Abstract
Preeclampsia is associated with autoimmune cells TH17, secreting interleukin-17, autoantibodies activating the angiotensin II type I receptor (AT1-AA), and placental oxidative stress (ROS). The objective of our study was to determine whether chronic IL-17 increases blood pressure by stimulating ROS and AT1-AAs during pregnancy. To answer this question four groups of rats were examined: normal pregnant (NP, n = 20), NP+IL-17 (n = 12), NP+tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) (n = 7) (a superoxide dismutase mimetic that scavenges ROS), and NP+IL-17+tempol (n = 11). IL-17 (150 pg/day) was infused into NP rats while tempol was administered via the drinking water ad libitum. On day 19 blood pressure (MAP) was recorded, and plasma, urine, and tissue were collected for isolation of ROS detected by chemilluminescent technique. Urinary isoprostane was measured by ELISA. AT1-AAs were determined via cardiomyocyte assay and expressed as beats per minute. MAP increased from 98 ± 3 mmHg in NP to 123 ± 3 mmHg in IL-17-infused NP rats. Urinary isoprostane increased from 1,029 ± 1 in NP to 3,526 ± 2 pg·mg−1·day−1 in IL-17-infused rats (P < 0.05). Placental ROS was 436 ± 4 RLU·ml−1·min−1 (n = 4) in NP and 702 ± 5 (n = 5) RLU·ml−1·min−1 in IL-17-treated rats. Importantly, AT1-AA increased from 0.41 ± 0.05 beats/min in NP rats (n = 8) to 18.4 ± 1 beats/min in IL-17 rats (n = 12). Administration of tempol attenuated the hypertension (101 ± 3 mmHg) ROS (459 ± 5 RLU·ml−1·min−1) and blunted AT1-AAs (7.3 ± 0.6 beats/min) in NP+IL-17+tempol-treated rats. Additionally, AT1 receptor blockade inhibited IL-17-induced hypertension and placental oxidative stress. MAP was 105 ± 5 mmHg and ROS was 418 ± 5 RLU·ml−1·min−1 in NP+IL 17-treated with losartan. These data indicate that IL-17 causes placental oxidative stress, which serves as stimulus modulating AT1-AAs that may play an important role in mediating IL-17-induced hypertension during pregnancy.
Keywords: autoimmunity, preeclampsia, reduced uterine perfusion pressure
preeclampsia is one of the major causes of maternal and perinatal mortality and morbidity, and yet, the pathophysiology of this disease remains incompletely understood (5, 14). The initiating event of the disease is associated with abnormal cytotrophoblast invasion resulting in inadequate remodeling of the uterine spiral arteries leading to significantly reduced uterine perfusion pressure (RUPP) of the uteroplacental unit (5, 6). This cytotrophoblast invasion is tightly regulated between various immune cell types, such as monocytes, macrophages, uterine natural killer cells, T lymphocytes, and endothelial cells (5, 6, 8, 14). As preeclampsia develops, a dysregulation among these immune cell types begins early in implantation, thus lending to the overactivation of a chronic inflammatory immune response (8). As a result the placenta becomes ischemic, releasing factors thought to contribute to the development of hypertension and other agents of disease expression such as proinflammatory cytokines, tumor necrosis factor (TNF)-α, and interleukin 6 (IL-6) being released into the maternal peripheral circulation (5, 6, 8, 14). Additionally, we have learned recently of autoimmune activities during preeclampsia such as the production of autoantibodies to the angiotensin II type I receptor (6, 8) and elevated circulating IL-17 and TH17 cells (16).
One important physiological function of IL-17 producing TH17 cells is to recruit leukocytes such as neutrophils to the site of infection as a major host defense mechanism against extracellular bacteria. At the site of recruitment, IL-17 stimulates a myriad of other cytokines such as TNF-α and IL-6, which are important for intercellular communication. Importantly, IL-17 also induces neutrophilic release of antimicrobial substances and/or phagocytosis of microbes and dead tissues. Macrophages and neutrophils convert molecular oxygen into reactive oxygen species (ROS) by the phagocyte oxidase system catalyzed by the enzyme NADPH oxidase. Once neutrophils are activated, they can cause injury to normal host tissues, such as the placental unit, by the release of lysosomal enzymes, ROS, or nitric oxide. Many studies have indicated that preeclamptic women have elevated oxidative stress within the placental unit measured by increased NADPH subunits as well as elevated urinary 8-isoprostanes as a measure of whole body oxidative stress.
