Abstract
The usefulness of endoscope-assisted microsurgical removal of vestibular schwannomas in the internal auditory canal (IAC) was evaluated. Microsurgical removal using the endoscope was done in 28 procedures and microsurgical removal without an endoscope was done in 43 procedures. A retrosigmoid approach was used. The tumor location in the IAC was classified as grade 1 (located up to the mid-portion), 2, 3, or 4 (located up to the fundus with bony destruction) according to the tumor extent, and residual tumor in the IAC was evaluated as grade A (remnant tumor was not observed), B, C, or D (remnant tumor was observed over the mid-portion) according to the extent of remnant tumor. The residual tumor in the IAC was less in the endoscope-assisted group than in the microsurgery group. There was a significant difference only in grade 2, that is, tumor located beyond the mid-portion of the IAC. There was no significant difference in the results of preservation of useful hearing, facial nerve function, and tumor recurrence between the two groups. The benefit of endoscope-assistance microsurgery was shown for those patients whose tumors extended beyond the mid-portion of the IAC but did not reach the fundus.
Keywords: vestibular schwannoma, endoscopy, retrosigmoid approach, internal auditory canal
The usefulness and problems of endoscope-assisted microsurgery for removal of vestibular schwannomas have been reported previously.1,2,3,4,5,6,7,8,9,10 The visualization of cranial nerves and vessels behind the tumor in the cerebellopontine angle and recognition of tumor located in the lateral internal auditory canal (IAC) are possible with endoscopic observation. These findings are useful for preservation of cranial nerve function and vessels and complete tumor removal. The opened air cells of the posterior wall of the IAC can be recognized with the angled endoscope, and this may be useful for prevention of cerebrospinal fluid (CSF) leakage.9 The problems are mechanical or heat injury to the cranial nerve and surrounding normal tissue during manipulation of the endoscope and heat generated by the endoscope’s light source.4,5,7
Although some benefits of endoscope usage have been reported previously, improved results of removal rate or cranial nerve function were not presented. We evaluated the usefulness of endoscope-assisted microsurgery for removal of vestibular schwannoma located in the IAC.
Materials and Methods
Among 76 surgeries (73 patients) from January 2000, 71 surgeries (68 patients) in whom preoperative and postoperative magnetic resonance (MR) imaging were performed were studied. Microsurgical removal with endoscopic observation was done in 28 procedures, and microsurgical removal without endoscope usage was done in 43 procedures. Surgery was performed mainly by one of the authors (Y.K.), and endoscope usage was decided case by case. The rigid type endoscope with a tip angle of 30 or 70 degrees (Olympus, Tokyo, Japan) was used. It was inserted by free hand or fixed using the Endo-arm (Olympus, Tokyo, Japan). A retrosigmoid, transmeatal approach was performed for all procedures.
Hearing function was considered useful in patients with pure tone average better than 50 dB and speech discrimination score better than 50% according to Gardner-Robertson grading system.11 Facial nerve function was evaluated with Yanagihara grading system.12 The classification of tumor localization in the IAC on MR images was grade 1 (tumor located up to the mid-portion of the IAC), grade 2 (tumor located over the mid-portion of the IAC), grade 3 (tumor located up to the fundus of the IAC), and grade 4 (tumor located up to the fundus of the IAC with bony destruction) (Fig. 1A–D). Residual tumor in the IAC on MR images was evaluated as grade A (remnant tumor not observed in the IAC), grade B (remnant tumor in the IAC observed slightly in the fundus), grade C (remnant tumor in the IAC observed up to the mid-portion of the IAC), and grade D (remnant tumor in the IAC located beyond the mid-portion of the IAC) (Fig. 2A–D).
Figure 1.
