Abstract
Neurohumoral activation, which includes augmented plasma levels of the neurohormone vasopressin (VP), is a common finding in heart failure (HF) that contributes to morbidity and mortality in this disease. While an increased activation of magnocellular neurosecretory cells (MNCs) and enhanced glutamate function in HF is well documented, the precise underlying mechanisms remain to be elucidated. Here, we combined electrophysiology and protein measurements to determine whether altered glial glutamate transporter function and/or expression occurs in the hypothalamic supraoptic nucleus (SON) during HF. Patch-clamp recordings obtained from MNCs in brain slices show that pharmacological blockade of astrocyte glutamate transporter 1 (GLT1) function [500 μM dihydrokainate (DHK)], resulted in a persistent N-methyl-d-aspartate receptor (NMDAR)-mediated inward current (tonic INMDA) in sham rats, an effect that was significantly smaller in MNCs from HF rats. In addition, we found a diminished GLT1 protein content in plasma membrane (but not cytosolic) fractions of SON punches in HF rats. Conversely, astrocyte GLAST expression was significantly higher in the SON of HF rats, while nonselective blockade of glutamate transport activity (100 μM TBOA) evoked an enhanced tonic INMDA activation in HF rats. Steady-state activation of NMDARs by extracellular glutamate levels was diminished during HF. Taken together, these results support a shift in the relative expression and function of two major glial glutamate transporters (from GLT1 to GLAST predominance) during HF. This shift may act as a compensatory mechanism to preserve an adequate basal glutamate uptake level in the face of an enhanced glutamatergic afferent activity in HF rats.
Keywords: N-methyl-d-aspartate, extrasynaptic, supraoptic, GLT1, GLAST
congestive heart failure (HF) is characterized by an increased neurohumoral drive, which involves augmented sympathetic tone, blunted cardiovascular reflexes, and elevated hormonal plasma levels, including vasopressin and ANG II, among others (39, 43, 70). Chronically elevated plasma VP levels have been reported both in animal models and human patients with HF (16, 19, 51, 61), being an important factor contributing to altered fluid/electrolyte balance, as well as detrimental myocardial effects (17, 18, 40, 44, 54).
Vasopressin (VP) [along with the neurohormone oxytocin (OT)], is produced by magnocellular neurosecretory cells (MNCs) located in the hypothalamic supraoptic (SON) and paraventricular nuclei (PVN). VP and OT peptides are then transported to the neurohypophyseal terminals, from where they are released into the circulation, according to the degree and pattern of electrical activity of MNCs (10, 50, 57). Thus, it is reasonable to speculate that the elevated VP levels in HF result from exacerbated activity of VP neurons. Indeed, a growing body of evidence supports augmented SON and PVN neuronal activation (23, 46, 47, 65, 68), as well as increased SON/PVN neuronal excitability during HF (23, 68). Still, the precise underlying mechanisms contributing to increased VP activity in HF remain to be elucidated.
It is well known that MNC firing activity is controlled by the combined action of intrinsic and extrinsic factors (8). Among the latter, the neurotransmitter glutamate acts as a major excitatory molecule stimulating OT and VP neuronal activity, an action that is largely mediated via activation of NMDA receptors (3, 25, 60, 66). We have recently shown that in addition to its classical fast synaptic actions, extracellular glutamate can also evoke a tonic, persistent form of excitation, via activation of extrasynaptic NMDA receptors (eNMDARs) (15). We found this novel glutamate-mediated excitatory modality to potently stimulate SON firing activity, and its strength to be tightly controlled by surrounding astrocytes. Thus, by efficiently clearing glutamate from the extracellular space, astrocyte glutamate transporters (53) restrain the degree of eNMDAR activation, preventing, in turn, overactivation of SON neurons (15). Recent in vitro and in vivo studies support an enhanced glutamate excitatory action in the SON and PVN of HF rats (29, 31, 34, 49, 69). However, whether an altered glial regulation of extracellular glutamate levels, and the concomitant activation of eNMDARs, occurs in HF, either as a pathogenic or compensatory mechanism, has not yet been investigated. To address this question, we obtained patch-clamp recordings from SON neurons in brain slices obtained from sham and HF rats, as well as immunohistochemical/Western blot studies to assess protein expression of two of the most predominant astrocytes glutamate transporter isoforms, GLT1 and GLAST. Our results support a diminished GLT1 function/expression in HF rats. This, however, was accompanied by a concomitant increase in GLAST expression and function, leading altogether to a more efficient clearance of ambient glutamate levels, and consequently, diminished tonic activation of NMDARs in HF rats. Thus, the shift in the regulation of extracellular glutamate levels from GLT1 to GLAST transporters may act as a compensatory mechanism in the face of an enhanced glutamatergic afferent activity in HF rats.
MATERIALS AND METHODS
Animals and induction of HF.
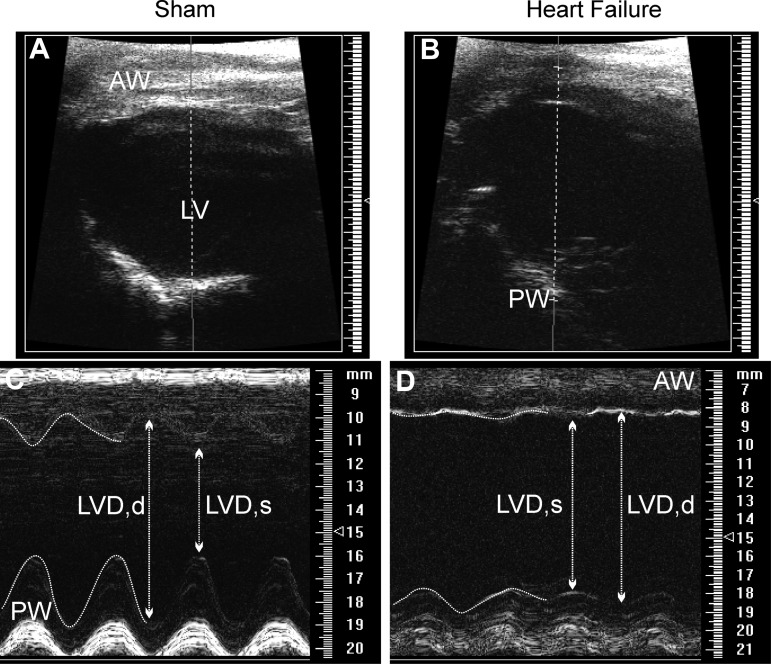
Male Wistar rats (150–180 g) were purchased from Harlan Laboratories (Indianapolis, IN). Rats were housed at room temperature (24–26°C) in a 12:12-h light-dark cycle room and given free access to food and water. All procedures were carried out in agreement with the Georgia Health Sciences University Institutional Animal Care and Use Committee guidelines. HF was induced by coronary artery ligation as previously described (5, 49). Briefly, animals were anesthetized with isoflurane 4% and intubated for mechanical ventilation. A left thoracotomy was performed, and the heart was exteriorized. The ligation was placed on the main diagonal branch of the left anterior descending coronary artery. Buprenorphine (Bruprenex C3 0.3 mg/kg sc; Butler Schein/NLS, Dublin, OH) was given immediately after surgery to minimize postsurgical pain. Sham animals underwent the same procedure, but the coronary artery was not occluded. All animals were used 6 to 7 wk after surgery. Transthoracic echocardiography (Vevo 770 system; Visual Sonics, Toronto, Ontario, Canada) was performed 4 wk after surgery under light anesthesia. The left ventricle internal diameter, as well as the left ventricle posterior and anterior walls' diameter, was obtained throughout the cardiac cycle from the short-axis, motion-imaging mode. Automatic calculation using the parameters measured was obtained for ejection fraction and fractional shortening. Representative echocardiography images are shown in Fig. 1, and mean cardiac function values obtained from sham and HF rats are summarized in Table 1.
