Abstract
Homeostatic eating cannot explain overconsumption of food and pathological weight gain. A more likely factor promoting excessive eating is food reward and its representation in the central nervous system (CNS). The anorectic hormones leptin and insulin reduce food reward and inhibit related CNS reward pathways. Conversely, the orexigenic gastrointestinal hormone ghrelin activates both homeostatic and reward-related neurocircuits. The current studies were conducted to identify in rats the effects of intracerebroventricular ghrelin infusions on two distinct aspects of food reward: hedonic valuation (i.e., “liking”) and the motivation to self-administer (i.e., “wanting”) food. To assess hedonic valuation of liquid food, lick motor patterns were recorded using lickometry. Although ghrelin administration increased energy intake, it did not alter the avidity of licking (initial lick rates or lick-cluster size). Several positive-control conditions ruled out lick-rate ceiling effects. Similarly, when the liquid diet was hedonically devalued with quinine supplementation, ghrelin failed to reverse the quinine-associated reduction of energy intake and avidity of licking. The effects of ghrelin on rats' motivation to eat were assessed using lever pressing to self-administer food in a progressive-ratio paradigm. Ghrelin markedly increased motivation to eat, to levels comparable to or greater than those seen following 24 h of food deprivation. Pretreatment with the dopamine D1 receptor antagonist SCH-23390 eliminated ghrelin-induced increases in lever pressing, without compromising generalized licking motor control, indicating a role for D1 signaling in ghrelin's motivational feeding effects. These results indicate that ghrelin increases the motivation to eat via D1 receptor-dependent mechanisms, without affecting perceived food palatability.
Keywords: reward, taste, dopamine
although major research efforts prompted by the global rise in obesity have led to the identification of humoral and neural mechanisms mediating short- and long-term energy homeostasis (10, 15, 51), it is now evident that hedonically driven nonhomeostatic feeding behaviors, exacerbated in environments with easily accessible high-calorie foods, contribute significantly to the escalating obesity pandemic (5, 43, 54, 61). Thus, it is critical to understand the motivational and sensory determinants of food intake that promote eating beyond the amount needed to fulfill nutritional requirements. Consumption of highly palatable foods is thought to be influenced by central nervous system (CNS) reward circuits that control both the hedonic valuation (i.e., “liking”) of food and the motivation (i.e., “wanting”) to eat (4, 43). In addition to this intrinsic circuitry, in which dopamine and opioids play important signaling roles (63), hormones reflecting short- and long-term caloric deficit or abundance interact with CNS reward circuitry to modulate its function (5, 43, 55). For example, receptors for leptin and insulin—metabolic hormones traditionally regarded primarily as regulators of homeostatic feeding—have been identified in key brain sites that mediate motivation, such as the ventral tegmental area (VTA) and lateral hypothalamus (28, 29, 45). Furthermore, we have demonstrated that CNS administration of leptin or insulin decreases performance in behavioral tasks that assess different aspects of reward, such as food-conditioned place preference for a high-fat treat (26), opioid-stimulated sucrose intake (30), and sucrose self-administration in fed rats (27).
The orexigenic gastrointestinal peptide ghrelin also appears to influence feeding behavior related to both energy homeostasis and reward, and it is a candidate contributor to reward-related overconsumption. Elucidation of the behavioral mechanisms that mediate the acute orexigenic effects of ghrelin is currently incomplete, in part, because there are numerous areas in the brain and periphery that express ghrelin receptors and/or are neurally activated following ghrelin administration. Within the CNS, ghrelin-responsive areas overlap with circuits involved in both energy homeostasis and food reward (44, 78). We and our colleagues have shown that ghrelin powerfully stimulates food intake when microinjected into brain areas involved in reward-related feeding (such as the VTA or nucleus accumbens) (53), with comparable efficacy as when it is delivered to areas primarily controlling homeostatic feeding (such as the hypothalamus or hindbrain) (25, 77).
In the present study, we hypothesized that an orexigenic, 3rd intracerebroventricular dose of ghrelin would increase reward-related feeding behaviors in non-food-deprived rats. Two aspects of ghrelin-induced food reward—hedonic valuation (i.e., palatability) and the motivation to eat (i.e., willingness to work for food)—were examined in separate sets of studies. In the first, we used lickometers to measure the avidity of ingestive motor patterns, as quantified by the initial licking rate and average size of licking bouts. On the basis of previously observed correlations of these lick parameters with positive orosensory stimulation by a range of foods (16, 34, 36, 60), we reasoned that their magnitude should increase if ghrelin administration increased rodents' hedonic valuation of a test food (17, 35, 37, 64). In the second set of studies, the effect of ghrelin on rats' motivation to eat was assessed by the number of lever presses animals were willing to execute to obtain food in a progressive-ratios, sucrose self-administration protocol. In this paradigm, rats self-administer small quantities of food by lever pressing, and a steadily increasing number of lever presses is required to continue to obtain food. The maximal number of lever presses a rat makes to acquire food reflects its motivation to eat (27). We focused on the acute behavioral effects of ghrelin because although the peptide can increase body weight when injected chronically (71, 77), the orexigenic effects of single central ghrelin injections similar to those used herein are primarily observed within the first 1–2 h (44).
The principal neurotransmitters that mediate food reward are opioids, which are primarily involved in hedonic valuation, and dopamine, which is implicated in both hedonic and motivational aspects of food ingestion (4, 43, 46, 62, 63). It seems plausible that these signals might be involved in ghrelin-induced feeding behaviors. However, central opioid blockade does not influence the orexigenic effects of ghrelin injected into the VTA (53). This observation suggests that VTA-ghrelin-induced feeding may not involve an enhanced perception of food palatability (31). Zigman and colleagues have demonstrated effects of ghrelin on food reward tasks in the mouse, using a high-fat diet reward (56), although palatability of the food reward per se was not evaluated. To this date, the dopamine dependence of ghrelin's orexigenic effects has not been explored systematically. Exposure to highly palatable foods triggers dopamine release in the mesolimbic reward system, including the nucleus accumbens (36, 48, 62). In these brain areas, dopamine acts primarily on dopamine receptors D1 and D2, which are present in several nodes of the reward network. Importantly, anatomical and functional data show that circulating endocrine factors, such as leptin, insulin, and ghrelin, can modulate mesolimbic function and aspects of food reward (2, 43). Therefore, we also examined the impact of dopaminergic blockade on food intake and the motivation to obtain food following central ghrelin administration.
MATERIALS AND METHODS
Experimental Animals and Cannula Placement
Male Sprague-Dawley rats (350–450 g; Harlan, San Diego, CA) were subjects in all experiments. They were housed individually, had ad libitum excess to regular Purina rat chow, and were exposed to a 12:12-h light-dark cycle, with lights on at 0600. All animal procedures followed National Institutes of Health guidelines for animal care and were approved by the Animal Care and Use Subcommittee of the Research and Development Committee at the VA Puget Sound Health Care System. Chronic indwelling cannulas were surgically implanted into the 3rd cerebral ventricle, as previously described (27). Generally, intracerebroventricular administration of appetite-regulating hormones reliably alters activity in CNS areas involved in homeostatic and reward feeding, consistent with their anorexigenic or orexigenic actions (27, 77). This route of administration was, therefore, adopted to test the rewarding effects of ghrelin. Cannula placement was functionally verified by monitoring the drinking response to an intracerebroventricular injection of ANG II (10 ng/μl, 2 μl per injection). Water intake of ≥5 ml within 30 min after injection was considered indicative of a functional cannula. Following surgery, experimental procedures were conducted after animals had returned to their preoperative body weight. Each cannulated rat participated in all conditions. Successful infusion was verified by examining displacement of an air bubble that was purposely positioned between the infusate and propelling fluid (saline) during loading. Unsuccessful infusions were reattempted at later sessions.
Effects of Ghrelin Administration on the Hedonic Valuation of Liquid Food
Lickometry apparatus and measured lick parameters.
Eight-channel lickometers (Dilog, Tallahassee, FL) were used to record the number and timing of individual licks with millisecond resolution. [For technical details, see Ref. (32)]. While ingesting a meal, rats lick in bouts, during which lick rates average 6–8 licks/s. These lick bouts (clusters) are separated by pauses (interlick intervals) larger than 500 ms and smaller than the threshold period set to define meal termination (commonly 5 or 10 min). Physiological state, as well as food-related and other factors influence lick rates in rodents, changing cluster size (i.e., the number of licks per cluster), and/or the duration of breaks between clusters (17, 64).
