Abstract
Objective The superior transvelar approach is used to access pathologies located in the fourth ventricle and brainstem. The surgical path is below the venous structures, through the superior medullary velum. Following splitting the tentorial edge, near the tentorial apex, the superior medullary velum is split in the cerebello-mesencephalic fissure. Using the supracerebellar infratentorial, transtentorial or parietal interhemispheric routes, the superior medullary velum is approached. Splitting this velum provides a detailed view of the fourth ventricle and its floor.
Materials and Methods A total of 10 formalin-fixed specimens were dissected in a stepwise manner to simulate the superior transvelar approach to the fourth ventricle. The exposure gained the distance from the craniotomy site and the ease of access was assessed for each of the routes. We also present an illustrative case, operated by the senior author (AN).
Results The superior transvelar approach provides access to the entire length of the fourth ventricle floor, from the aqueduct to the obex, when using the parietal interhemispheric route. In addition, this approach provides access to the entire width of the floor of the fourth ventricle; however, this requires retracting the superior cerebellar peduncle. Using the supracerebellar infratentorial route gives a limited exposure of the superior part of the fourth ventricle. The occipital interhemispheric route is a compromise between these two.
Conclusion The superior transvelar approach to the fourth ventricle provides a route for approaching the fourth ventricle from above. This approach does not require opening the posterior fossa in the traditional way, and provides a reasonable alternative for accessing the superior fourth ventricle.
Keywords: superior medullary velum, approach, fourth ventricle, brain stem, superior transvelar
The fourth ventricle is usually approached using either the transvermian (median suboccipital) or the telovelar approach.1 These “Standard” posterior fossa surgeries are not to be taken lightly. Posterior fossa surgery carries 31.8% chance of complications, which include: new neurological deficit, posterior fossa syndrome, cerebrospinal fluid leak, meningitis, and wound infection.2 The mortality rate for posterior fossa surgery is 2.6%.2 Although the mutism part of the posterior fossa syndrome is reversible, this mutism is the reversible part of a “persistent neurologic syndrome.”3 The above-mentioned complications are partly due to the pathology itself, its nature, and location, but partly due to the surgical approach selected for the pathology. Surgical dissection of the suboccipital region carries higher rates of CSF leak and meningitis, compared with supratentorial craniotomies.4
Also, although the exact mechanism of the posterior fossa syndrome remains unknown,3 splitting the vermis has been proposed as the cause of at least the mutism part of this syndrome.5 Using the telovelar approach may minimize vermis resection; however, it still might require retraction of this delicate structure.1
Part of the rationale for the current study, is trying to decrease these complication rates by changing the surgical approach selected for a certain posterior fossa lesion. Instead of using the suboccipital route, for a fourth ventricular pathology, we used the occipital (or parietal) transtentorial approach.
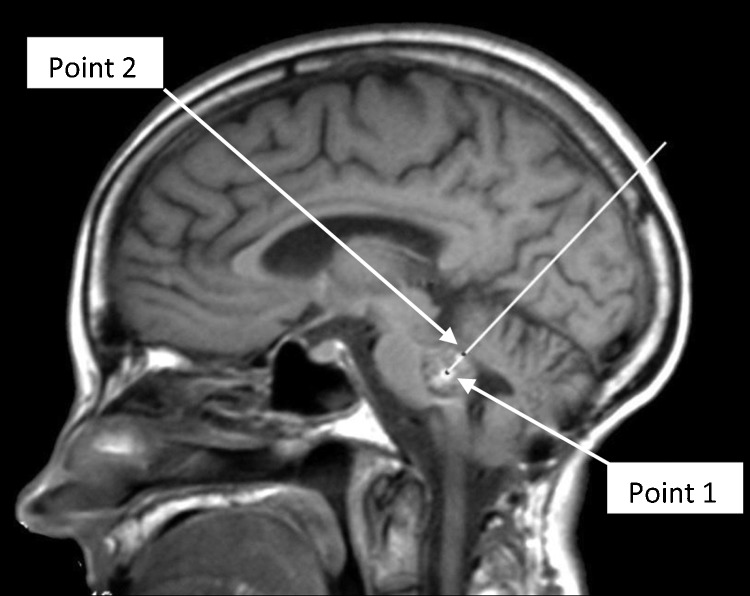
Also, there may come times, in which, a fourth ventricular pathology, would be best approached from above. For example, a pontine cavernous malformation, with its superficial part in the floor of the fourth ventricle (Fig. 1), will be best approached from above. This selection of approach is based on “The two-point method, for brainstem lesions.”6 The two-point method, selects the surgical approach to brainstem lesions that provides the best exposure, while minimizing tissue retraction. In this system, a line is drawn from the center point of the lesion (point 1) through the superficial point (point 2) and out, toward the skull, subcutaneous tissues, and skin. This line dictates the best approach to be used for resection of the lesion.6
Figure 1.
“The two-point method” for brainstem lesions.”6 The two point method, selects the surgical approach to brainstem lesions that provides the best exposure, while minimizing tissue retraction. In this system, a line is drawn from the center point (point 1) through the superficial point (point 2) and out, toward the skull, subcutaneous tissues, and skin. This line dictates the approach to be used for resection of the lesion.6
We present the superior transvelar approach (Fig. 2) to the fourth ventricle, which accesses the fourth ventricle through the superior medullary velum. The superior transvelar approach uses the interhemispheric transtentorial or supracerebellar infratentorial routes to approach the posterior incisural space. Following an incision in the tentorium, the superior medullary velum is exposed between the lingula of the cerebellum and the inferior colliculus. No venous structures are involved in this approach, and the trajectory is usually 1 or 2 cm below the vein of Galen complex. Following an incision in the superior medullary velum, the fourth ventricle is accessed, revealing its floor, the superior and middle cerebellar peduncles, and the inferior medullary velum, from above.
