Abstract
We showed previously that, at ambient room temperature (22°C), mice maintained at 20% below their initial body weight by calorie restriction expend energy at a rate below that which can be accounted for by the decrease of fat and fat-free mass. Food-restricted rodents may become torpid at subthermoneutral temperatures, a possible confounding factor when using mice as human models in obesity research. We examined the bioenergetic, hormonal, and behavioral responses to maintenance of a 20% body weight reduction in singly housed C57BL/6J +/+ and Lepob mice housed at both 22°C and 30°C. Weight-reduced high-fat-fed diet mice (HFD-WR) showed similar quantitative reductions in energy expenditure—adjusted for body mass and composition—at both 22°C and 30°C: −1.4 kcal/24 h and −1.6 kcal/24 h below predicted, respectively, and neither group entered torpor. In contrast, weight-reduced Lepob mice (OB-WR) housed at 22°C became torpid in the late lights-off period (0200–0500) but did not when housed at 30°C. These studies indicate that mice with an intact leptin axis display similar decreases in “absolute” energy expenditure in response to weight reduction at both 22°C and 30°C ambient temperature. More importantly, the “percent” decrease in total energy expenditure observed in the HFD-WR compared with AL mice is much greater at 30°C (−19%) than at 22°C (−10%). Basal energy expenditure demands are ∼45% lower in mice housed at 30°C vs. 22°C, since the mice housed at thermoneutrality do not allocate extra energy for heat production. The higher total energy expenditure of mice housed at 22°C due to these increased thermogenic demands may mask physiologically relevant changes in energy expenditure showing that ambient temperature must be carefully considered when quantifying energy metabolism in both rodents and humans.
Keywords: obesity, metabolism, thermoneutrality, leptin
we are interested in the physiology of the weight-reduced state (16, 21). Understanding the neurobiological basis of the reduced energy expenditure that accompanies maintenance of a reduced body weight is important for developing long-term treatment of obesity (23). Although the mouse is a useful model for many aspects of this problem, their bioenergetics are, of course, not entirely comparable to those in humans. One important difference—in part, a consequence of their higher somatic surface-to-volume ratio—is the mouse's higher range of ambient thermoneutrality (30–40°C) relative to that of a clothed human (22–25°C) (18) [the thermoneutrality range in rats (25–30°C) is between that of humans and mice (28)]. This difference in thermoneutrality between mice and humans contributes to the mouse's use of torpor (see below) to conserve energy in the face of metabolic/thermal stress (20, 26). Furthermore, this high surface-to-volume ratio obligates substantial energy expenditure to maintain body temperature, resulting in increased basal metabolic rates that could mask changes in metabolic efficiency that accompany weight loss. In the studies described here, we have attempted to characterize and quantify the contribution of such potential confounds to studies of energy homeostasis in weight-reduced mice.
Torpor, usually defined as a hypometabolic state (>50% decline in total energy expenditure) followed by hypothermia (core body temperature <30°C), is a common adaptation of small mammals to conditions of low food availability and/or decreased ambient temperature (26). The initiation of daily torpor in mice is thought to result from decreased availability of calories in conjunction with subthermoneutral ambient temperatures (20).
In most rodent vivaria, the ambient temperature (22–24°C) is set for comfort of personnel working in the facility. This ambient temperature, however, constitutes a constant thermal stress on these animals, requiring higher energy expenditure, energy intake, and sympathetic nervous system tone to maintain core body temperature (20, 27). In mice, the relationship of total energy expenditure (TEE) to ambient temperature is U-shaped, with the lowest TEE for mice occurring between 30 and 40°C (“thermoneutral zone”), although this relationship can be altered by many factors (e.g., strain, overall body size, and genotype). Above 40°C and below 30°C, increased metabolic rate is required to maintain stable body temperature through active cooling and thermogenic mechanisms, respectively (4). These responses are frequently inadequately controlled for or ignored in metabolic studies of mice (4, 20). For example, mice deficient in UCP1 protein (Ucp1−/−) are resistant to diet-induced obesity (DIO) at 22°C (7) but are highly susceptible to the same diet when housed at 30°C (8). Likewise, mice lacking type 2 deiodinase (DioII−/−), a protein involved in the conversion of T4 to T3 in brown adipose tissue and other tissues, are susceptible to DIO at 30°C but not at 22°C (5). Thus, ambient room temperature can clearly affect inferences reached with regard to energy homeostasis in rodents (4, 8, 14, 20).
The leptin axis is implicated in fasting-related phenotypes, including torpor (1, 26). At 22°C ambient temperature, Lepob mice maintain a 2–2.5°C lower body temperature than +/+ animal and display reduced sympathetic nervous system tone (29). At ambient temperatures of 12°C or lower, leptin administration protects mice null for both Ucp1 and Lep from hypothermia and death (31). Leptin administration also increases energy expenditure in food-restricted lean mice (6) and inhibits daily torpor in a 25-g marsupial (Sminthopsis macroura) (10).
To further investigate the interplay of ambient room temperature, leptin, and metabolic adaptation to weight reduction, we examined the bioenergetics, hormonal and behavioral responses to weight reduction of +/+ (WT) diet-induced obese and Lepob mice housed at both 22°C and 30°C. DIO mice were used as a model for human obesity since we have previously shown that C57BL/6J male mice fed a high-fat diet—and subsequently weight-reduced—showed decreases in energy expenditure similar to those shown by never-obese weight-reduced mice, suggesting an upward resetting of defended body weight in the high fat-fed animals (21). We quantified total, resting, and nonresting energy expenditure, ambulatory movement, and core body temperatures in five groups of animals: low (10%)-fat diet fed WT; high (60%)-fat diet fed WT; weight-reduced high-fat diet fed WT; high-fat diet fed Lepob; and weight-reduced high-fat diet fed Lepob mice housed at both subthermoneutral (22°C) and thermoneutral (30°C) ambient temperatures. We also measured serum concentrations of T3, leptin, glucose, and insulin in the WT mice. We assessed the same parameters in the weight-reduced and low-fat diet fed never-obese WT mice following a switch to ad libitum access to a high-fat diet (HFD) to determine whether the hypometabolic phenotype observed in the weight-reduced WT mice could be resolved following refeeding and weight regain. We hypothesized that torpor would not account for a significant portion of the reduced energy expenditure anticipated in our weight-reduced WT animals (21) but would play a major role in Lepob mice and that the metabolic adaptation observed in mice at 30°C would be more pronounced as a percentage of total 24-h energy expenditure than in mice at 22°C since thermogenic stress is greatly reduced at thermoneutrality.
MATERIALS AND METHODS
To assess the role of ambient temperature, caloric restriction/diet composition, and leptin deficiency on bioenergetic, behavioral, and hormonal responses to weight perturbation in mice, we examined wild-type (WT) C57BL/6J (experiment 1) and Lepob (experiment 2) mice before and during maintenance of a reduced body weight at both thermoneutral (30°C) and standard housing subthermoneutral (22°C) ambient temperatures. All mice in the study were in rooms with 12:12-h dark-light cycle (lights on at 0700), housed with wood-chip bedding, and given ad libitum access to food and water unless otherwise specified. Ad libitum mice in both experiments were provided food through specially constructed feeding baskets designed to minimize spillage and, therefore, allow accurate measurements of food intake (21). Weight-reduced animals in both experiments received one-third of their individually calculated food ration (± 0.1 g) in the morning (0745–0815) and two-thirds of their food ration in the evening (1830–1900).
All protocols were approved by the Columbia University Institutional Animal Care and Use Committee.