In the current study, durably higher lick rates were interpreted to indicate heightened avidity of ingestion associated with increased hedonic valuation of the ingested food (17, 37). In other words, lick rates reflected the animals' perception of food palatability (i.e., their “liking” of it). Four lick parameters served as output data. First, total number of licks reflected the amount ingested. Secondly, initial lick rate was calculated as the number of licks/min during the first 2 min after meal initiation. Shifts in this parameter occur largely before nutrients are absorbed from the gastrointestinal tract; they are controlled by sensory feedback and hedonic valuation (17, 35). Correlations of initial lick rate have been found with sweetness and the attractiveness of food (7, 18, 19). Thus, the initial lick rate was used in the present study as an index of rats' hedonic valuation of food, and hence, its perceived palatability. The third parameter, average lick-cluster size, was calculated as the average size of lick trains (in number of licks) with interlick intervals of <500 ms, measured during a 30-min food presentation, i.e., the period in which central ghrelin administration maximally stimulates solid food intake (44). Cluster size is a measure of the persistence of licking and, like initial lick rate, is considered an index of the ability of orosensory properties of food to stimulate or maintain intake (37, 66). Cluster size increases with concentration of substances that are readily ingested by rats, such as glucose, Polycose solutions, and Intralipid (17, 37). These associations may occur independently of metabolic effects of foods, as demonstrated by positive correlations between cluster size and both the concentration of the noncaloric sweetener saccharin and the ingested volume during sham feeding of a liquid diet in rats with open gastric fistulas (17). Finally, where relevant, the number of lick clusters in the 30-min period was evaluated as another index of the orexigenic action of infusions.
Experimental Protocol
Verification of the orexigenic effect of ghrelin on solid food intake.
Ten rats were implanted with 3rd ventricular cannulas. Two weeks after surgery, when animals had regained their preoperative body weight, the effects of two doses of ghrelin on solid chow intake were determined. During a 4-day habituation period, animals were deprived of food each day for 1 h during the mid-light period, then removed from their home cage, handled, administered 2 μl of artificial cerebrospinal fluid (ACSF) intracerebroventricularly, and returned to their home cages, where food was again provided. In the week thereafter, this food deprivation/injection protocol was repeated during three sessions, each spaced 2 days apart. Artificial cerebrospinal fluid or ghrelin (0.1 or 1.0 nM) was administered in random order during these separate sessions. Thirty-minute chow intake was recorded using electronic scales with 0.1-g resolution to test the acute orexigenic effects of the ghrelin injections. This procedure was included to verify the orexigenic effects of previously aliquoted ghrelin in our current cohort of rats. We and others have observed acute, distinct, and replicable orexigenic effects of ghrelin administration in studies using chow diet (44, 74, 77).
Habituation to experimental procedure and liquid food.
For 1 wk, rats were habituated to the daily experimental procedure. Animals were removed from their home cages at 1100 (mid-light period) and placed individually in shoebox cages fitted with lickometers. Food and water were withheld for 50 min, after which rats were handled, received a bolus intracerebroventricular injection of 2 μl ACSF, then returned to their experimental cages. Ten minutes later, they received 30-min ad libitum access to freshly prepared liquid food, with a caloric density of 1.1 kcal/g, and with an energy composition by kcal of 20% fats (Intralipid; Baxter Healthcare), 60% carbohydrates (maltodextrin and sucrose), and 20% protein (casein hydrolysate; Baxter Healthcare). We decided to use a mixed-macronutrient test food because ghrelin's orexigenic effects could be readily demonstrated with mixed-macronutrient foods such as chow, but not with simple sucrose solutions (6). Lick patterns were monitored using lickometers, and 30-min liquid food intake was measured using electronic scales with 0.1-g resolution. Habituation training established a robust ingestive pattern in all rats, characterized by a prompt meal lasting 5–15 min, followed by relaxation and sleep, i.e., the expected postprandial satiety sequence (3).
Rats' avid intake of hydrated foods has been previously observed (59), and it was also evident in our subjects (15–30 ml of liquid food within 30 min). To ensure that high baseline liquid intakes during test sessions would not obscure ghrelin's orexigenic potency due to a ceiling effect, rats were made accustomed to the liquid diet by being given daily access to a larger quantity of it outside of the experimental sessions (60 ml liquid food per day, provided during the late light phase). For all rats, this daily exposure to the liquid food decreased 30-min liquid intakes during the experimental sessions.
Test sessions.
The effect of exogenous ghrelin on lick microstructure was tested using the experimental procedure described above (main conditions). Using a within-subjects design, we also tested rats' responses in positive and negative control conditions, in which the palatability and/or intake of the liquid foods were raised or lowered by means other than ghrelin administration.
Main conditions.
We compared the effects on lick patterns and calorie intake of two orexigenic doses of ghrelin (0.1 and 1.0 nM) or equivolemic vehicle (ACSF), all administered as 2-μl bolus intracerebroventricular injections. Animals received injections 10 min before liquid food presentation, during separate sessions run in random order. Procedures were performed daily, and experimental sessions were alternated with sessions in which animals were handled and received liquid food but no actual injections.
Positive control conditions.
The hedonic value and/or intake of food were increased by means other than ghrelin administration, using the following interventions: 1) Intracerebroventricular injection of 5 μg of the orexigenic neuropeptide Y (NPY) was given 10 min before liquid presentation. 2) The rats were treated with 24-h food deprivation combined with sweetening of the liquid food using saccharin and vanilla flavor, to enhance palatability, as well as the motivation to eat (52). Animals were handled but received no injections. 3) Elevation of 30-min intake of liquid food during test sessions by withholding of the usual 60 ml/day liquid food supplement during the previous day. Before food presentation, animals received an intracerebroventricular injection of 2 μl ACSF.
Negative control condition.
Palatability of the liquid food was decreased by adding a small dose (0.03% wt/vol) of the bitter compound quinine (38), and intake of this bitter test liquid was assessed following intracerebroventricular injection of 2 μl ACSF. Intake was also assessed following intracerebroventricular administration of 1 nM ghrelin, to test whether hedonic devaluation of the bitter food could be reversed by ghrelin. Before experimental measurements, rats had been presented with quinine-supplemented liquid food for two consecutive days.
Data analyses.
Lick data were converted into structured lick parameters (total number of licks, initial 2-min lick rate, and average cluster size, and number of clusters) using software designed specifically for this purpose (written in Visual Basic, Microsoft, Redmond, WA). Statistical analyses were conducted using Microsoft Excel 2003 (Microsoft) and SYSTAT 11.0 statistical software (Systat, Richmond, CA). Repeated-measures ANOVAs were conducted to test for differences across conditions, followed, where appropriate, by pairwise, between-condition comparisons.
Effects of Ghrelin Administration on Progressive-Ratio Responding for Food
Test food and bar-pressing apparatus.
A 5% (wt/vol) sucrose solution was used as the test food. In prior studies with rats, we found that this food elicits moderately intense bar-pressing rates, which were decreased after intracerebroventricular administration of the anorexigenic agents leptin and insulin (27). We did not select a liquid mixed-macronutrient food (e.g., Ensure) because such foods may evoke high rates of lever pressing in control conditions (unpublished observations), thereby potentially obscuring detection of the hypothesized ghrelin-induced increases in motivation due to ceiling effects.
Operant boxes (Med Associates, Georgia, VT), in which animals were placed individually for the duration of experimental sessions, contained two levers hanging on opposite sides of the box. Pressing of the active, retractable lever delivered a small amount (0.4 ml) of the sucrose solution into a receptacle accessible to the rat. Presses on the inactive, stationary lever did not lead to food presentation but were recorded by a separate counter as an index of nonspecific motor activity. The number of presses on the inactive lever was low in all animals under all conditions (less than 10 lever presses/session). The timing and number of presses, as well as food delivery, were recorded and transmitted to a computer connected to the operant box for off-line analysis.
Experimental Protocol
There were four phases in this study, as previously described (27): fixed-ratio (FR) training, surgery and recovery, progressive ratio (PR) training, and experimental sessions. During the FR training phase, rats learned to self-administer very small doses of sucrose (0.4 ml, 5% solution). Individual FR training sessions lasted 1 h, and daily sessions were conducted for 10 consecutive days, according to a FR-1 reinforcement schedule, in which every lever press produces a sucrose treat. Each reward delivery was accompanied by a discrete, compound (i.e., multicomponent) cue, consisting of a 5-s tone (2900 Hz, 20 dB above background) + light (7.5 W white light above the active lever), which continued for 20 s. On day 11, animals were surgically implanted with 3rd-ventricular cannulas, followed by a 10-day recovery period, after which body weights and food intake had returned to preoperative levels. The procedure subsequently continued with 2 more days of FR recovery training, after which the PR training phase commenced. During PR training sessions, to obtain successive samples of sucrose solution, animals were required to press the active lever a progressively increasing number of times, as prescribed by Richardson and Roberts' algorithm, i.e., 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, 63, 83, 110, 145, 191, 251, 331, 437, 575, 759, and 999 times in sequence (57). PR training continued during daily 3-h sessions over a period of 14 days. These sessions started with the emergence of an active lever from the cage wall and illumination with a white house light that remained on for the entire session. Each reward delivery was accompanied by the same discrete, compound cue as that used during the FR phase, but with a 5-s timeout. The active lever was retracted during the timeouts in the PR sessions. PR sessions ended after either 3 total hours or after 30 min of no active lever-press responding, whichever came first. At termination, the house light was turned off and the active lever retracted.
