Abstract
Intravenous bolus injection of morphine causes a vagal-mediated brief apnea (∼3 s), while continuous injection, via action upon central μ-opioid receptor (MOR), arrests ventilation (>20 s) that is eliminated by stimulating central 5-hydroxytryptamine 1A receptors (5HT1ARs). Bronchopulmonary C-fibers (PCFs) are essential for triggering a brief apnea, and their afferents terminate at the caudomedial region of the nucleus tractus solitarius (mNTS) that densely expresses 5HT1ARs. Thus we asked whether the vagal-mediated apneic response to MOR agonists was PCF dependent, and if so, whether this apnea was abolished by systemic administration of 8-hydroxy-2-(di-n-propylamino)tetral (8-OH-DPAT) largely through action upon mNTS 5HT1ARs. Right atrial bolus injection of fentanyl (5.0 μg/kg, a MOR agonist) was performed in the anesthetized and spontaneously breathing rats before and after: 1) selective blockade of PCFs' conduction and subsequent bivagotomy; 2) intravenous administration of 5HT1AR agonist 8-OH-DPAT; 3) intra-mNTS injection of 8-OH-DPAT; and 4) intra-mNTS injection of 5HT1AR antagonist WAY-100635 followed by 8-OH-DPAT (iv). We found the following: First, fentanyl evoked an immediate apnea (2.5 ± 0.4 s, ∼6-fold longer than the baseline expiratory duration, TE), which was abolished by either blocking PCFs' conduction or bivagotomy. Second, this apnea was prevented by systemic 8-OH-DPAT challenge. Third, intra-mNTS injection of 8-OH-DPAT greatly attenuated the apnea by 64%. Finally, intra-mNTS microinjection of WAY-100635 significantly attenuated (58%) the apneic blockade by 8-OH-DPAT (iv). We conclude that the vagal-mediated apneic response to MOR activation depends on PCFs, which is fully antagonized by systemic 8-OH-DPAT challenge largely via acting on mNTS 5HT1ARs.
Keywords: blood pressure, heart rate, opiods, ventilation
bronchopulmonary c-fibers (PCFs) in the vagus nerve constitute 75–90% of the sensory fibers innervating the airways and lungs. PCFs are sensitive to various exogenous chemical substances and endogenously released mediators and play an important role in the control of respiratory rhythm (see review paper in Ref. 28). Pharmacological activation of PCFs in laboratory experiments with capsaicin, a pungent active ingredient of hot peppers, can evoke an expiratory apnea associated with a bradycardia and hypotension in both anesthetized and awake animals. This apnea, lasting for several seconds, is concomitant with a reflexive prolongation of medullary postinspiratory neuronal excitation (20, 35, 37) that is sufficiently powerful to globally inhibit inspiratory activity (39). Interestingly, a bolus injection of μ-opioid receptor (MOR) agonists into the vein or right atrium also evokes a vagal-mediated expiratory apnea (21, 54, 55, 58) followed by a respiratory depression resulting from action on the medullary respiratory neurons in the so called “ventral respiratory group” (2). The vagal nerve fibers and their cell bodies residing in the nodose and jugular ganglia are endowed with opioid receptors (9, 29, 60), and opioids can increase PCF activity and induce a vagal-mediated apnea (45, 54, 55). Furthermore, opioids reportedly facilitate the apneic response to stimulation of pulmonary stretch receptor in anesthetized cats (32) and rabbits (7). Thus the information as to what extent PCFs contribute to the vagal-mediated apneic response to MOR activation is lacking.
Serotonin (5-hydroxytryptamine, 5HT) is an important neurotransmitter involved in the central control of respiration by acting on a number of different 5HT receptor isoforms (5). A recent report revealed that intravenous administration of 5HT 1A receptor (5HT1AR) agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) eliminated a ventilatory arrest (>20 s) induced by central action of morphine in anesthetized and vagotomized rats (43). To date, however, it has not been established whether the brief apneic response to right atrial bolus injection of a MOR agonist can also be blocked by systemic 8-OH-DPAT challenge.
The caudomedial region of the nucleus tractus solitarius (mNTS) receives the PCF afferents and is essential for the PCF-mediated apnea (34, 36, 51, 57). Although the further direct projections from the mNTS have not been determined, activation of PCFs excites postinspiratory and suppresses inspiratory neurons in the ventral respiratory group (37, 56). Importantly, the mNTS densely expresses 5HT1ARs (30, 49), and activating these receptors in the medulla inhibits expiratory neuronal activity in anesthetized rats and cats (25, 53). These results, along with our pilot observation that systemic 8-OH-DPAT challenge fully blocked the apneic response to right atrial bolus injection of fentanyl, a selective MOR agonist, raised a fundamental question as to whether activation of mNTS 5HT1ARs was essential for this blockade.
In the present study, we hypothesized that the apneic response to right atrial bolus injection of fentanyl was PCF mediated. We further hypothesized that this apneic response was antagonized by systemic administration of 5HT1AR agonist 8-OH-DPAT, in which 5HT1ARs in the mNTS played a key role.
MATERIALS AND METHODS
Fifty-seven pathogen-free male Sprague-Dawley rats (300–400 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed in the animal facility at Lovelace Respiratory Research Institute in filter top cages and provided with water and food ad libitum. The room was constantly ventilated and the temperature was kept at 23°C. The animals were quarantined for 2 wk before experiments. The experimental protocols were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
General animal preparation.
The rats were anesthetized with urethane (1,200 mg/kg ip). As needed, supplemental urethane (300 mg/kg ip) was administered to completely eliminate eye-blink and limb-withdrawal reflex throughout the experiment. As previously reported (61), the trachea below the larynx was exposed through a midline incision, tracheotomized by blunt dissection, and cannulated. The right jugular vein was isolated, a catheter (PE-50 tubing, ∼15 cm length) was inserted with the tip advanced close to the right atrium for injection of agents, and the tip location was confirmed after necropsy. The right femoral artery was cannulated for monitoring of mean arterial blood pressure (MABP) and heart rate (HR). The tracheal cannula was connected to a pneumotachograph to record airflow. The pneumotachograph had a linear flow-pressure relationship in the range of 2–20 ml/s, a flow resistance of 0.046 cmH2O·ml−1·s, and a dead space of 0.2 ml. Another end of the pneumotachograph was placed (∼5 mm deep) in a plastic tube with a diameter fivefold greater than the pneumotachograph. The other side of the plastic tube was connected to a supplemental gases device through a gas mixing flowmeter (GF-3MP, Cameron Instrument, Port Aransas, TX), by which the animal was exposed to a gas mixture of 40% O2 in nitrogen. End-tidal pressure of carbon dioxide (PetCO2) was measured via a CO2 analyzer (model 15–10000, MicroCapStar end-tidal CO2 analyzer, CWE, Ardmore, PA) connected to a sideport of the tracheal cannula. Animals were placed into a rigid metal frame with the head fixed and centered in a stereotaxic apparatus (model 1404, Kopf, Tujunga, CA) with the dorsal brain stem being approximately horizontal. The area postrema was exposed by removing the dura and arachnoid membranes. In several rats, bilateral cervical vagus nerves were isolated and loosely looped by a thread for later blocking C-fibers' conduction by perineural treatment of both cervical vagi with capsaicin (see Selective blockade of vagal fibers' conduction) and subsequent transection. The animal's core temperature was monitored with a rectal probe and maintained at 36.5–37.5°C by a water heating pad and radiant heat lamp.
Selective blockade of vagal fibers' conduction.
To reversibly block vagal C-fibers' conduction, cotton strips soaked with capsaicin solution (100–250 μg/ml) were wrapped around a 2- to 3-mm segment of the isolated cervical vagi for 20 min and then removed as reported before (27). The criterion for a successful perineural capsaicin treatment was confirmed by the absence of the apneic responses induced by right atrial bolus injection of capsaicin (0.5 μg/kg). As reported previously (27), intravenous injection of capsaicin at this low dose stimulates PCF afferents to elicit the apnea, while the perineural treatment of both cervical vagi with the high concentration capsaicin (100–250 μg/ml) could result in a reversible blockade of the C-fibers' conduction.