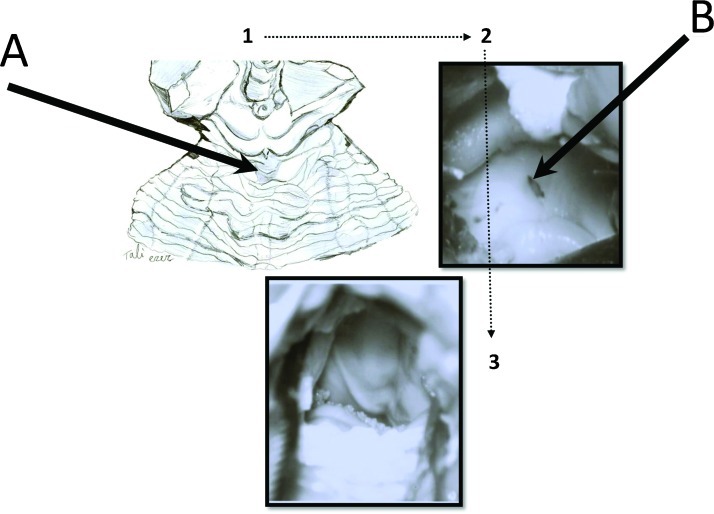
Figure 2.
The superior transvelar approach. Following dissection of the cerebello-mesencephalic fissure (1), the microsurgical anatomy of the superior medullary velum (2), and the two superior cerebellar peduncles are exposed, (3) the fourth ventricular floor, from above. The superior medullary velum was split using a single midline cut, to expose the rhomboid fossa (fourth ventricle floor). (A) The cerebello-mesencephalic fissure; (B) the superior medullary fissure cut.
Approaching the fourth ventricle or the brainstem by splitting the superior medullary velum has been mentioned in the literature, and is probably performed in many institutes around the world.7,8 However, a detailed description of this approach, the extent of fourth ventricle exposure gained, its advantages, disadvantages, pitfalls, and anatomical landmarks encountered, has not been performed until this date.
Materials and Methods
A total of 10 formalin-fixed specimens were dissected in a stepwise matter. In five of these heads, the arteries were perfused with red silicone dye and the veins with blue dye, as described before.9 The specimens were dissected, with the aid of an operating microscope, to simulate exposures gained using the superior transvelar approach. Special attention was paid to anatomical structures that limit visualization using this approach. The surgical exposure gained and the distance from the surface was measured in every head, for the three routes mentioned and compared with the other routes. We also performed the median suboccipital approach (transvermian approach)1 in three of these heads, to compare the view achieved to the view achieved using the superior transvelar approach.
Relevant Anatomy
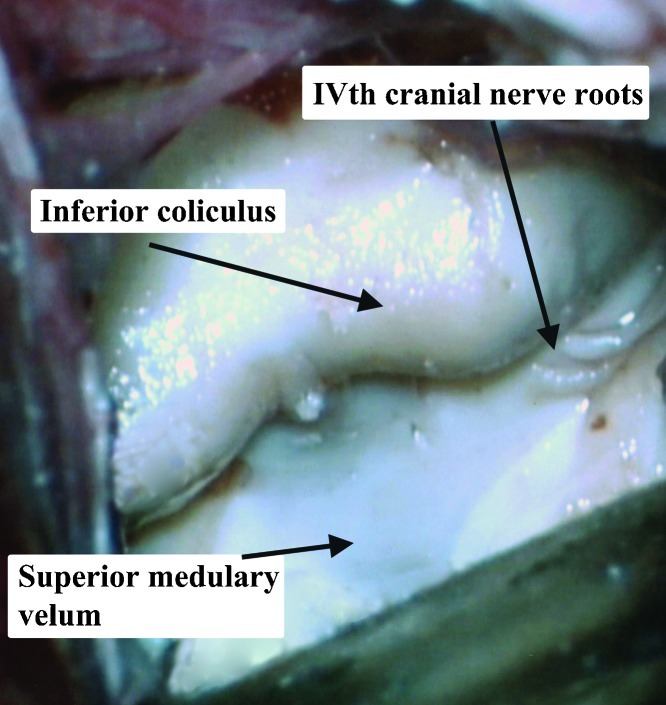
The superior medullary velum (velum medullare anterius; valve of Vieussens; anterior medullary velum) is a thin membrane connecting the two superior cerebellar peduncles.10 It is located caudal to the exit of the fourth cranial nerve, and is covered by the lingula of the cerebellum. Separating the lingula from the superior medullary velum creates a working corridor (the cerebellomesencephalic fissure).11,12 There are only few “essential” neural structures in the composition of the superior medullary velum, making its sacrifice not clinically significant, especially caudal to the decussation of the