During the experimental phase that followed completion of PR training, we assessed the effect on sucrose self-administration of 3rd intracerebroventricular injections of ghrelin (0.1 and 1.0 nM) or ACSF, all delivered in 2-μl bolus injections. The two ghrelin doses were selected for their acute and graded orexigenic effects on chow intake, as observed previously (71, 77). On experimental days, rats received these 3rd intracerebroventricular injections 10 min before being placed in the self-administration chamber. Each rat participated in all conditions. Successful infusion was verified by examining displacement of an air bubble that was purposely positioned between the infusate and propelling fluid (saline) during loading. Unsuccessful infusions were reattempted at later sessions. Rats were returned to 2 days of PR training between experimental sessions.
Effects of Dopamine Receptor Blockade on Ghrelin's Orexigenic and Motivational Effects
To assess dopamine-receptor involvement in ghrelin's behavioral effects, selective antagonists to either the D1 subtype (SCH-23390; 50 μg/kg; Sigma-Aldrich, St. Louis, MO) or D2 subtype (raclopride, 100 μg/kg; Sigma-Aldrich) or vehicle were injected intraperitoneally 10 min before intracerebroventricular ghrelin administration. The doses of SCH-23390 and raclopride were chosen to be moderately above the threshold levels to inhibit food intake in different rat feeding paradigms (60, 73). Both antagonists were administered systemically (intraperitoneally) rather than locally (intracerebroventricularly) to attain uniform blockade of dopaminergic sites throughout the CNS, i.e., at sites affected both directly and indirectly by intracerebroventricular ghrelin.
Data Analysis
Active lever responses and sucrose rewards were analyzed as within-subjects' repeated-measures ANOVAs and paired Student's t-tests. Group data are presented as means ± SE in the text and figures. Significance was defined as P ≤ 0.05.
RESULTS
Effects of Ghrelin Administration on Solid Food Intake and the Hedonic Valuation of Liquid Food, as Measured by Lickometer
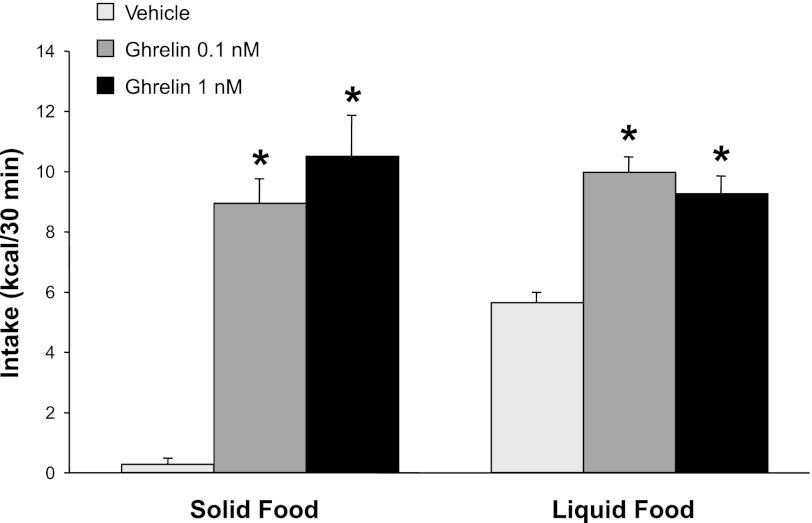
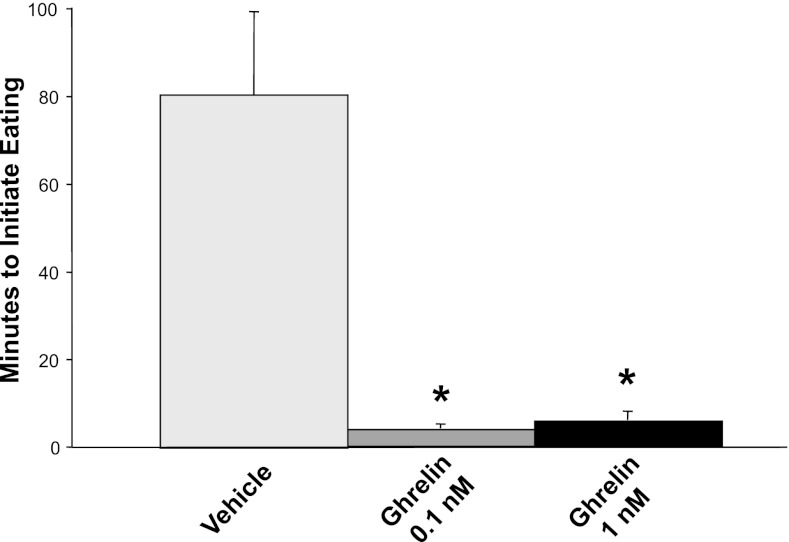
To confirm that the batch and doses of ghrelin peptide that were used in our studies of reward-related feeding behaviors were adequate to stimulate overall food intake as expected, we first examined their effects on intake of solid rat chow. Both of our doses of 3rd intracerebroventricularly injected ghrelin markedly stimulated 30-min chow intake, which increased from near 0 kcal following ASCF injection to 8.9 and 11.0 kcal following 0.1 and 1.0 nM of ghrelin, respectively [P < 0.001 for both ghrelin doses, Fig. 1]. Statistically, a main effect of ghrelin dose was found, reflecting the orexigenic potency of ghrelin relative to ACSF injections [F(2,19) = 66.3, P < 0.001]. The effects on energy intake were similar for the two ghrelin doses administered [F(1,9) = 3.96, not significant (NS)]. In addition to stimulating the overall amount of solid food consumed, ghrelin administration also greatly increased the speed with which rats initiated feeding. Rats initiated chow intake between 3 and 8 min after receiving ghrelin injections, whereas the latency to feed was 80 min after intracerebroventricular vehicle injections (ghrelin vs. vehicle effect P < 0.001 for both ghrelin doses, Fig. 2). Together, these results confirmed the acute orexigenic effects of our batch and doses of ghrelin on the intake of standard rat chow, in line with previous reports (44, 77).
Fig. 1.
Thirty-minute calorie intake of either solid food (standard rat chow) or liquid food after 3rd intracerebroventricular, 2-μl bolus injections of ghrelin or ACSF vehicle in rats (n = 10). Injections were scheduled during the mid-light cycle. Relative to vehicle, ghrelin administration at both doses increased intake of both solid and liquid food. Once animals were habituated to the injection procedure, liquid food presentation triggered acute meal intake in all conditions; with solid food, acute meals occurred only after ghrelin injection. *Compared with comparable vehicle injections, P < 0.001 for solid food at both ghrelin doses, P = 0.01 for liquid food at both ghrelin doses.
Fig. 2.
Latency to eat solid rat chow after third intracerebroventricular, 2-μl bolus injections of ghrelin or ACSF vehicle in rats (n = 10). The large latency to eat after vehicle injection reflects the low meal frequency at the time of injection (mid-light cycle) in these nocturnal rats. Ghrelin at both doses significantly decreased the latency to eat. *P < 0.001 for each dose of ghrelin compared with vehicle.
Having verified ghrelin-induced stimulation of chow intake, we next tested the effects of ghrelin administration on intake of the liquid food that was designed for this study of lick microstructure. As seen in Fig. 1, relative to ACSF injections, 3rd intracerebroventricular injections of ghrelin approximately doubled 30-min intake of liquid food [F(2,18) = 5.5; P = 0.01]; no differences were found between the two ghrelin doses [F(1,9) = 0.73, NS]. This pattern was reflected in the total number of licks [F(2,16) = 3.6; P = 0.05 and F(1,8) = 0; NS, respectively]. In contrast to our results with solid food, significant intake of liquid food occurred in the ACSF injection control condition, presumably reflecting the known preference among rats for liquid over solid food (59). Following ghrelin injections, 30-min energy intakes were comparable for solid and liquid foods. These results confirmed the stimulatory effect of ghrelin on liquid food intake and provided a starting point for measuring ghrelin's effects on hedonic valuation via lick microstructure.
