Abstract
Exercise training (ExT) normalizes the increased sympathetic outflow in heart failure (HF), but the underlying mechanisms are not known. We hypothesized ExT would normalize the augmented activation of the paraventricular nucleus (PVN) via an angiotensinergic mechanism during HF. Four groups of rats used were the following: 1) sham-sedentary (Sed); 2) sham-ExT; 3) HF-Sed, and 4) HF-ExT. HF was induced by left coronary artery ligation. Four weeks after surgery, 3 wk of treadmill running was performed in ExT groups. The number of FosB-positive cells in the PVN was significantly increased in HF-Sed group compared with the sham-Sed group. ExT normalized (negated) this increase in the rats with HF. In anesthetized condition, the increases in renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR) in response to microinjection of angiotensin (ANG) II (50∼200 pmol) in the PVN of HF-Sed group were significantly greater than of the sham-Sed group. In the HF-ExT group the responses to microinjection of ANG II were not different from sham-Sed or sham-ExT groups. Blockade of ANG II type 1 (AT1) receptors with losartan in the PVN produced a significantly greater decrease in RSNA, MAP, and HR in HF-Sed group compared with sham-Sed group. ExT prevented the difference between HF and sham groups. AT1 receptor protein expression was increased 50% in HF-Sed group compared with sham-Sed group. In the HF-ExT group, AT1 receptor protein expression was not significantly different from sham-Sed or sham-ExT groups. In conclusion, one mechanism by which ExT alleviates elevated sympathetic outflow in HF may be through normalization of angiotensinergic mechanisms within the PVN.
Keywords: blood pressure, central nervous system, at1 receptors, renal nerve activity
a hallmark feature of chronic heart failure (HF) is increased sympathoexcitation, which correlates with the severity of the disease (30, 32). The sympathoexcitatory state increases the progression and risk of mortality in patients with HF (22). The mechanism(s) underlying these abnormalities are poorly understood. Sinoaortic or cardiovagal denervation does not normalize plasma norepinephrine levels in pacing-induced HF dogs compared with nondenervated HF dogs (4, 23). Plasma norepinephrine is also not altered in HF dogs by β-adrenergic blockade (23). Increasing number of studies have suggested that altered central nervous system mechanisms may be responsible for the elevated neurohumoral drive in HF (16, 21, 32).
The paraventricular nucleus (PVN) of the hypothalamus is an important central site for the integration of sympathetic nerve activity (39). The PVN is reciprocally connected to other areas of the brain including the nucleus tractus solitarii (NTS) and the rostral ventrolateral medulla (RVLM) in the brain stem, which are involved in control of cardiovascular function (14). The PVN also contains preautonomic neurons that affect sympathetic outflow either directly by projections to the intermediolateral cell column in the spinal cord or via the RVLM (36). Previous data from this laboratory suggest that increased activity of neurons in the PVN associated with HF (33, 41) may be due to an increased angiotensinergic mechanism within the PVN (47).
Angiotensin (ANG) II has been found to act as a neurotransmitter in the central nervous system and is involved in the regulation of sympathetic activity to the cardiovascular system (19). In various autonomic areas of the brain, such as the PVN and RVLM, ANG II has been shown to contribute to tonic sympathoexcitatory input as well as participate in sympathoexcitatory reflexes, such as the cardiac sympathetic afferent reflex, baroreflex, and arterial chemoreflex (1, 8, 40). The increase in renal sympathetic nerve activity (RSNA) in response to microinjection of ANG II into the PVN is greater in HF rats compared with the sham rats, which correlates with an increased expression of ANG II type 1 (AT1) receptor within the PVN (47). These results suggest that enhanced AT1 receptor-mediated angiotensin action in the PVN on sympathetic outflow may contribute to sympathetic dysfunction in HF.
Exercise training (ExT) increases survival, decreases complications, and decreases muscle sympathetic nerve activity in human HF patients (3). In a rapid pacing model of HF in rabbits, ExT decreases RSNA, plasma ANG II, and central AT1 receptor expression and enhanced baroreflex sensitivity (9, 26, 27). However, the mechanism by which ExT normalizes sympathetic outflow in HF is not known. In the current study, we hypothesized that ExT restored sympathetic outflow to normal levels in HF possibly via normalization of an angiotensinergic mechanism. Specifically, we determined whether ExT: 1) attenuated the increased activation of PVN; 2) normalized the RSNA responses to ANG II microinjected into the PVN; and 3) normalized (negated) the elevated expression of AT1 receptor within the PVN in rats with HF.
METHODS
Animals.
The study was approved by the University of Nebraska Institutional Animal Care and Use Committee and was in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing 220 to 250 g (SASCO Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. Rats were given rat chow and water ad libitum and were housed in a room with a 12-h light-dark cycle. Rats were allowed to acclimatize for 1 wk before cardiac surgery.
Induction of heart failure.
