Abstract
Myogenic tone (MT) is a primary modulator of blood flow in the resistance vasculature of the brain, kidney, skeletal muscle, and perhaps in other high-flow organs such as the pregnant uterus. MT is known to be regulated by endothelium-derived factors, including products of the nitric oxide synthase (NOS) and/or the cyclooxygenase (COX) pathways. We asked whether pregnancy influenced MT in myometrial arteries (MA), and if so, whether such an effect could be attributed to alterations in NOS and/or COX. MA (200–300 μm internal diameter, 2–3 mm length) were isolated from 10 nonpregnant and 12 pregnant women undergoing elective hysterectomy or cesarean section, respectively. In the absence of NOS and/or COX inhibition, pregnancy was associated with increased MT in endothelium-intact MA compared with MA from nonpregnant women (P < 0.01). The increase in MT was not due to increased Ca2+ entry via voltage-dependent channels since both groups of MA exhibited similar levels of constriction when exposed to 50 mM KCl. NOS inhibition (Nω-nitro-l-arginine methyl ester, l-NAME) or combined NOS/COX inhibition (l-NAME/indomethacin) increased MT in MA from pregnant women (P = 0.001 and P = 0.042, respectively) but was without effect in arteries from nonpregnant women. Indomethacin alone was without effect on MT in MA from either nonpregnant or pregnant women. We concluded that MT increases in MA during human pregnancy and that this effect was partially opposed by enhanced NOS activity.
Keywords: fetal growth restriction, human myometrium, pressure-induced constriction, preeclampsia, uteroplacental blood flow
human pregnancy is associated with significant cardiovascular changes to meet the metabolic demands of the placenta and fetus as well as the pregnant woman. Heart rate and stroke volume increase ∼35% and ∼15%, respectively, plasma volume expands ∼40%, and unilateral uterine artery blood flow rises as much as 10-fold from ∼34 ml/min in nonpregnant women to ∼332 ml/min at term pregnancy (15, 16, 36). These changes are accompanied by a pronounced reduction in maternal peripheral vascular resistance that results in a modest lowering of blood pressure near term in healthy pregnancies (5). Despite these dramatic changes in cardiovascular function, little is known in women about how pregnancy affects the intrauterine vasculature and, in particular, how it responds to changes in pressure and workload. A vessel of particular interest is the myometrial artery (MA) because it is a principal site of uterine vascular resistance and possibly endothelial dysfunction in pregnancies complicated by preeclampsia and hence may contribute to the regulation of maternal uteroplacental blood flow (21, 33, 34).
One mechanism by which the body regulates blood flow to end organs is by pressure-induced constriction, also known as myogenic tone (MT). MT was first described in the canine carotid artery by Bayliss in 1902 (4) and has been frequently observed in resistance-sized arteries (≤300 μm) that exist in a partially constricted state from which they can constrict or dilate to meet the metabolic demands and/or perfusion requirements of their end organs. In the uterine circulation, MT has been observed in main uterine arteries in nonpregnant mice and second-/third-order uterine arteries from nonpregnant sheep, but this MT is attenuated as pregnancy progresses (51, 52). In contrast, second-order radial uterine arteries do not exhibit MT in nonpregnant rats, but MT is present as pregnancy progresses to term (11, 50). To our knowledge, there are no studies of MT in uterine arteries from nonpregnant women, and most studies in pregnant women are limited by the use of a single physiological intraluminal pressure (80 mmHg) (21) versus the broad pressure range used in other species (11, 51, 52). Therefore, the effect of pregnancy on MT in human uterine vessels is unclear.
Increased uteroplacental blood flow during pregnancy is associated with augmented endothelium-derived vasodilator production in experimental animals (11, 12, 18, 25, 47, 51) and women (8, 45, 49), with much of the emphasis placed on the role of increased synthesis of endothelium-derived nitric oxide (NO) and/or its downstream signaling. Inhibitors of NO synthase (NOS) augment MT in uterine vessels from experimental animals (11, 13, 51) and in MA from pregnant women (19–21), but less is known about the contribution of vascular cyclooxygenase (COX) products, which are also upregulated in pregnancy (14, 19). Furthermore, MA MT and its regulation by NOS and/or COX in nonpregnant women have yet to be studied.
The objectives of this study were to determine 1) whether pregnancy alters MT in human MA, and if so, 2) whether alterations in NOS/COX signaling are involved. To assess MT across the full range of blood pressures, all experiments were performed at intraluminal pressures ranging from 10 to 100 mmHg.
MATERIALS AND METHODS
All experimental protocols were performed in accordance with NIH guidelines for the use of human tissue and followed procedures approved by the Human Research Protection Program at Wake Forest University School of Medicine for obtaining informed consent.
Subjects.
Twelve healthy pregnant women undergoing elective cesarean section and 10 nonpregnant women undergoing elective hysterectomy for benign disorders (see Table 1) were included in the present study. We excluded those women with preexisting cardiovascular disease (e.g., hypertension, diabetes), a history of current smoking, and those with multiple births. The nonpregnant women included were the following: 1) premenopausal, 2) undergoing an elective hysterectomy for a benign disorder later confirmed by pathology report (i.e., fibroids, menorrhagia, uterine prolapsed, ovarian teratoma, and/or uterine leiomyomata), and 3) without the above cardiovascular risk parameters. The primary anesthetics or analgesics used in the pregnant women were bupicicaine, fentanyl, and duramorph. Patients undergoing hysterectomies received general anesthesia with isoflurane or desflurane.
Table 1.