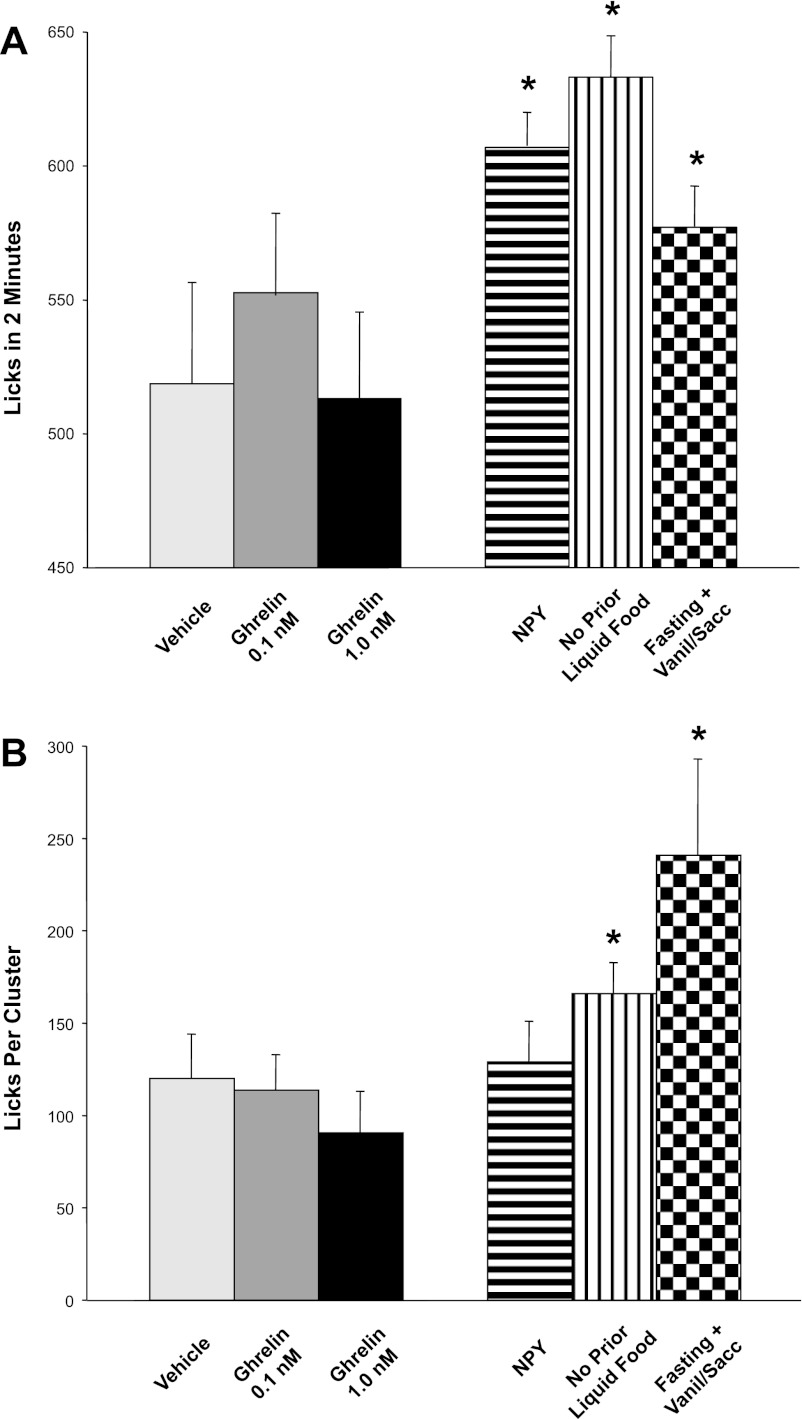
We first tested the effects of CNS ghrelin injections on initial lick rates of liquid food, as an index of hedonic valuation (i.e., perceived palatability) of this food. Relative to vehicle administration, 3rd-intracerebroventricular ghrelin injections, at both doses tested above, exerted absolutely no effect on either 2-min initial lick rates [F(2,16) = 0.90, NS; Fig. 3A] or average lick cluster sizes [F(2,14) = 1.9, NS; Fig. 3B]. Accordingly, the total number of lick clusters in the different conditions reflected the pattern of intake [F(2,14) = 9.8; P = 0.002]. Importantly, the considerable initial lick rates (Fig. 3A) and lick clusters size (Fig. 3B) in the control (ACSF injection) condition could theoretically have constituted a “ceiling effect” underlying the observed absence of increases in these parameters after ghrelin administration.
Fig. 3.
Lack of effect of 3rd intracerebroventricular injections of ghrelin on two lick parameters: initial 2-min lick rate and lick cluster size. Lick parameters were used as indicators of the avidity with which rats (n = 10) consumed liquid test foods. A: initial lick rate of liquid food during the first 2 min of consumption. Intracerebroventricular ghrelin did not affect lick rate relative to vehicle injection (solid bars). In contrast, positive control procedures (5 μg icv NPY, limited prior daily access to liquid food, and 24-h prior fasting plus attractively flavored liquid food; patterned bars) significantly increased lick rate, ruling out a ceiling effect on lick rates to explain ghrelin's lack of effect on this parameter. *P ≤ 0.05 compared with vehicle control condition. B: lick cluster size. Ghrelin did not increase lick cluster size compared with vehicle (solid bars). In contrast, two out of three positive orexigenic control procedures (limited daily access to liquid food and 24-h fasting plus attractively flavored liquid food; patterned bars) significantly increased cluster size, as did the three positive-control conditions considered together, again ruling out a ceiling effect to explain ghrelin's lack of impact on this lick parameter.
To examine this possibility, we ran three positive-control conditions, all of which could be expected to increase the avidity of licking: intracerebroventricular injection of the orexigenic neuropeptide NPY; 24-h food deprivation combined with flavor enhancement of the liquid food (with the addition of vanilla plus saccharine); and prior removal of the daily 60-ml liquid food aliquot that was provided to the animals to acclimatize them to this treat. As intended, these conditions increased 30-min liquid food intake relative to control conditions [F(3,21) = 24.9, P < 0.001 for intake by weight and F(3,21) = 29.1; P < 0.001 for total number of licks], with highly significant increases noted for each of them individually [F(1,8) = 169 for NPY; F(1,7) = 31.2 for prior withholding of liquid food from daily diet; F(1,7) = 54.5 for food deprivation plus vanilla flavoring; P < 0.001 for each condition]. In these three orexigenic conditions, the avidity of licking, as reflected in initial lick rate, was also increased relative to baseline [F(3,21) = 3.49, P = 0.034] (Fig. 3A). Considered in isolation, each of the three positive-control interventions significantly increased initial lick rate [F(1,7) = 71.7, P = 0.014 for NPY; F(1,7) = 5.5, P = 0.05 for prior withholding of liquid food from daily diet; F(1,7)=7.8, P = 0.03 for food deprivation plus vanilla; Fig. 3A]. Similar to the effects on initial lick rates, lick cluster size was also increased in vehicle-injected animals by these orexigenic positive-control interventions [for all conditions, F(3,21) = 5.16, P < 0.01; food deprivation plus vanilla F(1,7) = 6.05, P = 0.04; removal of prior liquid food exposure F(1,7) = 4.7, P = 0.07 trend; NPY F(1,8) = 3.3 NS; Fig. 3B]. Together, the lick patterns in these positive-control conditions demonstrate that the inability of ghrelin to increase the avidity of licking was not due to ceiling levels of lick rate or cluster size with our liquid food. Thus, despite the relatively high basal rates of these parameters in vehicle-injected rats, several nonghrelinergic orexigenic stimuli revealed rats' physical ability to elevate both measures of licking speed even further. Ghrelin administration, however, failed to elicit these effects.