Heart failure was induced by ligation of the left coronary artery as described previously (43). Rats were randomly assigned to either the sham-operated control group or the HF group. Left ventricular dysfunction was assessed using hemodynamic and anatomic criteria. Echocardiograms were performed at 7–8 wk after surgery. Left ventricular end-diastolic pressure (LVEDP) was measured by using a Mikro-Tip catheter (Millar Instruments, Houston, TX) at the time of the terminal experiment. To measure infarct size, the heart was dissected and the atria and right ventricle were removed. A digital image of the left ventricle was captured using a digital camera (Kodak, Rochester, NY). The infarcted area and total left ventricle area were quantified using SigmaScan Pro (Aspire Software International, Ashburn, VA). Infarct size (%) was determined by dividing the size of the infarcted area by the total area of the left ventricle. Rats with elevated LVEDP (>15mmHg) and infarct size >30% of total left ventricle wall were considered to be in HF.
Exercise training.
Three to four weeks after coronary artery ligation surgery, rats were randomly assigned to either ExT or sedentary (Sed) groups to produce four experimental groups: sham-Sed, HF-Sed, sham-ExT, and HF-ExT. For ExT, rats ran for specific times on a motor-driven treadmill (Columbus Instruments, Columbus, OH) for a period of 3 wk according to a modified protocol of Musch and Terrell (28). Low speed (10 m/min), grade (0%), and short duration (10 min/day) were initially used to familiarize the rats with running. The speed, duration, and grade were gradually increased to 20–25 m/min, 60 min/day, and 5–10%, respectively. This level of exercise is considered moderate for the sham rats. Only rats that ran steadily with little or no prompting were used in the study. To ensure a similar level of ExT between groups, citrate synthase activity assays on the soleus muscle were performed according to the protocol of Srere (38).
Metabolic cage assessment of urinary norepinephrine excretion.
Rats were placed in a metabolic cage to measure water and food intake, urine, and feces excretion, and body weight for 72- to 96-h intervals pre- and post-ExT. The urine was collected and frozen (−80°C) until it was used for the measurement of norepinephrine concentration. Urinary norepinephrine concentration was measured by an enzyme immunoassay kit (Labor Diagnostika Nord, Montreal, Quebec, Canada) according to the manufacturer's instruction.
FosB immunohistochemistry.
The rats were anesthetized with pentobarbital (65 mg/kg) and perfused transcardially with 150 ml of heparinized saline followed by 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. The brains were removed and postfixed at 4°C for 4 h in 4% paraformaldehyde solution and then placed in 20% sucrose. The brains were blocked in the coronal plane, and sections 30 μm in thickness were cut with a cryostat.
Floating sections were rinsed in 0.1 M phosphate-buffered saline (PBS) and then incubated in 1% hydrogen peroxide for 30 min at room temperature. After rinsing was completed, sections were incubated in PBS containing 3% normal horse serum and 0.25% Triton X-100 for 2 h. Sections were then incubated with a polyclonal rabbit anti-FosB antibody (1:5,000, Santa Cruz) for 48 h at 4°C. Sections were rinsed and incubated with biotinylated goat anti-rabbit IgG for 2 h at room temperature (1:100), incubated with avidin-biotin complex (1:200; Vectastain Elite ABC kit; Vector Laboratories, CA) for 1 h, and then rinsed in PBS for 30 min. Immunostaining was developed by incubation in diaminobenzidine kit (DAB kit, Vector Laboratories). Finally, they were mounted on gelatin-coated glass slides, dried, and coverslipped.
The presence of FosB-positive cells in discrete regions of the PVN was examined with a Leica microscope under bright field from all four groups of rats. Images were captured with a Qimaging digital camera (Canada). The staining was evaluated by counting the number of nuclei positively stained for FosB by ImageJ software (National Institutes of Health). FosB-positive cells were counted in the different regions of the PVN (dorsomedial, ventromedial, and magnocellular PVN) at approximately the same coronal level. Three adjacent sections were considered to represent one coronal level. The average number of cells in the sections (1.80 ± 0.1 mm posterior to bregma) was taken to represent the number of cells, unilaterally within the PVN. An independent observer blind to the four experimental groups counted cells within the identified boundaries of the PVN.
General surgery for hemodynamic and RSNA measurements and microinjection.
On the day of the experiment (7–8 wk after cardiac surgery), the rat was anesthetized with urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip) and instrumented for recording arterial pressure (AP) and heart rate (HR) as previously described (44).
The left kidney was exposed through a retroperitoneal flank incision. A branch of the renal nerve was isolated from fat and connective tissue. The central end of the nerve was placed on thin bipolar platinum electrodes. The nerve-electrode junction was fixed and electrically insulated from surrounding tissues with a Wacker Silgel mixture (604 and 601). The electrical signal was amplified with a Grass amplifier with high- and low-frequency cutoffs of 1,000 and 100 Hz, respectively. The rectified output from the amplifier was displayed using the PowerLab system to record and integrate the raw nerve discharge. Basal nerve activity was determined at the beginning of the experiment. The RSNA recorded at the end of the experiment (after the rat was injected with hexamethonium, 30 mg/kg iv) was defined as background noise. The value of RSNA was calculated by subtracting the background noise from the actual recorded value, and changes found in integration of the nerve discharge during the experiment were expressed as a percentage of the basal value. Responses of mean AP (MAP) and HR were expressed as the difference between the basal value and the value after each microinjection.
