Abstract
Endoscope, microscope, and neuronavigation systems are integrated in neurosurgical procedures mainly by using a serial combination algorithm, where, the user must switch his/her field of view from one platform display to another. The integration of theses devices could be optimized by incorporating different displays into one viewing platform thus achieving a parallel combination. In this study, we investigated the feasibility and the applicability of parallel integration of microscopic, endoscopic, and neuronavigation images by real time displaying the endoscope and neuronavigation image datasets in the main operative microscope oculars. The proposed set-up was effective in displaying the three images dataset in an operationally actionable mode. Ergonomically, the ability of using the different image dataset without the need of taking the eyes off the microscope oculars did not disrupt the flow or the tempo of the operative procedure. However, new endoscopes specific to this application are recommended.
Keywords: endoscope, image-guided surgery, microneurosurgery, medical technology, skull base surgery
Endoscopes and neuronavigation systems are becoming more and more popular as adjunctive tools during microscopic neurosurgical operations.1,2,3,4,5,6,7,8,9,10,11 However, the current integration of the endoscope or the neuronavigation system in a microneurosurgical environment is still based on what can be described as a serial combination. This type of combination is not ergonomically efficient due to the need of changing the visual field of the operating surgeon from the microscopic image displayed in the microscope oculars to the endoscopic images displayed on the endoscopy monitor, or to the neuronavigation’s screen. A more ergonomically efficient combination of these imaging platforms is what could be called the parallel integration, where about these image modalities are displayed simultaneously and at will on a single main viewing platform.
In this report, we tested and analyzed, in a cadaveric model, the technical features and the potential applicability of a new way of integrating the microscope, the endoscope, and the neuronavigation; in a parallel fashion by utilizing a new technology, the BiOpix (CCA Medical, Milford, OH) high definition (HD) picture-in-picture image injection system. This new technology allows parallel combination of the endoscope and microscope by allowing real-time display of high-resolution endoscopic and/or neuronavigation images in both microscope oculars, thus theoretically making the use of the endoscope and of the neuronavigation system more efficient and more ergonomic during microsurgery.
Materials and Methods
Preparation of Cadaveric Heads
We used six fresh cadaver heads injected with colored silicone, and implanted with eight adhesive scalp fiducials. The specimens then underwent computed tomographic imaging using the following protocol: slice thickness, 1-mm, contiguous nonoverlapping slices; gantry setting, 0 degrees; scan window diameter, 225 mm; pixel size, >0.44 × 0.44).
Image Guidance System
A Stryker Navigation System was used (Stryker Instruments, Kalamazoo, MI).
Microscope and Endoscope
We used the Moeller-Wedel neurosurgical microscope (Möller-Wedel GmbH, Wedel, Germany) with an integrated binocular data injection system and integrated video camera (Ikegami 1/3” HD camera; Ikegami, Redondo Beach, CA). A 2.7 mm diameter, 18 cm, rigid endoscope with 0 and 30 lenses (MINOP; Aesculp, Tuttlingen, Germany); and a 4 mm diameter, 18 cm, rigid endoscope with 0 and 30 lenses (the Aesculp Pernescky endoscpe; Aesculp, Tuttlingen, Germany) were used during the surgical procedures.
BiOpix® Injection System
The BiOpix HD picture-in-picture image injection system (CLA Medical, Milford, OH) is a component of the Möeller–Wedel neurosurgical microscope providing the neurosurgeon with the ability to observe in both microscopic oculars up to two HD (1280 × 1024 HD) image feeds.
System Set-Up
The system is integrated onto the microscope (Fig. 1). In addition, the endoscope camera control unite can be positioned on the base of the microscope so that the footprint of the endoscope cart is eliminated. The endoscope was hand held by the surgeon and its tip was navigated using the Stryker neuronavigation system (Stryker Instruments, Kalamazoo, MI) (Fig. 2).
Figure 1.
General set-up of the BiOpix® system. (A) The system is positioned in a cart next to the microscope. (B) The red arrow indicates the main control screen, and the green arrow points to the secondary control screen.
Figure 2.