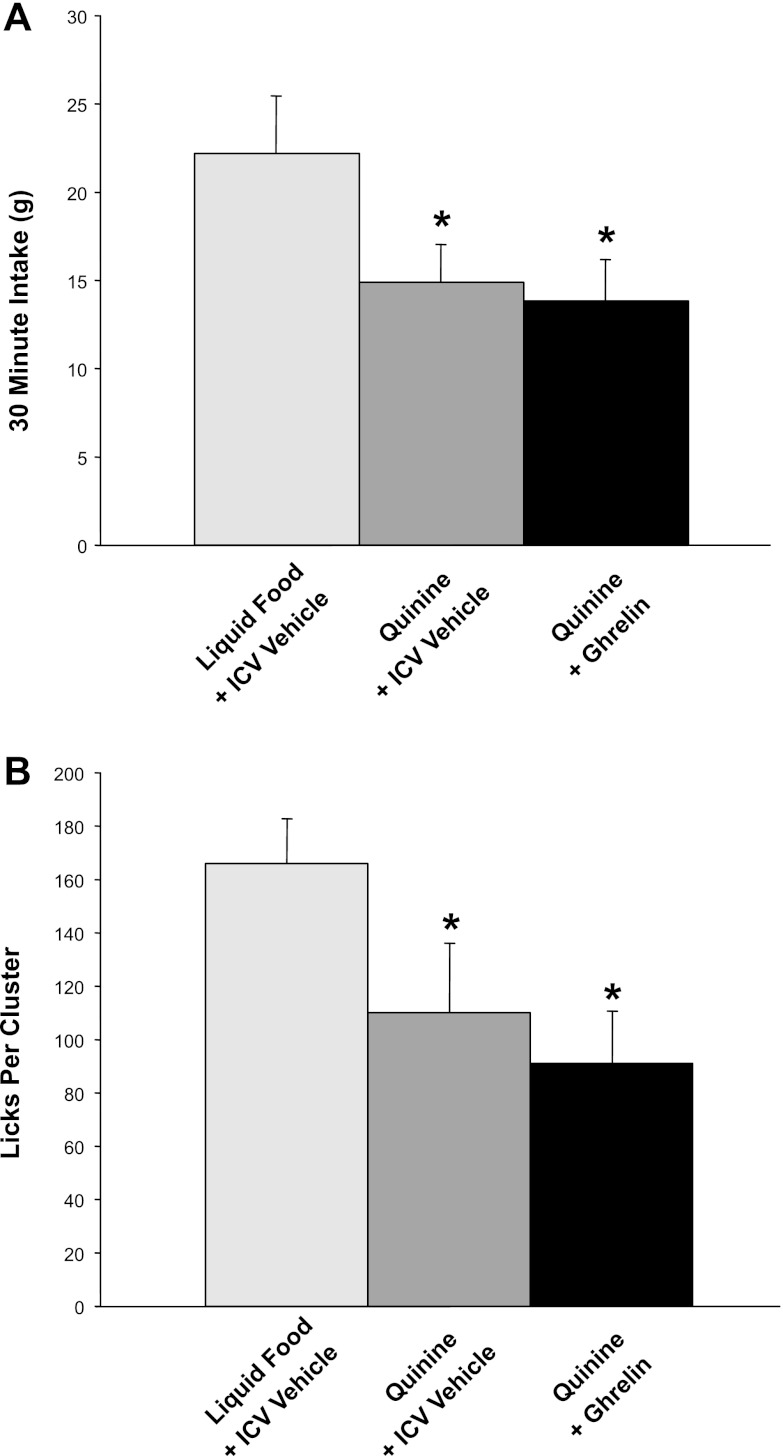
Another potential explanation for the inability of ghrelin to affect our measures of hedonic valuation might be that the hedonic value of our liquid food in the control condition (ACSF injection) was already high enough to make further, ghrelin-induced augmentation of palatability difficult to attain. To examine this issue, we repeated our basic experiment with a low-palatability version of the liquid food created by supplementing it with a small dose of quinine, a noncaloric, bitter-tasting compound. As intended, quinine supplementation reduced 30-min intake relative to baseline [F(1,8) = 8.7, P < 0.02, ACSF conditions, quinine vs. regular test diet; Fig. 4A]. Importantly, ghrelin did not restore 30-min intake of quinine-supplemented liquid food to baseline levels, suggesting that the hedonic devaluation by bitterness was not overcome [F(1,8) = 0.2, NS, quinine conditions, ghrelin vs. ACSF injection] (Fig. 4A). This pattern of results for food intake was confirmed by a similar lack of impact of ghrelin administration on lick-cluster size (Fig. 4B). Quinine supplementation of the test food decreased lick cluster size in ACSF-injected animals [F(1,8) = 17.7, P = 0.003; quinine-supplemented vs. regular test food], and intracerebroventricular ghrelin administration showed no hint of reversing this effect [F(1,8) = 1.5, NS]. In line with decreased total intake and reduced cluster size in the quinine conditions, the number of lick clusters did not differ across conditions [F(2,14) = 2.7; NS]. A quinine-associated decrease in average lick rate became apparent in the second minute and turned statistically significant at 5 min after meal onset [F(1,8) = 5.6, P < 0.05; ACSF conditions, quinine vs. regular test diet]. Consistent with the data for energy intake and lick cluster size, intracerebroventricular ghrelin administration had no effect on this decrease [F(1,8) = 0.4, NS, quinine conditions, ghrelin vs. ACSF-injected]. In summary, the addition of quinine to our liquid test food decreased energy intake, lick cluster size, and lick rate, indicating hedonic devaluation, which ghrelin administration failed to reverse, as reflected by its lack of impact on any of these three measurements.
Fig. 4.
Effects of intracerebroventricular ghrelin on intake and avidity of licking of liquid test foods, hedonically devalued by the bitter tastant quinine (0.03% wt/vol) in 10 rats. A: 30-min energy intake. Prior intracerebroventricular administration of ghrelin did not reverse the quinine-induced reduction of food intake. *P < 0.02 for quinine vs. regular test diet, equivalent with or without ghrelin injection. B: lick cluster size. Quinine reduced the lick cluster size, indicating decreased hedonic value. Analogous to our observations of energy intake, prior intracerebroventricular administration of ghrelin did not restore cluster size, indicating that ghrelin did not affect hedonic value. *P = 0.003 for quinine vs. regular test diet, equivalent with or without ghrelin injection. The low dose of quinine did not affect lick rate until after the initial 2 min (data not shown).
Effects of Ghrelin on the Motivation to Eat, as Measured by Lever Pressing in a Progressive-Ratio Task
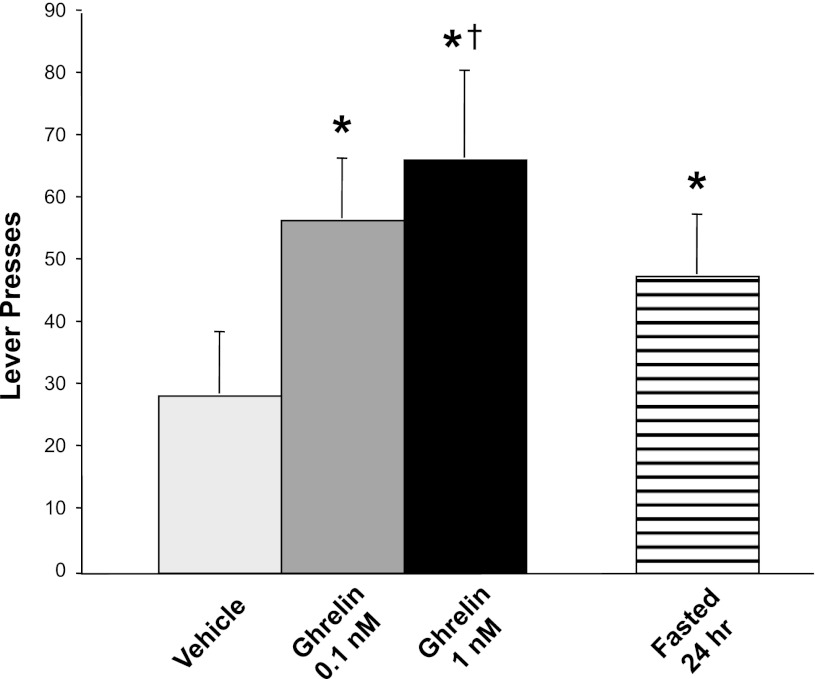
In contrast to our negative results regarding any effects of ghrelin on the hedonic valuation of food, as judged by lick microstructure, ghrelin administration markedly increased rats' motivation to obtain food, as judged by the number of lever presses they would execute to acquire it. Relative to ACSF, ghrelin administration markedly increased the number of lever presses in a PR reward schedule (Fig. 5). This was reflected in a main effect of condition [F(2,18) = 8.33, P = 0.003]. Each of the ghrelin doses, 0.1 and 1.0 nM, induced substantially more lever presses than those observed after ACSF administration [F(1,9) = 10.8, P = 0.009, and F(1,9) = 17.5, P = 0.002, respectively].
Fig. 5.
Number of lever bar presses during a progressive-ratios self-administration of a 5% sucrose solution. The results indicate that ghrelin administration enhanced rats' motivation to eat. Depending on the dose administered, the behavioral effect of ghrelin was equal to or greater than that of 24 h of food deprivation immediately preceding the test. *P < 0.01 relative to the vehicle control condition. †P < 0.01 relative to prior 24-h fasting.
To judge the relative magnitude of ghrelin's stimulatory effect on lever responding, we compared it to the effects of a known major stimulus of this response: a 24-h period of food deprivation imposed immediately before the lever bar-pressing test. After 24 h of fasting, rats pressed the lever to obtain food more than they did in the non-food-deprived condition [F(1,9) = 11.4, P = 0.008, with both groups receiving ACSF injections just before the test] (Fig. 5). In comparison, the number of lever presses after intracerebroventricular ghrelin administration in non-food-deprived rats was even higher than that in rats subjected to 24 h of food deprivation followed by intracerebroventricular ACSF injection (although differences between these conditions were only statistically significant for the higher ghrelin dose, P < 0.01, Fig. 5). Thus, the acute effects of ghrelin administration on animals' motivation to eat were at least as great, or greater, than those induced by 24 h of prior food deprivation.