The rat was placed in a stereotaxic apparatus, and a cannula connected to a microsyringe (0.5 μl) was introduced into the PVN (1.5 mm posterior, 0.4 mm lateral to the bregma and 7.8 mm ventral to the dura). A thin needle (0.2 mm OD) connected to microsyringe (Hamilton, Reno, NV) was lowered into the PVN. ANG II (50, 100, and 200 pmol in 100 nl) or AT1 receptor antagonist losartan (50, 100 nmol in 100 nl) was microinjected into the PVN in random order. Each animal received all three doses of ANG II or two doses of losartan in random order. Subsequent microinjections were made at least 20 min after prior injections to allow MAP, HR, and RSNA to return to basal levels.
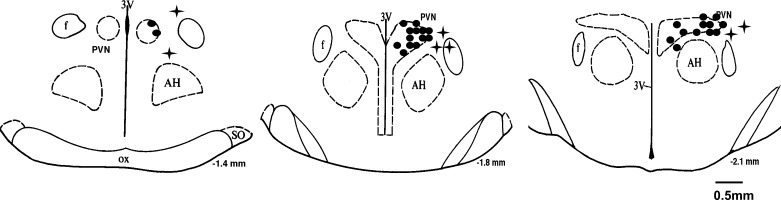
For histological verification of the microinjections sites in the PVN, Chicago blue dye (50 nl) was microinjected at the end of the experiment. The brain was removed, frozen, sectioned, and processed for histology as described previously (44). The location of the microinjection was visualized on a microscope, and microinjections with terminations within the boundaries of the PVN were considered to appropriately target to the PVN. Injections located outside of the PVN were excluded from data for the PVN and were analyzed as anatomical controls (Fig. 1).
Fig. 1.
Schematic representations of microinjection sites in serial coronal sections from the rostral (−1.4 mm) to caudal (−2.1 mm) regions of the paraventricular nucleus (PVN). Each filled circle represents the site of termination of a microinjection within the PVN. Each plus sign represents the site of termination of a microinjection outside of the PVN. AH, anterior hypothalamus; f, fornix; 3V, third ventricle; ox, optic chiasm; SO, supraoptic nucleus.
Micropunch of the PVN and isolation of protein for Western blot analysis.
The following experiments were performed in a separate group of animals. After the animal was euthanized (pentobarbital, 150 mg/kg ip), the brain was removed and quickly frozen on dry ice. Six serial coronal sections (100 μm) were cut through the hypothalamus at the level of the PVN using a cryostat. Following the Palkovits technique (31), we bilaterally punched the PVN using a blunt 18-gauge needle. The punches for each brain were placed in 100 μl protein extraction buffer [10 mM Tris, 1 mM EDTA, 1% sodium dodecyl sulfate (SDS), 0.1% Triton-X-100, and 1 mM phenylmethylsulfonyl fluoride], sonicated, and incubated for 30 min at 37°C to extract the protein.
Western blot measurement of AT1 receptor protein.
The total protein concentration from the extracted protein was measured using a BCA Assay Kit (Pierce, Rockford, IL). Samples were adjusted to contain the same concentration of total protein, and then equal volumes of 2 × 4% SDS sample buffer were added. The samples were boiled for 3 min and then loaded onto a 7.5% SDS-PAGE gel. Gels were subjected to electrophoresis at 40 mA/gel for 60 min. The fractionated proteins on the gel were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA) at 300 mA for 90 min. The membrane was probed with primary antibody: rabbit anti-AT1 receptor (1:500 dilution, Santa Cruz, CA) or rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2,000, Santa Cruz) and then probed with secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, 1:5,000 dilution, Pierce). An enhanced chemiluminescence substrate (Pierce) was applied to the membrane for 5 min followed by a 30-s exposure within an Epi Chemi II Darkroom (UVP BioImaging, Upland, CA) for visualization using the Worklab digital imaging system. Kodak 1D software was used to highlight the bands and quantify the signal. The expression of AT1 receptor was calculated as the ratio of intensity of AT1 receptor band relative to the intensity of the GAPDH band.
Data analysis.
Data are presented as means ± SE. The data were subjected to two-way ANOVA followed by comparison for individual group differences using the Newman-Keuls test. Statistical significance was indicated by a value of P < 0.05.
RESULTS
General data.
Table 1 summarizes the baseline RSNA, MAP, and HR data as well as morphological characteristics and left ventricular function data among the four experimental groups. Rats with >30% infarct of the left ventricular wall were included in the HF group. Sham rats had no visible myocardial damage. Ejection fraction was also significantly lower in both HF groups compared with the sham groups and was not altered by ExT. LVEDP was significantly increased in both HF-Sed and HF-ExT rats compared with both sham groups. LVEDP in HF-ExT group was not significantly reduced by ExT. HF-Sed rats had significantly lower change in pressure over time (dP/dt) compared with sham rats, which was partially improved by ExT. These data confirm that rats in the HF groups were experiencing cardiac dysfunction and that ExT did not normalize cardiac function per se.