The tip of the endoscope was navigated using the Stryker neuronavigation system.
Surgical Procedures
We performed on each cadaver bilaterally the suboccipital retrosigmoid approach targeting the jugular foramen, the foramen of Luschka, the origin of 7th and 8th complex nerves, the internal auditory meatus, and the 5th nerve. Also, we performed the subtemporal approaches bilaterally in all the cadavers targeting the tip of the basilar artery and its main trunk, the origin of the superior cerebellar arteries and posterior cerebral arteries, and the mamillary bodies.12,13,14,15,16,17 Every dissection was conducted first with the microscope alone and then with the microscope aided by the endoscope and neuronavigation. When the endoscope was used ,we injected the endoscopic view and the neuronavigation view into the microscope oculars in real time using the BiOpix system. All the surgical procedures were video documented.
Images Acquisition and Display
A touch screen mounted on the side of the microscope controls all BiOpix functions. By pressing a button on the touch screen, the corresponding image is selected to be displayed. Connections on the side of the BiOpix enclosure allow for connection of the endoscope and surgical navigation system. The touch screen allows for either one of these images to be display singularly, or simultaneously side-by-side.
In addition, the touch screen allows for positioning of the image anywhere in the field of view. The image can be resized as necessary using buttons on the touch screen.
Resolution of The Displayed Images
The BiOpix is optimized to work with endoscopes images that are HD 1080P. Images are acquired from a video connection on the BiOpix enclosure. The enclosure mounts to the side of the microscope or in a module which is housed inside the microscope floor stand. Currently, the BiOpix enclosure is adaptable for the Moeller 20 to 1000 g microscope.
Operational Evaluation
During each dissection, we evaluated qualitatively the effectiveness of the fed of endoscopic and neuronavigation dataset while performing the surgery using the microscope.
This was done by assessing our ability of achieving the full exposure of the selected surgical landmarks (exposure of the circumference of the target as well as target multiangled exposure). In addition, we evaluated the ability to perform basic surgical maneuvers on the exposed targets (dissecting the arachnoid, dissecting arteries and nerve, and drilling selected area of bone).
Results
The following advantages were evident.
Enhancement of the Surgical Orientation
The surgical orientation was enhanced through visualization of different structures in different planes at the same time, as well as by the visualization of hidden corner by combining the microscopic, endoscopic, and neuronavigation display in real time (Figs. 3 and 4). The endoscopic view is a “look ahead” view that might assist in showing the surgeon what is coming next in the operative field.
Figure 3.
(A, B) Subtemporal approach. Visualization of hard to see structures in the injected endoscopic image. (A) The area of the basilar artery between the origin of the superior cerebellar (*) and posterior cerebral artery (**) can be exposed and verified without cutting the tentorium. (B) The origin of cranial nerve III can be verified without cutting the tentorium.
Figure 4.
Suboccipital retrosigmoid approach. The foramen of Luschka can be visualized and identified without any retraction. In this picture, we injected only the endoscope display in the microscope ocular.
Educational Advantage
This system can be useful for the education of residents and fellows in that it demonstrates the full picture (microscopic and endoscopic) of the local surgical anatomy.
Ergonomic Advantages
The ability to operationally use different dataset (endoscopic and neuronavigation) without the need of removing the eyes from the microscope oculars allows for a more ergonomically efficient operating environment by decreasing distraction and unnecessary movements.
Flexibility of the System
The system has features that enable the neurosurgeon to change the location and the size of the overlaid-injected images thereby eliminating any potential obscuration of anatomical structures (Fig. 5). This feature can be controlled by the neurosurgeon itself or by an assistant. Also, the endoscope display can be injected alone without the neuronavigation images, and vice versa (Fig. 4).
Figure 5.