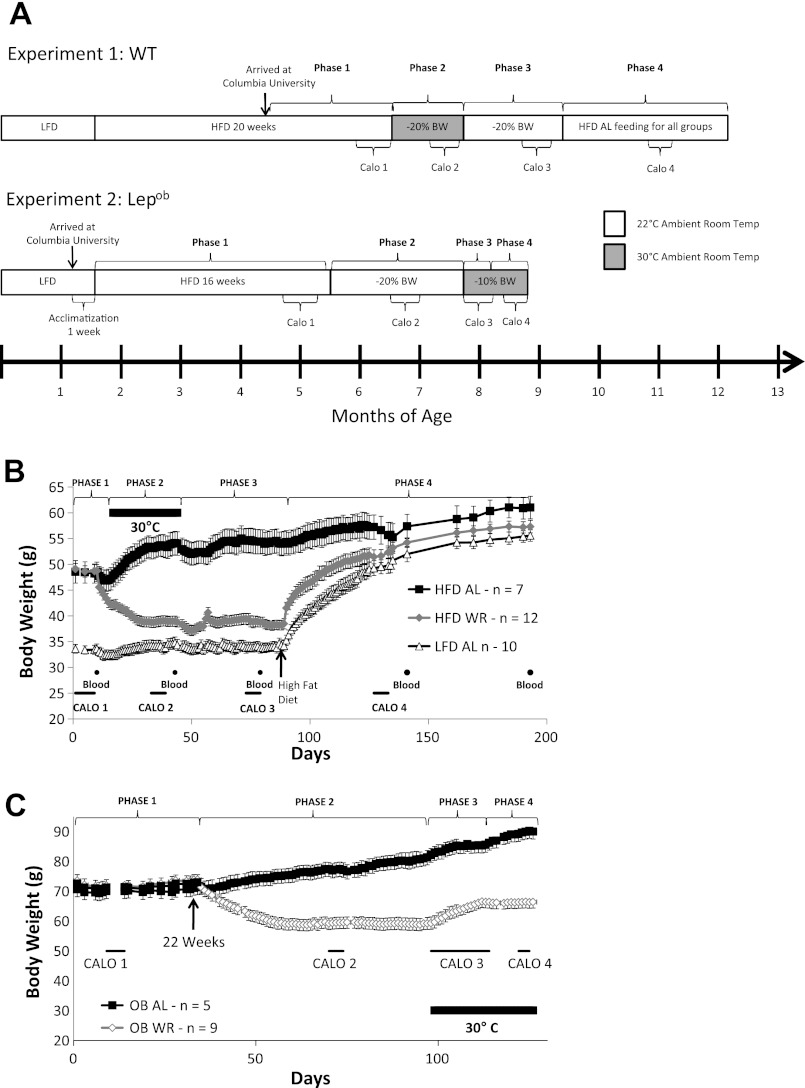
Figure 1A provides an overview and experimental timeline of the two experiments. Experiment 1 used wild-type C57BL/6J mice; experiment 2 used homozygous B6.V Lepob /J- mice that are deficient in leptin production. The materials and methods and results sections describe these experiments separately.
Fig. 1.
Experimental timeline and body weight data from experiments 1 and 2. A: experimental timeline of experiments 1 (C57BL/6J = wild type, WT) and 2 (Lepob). Experiment 1 was divided into four phases: phase 1 = ad libitum access to a high-fat diet (HFD), phase 2 = maintenance of weight reduction [−20% body weight (BW)] at 30°C, phase 3 = maintenance of weight reduction (−20% BW) at 22°C, phase 4 = ad libitum access to high-fat diet (HFD) for all groups. Indirect calorimetry and 4-h fasting bleeds were conducted during each of the four phases. Experiment 2 was divided into four phases: phase 1 = ad libitum access to HFD, phase 2 = maintenance of weight-reduction (−20% BW) at 22°C, phase 3 = active weight gain at 30°C, phase 4 = maintenance of weight-reduction (−10% BW) at 30°C. Indirect calorimetry (Calo) was conducted during each phase. B: means ± SE body weights of WT mice (experiment 1). Housed at 22°C at all times except where marked by thick black line during which ambient room temperature was maintained at 30°C. Indirect calorimetry (thin black lines) was conducted during each of the four phases, and blood was taken for hormone analyses. C: mean ± SE body weights of Lepob mice (experiment 2).
Experiment 1 Study Design: Wild-Type Mice
Eighteen-week-old C57BL/6J male mice were obtained from Jackson Laboratory (Bar Harbor, ME): 20 diet-induced obese (HFD mice fed a high-fat diet starting at 6 wk of age; D12492i, 60% kcal as fat; Research Diets, New Brunswick, NJ) and 12 low-fat diet-fed (LFD mice, fed a low-fat diet starting at 6 wk of age; Research Diets, D12450Bi, 10% kcal as fat) were used. Diet-induced-obese mice were used as a model for human obesity (21). This study was divided into four phases: phase 1 was preweight reduction, phase 2 was weight-reduced (−20% body weight) at 30°C, phase 3 was weight-reduced (−20% body weight) at 22°C, and phase 4 was high-fat diet feeding for all groups (Fig. 1A).
Phase 1, 22°C (60 days total).
Upon receipt, animals were singly housed and given ad libitum access to diet identical to that provided at Jackson Laboratory and maintained 60 days before the start of the weight reduction protocol (Fig. 1A, phase 1). During this period, body weight was measured every 2 or 3 days. One HFD mouse and two LFD mice died during this period from unknown causes. At the end of phase 1, mice in the HFD group were separated into two groups: 7 mice were assigned to the ad libitum-fed group (HFD-AL, final n = 7) and 12 mice were assigned to the weight-reduced group (HFD-WR, final n = 12). Group assignments were made so that body weights were similar between the two groups.
Phase 2: weight-reduced at 30°C (34 days total).
Room temperature was raised to 30°C, and HFD-WR mice were reduced to a weight 20% below their initial body weight, as previously described (21), and then they were maintained between 79 and 81% of initial body weight by subsequent adjustments in calories fed (Fig. 1, A and B). HFD-WR mice were fed as described above throughout phases 2 and 3; food intake was recorded daily.
Phase 3: weight-reduced at 22°C (44 days total).
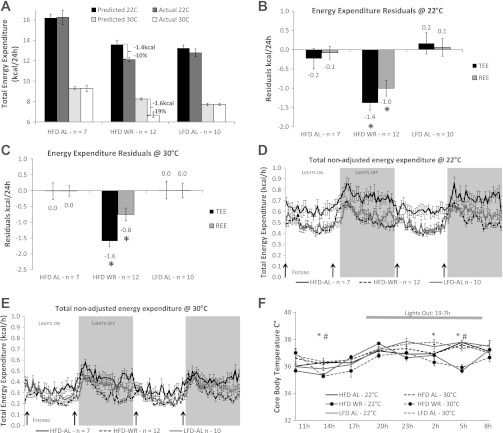
The ambient room temperature was lowered from 30°C to 22°C (Fig. 1, A and B). Food rations were adjusted so that HFD-WR mice maintained body weights between 79 and 81% of initial body weight. The number of calories required to maintain body weight in the HFD-WR mice at 22°C was approximately twice those required at 30°C. Energy expenditure was ≈2-fold higher at 22°C (Table 2 and Fig. 2A).
Table 2.
Energy expenditure and movement: energy expenditure
| TEE, kcal/24 h | REE, kcal/24 h | NREE, kcal/24 h | Movement, 1000×) | |
|---|---|---|---|---|
| Calo 2: 30°C, WR | ||||
| HFD-AL | 9.3 ± 0.3A | 5.6 ± 0.3A | 3.7 ± 0.1A | 158.8 ± 28.6A |
| HFD-WR | 6.7 ± 0.2C | 4.0 ± 0.1B | 2.7 ± 0.1B | 162.8 ± 21.9A |
| LFD-AL | 7.7 ± 0.1B | 4.1 ± 0.1B | 3.6 ± 0.1A | 251.7 ± 24.0B |
| Calo 3: 22°C, WR | ||||
| HFD-AL | 16.3 ± 0.7A€ | 12.4 ± 0.5A€ | 3.9 ± 0.1A∈ | 106.7 ± 16.4A |
| HFD-WR | 12.1 ± 0.2B€ | 8.6 ± 0.2B€ | 3.5 ± 0.1A∈ | 175.0 ± 12.5B |
| LFD-AL | 12.8 ± 0.4B€ | 8.7 ± 0.3B€ | 4.1 ± 0.1A∈ | 210.1 ± 13.7B |
| Calo 4: 22°C, Post-WR | ||||
| HFD-AL | 15.8 ± 0.7A | 11.7 ± 0.7A | 4.1 ± 0.2A$ | 88.5 ± 15.9A |
| HFD-WR | 15.8 ± 0.4A$ | 11.9 ± 0.4A$ | 3.9 ± 0.3A | 131.0 ± 12.7A |
| LFD-AL | 15.8 ± 0.5A$ | 12.0 ± 0.2A$ | 3.8 ± 0.3A | 139.6 ± 14.0A |
Values are expressed as means ± SE.TEE, total energy expenditure; REE, resting energy expenditure (lowest 1-h TEE period); NREE, nonresting energy expenditure (calculated as TEE − REE) and ambulatory movement (1000×) for all three mouse groups at 30°C (Calo 2, Phase 2), 22°C (Calo 3, Phase 3) and following diet switch to HFD at 22°C (Calo 4, Phase 4).
