Abstract
With the advent of microsurgery and surgical techniques, along with the improvement in neuroimaging techniques and the microanatomy in cadaver study, improvement in terms of surgical morbidity and mortality has been remarkable; however, controversy still exists regarding the optimal surgical strategies for giant petroclival meningiomas (GPMs). We report a study of clinical and radiological features as well as the surgical findings and outcomes for patients with GPM treated at our institution over the past 6 years. During a 6-year period (April 2004 to March 2010), 16 patients with GPM underwent surgery by subtemporal transtentorial petrosal apex approach during which electrophysiological monitoring of cranial nerves and brainstem function were reviewed. There were nine females and seven males with a mean age of 56.9 years (range from 32 to 78 years). The most frequent clinical manifestations were headache (93.7%) and dizziness (93.7%). Regions and directions of tumor extension include clivus, parasellar, and cavernous sinus, as well as compression of brainstem, and so on. The trochlear nerve was totally wrapped in nine cases (56.2%). The postoperative Karnofsky Performance Scale (KPS) score was 76.3 ± 13.1. Mean maximum diameter of the tumors on magnetic resonance imaging was 5.23 cm (range, 4.5 to 6.2 cm). Subtemporal transtentorial petrosalapex approach was performed in all 16 cases. Gross total resection was achieved in 14 cases (87.5%) and subtotal resection in 2 cases (12.5%) with no resultant mortality. Follow-up data were available for all 16 patients, with a mean follow-up period of 28.8 months (range from 4 to 69 months), of which 11 (68.75%) lived a normal life (KPS, 80–100). Our suggestion is that GPM could be completely resected by subtemporal transtentorial petrosalapex approach. The surgical strategy of GPM should be focused on survival and postoperative quality of life. Microneurosurgical technique plays a key role in tumor resection and preservation of nerve function. Intraoperative electrophysiological monitoring also contributes dramatically to the preservation of the nerve function. Complete resection of the tumor should be attempted at the first operation. Any remnant is treated by radiosurgery.
Keywords: subtemporal transtentorial petrosalapex approach, petroclival areas, meningioma, surgical approach, electrophysiological monitoring
Meningiomas arising from the petroclival region present one of the daunting surgical challenges in neurosurgery, primarily because of their propensity to engulf nerves and blood vessels, the difficulty of obtaining total resection, and the severe complications that can arise.1,2,3,4,5 Microsurgical treatment is still the first choice for giant petroclival meningiomas (GPMs). Complete tumor resection often brings good outcomes. However, patients with GPM often present with symptoms of cranial nerve impairment and brainstem compression. Achieving a good outcome is a challenge for the neurosurgeons. During the last 10 years, the development of microsurgery techniques and the minimal invasive concept, the choice and improvement of the skull base approaches, the accumulated expertise of the subspecial neurosurgeons, and the overwhelming use of intraoperative monitoring of cranial nerves and brainstem function have contributed significantly to successful operations.1,2,3,6,7 However, a gross total resection (GTR) rate of 43% reported by one study8 was still not satisfactory. The subtemporal transtentorial petrosalapex approach has obvious advantages, such as tendency of microinvasive operation and better outcome. In this article, 16 patients with GPMs with integrity proved by operation procedure and pathology were retrospectively reviewed on the basis of microanatomy by the senior author (J.Y.) from April 2004 to March 2010.
Patients and Methods
Patient Characteristics
In this study, 16 patients harboring petroclival meningiomas were treated surgically from April 2004 to March 2010 by the senior author (J.Y.). The study group consisted of seven males and nine females. The mean patient age was 56.9 years and ranged from 32 to 78 years.
Clinical Symptoms and Signs
The most frequent clinical manifestations were headache (93.7%) and dizziness (93.7%). Clinical manifestations and characteristics and extension direction of tumor are listed in Table 1. The mean duration of symptoms was 47 months, ranging from 4 to 120 months.
Table 1. Clinical Manifestations and Characteristics and Extension Direction of Tumor.
