% EtOAc in Hexane gradient; m.p. 198 – 199 °C; 1H NMR (400 MHz, CDCl3) δ ppm 9.49 (s, 1H), 7.97 (dd, J = 8.0, 1.2 Hz, 1H), 7.55 (td, J = 7.6, 1.2 Hz, 1H), 7.43 (td, J = 7.6, 0.9 Hz, 1H), 7.30 – 7.11 (m, 5H), 7.02 (d, J = 6.8 Hz, 1H), 4.79 (s, 1H), 2.89 (d, J = 10.8 Hz, 1H), 2.33 (d, J = 6.8 Hz, 2H), 2.21 – 1.90 (m, 3H), 1.69 – 1.33 (m, 3H), 0.98 – 0.49 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 168.4, 150.7, 140.4, 130.8, 129.6, 129.3, 128.6, 126.4, 126.3, 126.2, 122.8, 121.5, 66.1, 51.7, 51.3, 43.0, 37.7, 32.0, 31.9; [M+H]+ = 375.2; HRMS (ESI): m/z calcd for C21H23N6O [M+H]+ : 375.1928, found: 375.1924.
8,9-dimethyl-4-(piperidin-1-yl)-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-5(6H)-one (8c): white solid, 34% yield; product purified using 5 to 45% EtOAc in Hexane gradient; m.p. >250 °C; 1H NMR (400 MHz, CDCl3) δ ppm 9.16 (s, 1H), 7.72 (s, 1H), 7.00 (s, 1H), 4.76 (s, 1H), 2.54 – 2.24 (m, 2H), 2.38 (s, 6H), 2.20 – 2.02 (m, 2H), 1.26 (m, 6H); 13C NMR (100 MHz, CDCl3) δ ppm 168.3, 150.4, 140.0, 135.3, 127.0, 123.7, 123.1, 122.1, 66.5, 52.3, 25.7, 24.1, 20.1, 19.7; [M+H]+ = 313.3; HRMS (ESI): m/z calcd for C16H21N6O [M+H]+ : 313.1771, found: 313.1766.
4-(4-benzylpiperidin-1-yl)-8,9-dimethyl-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-5(6H)-one (8d): white solid, 28% yield; product purified using 5 to 50% EtOAc in Hexane gradient; m.p. 207 – 208 °C; 1H NMR (400 MHz, CDCl3) δ ppm 9.27 (b, s, 1H), 7.71 (s, 1H), 7.27 – 7.13 (m, 4H), 7.07 – 6.97 (m, 3H), 4.75 (s, 1H), 2.90 (d, J = 10.9 Hz, 1H), 2.39 (s, 6H), 2.35 (s, 1H), 2.22 – 1.91 (m, 3H), 1.57 – 1.29 (m, 3H), 0.97 – 0.61 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 168.2, 150.3, 140.5, 140.0, 135.4, 129.4, 128.6, 126.9, 126.3, 123.60, 123.1, 122.1, 66.1, 51.8, 51.5, 43.0, 37.8, 31.9, 31.8, 20.1, 19.7; [M+H]+ = 403.2; HRMS (ESI): m/z calcd for C23H27N6O [M+H]+ : 403.2241, found: 403.2245.
8,9-dimethyl-4-(pyrrolidin-1-yl)-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-5(6H)-one (8e): white solid, 39% yield; product purified using 5 to 80% EtOAc in Hexane gradient; m.p. >250 °C; 1H NMR (400 MHz, CDCl3) δ ppm 9.39 (s, 1H), 7.74 (s, 1H), 7.01 (s, 1H), 4.78 (s, 1H), 2.63 – 2.52 (b, m, 4H), 2.36 (s, 6H), 1.67 – 1.56 (b, m, 4H); 13C NMR (100 MHz, CDCl3) δ ppm 168.5, 150.7, 140.1, 135.4, 126.8, 123.34, 123.28, 122.4, 64.5, 52.1, 24.0, 20.1, 19.7. [M+H]+ = 299.1; HRMS (ESI): m/z calcd for C15H19N6O: 299.1615, found: 299.1613.