Tests of Dopamine Receptor Involvement in the Effect of Ghrelin to Increase Feeding Motivation
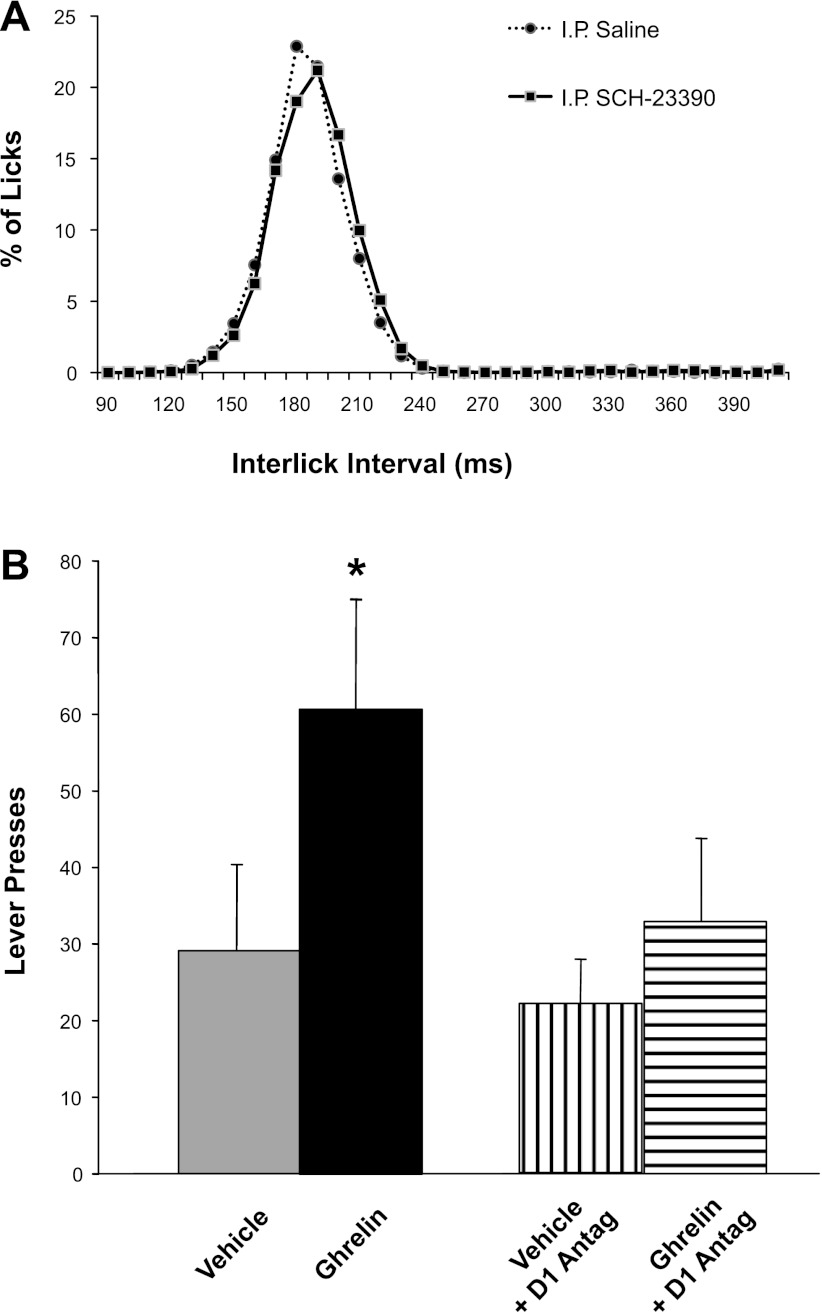
Our observation that ghrelin administration stimulated a behavioral measure of motivation (i.e., bar-pressing for food) predicts that central dopamine receptor antagonism should attenuate this effect, as CNS dopamine is a mediator of motivated behaviors (43, 63). To test this hypothesis, we assessed the effects of dopamine blockade on ghrelin-induced lever pressing for food. In a pilot study, we explored D1 and D2 involvement in the effect of ghrelin (1.0 nM icv) to stimulate chow feeding. We found in six rats that ghrelin-induced 30-min chow intake during the light phase (means ± SE; 2.6 ± 0.3 g) was significantly inhibited by prior intraperitoneal administration of 50 μg/kg of the D1 receptor antagonist SCH-23390 (intake 1.03 ± 0.7 g, P < 0.05). In contrast, D2 blockade by a 100 μg/kg intraperitoneal raclopride pretreatment did not affect ghrelin-induced chow intake (mean intake 2.5 ± 0.3 g, P = NS). Because dopamine signaling is involved in motor function, we sought to verify that the antiorexigenic effect of SCH-23390 in these rats was not based on drug-induced impairments of general ingestive motor behaviors. Toward that end, we examined the interlick-interval distribution during a liquid food intake test with Intralipid, and we found this to be unaltered by the drug (Fig. 6A).
Fig. 6.
Effects of intraperitoneal administration of the D1 dopamine receptor antagonist SCH-23390 (50 μg/kg ip) on fine motor behavior in the tongue (interlick interval distribution) and progressive-ratios self-administration of food. A: interlick interval distribution during consumption of a liquid meal (Intralipid, Baxter Healthcare) was comparable after administration of vehicle or SCH-23390. Thus, this dose of D1-antagonist did not cause any gross motor impairment in licking that could confound the assessment of the compound's impact on progressive ratios responding shown in B. B: effects of SCH-23390 on progressive-ratios responding. Prior administration of SCH-23390 eliminated ghrelin-induced increases in progressive-ratio bar-press responding. The same intervention had no effect on baseline bar pressing (i.e., there was no difference between vehicle injections with vs. without SCH-23390). These results indicate an involvement of dopamine D1 receptor signaling in the food-related motivational effects of ghrelin.
On the basis of these data, we examined the effects of D1 receptor antagonism on food self-administration in ghrelin-injected animals. As predicted, prior administration of SCH-23390 (50 μg/kg ip) attenuated the stimulatory impact of 3rd-intracerebroventricular injection of ghrelin (1.0 nM) on lever pressing for food, without exerting any effect on bar pressing in vehicle-injected controls (Fig. 6B). The number of lever presses following SCH-23390 plus ghrelin administration was significantly smaller than that following ghrelin alone [F(1,8) = 15.9, P = 0.004], whereas no differences were found between the respective baseline conditions of SCH-23390 plus ACSF vs. ACSF alone [F(1,8) = 0.24, NS]. Thus, although baseline lever pressing was not affected by this dose of SCH-23390, the drug did eliminate ghrelin-induced elevations in this measure of the motivation to obtain food.
DISCUSSION
Because excessive eating in modern societies is typically driven more by hedonic and behavioral factors than by homeostatic mechanisms, a full characterization of reward-related food intake is critical to understanding the determinants of the current obesity pandemic. Food reward and its representation in the CNS are thought to be tuned by extrinsic neural and hormonal signals that reflect nutritional state. For example, the anorexigenic hormones leptin and insulin, which circulate in proportion to body fat, have each been shown to attenuate food reward (28, 43). The current studies were performed to determine in vivo whether, in contrast, the orexigenic hormone ghrelin enhances the various discrete aspects of food reward. A stimulatory effect of ghrelin on food reward has previously been suggested by in vitro neuroanatomical findings and in vivo studies in mice (2, 21, 22, 39, 40, 53, 56). Because food reward is not a unitary concept (4, 43, 46), however, we sought to determine specifically whether ghrelin can influence the perceived palatability of food (i.e., liking), the motivation to obtain food (i.e., wanting), or both.
The impact of ghrelin on hedonic valuation (palatability) was evaluated by monitoring the avidity of ingestion of liquid test food via lickometry, while its effects on the motivation to acquire food were assessed via self-feeding tests that require rats to press a lever a successively increasing number of times to obtain small samples of food. The general outcome of these experiments was that ghrelin administration increased rats' motivation to work for food, without affecting their hedonic valuation of that food. In other words, ghrelin made rats more motivated to eat, but it did not affect their perception of food palatability. The primary neurochemical mediators of food motivation and palatability are thought to be dopamine and endogenous opioids, respectively (43, 63). Consistent with this model, our work also revealed a role of dopaminergic (D1-mediated) signaling in the motivational and feeding effects of ghrelin, whereas we and our colleagues have previously shown no role for opioid signaling in the orexigenic effects of ghrelin administered to nodes of the CNS food-reward circuitry (53).