| Clinical Manifestations and Symptoms/Signs before Surgery | Patient No. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | C | R%/ (Ave) |
|
| Age(years) | 44 | 64 | 53 | 49 | 32 | 51 | 52 | 63 | 71 | 54 | 49 | 57 | 73 | 55 | 78 | 65 | 16 | 56.9 |
| Sex (M/F) | F | M | M | F | F | M | M | M | M | F | F | F | F | F | M | F | 7/9 | |
| Headache | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | 15 | 93.7 |
| Dizziness | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | 15 | 93.7 |
| Facial numbness | − | + | + | + | − | + | + | − | − | − | − | + | − | + | − | + | 8 | 50 |
| Diplopia | + | − | + | + | − | + | + | − | − | + | − | + | − | + | − | + | 9 | 53.6 |
| Symptoms of CN impairment (V) | + | − | + | + | − | − | + | − | + | + | − | + | − | + | − | − | 8 | 50 |
| VI | − | − | + | − | − | + | + | − | − | − | − | + | − | + | − | + | 6 | 37.4 |
| VII | + | − | + | − | − | − | + | − | − | + | − | + | + | + | − | − | 7 | 43.7 |
| VIII | − | + | − | + | − | − | − | − | + | − | − | + | − | − | + | − | 5 | 31.2 |
| Lower CN (IX, X, XI, XII) | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | 1 | 6.2 |
| Myodynamia decreascence | + | + | − | − | − | + | + | + | + | + | − | − | + | − | + | + | 10 | 62.5 |
| Coordination disturbance | + | − | − | − | + | + | − | − | + | + | − | + | + | − | + | + | 9 | 56.2 |
| Characteristics and extension direction of tumor | ||||||||||||||||||
| Upper clivus | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | 1 | 6.2 |
| Middle clivus | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | + | 14 | 87.5 |
| Lower clivus | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | 1 | 6.2 |
| Parasellar | + | − | + | + | + | + | + | + | − | + | + | + | + | + | + | + | 14 | 87.5 |
| Suprasellar | + | − | + | − | − | + | + | − | − | − | − | + | − | + | − | + | 7 | 43.7 |
| Cavernous sinus | + | − | + | − | − | − | − | + | + | − | + | + | + | + | + | + | 10 | 62.5 |
| Orbital apex | − | − | + | − | − | + | + | + | − | − | − | + | − | + | + | − | 7 | 43.7 |
| Supratentorial extension | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 16 | 100 |
| Brainstem compression | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 16 | 100 |
| CN or artery encasement | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 16 | 100 |
| Blood supply | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 16 | 100 |
| Bone involvement | + | − | + | + | + | + | + | − | + | + | + | + | + | + | + | − | 13 | 81.2 |
| (IV) trochlear nerve wrapped | ||||||||||||||||||
| IV PW | − | + | − | + | + | − | + | + | − | + | + | − | − | − | − | − | 7 | 43.7 |
| IV TW | + | − | + | − | − | + | − | − | + | − | − | + | + | + | + | + | 9 | 56.2 |
| IV TW(e) | + | − | − | − | − | − | − | − | + | − | − | − | + | + | + | + | 6 | 37.4 |
| IV TW(une) | − | − | + | − | − | + | − | − | − | − | − | + | − | − | − | − | 3 | 18.7 |
| TCAA | − | − | − | − | − | − | + | + | − | − | − | − | + | + | − | − | 4 | 25 |
| TCPA | + | + | + | + | + | + | − | − | + | + | + | + | − | − | + | + | 12 | 75 |
Ave, average; C, cases; CN, cranial nerve; IV PW, trochlear nerve partly wrapped; IV TW, IV totally wrapped; IVTW(e), totally wrapped and explored tentorium; IVTW(une), totally wrapped and unexplored tentorium; R, rate of incidence; TCAA, tentorium cut along anterior apex; TCPA, tentorium cut along posterior apex.
Neuroradiological Evaluation
The preoperative evaluation included contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). CT angiography, magnetic resonance angiography, or magnetic resonance venography (MRV) were also performed in some cases to evaluate the blood supply of the tumors. According to Sekhar et al’s criteria,9 giant tumor refers to tumor with diameter larger than 4.5 cm. In our study, all 16 cases were giant tumors. The clivus was divided into three parts9 by two anatomical markers—Dorello’s canal and the nerve part of jugular foramen. The upper clivus is superior to the level of Dorello’s canal, the lower clivus is inferior to the level of nerve part of jugular foramen, and the middle clivus is between two parts. The inferior part of most GPMs (87.5%) were confined within the middle clivus, but one was confined within the upper clivus. Areas of tumor extension went beyond the clivus, sellar, and petrous apex. Brainstem compression was observed in all 16 cases and bone involvement in 13 cases (81.25%) (Table 1).
Surgical Approaches
All 16 cases were treated by subtemporal transtentorial petrosalapex approach. Surgical goals were discussed with each patient preoperatively. The patient was placed in the lateral decubitus position with the ipsilateral shoulder slightly elevated. The image of temporal bone was evaluated preoperatively. This approach is made through a temporal craniotomy that extends down to the floor of the middle fossa or the cerebellum tentorium. The mastoid air cell should be sealed by bone wax, fibrin glue, or temporal muscle strip. Cerebrospinal fluid (CSF) was evacuated before cerebellar retraction, and ventricular puncture was utilized when exposure was not satisfactory. Great attention was given to protect the vein of Labbé during exposure. After the exposure, coagulation of the tumor base was performed before tumor resection, and careful identification of the vessels passing through was completed. The supratentorial part of the tumor was resected after coagulating the cerebellum tentorium at the base of tumor, and tumor debulking was then performed. The incision on the cerebellum tentorium was performed parallel to the posterior part of superior petrous sinus to the free edge of the cerebellum tentorium. To have a wider exposure of the subtemporal structures, the bone of the petrous apex between the trigeminal nerve and the internal acoustic meatus was removed to expose the side of the clivus; drilling just in front of the petrous ridge in the area medial to the arcuate eminence, the exposure was under the trigeminal nerve to the edge of the inferior petrosal sinus (IPS) (Fig. 1). The cranial nerves III and IV, and branches of posterior cerebral artery (PCA) and superior cerebral artery (SCA) were dissected and protected after the exposure of the cerebellum tentorium. After debulking of the tumor, structures such as cranial nerves, vessels, and brainstem displaced or encased by tumor were visualized clearly. The brainstem was then dissected from the tumor, the tentorium involved by the tumor was resected, and then the tumor was totally resected (Fig. 2).