General procedure for the preparation of benzotetrazolodiazepines 10 and 11
Aryl glyoxaldehyde (1 mmol), amine (1 mmol), azidotrimethylsilane (1 mmol, 115 mg) and isocyanide (1 mmol) were dissolved in methanol (1 mL) in a 10 mL MW vial. The reaction was allowed to run at room temperature for 24 h. Crude mixture was concentrated in vacuo and purified by flash chromatography (Hexane/EtOAc). Ugi adduct was then dissolved in a 10% TFA/DCE solution and heated at 80 °C for 10 min by means of microwave irradiation. Reaction mixture was diluted with EtOAc (15 mL) and washed with NaHCO3 sat. solution (15 mL). The aqueous phase was then re-extracted with EtOAc (15 mL), combined organics were dried over MgSO4 and crude mixture was purified by flash chromatography (Hexane/EtOAc or EtOAc/MeOH) to afford title compounds.
5-(benzo[d][1,3]dioxol-5-yl)-4-(piperidin-1-yl)-6H-benzo[b]tetrazolo[1,5-d][1,4]diazepine (10a): yellow solid, 44% yield; product purified using 5 to 40% EtOAc in Hexane gradient; m.p. 203 – 204 °C; 1H NMR (400 MHz, CDCl3) δ ppm 7.76 (d, J = 8.0 Hz, 1H), 7.30 – 7.25 (m, 1H), 7.17 – 7.12 (m, 1H), 6.97 (d, J = 1.2 Hz, 1H), 6.93 (dt, J = 8.0, 1.5 Hz, 1H), 6.86 (dd, J = 8.0, 1.2 Hz, 1H), 6.78 (d, J = 8.0 Hz, 1H), 6.03 (s, 1H), 4.77 (s, 1H), 2.90 (s, 4H), 1.40 (s, 6H); 13C NMR (100 MHz, CDCl3) δ ppm 153.1, 149.4, 148.0, 147.3, 140.6, 131.9, 130.3, 127.3, 124.6, 123.5, 121.5, 120.7, 120.1, 108.8, 108.0, 101.4, 52.6, 26.1, 23.80; [M+H]+ = 389.1; HRMS (ESI): m/z calcd for C21H21N6O2 [M+H]+ : 389.1721, found: 389.1724.
5-phenyl-4-(piperidin-1-yl)-6H-benzo[b]tetrazolo[1,5-d][1,4]diazepine (10b): yellow solid, 51% yield; product purified using 10 to 40% EtOAc in Hexane gradient; m.p. 195 – 197 °C; 1H NMR (400 MHz, CDCl3) δ ppm 7.76 (dd, J = 8.0, 1.5 Hz, 1H), 7.47 – 7.38 (m, 5H), 7.29 – 7.24 (m, 1H), 7.16 – 7.11 (m, 1H), 6.76 (dd, J = 7.9, 1.3 Hz, 1H), 4.82 (s, 1H), 3.02 – 2.79 (m, 4H), 1.40 – 1.22 (m, 6H); 13C NMR (100 MHz, CDCl3) δ ppm 153.2, 150.3, 140.5, 138.2, 130.4, 128.9, 128.3, 127.7, 127.2, 124.6, 123.6, 120.8, 120.2, 52.6, 26.1, 23.8; [M+H]+ = 345.3; HRMS (ESI): m/z calcd for C20H21N6 [M+H]+ : 345.1822, found: 345.1819.