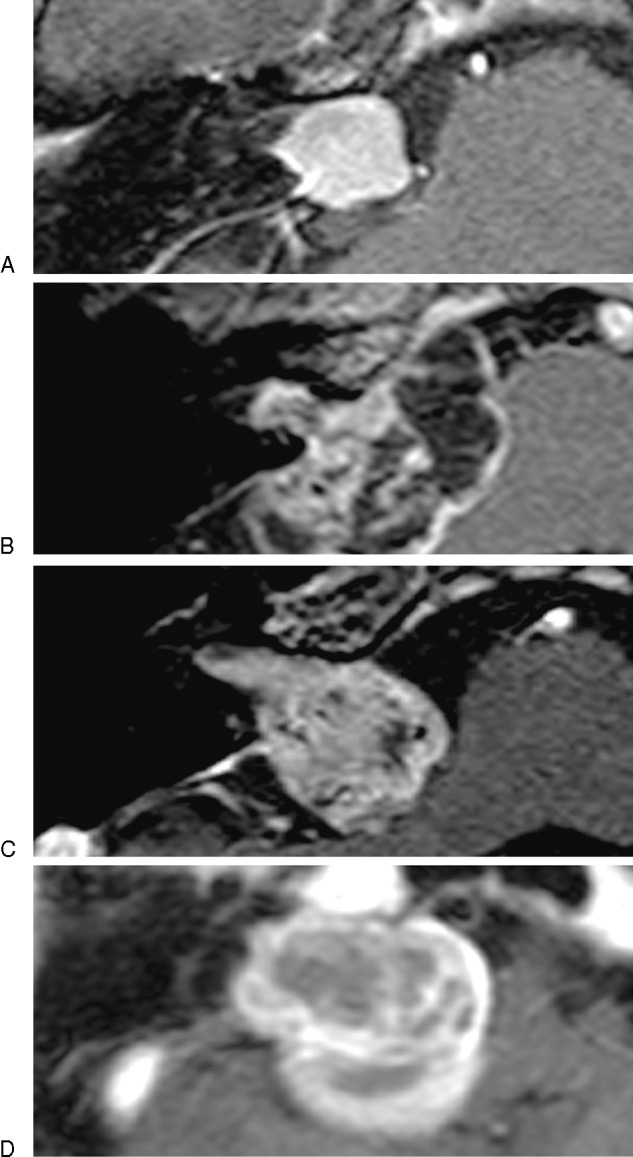
The classification of tumor localization in the internal auditory canal (IAC) on magnetic resonance images was grade 1: tumor located up to the mid-portion of the IAC (A), grade 2: tumor located over the mid-portion of the IAC (B), grade 3: tumor located up to the fundus of the IAC (C), and grade 4: tumor located up to the fundus of the IAC with bony destruction (D).
Figure 2.

The evaluation of residual tumor in the internal auditory canal (IAC) on magnetic resonance images was grade A: remnant tumor not observed in the IAC (A), grade B: remnant tumor in the IAC observed slightly in the fundus (B), grade C: remnant tumor in the IAC observed up to the mid-portion of the IAC (C), and grade D: remnant tumor in the IAC located beyond the mid-portion of the IAC (D).
The patients’ characteristics are shown in Table 1. The tumor size was significantly smaller in the endoscope-assisted group than in the no endoscope group. Other factors of useful hearing, facial nerve function, and grading of tumor location were same between the two groups.
Table 1. Patient’s Characteristics.
| ES-Assisted (n = 28) |
No Assisted (n = 43) |
|
|---|---|---|
| Age (years) | 51.8 | 54.3 |
| Gender (M/F) | 10/18 | 17/26 |
| Size (mm) | 21.4 | 26.0a |
| Useful hearing | 18 (64%) | 22 (51%) |
| Facial nerve score | 39.9 | 38.7 |
| Location of tumor | ||
| Grade 1 | 3 (11%) | 4 (9%) |
| Grade 2 | 12 (43%) | 17 (40%) |
| Grade 3 | 11 (39%) | 18 (42%) |
| Grade 4 | 2 (7%) | 4 (9%) |
Statistical difference was significant between endoscope-assisted group and no assisted group in tumor size (p < 0.05, Mann-Whitney U test).
ES, endoscope.
Residual tumor in the IAC, preservation of useful hearing, preservation of facial nerve function, and tumor recurrence were compared between the two groups. Statistical analysis was performed using Mann-Whitney U test and p values less than 0.05 were considered statistically significant.