A,B,C Levels not connected by same letter within Calo measurement period are significantly different (P < 0.05).
Significantly different (P < 0.05) between Calo 3 and Calo 2 (ambient temperature comparison).
Significantly different (P < 0.05) between Calo 4 and Calo 3. HFD feeding for all group comparison.
Fig. 2.
Energy expenditure and body temperature phenotypes of WT mice (experiment 1). Predicted values were obtained from multivariate regressions relating energy expenditure to fat-free mass (FFM) and fat mass (FM) of ad libitum fed mice (see Calculations in materials and methods for equations). Predictions of total energy expenditure (TEE) and resting energy expenditure (REE) based on measured FM and FFM were calculated for each mouse at both ambient temperatures. Means ± SE actual and predicted (based on FM and FFM) TEE for each group at either 22°C or 30°C A: means ± SE observed-minus-predicted values for each group 24-h TEE (black bars) and 24-h REE (gray bars) (kcal/24 h) for mice at 22°C (B) and 30°C (C) ambient room temperatures for experiment 1. D and E: absolute nonadjusted group mean (± SE) hourly TEE measured every 26 min over 48-h period at 22°C (D) and 30°C (E). Black arrows represent feeding times. F: mean 24-h core body temperatures (°C ± SE) measured every 3 h. Solid lines represent 22°C (phase 3) and dashed lines 30°C (phase 2) ambient temperatures. *Significantly lower body temperatures in WR vs. AL groups at 30°C; P < 0.05. #Significantly lower body temperatures in WR vs. AL groups at 22°C; P < 0.05.
Phase 4: ad libitum HFD for all groups (104 days total).
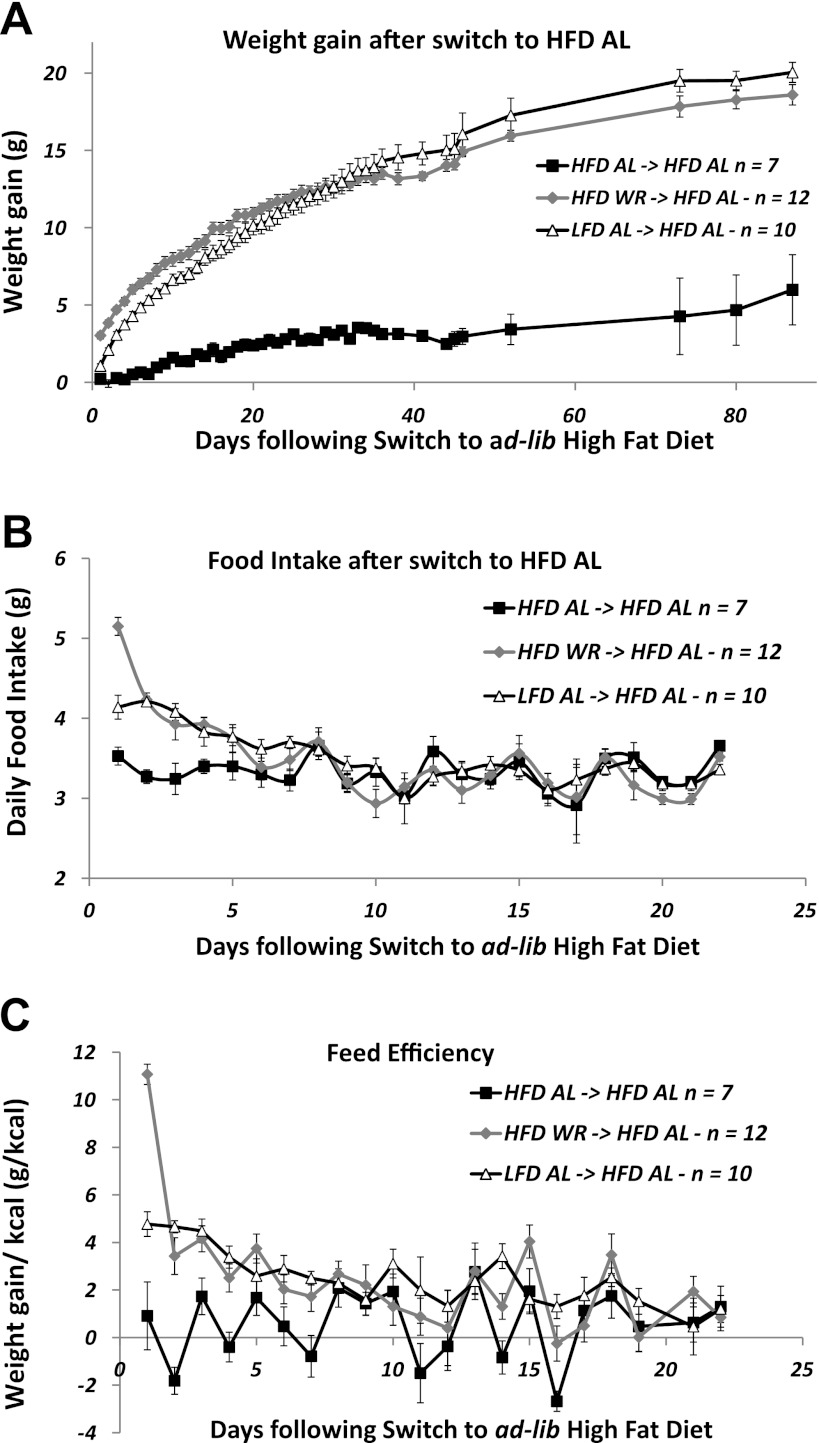
Phase 4 was designed to assess the effects of ad libitum access to a HFD on both the resolution of the metabolic adaptation observed in the HFD-WR mice (21) and on body weight homeostasis in mice that had never ingested a HFD (LFD-AL). Mice were given ad libitum access to HFD provided in the specialized feeding baskets and body weight (Fig. 3A) and food intake (Fig. 3B) were measured every 24 h for the following 23 days. Feed efficiency was estimated by dividing the change in body weight (g) by the number of calories consumed (kcal) in a 24-h period (see Fig. 3C).
Fig. 3.
Weight regain following ad libitum access to a HFD (experiment 1, phase 4). Body weight gain (A), food intake (B), and feed efficiency (C) (defined as grams of weight gain per kilocalories ingested per 24 h) were collected every 24 h for the first 23 days following a switch to a HFD for all mice. Body weight (A) was subsequently followed until the end of the experiment. Data are presented as group means ± SE.
Experiment 2 Study Design: Lepob Mice
Sixteen 5-wk-old homozygous B6.V Lepob/J- male mice were obtained from Jackson Laboratory, housed two per cage, acclimatized for 1 wk on standard breeder chow (Purina PicoLab 5058), and then switched to the high-fat diet starting at 6 wk of age so as to be comparable to the animals in experiment 1 (Research Diets, D12492i, 60% kcal as fat). Two mice died during this period of unknown causes. Experiment 2 was divided into four phases: phase 1 was preweight reduction, phase 2 was weight-reduced (−20% body weight) at 22°C, phase 3 was weight regain at 30°C, and phase 4 was weight-reduced (−10% body weight) at 30°C (Fig. 1, A and C).
Phase 1, 22°C high-fat diet feeding (16 wk total).
Following the 1-wk acclimatization period, all OB mice were given access to HFD in the specialized feeding baskets for the following 16 wk. At the end of phase 1, mice were separated into two groups: 1) ad libitum fed (OB-AL, final n = 5) and 2) weight-reduced (OB-WR, final n = 9). The animals were assigned, so that body weights were similar between the two groups.
Phase 2: weight-reduced (−20%) at 22°C (65 days total).
Room temperature was maintained at 22°C, and OB-WR mice were weight-reduced 20% below their body weight, as previously described (Ref. 21) and then maintained between 79 and 81% of initial body weight by subsequent adjustments in calories provided (Fig. 1C).
Phase 3: weight regain at 30°C (14 days total).
Following phase 2, room temperature was increased to 30°C (thermoneutrality). Food intake for the OB-WR mice during the subsequent 2 wk was “clamped” to their intake at 22°C, on which regimen at 30°C ambient temperature, as anticipated, they gained weight (see OB-WR, Fig. 1C). Total calories provided to OB-WR were decreased at the end of this 2-wk period to match total energy expenditure measured by calorimetry, halting their weight gain at 90 ± 0.8% of initial preweight loss body weight (see phase 4).