Microinjection into the mNTS.
Two double-barrel unfilamented glass micropipettes (1B100–3, 1/0.58 mm OD/ID, WPI, Sarasota, FL) with a tip size ∼30 μm (OD) were separately prepared using a vertical pipette puller (PE-21 Puller, Narishige Group, Japan). They were tightly glued side-by-side to make a paired-micropipette with the tips at the same level and 0.4 mm apart under an operating microscope (Photo-Zusatz, Germany). The two barrels of each micropipette were filled with vehicle and 5HT1AR agonist 8-OH-DPAT or 5HT3R agonist phenylbiguanide, respectively, or with 8-OH-DPAT and 5HT1AR antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane-carboxamide maleate salt (WAY-100635). These agents were dissolved in normal saline. One barrel in each micropipette contained red florescent microbeads (Lumafluor) for bilaterally identifying the injection locations. The paired micropipette, as seen under the microscope, was advanced by a micromanipulator simultaneously into the bilateral mNTS (0.2 mm lateral from midline, 0.2 mm rostral to the obex, and 0.6–0.7 mm depth). We chose this region relatively close to the midline for several reasons. First, the highest density of 5HT expression within the NTS is located at 0.2–0.3 mm lateral to the midline (23). Second, this region contains the PCF-driven neurons susceptible to modulating the PCF-mediated responses (34, 36). Third, the full expression of the pulmonary C fiber reflex requires neurons in the vicinity of the commissural NTS (cNTS) (6). For pressure injection, polyethylene tubing was sealed over each barrel end and connected to an infusion pump (model 55-1111, Harvard Apparatus). The individual injection volume (∼10 nl for 3–5 s) was monitored by measuring the movement of the fluid meniscus in the micropipette under a microscope with a fine reticule. At the end of the experiment the brain was fixed in situ by perfusing 0.1 M phosphate-buffered saline at a pH of 7.4 and then 4% paraformaldehyde in PBS through the left ventricle of the heart. The brain stem was removed and subsequently sectioned at a 50-μm thickness with a cryostat microtome (Leica, CM 1850, Microsystems, Nussioch, Germany). The area marked by fluorescent beads was identified under a fluorescence microscope (Axioskop 2 FS Plus, Zeiss, Germany).
Experimental protocol.
Study series I was designed to test whether the fentanyl-evoked apnea depended on PCFs. Right atrial bolus injection of fentanyl (5 μg/kg) was performed before and 5 min after blockade of vagal C-fiber conduction with perineural capsaicin treatment. When the apneic response to fentanyl recovered, fentanyl injection was repeated 5 min following subsequent bilateral transection of vagi (n = 7). The protocols and animals used in different study series are briefly summarized in Table 1. The interval for the following each fentanyl injection was ∼1 h. This fentanyl dose previously used in patients (11, 46) could markedly and consistently produce an apnea followed by ventilatory depression that recovered approximately within 20 min in our studies. Six other rats served as sham-operation control in which right atrial bolus injections of fentanyl was repeated three times with a 1-h interval between the two neighboring injections. The first injection was made before perineural vehicle treatment of vagi, and the second and third injections were performed 5 min after the treatments, respectively.
Table 1.
Experimental protocols and animals used
| Fentanyl Injection Into the Right Atrium Repeated After: |
|||
|---|---|---|---|
| Series | n | A | B |
| I | 7 | Perineural capsaicin treatment | Bivagotomy |
| 6 | First perineural vehicle treatment | Second perineural vehicle treatment | |
| II | 9 | 8-OH-DAPT (iv) | |
| III | 7 | 8-OH-DAPT (mNTS) | WAY (mNTS) +8-OH-DAPT (mNTS) |
| 7 | Phenylbiguanide (mNTS) | ||
| IV | 8 | 8-OH-DAPT (iv) | WAY (mNTS) +8-OH-DAPT (iv) |
| 6 | 8-OH-DAPT (iv) | WAY (iNTS) +8-OH-DAPT (iv) | |
| 3 | 8-OH-DAPT (iv) | 8-OH-DAPT (iv) | |
| 4 | WAY (mNTS) | ||
n, animal numbers. Fentanyl (iv) was injected into the right atrium before and sequentially after the procedures described in A and B columns, respectively. 8-OH-DAPT (5HT1AR agonist); WAY, WAY-100635 (5HT1AR antagonist); phenylbiguanide (5HT3R agonist); mNTS and iNTS, local microinjection into the caudomedial (mNTS) or the interstitial region of the nucleus tractus solitarius (iNTS). Note: local microinjection of WAY was performed 1 h after 8-OH-DAPT+ fentanyl (iv) when the apneic response to fentanyl (iv) recovered.
Study series II was carried out to determine whether the apneic response to fentanyl was prevented by systemic administration of 5HT1AR agonist 8-OH-DPAT. Right atrial bolus injection of fentanyl was performed before and 30 s after intravenous administration of vehicle and 8-OH-DPAT (10 μg/kg), respectively, in nine animals. This 8-OH-DPAT dose was similar to the lowest dose intravenously injected in other studies (25, 43). This 30-s period was chosen because systemic 8-OH-DPAT challenge-induced hyperventilation reached a plateau at 30 s that lasted for ∼2 min in our pilot study.
Study series III was performed to compare the apneic response to fentanyl before and 5 min after bilateral intra-mNTS injection of vehicle or 8-OH-DPAT (0.4 nmol/side) alone and coupled with local pretreatment of 5HT1AR antagonist WAY-100635 (0.4 nmol/side) (n = 7). In the latter, WAY-100635 was bilaterally microinjected into the mNTS, which was followed by local 8-OH-DPAT injection. Five minutes later, the apneic response to fentanyl was repeated. The doses of 8-OH-DPAT and WAY-100635 were similar to those used in previous reports (42, 47). 5HT3Rs are present in the mNTS (19), but these receptors' impact on ventilation is unknown. To establish the unique effect of mNTS 5HT1ARs, we also compared the apneic response to fentanyl before and 5 min after bilaterally microinjecting 5HT3R agonist phenylbiguanide (1.0 nmol/side) into the mNTS as reported before (33) (n = 7).
Study series IV was designed to define whether mNTS 5HT1ARs play a substantial role in the blocking effect of systemic 8-OH-DPAT challenge on the apneic response to fentanyl (n = 8). Right atrial bolus injection of fentanyl was performed before and after 8-OH-DPAT (10 μg/kg iv) alone and coupled with WAY-100635 (0.4 nmol/side) pretreatment in the mNTS. Subsequently, we verified whether the modulatory effects on the apneic response to fentanyl, if it occurred, resulted from the time-course effect. To this end, intravenous injection of fentanyl before and after systemic 8-OH-DPAT challenge was conducted twice in three other rats. Again, a 1-h interval was allowed between two neighboring fentanyl injections as mentioned above. This small sample size was selected here because of the consistency of apneic blockade by systemic 8-OH-DPAT challenge with little variance (see results section). Since 5HT1ARs are expressed not only in the mNTS but also in the interstitial portion of the NTS (30, 49), we further determined the site dependency of the mNTS 5HT1ARs on the apneic blockade by systemic 8-OH-DPAT. Right atrial injection of fentanyl was performed before and after 8-OH-DPAT (iv) alone and coupled with WAY-100635 pretreatment in the interstitial portion of the NTS located 1-mm lateral to the mNTS, respectively, in six other rats. To estimate the effects of blocking endogenous mNTS 5HT1AR activation on the apneic response to right atrial bolus injection of fentanyl, we compared the cardiorespiratory responses to the fentanyl before and after microinjection of WAY-100635 (0.4 nmol/side) into the mNTS in four other rats.
Data acquisition and statistical analysis.