Results
The purpose of endoscope usage was mainly observation of the lateral part of the IAC in 27 procedures. Of these, extent of tumor removal in the IAC was observed in 14 procedures, and remnant tumor in the IAC was removed as far as possible under endoscopic observation in 13 procedures. Observation of the facial nerve behind the tumor in the cerebellopontine angle was performed in three procedures. Opened air cells in the posterior wall of the IAC were observed to close for preventing leakage of CSF in one patient. This patient had been operated on previously through the middle fossa approach, and residual tumor recurred toward the cerebellopontine angle cistern. Following tumor removal through the retrosigmoid approach, careful observation of the removed wall of the IAC was necessary to occlude the opened air cells.
The result of residual tumor classification in the endoscope-assisted group was significantly better than those in the no endoscope group (p < 0.05) (Table 2). When the results of the residual tumor classification were compared in each grade of tumor localization, there was a significant difference only in grade 2, that is, tumor located beyond the mid-portion of the IAC (p < 0.05) (Table 2). But there was not a significant difference in grade 3 or 4, that is, tumor located up to the fundus of the IAC with or without bony destruction.
Table 2. Residual Tumor in the IAC.
| A (n = 17) |
B (n = 35) |
C (n = 14) |
D (n = 5) |
|
|---|---|---|---|---|
| ES-assisted (n = 28) | 11 (39%) | 11 (39%) | 5 (18%) | 1 (4%) |
| Grade 1 | 3 (100%) | 0 | 0 | 0 |
| Grade 2 | 7 (58%) | 4 (33%) | 1 (8%) | 0 |
| Grade 3 | 1 (9%) | 7 (64%) | 2 (18%) | 1 (9%) |
| Grade 4 | 0 | 0 | 2 (100%) | 0 |
| No-assisted (n = 43)a | 6 (14%) | 24 (56%) | 9 (21%) | 4 (9%) |
| Grade 1 | 4 (100%) | 0 | 0 | 0 |
| Grade 2a | 2 (12%) | 15 (88%) | 0 | 0 |
| Grade 3 | 0 | 9 (50%) | 9 (50%) | 0 |
| Grade 4 | 0 | 0 | 0 | 4 (100%) |
Statistical differences were significant between the endoscope-assisted group and no-assisted group, in total and grade 2 cases (p < 0.05, Mann-Whitney U test).
ES, endoscope; IAC, internal auditory canal.
Table 3 shows the surgical results in both groups. There were no significant differences in the useful hearing, facial nerve function score, and tumor recurrence between the two groups. Even when tumor recurrences in each grade of tumor localization were compared between the two groups, there were no significant differences.
Table 3. Postoperative Useful Hearing, Facial Nerve Function, and Recurrence.
| ES-Assisted (n = 28) |
No Assisted (n = 43) |
|
|---|---|---|
| Useful hearing | 9/18 (50%) | 11/22 (50%) |
| Facial nerve score | ||
| Preoperative | 39.9 | 38.7 |
| Postoperative | 38.8 | 36.0 |
| Recurrence | 3/28 (10.7%) | 7/43 (16.3%) |
| Reoperation or GK | 3/28 (10.7%) | 6/43 (14.0%) |
| Follow-up period (mo) | 55.6 (2∼125) | 60.9 (2∼121) |
Note: Facial nerve score: Yanagihara grading system.
ES, endoscope; GK, gamma knife; mo, months.
The tumor recurrence rate was related to the residual tumor in the IAC. It was 0% (0/17) in grade A, 6% (2/35) in grade B, 36% (5/14) in grade C, and 60% (3/5) in grade D. The recurrence rate was significantly lower in grade A than in grades B, C, or D (p < 0.01), and that in grade B was significantly lower than in grade C or D (p <0.01).
Representative Cases
A 57-year-old man complained of right hearing disturbance (useful hearing), and a preoperative MR image showed that the tumor was located up to the fundus of the IAC (Fig. 3A). The tumor was removed mainly under microscopic observation and the remnant tumor in the fundus of the IAC was recognized under endoscopic observation. The remnant tumor was observed and removed piece by piece using a microcurette under endoscopic observation as well as microscopic observation (Fig. 4A), and complete removal was identified on endoscopy (Fig. 4B). The patient’s postoperative course was uneventful, except for loss of useful hearing. The postoperative MR image showed total tumor removal (grade A) (Fig. 3B), and postoperative computed tomography demonstrated the removed posterior wall of the IAC (Fig. 3C).