Phase 4: weight reduced (−10%) at 30°C (14 days total).
Mice were maintained at −10% initial body weights until time of death at 30°C ambient temperature.
During each of the four phases of experiments 1 and 2, mice underwent a round of indirect calorimetry (see Energy Expenditure by Indirect Calorimetry) to determine energy expenditure and ambulatory activity parameters. Twenty-four-hour core body temperature was recorded during the weight reduction period at 22°C and 30°C ambient temperatures (see Core Body Temperature). In experiment 1, serum was obtained for hormone and metabolite analysis during each of the four phases (see Serum Hormone and Metabolite Profiles).
Body weight, body composition, and food intake.
Body weight was measured (± 0.1 g) before the morning feeding using an Ohaus Scout Pro 200g scale (Nänikon, Switzerland, between 0800 and 0830). Body composition (FM, fat mass; FFM, fat-free mass, and extracellular fluid) was estimated by time-domain-NMR (Minispec Analyst AD; Bruker Optics, Silberstreifen, Germany) (11) before the morning feeding: every 2–3 wk; before and after calorimetry measurements; before start of the weight reduction protocol; and on the day prior to being euthanized. Food intake ( ± 0.1 g) was recorded daily for WR animals in both experiments 1 and 2 (HFD-WR and OB-WR) and for all animals of experiment 1 during the first 23 days of the diet switch protocol (phase 4). Because of a 60-g upper body mass limit for the mini-Bruker NMR, we were unable to collect body composition data on some of the animals in experiment 2. For that reason, we have not included any body composition data of Lepob animals.
Energy expenditure by indirect calorimetry.
Energy expenditure was measured with a LabMaster-CaloSys-Calorimetry System (TSE Systems, Bad Homburg, Germany). O2 and CO2 measurements were taken every 26 min during a 72-h period, while mice were maintained on their respective weight-maintenance feeding schedules. TSE has the benefit of using home cages with normal bedding, thereby decreasing the amount of stress incurred by the animals. Mice were acclimatized to the specialized water bottles used by the system during a minimum of 3 days prior to entering the chambers. Because of possible stress related to transfer to the calorimetry system, only the last 48 h of measurements were used to calculate total 24-h energy expenditure (TEE; expressed in kcal/day) and respiratory quotient (RQ = V̇co2 / V̇o2). No significant decreases in body weight were noted in any of the experiments. Resting energy expenditure (REE in kilocalories per day) was defined as the lowest 1-h period of energy expenditure, and this value was extrapolated to 24 h. This almost always coincided with the lowest 1 h of total ambulatory activity (generally 1300–1400), during the 48-h period. Nonresting energy expenditure (NREE) was calculated as the difference between TEE and REE (NREE = TEE − REE). Physical activity was measured by infrared beams integrated with the LabMaster system with x, y, and z axes.
Energy expenditure was assessed in all mice in all four phases of both experiments. Only the most relevant data from these different timepoints are presented.
Core body temperature.
Rectal core body temperature (± 0.1°C) of mice was measured every 3 h for 24 h using a Thermalert Monitoring Thermometer starting at 0800 (TH5 model; Raytek, Santa Cruz, CA). Temperature measurements (at 22 and 30°C ambient temperature) were obtained at time points at least 5 days away from any indirect calorimetry measurements or bleeds for both experiments during the weight-reduced phases only (phases 2 and 3 for experiment 1 and phases 2 and 4 for experiment 2).
Calculations.
Energy expenditure is proportional to body mass and composition [fat-free (FFM) and fat mass (FM)] (12, 21, 30). Because we had body composition data from experiment 1 (WT mice) and there was no significant effect of diet composition on TEE per se, total energy expenditure (TEE; kcal/24 h) of HFD-AL and LFD-AL mice was related to both FFM and FM by multiple regression analysis (2, 16, 30). We pooled the data from ad libitum-fed mice to create separate baseline regression equations relating TEE (kcal/24 h) to FFM and FM (grams) using the Calo 2 (Fig. 1, A and B: 30°C ambient temperature) and Calo 3 (Fig. 1, A and B: 22°C ambient temperature) timepoints at 30°C: TEE = 4.3 + 0.13 × FFM + 0.09 × FM; R2 = 0.79, P < 0.0001; at 22°C, TEE = 1.2 + 0.45 × FFM + 0.15 × FM; R2 = 0.87, P < 0.0001. These multiple-regression equations were used to predict TEE for all mice following experimental weight perturbation (at the respective ambient temperatures), as we have done in similar studies of human subjects (16, 25) and mice (21) and as recently suggested by Tschop et al. (30). The residuals (i.e., the differences between measured and predicted values) were calculated for each animal and were tested against the null hypothesis that they were equal to 0 (Fig. 2, B and C). Baseline regression equations relating REE to FFM and FM were used to predict REE values, and residuals were calculated using the same method described above (Fig. 2, B and C). REE = lowest 1-h period of energy expenditure extrapolated to 24 h; at 30°C; REE = 3.6 − 0.01 × FFM + 0.11 × FM; R2 = 0.78, P < 0.0001; and at 22°C, REE = 1.02 + 0.28 × FFM + 0.21 × FM; R2 = 0.93, P < 0.0001. Because FFM and FM did not significantly predict NREE (P = 0.55 at 30°C and P = 0.19 at 22°C), residuals were not calculated using the equations above but instead determined for each calorimetry timepoint by subtracting REE from TEE (NREE = TEE − REE).
Serum hormone and metabolite profiles.
For experiment 1, blood glucose (by tail bleed − FreeStyle Lite, Abbott, Alameda, CA) and circulating serum insulin, leptin, and bioactive thyroid hormone (triiodothyronine: T3) concentrations (by retro-orbital bleed under 4% isoflurane) were determined after a 4-h fast following each of the calorimetry experiments and at time of being euthanized (see circles on Fig. 1B). Serum was collected and frozen at −80°C. Insulin was assayed using the Mercodia Mouse Insulin ELISA (Mercodia AB, Uppsala, Sweden); T3 using RIA at Hormone Assay & Analytical Services Core at Vanderbilt University (Vanderbilt University, Nashville, TN) and leptin using Quantikine ELISA kit (R&D Systems, Minneapolis, MN). All assays were conducted according to manufacturer's protocols. HOMA2 (calculator developed by University of Oxford based on Ref. 17) was used to estimate insulin resistance (HOMA IR) and insulin sensitivity (HOMA% S).
Statistical analysis.
Data are expressed as group means ± SE. Statistical analyses were performed using JMP (version 7; SAS, Cary, NC). Statistical significance was prospectively defined as Pα < 0.05. Student's t-tests and ANOVAs were used where applicable.
RESULTS
Experiment 1
Effects of weight perturbation and ambient room temperature on body weight/composition.
HFD mice weighed 45 ± 3% more than ad libitum LFD-fed mice and had significantly higher fractional body fat (HFD, 36 ± 1%; LFD, 17 ± 1% fat) at the end of phase 1 phase 1 (Fig. 1B, body composition data not shown). On the first day of calorie restriction (start of phase 2), ambient room temperature was elevated to 30°C and maintained at that temperature for the subsequent 34 days. During the active weight loss period of phase 2, HFD-WR mice lost 9.8 ± 0.3 g (72% accounted for by decreased FM), and HFD-AL mice gained 4.9 ± 0.3 g (100% accounted for by increased FM); LFD-AL showed no significant changes in body weight or composition during this time period (Fig. 1B). During Calo 2 and Calo 3 studies, HFD-AL had the highest body weight, fat mass, and fat mass percentage, LFD-AL had the lowest, and HFD-WR had intermediate phenotypes that were closer to—but still significantly higher than—those of LFD-AL (Table 1). At Calo 4, (during phase 4), following access to HFD for all groups, BW and FM were still slightly lower in the LFD-AL and HFD-WR than HFD-AL (Table 1), although by the time of euthansia no significant differences were observed in any of these parameters among the three groups (data not shown).
Table 1.