Raw data of the airflow, arterial blood pressure, HR, PetCO2, and rectal temperature were digitized, monitored, and recorded by a PowerLab/8sp (model ML 785; ADInstruments, Colorado Springs, CO) and a computer with the PowerLab Chart 5 software. Respiratory variables including expiratory duration (TE), tidal volume, respiratory frequency, and minute ventilation (VE) were derived by the on-line calculations. Baseline values (control) and their changes induced by different treatments (vagal perineural capsaicin treatment, vagotomy, systemic, or local injection of 5HT agonists/antagonist) were represented by absolute values. They were collected and averaged during the 10-s period immediately before fentanyl injection. In contrast, the responses to fentanyl were collected during the evoked apnea (prolonged TE) immediately after injection and expressed by percent change from the values obtained before fentanyl (Δ%). A TE threefold longer than baseline TE was defined as an apnea (62). If the apnea is blocked or shortened after a given treatment, the greatest TE response to fentanyl within 4 s after intravenous administration of fentanyl was measured. Group data were reported as means ± SE. One-way ANOVA with repeated measures was used to analyze the significant differences between the cardiorespiratory values or the fentanyl-induced apneic (cardiovascular) responses (Δ%) before and after the different treatments. If an overall test was significant, a Tukey test was utilized for specific comparisons between individual groups. SAS/STAT software (SAS Institute, Cary, NC) was used for statistical analysis. The difference was considered significant at a P value < 0.05.
RESULTS
Right atrial bolus injection of fentanyl produces a PCF-dependent apnea.
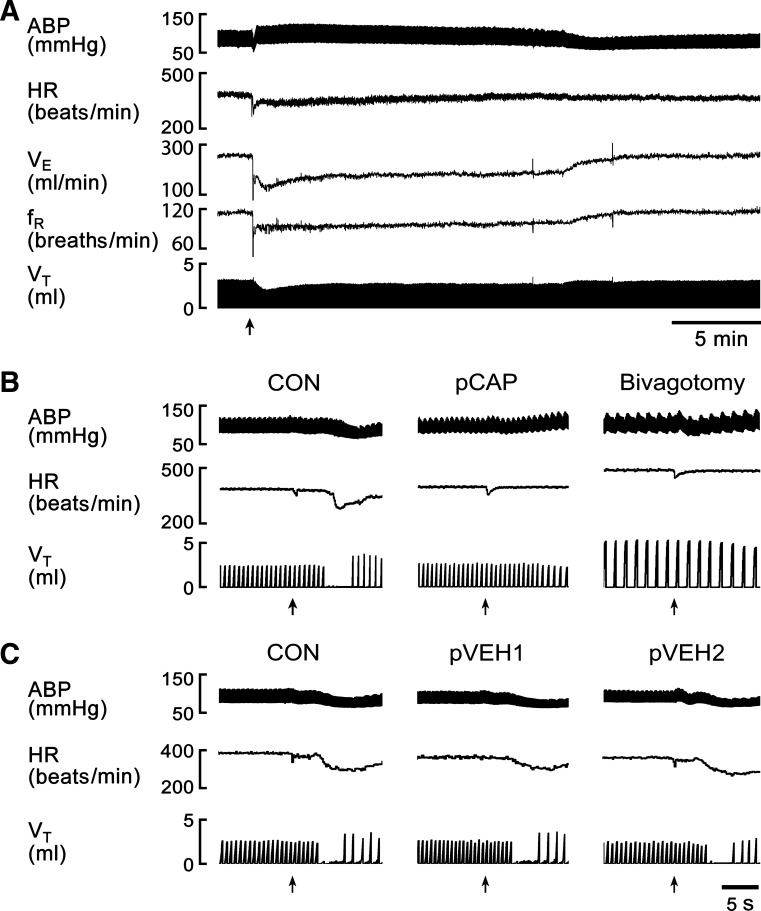
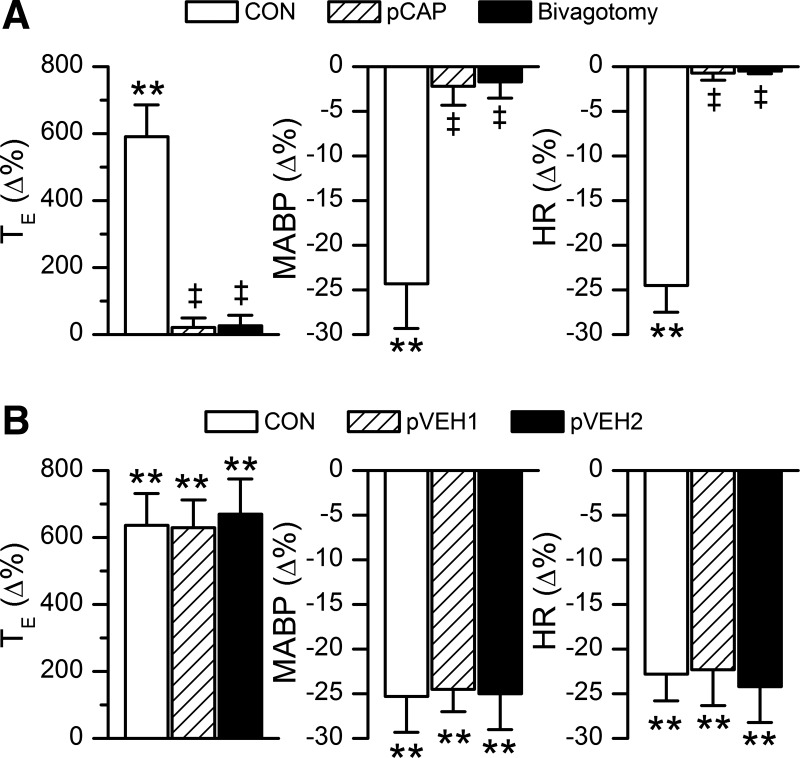
In anesthetized and spontaneously breathing rats, right atrial bolus injection of fentanyl (5.0 μg/kg), but not vehicle (not shown), evoked an immediate apnea lasting for 2.5 ± 0.4 s with TE 5.9-fold longer than the control immediately followed by a hypoventilation with a VE decrease to 52 ± 5% (Figs. 1 and 2). A bradycardia and hypotension were also observed during this apnea. The latency for the apneic response was 3.9 ± 0.4 s. These evoked cardiorespiratory activities returned to the control levels ∼20 min later as shown in Fig. 1A. We subsequently compared the fentanyl-evoked apnea before and after blocking vagal C-fibers' conduction by using perineural capsaicin treatment and subsequently after bivagotomy. The result showed that the fentanyl-evoked apnea, bradycardia, and hypotension were eliminated by either perineural capsaicin treatment of vagi or bivagotomy but unaffected by perineural vehicle treatment of vagi (Figs. 1 and 2). The similar cardiorespiratory responses to right atrial bolus injections of fentanyl after twice perineural vehicle treatments of vagi indicated a high reproducibility of the fentanyl-induced responses. The hypoventilation following the fentanyl-induced apnea was not eliminated and significantly attenuated by perineural capsaicin treatment of vagi or bivagotomy. As shown in Table 2, perineural treatment of vagi with capsaicin or vehicle did not significantly alter the baseline cardiorespiratory variables, which is similar to the previous reports (26, 27). Bivagotomy strikingly lowered respiratory frequency and increased tidal volume, leading to no change in VE with a significantly prolonged TE and elevated HR.
Fig. 1.
A: typical recordings showing the fentanyl-evoked cardiorespiratory activities and their recoveries. fR, respiratory frequency; VE, minute ventilation. B: typical recordings showing the fentanyl-evoked apneic and cardiovascular responses before (CON) and after perineural treatment of the vagi with capsaicin (pCAP) and subsequent bivagotomy in an anesthetized rat. C: typical recordings of the cardiorespiratory responses to fentanyl before and after perineural treatment of the vagi with vehicle for twice (pVEH1 and pVEH2) in another rat. The traces are arterial blood pressure (ABP), heart rate (HR), and tidal volume (VT). Arrows point to the onset of bolus injection of fentanyl (5.0 μg/kg) into the right atrium.