Comparison of nonpregnant and pregnant patient characteristics
| Nonpregnant | Pregnant | PValue | |
|---|---|---|---|
| Total number of patients | 10 | 12 | |
| Age (yr) or range (in parentheses) | 38.5 ± 1.8 (28 to 48) | 29.5 ± 1.6 (21 to 42) | P < 0.001 |
| African American | 3 | 4 | NS |
| Caucasian | 7 | 8 | |
| Heart rate, beats/min | 80.6 ± 3.5 | 92.6 ± 3.5 | P = 0.027 |
| Systolic BP, mmHg | 125 ± 6.7 | 128 ± 2.9 | NS |
| Diastolic BP, mmHg | 75.4 ± 3.7 | 75.9 ± 2.9 | NS |
| Height, in | 64.7 ± 0.7 | 64.1 ± 1.0 | NS |
| Weight at time of hysterectomy or prepregnancy, lbs | 197 ± 16.9 | 184 ± 14.5 | NS |
| BMI at time of hysterectomy or prepregnancy | 32.9 ± 2.6 | 31.8 ± 2.9 | NS |
| Weight at time of hysterectomy or C/S, lbs | 197 ± 16.9 | 208 ± 16.1 | NS |
| Gestational age, week | N/A | 39.0 ± 0.2 | |
| Birth weight, g | N/A | 3585 ± 61.2 | |
| Gravidity (no. pregnancies) | 3.2 ± 0.4 | 2.9 ± 0.3 | NS |
| Parity (no. live births) | 2.5 ± 0.3 | 2.3 ± 0.2 | NS |
Values are means ± SE. BP, blood pressure; BMI, body mass index; C/S, cesarean section. P values based on two-sample Student's t-tests for continuous data and Fisher's Exact test for proportions.
Preparation of myometrial arteries.
Full-thickness 1 cm wide × 1 cm deep biopsies were obtained at time of cesarean section from the upper lip of the uterine incision away from the placenta to prevent unwanted hemorrhage. Fundal placentation was observed in all participants. Tissue collection at the time of hysterectomy was from the anterior portion of the uterus, which closely approximated the region collected from pregnant women. These methods for tissue collection were similar to those described by others (17, 19–21). The tissue was immediately placed in cooled physiological saline solution [PSS composed of (in mmol/l): 118 NaCl, 4.5 KCl, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 11 dextrose, 2.5 CaCl2, and 0.26 EDTA at pH 7.4] and brought to the laboratory for isolation of MA.
All tissue samples were maintained in 4°C PSS and aerated with gas containing 21% O2, 74% N2, and 5% CO2 while MA segments were dissected from the outer third (maternal side) of the uterine biopsy to isolate similar-sized vessels (200 to 300 μm internal diameter, 2 to 3 mm length). Arterial segments were transferred to a dissection chamber where they were allowed to equilibrate for 6–8 h in oxygenated Krebs maintained at 3°C to approximate the same timing interval for all studies. All studies were completed within 24 h of receiving the tissue from the operating room. We observed no differences in the control pressure-response relationship in arteries studied in the first 12 h versus the last 12 h following tissue collection (pregnant, P = 0.356; nonpregnant, P = 0.622). After equilibration, vessels were placed into the well of a 2-ml arteriograph (Instrumentation and Model Facility, University of Vermont, Burlington, VT) fitted with a borosilicate (FHC, Bowdoinham, ME) micropipette at both ends. Both the proximal and distal micropipettes were attached to three-way Luer Stopcocks (Cole-Parmer, Vernon Hills, IL). The arteries were cannulated on the proximal pipette, secured with suture, gently flushed free of blood, and then cannulated and secured on the distal pipette. The arteriograph containing the cannulated vessel was then transferred to the stage of an inverted microscope (Nikon) where the internal diameter of the artery was viewed, measured, and continuously recorded using video edge detection equipment and data acquisition software, respectively (IonOptix, Milton, MA). After the arteriograph was placed on the microscope stage, the proximal cannula was attached to the pressure reservoir, and the intraluminal pressure increased to 10 mmHg to allow a gentle continuous flushing of the intraluminal contents from the vessel for 2 min. The three-way stopcock on the distal cannula was then closed to pressurize the artery; all experimental protocols were conducted under “no-flow” conditions. In all experiments, pressurized arteries were continuously superfused with PSS (8–10 ml/min) aerated with gas containing 21% O2, 74% N2, and 5% CO2 at 37°C.
Study protocol.
All experiments were conducted in a manner similar to that previously described (7). Briefly, the cannulated arteries were initially pressurized to 20 mmHg and allowed to equilibrate for 20 min at 37°C. The intraluminal pressure was then increased to 60 mmHg for ∼90 min during which the vessel was challenged with 50 mM KCl three times to confirm artery viability and assess maximal constriction, defined as smallest diameter recorded over a 2-min period in the presence of 50 mM KCl. Vessels that did not constrict to at least 30% of their passive diameter after exposure to 50 mM KCl were discarded. The constriction responses to 50 mM KCl for the MA from nonpregnant and pregnant women are shown in Table 2. Endothelial integrity was assessed in vessels using the endothelium-dependent vasodilator bradykinin (BK, 1 μM). Nonpregnant MA were pressurized at 60 mmHg and, because of the lack of MT, preconstricted with phenylephrine (PE, 10–100 μM) before dilation with BK. MA from pregnant women also were pressurized at 60 mmHg, and upon obtaining stable MT, they were challenged with BK to confirm endothelial function. PE-induced constriction and MT were similar in nonpregnant (n = 3) and pregnant (n = 5) MA, 40.4 ± 6.0% vs. 33.3 ± 4.9% maximal constriction (P = 0.4, t-test), respectively. BK reversal of PE-induced tone in nonpregnant MA or MT-induced tone in pregnant MA did not differ (92.9 ± 1.5% vs. 87.8 ± 3.8% reversal of constriction, P = 0.4 t-test, respectively). After smooth muscle viability was affirmed, vessels were returned to Krebs and intraluminal pressure was set to 10 mmHg. The effects of pressure-induced vasoconstriction (MT) were studied by generating two sequential pressure curves for each arterial segment at pressure steps from 10 to 100 mmHg intraluminal pressure such that each artery segment acted as its own control (a series experimental design). After the first curve was generated, the tissues were allowed to recover for 10 min at 10 mmHg pressure. The vessels were then repressurized to 60 mmHg (to confirm reproducible myogenic tone) and incubated for 30 min in either the nonspecific NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 200 μmol/l), the nonspecific COX inhibitor indomethacin (Indo; 10 μmol/l), or the two inhibitors together before starting the second pressure-diameter curve to determine the contribution of NOS-, COX-, or combined NOS/COX inhibition on MT in MA. Tissues were then returned to 10 mmHg, incubated in Ca2+-free PSS with a L-type Ca2+-channel inhibitor diltiazem (80 μmol/l) for 45 min, and followed by a third pressure-diameter relationship to obtain fully dilated (passive) diameters. MT was expressed as a percent constriction of the passive diameter of the individual MA at the same intravascular pressure using the equation:
where DP is the “passive” diameter of the artery in Ca2+-free PSS with diltiazem and DA is the “active” diameter of the artery in response to the stimulus (change in intraluminal pressure) in Ca2+-containing PSS in the absence/presence of l-NAME, Indo, or combined l-NAME/Indo.