Table 1.
Baseline and left ventricular function data
| Sham-Sed | HF-Sed | Sham-ExT | HF-ExT | |
|---|---|---|---|---|
| Body weight, g | 406 ± 21 | 397 ± 12 | 378 ± 10† | 373 ± 16 |
| Heart weight, g | 1.4 ± 0.2 | 2.1 ± 0.3* | 1.3 ± 0.2 | 2.0 ± 0.3* |
| Infarct size, % of epicardial LV | 0 | 37 ± 8* | 0 | 35 ± 6* |
| Ejection fraction, % | 83 ± 5 | 48 ± 3* | 82 ± 4 | 51 ± 4* |
| LVEDP, mmHg | 0.8 ± 0.6 | 25.9 ± 3.5* | 1.2 ± 0.6 | 21.2 ± 3.9* |
| dP/dt, mmHg/S | 8,334 ± 721 | 5,069 ± 749* | 8,399 ± 683 | 6,954 ± 896† |
| Basal MAP, mmHg | 92 ± 3 | 91 ± 3 | 90 ± 2 | 89 ± 3 |
| Basal HR, beats/min | 353 ± 21 | 369 ± 18 | 335 ± 19 | 350 ± 17 |
| Basal int. RSNA, μV•S | 46.8 ± 7.7 | 66.5 ± 6.7* | 48.1 ± 6.1 | 67.8 ± 7.8* |
Data are means ± SE. LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; MAP, mean arterial pressure; HR, heart rate; int. RSNA, integrated renal sympathetic nerve activity.
P < 0.05 compared with sham;
P < 0.05 compared with sedentary (Sed) group (n = 8/group).
Basal RSNA was significantly increased in HF rats compared with sham rats. After ExT, basal RSNA was not reduced in HF-ExT group compared with the HF-Sed group. It is recognized that comparison of raw RSNA between animals is wrought with many problems, preparation-to-preparation variation, contact point variations, etc. (6, 11). For sake of completion we have provided basal integrated RSNA values in the different groups (Table 1).
Citrate synthase activity of the soleus muscle was significantly higher in both ExT groups (sham-ExT: 9.8 ± 2.3 μmol·g−1·min−1; HF-ExT: 7.8 ± 1.4 μmol·g−1·min−1, n = 8/group) compared with the Sed groups (sham-Sed: 6.2 ± 0.6 μmol·g−1·min−1; HF-Sed: 5.8 ± 0.6 μmol·g−1·min−1, n = 8/group), demonstrating a significant effect of ExT. The level of citrate synthase activity in the soleus muscle was comparable in the two exercise groups indicative of similar levels of ExT.
Urinary norepinephrine levels were elevated in HF-Sed rats compared with sham-Sed rats (750 ± 120 vs. 348 ± 85 μg/24 h, n = 8/group, P < 0.05), indicative of increased overall sympathoexcitation. In HF-ExT (405 ± 125 μg/24 h, n = 8) rats, urinary norepinephrine levels were not different from sham-Sed (348 ± 85 μg/24 h, n = 8) or sham-ExT (440 ± 120 μg/24 h, n = 8, P < 0.05). ExT had no effect on urinary nonepinephrine in the sham group. It should be noted that moderate level of ExT was used in this study. The result suggested that this level of ExT normalized the increased overall sympathetic outflow associated with HF.
Effect of ExT on FosB-positive cell in rats with HF.
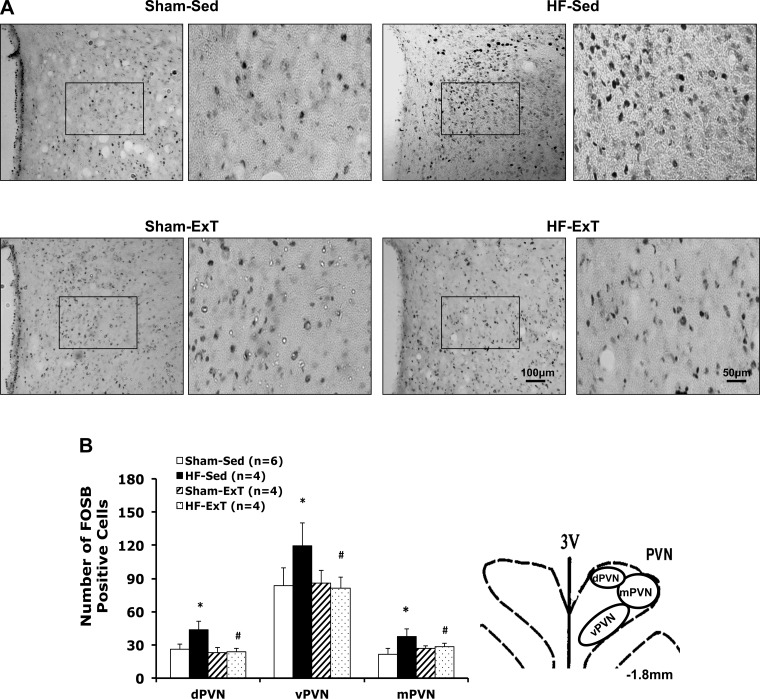
Significant increases in the numbers of FosB-positive cells were produced at 7–8 wk after coronary ligation surgery. In the PVN, the number of FosB-positive cells was significantly increased in the dorsomedial, ventromedial, and magnocellular areas (Fig. 2, A and B) of HF-Sed rats. Exercise training normalized the number of FosB-positive cells in the HF-ExT group. Increased FosB may indicate chronic neuronal activation within the PVN in HF rats. Exercise training reduced the neuronal activity within the PVN of HF group. Figure 2A shows the representative images of FosB staining from the PVN of the four groups of rats.