Fig. 2.
Group data of the fentanyl-evoked expiratory duration (TE) prolongation and cardiovascular responses before and after perineural treatment of the vagi with capsaicin (pCAP) and subsequent bivagotomy (A, n = 7) or after twice perineural treatments of the vagi with vehicle (pVEH1 and pVEH2; B, n = 6). Values are means ± SE; **P < 0.01, compared with the baseline values (before injecting fentanyl into the right atrium); ‡P < 0.01, compared with control (CON = without manipulation of vagi). MABP, mean arterial blood pressure.
Table 2.
Cardiorespiratory activities before and after perineural capsaicin or vehicle treatment of vagi and subsequent bivagotomy
| VE, ml/min | VT, ml | fR, breaths/min | MABP, mmHg | HR, beats/min | PetCO2, Torr | TE, s | |
|---|---|---|---|---|---|---|---|
| CON | 257 ± 13 | 2.4 ± 0.1 | 107 ± 5 | 97 ± 6 | 395 ± 15 | 37 ± 2 | 0.36 ± 0.02 |
| pCAP | 265 ± 36 | 2.8 ± 0.7 | 102 ± 6 | 99 ± 12 | 380 ± 7 | 38 ± 2 | 0.37 ± 0.04 |
| Bivagotomy | 223 ± 17 | 5.8 ± 0.4* | 40 ± 1* | 95 ± 12 | 507 ± 22* | 40 ± 3 | 0.93 ± 0.03* |
| CON | 260 ± 23 | 2.5 ± 0.4 | 112 ± 8 | 110 ± 9 | 386 ± 12 | 38 ± 2 | 0.33 ± 0.03 |
| pVEH1 | 258 ± 21 | 2.5 ± 0.4 | 113 ± 8 | 108 ± 10 | 384 ± 11 | 40 ± 2 | 0.35 ± 0.02 |
| pVEH2 | 264 ± 11 | 2.3 ± 0.1 | 115 ± 4 | 99 ± 6 | 394 ± 9 | 40 ± 3 | 0.34 ± 0.04 |
Values are means ± SE; n = 7 for perineural capsaicin (pCAP) and subsequent bivagotomy; pVEH1 and pVEH2, first and second perineural vehicle treatment of vagi in 6 rats, respectively;
P < 0.01 vs. control (CON = vagal intact). VE, minute ventilation; VT, tidal volume; fR, respiratory frequency; TE, expiratory duration; MABP, mean arterial blood pressure; HR, heart rate; and PetCO2, end-tidal pressure of carbon dioxide.
Systemic 8-OH-DPAT challenge abolishes the apneic response to fentanyl.
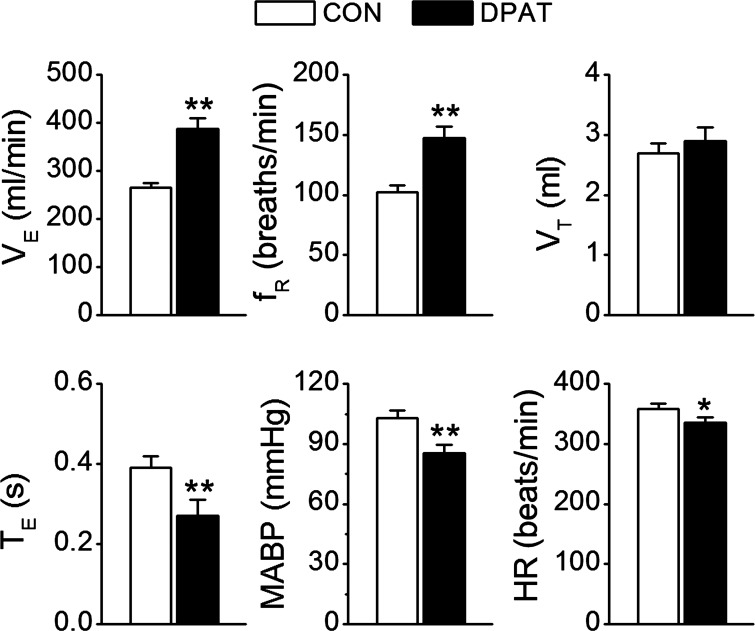
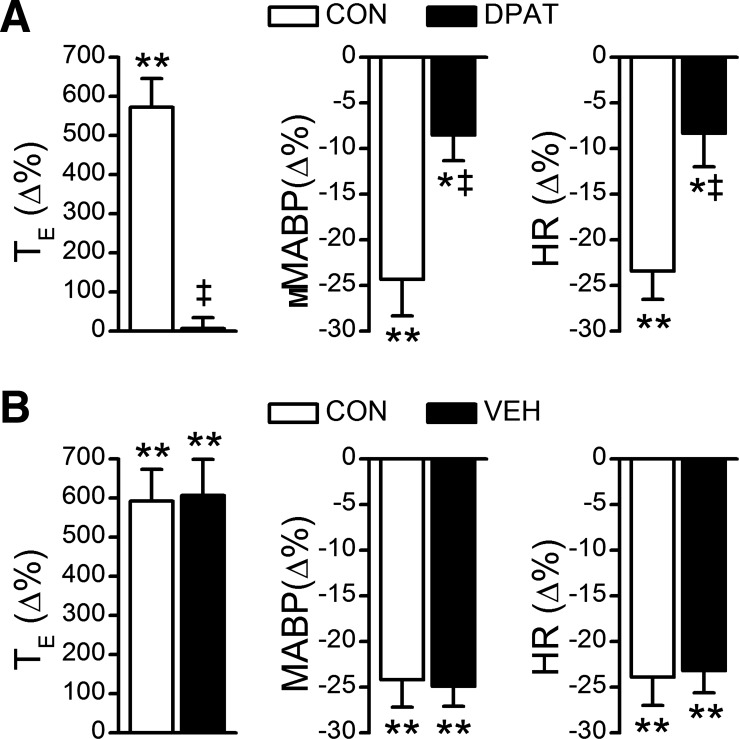
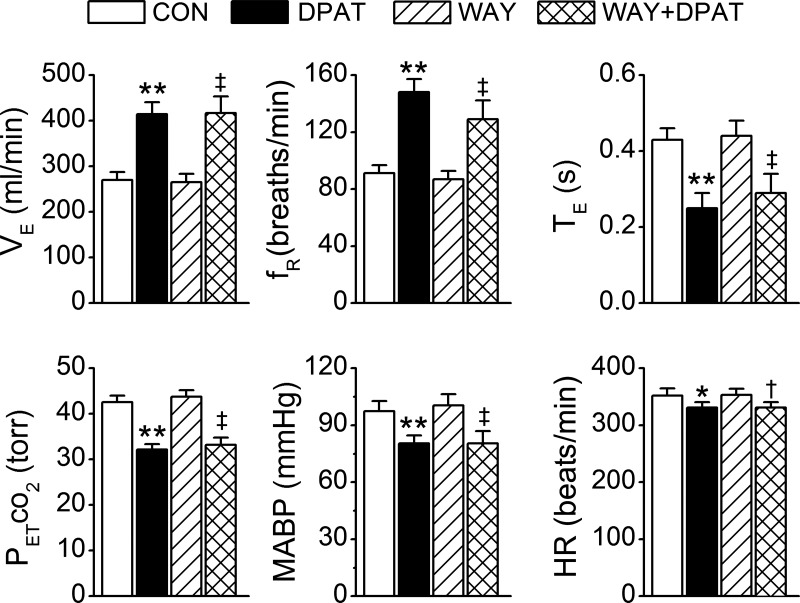
To determine whether systemic administration of 8-OH-DPAT prevents the apneic response, we compared the fentanyl-evoked apnea before and after intravenous administration of vehicle or 8-OH-DPAT. First, we tested the influences of systemic administration of 8-OH-DPAT on cardiorespiratory variables. Systemic administration of 8-OH-DPAT but not vehicle markedly increased baseline VE (Fig. 3). Compared with control, ventilation was increased by 47% mainly via elevating respiratory frequency by 45%. This excitatory ventilatory response was associated with a hypocapnia (from 40 ± 1.2 to 32 ± 2 Torr, P < 0.01), hypotension (from 103 ± 4 to 85 ± 4 mmHg, P < 0.01), and bradycardia (from 358 ± 9 to 336 ± 8 beats/min, P < 0.05). Systemic 8-OH-DPAT challenge-induced hyperventilation reached a plateau at ∼30 s that lasted for ∼2 min, and these changes returned to control levels within 1 h. Second, we compared the fentanyl-induced apnea before and after systemic administration of 8-OH-DPAT (Fig. 4A) or vehicle (Fig. 4B). Interestingly, intravenous administration of 8-OH-DPAT but not vehicle abolished the apneic response to fentanyl. In addition, systemic application of 8-OH-DPAT did not completely abolish, but significantly attenuated, MABP and HR responses to fentanyl.