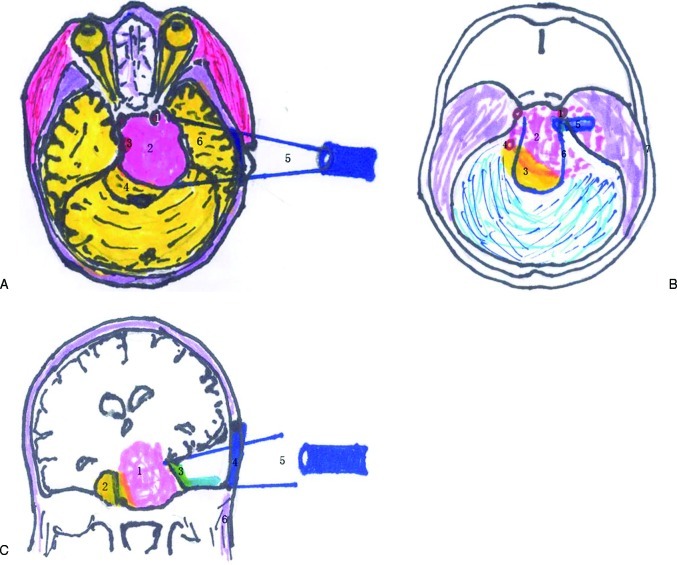
Figure 1.
(a) The schematic diagram of operation approach (axial view). 1. Internal carotid artery (ICA); 2. tumor; 3. basilar artery; 4. brainstem; 5. subtemporal approach; 6. temporal lobe; 120 × 94 mm (130 × 130 DPI). (b) The schematic diagram of interrelation between the tumor and the normal arteries (axial view). 1. ICA; 2. tumor; 3. brainstem; 4. basilar artery; 5. tentorial marginal incision; 6. tentorial margin; 7. temporal bone; 95 × 109 mm (131 × 131 DPI). (c) The schematic diagram of operation approach (coronal view). 1. Tumor; 2. brainstem; 3. tentorial margin; 4. bone window; 5. subtemporal approach; 6. infratemporal region; 124 × 88 mm (131 × 131 DPI).
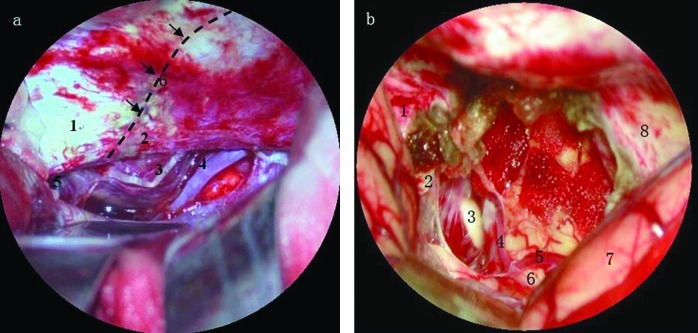
Figure 2.
(a) Intraoperative picture. 1. Petrous apex; 2. edge of tentorium of cerebellum; 3. cranial nerve IV; 4. arteria cerebelli superior; 5. cranial nerve III; 6. tentorial marginal incision (dash line was designed cut on tentorium of cerebellum along posterior surface of the petrous bone); 78 × 76 mm (148 × 148 DPI). (b) Intraoperative picture. 1. Petrous apex; 2. clivus; 3. abducent nerve; 4. anterior inferior cerebellar artery; 5. posterior cerebral artery; 6. brainstem; 7. temporal lobe; 8. tentorium of cerebellum; 82 × 79 mm (141 × 141 DPI).
Electrophysiologic Monitoring of Cranial Nerves and Brainstem Function
An electromyography (EMG) evoked potential monitoring system was used, and monitoring electrodes were placed at target points of the operative side to monitor cranial nerves V and VII, and the lower cranial nerve’s function, respectively. Intraoperative brainstem auditory evoked potentials (BAEP) monitoring was performed in all 16 cases. Standard stimulation and recording parameters were applied. An ear piece stimulator with a built-in tube extension was used routinely for intraoperative recordings. BAEP were continuously obtained throughout the surgical procedure. During the surgical procedure, all reproducible and progressive BAEP deteriorations were reported to the surgical team.
Criteria of Tumor Resection
According to intraoperative view and postoperative MRI findings (Fig. 3), the outcomes were clarified into four grades: (1) GTR: no tumor residual showing on the immediate postoperative MRI as well as the surgeon’s observation under the microscope during the operation; (2) subtotal resection (STR): tumor residual <10%; (3) large partial resection: tumor residual <50% but >10%; and (4) partial resection: tumor residual >50%. Residual tumor was treated by radiosurgery (gamma knife).