5-phenyl-N-(4-(trifluoromethoxy)phenyl)-6H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-4-amine (10c): yellow solid, 48% yield; product purified using 0 to 30% MeOH in EtOAc gradient; m.p. 193 – 195°C; 1H NMR (400 MHz, CDCl3) δ ppm 7.79 (dd, J = 8.0, 1.4 Hz, 1H), 7.70 – 7.36 (m, 5H), 7.32 (td, J = 7.7, 1.5 Hz, 1H), 7.19 (td, J = 7.9, 1.3 Hz, 1H), 6.89 (d, J = 8.9 Hz, 2H), 6.83 (dd, J = 8.0, 1.2 Hz, 1H), 6.58 (d, J = 9.0 Hz, 2H).5.30 (s, 1H), 5.19 (s, 1H); 13C NMR (400 MHz, CDCl3) δ ppm 151.5, 152.9, 144.6, 138.4, 136.1, 133.8 – 131.2 (m), 130.8, 130.1, 129.0, 128.8, 127.6, 127.2, 127.0, 126.6, 125.3, 123.6, 122.1, 121.9, 121.6, 121.2, 114.5, 105.5; [M+H]+ = 437.1; HRMS (ESI): m/z calcd for C22H16F3N6O [M+H]+ : 437.1332, found: 437.1331.
N,5-bis(4-methoxyphenyl)-6H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-4-amine (10d): yellow solid, 62% yield; product purified using 0 to 30% MeOH in EtOAc gradient; m.p. 195 – 197°C; 1H NMR (400 MHz, CDCl3) δ ppm 9.65 (s, 1H), 7.40 (d, J = 8.9 Hz, 1H), 7.06 – 6.86 (m, 6H), 6.76 (d, J = 11.7 Hz, 2H), 6.64 – 6.48 (m, 4H), 3.82 (s, 3H), 3.77 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 158.5, 155.0, 149.6, 141.3, 132.2, 131.31, 131.1, 130.0, 127.7, 126.1, 125.4, 121.3, 120.0, 118.6, 117.4, 116.2, 116.1, 114.3, 114.2, 112.8, 101.0, 55.8, 55.4; [M+H]+ = 413.3; HRMS (ESI): m/z calcd for C23H21N6O2 : 413.1721, found: 413.1723.
N-(3,4-dimethoxyphenyl)-5-phenyl-6H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-4-amine (10e): yellow solid, 59% yield; product purified using 0 to 30% MeOH in EtOAc gradient; m.p. 126 – 128°C; 1H NMR (400 MHz, CDCl3) δ ppm 10.17 (s, 1H), 7.28 – 7.14 (m, 4H), 7.04 (s, 1H), 7.03 – 6.96 (m, 3H), 6.93 (s, 1H), 6.61 – 6.35 (m, 3H), 3.93 (s, 3H), 3.82 (s, 3H), 3.30 (b, s, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 149.8, 149.5, 146.3, 141.34, 141.27, 133.4, 132.1, 130.9, 128.8, 128.71, 128.67, 126.7, 126.1, 121.9, 120.1, 119.9, 118.5, 117.4, 113.9, 100.7, 94.4, 56.2, 56.1; [M+H]+ = 413.3; HRMS (ESI): m/z calcd for C23H21N6O2 [M+H]+ : 413.1721, found: 413.1717.
5-(benzo[d][1,3]dioxol-5-yl)-8,9-dimethyl-4-(piperidin-1-yl)-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepine (11a): yellow solid, 59% yield; product purified using 10 to 40% EtOAc in Hexane gradient; m.p. 195 – 197 °C; 1H NMR (400 MHz, CDCl3) δ ppm 7.80 (s, 1H), 7.58 (dd, J = 8.3, 1.9 Hz, 1H), 7.52 (d, J = 1.8 Hz, 1H), 7.33 (s, 1H), 6.86 (d, J = 8.2 Hz, 1H), 6.03 (dd, J = 3.6, 1.3 Hz, 2H), 5.58 (s, 1H), 2.40 (s, 3H), 2.39 (s, 3H), 2.40 – 2.33 (m, 2H), 2.05 – 1.96 (m, 2H), 1.29 – 1.20 (m, 3H), 1.17 – 1.09 (m, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 159.7, 150.6, 150.1, 148.4, 138.1, 136.9, 135.1, 132.4, 127.6, 123.4, 121.4, 121.2, 108.2, 108.00, 101.8, 60.1, 51.9, 25.5, 24.00, 19.6, 19.5; [M+H]+ = 417.2; HRMS (ESI): m/z calcd for C23H25N6O2 [M+H]+: 417.2034, found: 417.2034.