Fig. 3.
Baseline cardiorespiratory activities (CON) and their responses to systemic 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) challenge (DPAT). n = 9; values are means ± SE; *P < 0.05 and **P < 0.01, vs. CON.
Fig. 4.
Fentanyl-induced TE prolongation and cardiovascular responses before and after intravenous administration of DPAT (A) or vehicle (VEH, B). n = 9; values are means ± SE; *P < 0.05 and **P < 0.01, compared with the baseline values (before injecting fentanyl into the right atrium); ‡P < 0.01, compared with CON (before systemic DPAT challenge).
Intra-mNTS injection of 8-OH-DPAT greatly attenuates the apneic response to fentanyl.
To reveal the role of mNTS 5HT1ARs in modulating the fentanyl-induced apnea, we compared the apnea before and after local injection of 8-OH-DPAT alone and coupled with WAY-100635 pretreatment. We found that intra-mNTS microinjection of 8-OH-DPAT rather than vehicle (not shown) markedly attenuated the apneic response to fentanyl by 64%, and this attenuation disappeared after pretreating the mNTS with WAY-100635 (Fig. 5). Intra-mNTS injection of 8-OH-DPAT also significantly diminished the hypotension and bradycardia response to fentanyl, which was reversed by local WAY-100635 pretreatment. In sharp contrast, stimulating mNTS 5HT3Rs by local microinjection of phenylbiguanide had no significant effect on the cardiorespiratory responses to fentanyl. The responses of TE, MABP, and HR to intravenous fentanyl before and after local 5HT3Rs blockade were 564 ± 133 vs. 574 ± 148%, −26.6 ± 3.0 vs. −23.7 ± 2.8%, and −22.1 ± 2.1 vs. −21.3 ± 1.4%, respectively (all P > 0.05). With respect to baseline values, local injection of 8-OH-DPAT or phenylbiguanide did not significant alter baseline cardiorespiratory values (Table 3).
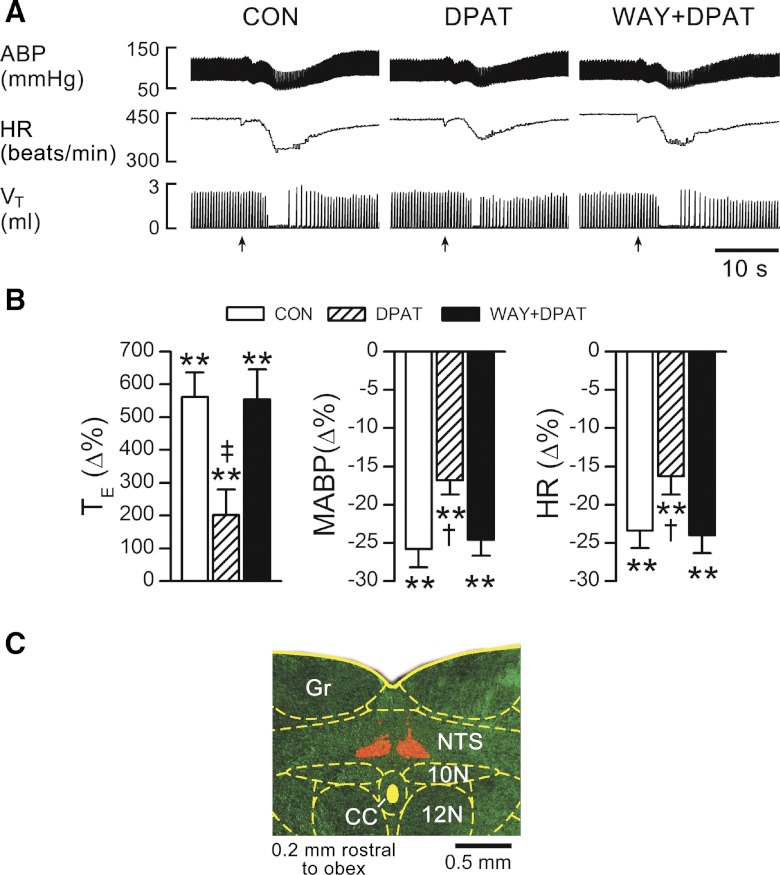
Fig. 5.
Fentanyl-evoked TE prolongation before and after intra-caudomedial nucleus of solitary tract (mNTS) microinjection of DPAT without and with local WAY-100635 pretreatment (WAY+DPAT). A: typical recordings with arrows indicating the onset of bolus injections of fentanyl via the right atrium. B: group data. n = 7; values are means ± SE; **P < 0.01, compared with baseline values (“0”) obtained before injecting fentanyl into the right atrium; †P < 0.05 and ‡P < 0.01, compared with control (CON = without systemic or local treatment) or WAY+DPAT. C: bilateral microinjection locations labeled by fluorescent microbeads. 10N, dorsal motor nucleus of vagus; 12N, hypoglossal nucleus; CC, central canal; Gr, gracile nucleus.
Table 3.
Effects of intra-mNTS microinjection of 5HT1AR agonist 8-OH-DPAT or 5HT3R agonist phenylbiguanide on the cardiorespiratory activities
| Microinjection | VE, ml/min | VT, ml | fR, breaths/min | MABP, mmHg | HR, beats/min | PetCO2, Torr | TE, s |
|---|---|---|---|---|---|---|---|
| 8-OH-DPAT (n = 7) | |||||||
| Before | 260 ± 9 | 2.3 ± 0.2 | 117 ± 6 | 94 ± 2 | 376 ± 15 | 38 ± 1 | 0.33 ± 0.02 |
| After | 240 ± 21 | 2.3 ± 0.1 | 104 ± 6 | 97 ± 4 | 377 ± 17 | 41 ± 3 | 0.39 ± 0.04 |
| PBG (n = 7) | |||||||
| Before | 266 ± 12 | 2.5 ± 0.1 | 109 ± 6 | 98 ± 5 | 351 ± 12 | 39 ± 2 | 0.33 ± 0.03 |
| After | 278 ± 11 | 2.5 ± 0.1 | 113 ± 3 | 92 ± 1 | 367 ± 10 | 38 ± 1 | 0.31 ± 0.01 |
Values are means ± SE. PBG, phenylbiguanide (5HT3R agonist).
Intra-mNTS injection of WAY-100635 diminishes the apneic blockade by intravenous 8-OH-DPAT.