In accord with exacerbated immune function during preeclampsia, recent evidence implicates an imbalance between regulatory (Treg) and effector T cells, subclasses of CD4+ T lymphocytes, in preeclamptic women (15, 16). There is considerable data both in humans and in mice for the importance of interleukin 17 (IL-17) in the development and progression of chronic inflammatory and autoimmune diseases (4). IL-17-producing CD4+ T cells (TH17 cells) are the dominant pathogenic cellular component in autoimmune inflammatory diseases, including autoimmune arthritis, psoriasis, and multiple sclerosis. In contrast, Tregs (foxp3+Tcells) have anti-inflammatory properties and can cause quiescence of immune activation (4, 16). In agreement with recent clinical studies performed in preeclamptic versus normal pregnant patients, we have demonstrated abnormal ratios of circulating Tregs (foxp3+CD4+Tcells)/TH17 (ROR γ+CD4+Tcells) occurring in response to RUPP in the pregnant rat. In addition, we have shown that adoptive transfer of total CD4+ T cells from RUPP control rats into normal pregnant (NP) recipient rats causes hypertension associated with elevated IL-17 and AT1-AA. Therefore, the objective of this study was to determine whether chronic IL-17 during pregnancy leads to increased blood pressure, placental oxidative stress, and autoantibody production (AT1-AA) as mediators of the pathogenicity of preclampsia.
MATERIALS AND METHODS
Pregnant Sprague-Dawley rats purchased from Harlan-Sprague Dawley (Indianapolis, IN) were used in the study. Animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle. All experimental procedures executed in this study were in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Protocol 1a: Effect of IL-17 Infusion on MAP in Pregnant Rats
Recombinant mouse IL-17 (150 pg/day) (RnD Systems, Minneapolis, MN) was infused intraperitoneally from day 14 to day 19 of gestation via mini-osmotic pumps (model 2002, Alzet Scientific) into NP rats. IL-17 (150 pg/day) was also infused into virgin rats via mini-osmotic pumps for 5 days.
Measurement of mean arterial pressure in chronically instrumented conscious rats.
Under isoflurane anesthesia on day 18 of gestation or the fifth day of IL-17 infusion for virgin rats, carotid arterial catheters were inserted for blood pressure measurements. The catheters inserted are V3 tubing (SCI), which is tunneled to the back of the neck and exteriorized. On day 19 of gestation mean arterial blood pressure (MAP) was analyzed after placing the rats in individual restraining cages. MAP was monitored with a pressure transducer (Cobe III Transducer CDX Sema) and recorded continuously after a 1-h stabilization period. Subsequently, a blood and urine sample was collected, kidneys and placentas were harvested, and litter size and pup weights were recorded under anesthesia (9, 12).
Determination of circulating T lymphocytes.
Circulating CD4+ T cell populations were measured from peripheral blood leukocytes (PBL) collected at day 19 of gestation from NP rats and from pregnant IL-17-infused rats. We utilized flow cytometry analysis to detect specific CD4+ T cell populations; CD4+RORγ+ (retinoic acid receptor-related organ receptor gamma) isolated from chronic IL-17-treated and NP rats PBLs. At the time of tissue harvest, plasma was collected and PBLs were isolated from plasma by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical) according to the manufacturer's directions. For flow cytometric analysis equal numbers of leukocytes (1 × 106) were incubated for 30 min at 4°C with antibodies against mouse CD4 (BD Biosciences, San Jose, CA). After washing was completed, cells were labeled with the secondary fluorescein isothiocyanate (FITC) antibody (Southern Biotech, Birmingham, AL) for 30 min at 4°C. Cells were washed and permeabilized and stained with anti-rat RORγ conjugated to PE (BD Pharmingen) for 30 min at 4°C. As a negative control, for each individual rat, cells were treated exactly as described above except they were incubated with anti-FITC and anti-PE secondary antibodies alone. Subsequently, cells were washed and resuspended in 500 μl of Roswell Park Memorial Institute medium (RPMI) and analyzed for single and double staining on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The percentage of positive staining cells above the negative control was collected for each individual rat and mean values for each experimental group (NP and NP+IL-17) was calculated.
Determination of IL-6.
An important function of IL-17 is induced cytokines such as IL-6, which would induce expansion of the TH17 and B lymphocytes; therefore, we utilized the rat IL-6 Quantikine ELISA. The assay displayed a sensitivity of 21 pg/ml, intra-assay variability is 7.4%, and interassay is 8.4%.
Determination of urinary isoprostane.
On day 19 of gestation, urine was collected and utilized for determination of excreted isoprostanes measured via ELISA from Oxford Biomedical Research (Oxford, MI). The assay displayed a sensitivity of 0.05 ng/ml, inter-assay variability of 4.2%, and intra-assay variability of 4.7%.
Determination of tissue ROS.