The effect of ghrelin on hedonic valuation was assessed via lickometer measurements of rats consuming a liquid test diet. The microstructure of licking is primarily thought to reflect hedonic valuation, especially during preabsorptive phases of a meal when food-related orosensory stimuli dominate over gastrointestinal feedback (17, 64). Earlier studies have shown that palatable (i.e., sweet, fatty, energy-dense) foods evoke an avid ingestive pattern in rodents, characterized by high initial lick rates, few pauses, and long trains (clusters) of licks (17, 37, 64). In the present study, intracerebroventricular administration of ghrelin increased 30-min food intake relative to vehicle injection. However, neither lick rate nor lick-cluster size was elevated during ingestion, indicating that ghrelin did not enhance rats' hedonic valuation of the food.
Several tests were conducted to validate this conclusion. To rule out a ceiling effect, i.e., the occurrence of maximal lick rates in vehicle-injected controls, three positive control conditions were added to heighten lick rates by alternative interventions: 1) intracerebroventricular NPY administration; 2) 24-h food deprivation plus test-food palatability enhancement with vanilla and saccharin; 3) restriction of daily access to the liquid test-diet treat (with permanent access to regular solid chow). These interventions increased rats' initial lick rates and lick cluster sizes, showing that our key observation that ghrelin failed to do so did not result from a ceiling effect on lick rates with our liquid test food. We further tested ghrelin's ability to increase hedonic valuation under negative control conditions. To lower palatability of the test food, we supplemented it with a small dose of the bitter tastant, quinine. The quinine reduced 30-min food intake, lick cluster size, and 5-min lick rate, as intended and in line with earlier reports (66, 68). Prior ghrelin administration at our maximum dose failed to reverse quinine's effects on intake and lick parameters, again confirming ghrelin's inability to heighten hedonic valuation of the test food (37). Our findings related to taste perception complement a recent publication pertaining to the sense of smell. In that study, although ghrelin administration lowered olfactory detection thresholds in rodents and humans, it did not affect the perceived pleasantness of odors (69).
In separate experiments, we used a food self-administration paradigm to test the ability of intracerebroventricular ghrelin to enhance the motivation to eat. Compared with vehicle administration, ghrelin doubled the number of lever presses, reflecting rats' increased motivation to eat. Ghrelin-induced lever pressing exceeded that induced by 24 h of prior food deprivation. Participation of dopaminergic signaling in the motivational actions of ghrelin was demonstrated by the effect of pretreatment with the D1-receptor antagonist SCH-23390, which abolished ghrelin-induced lever pressing and attenuated overall ghrelin-induced feeding.
Collectively, our current data and our previous work (53) support the view that ghrelin primarily exerts motivational and dopamine-related effects on feeding, rather than hedonic or opioid-related effects. These findings extend and complement prior investigations. For example, recent studies reported a ghrelin-induced increase in operant responding to high-fat food pellets (energy density 5.6 kcal/g) and peanut butter (energy density 5.9 kcal/g) in mice (23, 56). Apart from differences in test animal species and sites of ghrelin administration, a major difference between these studies and ours is that we demonstrated the motivational effects of ghrelin with a calorie-dilute food (5% sucrose solution; energy density 0.2 kcal/g, which is 15 times less energy-dense than our regular rat chow). In rats, intra-VTA ghrelin administration increased dopamine turnover in the nucleus accumbens (2), and in mice, ghrelin injections in the 3rd cerebral ventricle exerted comparable effects (39). These and other studies (1) show that ghrelin can increase mesolimbic dopamine release; our work implicates this action in the food-motivating effects of ghrelin, and it further reveals the D1 receptor as the relevant subtype mediating that action. We found that the D1-receptor antagonist SCH-23390 blocked ghrelin-induced stimulation of lever pressing for food and attenuated ghrelin-induced overall food intake, at a dose that affected neither basal food intake nor licking motor movements per se.
Dopamine D1 receptor mediation of ghrelin effects might provide an explanation for the earlier finding that short-term fasting (a condition that stimulates ghrelin secretion) increases D1 agonist-induced c-fos expression, motor activity, and operant behavior (9). It is possible that one neural substrate in this case might be the hybridization of ghrelin receptors and D1 receptors, which amplifies D1 signaling (40). We observed no impact of the D2-receptor antagonist raclopride on ghrelin-induced food intake. For raclopride (as well as SCH-23390), we selected a dose that by itself was modestly above the threshold level for food-intake suppression in several studies, but our experiments did not involve a full dose-response range of dopaminergic antagonists. Therefore, we cannot entirely disregard the possibility that higher doses of raclopride might have attenuated ghrelin-induced increases in lever pressing. It does seem evident, however, that the origin of the observed dopaminergic involvement in ghrelin-induced motivation to feed did not lie in an inadvertent, experimentally induced flavor-nutrient conditioning (49). For this, the glycemic and caloric stimuli that we delivered during bar-pressing sessions (i.e., a small number of droplets containing 0.02 g of sucrose and 0.08 kcal each) were too insignificant, both calorically and glycemically.
Some caveats are warranted regarding the strictly dichotomous distinction of reward-relating feeding into behaviors based on liking (mediated by endogenous opioids) vs. wanting (mediated by dopamine), as well as the assertion that lickometry patterns solely reflect the former. Although these distinctions and mediators are supported by large bodies of data, overlap elements likely exist. For example, while it is generally agreed that lickometer patterns reflect hedonic liking, those patterns may also involve an incentive motivation component to ingestion. For example, classic studies of G. P. Smith (62) and J. D. Davis and colleagues (7, 17, 70) concluded that dopamine mediated a component of hedonic palatability, because dopamine antagonists suppressed terminal licks in clusters in a manner similar to sucrose dilution. Because subsequent studies largely implicated dopamine in motivational aspects of eating, rather than palatability, the implication is that lickometry can sometimes give false-positive results regarding palatability changes. Fortunately, false positives are not a concern for the current work because all of our lickometry data pertaining to ghrelin were negative. An alternate explanation is that although lickometry patterns primarily track hedonic palatability properties of food, there may also be some motivational aspects reflected in lick patterns. Conversely, although lever bar pressing in the PR paradigm that we used generally reflects the motivation to eat, hedonic palatability might play a role in such self-administration of food. Acknowledging these complexities, however, our data as a whole support the conclusion that ghrelin primarily affects the motivation to eat, more than food palatability.
Ghrelin has traditionally been viewed as a meal-initiation signal (16), as reaffirmed in the current study by the very rapid induction of a chow meal after ghrelin injection. However, we found that ghrelin also increased meal duration and size in the context of regularly scheduled meals, as has previously been shown in both free-feeding rats (34) and humans provided with a buffet test meal (76). The increased number of lick clusters following ghrelin administration reflects a heightened chance that during meals, pauses in ingestion are followed by reinitiation of licking, and this could be interpreted as a nonhedonic appetitive response (70). Thus, our studies suggest a role for ghrelin in both key determinants of meals—initiation and continuance—although the former role is probably dominant (see Perspectives and Significance). Given these findings, it is noteworthy that dopaminergic signaling, which is stimulated by ghrelin, has been shown to have an analogous dual role in both meal initiation and the maintenance of operant, goal-directed behavior (8, 58) and that during food intake, inhibitory effects of dopamine-receptor blockade become evident only after completion of the initial eating bouts (65).
Perspectives and Significance
Together with prior work, our observation that ghrelin increases the amount of work that animals will perform to obtain food supports the notion that a prominent role for ghrelin is to enhance appetitive aspects of ingestive behavior, i.e., the drive to seek out and locate food, then initiate eating. Ghrelin has previously been shown to stimulate perceived hunger (76), foraging for, and hoarding food (41, 42), exploratory sniffing, and olfactory sensitivity (69), meal initiation (24), and overall food intake (71). Consistent with a role in the anticipation of eating, circulating ghrelin levels surge prior to habitual meal times (13, 16), and the magnitude of these surges correlates inversely with the number of regularly scheduled daily meals (12, 67).
The observed impact of ghrelin on motivational rather than hedonic determinants of eating has significance beyond a strictly academic context. Instinctively, one might question whether our finding that ghrelin does not affect food palatability would lessen the likelihood that ghrelin antagonism could reduce food intake to promote weight loss. However, nonhedonic stimulation of energy consumption probably plays an extensive role in common eating. One example is so-called “mindless eating,” an umbrella term describing various behaviors related to energy overconsumption, including urges to nibble snack foods, as well as excessive energy intake facilitated by subtle environmental cues (72). Furthermore, metabolic properties of food can induce food preferences even in the absence of taste perception (20). Similarly, some pathological states involve a pervasive motivation to seek and consume foods regardless of taste, such as in Prader-Willi syndrome and uncontrolled diabetes, both of which are characterized by extremely high ghrelin levels (11, 33). Finally, as the food industry has increasingly noted, initial hedonic ratings of novel foods correlate notoriously poorly with customers' long-term appreciation and repeat purchase of those items (47, 50).