Body weight and composition of experiment 1 calorimetry timepoints: body weight and composition
| Body Weight | Fat-Free Mass | Fat Mass | % Fat Mass | |
|---|---|---|---|---|
| Calo 2: 30°C, WR | ||||
| HFD-AL | 53.4 ± 2.1A | 24.9 ± 0.3A | 21.2 ± 1.5A | 39 ± 1A |
| HFD-WR | 38.9 ± 2.1B | 23.2 ± 0.5B | 11.6 ± 0.5B | 30 ± 1B |
| LFD-AL | 34.2 ± 1.1C | 22.4 ± 0.4B | 6.4 ± 0.8C | 18 ± 2C |
| Calo 3: 22°C, WR | ||||
| HFD-AL | 54.4 ± 2.4A | 26.5 ± 0.5A€ | 21.2 ± 1.7A | 38 ± 2A |
| HFD-WR | 38.9 ± 1.1B | 23.4 ± 0.5B | 11.0 ± 0.6B | 28 ± 1B |
| LFD-AL | 33.9 ± 1.0C | 23.0 ± 0.4B | 6.3 ± 0.7C | 18 ± 2C |
| Calo 4: 22°C, Post-WR | ||||
| HFD-AL | 56.3 ± 2.6A | 26.8 ± 0.6A | 22.2 ± 1.7A | 39 ± 1A |
| HFD-WR | 51.8 ± 1.2AB$ | 25.6 ± 0.4A$ | 19.5 ± 0.6AB$ | 38 ± 1A$ |
| LFD-AL | 49.6 ± 1.4B$ | 25.4 ± 0.3A$ | 18.0 ± 0.8B$ | 36 ± 1A$ |
Values are expressed as means ± SE. Body weight and body composition [fat mass (FM) and fat-free mass (FFM)] of the three mouse groups at 30°C (Calo 2, Phase 2), 22°C (Calo 3, Phase 3), and the following diet switch to a high-fat diet (HFD) (Calo 4, Phase 4).
A,B,CPhenotypes not connected by same letter within Calo measurement period are significantly different (P < 0.05).
Significantly different (P < 0.05) between Calo 3 and Calo 2 (ambient temperature comparison).
Significantly different (P < 0.05) between Calo 4 and Calo 3. HFD feeding for all group comparisons. LFD, low-fat diet; WR, weight-reduced; AL, ad libitum.
Effects of ambient room temperature and weight perturbation on energy expenditure.
TEE (40–45% lower) and REE (55% lower) were significantly decreased in all mice housed at 30°C (Calo 2) compared with 22°C (Calo 3: Table 2 and Fig. 2A). Residuals for 24-h TEE and REE of HFD-WR mice were significantly and proportionately equivalent below predicted at both 22°C (−1.4 kcal/24 h, P < 0.01 and −1.0 kcal/24 h, P < 0.01, respectively; Fig. 2, A and B) and at 30°C (−1.6 kcal/24 h, P < 0.01 and −0.8 kcal/24 h, P < 0.01, respectively; Fig. 2, A and C), indicating that—irrespective of ambient temperature—these components of EE were reduced beyond what could be attributed to changes in body mass and composition. Nonadjusted (for body mass and composition) TEE was significantly lower in HFD-WR and LFD-AL compared with HFD-AL at both 22°C (Fig. 2D) and 30°C (Fig. 2E) ambient for most measurement periods. Although HFD-WR mice weighed on average 14% more than LFD-AL and had 1 g (5%) higher FFM (Table 1), they had significantly lower nonadjusted (for FFM and FM) TEE and similar REE at 30°C (Table 2, Calo 2), differences that are abolished when measured at 22°C (Table 2, Calo 3). When phase 3 was started and the room temperature was lowered back to 22°C (day 44, Fig. 1B), calories required to stabilize body weight of the HFD-WR mice were ∼50% higher than at 30°C. When HFD-WR and LFD-AL mice were given 38 days of ad libitum access to HFD (Calo 4, 22°C), both groups significantly increased their body weights, resulting from increases in both FFM and FM (Table 1 and Fig. 3A). FFM, the major contributor to metabolic rate, was no longer significantly different among groups at the Calo 4 timepoint (Table 1), which was reflected in significant increases in absolute TEE and REE compared with the preweight gain measurements; TEE and REE were no longer significantly lower than those observed in HFD-AL (Table 2), indicating a normalization of energy expenditure parameters.
Effects of ambient room temperature and weight perturbation on 24-h body temperature.
At both 22°C (phase 3) and 30°C (phase 2) ambient temperatures, core body temperature was significantly lower (at the 1400 and 0500) in HFD-WR mice compared with AL-fed groups (Fig. 2F). There was no effect of ambient temperature per se on core body temperature of HFD-WR, and none of the mice entered torpor.
Effects of high-fat diet on body weight and composition in HFD-WR and LFD-AL mice.
HFD-WR and LFD-AL mice were switched to HFD (phase 4, Fig. 1, A and B) to evaluate effects of ad libitum access to a high-fat diet on food intake, weight gain, metabolic efficiency [as reflected by the ratio of weight gain (g) to calories ingested (kcal) − phase 4 conducted at 22°C] and to determine whether the decreased components of energy expenditure (TEE and REE) observed in HFD-WR mice were resolved by weight regain. At the end of phase 4, both HFD-WR and LFD-AL mice had gained similar amounts of weight (19.0 ± 1.0 and 20.9 ± 0.3 g, respectively), with FM accounting for 64 ± 2% and 66 ± 2% of the weight increments (Fig. 3A and Table 1). Body weights and body composition at time of euthansia were not significantly different among groups (i.e., HFD-AL, HFD-WR, and LFD-AL). For the first 5 days following the diet switch, 24-h energy intake was significantly increased in HFD-WR and LFD-AL mice compared with HFD-AL mice (Fig. 3B). During the first 24 h, HFD-WR ingested 25% more calories than LFD-AL. After day 5, there was no significant difference in 24-h food intake among the three groups. Since HFD-WR and LFD-AL mice gained similar absolute amounts of body weight, and 64% and 66% of that weight gain was fat, respectively, we estimated feed efficiency by dividing the weight gain (g) by the cumulative food intake (g) for each 24-h period. Feed efficiency was significantly greater in HFD-WR and LFD-AL mice than the HFD-AL during the first 7 days post-diet switch (Fig. 3C). Compared with LFD-AL, the HFD-WR mice showed more than a two-fold higher feed efficiency during the first 24 h. Food energy intake was no longer significantly different among the three groups (except on days 11, 14, and 16) after the first 7 days. However, although not significantly higher on a 24-h basis, mean feed efficiency from days 7 to 23 was >2.5-fold higher in HFD-WR (1.6 g/kcal) and more than three-fold higher in LFD-AL (1.9 g/kcal) than HFD-AL (0.6 g/kcal).
Effects of weight perturbation and ambient room temperature on blood hormone and metabolites.
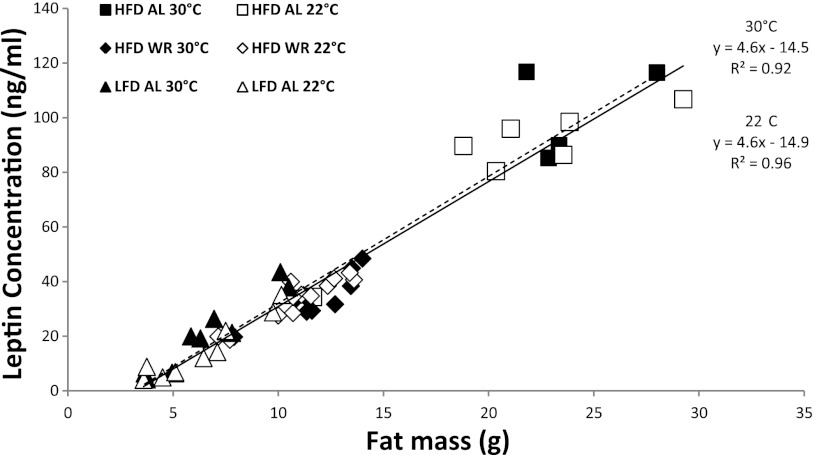
At both 30°C and 22°C ambient temperatures, serum insulin (Table 3) and leptin (Fig. 4 and Table 3) concentrations were highest in the most obese mice (HFD-AL) and lowest in the leanest mice (LFD-AL). Serum leptin concentrations were highly correlated with total FM (by NMR) with nearly identical regression equations at both 22°C (r2 = 0.96; leptin concentration = 4.6 × FM − 14.9) and 30°C (r2 = 0.92; leptin concentration = 4.6 × FM − 14.5). Insulin sensitivity, assessed by HOMA% S, was lowest in HFD-AL mice and highest in LFD-AL mice, and weight reduction significantly improved insulin sensitivity in the HFD-WR group at the Calo 2 and Calo 3 time points (Table 3). At 30°C ambient (Calo 2), circulating T3 concentrations were significantly lower in the LFD-AL group compared with HFD-AL (2.8 ± 0.1 ng/ml vs. 3.3 ± 0.1 ng/ml, respectively; P < 0.05). At 22°C ambient, T3 concentrations in all three groups were higher than at 30°C, with the greatest relative increase observed in HFD-AL: HFD-AL +57%; HFD-WR +26%, LFD-AL +18%. In phase 4, following 40 days of HFD feeding (Fig. 1B), insulin concentrations doubled in the HFD-WR mice and quadrupled in the LFD-AL mice, while glucose concentrations rose significantly in both groups to concentrations comparable to those of HFD-AL mice (Calo 4; Table 3). These changes in insulin and glucose concentrations seen in HFD-fed animals that were previously HFD-WR and LFD-AL are reflected in twofold and four-fold increases in insulin resistance, respectively, as reflected by HOMA IR (Table 3).