Table 2.
Comparison of myometrial artery internal diameters in the absence and presence of 50 mM KCl-Krebs
| Nonpregnant (n = 10) | Pregnant (n = 10) | P Value | |
|---|---|---|---|
| Diameter | |||
| Resting diameter, μm | 213.5 ± 15.8 | 194.1 ± 17.9 | NS |
| 50 mM KCl diameter, μm | 114 ± 9.9 | 128.9 ± 17.5 | NS |
| Passive diameter, μm | 244.2 ± 15.1 | 239.1 ± 13.9 | NS |
| % Constriction | |||
| Constriction before 50 mM KCl | 12.8 ± 2.6 | 19.4 ± 4.5 | NS |
| Constriction with 50 mM KCl | 52.8 ± 3.8 | 45.4 ± 6.4 | NS |
Values are means ± SE. Tests for statistical significance were based on two-sample Student's t-tests for continuous data.
Chemicals.
All chemicals used for PSS were purchased from Fischer Scientific (Pittsburgh, PA), and all other chemicals were from Sigma Chemicals (St. Louis, MO). Drug stock solutions were prepared as follows: l-NAME (200 μmol/l) and Indo (10 μmol/l). Both phenylephrine (PE, 10 μM) and bradykinin (BK, 1 μM) were prepared daily from 1.0 mmol/l aliquots made in an aqueous stock solution.
Statistical analyses.
Data are expressed as means ± SE. In instances where multiple segments from a single MA were used for a particular experimental protocol, the results from all experiments were averaged for a single “n,” and hence, n values reported in the figures and tables indicate numbers of subjects, not vessels. Comparisons between pregnant and nonpregnant women for the continuous measures in Tables 1 and 2 and between two groups where indicated elsewhere were made using a two-sample (“Student's”) t-test. Dichotomous measures were compared using Fisher's exact test. All P values are based on two-sided comparisons. All analyses of MA MT were performed using analysis of variance (ANOVA) with repeated measures. The between-subject factor was pregnancy status (yes, no) and the within-subject factors were treated as 12 repeated measures in a 2 × 6 doubly repeated measures design for each woman's vessel, given that MT was measured in MA on 12 times [i.e., at 6 different pressures and with or without the drug(s) of interest]. An unstructured inhibitor-repeated factor and compound-symmetry pressure-repeated factor model provided the best fit for these measures. All repeated measures analyses were performed using SAS PROC MIXED procedures (SAS, Carey, NC). Two approaches were used to test whether the shape of the MT pressure curve differed by pregnancy or drug (L-NAME, Indo, diltiazem) status. The first was a global test of interaction with pressure treated as a six-category factor. Since this test has very low power to detect specific types of trends, a second approach was used in which pressure was treated as a continuous factor such that tests of interactions could determine whether the first factor affected the general upward or downward trend in MT or a quadratic curvature in the plots. If there was a significant pressure (categorized) by inhibitor interaction, then pairwise comparisons between groups at individual pressures were analyzed using Bonferroni adjustment for post hoc multiple comparisons. Comparisons between groups were considered statistically significant when the P < 0.05. Asterisks are placed above the symbols to designate comparisons that are significant at specific pressures and are located to the right of curves to designate significant overall main effects at P values of <0.05, 0.01, and 0.001, respectively.
RESULTS
Subject characteristics.
Nonpregnant women (n = 10) ranged from 28 to 48 yr of age and as a group were older than the pregnant women (n = 12), who ranged from 21 to 42 yr (Table 1). Eight of the 10 nonpregnant women fell within the same age range as the pregnant group (≤42 yr). The groups did not differ in blood pressure, height, weight, body mass index (BMI), or parity. Nonpregnant women underwent elective hysterectomy for benign uterine complications (see materials and methods). Women undergoing cesarean section fell within three groups: 10 of 12 were repeat cesarean section without complications, one underwent a repeat cesarean delivery due to shoulder dystocia, and the remaining was a first-time cesarean delivery due to maternal Herpes infection.
MT in MA.