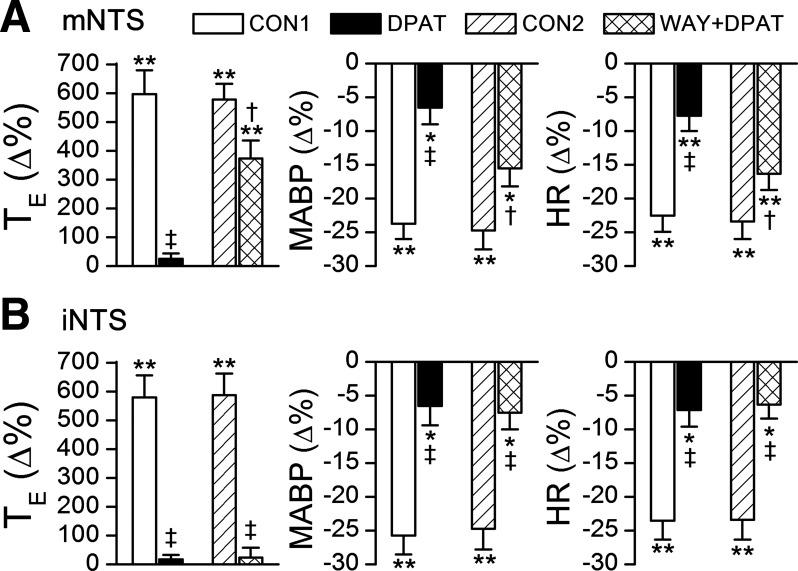
The purpose of this study series was to evaluate the contribution of mNTS 5HT1ARs to the apneic blockade by systemic 8-OH-DPAT challenge. Our data showed that after pretreating the mNTS with WAY-100635, systemic 8-OH-DPAT challenge was unable to completely abolish fentanyl-induced apnea but still shortened it by 58% (Figs. 6A and 7A). Moreover, the effects of systemic administration of 8-OH-DPAT on reducing the hypotension and bradycardia responses to fentanyl became significantly smaller after pretreating the mNTS with WAY-100635. In six other rats, the microinjections of WAY-100635 were made in the interstitial portion of the NTS, in which the apneic blockade by systemic 8-OH-DPAT challenge was not significantly changed (Figs. 6, B and C, and 7B). To verify whether the modulatory effects of mNTS 5HT1Rs on the apneic response to fentanyl resulted from the time-course effect, intravenous injection of fentanyl before and after systemic 8-OH-DPAT challenge was performed twice in three other rats. TE responses to fentanyl were 5.7-fold and 5.9-fold longer than the baseline for the first and second test, respectively, and both responses were fully abolished by systemic 8-OH-DPAT challenge (−6 ± 5% and −3 ± 4%). These data were not supportive of this assumption.
Fig. 6.
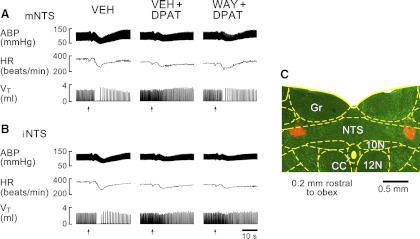
Typical recordings showing the effects of microinjecting WAY into the mNTS (A) or the interstitial NTS (iNTS, B) on the apneic blockade by systemic DPAT challenge in two rats, respectively. VEH, local microinjection of vehicle; VEH+DPAT or WAY+DPAT, local microinjection of vehicle or WAY followed by systemic DPAT challenge. Arrows point to the onset of bolus injection of fentanyl into the right atrium. C: locations of microbeads microinjected into bilateral iNTS.
Fig. 7.
Effects of microinjection of WAY into the mNTS (A) or the iNTS (B) on the apneic blockade by systemic DPAT challenge. CON1, the control of apneic response to fentanyl before systemic DPAT challenge; CON2, the recovery of the apneic response from systemic DPAT challenge; DPAT, intravenous injection of 8-OH-DPAT; WAY+ DPAT, intra-mNTS or -iNTS microinjection of WAY-100635 (WAY) followed by systemic DPAT challenge. n = 8; values are means ± SE; *P < 0.05 and **P < 0.01, compared with the baseline values (before injecting fentanyl into the right atrium); †P < 0.05 and ‡P < 0.01, compared with CON1/CON2 and DPAT.
It is noteworthy that local WAY-100635 pretreatment in the mNTS (Fig. 8) or in the sites outside of the mNTS (not shown) did not significantly alter baseline cardiorespiratory values and their responses to systemic 8-OH-DPAT challenge. In addition, we evaluated the role of endogenous mNTS 5HT1AR activation in the apneic response to right atrial bolus injection of fentanyl. We found that microinjection of WAY-100635 into the mNTS did not significantly change the fentanyl-induced apnea (602 ± 89 vs. 719 ± 106%; P > 0.05), hypotension (−24 ± 2 vs. −27 ± 3%; P > 0.05), and bradycardia (−26 ± 3 vs. −28 ± 3%; P > 0.05).
Fig. 8.
Comparison of baseline cardiorespiratory activities and their responses to systemic DPAT challenge before and after pretreating the mNTS with WAY. CON, before intra-mNTS injection of WAY; DPAT, systemic DPAT challenge; WAY, intra-mNTS injection of WAY; WAY+DPAT, intra-mNTS injection of WAY followed by systemic DPAT challenge. n = 8; values are means ± SE; *P < 0.05 and **P < 0.01, compared with CON; †P < 0.05 and ‡P < 0.01, compared with WAY. Note: all data of WAY+DPAT are not significantly different from those of DPAT alone (P > 0.05). PetCO2, end-tidal pressure of carbon dioxide.
DISCUSSION
There are three major findings in this study: 1) the apneic response to right atrial bolus injection of fentanyl is fully PCF dependent; 2) intravenous administration of 5HT1AR agonist 8-OH-DPAT prevents this apnea; and 3) 5HT1ARs in the mNTS play an important role in the apneic blockade by systemic 8-OH-DPAT challenge.
Several studies have demonstrated the presence of MORs on vagus nerve (1, 3, 21) and a vagal-mediated apneic response to opioids (21, 54, 55, 58). Our results reveal that similar to bivagotomy, blocking PCFs' conduction fully abolished the apneic response to right atrial bolus injection of fentanyl, providing direct functional evidence of a dominant role PCFs play in generating this apnea. This novel finding is in line with an enhanced PCF activity in response to intra-atrium perfusion of opioids (45, 54, 55), and vagal afferent neurons' colabeling with MORs and substance P/calcitonin gene-related peptide (9) that are the common markers for PCFs (28). In the anesthetized cat (32) and rabbit (7), opioids reportedly facilitate the apneic response to stimulating pulmonary stretch receptor. However, the full dependency of the apneic response to fentanyl on PCFs minimizes the possible contribution of the pulmonary stretch receptors to the apnea observed in this study. Fentanyl-evoked apnea in this study has a latency of 3.9 s, significantly longer than that previously reported where intra-atrium injection of opioids induced an apnea within 2 s (54, 55). This discrepancy may be because our study was performed in anesthetized animals, whereas their experiments were conducted on decerebrate and unanesthetized rats.
An important finding in this study is that the PCF-mediated apneic response to fentanyl was fully prevented by systemic 8-OH-DPAT challenge. Previous studies have shown that an immediate expiratory apneic response to bolus injection of opioids into the vein or right atrium is vagal mediated (21, 54, 55, 58). The respiratory depression following the apnea presumably results from opioids directly acting on the medullary respiratory neurons (2). Interestingly enough, 8-OH-DPAT administered intravenously was reported to be able to restore the respiratory rhythm from a ventilatory arrest induced by central action of morphine in anesthetized rats (43).Our data, along with these results, lead to the conclusion that systemic 8-OH-DPAT challenge is capable of antagonizing both PCF- and central-mediated apneic (ventilatory arrest) responses to MOR agonists.