trochlear nerves.13 However, in our studies, we tried to preserve it as much as possible by performing a midline cut with an arachnoid knife, and then anchoring the two separated leaves, with a delicate suture. The superior medullary velum is a relatively avascular thin structure, roofing the fourth ventricle. This structure allows entrance to the fourth ventricle and visualization of its floor once divided.13 The superior medullary velum receives its arterial supply from the superior cerebellar artery via its precerebellar branch.14 Venous drainage is by the superior cerebellar peduncle veins, which unite superiorly at the emergence of the trochlear nerve from the brainstem, to form the vein of the cerebello-mesencephalic fissure.15
The precentral cerebellar fissure, also known as the cerebello-mesencephalic fissure, extends between the cerebellum and the midbrain and has an anterior wall formed by the superior cerebellar peduncles, lingula of the vermis overlying the superior medullary velum, trochlear nerve origin, quadrigeminal plate, and pineal body.16,17 This cerebellomesencephalic fissure actually extends downward between the midbrain and the cerebellum. Also, the quadrigeminal cistern, extends caudally from the pineal gland, into the cerebello-mesencephalic fissure.11
The trochlear (or fourth cranial) nerve is the smallest of the cranial nerves, and yet has the longest intracranial course. It arises from the dorsal midbrain, just above the superior medullary velum, and its fibers decussate in the anterior medullary velum to end in the contralateral superior oblique muscle.18 These decussating fibers are in its most rostral portion just caudal to the inferior colliculus.13
The Superior Transvelar Approach, Surgical Technique
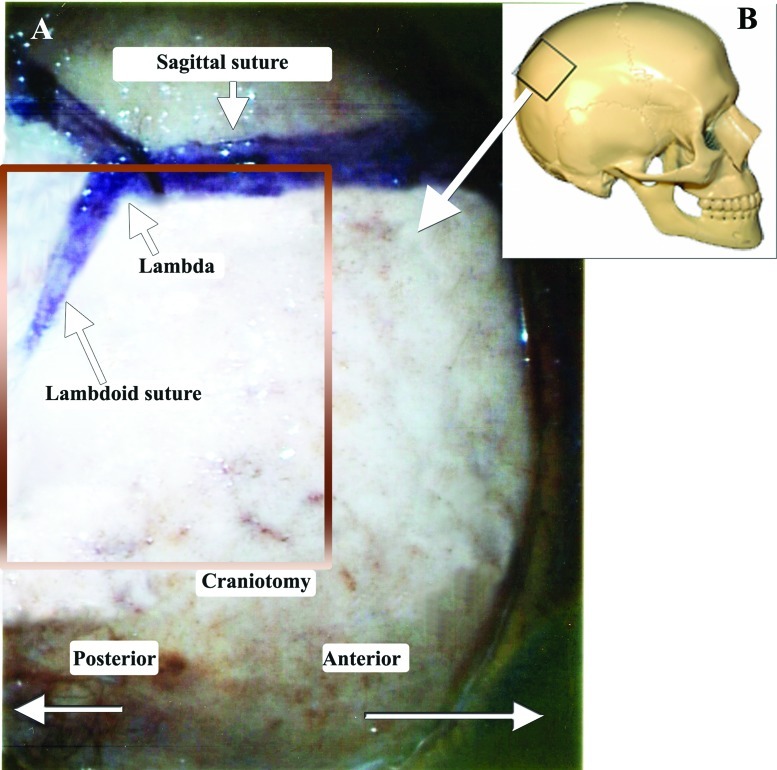
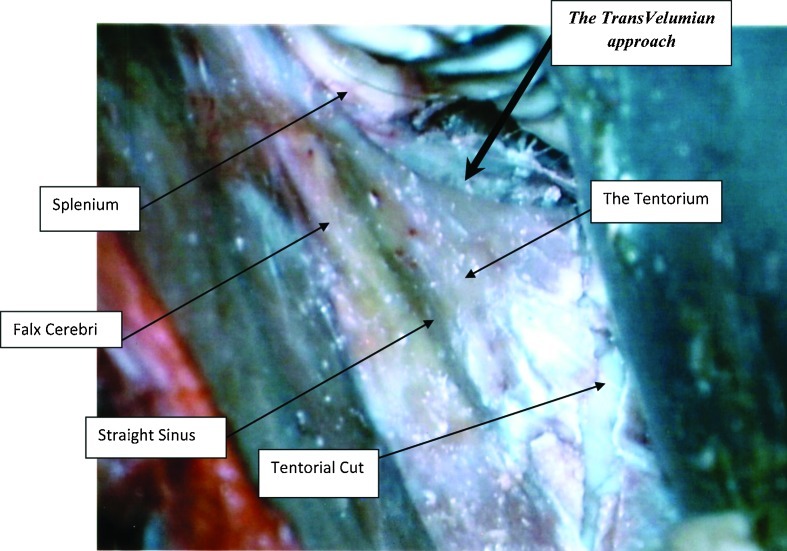
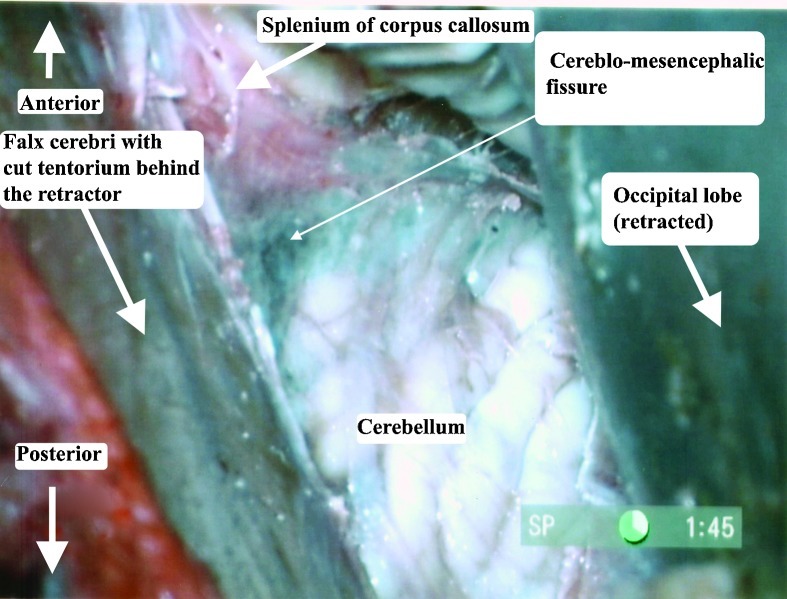
The heads were fixed in a position, placing the sagittal suture parallel to the floor and the contralateral zygomatic arch in the highest position. For the occipital transtentorial route, a midline 10 cm coronal skin incision, with its middle on the lambda, was performed. Two burr holes (Fig. 3) were placed on the superior sagittal sinus, 2.5 cm anterior and posterior to the lambda. A 8 × 5 cm craniotomy was performed, followed by cutting the dura in a U shape, facing the superior sagittal sinus. Following arachnoid