Superoxide production in the placenta was measured by using the lucigenin technique as we have recently described (10, 13). Rat placentas were snap frozen in liquid nitrogen directly after collection and stored at −80°C until further processing. Placentas were removed and homogenized in RIPA buffer (phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail; Santa Cruz, Santa Cruz, CA) as described previously (10, 13). The samples were centrifuged at 12,000 g for 20 min, the supernatant aspirated, and the remaining cellular debris was discarded. The supernatant was incubated with lucigenin at a final concentration of 5 μmol/l. The samples were allowed to equilibrate for 15 min in the dark, and luminescence was measured every second for 10 s with a luminometer (Berthold, Oak Ridge, TN). Luminescence was recorded as relative light units (RLU) per minute. An assay blank with no homogenate but containing lucigenin was subtracted from the reading before transformation of the data. Each sample was repeated five times and the average used for data transformation. The protein concentration was measured using a protein assay with BSA standards (Pierce, Rockford, IL; expressed as RLU·min−1·mg protein−1).
Determination of circulating AT1-AA.
On day 18 of gestation blood was collected and immunoglobulin was isolated from 1 ml of serum by specific anti-rat IgG column purification. AT1-AA was purified from rat IgG by epitope binding to the amino acid sequence corresponding to the second extracellular loop of the AT1 receptor covalently linked to Sepharose 4B CNBr-activated gel. Unbound IgG was washed away and bound IgG was eluted with 3 M potassium thiocyanate. AT1-AA activity was measured utilizing a bioassay that evaluates the beats per minute (bpm) of neonatal cardiomyocytes in culture (2, 3, 9–12). AT1-AAs were assessed in NP controls and NP+IL-17-infused rats. Since AT1-AA is not present in nonpregnant rats, it was not determined in this study.
Protocol 2: Effect of Tempol on IL-17-Induced Hypertension
This protocol was performed to determine whether the effect of placental and cortical oxidative stress was generated in response to chronic IL-17 to increase blood pressure by administering a superoxide dismutase mimetic (tempol) to NP and IL-17-infused rats.NP rats treated with tempol added to the drinking water were used as controls, whereas IL-17-infused rats treated with tempol served as the experimental group. On day 14 of gestation, tempol (30 mg·kg−1·day−1) was administered in the drinking water. Mini-osmotic pumps (Alzet 2002) infusing 150 pg/day of IL-17 were surgically implanted intraperitoneally on day 14 into 12 pregnant rats. Arterial pressure was determined in all groups of pregnant rats at day 19 of gestation as previously described in protocol 1a.
Protocol 3: Effect of AT1 Receptor Blockade on IL-17-Induced Hypertension
This protocol was performed to determine whether activation of the AT1 receptor response to chronic IL-17 caused an increase blood pressure and placental ROS. In this protocol losartan (5 mg·kg−1·day−1) was administered to NP and IL-17-infused rats via the drinking water beginning on day 14 of gestation. Mini-osmotic pumps (Alzet 2002) infusing 150 pg/day of IL-17 were surgically implanted intraperitoneally on day 14 into five pregnant rats; losartan-treated NP served as controls (n = 4).Arterial pressure was determined in all groups of pregnant rats at day 19 of gestation as previously described in protocol 1a.
Protocol 4: B Cell Depletion Attenuates IL-17-Induced Increases in Blood Pressure
NP rats were treated with Rutiximab (250 mg/kg) from day 14 to day 19 of gestation (n = 7) via osmotic minipump insertion. Rituximab is a chimeric monoclonal anti-CD20 antibody that is used to induce B cell depletion in vivo (10, 17). The dose used was based on the efficacy demonstrated in previous studies from our lab illustrating that administration of Rituximab depleted circulating B cells and suppressed AT1-AA in RUPP rats and RUPPCD4+ T cell-induced hypertensive pregnant rats. Arterial pressure and circulating TH17 cells were determined in pregnant rats at day 19 of gestation as described in protocol 1.
Statistical Analysis
All data are expressed as means ± SE. ANOVA was used to determine differences among blood pressure and TH17 cells between the groups the multiple experimental groups. Difference between control and experimental tissues were analyzed using the t-test and was considered statistically significant at P values < 0.05. NP was compared with IL-17 infused, whereas tempol-treated NP was compared with tempol-treated IL-17-infused rats. Losartan-treated NP were compared with losartan-treated IL-17-infused pregnant rats.
RESULTS
Protocol 1: IL-17 Infusion into NP Rats is Associated With Hypertension and Oxidative Stress
Effect of IL-17infusion on TH17 cells and blood pressure during pregnancy.