Two lines of evidence in this study support a crucial contribution of mNTS 5HT1ARs to antagonizing the apneic response to fentanyl. First, antagonizing mNTS 5HT1ARs largely attenuated (58%, see Figs. 6A and 7A) the apneic blockade by systemic administration of 8-OH-DPAT, consistent with a greatly attenuated (64%, see Fig. 5) apneic response to fentanyl after intra-mNTS injection of 8-OH-DPAT. These data demonstrate that activation of mNTS 5HT1ARs plays an important role in reversing the expiratory apnea by right atrial bolus injection of fentanyl. However, this incomplete reversing (∼60% shortening of the fentanyl-induced apnea by activation of mNTS 5HT1AR) suggests that other brain stem regions, aside from the mNTS, are also involved. In fact, in addition to the mNTS, other medullary nuclei, such as the nucleus ambiguous (24), raphe neurons (50), and pre-BötC (40) also express 5HT1ARs, and these receptors are involved in generating and modulating respiratory rhythm. Thus they may also participate in the blocking effect of systemic 8-OH-DPAT challenge. It should be noted that the effects of endogenous activation of central 5HT1ARs on the baseline cardiorespiratory activity may differ from those of exogenous activation of central 5HT1ARs. For example, we recently found that intravenous administration of 8-OH-DPAT-induced hyperventilation was central-mediated largely via action on pre-BötC 5HT1ARs (63). However, blocking endogenous 5HT1AR activation in the CNS by intracisternal infusion of WAY-100635 failed to change the baseline respiratory activity (22). This difference may also be injecting site related. Second, neither microinjection of 8-OH-DPAT into the interstitial portion of the NTS nor microinjection of 5HT3R agonist phenylbiguanide into the mNTS altered the PCF-mediated apneic response to fentanyl. These data agree with previous results showing that the mNTS is the key for PCF-mediated apnea due to its containing the second-order neurons of PCF inputs (51, 57) and densely expressing 5HT1ARs (30, 31, 49). The mechanisms by which activating mNTS 5HT1ARs antagonizes the PCF-mediated apneic response to fentanyl are unknown. One possible interpretation is that the mNTS neurons involved in the PCF-mediated TE response to fentanyl are directly or indirectly inhibited by activating local 5HT1ARs because 8-OH-DPAT was reportedly inhibitory to some medullary respiratory neural activity (25, 40).
The majority of previous reports have pointed out that systemic administration of 5HT1AR agonists excite ventilation in animals (10, 13, 25, 48). A similar excitatory effect of systemic 8-OH-DPAT challenge on ventilation and respiratory frequency was also observed in this study. Since fentanyl fails to produce the apnea after systemic 8-OH-DPAT challenge that causes a hyperventilation via elevating respiratory frequency, it becomes questionable whether the evoked hyperventilation per se is the key responsible for the apneic blockade. Our observation that microinjection of 8-OH-DPAT into the mNTS did not alter baseline ventilation but largely diminished the apneic blockade indicates the independence of the apneic blockade on this hyperventilation. In the present study, microinjection of 5HT1AR agonist or antagonist into the mNTS did not significantly alter baseline cardiorespiratory values and their changes after systemic 8-OH-DPAT challenge, pointing to a limited contribution of endogenous and exogenous activation of mNTS 5HT1ARs to eupneic breathing. Because multiple respiratory-related medullary regions contains 5HT1ARs (24, 40, 50) in addition to the mNTS, their involvement in the hyperventilation requires further clarification.
With respect to cardiovascular activity, this study generates several new findings. First, the fentanyl-induced hypotension and bradycardia were mediated by PCFs, extending the previous results from bivagotomy (21, 43). Second, these cardiovascular responses to fentanyl were significantly attenuated, but not eliminated, by systemic administration of 8-OH-DPAT. Similar to this, a previous study indicated a blocking effect of 8-OH-DPAT on the central action of morphine-induced apnea with attenuation of the associated hypotension and bradycardia (43). These data suggest a much more powerful impact of systemic activation of 5HT1ARs on antagonizing MOR-induced respiratory than cardiovascular responses. Third, the effect of systemic 8-OH-DPAT challenge on the apneic and hypotension/bradycardia responses to fentanyl was similarly reduced after intra-mNTS injection of 5HT1AR antagonist. This similarity supports the postulation that this local activation of 5HT1ARs may occur on the second-order neurons receiving PCF inputs within the mNTS. In addition, the effects of endogenous activation of central 5HT1ARs on the PCF-mediated bradycardia seem to be site dependent. The PCF-mediated bradycardia was unaffected by WAY-100635 microinjected into the mNTS in this study, but attenuated by intracisternal infusion of the same agent (22).
Perspectives and Significance
Our study is potentially relevant to clinical care. Opioids are the most powerful analgesics with side effects such as the risk of ventilatory depression and apnea (12, 14, 44, 52). The latter could be lethal (16, 44), especially in some patients with pulmonary diseases such as chronic obstructive pulmonary disease (4, 15, 18). Many studies have shown that pulmonary inflammatory mediators–hypoxemia and hypercapnia, congestion, and edema–that are common in patients with these diseases, can sensitize and/or stimulate PCFs to prolong PCF-mediated apnea (8, 17, 41). For example, activation of PCF-induced apneic duration is substantially prolonged to a lethal degree by acute hypoxia (59) or viral infection (pulmonary inflammation) (38) in animals. These results, along with our finding that intravenous injection of fentanyl induced an apnea fully dependent on PCFs, may provide one possible explanation for why these patients are more vulnerable to opioids. In addition, the antagonistic effect of 8-OH-DPAT on the PCF- (in the present study) and central-mediated apneic responses to opioids (reported before) may generate a clue to therapeutically prevent the respiratory disorders induced by opioids. Our findings that systemic 8-OH-DPAT challenge antagonizes the PCF-mediated apneic response to MOR agonists in which mNTS 5HT1ARs play an important role also extends respiratory physiology of PCFs. Further studies are warranted to elucidate the mechanism by which activation of mNTS 5HT1AR antagonizes the PCF-mediated apneic response to fentanyl.
GRANTS
This study is supported by National Institutes of Health Grants HL-107462 and ALA RG-191095-N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z., Z.Z., and F.X. conception and design of research; J.Z., Z.Z., and C.Z. performed experiments; J.Z. and C.Z. analyzed data; J.Z. and F.X. interpreted results of experiments; J.Z. prepared figures; J.Z. and F.X. drafted manuscript; J.Z., Z.Z., and F.X. edited and revised manuscript; F.X. approved final version of manuscript.