Fig. 1.
Echocardiographic measurement of left ventricular function. Representative echocardiograph images in brightness-mode, short-axis view in a sham (A) and a heart failure (HF; B) rat. Movement-mode, short-axis view in sham (C) and HF (D) rats. AW, anterior wall; LV, left ventricle; PW, posterior wall; LVD, d/s: left ventricle dimension in diastole and systole. Note the increased left ventricle internal dimension during HF, indicative of loss of contractility function.
Table 1.
Summary data of echocardiography measurements of left ventricular parameters obtained from sham and heart failure
| EF, % | FS, % | LVIDd, mm | LVIDs, mm | |
|---|---|---|---|---|
| Sham | 84.2 ± 1.6 | 55.4 ± 1.8 | 7.7 ± 0.2 | 3.5 ± 0.2 |
| HF | 36.8 ± 2.0* | 19.9 ± 1.4* | 10.2 ± 0.2* | 8.2 ± 0.2* |
Values are expressed as means ± SE; sham, n = 25; HF, n = 18. EF, ejection fraction; FS, fractional shortening; LVID, d and s: left ventricle internal dimension during diastole and systole.
P < 0.0001 vs. sham.
Hypothalamic slice preparation.
Hypothalamic brain slices were prepared according to methods previously described (49, 59). Briefly, rats were deeply anesthetized with pentobarbital sodium (80 mg/kg ip) and perfused through the heart with an ice-cold sucrose solution [containing in mM: 200 sucrose, 2.5 KCl, 3 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 1 CaCl2, and 2 pyruvic acid (290–310 mosmol/l)]. Rats were then quickly decapitated, brains were dissected out, and coronal slices were cut (300 μm thick) using a vibroslicer (D.S.K. Microslicer, Ted Pella, Redding, CA). An oxygenated ice-cold artificial cerebrospinal fluid (ACSF) was used during slicing (containing in mM: 119 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 2 CaCl2, and 2 pyruvic acid; at pH 7.4; 290–310 mosmol/l). Slices were placed in a holding chamber containing ACSF and kept at room temperature until used.
Patch-clamp electrophysiology.
Slices were bathed with solutions (∼2.0 ml/min) that were continuously bubbled with 95% O2-5% CO2 and maintained at 32°C. Thin-walled (1.5 mm O.D., 1.17 mm I.D.) borosilicate glass (G150TF-3; Warner Instruments, Sarasota, FL) was used to pull patch pipettes (3–4 MΩ) on a horizontal Flaming/Brown micropipette puller (P-97; Sutter Instruments, Novato, CA). The internal solution contained (in mM): 140 potassium gluconate, 0.2 EGTA, 10 HEPES, 10 KCl, 0.9 MgCl2, 4 MgATP, 0.3 NaGTP, and 20 phosphocreatine (Na+); pH 7.2–7.3. For voltage-clamp recordings, a low Mg2+ ACSF (20 μM MgSO4,) was used to facilitate measurements of NMDA-mediated currents. Recordings were obtained with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) from SON/PVN neurons using infrared differential interference contrast videomicroscopy. The voltage-output was digitized at 16-bit resolution, 10 kHz, and were filtered at 2 kHz (Digidata 1320A; Axon Instruments). Data were discarded if the series resistance were not stable throughout the entire recording (>20% change), or if neuronal input resistance were lower than 350 MΩ at the beginning of the recording (49, 59). The NMDA receptor-mediated tonic current (tonic INMDA) in SON neurons (15) was assessed by measuring changes in holding current (Iholding) and root mean square (RMS) baseline noise levels following either bath application of glutamate receptor blockers or after blockade of glutamate transporter function. The mean tonic INMDA amplitude was calculated as the difference in the holding current measured as the average of a 2-min segment of steady-state baseline obtained before and after a 5-min application of blockers (15). The tonic INMDA current density was determined by dividing the current amplitude by the cell capacitance, obtained by integrating the area under the transient capacitive phase of a 5-mV depolarizing step pulse, in voltage-clamp mode. RMS noise was measured using Pclamp software within seven different 100-ms segments of traces lacking PSCs, and values were averaged. d-(-)-2-amino-5-phosphonopentanoic acid (d-AP5), and dihydrokainic acid (DHK) were purchased from Ascent Scientific (Princeton, NJ). All other drugs were purchased from Sigma-Aldrich (St. Louis, MO).
Immunohistochemistry and imaging analysis.
Standard immunohistochemistry was performed as previously described (4). Briefly, rats were perfused transcardially with 150 ml of 0.01 M PBS followed by 350 ml of 4% paraformaldehyde (PFD). Brains were dissected out, postfixed overnight in 4% PFD, and then cryoprotected in 0.01 M PBS containing 30% sucrose for 3 days at 4°C. Sequential 30-μm hypothalamic coronal sections were collected and subsequently blocked for unspecific sites in 10% horse serum. Sections were then incubated in a mixture of primary antibodies against EAAT2 glutamate transporter (anti-rabbit GLT1, 1:1,000 for 48 h; Abcam, Cambridge, MA); glial fibrillary protein [anti-mouse glial fibrillary acidic protein (GFAP), 1:2,000 for 24 h; Chemicon, Temecula, CA] and (Arg8)-vasopressin (anti-guinea pig VP 1:50,000 for 24 h; Bachem, Torrance, CA) or a mixture of EAAT1 glutamate transporter (anti-rabbit GLAST, 1:2,000 for 48 h; Abcam, MA) and anti-mouse GFAP, as described previously. Immunoreactivity was visualized by secondary reaction with anti-rabbit Cy3 (1:250); anti-mouse Alexa Fluor 647 (1:50) and anti-guinea pig FITC (1:250) for 4 h. Blocking agent and all antibodies were diluted in PBS 0.01 M containing 0.1% Triton X-100 and 0.04% NaN3. All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). Preabsorption controls for EAAT2 antibody were performed using a five-fold molar excess (relative to IgG concentration) of synthetic antigen (Ab 41752; Abcam, Cambridge, MA), as previously described (58).