Figure 3.
Preoperative magnetic resonance (MR) image shows that the tumor was located up to the fundus of internal auditory canal (IAC) (A). Postoperative MR image shows no remnant tumor in the IAC (B). Postoperative computed tomography (CT) image demonstrates the extent of removed posterior wall of the IAC (C).
Figure 4.
Remnant tumor was observed in the fundus of the internal auditory canal by endoscopic observation, and totally removed using microcurette (A, B).
Discussion
Usefulness of Endoscope-Assisted Microsurgery
It has been reported that endoscope-assisted microsurgery is useful for clear visualization of the microscopic blind field, therefore, tumor could be removed safely and completely, in spite of some problems.1,2,3,4,5,6,7,8,9,10 The endoscope may be particularly useful when one is attempting to preserve hearing. In these cases, it is critical that the intraosseous endolymphatic sac and the posterior semicircular canal are not violated when the posterior wall of the IAC is removed.13 The limited access to the fundus of the IAC through the retrosigmoid approach, has been a reason that surgeons have chosen a middle fossa approach rather than retrosigmoid approach for removing some primary intracanalicular tumors.14 In the typical situation, microscopic exposure of the fundus of the IAC during retrosigmoid surgery can be impossible, and dissecting instruments are often passed blindly to remove the residual tumor consequently. The angled endoscope allows less bony removal of the posterior wall of the IAC while achieving excellent visualization of the fundus. In our series, the lateral part of the IAC that could not be observed under microscope could be identified in 27 procedures, and residual tumor was removed in 13 procedures.
Although these benefits have been reported previously, data such as improved removal rate were not presented in details. In this study, we could present the advantage of endoscope-assisted microsurgery for tumor removal. The remnant tumor in the IAC was decreased especially in grade 2, that is, tumor was located beyond the mid-portion of the IAC. As the grade of residual tumor in the IAC was related to tumor recurrence, endoscope usage to remove the tumor located in the lateral part of the IAC may contribute toward decreasing the tumor recurrence rate. The chance of endoscope usage was increased after introduction of the Endo-arm in our series, consequently there was a tendency that the endoscope-assisted group included recent cases. We cannot completely rule out that this is related to the improved removal rate in the endoscope-assisted group without worsening the cochlear or facial nerve function.
We could not completely remove the tumor extending all the way to the fundus even with the endoscope through the retrosigmoid approach. To remove the tumor located in the fundus completely, the middle fossa approach should be selected for hearing preservation,14 and the translabyrinthine approach when hearing preservation is not an issue, instead of the retrosigmoid approach.
Opened air cells of the temporal bone due to removal of the posterior wall of the IAC could also be recognized, and this information was useful to prevent postoperative leakage of CSF.4,8 In one case in whom the middle fossa approach was performed previously for removal of an intracanalicular vestibular schwannoma, the recognition of opened air cells using the endoscope was necessary to occlude this area.
Complication
The application of the endoscope is associated with risks of iatrogenic neural or vascular injuries.4,7 King et al5 reported the possibility of thermal injury caused by the heat generated by the light of the endoscope. They observed reversible changes of the auditory brainstem response, that is, diminution in amplitude of wave V, during positioning of the endoscope adjacent to the eighth nerve in 1 of 10 patients. On the other hand, thermographic evaluation by Hori et al did not reveal a significant increase in the local temperature due to use of the endoscope in an experimental study.4 Furthermore, Gerganov et al concluded that the application of the endoscope does not lead to heat-related or mechanical neural or vascular injuries, because the risk for loss of waves I, II, and V, both transiently or permanently, did not depend on the application of the endoscope and was similar in the endoscope-assisted group and the no endoscope group.1
Endoscope manipulation in the cerebellopontine angle cistern may lead to mechanical injury,7 and Hori et al reported mechanical injury of the facial nerve in 1 of 33 consecutive surgeries.4 To avoid this complication, we always introduce the endoscope under microscopic observation, and recently began using the Endo-arm for fixing the endoscope. The shift of the endoscope tip is small during the positioning procedure using the Endo-arm. Careful manipulation of the endoscope and careful case selection are necessary, as risks of endoscope usage, such as mechanical and/or heat-related injury, still exist.