4-(8,9-dimethyl-5-phenyl-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepin-4-yl)morpholine (11b): yellow solid, 51% yield; product purified using 10 to 100% EtOAc in Hexane gradient; m.p. 154 – 156°C; 1H NMR (400 MHz, CDCl3) δ ppm 8.05 – 7.94 (m, 2H), 7.84 (s, 1H), 7.56 – 7.43 (m, 3H), 7.38 (s, 1H), 5.72 (s, 1H), 3.26 (t, J = 4.5 Hz, 4H), 2.50 – 2.43 (m, 2H), 2.42 (s, 3H), 2.41 (s, 3H), 2.15 – 2.07 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 159.8, 149.4, 138.4, 137.7, 136.6, 136.0, 131.6, 128.9, 128.0, 127.9, 121.52, 121.50, 121.3, 66.3, 59.7, 50.9, 19.6, 19.5; [M+H]+ = 375.3; HRMS (ESI): m/z calcd for C21H23N6O [M+H]+ : 375.1928, found: 375.1935.
5-(benzo[d][1,3]dioxol-5-yl)-4-(3,4-dihydroisoquinolin-2(1H)-yl)-8,9-dimethyl-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepine (11c): yellow viscous oil, 23% yield; product purified using 10 to 100% EtOAc in Hexane gradient; 1H NMR (400 MHz, CDCl3) δ ppm 7.80 (s, 1H), 7.58 – 7.53 (m, 2H), 7.34 (s, 1H), 7.12 – 7.00 (m, 2H), 6.99 – 6.91 (m, 1H), 6.91 – 6.80 (m, 2H), 6.04 (dd, J = 4.2, 1.3 Hz, 2H), 5.82 (s, 1H), 3.62 (d, J = 14.9 Hz, 1H), 3.32 (d, J = 14.9 Hz, 1H), 2.59 – 2.41 (m, 4H), 2.34 (s, 3H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ ppm 158.6, 150.8, 149.6, 148.6, 138.4, 136.3, 135.7, 133.7, 133.0, 132.3, 128.4, 128.2, 126.6, 126.3, 125.9, 123.5, 121.5, 121.1, 108.3, 108.0, 101.9, 58.9, 53.3, 48.0, 28.1, 19.6, 19.5; [M+H]+ = 465.3; ; HRMS (ESI): m/z calcd for C27H25N6O2 [M+H]+: 465.2034, found: 465.2027.
5-(benzo[d][1,3]dioxol-5-yl)-8,9-dimethyl-4-(4-(pyridin-2-yl)piperazin-1-yl)-4H-benzo[b]tetrazolo[1,5-d][1,4]diazepine (11d): yellow solid, 41% yield; product purified using 10 to 100% EtOAc in Hexane gradient; m.p. 228 – 230°C; 1H NMR (400 MHz, CDCl3) δ ppm 8.10 (dd, J = 4.9, 1.1 Hz, 1H), 7.82 (s, 1H), 7.59 (dd, J = 8.3, 1.8 Hz, 1H), 7.54 (d, J = 1.8 Hz, 1H), 7.43 – 7.38 (m, 1H), 7.35 (s, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.58 (dd, J = 6.9, 5.0 Hz, 1H), 6.49 (d, J = 8.6 Hz, 1H), 6.03 (dd, J = 8.6, 7.6 Hz, 3H), 5.68 (s, 1H), 3.16 – 3.02 (m, 4H), 2.62 – 2.48 (m, 2H), 2.38 (s, 3H), 2.37 (s, 2H), 2.26 – 2.14 (m, 2H); 13C NMR (100 MHz, CDCl3) δ ppm 159.2, 158.8, 150.9, 149.7, 148.6, 147.9, 138.4, 137.4, 136.8, 135.6, 132.1, 127.7, 123.5, 121.4, 121.3, 113.7, 108.2, 108.0, 107.2, 101.9, 59.3, 50.3, 44.9, 19.7, 19.5; [M+H]+ = 495.3; HRMS (ESI): m/z calcd for C27H27N8O2 [M+H]+ : 495.2252, found: 495.2251.
Supplementary Material
Figure 1.