Acquisition and quantification of immunofluorescent images were done as previously described (4). A Zeiss LSM 510 confocal scanning microscope (Carl Zeiss, Oberkochen, Germany) was used to obtain 15 consecutive optical focal planes (30 μm thick, 2-μm interval) at various rostrocaudal levels of the SON. Images from HF and sham groups were digitized with identical acquisition settings. For quantification, a projection image of the sections was generated, and imaging analysis was performed using Image J software (NIH). The SON nucleus was manually traced using VP or GFAP immunoreactivity as an anatomical landmark. Subsequently, background fluorescence was subtracted from the images from areas lacking immunoreactive signal. The density of GLT1 and GFAP thresholded signal within the traced nucleus was obtained from the different channels using the same demarcated area and expressed as a percentage of threshold area (i.e., a percentage of total area containing thresholded immunoreactive signal). The mean immunoreactivity intensity was also obtained from the same regions and expressed as arbitrary units (4). Likewise, colocalization was calculated from both images superimposed within the same region previously measured and also expressed as the percentage of threshold area. Algorithms were provided by Image J software. A mean value of all sampled sections was calculated, and then values per animal were used for statistical analysis.
Western blots.
Brains from sham and HF animals (n = 4 each group) were dissected, fast frozen in isopentane −35°C for 30 s, and transferred to a cryostat. The SON was punched out from 300-μm hypothalamic slices (three sequential slices/rat) using a micropunch (Ted Pella, Redding, CA), and immediately frozen on liquid nitrogen. Membrane and cytosolic fractions were extracted from SON punches, according to methods described previously (38). Briefly, samples were homogenized in 40-μl ice-cold lysis buffer (RIPA buffer added PMSF; a cocktail of protease inhibitors and Na3VO4), extracted on ice for 1 h and then centrifuged at 4°C 1,000 g for 10 min for rough partition between cytosolic and membrane fractions. The supernatant was collected, recentrifuged at 16,000 g for 15 min to isolate any contaminating pellet materials and recollected as cytosolic fraction. The initial pellet was resuspended in 10-μl cell lysis buffer containing 1% Triton X-100, extracted on ice for 1 h, centrifuged at 4°C 16,000 g for 15 min, and the supernatant was collected as a membrane fraction. The Bradford method was used to measure protein concentration from each fraction using BSA as a standard; 7.5 μg of total protein from each fraction for sham and HF were separated on an SDS-PAGE 10% polyacrilamide gel and electrophoretically transferred to a nitrocellulose membrane. The nonspecific sites of the membrane were blocked with 5% nonfat dry milk in Tris-buffered saline solution with Tween 0.1% (TBS-T) for 1 h. The membranes were then incubated overnight at 4°C with the primary rabbit polyclonal antibody raised against EAAT2 (GLT, 1:500; Abcam) diluted in 5% nonfat milk in TBS-T. The membrane was washed and subsequently incubated with an anti-rabbit horseradish peroxidase secondary antibody. A chemiluminescent assay kit (ChemiGlow; Alpha Innotech, Santa Clara, CA) was used to detect immunoreactivity, and the intensity of all bands was estimated by densitometry analysis (Alpha View Software—Proteinsimple, Santa Clara, CA). All densitometry measurements were normalized using an antibody against β-actin (1:30,000; Sigma-Aldrich, St. Louis, MO) as a loading control and expressed as unit of change.
Statistical analysis.
All values are expressed as means ± SE. Student's paired t-test was used to compare the effects of a drug treatment on tonic INMDA. Between-group differences (e.g., sham vs. HF) were compared using unpaired t-tests or ANOVA, as indicated followed by Bonferroni post hoc tests. Differences were considered statistically significant at P < 0.05, and n refers to the number of cells. All statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Representative echocardiography images obtained from a total of 25 sham and 18 HF rats are shown in Fig. 1, and mean cardiac function values obtained are summarized in Table 1. Compared with sham rats, ligated rats showed a significant increased left ventricle internal dimension throughout the cardiac cycle, a decreased percentage of ejection fraction, and a decreased percentage fractional shortening (P < 0.0001 in all cases). Patch-clamp electrophysiological recordings were obtained from a total of 87 SON MNCs obtained from sham (n = 48 MNCs from 16 rats) and HF rats (n = 39 MNCs from 10 rats).
Blunted glial GLT1 transporter function and expression in the SON of HF rats.
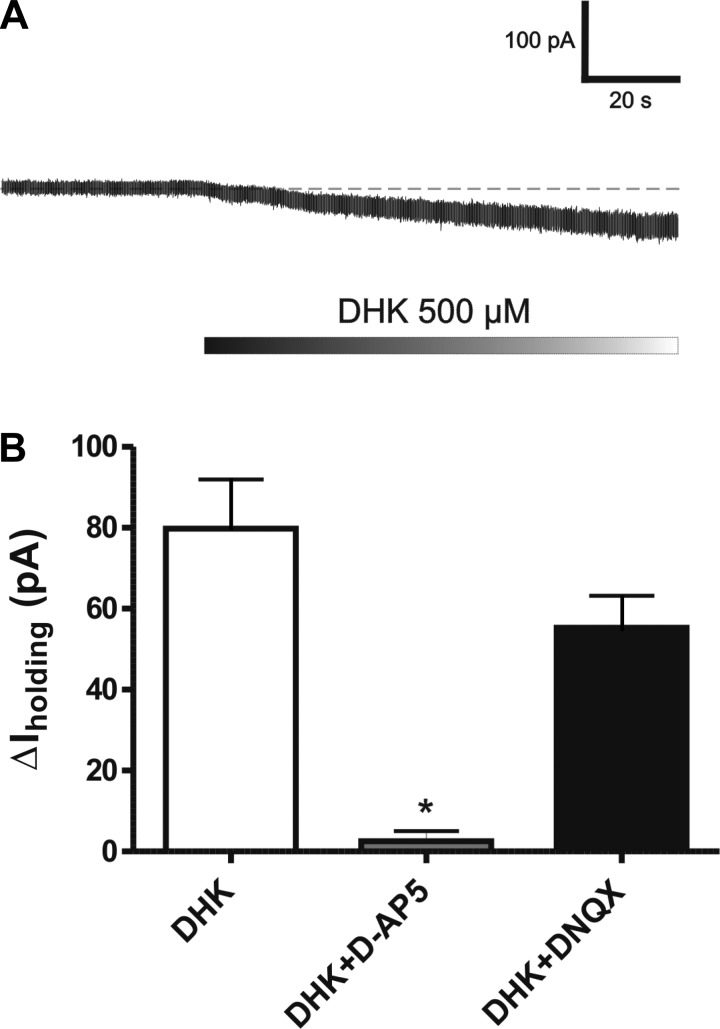
Blockade of the astrocyte glutamate transporter GLT1 by bath-applied dihydrokainate (DHK, 500 μM) resulted in a sustained inward shift in Iholding in SON neurons (Fig. 2A), as well as an increase in basal RMS noise level (basal: 5.9 ± 0.9 pA; DHK: 9.5 ± 1.4 pA; P < 0.01), due to increased stochastic channel activity (52). The DHK-evoked shift in Iholding was significantly and largely blocked when slices were preincubated with an NMDA receptor blocker (100 μM AP5, n = 6, P < 0.001) but not with an AMPA receptor blocker (10 μM DNQX, n = 5) (Fig. 2B). As we recently demonstrated, this current (termed also tonic INMDA) is mediated by activation of extrasynaptic NMDA receptors by extracellular glutamate (15).