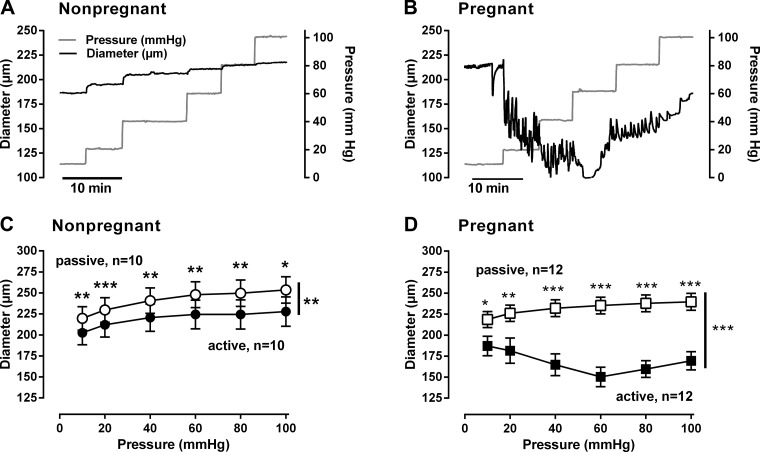
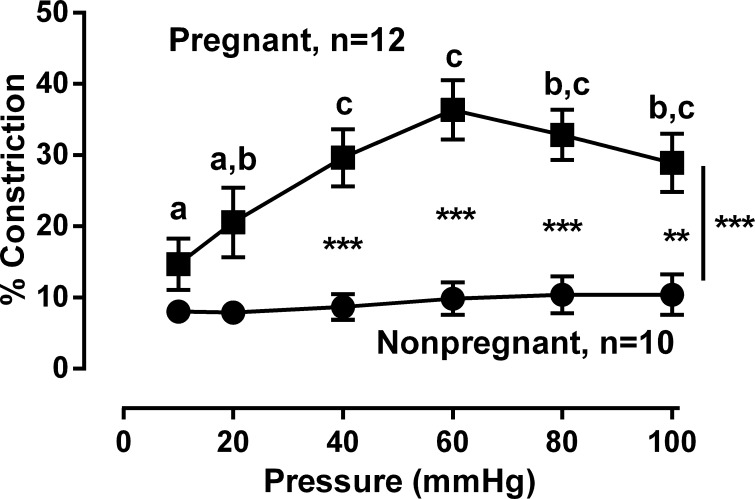
Representative responses to stepwise increases in intraluminal pressure from 10 to 100 mmHg in MA are illustrated in Figure 1, A and B. Although the passive diameters were greater than active diameters in both nonpregnant and pregnant MA (P < 0.001), those from pregnant women had smaller active diameters compared with nonpregnant (Fig. 1, C and D, P < 0.001). MT in pregnant MA was greater than in nonpregnant MA (overall P < 0.001), with the effect of pregnancy on MT being dependent on pressure (P < 0.001) and the largest differences present at pressures ≥40 mmHg (P < 0.001, Fig. 2). Comparisons within each group also showed that MT in pregnant MA at pressures ≥40 mmHg exceeded those observed at ≤20 mmHg (P < 0.05); there were no effects of pressure in the nonpregnant group (Fig. 2).
Fig. 1.
Relationship between intraluminal pressure and vascular diameter in myometrial arteries from nonpregnant and pregnant women. A and B: representative changes in vascular diameter (black line) observed with stepwise increases of intraluminal pressure (gray line) from 10 to 100 mmHg. C and D: summarized changes in diameter with increasing intraluminal pressures in Ca2+-containing Krebs (active) and Ca2+-free Krebs +40 μM diltiazem (passive) in arteries from nonpregnant (n = 10; ●, active and ○, passive) and pregnant (n = 12; ■, active and □, passive) women. Passive diameters were larger at all pressures (10 to 100 mmHg) than active diameters in arteries from both groups of women (**P < 0.01; ***P < 0.001). Data were analyzed by ANOVA doubly repeated measures where pregnancy status is the between-subjects factor and the active/passive and pressures are the two repeated factors.
Fig. 2.
Comparison of myogenic tone in myometrial arteries from pregnant vs. nonpregnant women. Myogenic tone in arteries from pregnant women (■, n = 12) was greater than in arteries from nonpregnant women (●, n = 10) across all values of intraluminal pressures (P < 0.001) and specifically at ≥40 mmHg (***P < 0.001 **P < 0.01). Letters signify values that differ in the MA from pregnant women at the designated pressures at P < 0.05. Data were analyzed by ANOVA repeated measures where pregnancy status was the between-subjects factor and pressure was the repeated factor.
KCl constriction.
MT is closely linked to membrane depolarization, resulting in Ca2+ entry via voltage-dependent Ca2+ channels. Thus our next series of experiments were designed to assess whether membrane depolarization using 50 mM KCl was different in MA from nonpregnant and pregnant women. Increasing extracellular KCl from 5 to 50 mM resulted in similar decreases in arterial diameter in arteries from both groups, indicating no differences in response to membrane depolarization (Table 2).
Contribution of NO to pressure-induced constriction.
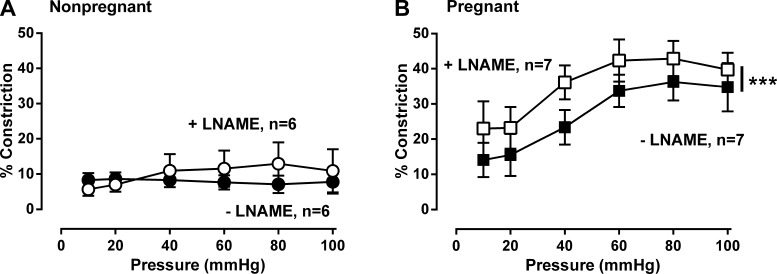
To assess the role of NO, we compared the effects of NOS inhibition on MT in MA from nonpregnant and pregnant women. NOS inhibition with l-NAME (200 μM) did not affect MT in nonpregnant MA (P = 0.8, Fig. 3A). In pregnant women, MT rose with increasing pressure in a quadratic fashion, but MT was greater in the presence than the absence of l-NAME (P < 0.001, Fig. 3B). Therefore, NOS inhibition augmented MT in pregnant arteries and acted in an additive manner to increase MT overall by 8.3 ± 3.2%.
Fig. 3.