Structurally close benzodiazepine-based drugs showing different activities.
Figure 2.
The pivotal collection of benzodiazepinones 8. Yields represent the combined two overall steps.
Figure 4.
Synthesized benzodiazepines 10 and 11. Yields represent the combined two overall steps.
Scheme 1.
Mechanism of the 4-component Ugi-Azide reaction (compatible with 1ry or 2ry amines).
Scheme 2.
Synthetic route toward tetrazolo-fused benzodiazepinones 8.
Scheme 3.
Synthetic route toward tetrazolo-fused benzodiazepines 10 and 11.
Acknowledgements
The authors thank Kristen Keck for compound purification, Alex Laetsch for compound management, Gary Nichol for X-ray crystallographic work and Nicole Schechter for proof-reading. National Institute of Health (NIH) is gratefully acknowledged for funding (P41GM086190).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found online at doi:10.1016/j.tet.2012.xx.xxx.
References and notes
- 1.Costantino L, Barlocco D. Curr. Med. Chem. 2006;13:65. [PubMed] [Google Scholar]
- 2.Rudolf U, Mohler H. Curr. Opin. Pharmacol. 2006;6:18. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.(a) Parks DJ, LaFrance LV, Calvo RR, Milkiewicz KL, Gupta V, Lattanze J, Ramachandren K, Carver TE, Petrella EC, Cummings MD, Maguire D, Grasberger BL, Lu T. Bioorg. Med. Chem. Lett. 2005;15:765. doi: 10.1016/j.bmcl.2004.11.009. [DOI] [PubMed] [Google Scholar]; (b) Koblish HK, Zhao S, Franks CF, Donatelli RR, Tominovich RM, LaFrance LV, Leonard KA, Gushue JM, Parks DJ, Calvo RR, Milkiewicz KL, Marugan JJ, Raboisson P, Cummings MD, Grasberger BL, Johnson DL, Lu T, Molloy CJ, Maroney AC. Mol. Cancer Ther. 2006;5:160. doi: 10.1158/1535-7163.MCT-05-0199. [DOI] [PubMed] [Google Scholar]; (c) Forso S. Mini–Rev. Med. Chem. 2010;13:68. [Google Scholar]
- 4.Breslin HJ, Kukla MJ, Ludovici DW, Mohrbacher R, Ho W, Miranda M, Rodgers JD, Hitchens TK, Leo G, Gauthier DA, Ho CY, Scott MK, De Clercq E, Pauwels R, Andries K, Janssen MAC, Janssen PA. J. Med. Chem. 1995;38:771. doi: 10.1021/jm00005a005. [DOI] [PubMed] [Google Scholar]
- 5.Ursini A, Capelli AM, Carr RAE, Cassara P, Corsi M, Curcuruto O, Curotto G, Dal Cin M, Davalli S, Donati D, Feriani A, Finch H, Finizia G, Gaviraghi G, Marien M, Pentassuglia G, Polinelli S, Ratti E, Reggiani A, Tarzia G, Tedesco G, Tranquillini ME, Trist DG, Van Amsterdam FTM. J. Med. Chem. 2000;43:3596. doi: 10.1021/jm990967h. [DOI] [PubMed] [Google Scholar]
- 6.Samanen JM, Ali FE, Barton LS, Bondinell WE, Burgess JL, Callahan JF, Calvo RR, Chen W, Chen L, et al. J. Med. Chem. 1996;39:4867. doi: 10.1021/jm960558a. [DOI] [PubMed] [Google Scholar]
- 7.Keenan RM, Miller WH, Kwon C, Ali FE, Callahan JF, Calvo RR, Hwang SM, Kopple KD, Peishoff CE, Samanen JM, Wong AS, Yuan CK, Huffman WF. J. Med. Chem. 1997;40:2289. doi: 10.1021/jm970205r. [DOI] [PubMed] [Google Scholar]
- 8.(a) Multicomponent Reactions; Zhu J, Bienaymè H, editors. Weinheim, Germany: Wiley-VCH; 2005. Doemling A. Chem. Rev. 2006;106:17. doi: 10.1021/cr0505728. Sunderhaus JD, Martin SF. Chem. Eur. J. 2009;15:1300. doi: 10.1002/chem.200802140.