Fig. 2.
Blockade of astrocyte glutamate transporters GLT1 induces an N-methyl-d-aspartate (NMDA)-mediated tonic inward current (tonic INMDA). A: bath application of the glial-selective glutamate transporter blocker dihydrokainate (DHK; 500 μM) induced an inward shift in Iholding. B: group data showing that the DHK effect was significantly blocked by the NMDA receptor blocker AP5 (100 μM; n = 6) but not by the AMPA receptor blocker DNQX (10 μM; n = 5). *P < 0.01 vs. DHK.
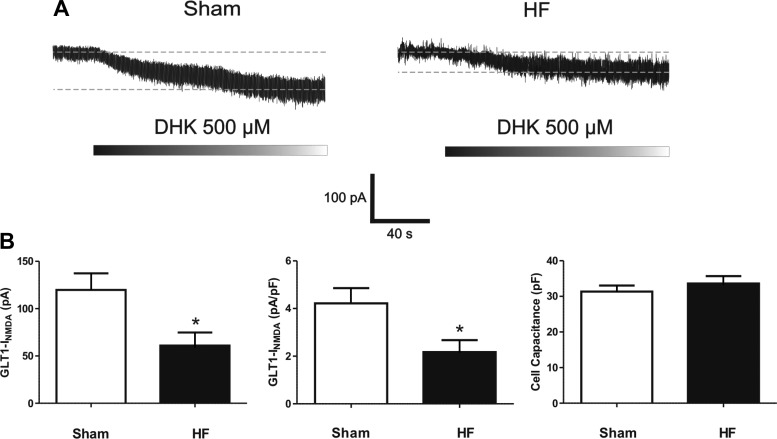
The magnitude of the DHK-evoked tonic INMDA was significantly smaller in SON neurons recorded in HF, compared with sham rats (P < 0.01). Representative examples and summary data are shown in Fig. 3. Similarly, a blunted DHK-evoked change in RMS was also observed in HF rats (sham: Δ3.6 ± 0.9 pA; HF: Δ1.6 ± 0.3, P < 0.05). To rule out that these effects were not secondary to potential changes in neuronal size in HF rats, we measured neuronal cell capacitance and compared current densities between groups. SON cell capacitance was similar between sham and HF rats. Accordingly, the DHK-evoked tonic INMDA current density was still significantly smaller in SON from HF, compared with sham rats (P < 0.01, Fig. 3B).
Fig. 3.
Blunted DHK-evoked tonic INMDA in supraoptic nucleus (SON) neurons from heart failure (HF) rats. A: representative traces showing a DHK-evoked tonic INMDA in a SON neuron from a sham and a HF rat. B: group data showing the mean DHK-evoked tonic INMDA current (left), INMDA current density (middle), and cell capacitance (right) in SON neurons from sham (n = 26) and HF (n = 27) rats. *P < 0.01 vs. sham.
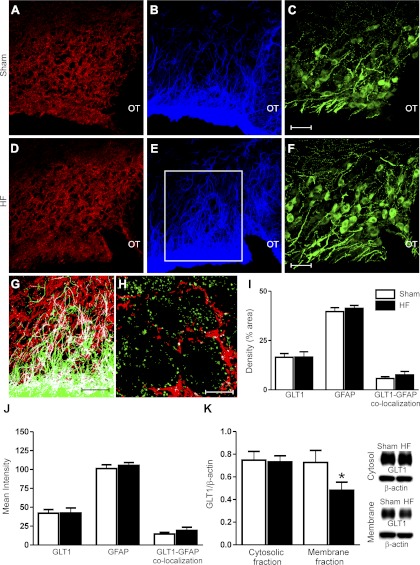
To determine whether a diminished GLT1 protein expression contributed to the observed blunted DHK-evoked tonic INMDA in HF rats, we measured GLT1 along with GFAP immunoreactivites in the SON of sham and HF rats (n = 4 rats in each group). Representative examples are shown in Fig. 4A–F. GLT1 immunoreactivity was localized in neuropilar elements, and in many cases, in close apposition to VP neuronal somata. As expected, we found a certain degree of colocalization between GLT1 and GFAP profiles, but not with VGLUT2, a marker of glutamatergic presynaptic terminals (Fig. 4, G and H), supporting a glial, rather than neuronal, localization of GLT1 in the SON. Preabsorption of the GLT1 antibody with the respective antigen protein resulted in complete absence of staining (not shown). No differences in GLT1, nor GFAP SON immunoreactivity densities or mean intensities, or in their degree of colocalization, were observed between sham and HF rats (Fig. 4, I and J). Given however, that the approach used did not distinguish membrane from cytosolic GLT1 distribution and that an altered trafficking of GLT1 to and from the surface membrane has been reported in several brain disease conditions (9, 67), we further investigated GLT1 protein expression by performing Western blot analyses of GLT1 protein in membrane and cytosolic fractions of SON punches. As shown in Fig. 4K, while GLT1 content in cytosolic fractions was similar between sham and HF rats, a significantly lower content was observed in SON membrane fractions in HF compared with sham rats (P < 0.02).
Fig. 4.