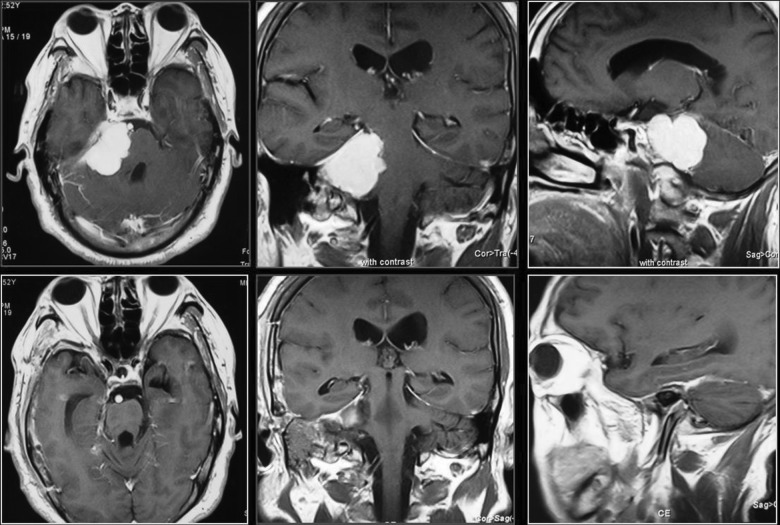
Figure 3.
Contrast of magnetic resonance imaging (MRI); classical case. Axial, coronal, and sagittal preoperative MRI (a, b, c); axial, coronal, and sagittal postoperative MRI (d, e, f); 187 × 125 mm (150 × 150 DPI).
Results
The subtemporal transtentorial petrosalapex approach was performed in all 16 cases. EMG and BAEP monitoring were also performed. The trochlear nerve was partly wrapped in seven cases, totally wrapped but could be explored in the initial segment of the cerebellum tentorium in six cases, and totally wrapped and could not be seen unless tumor was partly removed in three cases. The incision of the cerebellum tentorium was performed posterior to where cranial nerve IV merged and parallel to the junction of the cerebellum tentorium and posterior-interior part of the petrous bone. GTR was accomplished in 14 cases (87.5% of patients) and STR in 2 patients (12.5%) due to the encompassing of internal carotid artery (ICA) and the cavernous sinus invasion. Results of tumor resection was shown on the immediate postoperative MRI or CT as well as the surgeon’s observation under the microscope during the operation. Mean hospitalized days were 28 days (range from 15 to 75 days). Cranial nerves in all cases were kept intact anatomically. Postoperative complications included new neurological deficits or aggravations of preexisting deficit in six cases (37.5%), among which five cases (31.25%) for cranial nerve III, five cases (31.25%) for cranial nerve IV, three cases (18.7%) for cranial nerve V, three cases (18.7%) for cranial nerve VI, and one case (6.2%) for lower cranial nerves; temporary aphasia in two cases (12.5%); hemiplegia in one case (12.5%); brain infection in two cases (12.5%); temporary CSF leakage in one case (6.2%); and encephalorrhagia and high intracranial pressure (ICP) due to brain swelling in two cases (12.5%), among which one patient underwent craniectomy (6.2%), and two patients underwent tracheotomy. Follow-up data were available for all cases ranging from 4 to 69 months with a mean follow-up period of 28.8 months. MRI was performed through outpatient recheck at 3- to 6-month intervals to observe the recurrence or regrowth of the tumor; none was found. After 3 to 6 postoperative months, the recovery of cranial nerve III function was observed in three cases and there was improvement in one case; the recovery of cranial nerve IV function was observed in five; the recovery of cranial nerve V function was observed in three; the recovery of cranial nerve VI function in was observed in three; and the recovery of lower cranial nerve function was observed in one. Two cases with aphasia completely recovered within 0.5 to 3 months; House-Brackmann scale was applied for evaluation of nerve function. One case with hemiplegia still has motor defect (muscle strength 4 to 5 grade, can walk with help). One patient with CSF leakage was cured by lumbar cistern drainage. Two patients were treated with gamma knife radiosurgery due to tumor residue. Ventriculoperitoneal shunt was performed in one case with postoperative hydrocephalus. There was no resultant mortality or long-term coma cases. The Karnofsky Performance Scale (KPS) score was 76.3 ± 13.1 (Table 2).
Table 2. Clinical Results.
| Patient No. | Size of Tumor AP × ML × CC, cm |
Resection Extent | Cranial Nerve Morbidities | Clinical Complications | Karnofsky Score (P.O.) | Cranial Nerve Improvement (3–6 mo) |
Follow-Up (Months) |
|---|---|---|---|---|---|---|---|
| 1 | 4.2 × 4.5 × 3.8 | GTR | 80 | No recurrence (69) | |||
| 2 | 4.5 × 3.9 × 4.3 | STR | CN IV, VI | 60 | No regrowth (63) (Gamma knife) |
||
| 3 | 5.5 × 4.5 × 4.1 | GTR | CN III, V, VI | 80 | No recurrence (58) | ||
| 4 | 4.7 × 3.9 × 3.8 | GTR | 90 | No recurrence (51) | |||
| 5 | 4.5 × 4.0 × 4.1 | GTR | 80 | No recurrence (45) | |||
| 6 | 6.5 × 4.8 × 6.0 | STR | CN III, IV, V, VI | Aphasia, encephalorrhagia swelling, craniectomy, tracheotomy, brain infection | 50 | CN III | No regrowth (38) V-P shunt (Gamma knife) |
| 7 | 4.8 × 4.0 × 3.9 | GTR | 80 | No recurrence (31) | |||
| 8 | 4.6 × 4.1 × 3.8 | GTR | CN III, IV | 90 | No recurrence (25) | ||
| 9 | 6.2 × 4.7 × 5.9 | GTR | CN III, IV, V, IX–XII |
Aphasia, hemiplegia, swelling, treachoectomy | 50 | Motor defect MS 4–5 grade | No recurrence (20) |
| 10 | 4.6 × 4.2 × 3.8 | GTR | 90 | No recurrence (15) | |||
| 11 | 4.8 × 4.1 × 4.0 | GTR | 90 | No recurrence (12) | |||
| 12 | 5.7 × 4.8 × 5.4 | GTR | 80 | No recurrence (10) | |||
| 13 | 5.3 × 4.6 × 4.9 | GTR | 70 | No recurrence (9) | |||
| 14 | 5.2 × 4.6 × 3.9 | GTR | 80 | No recurrence (7) | |||
| 15 | 6.1 × 4.7 × 5.0 | GTR | CN III, IV | CSF leakage, brain infection |
70 | No recurrence (5) | |
| 16 | 5.9 × 4.5 × 6.1 | GTR | 80 | No recurrence (4) |
CN, cranial nerve; CSF, cerebrospinal fluid; GTR, gross total resection; MS, muscle strength; P.O., postoperation; STR, subtotal resection; V-P, ventriculoperitoneal.