Effect of the nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 200 μM) on myogenic tone in myometrial arteries from nonpregnant and pregnant women. l-NAME had no effect on myogenic tone in nonpregnant women (A: ●, −l-NAME vs. ○, +l-NAME; n = 6, P = 0.68,). In contrast, l-NAME augmented myogenic tone in pregnant women (B: ■, −l-NAME vs. □, +l-NAME; n = 7, P < 0.001). Data were analyzed by ANOVA doubly repeated measures analysis where pregnancy status is the between-subjects factor and absence or presence of l-NAME and pressure are the within-subject repeated factors. ***P < 0.001.
Contribution of COX to pressure-induced constriction.
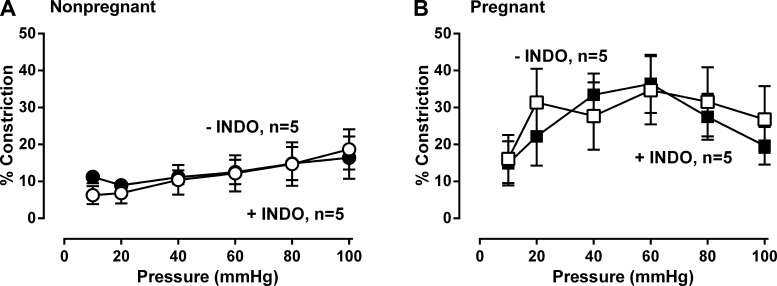
To determine the role of vascular prostaglandins, we treated the MA from nonpregnant and pregnant women with the COX inhibitor Indo (10 μM). Indo alone did not affect MT in MA from either nonpregnant (P = 0.26, Fig. 4A) or pregnant women (P = 0.26, Fig. 4B).
Fig. 4.
Effect of the cyclooxygenase (COX) inhibitor indomethacin (Indo, 10 μM) on myogenic tone in myometrial arteries from nonpregnant and pregnant women. Indo had no effect on myogenic tone in nonpregnant (A: ●, −Indo vs. ○, +INDO; n = 5, P = 0.66,) and pregnant women (B: ■, −Indo vs. □, +Indo; n = 5, P = 0.25). Data were analyzed by ANOVA doubly repeated measures analysis where pregnancy status is the between-subjects factor and the absence or presence of INDO and pressure are the within-subject repeated factors.
Effect of combined NOS and COX inhibition on pressure-induced constriction.
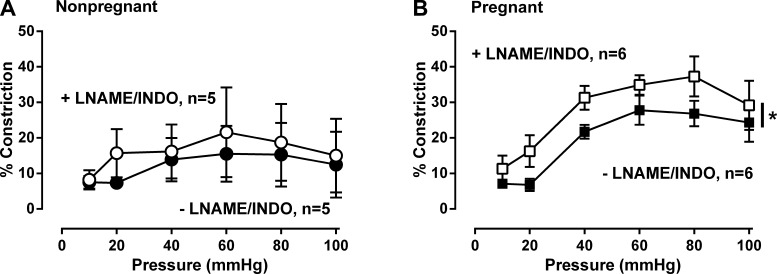
Because of potential redundancy in endothelium-dependent signaling, we also asked if combined NOS/COX inhibition modified the pressure-dependent changes in MA. As with l-NAME alone (Fig. 3A), combined NOS-COX inhibition did not have affect MT (P = 0.28) or have any interactive affect with pressure (P = 0.80) in MA from nonpregnant women (Fig. 5A). In the pregnant women, MA showed a significant quadratic relationship with pressure (P < 0.001, Fig. 5B), which was augmented 7.6 ± 2.8% by the presence of combined l-NAME/Indo (P < 0.04, Fig. 5B). The effect of combined l-NAME/Indo did not differ from that observed by l-NAME alone (7.6% vs. 8.3%, P = NS).
Fig. 5.
Effect of combined NOS (l-NAME, 200 μM) and COX (Indo, 10 μM) inhibition on myogenic tone in myometrial arteries from nonpregnant and pregnant women. Combined NOS/COX inhibition with l-NAME/Indo augmented myogenic tone in nonpregnant (A: ●, −l-NAME/Indo vs. ○, +l-NAME/Indo; n = 5, NS) and pregnant women (B: ■, −l-NAME/Indo vs. □, +l-NAME/Indo n = 6, P = 0.040). Data were analyzed by ANOVA doubly repeated measures analysis where pregnancy status is the between-subjects factor and placebo/inhibitor and pressure are the within-subject repeated factors (*P < 0.05).
DISCUSSION
Increasing evidence suggests that MT contributes to the regulatory mechanisms that control uteroplacental blood flow in pregnancy, but the extent of this contribution remains unclear (6, 11, 13, 19, 50). To the best of our knowledge, this is the first study to examine and compare MT in MA from nonpregnant and pregnant women. We observed that arteries from pregnant but not nonpregnant women demonstrated robust pressure-induced constriction and thus evidence of myogenic responses. The attenuated MT in arteries from nonpregnant women was not due to an inability to vasoconstrict since 50 mM KCl elicited similar vasoconstriction responses in arteries from both nonpregnant and pregnant women. Likewise, the attenuated MT response in nonpregnant arteries could not be attributed to greater NOS and/or COX activity because their inhibition, alone or in combination, did not alter MT. NOS inhibition enhanced MT in pregnant MA, consistent with the enhanced NOS activation described in myometrial and uterine arteries during pregnancy (11, 13, 19, 20, 51). In contrast, COX inhibition was without effect on MT in MA from pregnant women either when Indo was administered alone or combined with NOS inhibition. This suggests that COX inhibition has little influence on MT in MA from pregnant women. Thus we concluded that myogenic responses are present in MA from pregnant but not nonpregnant women and suggest that MT may contribute to the regulation of uteroplacental blood flow in pregnancy.