Diminished GLT1 expression in surface membrane, but not cytosolic fractions in the SON of HF rats. A–F: representative confocal photomicrographs of GLT1 (red; A, D), glial fibrillary acidic protein (GFAP) (blue; B, E), and vasopressin (VP; green; C, F) within the SON of a sham (A–C) and a HF rat (D–F), respectively. G: colocalization diagram from merged GLT1 and GFAP from the area delineated in E (red is GLT1, green is GFAP, and white represents the colocalized signal between both). H: confocal photomicrograph showing no colocalization between GLT1 (red) and VGLUT-positive boutons (green). I, J: summary data showing no differences in GLT1, GFAP, or GLT1-GFAP colocalization immunoreactivity density (I) or intensity (J) in HF rats compared with sham rats. K: representative Western blot bands for GLT1 protein levels on SON cytosolic and membrane fractions in a sham and HF rat. The summary data showed decreased membrane, but not cytosolic, GLT1 protein levels in HF compared with sham rats. OT: optic tract. *P < 0.05 vs. sham. n = 4 in each group. Scale bars: 50 μm (C, F, and G) and 10 μm (H).
Tonic persistent activation of eNMDARs by ambient glutamate levels is diminished in SON neurons from HF rats.
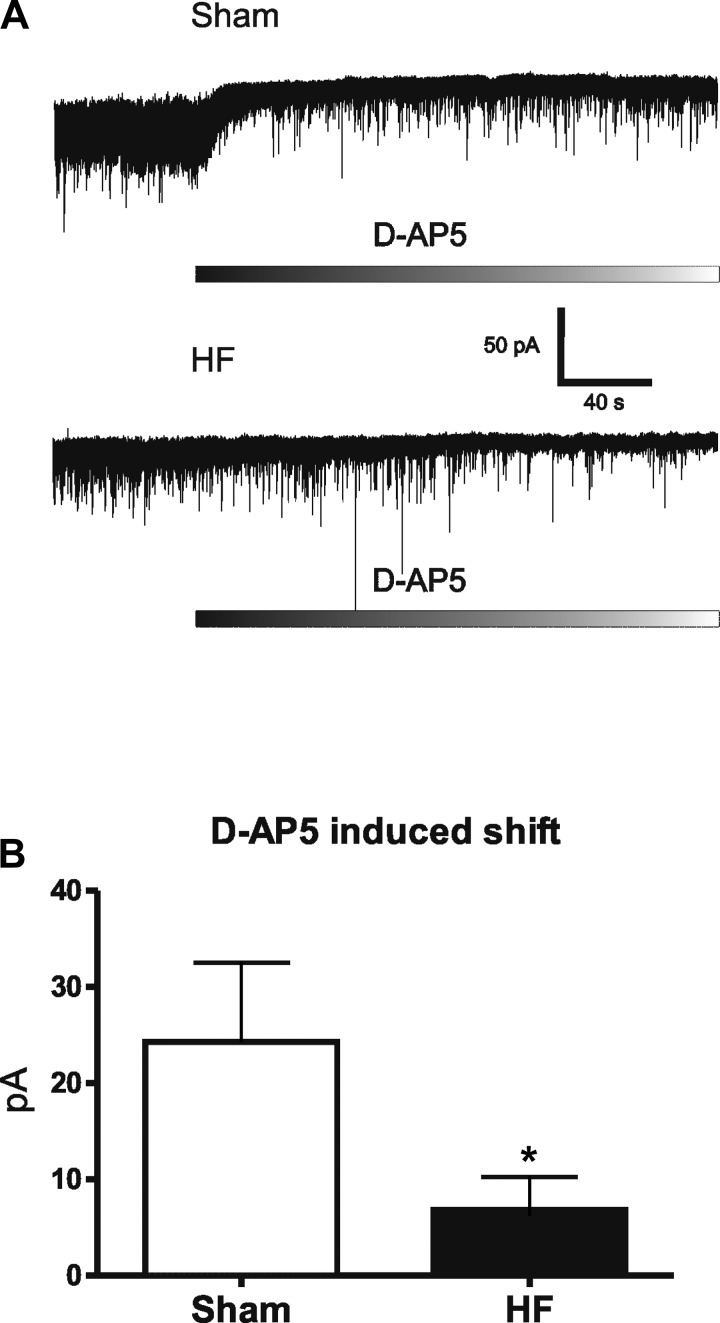
We have shown that the magnitude of the basal tonic INMDA, which results from activation by extracellular ambient glutamate levels under glial control, can be monitored as an outward shift in the holding current following NMDAR blockade (15). Thus, we used this approach to determine whether the blunted GLT1 expression/function described above resulted in an increased activation of basal tonic INMDA. As we previously reported (15), bath application of AP5 (100 μM) induced a sustained outward shift in Iholding in SON neurons. However, we unexpectedly found the AP5 effect to be significantly smaller in SON neurons from HF compared with sham rats (sham: Δ24.3 ± 8.24 pA; HF: Δ6.8 ± 3.4 pA, P < 0.05, n = 11/group) (Fig. 5, A and B). Similarly, a smaller AP5-evoked change in RMS was also observed in HF rats (sham: Δ4.5 ± 0.7 pA; HF: Δ2.4 ± 0.3 pA, P < 0.02, n = 11/group). These results indicate that despite the blunted contribution of GLT1 to glutamate clearance, there is an overall diminished basal tonic activation of INMDA in HF rats.
Fig. 5.
Diminished tonic NMDA receptor-mediated current (tonic INMDA) in SON neurons in HF rats. A: representative traces showing an outward shift in Iholding evoked by bath application of the NMDAR blocker d-AP5 (100 μM) in SON neurons recorded in a sham and in a HF rat. B: group data showing a significantly diminished d-AP5-evoked current in SON neurons from HF compared with sham rats (n = 11/group). *P < 0.05 vs. sham.
Enhanced glial GLAST transporter function and expression in SON of HF rats.