Discussion
Petroclival meningiomas arise from the upper two-thirds of the clivus at the petroclival junction, and medial to the trigeminal nerve.10 Petroclival meningiomas are formidable and represent a great challenge to the neurosurgeon because of their propensity to encase cranial nerves, basilar and carotid arteries, and their perforating arteries; their involvement of the cavernous sinus and parasellar region; their proximity to or their presence in the brainstem; and the natural history marked by clinical deterioration and fatal outcome. The mortality rate of petroclival meningioma was 53% before 1970s, and only one GTR was reported.11
Multiple surgical approaches have been developed over the last several decades to manage these lesions. They can be approached by the anterolateral, lateral, posterolateral, or combined approaches, depending on the location and extension of the tumor and the patient’s preoperative hearing status.12 The suboccipital retrosigmoid approach was a classic approach familiar to neurosurgeons, and it was used in petroclival meningiomas because of the advantages: it is easy to perform, it has fewer postoperative complications, and it has faster recovery. The exposure needs no drilling of the petrous bone; however, because of the disadvantages of a long surgical corridor and the risk of cranial nerves’ impairment (cranial nerves are interposed between the surgeon and the pathology), this approach was used for smaller tumors that were medially or laterally situated, with a limited area of dural attachment, and in patients with intact hearing. Lateral skull base approaches such as translabyrinthine, transcochlear, and total petrosal approaches provided wide exposure to the petrocival region, but could cause damage to cranial nerves VII and VIII, so these approaches were reserved for patients without hearing or with lesion invading the temporal bone.13,14 Drilling the petrous bone in these approaches was time-consuming, and some neurosurgeons even staged the total petrosal approach by performing the exposure on one day and tumor resection on the following day.7
Combined supra-infratentorial transpetrosal approach was considered to be the first option for petroclival meningiomas, then it was divided into kinds of subtypes by experts and was also refined by some scholars.15,16,17 Strategies to overcome the petrous bone as an obstacle14,18 have included resection of the petrous apex with the middle fossa approach; resection of presigmoid, retrolabyrinthine petrous bone with the posterior petrosal approach; and resection of the entire petrous bone with complete petrosectomy. It can bring a wider version, a shorter distance, a clear exposure of cranial nerves III to XII, PCA, and SCA, and also helps reduce the retraction of temporal lobe and preservation of the vein of Labbé.19 Complete resection rates were greatly improved. However, this approach also has disadvantages: complicated performance (which makes it time-consuming), more time used in tumor exposure than tumor resection, complex invasiveness, slow recovery, high risk of infection, impairment of hearing, and exposure not enough due to previa sigmoid sinus. The superficial location of cranial nerves, such as V, VII, and lower cranial nerves, makes it difficult to operate, and damage to the nerves is relatively easy; the incidence of surgical complications is as high as 50%.8
The presigmoid transpetrosal approach was once considered to be a good approach for petroclival tumors by the majority of neurosurgeons because it provides a wider surgical field, a short distance, a wide exposure of cranial nerves III to XII and main arteries of posterior circulation, and a higher chance in preservation of the vein of Labbé by less retraction of temporal lobe.19 However, the disadvantages of this approach have been recognized: it is time-consuming, has more complications, exposure was often affected by anatomic variations, and there is risk of impairment of cranial nerves due to their superficial location. Since this century, the concept of minimally invasive surgery has been booming, and more and more scholars are changing their goals from achieving complete tumor resection to balancing between maximized tumor resection and postoperative quality of life.20 Zhu et al21 reported a combined subtemporal and retrosigmoid keyhole approach that bears the advantages of being less time-consuming and minimally invasive, and uses well-adapted instruments and endoscope to provide satisfactory exposure. Comprehensive understanding of minimally invasive surgery makes the point and all the therapeutic strategies and the choice of approach relied on it.2,4,6,22,23 Optimal surgical approaches should be minimally invasive, simple, and effective.