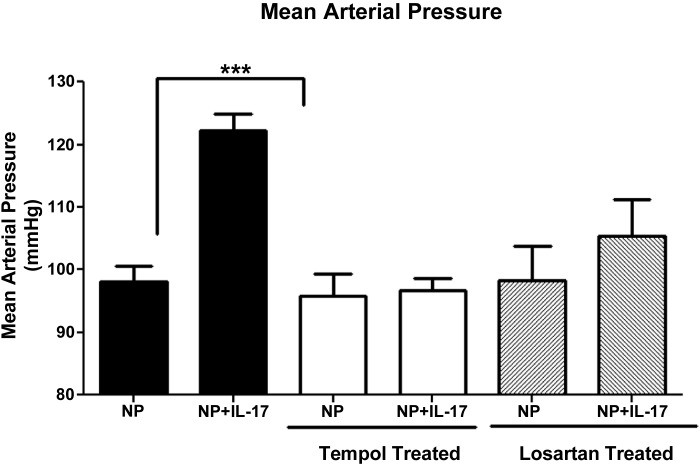
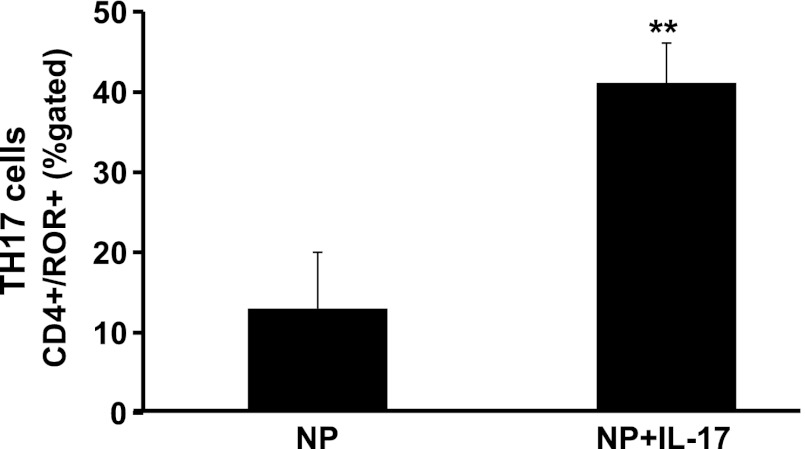
MAP was increased significantly in IL-17-infused NP rats 123 ± 3 mmHg (n = 12) compared with NP rats 98 ± 3 mmHg (n = 20) (Fig. 1; P = 0.001). As with other cytokine infusion studies, infusion of IL-17 into virgin rats had no effect on blood pressure, and MAP was 124 ± 2 mmHg in virgins versus 117 ± 3 mmHg in virgins treated with IL-17. We hypothesized that infusion of IL-17 would stimulate TH17 cells, which would be found elevated in the circulation of IL-17-treated rats; therefore, we utilized flow cytometry to determine levels of circulating TH17 cells in NP and IL-17-treated rats. Figure 2 demonstrates that circulating CD4+ TH17 cells, identified by expression of the RORγ transcription factor, are significantly elevated in response to chronic IL-17 infusion during pregnancy compared with NP rats (13 ± 7% NP vs. 41 ± 5% NP+IL-17, P < 0.02).
Fig. 1.
IL-17-induced hypertension is attenuated with a superoxide dismutase (SOD) mimetic (tempol) or AT1 receptor blockade. Chronic infusion of IL-17 into normal pregnant (NP) rats causes hypertension during pregnancy. This blood pressure (MAP) response is attenuated by administration of a SOD mimetic or losartan, an At1 receptor blocker. Data are expressed as means ± SE. ***P < 0.05 vs. NP controls.
Fig. 2.
Infusion of IL-17 into NP rats increased circulating CD4+/RORγ+T cells compared with NP control rats. Data are expressed as means ± SE. **P < 0.05 vs. NP controls.
Pup weight nor litter size nor placental weight were different between NP and NP+IL17-treated pregnant rats. NP litter size was 14, pup weight was 2.27 ± 0.05 g, and placental weight was 0.66 ± 0.07 g in NP rats. IL-17-treated litter size was 13, pup weight was 2.24 ± 0.133 g, and placental weight was 0.62 ± 0.11 g in IL-17-treated pregnant rats.
Effect of IL-17 on ROS.