Fig. 2.
A: representative images of FosB staining in the PVN (−1.8 mm from bregma) from one rat/group [sham-sedentary (Sed), exercised trained (ExT)-Sed, sham-ExT, and heart failure (HF)-ExT]. Right: higher magnification of FosB staining in the ventromedial PVN (square area in the left panel). B: number of FosB-positive cells in three subdivisions of the PVN in the four groups of rats (left); Schematic drawing of the PVN (at −1.8 mm level) depicting subdivision of the PVN being analyzed (right). Data are means ± SE. *P < 0.05 compared with sham; #P < 0.05 compared with Sed group. dPVN, dorsomedial PVN; vPVN, ventromedial PVN; mPVN, magnocellular PVN.
Effects of ExT on the sympathoexcitatory responses to ANG II in rats with HF.
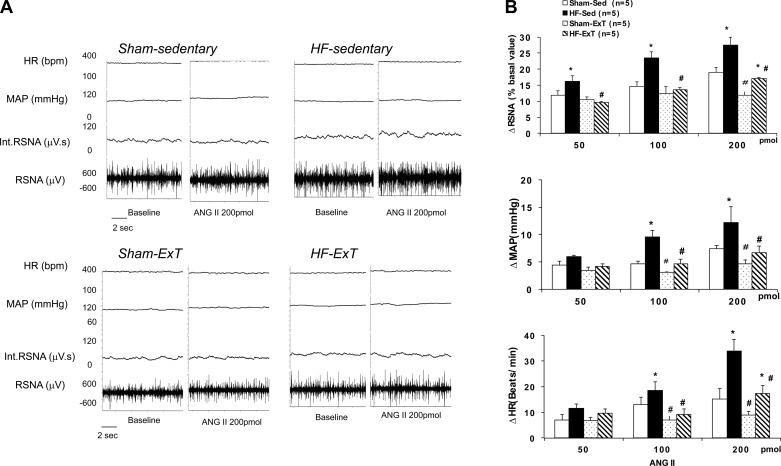
An example of the peak responses in RSNA, MAP, and HR to ANG II in the four groups is illustrated in Fig. 3. Administration of ANG II (50, 100, and 200 pmol) into the PVN elicited a dose-dependent increase in RSNA, MAP, and HR in the sham and HF groups (Fig. 3). The HF-Sed group showed an increase in RSNA, MAP, and HR that was significantly enhanced with all three doses compared with sham rats (P < 0.05). In HF-ExT group, the ANG II-induced changes in RSNA, MAP, and HR were similar to the sham groups. These data suggest that ExT normalizes changes in RSNA, MAP, and HR to microinjection of ANG II in the PVN. Exercise training also had significant effects on the RSNA, MAP, and HR responses in the sham group at the highest dose of ANG II. The sham-Sed group showed reduced responses in RSNA, MAP, and HR to ANG II compared with the sham-ExT group (P < 0.05). This result is interesting and suggests that ExT may reduce the general sympathetic tone also partially via the angiotensinergic mechanism in the normal control rats.
Fig. 3.
Renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR) responses to angiotensin (ANG) II microinjected into the PVN. A: segments of original recordings from individual rats from each experimental group showing responses of RSNA, integrated RSNA (int.RSNA), MAP, and HR to ANG II (200 pmol) microinjected into the PVN. B: mean changes in RSNA, MAP, and HR following microinjections of ANG II into the PVN. *P < 0.05 vs. sham with the same dose of ANG II; #P < 0.05 vs. Sed with the same dose of ANG II.