REFERENCES
- 1. Aicher SA, Goldberg A, Sharma S, Pickel VM. mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol 422: 181– 190, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Ballanyi K, Lalley PM, Hoch B, Richter DW. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J Physiol 504: 127– 134, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belvisi MG, Hele DJ. Cough Sensors. III. Opioid and cannabinoid receptors on vagal sensory nerves. In: Handbook of Experimental Pharmacology, Berlin, Germany: Springer-Verlag, 2009, vol. 187, p. 63– 76 [DOI] [PubMed] [Google Scholar]
- 4. Berrill JW, Linnasne SJ. Morphine for management of refractory dyspnoea: opiates should be used with caution. BMJ 327: 1288; author reply 1288–1289, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1– 45, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Bonham AC, Joad JP. Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol 441: 95– 112, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Budzinska K, Grieb P, Romaniuk JR. Morphine selectively facilitates the inspiratory-inhibitory vagal reflex in adult rabbits. Experientia 41: 458– 460, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Churchill ED, Cope O. The rapid shallow breathing resulting from pulmonary congestion and edema. J Exp Med 49: 531– 537, 1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding YQ, Li JL, Lu BZ, Wang D, Zhang ML, Li JS. Co-localization of mu-opioid receptor-like immunoreactivity with substance P-LI, calcitonin gene-related peptide-LI and nitric oxide synthase-LI in vagal and glossopharyngeal afferent neurons of the rat. Brain Res 792: 149– 153, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Edwards E, Whitaker-Azmitia PM, Harkins K. 5-HT1A and 5-HT1B agonists play a differential role on the respiratory frequency in rats. Neuropsychopharmacology 3: 129– 136, 1990 [PubMed] [Google Scholar]
- 11. Ellis DJ, Steward DJ. Fentanyl dosage is associated with reduced blood glucose in pediatric patients after hypothermic cardiopulmonary bypass. Anesthesiology 72: 812– 815, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth 41: 125– 132, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Garner SJ, Eldridge FL, Wagner PG, Dowell RT. Buspirone, an anxiolytic drug that stimulates respiration. Am Rev Respir Dis 139: 946– 950, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Goetz AM, Rogers PL, Schlichtig R, Muder RR, Diven WF, Prior RB. Adult respiratory distress syndrome associated with epidural fentanyl infusion. Crit Care Med 22: 1579– 1583, 1994 [PubMed] [Google Scholar]
- 15. Gruber EM, Tschernko EM. Anaesthesia and postoperative analgesia in older patients with chronic obstructive pulmonary disease: special considerations. Drugs Aging 20: 347– 360, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hall WD, Degenhardt LJ, Lynskey MT. Opioid overdose mortality in Australia, 1964–1997: birth-cohort trends. Med J Aust 171: 34– 37, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Hatridge J, Haji A, Perez-Padilla JR, Remmers JE. Rapid shallow breathing caused by pulmonary vascular congestion in cats. J Appl Physiol 67: 2257– 2264, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Horton R, Barber C. Opioid-induced respiratory depression resulting from transdermal fentanyl-clarithromycin drug interaction in a patient with advanced COPD. J Pain Symptom Manage 37: e2– e5, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Huang J, Spier AD, Pickel VM. 5-HT3A receptor subunits in the rat medial nucleus of the solitary tract: subcellular distribution and relation to the serotonin transporter. Brain Res 1028: 156– 169, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Jones JFX, Jordan D. Activity of medullary respiratorv neurones during the pulmonary chemoreflex in anaesthetized rabbits. J Physiol 459: 354P, 1993 [Google Scholar]
- 21. Kaczynska K, Szereda-Przestaszewska M. Involvement of vagal opioid receptors in respiratory effects of morphine in anaesthetized rats. J Physiol Pharmacol 56: 195– 203, 2005 [PubMed] [Google Scholar]
- 22. Kellett DO, Ramage AG, Jordan D. Central 5-HT7 receptors are critical for reflex activation of cardiac vagal drive in anaesthetized rats. J Physiol 563: 319– 331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kellett DO, Stanford SC, Machado BH, Jordan D, Ramage AG. Effect of 5-HT depletion on cardiovascular vagal reflex sensitivity in awake and anesthetized rats. Brain Res 1054: 61– 72, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lalley PM. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N,N, dimethyltryptamine. Brain Res 648: 87– 98, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol 476: 117– 130, 1994 [PMC free article] [PubMed] [Google Scholar]
- 26. Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol 91: 1318– 1326, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Lee LY, Morton RF, Lundberg JM. Pulmonary chemoreflexes elicited by intravenous injection of lactic acid in anesthetized rats. J Appl Physiol 81: 2349– 2357, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47– 65, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Li JL, Kaneko T, Mizuno N. Effects of peripheral nerve ligation on expression of mu-opioid receptor in sensory ganglion neurons: an immunohistochemical study in dorsal root and nodose ganglion neurons of the rat. Neurosci Lett 214: 91– 94, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience 165: 61– 78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol (Berl) 117: 257– 265, 2009 [DOI] [PubMed] [Google Scholar]
- 32. May AJ, Widdicombe JG. Depression of the cough reflex by pentobarbitone and some opium derivatives. Br J Pharmacol Chemother 9: 335– 340, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merahi N, Laguzzi R. Cardiovascular effects of 5HT2 and 5HT3 receptor stimulation in the nucleus tractus solitarius of spontaneously hypertensive rats. Brain Res 669: 130– 134, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Mutoh T, Bonham AC, Joad JP. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am J Physiol Regul Integr Comp Physiol 279: R1215– R1223, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Paton J. Apnoeic rhythm of respiratory neurons in mature mice. J Physiol 497: 4s, 1996. [Google Scholar]
- 36. Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365– 2373, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Paton JF. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. J Physiol 502: 623– 639, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng W, Zhuang J, Harrod KS, Xu F. Respiratory syncytial virus infection in anesthetized weanling rather than adult rats prolongs the apneic responses to right atrial injection of capsaicin. J Appl Physiol 102: 2201– 2206, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol 2: 788– 793, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514: 567– 578, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J: 3s– 14s, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther 323: 477– 487, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA. Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. J Pharmacol Exp Ther 292: 704– 713, 2000 [PubMed] [Google Scholar]
- 44. Santiago TV, Edelman NH. Opioids and breathing. J Appl Physiol 59: 1675– 1685, 1985 [DOI] [PubMed] [Google Scholar]
- 45. Sapru HN, Willette RN, Krieger AJ. Stimulation of pulmonary J receptors by an enkephalin-analog. J Pharmacol Exp Ther 217: 228– 234, 1981 [PubMed] [Google Scholar]
- 46. Scott BH. Opioids in cardiac surgery: cardiopulmonary bypass and inflammatory response. Int J Cardiol 64, Suppl 1: S35– S41, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Sporton SC, Shepheard SL, Jordan D, Ramage AG. Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anaesthetized rat. Br J Pharmacol 104: 466– 470, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Szereda-Przestaszewska M, Kaczynska K. Peripheral 5-HT1A receptors are not essential for increased ventilation evoked by systemic 8-OH-DPAT challenge in anaesthetized rats. Exp Physiol 92: 953– 961, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Thor KB, Blitz-Siebert A, Helke CJ. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse 10: 217– 227, 1992 [DOI] [PubMed] [Google Scholar]
- 50. Trulson ME, Arasteh K. Buspirone decreases the activity of 5-hydroxytryptamine-containing dorsal raphe neurons in-vitro. J Pharm Pharmacol 38: 380– 382, 1986 [DOI] [PubMed] [Google Scholar]
- 51. Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in the nucleus tractus solitarius mediate the responses to the stimulation of cardio-pulmonary vagal afferent C fiber endings. Brain Res 618: 23– 31, 1993 [DOI] [PubMed] [Google Scholar]
- 52. Walker JM, Farney RJ. Are opioids associated with sleep apnea? A review of the evidence. Curr Pain Headache Rep 13: 120– 126, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Ramage AG, Jordan D. In vivo effects of 5-hydroxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neuropharmacology 36: 489– 498, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Willette RN, Sapru HN. Peripheral versus central cardiorespiratory effects of morphine. Neuropharmacology 21: 1019– 1026, 1982 [DOI] [PubMed] [Google Scholar]
- 55. Willette RN, Sapru HN. Pulmonary opiate receptor activation evokes a cardiorespiratory reflex. Eur J Pharmacol 78: 61– 70, 1982 [DOI] [PubMed] [Google Scholar]
- 56. Wilson CG, Bonham AC. Effect of cardiopulmonary C fibre activation on the firing activity of ventral respiratory group neurones in the rat. J Physiol 504: 453– 466, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilson CG, Zhang Z, Bonham AC. Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii in rats. J Physiol 496: 773– 785, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wojciechowski P, Szereda-Przestaszewska M, Lipkowski AW. Supranodose vagotomy eliminates respiratory depression evoked by dermorphin in anaesthetized rats. Eur J Pharmacol 563: 209– 212, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Xu F, Gu QH, Zhou T, Lee LY. Acute hypoxia prolongs the apnea induced by right atrial injection of capsaicin. J Appl Physiol 94: 1446– 1454, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Zarbin MA, Wamsley JK, Kuhar MJ. Anterograde transport of opioid receptors in rat vagus nerves and dorsal roots of spinal nerves: pharmacology and sensitivity to sodium and guanine nucleotides. Exp Brain Res Exp Hirnforschung 81: 267– 278, 1990 [DOI] [PubMed] [Google Scholar]
- 61. Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107: 288– 297, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Zhuang J, Xu J, Zhang C, Xu F. IL-1beta acutely increases pulmonary SP and permeability without associated changes in airway resistance and ventilation in anesthetized rats. Respir Physiol Neurobiol 175: 12– 19, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Zhuang J, Zhang C, Xu F. Pre-Botzinger Complex (PBC) plays an important role in the hyperventilatory response to intravenous injection of 5HT1AR agonist in anesthetized rats (Abstract). FASEB J 895.4: 26, 2012. [Google Scholar]