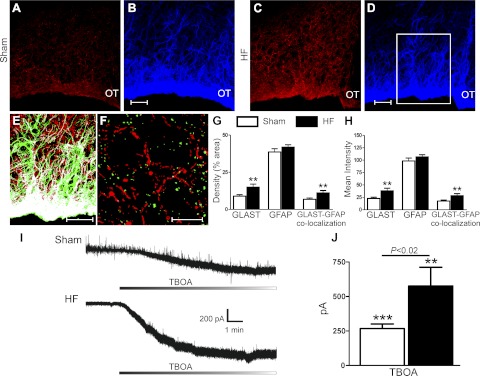
Our findings showing blunted GLT1 activity/expression along with a diminished tonic activation of INMDA suggests that an alternative glutamate transport mechanism, most likely GLAST, may be compensating in the clearance of extracellular glutamate for the compromised GLT1 activity during HF. It has been recently shown that a diminished GLT1 function/expression can lead to a compensatory response involving an increased function/expression of the alternative major glutamate transporter in the central nervous system, GLAST (55). To determine whether this was the case in HF rats, we measured GLAST immunoreactivity in the SON of sham and HF rats (n = 4 and 3 rats, respectively). Representative examples are shown in Fig. 6, A–D. Similar to GLT1, GLAST immunoreactivity displayed a glial-like pattern, with a predominant neuropilar distribution surrounding somata of large magnocellular neurons. Colocalization with GFAP, but not VGLUT2, immunoreactivities, was observed (Fig. 6, E and F). Compared with sham rats, GLAST immunoreactivity, density, mean intensity, as well as its colocalization with the glial marker GFAP, were significantly enhanced in HF rats (P < 0.01) (Fig. 6, G and H).
Fig. 6.
Enhanced SON GLAST expression and TBOA-evoked tonic INMDA in SON neurons from HF rats. A–D: representative confocal photomicrographs of GLAST (red; A, C) and GFAP (blue; B, D) within the SON of a sham (A, B) and a HF rat (C, D). E: colocalization diagram from merged GLAST and GFAP from the area delineated in D (red is GLAST, green is GFAP, and white represents the colocalized signal between both). F: confocal photomicrograph showing no colocalization between GLAST (red) and VGLUT-positive boutons (green). G, H: summary data showing a significant increase in the density (left) and intensity (right) of GLAST immunoreactivity, as well as an increased GLAST-GFAP colocalization in HF (n = 3) compared with sham rats (n = 4). I: representative traces showing a TBOA-evoked tonic INMDA in SON neurons from a sham and a HF rat. J: group data showing the mean TBOA-evoked tonic INMDA current in SON neurons from sham (n = 8) and HF (n = 6) rats. **P < 0.01 and ***P < 0.0001 vs. respective sham. OT, optic tract. Scale bars: 50 μm (B, D, and E) and 10 μm (F).
We then assessed whether the enhanced GLAST protein expression in the SON of HF rats translated into a more predominant functional contribution of this transporter to glutamate clearance. We attempted first to use a recently developed selective GLAST blocker compound, UCPH-101 (28). Unfortunately, however, in our hands, this compound was only soluble at high DMSO concentrations, which resulted per se in a change in holding current (not shown). Thus, as an alternative approach, we tested the effects of the nonselective glutamate transporter blocker DL-threo-β-benzyloxyaspartic acid (TBOA). We found that similar to DHK, bath application of TBOA (100 μM) induced a sustained inward shift in Iholding. This effect, however, was significantly larger in SON neurons from HF compared with sham rats (sham: Δ267.8 ± 33.1 pA; HF: Δ576.3 ± 136.0, P < 0.02, n = 8 and 6, respectively, Fig. 6, I and J). Similar differences were obtained when data were expressed as current density (sham: Δ9.2 ± 3.8 pA/pF; HF: Δ15.7 ± 6.7 pA/pF, P < 0.05). Similarly, a larger TBOA-evoked change in RMS noise level was also observed in HF rats (sham: Δ0.3 ± 5.9 pA; HF: Δ18.6 ± 5.1, P < 0.001).
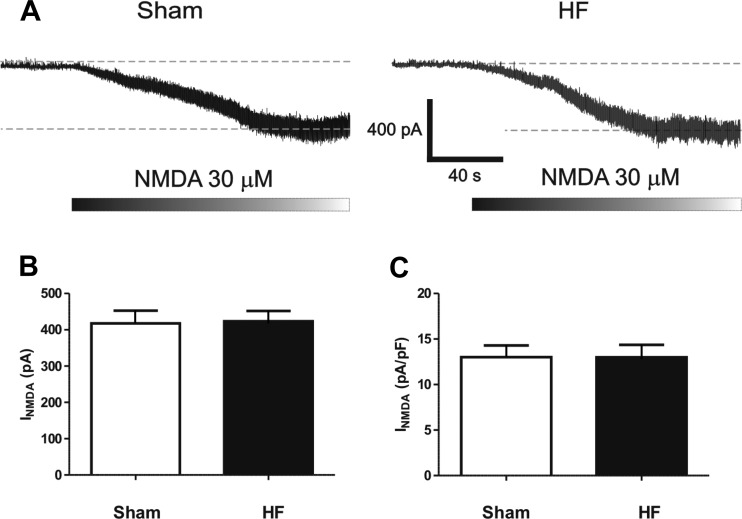
Finally, the fact that we observed opposing changes in tonic INMDA following selective and nonselective glutamate transporter blockade argues against a contribution of altered NMDAR function and expression per se to the reported changes in INMDA during HF. Nonetheless, given that previous reports demonstrated an altered expression of NR1 NMDAR subunits in PVN neurons of HF rats (34), we tested for this possibility by directly activating NMDARs via bath application of the agonist NMDA (30 μM), with the caveat that while this approach preferentially activates extrasynaptic NMDARs, synaptic receptors would also be activated. As shown in Fig. 7, no differences in evoked NMDA current or current density were observed between SON neurons from sham or HF rats (n = 26 and 22, respectively).
Fig. 7.
Direct NMDA receptor activation evoked similar currents in SON neurons in sham and HF rats. A: representative traces showing inward currents evoked by bath application of NMDA (30 μM) in SON neurons recorded in a sham and in a HF rat. B: group data showing the mean NMDA-mediated current (left) and current density (right) in SON neurons from sham (n = 26) and HF (n = 22) rats.
DISCUSSION
An augmented neuronal activation, along with a concomitant increase in firing activity (23, 46, 47, 65, 68), has been proposed to contribute to neurohumoral activation in HF rats. While an enhanced glutamate function has been demonstrated in the SON/PVN of HF rats (29, 31, 34, 49, 69), the precise mechanisms underlying regulation of glutamate function in HF remain unknown. We have recently demonstrated the presence of a novel glutamate excitatory modality in SON neurons (i.e., tonic INMDA), which is mediated by activation of eNMDARs by extracellular glutamate. We have also shown that by tightly regulating extracellular glutamate levels, astrocyte glutamate transporters influence the strength of tonic INMDA (15). In this study, we aimed to determine whether changes in astrocyte glutamate transporter function and/or expression occur in the SON of HF rats, contributing, in turn, to altered glutamate excitatory actions in HF rats.