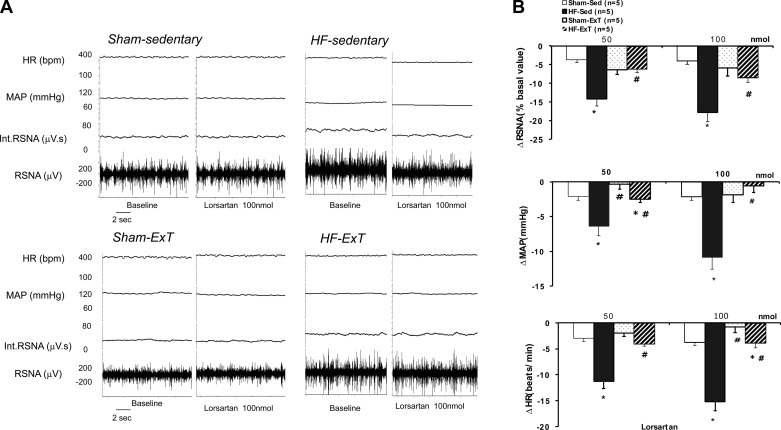
We also determined changes in RSNA upon microinjection of increasing concentrations of losartan (50∼100 nmol) into the PVN (Fig. 4). In response to losartan, there was a significantly greater decrease in RSNA, MAP, and HR in the HF-Sed group compared with the sham group. In the ExT-HF group, the change in RSNA in response to losartan was similar to that of the sham group. Similarly, changes in MAP and HR in response to losartan in the HF ExT group were also comparable to that of the sham group. These data strongly suggest an endogenous angiotensinergic component to the effect of ExT in rats with HF.
Fig. 4.
RSNA, MAP, and HR responses to losartan microinjected into the PVN. A: segments of original recordings from individual rats from each experimental group showing responses of RSNA, int.RSNA, MAP, and HR to losartan (100 nmol) microinjected into the PVN. B: mean changes in RSNA, MAP, and HR following microinjections of losartan into the PVN. *P < 0.05 vs. sham with the same dose of losartan; #P < 0.05 vs. Sed with the same dose of losartan.
Effects of ExT on the expression of AT1 receptors within the PVN in rats with HF.
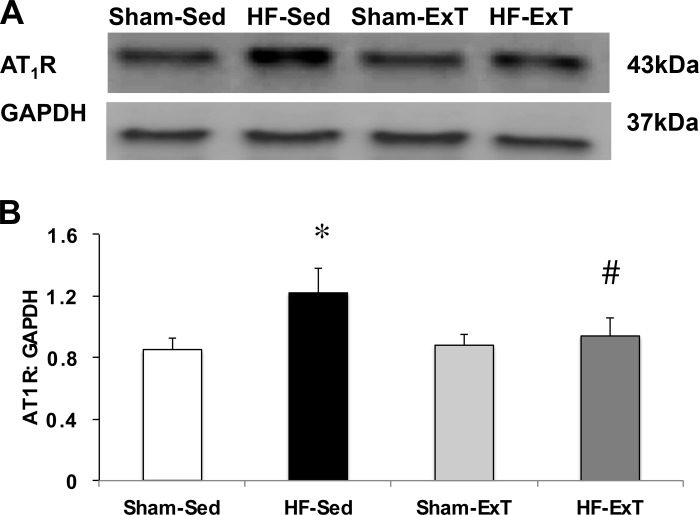
To test whether there was an increase in AT1 receptor expression in the PVN of HF-Sed animals and a subsequent decrease in AT1 receptor protein following ExT, we performed Western blot analysis on PVN micropunches and probed for AT1 receptor expression level (n = 4/group, Fig. 5). There was a significant increase in AT1 receptor protein expression level in the HF-Sed animals compared with the sham groups. Following ExT, AT1 receptor expression in HF animals was significantly decreased, comparable to sham levels.
Fig. 5.
Protein expression of AT1 receptor in punched PVN samples measured by Western blot. A: example of visualized electrophoresis bands of AT1 receptor and GAPDH protein in the four groups. B: mean data of band densities of AT1 receptor relative to GAPDH, n = 4/group. *P < 0.05 vs. sham; #P < 0.05 vs. Sed.
DISCUSSION
In the present study, the main finding was that a regimen of ExT for 3 wk normalized the activation of the PVN in rats with HF and abrogated the potentiated responses of RSNA, MAP, and HR to endogenous or exogenous ANG II in rats with HF. Consistent with these observations we saw that ExT is associated with normalization in AT1 receptor protein expression level within the PVN. Taken together, these results indicate that one mechanism by which ExT normalizes sympathetic outflow in HF is through normalization of PVN activation, possibly via an angiotensinergic mechanism.
FosB has been extensively used as an indicator of synaptic activation in the nervous system (12, 18). The present study mapped long-term neuronal activation (7–8 wk after HF surgery) in rats using immunohistochemical detection of FosB. The results show the increased number of FosB-positive cells in both parvocellular and magnocellular divisions of the PVN in rats with HF. This suggests that activation of both autonomic and neuroendocrine neurons within the PVN may play a major role in the processes leading to sympathetic hyperactivity in rats with HF. Normalization of FosB cells in the PVN after ExT indicates an inhibitory effect of ExT on the neuronal activity. It is presumed that ExT most likely inhibits the excitatory neuronal activity within the PVN, although FosB activation may not distinguish between excitatory and inhibitory neuronal activation. The correction of the overactivation of the PVN in rats with HF by ExT may contribute to the normalization of the general sympathoexcitation. This line of thought is consistent with the observation that global sympathoexcitation, as gauged by 24 h catecholamine excretion, is normalized by ExT.