dissection, the brain was separated from the superior sagittal sinus and the tentorium. (Fig. 4) When the brain was too stiff to mobilize, we enlarged the craniotomy, to include the lateral occipital bone, opened the dura and let the occipital lobe fall. No bridging veins were encountered in this area, in any of the specimens. The tentorial apex, the splenium part of the corpus callosum, and the posterior incisural space were then exposed. (Fig. 4) We performed a tentorial incision 2 cm lateral to the tentorial apex (Fig. 4) and lifted the tentorial cut part up, reflecting it on the falx cerebri. (Fig. 5). Following dissection of the cerebello-mesencephalic fissure (Figs. 6, 7) the microsurgical anatomy of the superior medullary velum, and the two superior cerebellar peduncles were studied. (Fig. 8) The superior medullary velum was split using a single midline cut. We then anchored delicate sutures to the two leaflets, and pull them apart. This was done to avoid damaging the velum. Care was taken, not to cut the superior part of the superior medullary velum, in between the two emerging trochlear nerves, which carries the decussating fibers of both trochlear nerves.13
Figure 3.
The craniotomy plan. The craniotomy used for the occipital interhemispheric route, resembles a craniotomy for a posterior parasagittal meningioma. (A) Right lateral view of the planed craniotomy, centered on the lambda, in the anteroposterior direction. (B) The skull image shows the place of the craniotomy.
Figure 4.
Superior view of the posterior left incisural space,29 following occipital craniotomy, showing the planned tentorial cut.
Figure 5.
Right parasagittal occipital craniotomy. The tentorial cut is placed 2 cm lateral to the midline, going posteromedially.
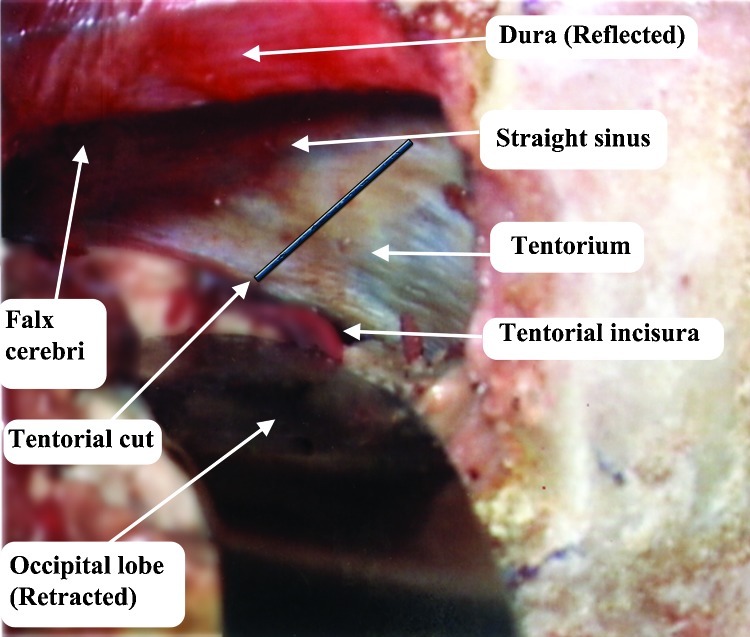
Figure 6.
Cutting of the right tentorium, 2 cm lateral to the midline and reflected medially, on the falx cerebri. The cerebello-mesencephalic fissure is exposed covered with arachnoid.
Figure 7.
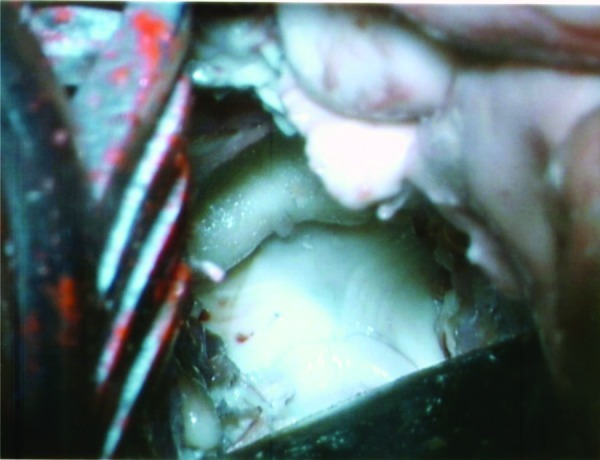
The cerebello-mesencephalic fissure, also known as the precentral cerebellar fissure, is the space between the lingula and superior medullary velum. This fissure was exposed after arachnoid dissection. Multiple minute distal branches of the superior cerebellar artery, are encountered in this region, and are retracted either anteriorly or posteriorly.