A major strength of our study was our ability to determine whether pregnancy altered MT in relevant human uterine arteries, overcoming obstacles faced in studies using animal models (11, 13, 26, 51, 52). The distinctive nature of human pregnancy stemming from the nature of human trophoblast invasion, vascular remodeling, placental architecture, and bipedal posture argues in favor of studying human rather than experimental animal tissues. We studied MA from the outer third of the uterine wall in pregnant women so as to be sampling from the same vascular region and to be studying similarly sized arteries in the nonpregnant and pregnant groups. In addition, we assessed alterations in MT across the full range of pressures (10 to 100 mmHg) to encompass both normal and pathophysiological pressures and examined the contributions of NOS and COX in a similar manner using well-established inhibitors separately and in combination. Although the nonpregnant group was 9 yr older than the pregnant women, the age range was similar in the two groups and eight of the 10 nonpregnant women were within the same age range. The uterine vascular bed is progressively remodeled throughout pregnancy in all species [see review by Osol and Mandala (35)], which results in a redistribution of blood flow within the pregnant uterus from the myoendometrial tissues to the uteroplacental vascular bed (i.e., spiral arterioles, intervillous space) (5, 15, 16, 36). Although the spiral arterioles contribute to the regulation of uteroplacental perfusion (27, 31, 37, 38), they are difficult to obtain and study and undergo remodeling that, by definition, is not present in nonpregnant vessels. Therefore, we studied MA of similar size from nonpregnant and pregnant women. We consider that these arteries may play one or more of three roles: 1) be responsible for myoendometrial blood flow, 2) be partially responsible for blood flow through the distal spiral arterioles, and/or 3) be representative of the downstream spiral arterioles. It is unclear which is correct, but any or all of these possibilities is (are) likely to contribute to the regulation of uteroplacental blood flow. Furthermore, MT is evident in pregnant MA across the full range of pressures (10 to 100 mmHg), which encompasses normal and pathological perfusion pressures, thus extending the observations of Kublickiene et al. (20), who focused on flow-dependent dilation in human MA and in that report, MT was therefore studied only at a single intraluminal pressure following norepinephrine preconstruction as required to assess flow-dependent dilation (19, 21, 22).
Although MT has been observed in MA from pregnant women (19–22, 48) and various generations of nonpregnant and pregnant UA from other species (11, 51, 52), it is unclear how MT is affected by human pregnancy (19–22). To the best of our knowledge, this is the first comparison of MT in nonpregnant and pregnant human MA. The observation that pregnancy raises MT in MA is somewhat unexpected, given the increased production of endothelium-dependent vasodilators (8, 11, 12, 19) that would be expected to decrease or attenuate MT. Furthermore, the shape of our pressure-response curves in pregnant MA differed from that observed by Kublickiene et al. (20) in a study in which MT was examined across a 20- to 120-mmHg pressure range. That study found that MT was greatest at lower intraluminal pressures (i.e., 20 mmHg) and decreased modestly as pressures rose to 100 mmHg (20), whereas our study found that MT increased from 10 to 60 mmHg and tapered off as pressures approached 100 mmHg. The reasons for the differences in the shape of the pressure dose-response curve are unclear. One possibility is that Kublickiene et al. (20) used a HEPES-based buffer while we used a non-HEPES buffer (PSS), since differences in vascular reactivity with different kinds of buffering solutions have been observed (1). A second reason may be the greater diameter of the MA studied by Kublickiene; for example, at 20 mmHg the vessels in that study averaged 295 ± 27 μm, whereas those in the present study averaged 229 ± 14 μm. Finally, Kublickiene and colleagues assessed myogenic tone in the absence and presence of NOS inhibition using a parallel experimental design (20), whereas our experiments were performed in series (e.g., pressure curves are obtained in the absence/presence of inhibitor in each artery studied). To ensure the myogenic response remained consistent throughout the experiment, we assessed pressures steps from 10 to 60 mmHg twice before control pressure curve, once at 60 mmHg for the control curve in the absence of the inhibitor, and finally just before obtaining the pressure curve in the presence of the inhibitor. We defend our approach on the basis that no difference was observed in MT at 60 mmHg across the four time points in the nonpregnant or pregnant MA (both P = NS).
From our observation that MT was attenuated in MA from nonpregnant women and enhanced in those from pregnant women, we sought to determine what mechanisms contributed to this pregnancy-associated change. Endothelium-derived NO is upregulated in the uterine vascular bed in the pregnancy of most species (8, 44, 46); thus it might contribute to the regulation of MT in MA in pregnancy (9, 19). Notably, NOS inhibition increased MT in MA from pregnant but not nonpregnant women; thus, NO appears to modulate myogenic responses in MA during pregnancy. This is consistent with prior observations in pregnant women (19, 22) and radial arteries from pregnant rats (13). They also are consistent with the vasodilating effects of increased NO during pregnancy in response, for example, to flow (8, 44). It is now evident that the increases in arterial NOS activity that occur in healthy pregnancy are also involved in modulating arterial MT. The contribution of NO-dependent signaling to MT in complicated pregnancies is not well understood. Kublickiene and colleagues (21) demonstrated that attenuated NO-dependent signaling may be responsible for decreased flow-induced vasodilation in MA from preeclamptic women, yet in these same studies MA from normal and preeclamptic women had similar levels of MT in the absence and presence of NOS inhibition (21). Since these were done at a single intraluminal pressure and MT was augmented with norepinephrine (21, 48), it is possible that the contribution of NO to MT may have been missed. Thus these studies should be repeated across the full pressure range to capture the relevant pressures present in normotensive and hypertensive states.
Having shown that local vascular NO modifies MT in pregnant human MA, we next sought to determine whether local prostaglandin synthesis might also play a role. It is known that prostaglandin synthesis and COX-1 are elevated in ovine uterine artery endothelium during pregnancy (14, 24). Interestingly, COX inhibition had no effect on MT or the pressure-induced increases in contractions in MA from pregnant and nonpregnant women; thus COX-dependent signaling in the uterine vasculature may differ between species or there is redundancy in endothelium-dependent signaling pathways. When we assessed myogenic reactivity in the presence of combined NOS/COX inhibition, MT in arteries from pregnant women was not different from that seen with NOS inhibition alone and was without effect in arteries from nonpregnant women. These results are similar to those previously obtained in MA from pregnant women (19, 22) and radial and uterine arteries from pregnant mice and rats (13, 51).