The central renin-angiotensin system is pivotal in the generation of central sympathoexcitation in HF (8, 13, 47). In the current study, the RSNA response to PVN microinjection of ANG II was potentiated in rats with HF in a dose-dependent manner, whereas administration of AT1 receptor antagonist losartan reduced RSNA to a greater extent in HF rats. These data strongly suggest that in the PVN, the endogenous angiotensin system contributes to the driving force for sympathoexcitation during the HF condition. The finding that administration of losartan reduced sympathetic nerve activity in sedentary animals with HF to a greater extent suggests a tonic endogenous increase in ANG II signaling in the PVN. Since ANG II signaling has been shown to participate in excitatory reflexes that produce sympathoexcitation, it is of particular importance that endogenous ANG II responses were increased in rats with HF in this study. Consistent with these observations, it has been demonstrated that ANG II signaling participates in sympathoexcitation and modulation of excitatory reflexes in HF (48). The reduced losartan response following ExT indicates a reduction in endogenous tonic angiotensinergic drive. Importantly, the fact that ExT reduced the responses to microinjection of both ANG II and losartan in rats with HF lends credence to the notion that the central renin-angiotensin system plays a significant role and is modulated by ExT.
The HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial has so far indicated an enhanced quality of life and a reduction in hospitalization and a reduction in all cause mortality for HF-ExT patients (29). Unfortunately, these clinical studies do not address underlying mechanisms. It is not clear if this contributes to central alterations as well. In a seminal study by Roveda et al. (35), direct recording of muscle sympathetic nerve activity in patients with HF provided strong support for the inhibition of peripheral sympathetic outflow following ExT. Consistent with these observations, elevated urinary norepinephrine levels in HF-Sed rats were normalized in HF-ExT rats, suggesting that ExT normalized the increased overall sympathetic outflow associated with HF. However, comparison of basal RSNA between animals showed no significant difference between HF-ExT group and HF-Sed group. This lack of difference is likely due to the well-recognized problems in comparison of raw RSNA between animals such as preparation-to-preparation variation, contact point variations, etc. (6, 11). In a rapid pacing model of HF in rabbits, ExT has been shown to decrease basal RSNA in conscious animals (9).
The mechanisms responsible for the ExT-induced decrease in angiotensinergic mechanism within the PVN remain unknown. We have previously observed an enhanced AT1 receptor-mediated sympathetic outflow (47) and concomitant downregulation of neuronal nitric oxide (NO) synthase (nNOS) with reduced NO-mediated inhibition (43) from the PVN in rats with HF. In addition we have shown that ExT results in an increase in nNOS protein in the PVN (46). Interestingly, there is a strong interaction between NO and ANG II in neuronal cells (37). The results show that NO exerts an inhibitory effect on AT1 receptor expression in the PVN (37). Based on this, one possible mechanism responsible for the ExT-induced decrease in angiotensinergic mechanism within the PVN may be via the inhibitory effects on AT1 receptor-mediated signaling by an NO mechanism.
Another possibility is that ExT reduces RSNA via a decrease in the generation of reactive oxidant species (2). Exercise training has been shown to reduce sympathetic outflow by an upregulation of antioxidant enzymes such as superoxide dismutase (9). An important consequence of a reduction in reactive oxygen species generation is the enhanced bioavailability of NO, which is sympathoinhibitory (10). Enhanced bioavailability of NO may have the inhibitory effect on the exaggerated angiotensin signaling in HF.
Additionally, since NO is presumably producing its inhibitory actions via a GABA mechanism (45), which are also altered in HF (44), it may well be that there is some interaction between the actions of ANG II and the GABA mechanisms as well (5, 24). Furthermore, another major excitatory neurotransmitter, glutamate, has been found to modulate sympathetic nerve activity in HF (25). Since there is interaction between glutamate and ANG II (20), it may well be that there is some cross-talk between these two excitatory pathways in HF, which may be modulated concommitantly or dependently in ExT-HF rats (17, 34).
Recently, the role of nonclassical pathway of renin-angiotensin system genes like angiotensin-converting enzyme 2 (ACE2) and Mas receptor in the central nervous system and their participations in central sympathetic activation has also been widely addressed (7, 15, 42). Activation of the central renin-angiotensin system in animals with HF involves a possible imbalance of ACE and ACE2 in regions of the brain that regulate autonomic function. Exercise training normalized the upregulation of ACE protein and mRNA in the cerebellum, medulla, hypothalamus, PVN, NTS, and RVLM of HF rabbits (15). Exercise training also increased ACE2 expression in these brain sites during HF (15). It is possible that ExT reverses/alters this imbalance and thus improves the ANG II-mediated sympathoexcitation in HF (15).
In conclusion, the results of the present study demonstrate that ExT attenuates the potentiated increased activation of neurons in the PVN and responses in RSNA to ANG II microinjected into the PVN in rats with HF. Normalization of expression of the AT1 receptors within the PVN may underlie this beneficial effect of ExT. Therefore, one mechanism by which ExT restores sympathetic outflow in HF is through normalization of angiotensinergic mechanisms within the PVN.