Figure 8.
The cerebello-mesencephalic fissure, also known as the precentral cerebellar fissure, is the space between the lingula and superior medullary velum. This fissure was exposed after arachnoid dissection. Multiple minute distal branches of the superior cerebellar artery, are encountered in this region, and are retracted either anteriorly or posteriorly.
Results
Using the supracerebellar infratentorial, occipital transtentorial and parietal interhemispheric with splenium cut, the fourth ventricle was exposed in all 10 heads. The view and extent of exposure achieved, in each of these routes, is summarized in Table 1. Using the supracerebellar infratentorial route gave the least exposure of the fourth ventricle floor. This exposure was limited to the superior part of the pontine part of the fourth ventricle floor. Using the occipital transtentorial approach and splitting the tentorium, enlarged the exposure gained, to include the facial colliculus on both sides. Using the parietal interhemispheric route, the whole floor of the fourth ventricle, to the foramen of Magendie was exposed. This route gave the largest exposure of the fourth ventricle floor, however, the distance from the craniotomy site to the fourth ventricle floor was the largest (actually at the end of the focus length of our microscope, in high magnification). Also, the splenium part of the corpus callosum had to be cut, to access the fourth ventricle, using this route. Using this parietal interhemispheric approach, we also encountered many bridging veins, which made it difficult to retract the brain from the falx. The parietal interhemispheric route, gives exposure to the whole fourth ventricle floor, however, the distance to the ventricle was the largest using this route.
Table 1. Comparison of the Three Routes Mentioned.
| Supracerebellar Infratentorial | Occipital Transtentorial | Parietal Interhemispheric | |
|---|---|---|---|
| Average distance | 53.31 mm | 80.13 mm | 103.77 mm |
| Standard deviation | 21.46 mm | 3.82 mm | 10.98 mm |
| Exposure length | Aqueduct to superior part of the fourth ventricle | Aqueduct to facial colliculus | Aqueduct to foramen Magendie |
| Splenium cut | No | No | Yes |
| Tentorial cut | No | Yes | No |
Calculated in terms of distance from the surface to the fourth ventricle, the standard deviation of these distances, the amount of exposure gained, with every route, the need to cut the splenium part of the corpus callosum and whether there is a need for a tentorial cut.
The superior transvelar approach proved to be an efficient and useful way to approach the fourth ventricle, without sacrificing any neural tissue. The superior medullary vellum itself was only split in its middle, without sacrificing it in most specimens.
Using the occipital and parietal interhemispheric routes, we used simple gravitational pooling of the occipital lobe, and retraction of the falx cerebri with sutures placed in the falx, just near its attachment to the straight sinus.
The venous structures, including the vein of Galen, were not encountered in any of the dissections. Using the superior transvelar approach, the surgical corridor is ~2 cm below these venous structures.
Table 1 compares the three routes mentioned in terms of distance from the surface to the fourth ventricle, the standard deviation of these distances, the amount of exposure gained with every route, the need to cut the splenium of the corpus callosum, and whether there is a need for a tentorial cut. As is depicted in Table 1, the supracerebellar infratentorial route is the shortest route to the fourth ventricle, however, it offers a limited exposure of the ventricular floor. The standard deviation values, depicted in Table 1, are quite high for the infratentorial supracerebellar route, probably due to large variation in the shape and angle of the posterior fossa, amongst different individuals. On the other hand, for the occipital transtentorial route and for the parietal interhemispheric routes, the standard deviation variations were much smaller, probably reflecting less interindividual variations, in this supratentorial space. These distances could be partly calculated using preoperative imaging, and thus providing an option to assess the distance from the craniotomy site, on an individual patient basis.
As could be expected, the steeper the angle of view, to the fourth ventricle, the larger the exposure gained. The parietal interhemispheric route offers an exposure of the whole fourth ventricle, from the aqueduct to the foramen Magendie, however, this exposure required a splenium cut, and also, the distance from the surface to the fourth ventricle was ~10 cm, which is at the end of the our microscope's field. Another disadvantage is that the increased angulation leads to inconvenience in dissection of the tumor, especially around the anteroinferior portion.
A tentorial cut was also utilized, only for the occipital interhemispheric route. This maneuver may be associated with a large hemorrhage, from the tentorial venous lakes, and may be performed using a monopolar coagulator or a laser device, to overcome this problem.
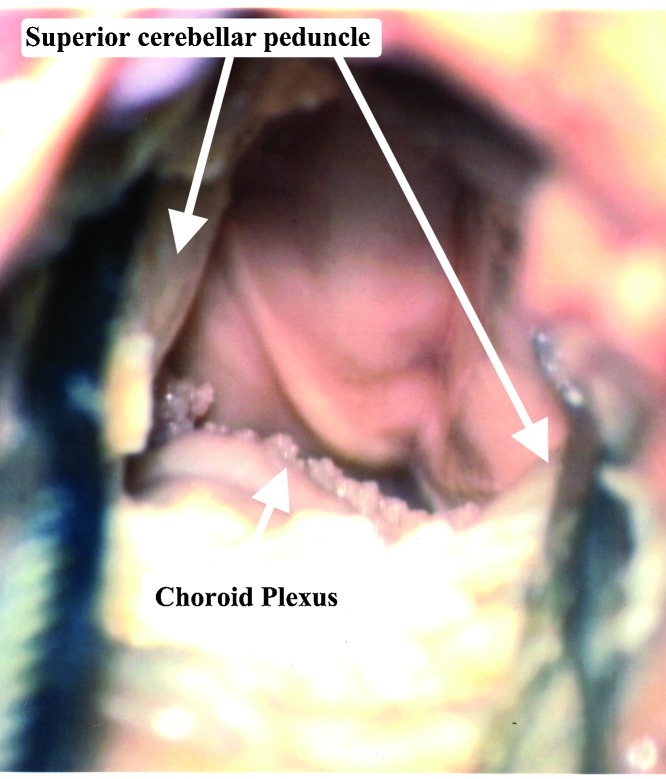
The width of exposure gained, using the superior transvelar approach, depended upon whether or not a retraction of the superior cerebellar peduncles was performed. Without retraction, the exposure was limited, from one sulcus limitans to the contralateral one. With retraction, the whole width of the fourth ventricle was exposed (Fig. 9). We do not recommend retracting the superior cerebellar peduncles, since damage to this structure, may cause severe ipsilateral intention tremor, dysmetria, and decomposition of movements.13
Figure 9.