Table 3.
Serum hormones and metabolites: blood hormone and metabolites
| Leptin, ng/ml | T3, ng/dl | Glucose, mg/dl | Insulin, μg/l | HOMA%S | HOMA IR | |
|---|---|---|---|---|---|---|
| Calo 2: 30°C, WR | ||||||
| HFD-AL | 102.1 ± 5.3A | 3.3 ± 0.1A | 135 ± 4.1A | 0.26 ± 0.05A | 136.3 ± 20.3A | 0.92 ± 0.17A |
| HFD-WR | 34.4 ± 2.6B | 3.1 ± 0.1AB | 118 ± 4.5AB | 0.16 ± 0.01B | 182.8 ± 9.5B | 0.56 ± 0.03B |
| LFD-AL | 18.9 ± 4.4C | 2.8 ± 0.1B | 126 ± 6.4B | 0.14 ± 0.01B | 209.4 ± 7.1B | 0.48 ± 0.02B |
| Calo 3: 22°C, WR | ||||||
| HFD-AL | 84.6 ± 7.5A | 5.2 ± 0.4A€ | 138.9 ± 4.6A | 0.30 ± 0.03A | 106.2 ± 15.2A | 1.07 ± 0.12A |
| HFD-WR | 33.5 ± 2.4B | 3.9 ± 0.1B€ | 118.2 ± 4.1B | 0.20 ± 0.01B | 154.8 ± 10.5B | 0.68 ± 0.05B |
| LFD-AL | 14.3 ± 3.4C | 3.3 ± 0.1C€ | 124.1 ± 3.8B | 0.14 ± 0.01C | 205.1 ± 6.9C | 0.49 ± 0.02B |
| Calo 4: 22°C, Post-WR | ||||||
| HFD-AL | 72.3 ± 7.3A | 4.5 ± 0.3A | 145.3 ± 10.4A | 0.29 ± 0.03A | 104.7 ± 12.3A | 1.05 ± 0.11A |
| HFD-WR | 89.4 ± 3.3A$ | 4.0 ± 0.1A | 151.7 ± 3.7A$ | 0.40 ± 0.08AB$ | 85.9 ± 10.6AB$ | 1.46 ± 0.27AB$ |
| LFD-AL | 91.7 ± 8.8A$ | 4.0 ± 0.3A$ | 155.7 ± 6.5A$ | 0.56 ± 0.08B$ | 61.5 ± 13.0B$ | 2.04 ± 0.30B$ |
Values are expressed as means ± SE. Serum hormones and metabolites for all three mouse groups at 30°C (Calo 2, Phase 2), 22°C (Calo 3, Phase 3), and following diet switch to HFD at 22°C (Calo 4, Phase 4). T3, triiodothyronine; HOMA, homeostasis model assessment; HOMA-IR, HOMA-insulin resistance.
A,B,CLevels not connected by same letter within Calo measurement period are significantly different (P < 0.05) by ANOVA with Tukey post hoc analysis.
Significantly different (P < 0.05) between Calo 3 and Calo 2 (ambient temperature comparison).
Significantly different (P < 0.05) between Calo 4 and Calo 3 (HFD feeding for all groups comparison).
Fig. 4.
Serum leptin concentration vs. fat mass. Leptin (ng/ml) vs. fat mass (g). Linear regression for all WT mice; 22°C (solid line) and 30°C (dashed line). Squares denote HFD-AL, diamonds denote HFD-WR, and triangles denote LFD-AL at either 30°C (solid symbols) or 22°C (open symbols).
Experiment 2
Effects of weight perturbation and ambient room temperature on body weight/composition.
By design, caloric restriction (from days 34 to 98; phase 2) resulted in a 20% decrease in body weight in OB-WR mice (time to achieve 20% weight reduction = 19 ± 2 days; Fig. 1, A and C). When room temperature was raised to 30°C (day 99) and food intake of OB-WR was “clamped” to their intake at 22°C, these animals gained weight at a rate of 0.7 g per day for the following 14 days (phase 3; Fig. 1C). By 14 days, the mice weighed 90% of their initial body weight, meaning that they had regained 50% of the original weight lost. Total calories provided to OB-WR were then decreased to match total energy expenditure (calculated from indirect calorimetry: phase 3, Calo 3), and body weight was maintained at 90 ± 0.6% of initial weight until time of death (day 126).
Effects of ambient room temperature and weight perturbation on energy expenditure.
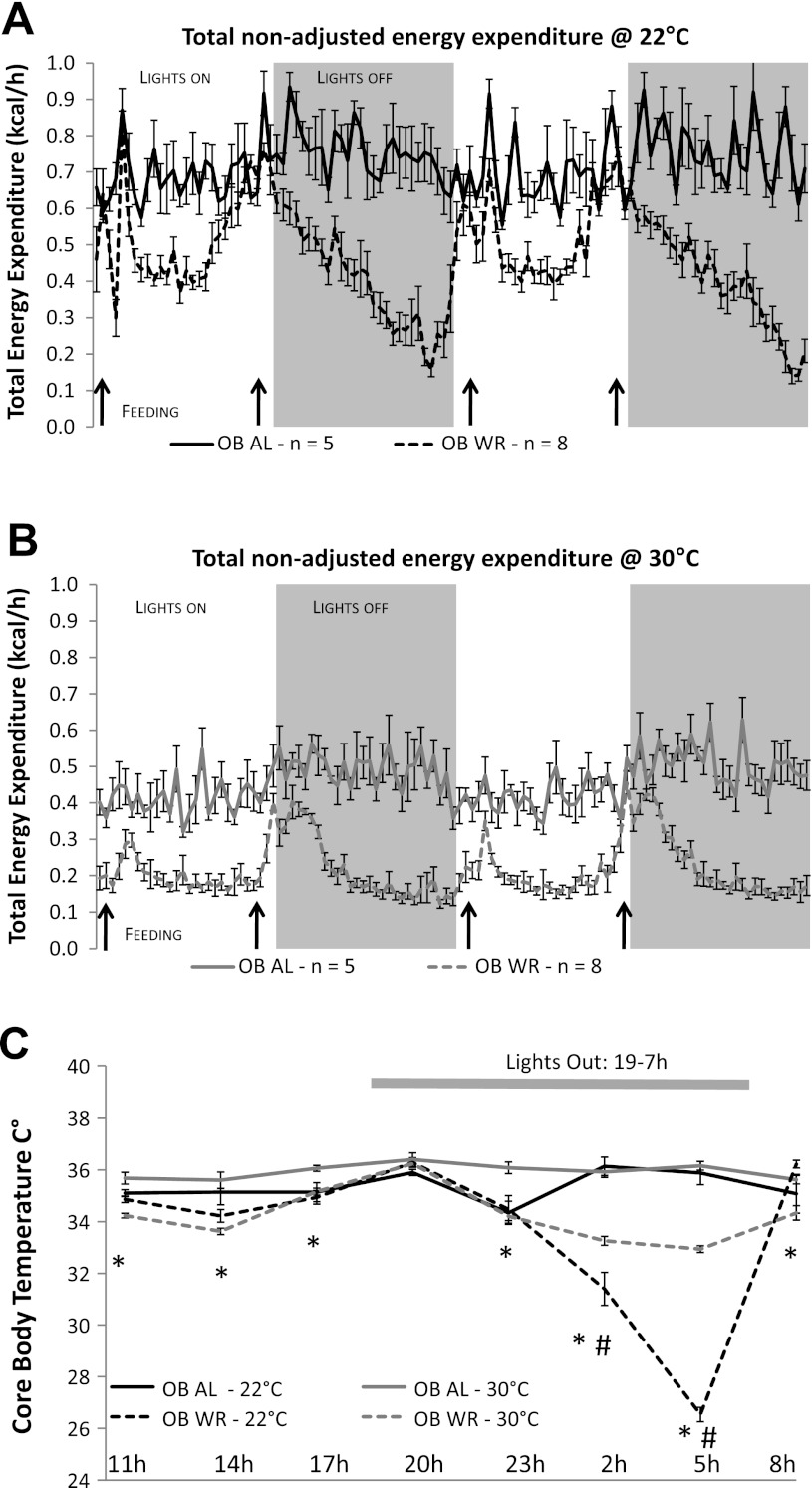
OB-WR mice had significantly lower absolute TEE at both 22°C (−20% body weight: Fig. 5A) and 30°C (−10% body weight: Fig. 5B) ambient temperatures compared with OB-AL mice. OB-WR TEE was decreased to ∼30% of maximal TEE during the late hours (0400–0600) of the lights-off period at 22°C (Fig. 5A), coinciding with a decline in core body temperature from ∼34°C to 26°C (Fig. 5C). Absolute TEE was also significantly lower in OB-WR mice at 30°C, except during some feeding periods (black arrows in Fig. 5B).