Perspectives and Significance
Heart failure is commonly characterized by an overall increase in sympathoexcitation, an effect, directed to compensate for the decrease in cardiac output but which ultimately exacerbates the condition. Uncovering the mechanism(s) involved in this increased sympathetic outflow may lead to improved treatment of this particular symptom of the disease, improving quality of life and survival. We have shown here that ExT normalizes the activation of the PVN in HF. We have demonstrated that alterations of angiotensinergic system within the PVN play a role in the hyperactivity of the renal nerves in HF, thus identifying this system as a potential therapeutic target. Furthermore, among the numerous beneficial effects of ExT on the HF state is alleviation of the increased sympathoexcitation. This understanding of the mechanisms of ExT-induced normalization of sympathoexcitation furthers our knowledge of the mechanisms underlying some of the symptoms of HF, offers a target in care of the disease, and emphasizes the importance of ExT in the treatment of HF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-62222 (to K. P. Patel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.Z. and K.P.P. conception and design of research; H.Z., N.M.S., and X.L. performed experiments; H.Z., N.M.S., and X.L. analyzed data; H.Z., N.M.S., X.L., and K.P.P. interpreted results of experiments; H.Z., N.M.S., X.L., and K.P.P. prepared figures; H.Z., X.L., and K.P.P. drafted manuscript; H.Z. and K.P.P. edited and revised manuscript; H.Z., N.M.S., X.L., and K.P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The technical assistance of Dr. Kurtis Cornish is greatly appreciated.
REFERENCES
- 1. Barbella Y, Cierco M, Israel A. Effect of Losartan, a nonpeptide angiotensin II receptor antagonist, on drinking behavior and renal actions of centrally administered renin. Proc Soc Exp Biol Med 202: 401–406, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Belardinelli R. Exercise training in chronic heart failure: how to harmonize oxidative stress, sympathetic outflow, and angiotensin II. Circulation 115: 3042–3044, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Brändle M, Patel KP, Wang W, Zucker IH. Hemodynamic and norepinephrine responses to pacing-induced heart failure in conscious sinoaortic-denervated dogs. J Appl Physiol 81: 1855–1862, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Coote JH, Sato Y. Reflex regulation of sympathetic activity in the spontaneously hypertensive rat. Circ Res 40: 571–577, 1977 [DOI] [PubMed] [Google Scholar]
- 7. Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher KP. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol 93: 694–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao L, Wang W, Li YF, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol 300: R818–R826, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Hopp FA, Seagard JL, Kampine JP. Comparison of four methods of averaging nerve activity. Am J Physiol Regul Integr Comp Physiol 251: R700–R711, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Howe BM, Bruno SB, Higgs KA, Stigers RL, Cunningham JT. FosB expression in the central nervous system following isotonic volume expansion in unanesthetized rats. Exp Neurol 187: 190–198, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res 79: 671–678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: Their possible involvement in neural control of the cardiovascular system in rats. Brain Res 329: 205–212, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol 108: 923–932, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaye DM, Lefkovits J, Cox H, Lambert G, Jennings G, Turner A, Esler MD. Regional epinephrine kinetics in human heart failure: Evidence for extra-adrenal, nonneural release. Am J Physiol Heart Circ Physiol 269: H182–H188, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumagai K, Reid IA. Angiotensin II exerts differential actions on renal nerve activity and heart rate. Hypertension 24: 451–456, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Latchford KJ, Ferguson AV. ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol 286: R894–R902, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Leenen FHH, Huang BS, Yu HL, Yuan BX. Brain ‘ouabain’ mediates sympathetic hyperactivity in congestive heart failure. Circ Res 77: 993–1000, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Leimbach WN, Gunner wallin B, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central symapthetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Levett JM, Marinelli CC, Lund DD, Pardini BJ, Nader S, Scott BD, Augelli NV, Kerber RE, Schmid PG., Jr Effects of β-blockade on neurohumoral responses and neurochemical markers in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 266: H468–H475, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure–A role for angiotensin II. Circulation 102: 1854–1862, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol 92: 2403–2408, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 29. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators HA. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439–1450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721–730, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Palkovits M, Brownstein M. Brain microdissection techniques. In: Brain Microdissection Techniques, edited by Cuello AE. Chichester: Wiley, 1983 [Google Scholar]
- 32. Patel KP. Neural regulation in experimental heart failure. Baillieres Clin Neurol 6: 283–296, 1997 [PubMed] [Google Scholar]
- 33. Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol 265: R923–R928, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol 302: H527–H537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Sharma NM, Zheng H, Li YF, Patel KP. Nitric oxide inhibits the expression of AT1 receptors in neurons. Am J Physiol Cell Physiol 302: C1162–C1173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srere PA. Citrate synthase. In: Methods in Enzymology 1969, vol. 13, p. 3–11 [Google Scholar]
- 39. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980 [DOI] [PubMed] [Google Scholar]
- 40. Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W, Zhu GQ. AT1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Auton Neurosci 121: 56–63, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Xu B, Zheng H, Patel KP. Enhanced activation of RVLM projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 302: H1700–H1711, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49: 926–931, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995–H1004, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol 297: R1364–R1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 287: H1828–H1835, 2004 [DOI] [PubMed] [Google Scholar]