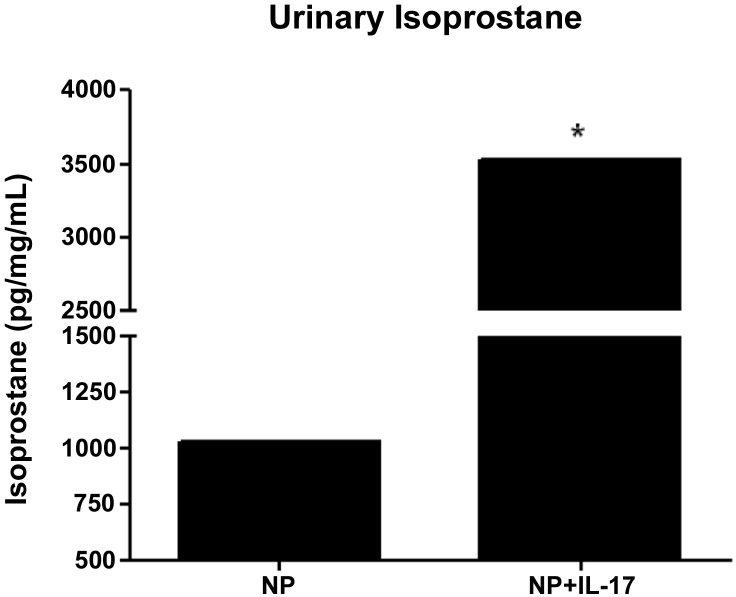
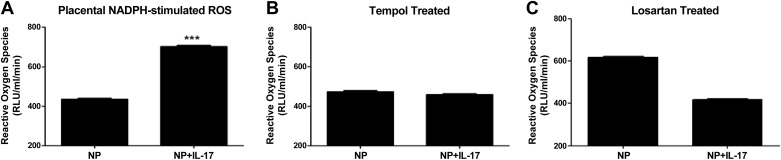
Urinary levels of isoprostane were found to be significantly elevated with chronic infusion of IL-17 from 1,029 ± 1 pg·mg−1·ml−1 in NP to 3,526 ± 2 pg·mg−1·ml−1 in NP+IL-17 rats (Fig. 3; P < 0.05). We observed an increase in placental ROS production with the addition of NADPH oxidase in IL-17-infused rats (702 ± 5 RLU·ml−1·mg−1) compared with NP controls (436 ± 4 RLU·ml−1·mg−1) (Fig. 4A, P < 0.001).
Fig. 3.
IL-17 infusion increases urinary isoprostane excretion. Urinary isoprostane excretion is increased in response to chronic IL-17 infusion, indicating that IL-17 increases oxidative stress. Data are expressed as means ± SE. *P < 0.05 vs. NP controls.
Fig. 4.
IL-17-infused placental oxidative stress is blunted by a SOD mimetic (tempol) or losartan. A: chronic infusion of IL-17 into NP rats significantly produces greater NADPH-stimulated placental reactive oxygen species (ROS). B: chronic administration of tempol to IL-17-infused rats decreases NADPH-stimulated placental oxidative stress to levels no longer significantly different from that of tempol-treated controls. C: chronic AT1 receptor blockade with losartan significantly decreased NADPH-stimulated placental oxidative stress IL-17-treated rats. Data are expressed as means ± SE. RLU, relative light units. ***P < 0.05 vs. NP controls.
Effect of IL-17 on renin angiotensin system.
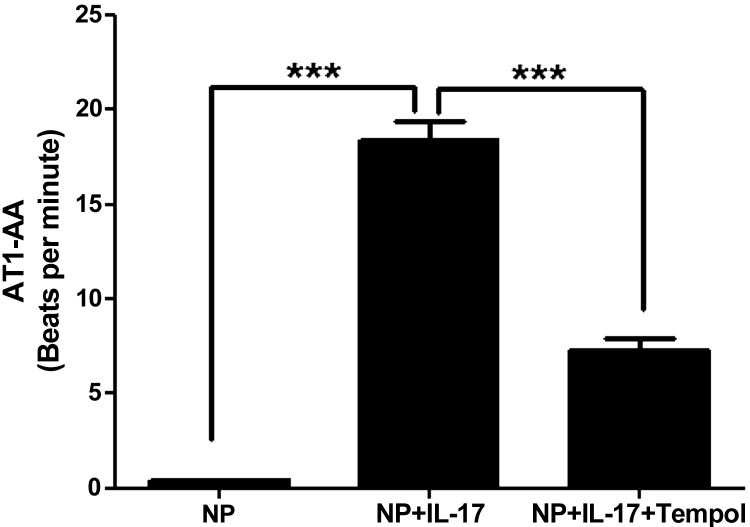
Infusion of IL-17 into NP rats increased production of the AT1-AA to 18.4 ± 1 bpm (n = 12) compared with NP control rats 0.41 ± 0.05 bpm (n = 8) (Fig. 5, P < 0.001). Plasma ANG II, measured at Wake Forest, increased slightly but was not significantly different with IL-17 infusion in pregnant rats. In control pregnant rats, plasma ANG II was 54 ± 16 versus 86 ± 24 pg/ml in IL-17-treated pregnant rats.
Fig. 5.
Tempol administration decreases AT1-AA in response to chronic infusion of IL-17. AT1-AA isolated from serum of rats chronically infused with IL-17 is significantly increased compared with NP rats but is blunted in response to tempol administration. Data are expressed as means ± SE. ***P < 0.001 vs. IL-17-infused rats.