Kawase et al16 reported 10 cases of petroclival meningioma that underwent surgery via subtemporal approach. Due to the poor exposure and difficulty in operation caused by the poor condition of the microscope, the surgical teams had to employ large bone scalp to improve the exposure to get the GTR. Microanatomy research on subtemporal transtentorial petrosalapex approach was reported by the senior author’s team in 2008.4 The subtemporal transtentorial petrosalapex approach is made through a temporal craniotomy that extends down to the floor of the middle fossa or the cerebellum tentorium. After discission of the cerebellum tentorium and dissection of the petrous apex, this approach offers the visibility of the structures around the parasellar and cavernous sinus to the lower cranial nerves with the help of microinvasive equipment and instruments such as high-resolution microscopes, neuroendoscopes, and well-adapted instruments. It can provide better version, because of wider exposure achieved by CSF evacuation, and elevation of the temporal lobe. The prominent advantages of this approach are simplicity, less time-consuming, safety, minimal invasive, more versatility (barely affected by anatomic variation of sigmoid sinus or glomus jugulare),1,4 and decreased risk of surgical complications such as CSF leak and brain infection. The blood supply to the tumor from the cerebellum tentorium can be coagulated at an early stage, and tumor resection could be started from the base of the tumor. Therefore, bleeding is less during the resection course. Separation, dissection, and preservation of surrounding nerves and vessels which were displaced or encased by the tumor becomes easier after PTR. The incision of cerebellum tentorium was performed posterior to where cranial nerve IV merged and parallel to the line where the cerebellum tentorium and posterior-interior part of the petrous bone fused. The approach is performed by dissection of the temporal bone to the level of petrous part of the carotid artery or the internal acoustic meatus, with drilling the petrous ridge if necessary. The drilling extent depends on the pathological and anatomical conditions. Structures such as cranial nerves, vessels, brainstem, and cerebellum will be visualized clearly after tumor debulking. Then GTR will be achieved. Mathiesen et al2 reported 29 cases of petroclival meningiomas in combined transpetrosal surgical approaches. Outcomes such as tumor exposure as well as preservation of cranial nerve function were also acquired well, as in our series. Our experience was that this approach is suitable for tumors located in the middle fossa, especially for regions from the upper two-thirds of the clivus to petrous bone, even in such cases with soft consistency of the tumor tissue diagnosed by MRI on T2-weighted and proton density images, though part of the tumor may be located within the lower one-third clivus.
The necessity of brain retraction increases the risk of venous infarction of the vein of Labbé15,24; the vein of Labbé can reach the transverse sinus through a “tentorial” sinus, and caution is necessary not to put excessive tension on the vein while retracting the temporal lobe.25 Several measures can be taken for preservation of the vein of Labbé, such as freeing a segment of it from the cortex or removing nearby nonfunctional brain tissue in the case of exposure. The vein of Labbé should be protected carefully; use of coagulation should be decreased because of complications caused by the damage of even little branches.4,22,25 Preoperative MRV of drainage patterns of veins is then evaluated, and the most ideal superior petrosal venous complex (SPVC) for this approach was that the SPVC emptied into the superior petrosal sinus (SPS) above and lateral to the boundaries of the inferior acoustic meatus.26 According to some studies,1 surgeons may have imagined themselves safe scarifying the petrosal vein; however, knowledge of partial or total occlusion of the SPS is mandatory for the surgeon, patency of the IPS on the lesion side is of clinical importance in cases having occluded SPS, and preservation of the petrosal venous complex should be attempted whenever possible to increase the safety of surgery. If preservation of superior petrosal veins is not feasible, effective brainstem decompression following tumor removal is essential for minimizing the risk of significant morbidity.27 The preoperative embolization of all the major feeding vessels of the tumor can make the surgical procedure easier.28 Cranial nerves and vessels were wrapped when part of parasellar, cavernous sinus, and suprasellar were filled with tumor. Two cases did not achieve GTR, and this was because the ICA was encompassed and there was cavernous sinus invasion. Risk of damage to cranial nerve IV is high if it is totally wrapped, and this can be avoided by exploration of the place where the cranial nerve joins the cerebellum tentorium. Great attention should be paid to bleeding when dissecting the residual of the tumor left in the lateral and posterior part of the cavernous sinus, and bleeding also causes difficulties in finding and preventing injury to cranial nerve IV, which is the thinnest and has no fixed location. The presence of edema on T2-weighted images indicates a disruption of the blood-brain barrier and invasion of or adherence of the tumor to the brainstem surface.28 Pial invasion by the tumor can also be related to the loss of the arachnoidal plane on T1-weighted magnetic resonance (MR) images and to the presence of blood supply to the tumor from the vertebrobasilar complex at the arterial phase of the angiography.29 Even in cases where the pia-arachnoid plane of the brainstem is partially lost, however, total dissection from the brainstem is possible without major neurological deficits unless the brainstem perforating arteries are obliterated. The senior author’s policy for managing such tumors is to obtain total removal of the intracranial portion of the meningiomas without attempting to excise the tenaciously adherent portion of the tumor. The possible remnant is treated by radiosurgery. However, several factors such as dense adhesions, which is a consequence of the first intervention, may significantly increase the surgical risk involved in a second operation. To avoid perforator injury, bipolar coagulation should be avoided as much as possible during brainstem dissection.