Fig. 5.
Energy expenditure and body temperature phenotypes of Lepob mice (experiment 2). Absolute nonadjusted group mean (± SE) hourly total energy expenditure measured every 26 min over a 48-h period for Lepob mice at 22°C (A) and at 30°C (B). Black arrows represent feeding times for A and B. C: mean 24-h core body temperatures (°C ± SE) measured every 3 h. Solid lines represent 22°C (phase 2) and dashed lines represent 30°C (phase 4) ambient temperatures. *Significantly lower body temperatures in WR vs. AL group at 30°C; P < 0.05. #Significantly lower body temperatures in WR vs. AL group at and 22°C; P < 0.05.
Effects of ambient room temperature and weight perturbation on 24-h body temperature.
When housed at 30°C, OB-WR mice had significantly lower core body temperatures than OB-AL at all time points except at 2000 (Fig. 5C). Interestingly, OB-WR mice maintained at 22°C ambient temperature showed decreased body temperature only at 0200 and 0500 compared with OB-AL mice housed at the same ambient temperature. However, the decreases (≈10°C torpor) in body temperature of OB-AL vs. OB-WR at these two time points were much greater than those observed when these mice were maintained at 30°C (≈ 3°C) (Fig. 5C). Meanwhile, body temperatures of the OB-AL and OB-WR were identical near feeding times (0800 and 2000) at either ambient temperature.
DISCUSSION
The studies reported here were designed to compare metabolic changes in WT and Lepob weight-reduced mice at two different ambient temperatures, either at subthermoneutrality (22°C), representing a constant thermal stress, or at thermoneutrality (30°C). We previously reported that maintenance of a 20% reduction in body weight in diet-induced obese and never-obese C57BL/6J mice resulted in decreased energy expenditure per unit of metabolic mass (21). That study was conducted at 22°C ambient temperature, and core body temperatures were ascertained only at one time of day (1000), at which time body temperatures were slightly lower in weight-reduced animals. In the current study, we show that the “absolute” decreases in energy expenditure observed in weight-reduced WT mice are similar at both 22°C and 30°C and that core body temperature does not fall below 35°C in either circumstance. These findings preclude torpor as a possible contributor to the increase in metabolic efficiency that characterizes these animals. Furthermore, we show that the “relative” reduction in energy expenditure in the HFD-WR (vs. AL) at 30°C is nearly twice that observed at 22°C (−19% and −10%, respectively). This nearly twofold difference in relative energy expenditure reflects the fact that at 22°C, maintenance of body temperature accounts for about half of total energy expenditure, partially masking the metabolic consequences of weight reduction. Many metabolic studies in mice attempt to quantify relatively small changes in energy expenditure, changes that—as demonstrated here—may be difficult to detect in mice studied at subthermoneutrality (e.g., 22°C). Furthermore, the torpor observed in the weight-reduced Lepob mice at 22°C (OB-WR) demonstrates the requirement of an intact leptin axis to prevent torpor onset. Bioenergetic studies in rodents with possible impairments of control of body temperature should consider torpor as a possible contributor to apparent changes in metabolic efficiency. Finally, we show that the decreased energy expenditure (i.e., increased metabolic efficiency) observed in weight-reduced mice is completely resolved once mice are given free access to HFD.
Even small differences between energy intake and energy expenditure, if sustained over time, can result in substantial changes in body weight and composition. Many rodent studies aim to quantify the relative contributions of a drug, genetic alteration, or behavioral modification on components of energy expenditure. Subtle, but important, differences may be missed if the animals are in environments that do not minimize the effects of thermal stress (4, 20). This study confirms the striking effects that ambient room temperature has on energy expenditure (20). WT mice housed at 30°C require 40–45% fewer calories to maintain a stable body weight than WT mice housed at 22°C (Table 2 and Fig. 2A). The effects of ambient temperature on energy expenditure and responses to weight perturbation in animals with genetic disruption of the leptin axis are even more striking. Manipulation of genes influencing energy intake and/or expenditure constitutes a major strategy for deconvoluting the molecular bases for the control of body weight. Failure to take account of the effects of ambient temperature on these phenotypes could lead to incorrect conclusions regarding the nature and magnitude of the effects under study. For example, compared with the HFD-AL mice, the 1.6 kcal/day reduction in total energy expenditure in HFD-WR mice housed at 30°C constitutes a 19% decrease of 24 h TEE, whereas the 1.4 kcal/day reduction in energy expenditure at 22°C constitutes a much smaller (9.6%) proportional decrease (Fig. 2A). The similarity of absolute decreases in the energy expenditure of HFD-WR mice maintained at 22° and 30°C ambient temperatures suggests that the metabolic adaptation to reduction in reduced body weight is dictated by the change in body mass and composition per se and is not related to differences in thermogenic demand and/or torpid behavior. However, the “relative” magnitude of such adjustments is diminished in WR animals at 22°C ambient temperature due to the higher rates of energy expenditure required to maintain body temperature. Torpor is not invoked—at either 22°C (subthermoneutral) or 30°C (thermoneutral) ambient temperatures—in metabolic adaptations of calorically restricted WT mice (HFD-WR) with a functionally intact leptin axis. Although not examined in the present study, we would anticipate a linear decrease in absolute TEE as the ambient temperature is increased from 22°C to 30°C (4). Meanwhile, the decreased energy expenditure observed in the HFD-WR mice (Fig. 2, A–C) would be similar at different ambient temperatures in absolute terms (i.e., residuals) but would increase in relative terms (i.e., residuals/TEE at that temperature) as the temperature increased toward the thermoneutral zone (i.e., 30°C).
Ambient temperature had no appreciable effect on circulating glucose and insulin concentrations (and by extension HOMA% S and HOMA IR measurements), indicating that glucose homeostasis parameters are determined primarily by body composition/mass and feeding behavior. Serum T3 concentrations were significantly increased at 22°C in all groups (Table 3). Increased T3 induces brown fat thermogenesis, and total T3 concentrations correlate with energy expenditure (3). Reduced circulating T3 concentrations probably play a role in the reduced energy expenditure that characterizes the weight-reduced state in animals (22) and humans (24). Although T3 concentrations were reduced in HFD-WR compared with HFD-AL at both ambient temperatures, the declines reached significance only at 22°C.