Probably, the type of ghrelin manipulation most likely to prove clinically useful with regard to food intake will be stimulation of ghrelin signaling in cases in which increased eating would be beneficial, such as poor nutritional status in the elderly or pathological disease-related anorexia (14, 75). Our data suggest that ghrelin agonists might increase the motivation to eat, which could be helpful in cases where this drive is insufficient. The conclusion that ghrelin signaling can bypass mechanisms underlying energy homeostasis via increased motivation to eat offers guidance for interventions in cases that may benefit from alterations in energy balance.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants RO1 DK61516 to David Cummings and DK40963 to Dianne Figlewicz Lattemann. Dr. Cummings is also supported by NIH grants RO1 DK517498, RO1 DK089528, UO1 DK66568, and P30 DK17047. Dr. Figlewicz Lattemann is a Senior Research Career Scientist, Biomedical Laboratory Research Program, Department of Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.O., D.P.F., and D.E.C. conception and design of research; J.O., J.L.B.-J., S.K., and D.E.C. performed experiments; J.O., D.P.F., and D.E.C. analyzed data; J.O., D.P.F., and D.E.C. interpreted results of experiments; J.O. and D.E.C. prepared figures; J.O. and D.E.C. drafted manuscript; J.O., D.P.F., and D.E.C. edited and revised manuscript; J.O., D.P.F., J.L.B.-J., S.K., and D.E.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We are very grateful to Kim Griffin for her valuable technical assistance.
REFERENCES
- 1. Abizaid A. Ghrelin and dopamine: new insights on the peripheral regulation of appetite. J Neuroendocrinol 21: 787–793, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116: 3229–3239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol 89: 784–790, 1975 [DOI] [PubMed] [Google Scholar]
- 4. Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav 81: 179–209, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Berthoud H, Morrison C. The brain appetite, obesity. Annu Rev Psychol 59: 55–92, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bomberg EM, Grace MK, Wirth MM, Levine AS, Olszewski PK. Central ghrelin induces feeding driven by energy needs not by reward. Neuroreport 18: 591–595, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Breslin PA, Davis JD, Rosenak R. Saccharin increases the effectiveness of glucose in stimulating ingestion in rats but has little effect on negative feedback. Physiol Behav 60: 411–416, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci 23: 10827–10831, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76: 353–364, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89: 71–84, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 8: 643–644, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: focus on current controversies. Curr Drug Targets 6: 153–169, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab 287: E297–E304, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Cummings DE, Overduin J. Circulating ghrelin levels in pathophysiological conditions. In: Ghrelin, edited by Ghigo E. Boston, MA: Kluwer Academic, 2004, p. 207–224 [Google Scholar]
- 15. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Davis JD. Some new developments in the understanding of oropharyngeal and postingestional controls of meal size. Nutrition 15: 32–39, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Davis JD, Breslin PA. A behavioral analysis of the ingestion of glucose, maltose and maltooligosaccharide by rats. Physiol Behav 69: 477–485, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol Regul Integr Comp Physiol 264: R97–R103, 1993 [DOI] [PubMed] [Google Scholar]
- 20. de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron 57: 930–941, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 340: 80–87, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Disse E, Bussier AL, Deblon N, Pfluger PT, Tschop MH, Laville M, Rohner-Jeanrenaud F. Systemic ghrelin and reward: effect of cholinergic blockade. Physiol Behav 102: 481–484, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, Andersson D, Bjursell M, Perrissoud D, Engel JA, Dickson SL. Ghrelin increases intake of rewarding food in rodents. Addict Biol 15: 304–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes 52: 2260–2265, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes 54: 1985–1993, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci 118: 479–487, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav 89: 611–616, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol 296: R9–R19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra of the rat. Brain Res 964: 107–115, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav 73: 229–234, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Frisina PG, Sclafani A. Naltrexone suppresses the late but not early licking response to a palatable sweet solution: opioid hedonic hypothesis reconsidered. Pharmacol Biochem Behav 74: 163–172, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav 51: 515–521, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Gelling RW, Overduin J, Morrison CD, Morton GJ, Frayo RS, Cummings DE, Schwartz MW. Effect of uncontrolled diabetes on plasma ghrelin concentrations and ghrelin-induced feeding. Endocrinology 145: 4575–4582, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Gilg S, Lutz TA. The orexigenic effect of peripheral ghrelin differs between rats of different age and with different baseline food intake, and it may in part be mediated by the area postrema. Physiol Behav 87: 353–359, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of tas1r3 polymorphisms. Chem Senses 30: 601–614, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286: R31–R37, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Higgs S, Cooper SJ. Evidence for early opioid modulation of licking responses to sucrose and intralipid: a microstructural analysis in the rat. Psychopharmacology (Berl) 139: 342–355, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Ishii Y, Blundell JE, Halford JC, Rodgers RJ. Palatability, food intake and the behavioural satiety sequence in male rats. Physiol Behav 80: 37–47, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 11: 45–54, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol 20: 1772–1785, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol 292: R1728–R1737, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 288: R716–R722, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Lattemann DF. Endocrine links between food reward and caloric homeostasis. Appetite 51: 452–455, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143: 155–162, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Munzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10: 89–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levine AS, Kotz CM, Gosnell BA. Sugars and fats: the neurobiology of preference. J Nutr 133: 831S–834S, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Levy C, MacRae A, Koster E. Perceived complexity and food preference development. Acta Psychol (Amst) 123: 394–413, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 291: R1236–R1239, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Mark GP, Smith SE, Rada PV, Hoebel BG. An appetitively conditioned taste elicits a preferential increase in mesolimbic dopamine release. Pharmacol Biochem Behav 48: 651–660, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Mela DJ. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite 47: 10–17, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Naim M, Brand JG, Christensen CM, Kare MR, Van Buren S. Preference of rats for food flavors and texture in nutritionally controlled semi-purified diets. Physiol Behav 37: 15–21, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26: 2274–2279, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav 91: 506–512, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology 68: 11–20, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry 67: 880–886, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137: 3–25, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Sclafani A. Oral and postoral determinants of food reward. Physiol Behav 81: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Sipols AJ, Stuber GD, Klein SN, Higgins MS, Figlewicz DP. Insulin and raclopride combine to decrease short-term intake of sucrose solutions. Peptides 21: 1361–1367, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes (Lond) 33 Suppl 2: S44–S48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite 43: 11–13, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Smith GP. Dopamine and food reward. Prog Psychobiol Physiol Psychol 16: 83–143, 1995 [PubMed] [Google Scholar]
- 64. Smith GP. John Davis and the meanings of licking. Appetite 36: 84–92, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Smith GP, Smith JC. The inhibitory potency of SCH 23390 and raclopride on licking for sucrose increases across brief-access tests. Physiol Behav 101: 315–319, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Spector AC, St, John SJ. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol Regul Integr Comp Physiol 274: R1687–R1703, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Sugino T, Yamaura J, Yamagishi M, Ogura A, Hayashi R, Kurose Y, Kojima M, Kangawa K, Hasegawa Y, Terashima Y. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem Biophys Res Commun 298: 785–788, 2002 [DOI] [PubMed] [Google Scholar]
- 68. Thornton-Jones ZD, Kennett GA, Vickers SP, Clifton PG. A comparison of the effects of the CB(1) receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology (Berl) 193: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Tong J, Mannea E, Aime P, Pfluger PT, Yi CX, Castaneda TR, Davis HW, Ren X, Pixley S, Benoit S, Julliard K, Woods SC, Horvath TL, Sleeman MM, D'Alessio D, Obici S, Frank R, Tschop MH. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci 31: 5841–5846, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Torregrossa AM, Davis JD, Smith GP. Orosensory stimulation is sufficient and postingestive negative feedback is not necessary for neuropeptide Y to increase sucrose intake. Physiol Behav 87: 773–780, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York: Bantam Books, 2006 [Google Scholar]
- 73. Weatherford SC, Greenberg D, Gibbs J, Smith GP. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmacol Biochem Behav 37: 317–323, 1990 [DOI] [PubMed] [Google Scholar]
- 74. Wisse BE, Frayo RS, Bowers CY, Merriam GR, Cummings DE. Ghrelin increases food intake in anorexic rats with prostate cancer. Proceedings of the Endocrine Society, Denver, CO, 2001. [Google Scholar]
- 75. Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142: 3292–3301, 2001 [DOI] [PubMed] [Google Scholar]
- 76. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86: 5992, 2001 [DOI] [PubMed] [Google Scholar]
- 77. Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50: 2540–2547, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]