The prognostic value of intraoperative BAEP is well recognized and established. Abrupt loss of BAEP is associated with permanent anacusis. Cranial nerves such as V, VII, and the lower cranial nerves can be monitored by EMG; the prolonged latency of wave I to V and the decline of amplitude of wave III to V are sensitive indications of brainstem dysfunction. Surgical reactions to BAEP changes included repositioning or transient removal of cerebellar retractor, pause of surgical dissection or shifting of dissection toward another tumor area, and mechanical manipulations of the capsule. Bipolar coagulation is limited to the necessary minimum, sharp dissection is favored, and Ringer solution is used for irrigation.30 The operation should be modified or even stopped when the waveforms change significantly. Postoperative cranial nerve dysfunction and motor deficits still occur in our series as well as in other series,5,16,20 although all cranial nerves were preserved anatomically. There were no significant differences in deficits of cranial nerves in these two approaches. We suggest that the overtraction of the nerves and the disturbance of blood supply could be the critical causes and should be avoided. The causes of aphasia may be related to temporal lobe edema and damage of the vein of Labbé or petrosal venous complex. Encephalorrhagia and high ICP caused by brain swelling may be caused by insufficient release of the CSF, cerebral contusion during exposure, and venous disorder. Blood vessel spasm and edema of the brainstem might be the cause of temporary aphasia hemiplegia.
Meningiomas, characterized by well-defined radiographic margins, no brain invasion in benign cases, and vascularity that may be obliterated with radiation therapy, are particularly well suited to stereotactic radiosurgery.10 Adjuvant therapy using gamma knife radiosurgery for meningiomas can safely achieve high rates of tumor growth control, improving patient outcomes. The combination of surgery and radiosurgery is a useful treatment strategy for ensuring good functional outcomes while maintaining long-term tumor growth control.31 We recommend a time interval of 3 to 6 months between surgery and radiosurgery for residual meningiomas.
Our experience is that the subtemporal transtentorial petrosalapex approach is an ideal approach for meningiomas located in middle and posterior fossa, especially for those located within the upper two-thirds of the clivus. The surgical approach needs to be diversified, and the choice of surgical approach should accord with the principle of excellent nerve function preservation, better surgical exposure, and fewer surgical complications. Microneurosurgical technique plays a key role in tumor resection and preservation of nerve function. Intraoperative electrophysiological monitoring also contributes dramatically to the preservation of the nerve function. Complete resection of the tumor should be attempted at the first operation. However, microsurgery is not the only way to solve the problem: other scholars6 proposed that stereotactic radiosurgery can also be used for the treatment of petroclival meningioma, and it should be used on an individualized basis. The subtotally removed petroclival meningiomas remain stationary for long periods, and radiosurgery can control eventual regrowth.32
Acknowledgment
This research has been supported by the Fund of Science and Technology of Beijing 2011.
Footnotes
This article was originally Published online in Skull Base on November 30, 2011 (DOI:10.1055/s-0031-1296037)
References
- 1.Gong J, Yu C J, Guan S S. et al. Modified surgical approaches to the petroclival region: an anatomic study. Chin J Minim Invasive Neurosurg. 2005;10:26–29. [Google Scholar]
- 2.Mathiesen T, Gerlich A, Kihlström L, Svensson M, Bagger-Sjöbäck D. Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery. 2008;62(6, Suppl 03):1213–1223. doi: 10.1227/01.neu.0000333787.06221.e5. [DOI] [PubMed] [Google Scholar]
- 3.Samii M, Tatagiba M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir (Wien) 1992;118(1-2):27–32. doi: 10.1007/BF01400723. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Yu C J, Qi Z. et al. Microsurgical management of giant petroclival meningiomas. Chin J Neurosurg. 2008;24(3):190–192. [Google Scholar]
- 5.Yu C J, Wang Z C, Guan S S, Sun H L. Microsurgical treatment of giant petroclival tumors (report of experiences with 15 cases) Chin J Neurosurg. 1997;13:205–207. [Google Scholar]
- 6.Bambakidis N C, Kakarla U K, Kim L J. et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery. 2007;61(5, Suppl 02):202–209, discussion 209–211. doi: 10.1227/01.neu.0000303218.61230.39. [DOI] [PubMed] [Google Scholar]
- 7.Erkmen K, Pravdenkova S, Al-Mefty O. Surgical management of petroclival meningiomas: factors determining the choice of approach. Neurosurg Focus. 2005;19(2):E7. doi: 10.3171/foc.2005.19.2.8. [DOI] [PubMed] [Google Scholar]
- 8.Ying M, Liang Z F, Rong Z, Wei Z. Microinvasive approach for surgery on petroclival meningiomas. Chin J Microsurg. 2005;28:99–102. [Google Scholar]
- 9.Sekhar L N, Jannetta P J, Burkhart L E, Janosky J E. Meningiomas involving the clivus: a six-year experience with 41 patients. Neurosurgery. 1990;27(5):764–781, discussion 781. [PubMed] [Google Scholar]