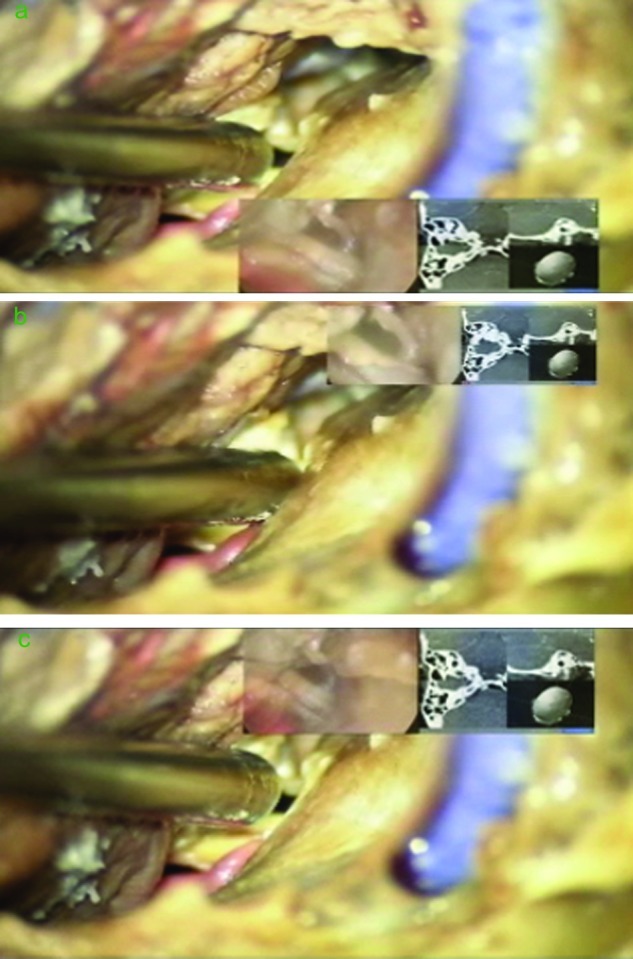
Suboccipital retrosigmoid approach. The location and the size of the injected image can be changed. The endoscope and navigation feed are injected simultaneously in the microscope oculars.
System Drawbacks
The current system is set up in such a way that moving the endoscope occasionally bumps the operating microscope requiring readjustment, especially when the neuronavigation tracker is attached to the endoscope. In that regard, using a smaller endoscope (2.7 mm) led to better ease of use than using a 4 mm endoscope.
Other drawbacks are the fact that the BiOpix device is only compatible with Moeller microscopes and that the overlaid image blurs slightly with the microscopic image. Moreover, a learning curve is necessary to make the neurosurgeon comfortable with having all the visual feedback in the microscope space.
Discussion
Endoscope-Assisted Microneurosurgery: The Current Problem
Minimal access techniques have recently become popular in neurosurgery.
The philosophical principle of minimal access surgery is straightforward to reduce tissue trauma and approach-related morbidity without compromising the effectiveness of the surgical procedure. The concept of minimal access surgery has been made possible by the continuous development of new technologies that have refined neurosurgical techniques. Aside from the historical impact of the operating microscope, endoscopes and neuronavigation tools have been the most recent technologies that have had an impact on neurosurgery.
Both the microscope and the endoscope have advantages and disadvantages.18,19,20,21,22,23,24,25,26 The main advantage of the microscope is the ability to provide stereoscopic images associated with high degree of maneuverability (by allowing the use of both hands). On the other hand, the endoscope provides a close-up view of the lesion, with capability of looking around the corners and, when angulated lenses are used, of providing a panoramic view of the surrounding area.17,18
Theoretically, one would stand to gain by combining the advantages of both technologies. Yet, in real practice this has not been the case. Until now, endoscopic and microscopic techniques have been, by and large, regarded as mutually exclusive, sometimes competitive, techniques.19,20,21,22,23,24,26
Even when one wanted to combine the microscope and the endoscope in the same setting, there has been a lack of tools to make this combination smooth and effective,20 mainly due to the fact that when using the endoscope in a microsurgical environment the surgeon has to switch from the microscope ocular view to the endoscope monitor view or vice versa.
Parallel Integration of the Endoscope and Microscope as a Possible Solution to the Previous Problem
We found that the integration provided by the BiOpix system allows effective and unencumbered use of the endoscopic image in a microneurosurgical environment by providing high quality, acceptable and easy to use display of endoscopic images in the microscope oculars. In addition, other image dataset, such as, in our study, neuronavigation images can be effectively and simultaneously displayed in the microscope oculars.