Glutamate transporters, particularly the astrocytic GLT1 and GLAST isoforms, constitute the major mechanism for glutamate clearance and maintenance of low ambient glutamate levels in the central nervous system (11, 53). As shown in Figs. 2 and 3 in this article, selective blockade of GLT1 function via bath application of DHK induced a persistent inward current in SON neurons, mediated by activation of NMDA, but not AMPA receptors (tonic INMDA). As we recently reported, tonic INMDA is mediated by activation of extrasynaptic NMDARs, which strongly stimulate SON firing activity (15). Taken together, these results indicate that under basal conditions, glial GLT1 transporters actively maintain extracellular glutamate at relatively low levels, restraining, in turn, the degree of extrasynaptic NMDAR activation, and its excitatory effect on SON neuronal activity.
Here, we found that the enhancement of tonic INMDA following glial GLT1 blockade was diminished in SON MNCs from HF rats, supporting a blunted contribution of GLT1 function to extracellular glutamate clearance during HF. To determine whether this was associated with a diminished GLT1 protein expression, we performed immunohistochemistry and Western blot analyses. Using conventional immunohistochemistry, we did not observe differences in overall GLT1 immunoreactivity between sham and HF rats. However, it is still possible that changes in GLT1 expression were below detectable levels of this technique, or alternatively, that changes in GLT1 expression were restricted to a specific cell compartment (e.g., surface plasma membrane), which conventional immunohistochemistry would fail to discriminate. This later option was indeed confirmed by our Western blot studies, which revealed a diminished GLT1 expression in membrane, but not cytoplasmic fractions in SON punches from HF rats. A selective decrease in protein expression at the surface membrane could reflect an altered trafficking of GLT1 to and from the surface membrane during HF. Trafficking and translocation are critical steps for proper transport activity, which are amenable to modulation by a variety of mechanisms, particularly protein kinases (2, 6, 20, 33). Importantly, reactive oxygen species (ROS) have been shown to prevent GLT1 trafficking, diminishing, in turn, GLT1 activity (38, 63, 64). This is particularly relevant to this study, given the well-documented increased oxidative stress in the hypothalamus of HF rats and its critical contribution to neurohumoral activation in this disease (22, 26, 27, 36, 37). Thus, future studies evaluating the role of ROS in altered GLT1 function and expression during HF are warranted.
Similar to what we recently reported in SON neurons of water-deprived rats (15) [see, however, (30)], we hypothesized that a diminished GLT1 activity in HF rats would result in an enhanced activation of tonic INMDA in SON neurons (due to excess of extracellular glutamate), contributing, in turn, to increased neuronal excitability in HF rats. Our results with the NMDAR blocker AP5, however, support the opposite phenomenon. AP5 under basal conditions in sham rats resulted in an outward shift in Iholding, reflecting the tonic activation of NMDARs by extracellular glutamate levels (15, 32). Unexpectedly, we found a blunted AP5 effect in SON neurons from HF rats, suggesting lower basal levels of extracellular glutamate, possibly due to a more efficient clearance mechanism during this condition.
It was recently shown that blockade of GLT1 in the striatum evokes a transient compensatory increase in GLAST activity, resulting in an overall increase in glutamate uptake (55). This response was proposed to provide an endogenous defense mechanism against excitotoxicity to the nigrostriatal pathway (55). Our results showing an increased GLAST expression in the SON of HF rats, along with a larger TBOA-evoked activation of tonic INMDA support a compensatory enhancement of GLAST participation in extracellular glutamate clearance in the SON of HF rats. Thus, one possible scenario to consider is that in the initial stages of HF, an enhanced glutamate afferent activity, along with a blunted GLT1 expression and activity, results in an excess of extracellular glutamate levels (29, 31, 34), which then triggers a compensatory increase in GLAST expression and activity, in an attempt to maintain extracellular glutamate levels within normal ranges. In this sense, astrocyte GLAST activity has been shown to be enhanced in response to increases in glutamate levels (14). Future studies evaluating the time-course of changes in GLT1/GLAST expression and activity during the progress of the HF syndrome would be necessary to more conclusively support this hypothesis. Finally, it is important to consider that a limitation of these studies is that extracellular glutamate levels in vitro may not necessarily reflect those in vivo, in which the activity of all glutamate afferent inputs is preserved. Thus, future whole animal studies would be needed to confirm the in vitro findings reported in our study.
In summary, results from this study support a shift in the relative expression and function of two major glial glutamate transporters (from GLT1 to GLAST predominance) during HF. Despite these changes, basal steady-state activation of eNMDARs by extracellular glutamate, rather than being exacerbated, was actually found to be diminished. Thus, our studies suggest that compromise in one of the major glutamate uptake mechanisms (GLT1) in heart failure leads to the compensatory enhancement of an alternative one (GLAST) in order to preserve an adequate basal glutamate uptake level.
Perspectives and Significance
Given the clinical relevance of neurohumoral activation in HF, there is a great deal of interest in elucidating its underlying mechanisms. While a growing body of evidence supports elevated excitatory glutamate function as a pivotal pathophysiological finding in HF, the precise mechanisms contributing to such elevated excitatory effects, as well as potential compensatory cellular and network responses, remain unclear.
Astrocytes have emerged in recent years as critical players in the regulation of neuronal and synaptic function in the central nervous system (1, 41). Moreover, an abnormal astrocyte function, including altered GLT1 modulation of extracellular glutamate levels, has been demonstrated in various neurological conditions, including Alzheimer's disease, stroke, and amyotrophic lateral sclerosis (12, 35, 56). Still, whether an altered astrocyte function also contributes to cardiovascular related disorders, particularly those displaying elevated neurohumoral activation, is still unknown.
The magnocellular neurosecretory system provides an ideal model to address this problem. It is a relatively simple, well-characterized system that displays a unique and highly plastic neuroglial microenvironment (24, 62). A growing body of evidence supports a major role of astrocytes in the regulation of SON/PVN neuronal function, via a variety of mechanisms, including release of neuroactive substances, such as ATP (21), taurine (13), d-serine (45), and nitric oxide (5), as well as via the activity of neurotransmitter transporters. Thus, glial GLT1 have been shown to strongly influence the efficacy of both metabotropic glutamate receptors (7, 42, 48), as well as extrasynaptic NMDA receptor activation (15). Results from the present study provide evidence for a change in glial regulation of glutamate function during HF, which involves a shift in the expression of different glutamate transporters, as well as in their relative contribution to glutamate uptake. Future studies evaluating the time course of such changes are necessary to determine their overall contribution to neurohumoral activation, either as a pathogenic or compensatory mechanism.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.S.P., V.C.B., and Y.Z. performed experiments; E.S.P., V.C.B., and J.E.S. analyzed data; E.S.P., V.C.B., and J.E.S. interpreted results of experiments; E.S.P. and V.C.B. prepared figures; E.S.P., V.C.B., and J.E.S. approved final version of manuscript; J.E.S. conception and design of research; J.E.S. drafted manuscript; J.E.S. edited and revised manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health R01 HL090948 (to J. E. Stern).