IL-6 is stimulated during IL-17-induced hypertension.
We hypothesized that as a result of elevated IL-17 during pregnancy, IL-6 would be induced and be an important player in intercellular communication to facilitate TH17 and potentially B cell activation. Therefore, we measured circulating IL-6 in IL-17-induced hypertensive pregnant rats.
IL-6 increased significantly in response to IL-17 infusion and was 323 ± 83 pg/ml in IL-17-infused rats (n = 5) and was 89 ± 13 pg/ml in NP rats (P < 0.005) (n = 8).
Protocol 2: Tempol Attenuates Placental Oxidative Stress, AT1-AA, and Hypertension During Pregnancy
Effect of tempol on placental oxidative stress.
Administration of tempol decreased NADPH-stimulated placental production of ROS from IL-17-infused pregnant rats (459 ± 5 RLU·min−1·mg−1) to levels no longer statistically different compared with placental ROS from tempol-treated NP controls (474 ± 5 RLU·min−1·mg−1; n = 3) (Fig. 4B; P = 0.096). In addition, tempol administration significantly decreased circulating AT1-AAs produced in response to IL-17 to 7.3 ± .6 bpm (n = 7) in NP+IL-17 rats (Fig. 5, P < 0.001). Importantly, the blood pressure increase to chronic IL-17 was completely attenuated with tempol administration and was no different in tempol-treated IL-17-infused rats compared with tempol-treated control rats. MAP was 102 ± 5 mmHg (n = 7) in tempol control NP rats versus 101 ± 2 mmHg (n = 7) in IL-17 tempol-treated pregnant rats (Fig. 1).
Protocol 3: AT1 Receptor Blockade Attenuates IL-17-Induced Increases in Blood Pressure and Placental Oxidative Stress
Importantly, losartan administration attenuated the blood pressure increase to chronic IL-17; MAP was no different in losartan-treated IL-17-infused rats compared with losartan-treated control rats. MAP was 98 ± 5 mmHg (n = 4) in losartan control NP rats versus 105 ± 2mmHg (n = 5) in IL-17 losartan-treated pregnant rats (Fig. 1). Furthermore, administration of losartan decreased both basal and NADPH-stimulated placental production of ROS from IL-17-infused pregnant rats (185 ± 38 RLU·min−1·mg−1 n = 3, basal) to levels no longer statistically different compared with basal placental ROS from losartan-treated NP controls (243 ± 36 RLU·min−1·mg−1; n = 4). NADPH-stimulated placental production of ROS from IL-17-infused pregnant rats (418 ± 5 RLU·min−1·mg−1; n = 5) to levels no longer statistically different compared with placental ROS from losartan-treated NP controls (619 ± 5 RLU·min−1·mg−1; n = 4).
Protocol 4: B Cell-Depleting Agent Rituximab Decreased Blood Pressure in Response to Chronic IL-17 During Pregnancy
Importantly, administration of Rituximab significantly decreased blood during IL-17 infusion; however, this decrease was not as profound as the blockade with either tempol or losartan. MAP was reduced from 123 ± 3 mmHg in IL-17-treated controls to 115 ± 4 mmHg in IL-17 Rituximab-treated rats (P < 0.05) compared with NP rats 98 ± 3 mmHg (P < 0.05). In addition, Rituximab decreased circulating TH17 cells to 21% ± 4.7, which was significantly less compared with IL-17-induced hypertension pregnant rats (P < 0.05). However, TH17 cells in IL-17 + Rituximab was still significantly greater than that in the NP control group (13 ± 7%, P < 0.05)
DISCUSSION
IL-17 producing TH17 cells is a newly discovered subset of T helper cell, which is believed to be a mediator for many autoimmune diseases such as Crohn's disease, psoriasis, rheumatoid arthritis, and multiple sclerosis. We are now learning of a potential role for IL17 and TH17 cells as mediators for the pathophysiology associated with preeclampsia (1, 7, 16). Although IL-17 and TH17 cells are elevated in preeclampsia, it remains unknown if either IL-17 or TH17 cells cause hypertension or any state of disease expression that is associated with preeclampsia. Therefore, in this study we demonstrate, for the first time, that chronic IL-17 increases blood pressure and TH17 cells in pregnant rats but not in virgin rats. It is important to note that infusion of this dose of IL-17 had no hypertensive effects on the nonpregnant rats, just as we have seen with both TNF-α and IL-6, indicating that moderate doses of cytokines cause hypertension during pregnancy most likely by stimulating some placental factor or factors to cause much of the disease characteristics.