The fourth ventricle is exposed, using retraction of the superior cerebellar peduncles. Using the retractors, clearly increases the width of the exposure, however we do not recommend retracting these delicate structures.
An Illustrative Case
A 60-year-old woman presented to the outpatient clinic of the senior author (A.N.) complaining of progressive difficulty in walking, balance problems, left-sided weakness, and blurred vision lasting more than a year. Her past medical history was positive for multiple intracranial cavernous malformation, arthritis, seizure disorder, and a hydrocephalus, she was treated with endoscopic third ventriculostomy a year before her admission. An MRI was performed (Fig. 10) showing a smooth, lobulated mass in the pons, and a small lesion in the right parietal, left parietal, and right frontal lobes, representing multiple cavernous hemangiomas. The patient was diagnosed with hemorrhagic brainstem cavernous malformation. Following discussion of the treatment options, with the patient, she decided to undergo a surgical intervention. The patient then underwent an occipital craniotomy with an interhemispheric approach and an infratentorial resection of the brainstem, cavernous hemangioma. It was a frameless, stereotactic-assisted craniotomy. Postoperatively, the patient gradually regained full consciousness, with persistent left-sided weakness. On postoperative day 6, the patient developed a DVT, and an IVC filter was placed. The patient was discharged, from our hospital on postoperative day 10, with mild left hemiparesis. About a year following the surgery, she was fully conscious; she still had mild left-sided weakness, but was getting better and was ambulating.
Figure 10.
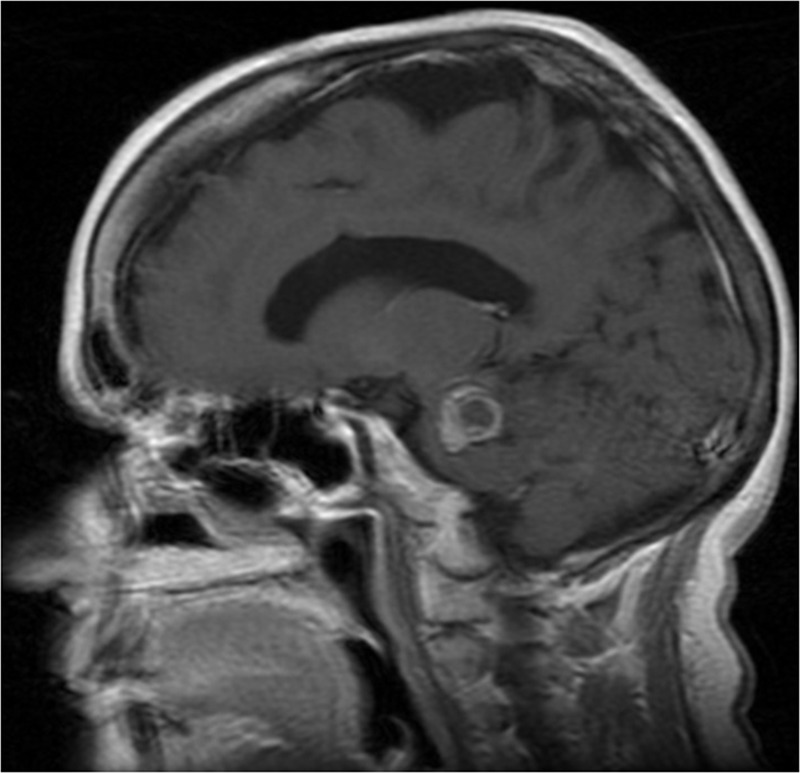
A T1 image with gadolinium of a 60-year-old woman, with brainstem cavernoma, operated, using the occipital interhemispheric approach.
Discussion
Selecting the best approach for a certain lesion depends on several factors. The exposure accomplished using this approach, the technical ease of this approach, the most direct (distance) approach, or the approach which transverses the least neural structures.6 Our current study aims to add another approach to the arsenal of approaches, directed at the fourth ventricle and the brainstem. We try to describe the exposure accomplished, using the superior transvelar approach. The transvermian approach is the most frequently used approach to the fourth ventricle.19 This fact makes most neurosurgeons feel at ease, using this approach instead of other approaches. Although a surgeon's familiarity with a certain approach is an important factor for selecting this approach, this familiarity should not restrain a surgeon, from using another approach, if it is more suitable for a safe resection of pathology.