Myogenic responses in MA from pregnant women exceeded those observed in nonpregnant MA and appeared to be attenuated by increased NO but not vasodilator prostaglandin production in the pregnant but not the nonpregnant state. Thus NOS or COX-independent mechanisms are likely involved in increasing myogenic tone during human pregnancy. One possibility is that alterations in the production or activity of endothelium-derived hyperpolarizing factor are involved, as it appears to contribute to endothelium-dependent dilation in human MA (10, 17, 23) and whose responses are attenuated in pressurized (17) or wire mounted (23) MA from preeclamptic women, suggesting it contributes to overall MA reactivity in normal and pathological conditions. Alternatively, MA may undergo remodeling in more proximal uterine arteries and therefore express increasing amounts of contractile proteins (2, 3, 35).This is unlikely since responses to 50 mM KCl did not differ in arteries from nonpregnant and pregnant women. There also may be differences in the expression and/or function of vascular smooth muscle K+ channels that contribute to myogenic responses and have been identified in the uteroplacental circulation, e.g., Kv and BKCa (32, 39, 43, 50). These channels have been identified in women (43), sheep (37), and rat (41, 50) uterine arteries and shown to modify maternal uterine blood flow (18, 42), but their expression and function in human MA is not known. Whereas BKCa channels appear to modulate uterine vasodilation and vasoconstriction in women and sheep (39, 41, 43), downregulation and/or inactivation of Kv channels is associated with increased myogenic responses in small uterine arteries from pregnant rats (50). Although not studied, downregulation of BKCa channels could result in similar changes. Thus further studies of these channels in human MA and other uterine vessels are warranted.
Perspectives and Significance
Our current understanding of the mechanisms regulating human uteroplacental blood flow in pregnancy remains limited and is primarily derived from various animal models. We and others (19, 39) are now able to obtain and study human arteries from the uterine micro- and macrovasculature. Although endothelial factors such as NO and prostaglandins contribute to vascular regulation, increasing evidence suggests that downstream cyclic nucleotides are also essential (18, 32, 40, 41) and that various smooth muscle membrane channels (18, 43, 50) are of major importance and need additional study. Furthermore, it is now evident that myogenic responses associated with these pathways may contribute not only to the regulation of total uterine blood flow but also its redistribution during pregnancy, accounting for the development of a high resistance (myometrium and endometrium) and low resistance vascular bed on the maternal side of the placenta (28, 28–30). If this is indeed correct, the arteries demonstrating myogenic reactivity might also play an important role in the pathophysiology of preeclampsia and other hypertensive disorders in pregnancy by decreasing perfusion of the maternal placental vascular bed and thus the availability of nutrients required for normal fetal growth and well being.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-079647 (L. G. Moore), departmental funds from Brenner Children's Hospital (D. M. Eckman), and by the George L. MacGregor Professorship in Pediatrics (C. R. Rosenfeld).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M.E. and L.G.M. conception and design of research; D.M.E., R.G., and S.M.C. performed experiments; D.M.E., C.R.R., T.M.M., and S.M.C. analyzed data; D.M.E., R.G., C.R.R., T.M.M., S.M.C., H.M., and L.G.M. interpreted results of experiments; D.M.E. prepared figures; D.M.E., C.R.R., and L.G.M. drafted manuscript; D.M.E., R.G., C.R.R., T.M.M., S.M.C., H.M., and L.G.M. edited and revised manuscript; D.M.E., R.G., C.R.R., T.M.M., S.M.C., H.M., and L.G.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kristi Lanier and Melissa Swain for assistance identifying patients and collecting tissue from the operating room, along with Crystal Butler and Mary K. Brewer for technical assistance conducting experiments. In addition, we thank Drs. Michael O'Shea and Qing Yang for reading of the manuscript and providing editorial comments.
REFERENCES
- 1.Altura BM, Carella A, Altura BT. Adverse effects of Tris. HEPES and MOPS buffers on contractile responses of arterial and venous smooth muscle induced by prostaglandins. Prostaglandins Med 5: 123–130, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Annibale DJ, Rosenfeld CR, Kamm KE. Alterations in vascular smooth muscle contractility during ovine pregnancy. Am J Physiol Heart Circ Physiol 256: H1282–H1288, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am J Physiol Cell Physiol 259: C484–C489, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231, 1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54: 2056–2063, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Cox BE, Roy TA, Rosenfeld CR. Angiotensin II mediates uterine vasoconstriction through α-stimulation. Am J Physiol Heart Circ Physiol 287: H126–H134, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Eckman DM, Kerr BA, Fuloria M, Simandle SA, Watt SE, Rose JC, Figueroa JP. Antenatal betamethasone alters vascular reactivity in adult female ovine cerebral arteries. Pediatr Res 68: 344–348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faber-Swensson AP, O'Callaghan SP, Walters WA. Endothelial cell function enhancement in a late normal human pregnancy. Aust NZ J Obstet Gynaecol 44: 525–529, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Faxen M, Nisell H, Kublickiene KR. Altered mRNA expression of ecNOS and iNOS in myometrium and placenta from women with preeclampsia. Arch Gynecol Obstet 265: 45–50, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Gillham JC, Myers JE, Baker PN, Taggart MJ. Regulation of endothelial-dependent relaxation in human systemic arteries by SKCa and IKCa channels. Reprod Sci 14: 43–50, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Gokina NI, Kuzina OY, Fuller R, Osol G. Local uteroplacental influences are responsible for the induction of uterine artery myogenic tone during rat pregnancy. Reprod Sci 16: 1072–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol 189: 1489–1493, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Janowiak MA, Magness RR, Habermehl DA, Bird