IL-17 stimulates neutrophillic reactions and the production of antimicrobial substances such as defensins and ROS. We demonstrate that IL-17 stimulates oxidative stress as illustrated by the increased in urinary isoprostanes and NADPH-stimulated placental oxidative stress. With preeclampsia being associated with autoantibodies, and IL-17 being primarily an autoimmune cytokine, we wanted to determine whether IL-17-induced hypertension was mediated through production of AT1-AA. Indeed, as with TNF-α and IL-6-induced hypertension during pregnancy, IL-17-induced hypertension in pregnant rats is associated with production of the AT1-AA. Other investigators have demonstrated a clear link with IL-17- and ANG II-induced hypertension (17), therefore, we measured plasma ANG II and found only a small increase that was not significantly different between NP controls and IL-17-infused pregnant rats. Although, elevations in ANG II are not associated with preeclampsia, a link with increased ANG II sensitivity is speculated to play a role in this disease, we believe this may be due to production of the AT1-AA acting in concert with ANG II to activate the AT1 receptor. To determine a role for activation of AT1 receptors, we treated a group of rats with losartan in the presence or absence of IL-17 infusion. We found that losartan attenuated the blood pressure response to IL-17 during pregnancy but also surprisingly inhibited placental oxidative stress in IL-17-treated pregnant rats. This could be due to blockade of both AT1-AA and ANG II activation of the AT1 receptor.
To determine whether the increase in oxidative stress caused the hypertension observed with IL-17 infusion, we repeated the infusion study in pregnant rats treated with the SOD mimetic in their drinking water (tempol). We found that administration of tempol not only attenuated the placental oxidative stress but also the increase in blood pressure in response to IL-17 infusion. Surprisingly, we found that administration of tempol and decreased ROS in the placenta was associated with significantly lowered AT1-AA in response to IL-17. These data indicate the importance of ROS as signaling molecules between immune cells and tissues to cause much of the pathology associated with preeclampsia and IL-17-induced hypertension during pregnancy.
In a previous study, we infused rat AT1-AA into NP rats and found that placental oxidative stress was significantly elevated when compare with the nonhypertensive control NP rats (13). In that study we administered tempol and found that we could attenuate the placental oxidative stress and the hypertension in that model. Therefore, in the current study, we examined the role of the AT1-AA to mediate the hypertension, stimulated in response to IL-17 as mediators of pathophysiology of preeclampsia. We have recently demonstrated that administration of Rituximab decreased circulating B cells and AT1-AA in two previous experiments. First, we administered the B cell-depleting agent to RUPP rats and found that AT1-AA, local ET-1, and blood pressure were decreased in response to placental ischemia in this rat model of preeclampsia (10). Most recently, we administered Rituximab to NP recipient rats of RUPP CD4+ T cells. In this T cell-induced preeclampsia rat model, blood pressure and circulating AT1-AA were significantly elevated compared with NP recipients of NP CD4+ T cells. Administration of losartan decreased the hypertension. Furthermore, administration of Rituximab was efficient at B cell depletion and AT1-AA suppression and attenuation of hypertension in response to CD4+ T cells from RUPP rats (17). We demonstrate, in this study, that administration of Rituximab significantly decreased the blood pressure in response to IL-17 infusion, suggesting an important role for the AT1-AA to cause hypertension in this model. However, it is important to note that as seen in RUPP rats, B cell depletion by administration of Rituximab did not attenuate but significantly blunted the blood pressure response to IL-17; MAP in IL-17+Rituximab-treated rats was still significantly elevated compared with NP control rats. Furthermore, we demonstrate that administration of Rituximab significantly decreased circulating TH17 cells stimulated in response to IL-17 infusion. However, this response was not completely attenuated, and circulating TH17cells stimulated in response to IL-17 infusion were still significantly higher than that of NP control rats. Therefore, we conclude that the remaining increased in blood pressure in this group could be due to the presence of excess circulating TH17 cells or ANG II or other inflammatory cytokines stimulated in response to IL-17 infusion. However, since the blood pressure response nor the TH17 stimulation was completely abolished, we did not examine these additional factors. Nevertheless, these data highlight the importance of many factors caused by IL-17 excess during pregnancy, such as IL-17-stimulated oxidative stress, other inflammatory cytokines, and TH17 cells to mediate the elevation in blood pressure and pathophysiology associated with preeclampsia.