Conclusion
The benefit of endoscopic assistance was shown for those patients whose tumors extended beyond the mid-portion of the IAC but did not reach the fundus. The significance of this observation was safe removal of the intracanalicular remnant tumor under recognizing clearly the boundary between tumor and cranial nerves.
Footnotes
This article was originally Published online in Skull Base on December 1, 2011 (DOI:10.1055/s-0031-1296035)
References
- 1.Gerganov V M, Giordano M, Herold C, Samii A, Samii M. An electrophysiological study on the safety of the endoscope-assisted microsurgical removal of vestibular schwannomas. Eur J Surg Oncol. 2010;36(4):422–427. doi: 10.1016/j.ejso.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Gerganov V M, Romansky K V, Bussarsky V A, Noutchev L T, Iliev I N. Endoscope-assisted microsurgery of large vestibular schwannomas. Minim Invasive Neurosurg. 2005;48(1):39–43. doi: 10.1055/s-2004-830171. [DOI] [PubMed] [Google Scholar]
- 3.Göksu N, Bayazit Y, Kemaloğlu Y. Endoscopy of the posterior fossa and dissection of acoustic neuroma. J Neurosurg. 1999;91(5):776–780. doi: 10.3171/jns.1999.91.5.0776. [DOI] [PubMed] [Google Scholar]
- 4.Hori T, Okada Y, Maruyama T, Chernov M, Attia W. Endoscope-controlled removal of intrameatal vestibular schwannomas. Minim Invasive Neurosurg. 2006;49(1):25–29. doi: 10.1055/s-2006-932125. [DOI] [PubMed] [Google Scholar]
- 5.King W A, Wackym P A. Endoscope-assisted surgery for acoustic neuromas (vestibular schwannomas): early experience using the rigid Hopkins telescope. Neurosurgery. 1999;44(5):1095–1100, discussion 1100–1102. doi: 10.1097/00006123-199905000-00084. [DOI] [PubMed] [Google Scholar]
- 6.McKennan K X. Endoscopy of the internal auditory canal during hearing conservation acoustic tumor surgery. Am J Otol. 1993;14(3):259–262. [PubMed] [Google Scholar]
- 7.Miyazaki H, Deveze A, Magnan J. Neuro-otologic surgery through minimally invasive retrosigmoid approach: endoscope assisted microvascular decompression, vestibular neurotomy, and tumor removal. Laryngoscope. 2005;115(9):1612–1617. doi: 10.1097/01.mlg.0000172038.22929.63. [DOI] [PubMed] [Google Scholar]
- 8.Tatagiba M, Matthies C, Samii M. Microendoscopy of the internal auditory canal in vestibular schwannoma surgery. Neurosurgery. 1996;38(4):737–740. [PubMed] [Google Scholar]
- 9.Valtonen H J, Poe D S, Heilman C B, Tarlov E C. Endoscopically assisted prevention of cerebrospinal fluid leak in suboccipital acoustic neuroma surgery. Am J Otol. 1997;18(3):381–385. [PubMed] [Google Scholar]
- 10.Wackym P A, King W A, Poe D S. et al. Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope. 1999;109(8):1193–1201. doi: 10.1097/00005537-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gardner G, Robertson J H. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55–66. doi: 10.1177/000348948809700110. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara N. Amstelveen, The Netherlands: Kugler Medical Publications; 1977. Grading of facial palsy; pp. 533–554. [Google Scholar]
- 13.Roland P S, Meyerhoff W L, Wright C G, Mickey B. Anatomic considerations in the posterior approach to the internal auditory canal. Ann Otol Rhinol Laryngol. 1988;97(6 Pt 1):621–625. doi: 10.1177/000348948809700608. [DOI] [PubMed] [Google Scholar]
- 14.Kumon Y, Sakaki S, Kohno K. et al. Selection of surgical approaches for small acoustic neurinomas. Surg Neurol. 2000;53(1):52–59, discussion 59–60. doi: 10.1016/s0090-3019(99)00199-8. [DOI] [PubMed] [Google Scholar]