Because the vision of the microscope can be supported by the endoscope vision in real time, the advantages of both the devices can be exploited simultaneously. This allowed us to use the endoscope as an assistant to the microscope in an efficient way. The endoscopic information, even when not usable to carry on surgical maneuvers, was useful to enhance the recognition of the surgical landscape.
The ability to navigate the endoscope tip and to display the navigation images in the microscope ocular, together and at the same time of the endoscopic images, was clearly beneficial in our dissections and may be helpful in clinical practice.
As with any new technology there are some drawbacks such as, amongst others, the applicability limited only to the Moller microscope. In addition, we think that using a flexible endoscope would avoid some of the difficulties encountered when the endoscope is brought in the surgical field, such as the collision between the endoscope and the microscope. As in any situation when endoscopic-assisted microsurgery is performed, use of a smaller endoscope is preferable to a bigger one.
Finally, we have to emphasize that there are other systems currently available in the market, which can provide—to some degree—a parallel integration similar to the one provided by the system we used. However, these systems have some drawbacks such as the low resolution of the injected images, and the fact that due to the injected image being displayed only in one of the two main microscope oculars, stereoscopic microscopic view is lost when the injected image is displayed.
Conclusion
Based on our laboratory evaluation, the real time parallel combination of microscopic, endoscopic, and neuronavigation images using the BiOpix® HD picture-in-picture image injection system is a promising medical technology that seems to be able to provide a solution for an efficient combination of different neurosurgical images dataset in a microscopic operating environment. As with all new technology, there are multiple nuances that are best learned in a controlled environment such as the one offered by anatomical microneurosurgical laboratories, as well as some technical drawbacks that needs to be addressed. Systematic evaluation of this technology in clinical settings needs to be conducted to validate its real usefulness.
Note
Funding and financial disclosure: Local Research Grant, Ohio State University.
Footnotes
This article was originally Published online in Skull Base on December 1, 2011 (DOI:10.1055/s-0031-1296034)
References
- 1.Cappabianca P, Cinalli G, Gangemi M. et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl 02):575–597, discussion 597–598. doi: 10.1227/01.neu.0000316262.74843.dd. [DOI] [PubMed] [Google Scholar]
- 2.Di Rocco F, Oi S, Samii A. et al. Neuronavigational endoscopic endonasal sellar and parasellar surgery using a 2-mm-diameter lens rigid-rod endoscope: a cadaver study. Neurosurgery. 2007;60(4, Suppl 02):394–400, discussion 400. doi: 10.1227/01.NEU.0000255381.64969.C8. [DOI] [PubMed] [Google Scholar]
- 3.Eloy J A, Carai A, Patel A B, Genden E M, Bederson J B. Combined endoscope-assisted transclival clipping and endovascular stenting of a basilar trunk aneurysm: case report. Neurosurgery. 2008;62(3, Suppl 01):142–143, discussion 143–144. doi: 10.1227/01.neu.0000317385.91432.df. [DOI] [PubMed] [Google Scholar]
- 4.Greenfield J P, Leng L Z, Chaudhry U. et al. Combined simultaneous endoscopic transsphenoidal and endoscopic transventricular resection of a giant pituitary macroadenoma. Minim Invasive Neurosurg. 2008;51(5):306–309. doi: 10.1055/s-0028-1082323. [DOI] [PubMed] [Google Scholar]