Although we recognize that ROS is a product of neutrophils and monocytes, we did not determine nor isolate and culture these cell types from our IL-17-infused pregnant rats. These phagocytic cells contain NADPH subunits and produce ROS, therefore, they could be the producers of elevated whole body oxidative stress indicated by the sharp increase in urinary 8-isoprostanes. We did, however, demonstrate that in response to chronic IL-17 infusion, TH17 cells are elevated in the circulation of pregnant rats. The stimulus for such could be a typical cellular response resulting from IL-6 and other cytokines such as IL-8, TNF-α, or IL-1, all of which synthesis is induced by elevated IL-17. Importantly, we found that indeed in response to chronic IL-17 administration, IL-6 is significantly elevated above that observed in NP rats. IL-17 is critical for function of TH17 cells, which are associated with immune and autoimmune phenomena. It is accepted that elevated cytokines stimulate immune cells and intercellular/intracellular communication between leukocytes. Therefore, as demonstrated here, one result of chronic IL-17 infusion is elevated TH17 cells, oxidative stress, and AT1-AA as mechanisms of hypertension during pregnancy. A secondary mediator could be elevated IL-6, which facilitates proliferation of both TH17 cells and B cells, and as we have previously shown, is an important player in hypertension and AT1-AA production in response to placental ischemia (19). Future studies of interest would be to determine what immune cell type is the source of oxidative stress in the placenta and what importance do TH17 cells play in causing hypertension and AT1-AA production from B cells when IL-17 is elevated or in response to placental ischemia. Importantly, this study highlights the importance of IL-17-stimulated ROS as signaling molecules linking immune activation with the development of hypertension during pregnancy. Future studies blocking IL-17 and possibly TH17 cells in response to placental ischemia will be important to determine a causal role for IL-17 in placental oxidative stress and hypertension during preeclampsia.
GRANTS
This work was supported by American heart Association Grant SDG0835472N and by National Institutes of Health Grants HD-67541 and HL-51971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.D., K.W., F.H., G.W., J.H., J.M., and B.L. performed experiments; P.D., K.W., J.S., G.W., J.M., and B.L. prepared figures; P.D., K.W., and B.L. drafted manuscript; K.W., F.H., J.S., G.W., J.H., J.M., R.D., and B.L. analyzed data; F.H., G.W., J.M., and B.L. interpreted results of experiments; G.W., J.N.M., and R.D. edited and revised manuscript; J.H., J.M., J.N.M., R.D., and B.L. approved final version of manuscript; B.L. conception and design of research.
REFERENCES
- 1.Crispin J, Tsokos G. Interleukin-17 producing T cells in lupus. Curr Opin Rheumatol 22: 499–503, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park J, Theuer J, Jeupner A, Gulba D, Mackman N, Haller H, Luft F. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 101: 2382–2387, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Dechend R, Homuth V, Wallukat G, Muller D, Krause M, Dudenhausen J, Haller H, Luft F. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Invest 13: 79–86, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Emamaullee J, Davis J, Merani S, Toso C, Elliott J, Thiesen A, Shapiro A. Inhibition of Th17 cells regulates autoimmune diabete4s in NOD mice. Diabetes 58: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher S, Martin J. Hypertensive disorders in pregnancy. In: Chelsey Hypertensive Disorders in Pregnancy, edited by Lindheimer MD, Roberts JM, Cunnigham FG. Amsterdam: Elselvier, 1998, p. 377–394 [Google Scholar]
- 6.Granger J, Alexander B, Bennett W, Khalil R. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens 14: 178S–185S, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Hisakata Y. Current prospective on the role of IL-17 in autoimmune disease. J Inflam Res 3: 33–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol 62: 105–120, 2010 [PMC free article] [PubMed] [Google Scholar]
- 9.LaMarca B, Parrish M, Ray L, Murphy S, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin J, Ryan M, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: a role of endothelin-1. Hypertension 54: 905–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamarca B, Wallace K, Herse F, Wallukat G, Martin J, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 864–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrish M, Murphy S, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray L, Dechend R, Martin J, Granger J, LaMarca B. The effect of immune factors, Tumor Necrosis Factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-Like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrish M, Ryan M, Glover P, Brewer J, Ray L, Dechend R, Martin J, LaMarca B. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gender Med 8: 184–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrish M, Wallace K, Tam Tam K, Herse F, Weimer A, Wenzel K, Wallukat G, Ray L, Arany M, Cockrell K, Martin J, Dechend R, Lamarca B. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens 24: 835–840, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Roberts J, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Sargent I, Borzychowski A, Redman C. Immunoregulation in normal pregnancy and preeclampsia: an overview. Reprod Biomed Online 13: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Santner-Nanan B, Peek M, Khanam R, Richarts L, Zhu E, FazekasdeSt G, Nanan R. Systemic increase in the ration between FoxP3+ and IL-17 producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183: 7023–7030, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Madhur MS, lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TH, Harrison DG. IL-17 promotes ANGII-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, LaMarca B. Activating autoantibodies to the angiotensin receptor are important in mediating hypertension in response to adoptive transfer of RUPP CD4+ T lymphocytes. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaMarca BB, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Intern J Interferon, Cytokine, Mediator Res 3: 65–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]