Weight-reduced mice with congenital leptin deficiency (OB-WR) display a 3°C decrease in core body temperature between 0200 and 0500 (lights off from 1900 to 0700) at 30°C, whereas they become torpid (core body temperature < 30°C) when housed at 22°C ambient (Fig. 5C). These results confirm previously published data showing that food-restricted Lepob mice are prone to entering torpor when housed at subthermoneutrality (9). Circulating leptin concentrations in both humans and rodents are highest from 0000 to 0600, the period when the OB-WR mice enter torpor at 22°C ambient temperature (3, 13, 14). Whether this normal diurnal rhythm of leptin plays a role in torpor inhibition and/or mouse temperature maintenance during these hours under normal circumstances is unknown, but most torpor observed in food-restricted mice occurs in this time period (i.e., 0200–0600) (6, 9). Furthermore, if core temperatures had been measured only at the morning feeding, torpor would not have been detected; a few hours prior to that time period, all OB-WR were torpid (Fig. 5C), suggesting that in the OB-WR mice, the lack of leptin coupled with the intermittent access to food may have synergistically triggered the onset of torpor. The rise in leptin concentrations expected from 0200 to 0600 in WT mice may protect the HFD-WR mice from torpor onset. If REE is defined as the lowest 1 h of TEE in an awake postdigestive nontorpid mouse, OB-WR mice have an REE of ∼10 kcal/24 h/mouse at 22°C ambient (Fig. 5A) and ∼5 kcal/24 h at 30°C (Fig. 5B) between 1100 and 1600. The twofold higher REE observed in OB-WR mice housed at 22°C vs. OB-WR at 30°C reflects the increased energy requirements for maintenance of body temperature at the lower ambient temperatures. Interestingly, TEE in OB-WR mice housed at 22°C while torpid (between 0200 and 0500) is similar to that in OB-WR animals housed at 30°C (≈5 kcal/24 h) (Fig. 5, A and B). The similarity of TEE within the OB-WR mice at 22°C vs. 30°C between 0200 and 0500 suggests that in the absence of a functional leptin axis, the maintenance of a reduced body weight at subthermoneutral temperature results in decreased metabolic rate and a decline in core body temperature likely resulting from a complete disengagement of thermogenic mechanisms. The OB-WR mice are reducing/disengaging adaptive thermogenesis at an ambient temperature that constitutes a constant thermogenic stress (i.e., 22°C). In contrast, the WT HFD-WR mice with intact leptin axis are capable of maintaining near-normal body temperatures at both 30° and 22°C ambient temperatures, which is reflected in the twofold higher TEE at the latter ambient temperature (i.e., 22°C) (Fig. 4).
We also examined whether the decreased EE observed in HFD-WR mice would be resolved if these animals were provided ad libitum access to the HFD. As a control, we assessed the effects of such a feeding regimen (switch to a HFD) on the never-obese LFD-AL mice. When nonobese control-diet fed mice (LFD-AL) and formerly obese weight-reduced mice (HFD-WR) were given ad libitum access to the HFD (both conducted at 22°C), both groups ingested significantly more calories, despite lower body weights, than the obese HFD-AL for the first 7 consecutive days (Fig. 3B: see arrow in Fig. 1B). This increased caloric intake per unit body mass has been well documented and resulted in rapid gains of body weight in both groups, probably reflecting hedonic drive to eat the more palatable high-fat diet once given ad libitum access (19). Although feed efficiency on a 24-h basis was not significantly higher in the HFD-WR and LFD-AL mice after day 7, mean feed efficiency during the subsequent 16-day period (day 7 to 23; Fig. 1B) was >2.5-fold higher in HFD-WR (1.6 g/kcal) and >3-fold higher in LFD-AL (1.9 g/kcal) than HFD-AL (0.6 g/kcal) underlying the rapid increase in body weight in the two diet-switched groups. The higher feed efficiency observed in HFD-WR vs. LFD-AL mice on the first day most likely reflects combined effects of 1) increased metabolic efficiency; and 2) lower initial day 0 body weight of the HFD-WR mice, since they would have been without food in the gut as opposed to the LFD-AL, which had ad libitum food access. LFD-AL would, therefore, have had some residual weight from nondigested food in the digestive tract as opposed to none in the HFD-WR.
Perspectives and Significance
Obesity results from persistent, relatively small positive imbalances of energy intake over expenditure. To the extent that rodent studies are intended to illuminate relevant molecular physiology in humans, metabolic stress resulting from housing of animals in nonthermoneutral environments may mask subtle but important bioenergetic phenotypes. For example, modest decreases in energy expenditure disproportionate to metabolic mass following weight-reduction contribute to subsequent weight regain (15). In the experiments reported here, we show that these effects are less apparent in animals housed in a subthermoneutral (22°C) than a thermoneutral (30°C) environment. The increased metabolic demand imposed by thermoregulation in mice studied at 22°C reduces the fractional decline (−10%) in energy expenditure of weight-reduced animals compared with animals studied at 30°C ambient (−19%). This difference is physiologically significant and constitutes an important potential confounding factor in studies of body weight homeostasis using rodents. These physiological considerations are, of course, also relevant to metabolic studies conducted in other species, including humans. In addition to careful consideration of ambient temperature in the design and analysis of bioenergetic studies in mice, a recent review highlights the impact of housing at subthermoneutral ambient temperatures on immune response (increased glucocorticoid production and immunosuppression), lipid homeostasis (upregulation of plasma clearance of triglyceride-rich lipoproteins via brown adipose tissue, compromising the utility of such animals as models for atherosclerosis), and neurobiology (activation of sympathetic nervous system) (13). The technical means exist to adjust ambient temperature without the need to alter temperature in an entire room.
GRANTS
This work was supported by National Institutes of Health Grants RO1-DK-066518, , R01 DK064773, P30-DK-26687, ADA-1–08-RA-36, Japan Society for the Promotion of Science, Manpei Suzuki Diabetes Foundation, and a research grant from AstraZeneca.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.R., C.A.L., and R.L.L. conception and design of research; Y.R., C.A.L., and K.W. performed experiments; Y.R. and C.A.L. analyzed data; Y.R., C.A.L., and R.L.L. interpreted results of experiments; Y.R. prepared figures; Y.R. drafted manuscript; Y.R., C.A.L., and R.L.L. edited and revised manuscript; Y.R., C.A.L., K.W., and R.L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the Vanderbilt University Hormone Assay and Analytical Services Core for performance of serum T3 assay.
REFERENCES
- 1. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250– 252, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30: 1322– 1331, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bianco AC, Maia AL, da Silva WS, Christoffolete MA. Adaptive activation of thyroid hormone and energy expenditure. Biosci Rep 25: 191– 208, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214: 242– 253, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Castillo M, Hall JA, Correa-Medina M, Ueta C, Won Kang H, Cohen DE, Bianco AC. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 60: 1082– 1089, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int J Obes Relat Metab Disord 22: 83– 88, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90– 94, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203– 209, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, Vinson C, Reitman ML. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci USA 96: 14623– 14628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geiser F, Kortner G, Schmidt I. Leptin increases energy expenditure of a marsupial by inhibition of daily torpor. Am J Physiol Regul Integr Comp Physiol 275: R1627– R1632, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Halldorsdottir S, Carmody J, Boozer C, LeDuc CA, Leibel RL. Reproducibility and accuracy of body composition assessments in mice by dual energy X-ray absorptiometry and time domain nuclear magnetic resonance. Int. J. Body Comp. Res. 7: 147– 154, 2009 [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657– 1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209: 1069– 1074, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 32 Suppl 7: S32– S38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32 Suppl 7: S98– S108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621– 628, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21: 2191– 2192, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab 9: 111– 112, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Manabe Y, Matsumura S, Fushiki T. Preference for High-Fat Food in Animals. In: Fat Detection: Taste, Texture, and Post-Ingestive Effects. edited by Montmayeur JP, le Coutre J. Boca Raton, FL: CRC Press, 2010 [Google Scholar]
- 20. Overton JM. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 34 Suppl 2: S53– S58, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Ravussin Y, Gutman R, Diano S, Shanabrough M, Borok E, Sarman B, Lehmann A, LeDuc CA, Rosenbaum M, Horvath TL, Leibel RL. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am J Physiol Regul Integr Comp Physiol 300: R1352– R1362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravussin Y, Gutman R, Diano S, Shanabrough M, Borok E, Sarman B, Lehmann A, Leduc CA, Rosenbaum M, Horvath TL, Leibel RL. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am J Physiol Regul Integr Comp Physiol 300: R1352– R1362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 34, Suppl 1: S47– S55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87: 2391– 2394, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 82: 3647– 3654, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Swoap SJ. The pharmacology and molecular mechanisms underlying temperature regulation and torpor. Biochem Pharmacol 76: 817– 824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294: H1581– H1588, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Szymusiak R, Satinoff E. Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav 26: 687– 690, 1981 [DOI] [PubMed] [Google Scholar]
- 29. Trayhurn P, James WP. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflügers Arch 373: 189– 193, 1978 [DOI] [PubMed] [Google Scholar]
- 30. Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Muller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57– 63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 147: 2468– 2480, 2006 [DOI] [PubMed] [Google Scholar]