There are a few routes to expose the superior medullary velum, and every route has its pros and cons. Using the parietal interhemispheric route, for the superior transvelar approach and splitting the splenium part of the corpus callosum, offers the largest exposure of the fourth ventricle. Using this route, the whole fourth ventricle was exposed in all 10 cadaveric heads dissected from the aqueduct to the obex. However, using this route, the Vein of Trolard had to be divided in two of the heads. Also, cutting the splenium of the corpus callosum might lead to disconnection syndrome.20 Using this route, the distance from the craniotomy site to the obex was 15.2 cm on average, making it very difficult to dissect structures near the obex.
Using the occipital-transtentorial route and approaching the fourth ventricle using the superior transvelar approach, exposed the upper part of the fourth ventricle in all the heads studied.
In seven of the heads, the exposure gained was from the aqueduct to the striae medullares (the upper half of the fourth ventricle was exposed), and in the other three heads, the striae medullares was clearly visible. No bridging veins were encountered, in any of the heads and the Vein of Galen complex was about 1 to 2 cm above the operative corridor.
Using the supracerebellar route for the superior transvelar approach, would probably make most surgeons at ease, using this approach. However, using this route for the superior transvelar approach yields the least amount of fourth ventricle exposure, of all three routes. In average, only the upper 1 to 2 cm of the fourth ventricle floor were exposed. Different maneuvers could be implemented to “steepen” (make it more perpendicular to the base) the angle, and get a larger exposure of the fourth ventricle, using the supracerebellar infratentorial route. A well-known maneuver is the lifting of the torcula, and transverse sinuses, mentioned in a recent manuscript.21 However, we used the occipital interhemispheric route instead.
Many neurosurgeons are reluctant to use the occipital interhemispheric route because of its complications, mainly homonymous hemianopsia due to occipital lobe retraction. Early postoperative visual field loss has been reported in 19 to 100% of patients, and long-term visual field loss has been reported in up to 17% of patients.22,23,24,25,26 However, to overcome this problem, various authors suggested large craniotomies, with gravitational pull of the occipital lobe, without retraction.22,23,27,28 Chi and Lawton describe the effect of experience on the selection of positioning, for the occipital interhemispheric route, and state that with experience, one tends to move from the prone position to the lateral position, with no retraction on the occipital lobe.23
With all this in mind, approaching the fourth ventricle using the superior transvelar approach may offer an alternative to cutting or retracting the vermis, and hence may decrease the likelihood of the posterior fossa syndrome.
Another important advantage of the superior transvelar approach, compared with the “traditional” suboccipital approaches, is its CSF diversion. Patients harboring fourth ventricle pathology often have concomitant noncommunicating hydrocephalus. Using the superior transvelar approach to the fourth ventricle would deal with the hydrocephalus too, besides dealing with the fourth ventricular pathology. Opening the superior medullary velum creates a pathway for CSF flow via the quadrigeminal cistern. Fenestration of the superior medullary velum was suggested, in the literature, using an endoscope to treat noncommunicating hydrocephalus.13
Lesions located in the upper part of the fourth ventricle may expand, and in doing so, stretch the superior medullary velum, increasing its size. This fact would make it more feasible, to use this approach, with no need for retraction to reach the lateral fourth ventricle.
Superior approach to the fourth ventricle has been mentioned in the literature.7,8 However, no detailed anatomical study exists, which describes this approach and its advantages and disadvantages. It seems plausible to approach the fourth ventricle from above, for lesions located in the upper part of the fourth ventricle, and for lesions “directed” at the superior aspect of the fourth ventricle. (Fig. 2) In 1996, Brown et al6 described an intuitive method, “used by experienced surgeons” to dictate the surgical approach to brainstem lesions. This method is used to select an approach “that leads to the pathology, while avoiding incision of brainstem tissue”.6 In this method, one draws a line from the center point of the lesion, through the superficial point, and out toward the skull. In many instances, the lesion is directed superiorly, making the superior transvelar approach suitable for this pathology.
Conclusion
The superior transvelar approach to the fourth ventricle is a valid option, for accessing pathologies in the fourth ventricle, and brainstem. It does not involve sacrifice of neural tissue, nor does it require a suboccipital craniotomy. The occipital transtentorial superior transvelar approach offers the balance between surgical exposures gained, neural tissue sacrificed and ease of access to the fourth ventricle. Using this approach enables the surgeon to overcome the common disadvantages of posterior fossa surgeries, (CSF leak, infections) while accessing a posterior fossa lesion. The superior transvelar approach offers CSF diversion, inherent in the procedure, and replaces the need for shunts or third ventriculostomy, in the advent of an obstructive hydrocephalus.