REFERENCES
- 1. Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63: 795–813, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci 17: 2106–2118, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol 499: 733–746, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol 518: 567–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension 58: 454–463, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4–2 is impacted by SGK kinases. J Neurochem 97: 911–921, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol 551: 815–823, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bourque CW, Oliet SH, Kirkpatrick K, Richard D, Fisher TE. Extrinsic and intrinsic modulatory mechanisms involved in regulating the electrical activity of supraoptic neurons. Ann NY Acad Sci 689: 512–519, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Bradesi S, Golovatscka V, Ennes HS, McRoberts JA, Karagiannides I, Bakirtzi K, Pothoulakis C, Mayer EA. Role of astrocytes and altered regulation of spinal glutamatergic neurotransmission in stress-induced visceral hyperalgesia in rats. Am J Physiol Gastrointest Liver Physiol 301: G580–G589, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol 369: 45–60, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danbolt NC. Glutamate uptake. Prog Neurobiol 65: 1–105, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Davalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke 28: 708–710, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol 507: 463–471, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19: 10193–10200, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol 589: 3929–3941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 82: 1724–1729, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Goldsmith SR, Francis GS, Cowley AW., Jr Arginine vasopressin and the renal response to water loading in congestive heart failure. Am J Cardiol 58: 295–299, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Goldsmith SR, Francis GS, Cowley AW, Jr, Goldenberg IF, Cohn JN. Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol 8: 779–783, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Goldsmith SR, Francis GS, Cowley AW, Jr, Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol 1: 1385–1390, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez MI, Bannerman PG, Robinson MB. Phorbol myristate acetate-dependent interaction of protein kinase Cα and the neuronal glutamate transporter EAAC1. J Neurosci 23: 5589–5593, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8: 1078–1086, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-α blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol 293: H599–H609, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol 299: R129–R139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol 34: 437–504, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol 458: 667–687, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang BS, Zheng H, Tan J, Patel KP, Leenen FH. Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Exp Physiol 96: 1028–1038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763–1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen AA, Erichsen MN, Nielsen CW, Stensbol TB, Kehler J, Bunch L. Discovery of the first selective inhibitor of excitatory amino acid transporter subtype 1. J Med Chem 52: 912–915, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 83: 737–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. King TS, Toney GM, Tian PY, Javors MA. Dehydration increases sodium-dependent glutamate uptake by hypothalamic paraventricular nucleus synaptosomes. Neuroendocrinol Lett 32: 763–768, 2011 [PMC free article] [PubMed] [Google Scholar]
- 31. Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol 580: 373–383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem 97: 759–771, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20: 589–602, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res 103: 186–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu M, Hu LF, Hu G, Bian JS. Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic Biol Med 45: 1705–1713, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Mancia G. Sympathetic activation in congestive heart failure. Eur Heart J 11 Suppl A: 3–11, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Nakamura Y, Haneda T, Osaki J, Miyata S, Kikuchi K. Hypertrophic growth of cultured neonatal rat heart cells mediated by vasopressin V1A receptor. Eur J Pharmacol 391: 39–48, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26: 523–530, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292: 923–926, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721–730, 1988 [DOI] [PubMed] [Google Scholar]
- 44. Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 75: IV80–IV92, 1987 [PubMed] [Google Scholar]
- 45. Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell 125: 775–784, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of Fos protein in the hypothalamus of rats with heart failure. Brain Res 865: 27–34, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol 265: R923–R928, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci USA 101: 2151–2155, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol 106: 1545–1557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience 7: 773–808, 1982 [DOI] [PubMed] [Google Scholar]
- 51. Riegger GA, Liebau G, Bauer E, Kochsiek K. Vasopressin and renin in high output heart failure of rats: hemodynamic effects of elevated plasma hormone levels. J Cardiovasc Pharmacol 7: 1–5, 1985 [DOI] [PubMed] [Google Scholar]
- 52. Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci 15: 2788–2795, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Rouleau JL, Packer M, Moye L, de Champlain J, Bichet D, Klein M, Rouleau JR, Sussex B, Arnold JM, Sestier F, Parker JO, McEwan P, Bernstein V, Cuddy TE, Lamas G, Gottlieb SS, McCans J, Nadeau C, Delage F, Wun C-CC, Pfeffer MA. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol 24: 583–591, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Salvatore MF, Davis RW, Arnold JC, Chotibut T. Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at Ser (19). Exp Neurol 234: 428–436, 2012 [DOI] [PubMed] [Google Scholar]
- 56. Sasaki K, Shimura H, Itaya M, Tanaka R, Mori H, Mizuno Y, Kosik KS, Tanaka S, Hattori N. Excitatory amino acid transporter 2 associates with phosphorylated tau and is localized in neurofibrillary tangles of tauopathic brains. FEBS Lett 583: 2194–2200, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Silverman AJ, Zimmerman EA. Magnocellular neurosecretory system. Annu Rev Neurosci 6: 357–380, 1983 [DOI] [PubMed] [Google Scholar]
- 58. Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol 582: 1219–1238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Swenson KL, Badre SE, Morsette DJ, Sladek CD. N-methyl-d-aspartic acid stimulation of vasopressin release: role in osmotic regulation and modulation by gonadal steroids. J Neuroendocrinol 10: 679–685, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med 305: 263–266, 1981 [DOI] [PubMed] [Google Scholar]
- 62. Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol 468: 175–182, 1999 [DOI] [PubMed] [Google Scholar]
- 63. Trotti D, Nussberger S, Volterra A, Hediger MA. Differential modulation of the uptake currents by redox interconversion of cysteine residues in the human neuronal glutamate transporter EAAC1. Eur J Neurosci 9: 2207–2212, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci 9: 1236–1243, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol 275: H2140–H2146, 1998 [DOI] [PubMed] [Google Scholar]
- 66. van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science 250: 1276–1278, 1990 [DOI] [PubMed] [Google Scholar]
- 67. Vanoni C, Massari S, Losa M, Carrega P, Perego C, Conforti L, Pietrini G. Increased internalisation and degradation of GLT-1 glial glutamate transporter in a cell model for familial amyotrophic lateral sclerosis (ALS). J Cell Sci 117: 5417–5426, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol 84: 217–232, 2004 [DOI] [PubMed] [Google Scholar]