- 10.Couldwell W T, Fukushima T, Giannotta S L, Weiss M H. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84(1):20–28. doi: 10.3171/jns.1996.84.1.0020. [DOI] [PubMed] [Google Scholar]
- 11.Mayberg M R, Symon L. Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg. 1986;65(2):160–167. doi: 10.3171/jns.1986.65.2.0160. [DOI] [PubMed] [Google Scholar]
- 12.Iaconetta G, Fusco M, Samii M. The sphenopetroclival venous gulf: a microanatomical study. J Neurosurg. 2003;99(2):366–375. doi: 10.3171/jns.2003.99.2.0366. [DOI] [PubMed] [Google Scholar]
- 13.Hakuba A, Nishimura S, Tanaka K, Kishi H, Nakamura T. Clivus meningioma: six cases of total removal. Neurol Med Chir (Tokyo) 1977;17(1 Pt 1):63–77. doi: 10.2176/nmc.17pt1.63. [DOI] [PubMed] [Google Scholar]
- 14.Hakuba A, Nishimura S, Jang B J. A combined retroauricular and preauricular transpetrosal-transtentorial approach to clivus meningiomas. Surg Neurol. 1988;30(2):108–116. doi: 10.1016/0090-3019(88)90095-x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mefty O, Fox J L, Smith R R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22(3):510–517. doi: 10.1227/00006123-198803000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Kawase T, Shiobara R, Toya S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28(6):869–875, discussion 875–876. [PubMed] [Google Scholar]
- 17.Malis L I. Baltimore: Williams & Wilkins; 1992. Suboccipital subtemporal approach to petroclival tumors; pp. 41–51. [Google Scholar]
- 18.Cho C W, Al-Mefty O. Combined petrosal approach to petroclival meningiomas. Neurosurgery. 2002;51(3):708–716, discussion 716–718. [PubMed] [Google Scholar]
- 19.Goel A, Muzumdar D. Conventional posterior fossa approach for surgery on petroclival meningiomas: a report on an experience with 28 cases. Surg Neurol. 2004;62(4):332–338, discussion 338–340. doi: 10.1016/j.surneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Samii M, Tatagiba M, Carvalho G A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6(1):27–30. doi: 10.1054/jocn.1997.0201. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W, Mao Y, Zhou L F, Zhang R, Chen L. Combined subtemporal and retrosigmoid keyhole approach for extensive petroclival meningioma surgery: report of experience with 7 cases. Minim Invasive Neurosurg. 2007;50(2):106–110. doi: 10.1055/s-2007-984384. [DOI] [PubMed] [Google Scholar]
- 22.Shen T, Friedman R A, Brackmann D E. et al. The evolution of surgical approaches for posterior fossa meningiomas. Otol Neurotol. 2004;25(3):394–397. doi: 10.1097/00129492-200405000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Spallone A, Makhmudov U B, Mukhamedjanov D J, Tcherekajev V A. Petroclival meningioma. An attempt to define the role of skull base approaches in their surgical management. Surg Neurol. 1999;51(4):412–419, discussion 419–420. doi: 10.1016/s0090-3019(98)00100-1. [DOI] [PubMed] [Google Scholar]
- 24.Tahara A, de Santana P A Jr, Calfat Maldaun M V. et al. Petroclival meningiomas: surgical management and common complications. J Clin Neurosci. 2009;16(5):655–659. doi: 10.1016/j.jocn.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Koperna T, Tschabitscher M, Knosp E. The termination of the vein of “Labbé” and its microsurgical significance. Acta Neurochir (Wien) 1992;118(3-4):172–175. doi: 10.1007/BF01401304. [DOI] [PubMed] [Google Scholar]
- 26.Tanriover N, Abe H, Rhoton A L Jr, Kawashima M, Sanus G Z, Akar Z. Microsurgical anatomy of the superior petrosal venous complex: new classifications and implications for subtemporal transtentorial and retrosigmoid suprameatal approaches. J Neurosurg. 2007;106(6):1041–1050. doi: 10.3171/jns.2007.106.6.1041. [DOI] [PubMed] [Google Scholar]
- 27.Koerbel A, Gharabaghi A, Safavi-Abbasi S. et al. Venous complications following petrosal vein sectioning in surgery of petrous apex meningiomas. Eur J Surg Oncol. 2009;35(7):773–779. doi: 10.1016/j.ejso.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Roberti F, Sekhar L N, Kalavakonda C, Wright D C. Posterior fossa meningiomas: surgical experience in 161 cases. Surg Neurol. 2001;56(1):8–20, discussion 20–21. doi: 10.1016/s0090-3019(01)00479-7. [DOI] [PubMed] [Google Scholar]
- 29.Sekhar L N, Javed T. Meningiomas with vertebrobasilar artery encasement: review of 17 cases. Skull Base Surg. 1993;3(2):91–106. doi: 10.1055/s-2008-1060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neu M, Strauss C, Romstöck J, Bischoff B, Fahlbusch R. The prognostic value of intraoperative BAEP patterns in acoustic neurinoma surgery. Clin Neurophysiol. 1999;110(11):1935–1941. doi: 10.1016/s1388-2457(99)00148-0. [DOI] [PubMed] [Google Scholar]
- 31.Iwai Y, Yamanaka K, Morikawa T. Adjuvant gamma knife radiosurgery after meningioma resection. J Clin Neurosci. 2004;11(7):715–718. doi: 10.1016/j.jocn.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Linskey M E, Davis S A, Ratanatharathorn V. Relative roles of microsurgery and stereotactic radiosurgery for the treatment of patients with cranial meningiomas: a single-surgeon 4-year integrated experience with both modalities. J Neurosurg. 2005;102(Suppl):59–70. doi: 10.3171/jns.2005.102.s_supplement.0059. [DOI] [PubMed] [Google Scholar]