References
- 1.Tanriover N, Ulm A J, Rhoton A L, Yasuda A. Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg. 2004;101(3):484–498. doi: 10.3171/jns.2004.101.3.0484. [DOI] [PubMed] [Google Scholar]
- 2.Dubey A, Sung W S, Shaya M. et al. Complications of posterior cranial fossa surgery—an institutional experience of 500 patients. Surg Neurol. 2009;72(4):369–375. doi: 10.1016/j.surneu.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Doxey D, Bruce D, Sklar F, Swift D, Shapiro K. Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg. 1999;31(3):131–136. doi: 10.1159/000028848. [DOI] [PubMed] [Google Scholar]
- 4.Patir R, Mahapatra A K, Banerji A K. Risk factors in postoperative neurosurgical infection. A prospective study. Acta Neurochir (Wien) 1992;119(1-4):80–84. doi: 10.1007/BF01541786. [DOI] [PubMed] [Google Scholar]
- 5.Dailey A T, McKhann G M, Berger M S. The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg. 1995;83(3):467–475. doi: 10.3171/jns.1995.83.3.0467. [DOI] [PubMed] [Google Scholar]
- 6.Brown A P, Thompson B G, Spetzler R F. The two-point method: evaluating brainstem lesions. BNI Q. 1996;12(1):20–24. [Google Scholar]
- 7.Dammers R Delwel E J Krisht A F Cavernous hemangioma of the mesencephalon: tonsillouveal transaqueductal approach Neurosurgery 2009645, Suppl 2296–299., discussion 299–300 [DOI] [PubMed] [Google Scholar]
- 8.Porter R WDP, Spetzler R F. Philadelphia: Saunders; 2004. Infratentorial cavernous malformations; p. 2327. [Google Scholar]
- 9.Tompsett D H Anatomical Techniques. 2nd ed Edinburgh: E & S Livingstone; 1970xvii, 283 [Google Scholar]
- 10.Gray H, Lewis W H. New York: Bartleby.com; 2000. Anatomy of the Human Body. 20th ed. [Google Scholar]
- 11.Rhoton A L. Cerebellum and fourth ventricle. Neurosurgery. 2000;47(3, Suppl):S7–S27. doi: 10.1097/00006123-200009001-00007. [DOI] [PubMed] [Google Scholar]
- 12.Gray H Williams P L Bannister L H Gray's Anatomy: The Anatomical Basis of Medicine and Surgery. 38th ed New York: Churchill Livingstone; 1995xx, 2092 [Google Scholar]
- 13.Tubbs R S, Wellons J C, Salter G, Oakes W J. Fenestration of the superior medullary velum as treatment for a trapped fourth ventricle: a feasibility study. Clin Anat. 2004;17(2):82–87. doi: 10.1002/ca.10185. [DOI] [PubMed] [Google Scholar]
- 14.Rhoton A L. The cerebellar arteries. Neurosurgery. 2000;47(3, Suppl):S29–S68. doi: 10.1097/00006123-200009001-00010. [DOI] [PubMed] [Google Scholar]
- 15.Rhoton A L. The posterior fossa veins. Neurosurgery. 2000;47(3, Suppl):S69–S92. doi: 10.1097/00006123-200009001-00012. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima M Rhoton A L Matsushima T Comparison of posterior approaches to the posterior incisural space: microsurgical anatomy and proposal of a new method, the occipital bi-transtentorial/falcine approach Neurosurgery 20025151208–1220., discussion 1220–1221 [DOI] [PubMed] [Google Scholar]
- 17.Rhoton A L, Yamamoto I, Peace D A. Microsurgery of the third ventricle: Part 2. Operative approaches. Neurosurgery. 1981;8(3):357–373. doi: 10.1227/00006123-198103000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Leblanc A. Berlin, New York: Springer; 1995. The Cranial Nerves: Anatomy, Imaging, Vascularisation. 2nd enl. ed; p. 297. [Google Scholar]
- 19.Schmidek H H Roberts D W Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results. 5th ed Philadelphia: Saunders Elsevier; 2006xxxix, 2337, 2367 [Google Scholar]
- 20.Greenberg M S Handbook of Neurosurgery. 5th ed New York: Greenberg Graphics; Thieme Medical Publishers; 2001. x, 971 [Google Scholar]
- 21.de Oliveira J G, Lekovic G P, Safavi-Abbasi S. et al. Supracerebellar infratentorial approach to cavernous malformations of the brainstem: surgical variants and clinical experience with 45 patients. Neurosurgery. 2010;66(2):389–399. doi: 10.1227/01.NEU.0000363702.67016.5D. [DOI] [PubMed] [Google Scholar]
- 22.Ausman J I, Malik G M, Dujovny M, Mann R. Three-quarter prone approach to the pineal-tentorial region. Surg Neurol. 1988;29(4):298–306. doi: 10.1016/0090-3019(88)90161-9. [DOI] [PubMed] [Google Scholar]
- 23.Chi J H Lawton M T Posterior interhemispheric approach: surgical technique, application to vascular lesions, and benefits of gravity retraction Neurosurgery 2006591, Suppl 1ONS41–ONS49., discussion ONS41–ONS49 [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa Y, Uede T, Hashi K. Operative approach to mediosuperior cerebellar tumors: occipital interhemispheric transtentorial approach. Surg Neurol. 1999;51(4):421–425. doi: 10.1016/s0090-3019(98)00123-2. [DOI] [PubMed] [Google Scholar]
- 25.Nazzaro J M, Shults W T, Neuwelt E A. Neuro-ophthalmological function of patients with pineal region tumors approached transtentorially in the semisitting position. J Neurosurg. 1992;76(5):746–751. doi: 10.3171/jns.1992.76.5.0746. [DOI] [PubMed] [Google Scholar]
- 26.Moshel Y A Parker E C Kelly P J Occipital transtentorial approach to the precentral cerebellar fissure and posterior incisural space Neurosurgery 2009653554–564., discussion 564 [DOI] [PubMed] [Google Scholar]
- 27.Clark W K. Baltimore: Williams & Wilkins; 1987. Occipital transtentorial approach; pp. 591–625. [Google Scholar]
- 28.Lawton M T Golfinos J G Spetzler R F The contralateral transcallosal approach: experience with 32 patients Neurosurgery 1996394729–734., discussion 734–735 [DOI] [PubMed] [Google Scholar]
- 29.Rhoton A L. Tentorial incisura. Neurosurgery. 2000;47(3, Suppl):S131–S153. doi: 10.1097/00006123-200009001-00015. [DOI] [PubMed] [Google Scholar]