- 9.Banfi L, Riva R, Basso A. Synlett. 2010:23. [Google Scholar]
- 10.(a) Kennedy AL, Fryer AM, Josey JA. Org. Lett. 2002;4:1167. doi: 10.1021/ol0256015. [DOI] [PubMed] [Google Scholar]; (b) Shaabani A, Maleki A, Mofakham H. J. Comb. Chem. 2008;10:595. doi: 10.1021/cc8000635. [DOI] [PubMed] [Google Scholar]; (c) Hulme C, Ma L, Kumar NV, Krolikowski PH, Allen AC, Labaudiniere R. Tetrahedron Lett. 2000;41:1509. [Google Scholar]; (d) Salcedo A, Neuville L, Rondot C, Retailleau P, Zhu J. Org. Lett. 2008;10:857. doi: 10.1021/ol7029799. [DOI] [PubMed] [Google Scholar]; (e) Hulme C, Chappeta S, Dietrich J. Tetrahedron Lett. 2009;50:4054. doi: 10.1016/j.tetlet.2010.06.131. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Xu Z, Dietrich J, Shaw AY, Hulme C. Tetrahedron Lett. 2010;51:4566. doi: 10.1016/j.tetlet.2010.06.116. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Sanudo M, Garcia-Valverde M, Marcaccini S, Delgado J, Rojo J, Torroba T. J. Org. Chem. 2009;74:2189. doi: 10.1021/jo8025862. [DOI] [PubMed] [Google Scholar]; (h) Banfi L, Basso A, Guanti G, Kielland N, Repetto C, Riva R. J. Org. Chem. 2007;72:2151. doi: 10.1021/jo062626z. [DOI] [PubMed] [Google Scholar]; (i) Mossetti R, Seggiorato D, Tron GC. J. Org. Chem. 2011;76:10258. doi: 10.1021/jo2015054. [DOI] [PubMed] [Google Scholar]; (j) Lecinska P, Corres N, Moreno D, Garcia-Valverde M, Marcaccini S, Torroba T. Tetrahedron. 2010;66:6783. [Google Scholar]; (k) Donald JR, Martin SF. Org. Lett. 2011;13:852. doi: 10.1021/ol1028404. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Donald JR, Wood RR, Martin SF. ACS Comb. Sci. 2012;14:135. doi: 10.1021/co2002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For a review see: Hulme C, Nixey T, Bienaymé H, Chenera B, Jones W, Tempest P, Smith AL. Meth. Enzymol. 2003;369:469. doi: 10.1016/S0076-6879(03)69024-5.
- 12.See for instance: Gunawan S, Nichol G, Hulme C. Tetrahedron Lett. 2012;53:1664. doi: 10.1016/j.tetlet.2012.01.080.
- 13.Zabrocki J, Smith GD, Dunbar JB, Jr, Iijima H, Marshall GR. J. Am. Chem. Soc. 1988;110:5875. [Google Scholar]
- 14.Ugi I, Steinbruckner C . Chem. Ber. 1961;94:734. [Google Scholar]
- 15.(a) Wang W, Doemling A. J. Comb. Chem. 2009;11:403. doi: 10.1021/cc9000136. [DOI] [PubMed] [Google Scholar]; (b) Westermann B, Neuhaus C. Angew. Chem. Int. Ed. 2005;44:4077. doi: 10.1002/anie.200500297. [DOI] [PubMed] [Google Scholar]
- 16. Nichol GS, Xu Z, Kaiser CE, Hulme C. Acta Crystallogr. Sect. E. 2011;67:23. doi: 10.1107/S1600536810049950.. Crystallographic data can also be found at CDCC 807314
- 17.For the only reported example of a closely related structure see: Fetter J, Nagy I, Giang LT, Kajtar-Peredy M, Rockenbauer A, Korecz L, Czira G. J. Chem. Soc Perkin Trans. 1. 2001:1131.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.