IM. Pregnancy increases ovine uterine artery endothelial cyclooxygenase-1 expression. Endocrinology 139: 765–771, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol 295: R906–R915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 296: R1564–R1575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 103: 67–73, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab 298: E222–E228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kublickiene KR, Cockell AP, Nisell H, Poston L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am J Obstet Gynecol 177: 1263–1269, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kublickiene KR, Kublickas M, Lindblom B, Lunell NO, Nisell H. A comparison of myogenic and endothelial properties of myometrial and omental resistance vessels in late pregnancy. Am J Obstet Gynecol 176: 560–566, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Kublickiene KR, Lindblom B, Kruger K, Nisell H. Preeclampsia: evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am J Obstet Gynecol 183: 160–166, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Kublickiene KR, Nisell H, Poston L, Kruger K, Lindblom B. Modulation of vascular tone by nitric oxide and endothelin 1 in myometrial resistance arteries from pregnant women at term. Am J Obstet Gynecol 182: 87–93, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Luksha L, Luksha N, Kublickas M, Nisell H, Kublickiene K. Diverse mechanisms of endothelium-derived hyperpolarizing factor-mediated dilatation in small myometrial arteries in normal human pregnancy and preeclampsia. Biol Reprod 2010 [DOI] [PubMed] [Google Scholar]
- 24.Magness RR, Osei-Boaten K, Mitchell MD, Rosenfeld CR. In vitro prostacyclin production by ovine uterine and systemic arteries. Effects of angiotensin II. J Clin Invest 76: 2206–2212, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am J Physiol Heart Circ Physiol 280: H1692–H1698, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Mateev SN, Mouser R, Young DA, Mecham RP, Moore LG. Chronic hypoxia augments uterine artery distensibility and alters the circumferential wall stress-strain relationship during pregnancy. J Appl Physiol 100: 1842–1850, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Misenhimer HR, Margulies SI, Panigel M, Ramsey EM, Donner MW. Effects of vasoconstrictive drugs on the placental circulation of the Rhesus monkey. A preliminary report. Invest Radiol 7: 496–499, 1972 [DOI] [PubMed] [Google Scholar]
- 28.Moll W, Kunzel W. Blood pressures in the uterine vascular system of anaesthetized pregnant guinea pigs. Pflügers Arch 330: 310–322, 1971 [DOI] [PubMed] [Google Scholar]
- 29.Moll W, Kunzel W. The blood pressure in arteries entering the placentae of guinea pigs, rats, rabbits, and sheep. Pflügers Arch 338: 125–131, 1973 [DOI] [PubMed] [Google Scholar]
- 30.Moll W, Kunzel W, Stolte LAM, Kleinhout J, de Jong P, Veth AFL. The blood pressure in the decidual part of the uteroplacental arteries (spiral arteries) of the Rhesus monkey. Pflügers Arch 346: 291–297, 1974 [Google Scholar]
- 31.Moll W, Wallenburg HC, Kastendieck E, Voslar M. The flow resistance of the spiral artery and the related intervillous space in the rhesus monkey placenta. Pflügers Arch 377: 225–228, 1978 [DOI] [PubMed] [Google Scholar]
- 32.Nagar D, Liu XT, Rosenfeld CR. Estrogen regulates β1-subunit expression in Ca2+-activated K+ channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol 289: H1417–H1427, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Ong SS, Baker PN, Mayhew TM, Dunn WR. Remodeling of myometrial radial arteries in preeclampsia. Am J Obstet Gynecol 192: 572–579, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Ong SS, Moore RJ, Warren AY, Crocker IP, Fulford J, Tyler DJ, Gowland PA, Baker PN. Myometrial and placental artery reactivity alone cannot explain reduced placental perfusion in pre-eclampsia and intrauterine growth restriction. BJOG 110: 909–915, 2003 [PubMed] [Google Scholar]
- 35.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000–1006, 1992 [PubMed] [Google Scholar]
- 37.Ramsey EM. Vascular adaptations of the uterus to pregnancy. Ann NY Acad Sci 75: 726–745, 1959 [DOI] [PubMed] [Google Scholar]
- 38.Ramsey EM. The story of the spiral arteries. J Reprod Med 26: 393–399, 1981 [PubMed] [Google Scholar]
- 39.Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E(2)β-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol 281: H422–H431, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest 98: 2158–2166, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol 296: H1878–H1887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenfeld CR, Roy T, Cox BE. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul Pharmacol 38: 115–125, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Rosenfeld CR, Word RA, DeSpain K, Liu XT. Large conductance Ca2+-activated K+ channels contribute to vascular function in nonpregnant human uterine arteries. Reprod Sci 15: 651–660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salhab WA, Shaul PW, Cox BE, Rosenfeld CR. Regulation of types I and III NOS in ovine uterine arteries by daily and acute estrogen exposure. Am J Physiol Heart Circ Physiol 278: H2134–H2142, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 361: 1511–1517, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441–R463, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Stanley JL, Cheung CC, Rueda-Clausen CF, Sankaralingam S, Baker PN, Davidge ST. Effect of gestational diabetes on maternal artery function. Reprod Sci 18: 342–352, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Svedas E, Nisell H, Vanwijk MJ, Nikas Y, Kublickiene KR. Endothelial dysfunction in uterine circulation in preeclampsia: can estrogens improve it? Am J Obstet Gynecol 187: 1608–1616, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Takata M, Nakatsuka M, Kudo T. Differential blood flow in uterine, ophthalmic, and brachial arteries of preeclamptic women. Obstet Gynecol 100: 931–939, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Telezhkin V, Goecks T, Bonev AD, Osol G, Gokina NI. Decreased function of voltage-gated potassium channels contributes to augmented myogenic tone of uterine arteries in late pregnancy. Am J Physiol Heart Circ Physiol 294: H272–H284, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol 283: H2226–H2233, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol 290: H2337–H2343, 2006 [DOI] [PubMed] [Google Scholar]