- 5.Rhoten R L, Luciano M G, Barnett G H. Computer-assisted endoscopy for neurosurgical procedures: technical note. Neurosurgery. 1997;40(3):632–637, discussion 638. doi: 10.1097/00006123-199703000-00042. [DOI] [PubMed] [Google Scholar]
- 6.Kalavakonda C, Sekhar L N, Ramachandran P, Hechl P. Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery. 2002;51(5):1119–1126, discussion 1126–1127. doi: 10.1097/00006123-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rak R, Sekhar L N, Stimac D, Hechl P. Endoscope-assisted microsurgery for microvascular compression syndromes. Neurosurgery. 2004;54(4):876–881, discussion 881–883. doi: 10.1227/01.neu.0000115151.52925.37. [DOI] [PubMed] [Google Scholar]
- 8.Gerganov V M, Romansky K V, Bussarsky V A, Noutchev L T, Iliev I N. Endoscope-assisted microsurgery of large vestibular schwannomas. Minim Invasive Neurosurg. 2005;48(1):39–43. doi: 10.1055/s-2004-830171. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder H W, Oertel J, Gaab M R. Endoscope-assisted microsurgical resection of epidermoid tumors of the cerebellopontine angle. J Neurosurg. 2004;101(2):227–232. doi: 10.3171/jns.2004.101.2.0227. [DOI] [PubMed] [Google Scholar]
- 10.Teo C, Nakaji P, Mobbs R J. Endoscope-assisted microvascular decompression for trigeminal neuralgia: technical case report. Neurosurgery. 2006;59(4, Suppl 02):E489–E490, discussion E490. doi: 10.1227/01.NEU.0000232768.47615.82. [DOI] [PubMed] [Google Scholar]
- 11.Charalampaki P, Filippi R, Welschehold S, Perneczky A. Endoscope-assisted removal of colloid cysts of the third ventricle. Neurosurg Rev. 2006;29(1):72–79. doi: 10.1007/s10143-005-0419-0. [DOI] [PubMed] [Google Scholar]
- 12.Matula C, Diaz Day J, Czech T, Koos W T. The retrosigmoid approach to acoustic neurinomas: technical, strategic, and future concepts. Acta Neurochir (Wien) 1995;134(3-4):139–147. doi: 10.1007/BF01417681. [DOI] [PubMed] [Google Scholar]
- 13.Mangham C A Jr. Retrosigmoid versus middle fossa surgery for small vestibular schwannomas. Laryngoscope. 2004;114(8):1455–1461. doi: 10.1097/00005537-200408000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Ciric I, Zhao J C, Rosenblatt S, Wiet R, O’Shaughnessy B. Suboccipital retrosigmoid approach for removal of vestibular schwannomas: facial nerve function and hearing preservation. Neurosurgery. 2005;56(3):560–570, discussion 560–570. doi: 10.1227/01.neu.0000154059.34990.b8. [DOI] [PubMed] [Google Scholar]
- 15.Hernesniemi J, Ishii K, Niemelä M, Kivipelto L, Fujiki M, Shen H. Subtemporal approach to basilar bifurcation aneurysms: advanced technique and clinical experience. Acta Neurochir Suppl (Wien) 2005;94:31–38. doi: 10.1007/3-211-27911-3_6. [DOI] [PubMed] [Google Scholar]
- 16.Kakino S, Ogasawara K, Kubo Y, Nishimoto H, Ogawa A. Subtemporal approach to basilar tip aneurysm with division of posterior communicating artery: technical note. Vasc Health Risk Manag. 2008;4(4):931–935. doi: 10.2147/vhrm.s2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youssef S, Kim E Y, Aziz K M, Hemida S, Keller J T, Loveren H R van. The subtemporal interdural approach to dumbbell-shaped trigeminal schwannomas: cadaveric prosection. Neurosurgery. 2006;59(4, Suppl 02):ONS270–ONS277, discussion ONS277–ONS278. doi: 10.1227/01.NEU.0000227590.70254.02. [DOI] [PubMed] [Google Scholar]
- 18.Filipce V, Pillai P, Makiese O, Zarzour H, Pigott M, Ammirati M. Quantitative and qualitative analysis of the working area obtained by endoscope and microscope in various approaches to the anterior communicating artery complex using computed tomography-based frameless stereotaxy: a cadaver study. Neurosurgery. 2009;65(6):1147–1152, discussion 1152–1153. doi: 10.1227/01.NEU.0000359328.90826.97. [DOI] [PubMed] [Google Scholar]
- 19.Frank G, Pasquini E, Farneti G. et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83(3-4):240–248. doi: 10.1159/000095534. [DOI] [PubMed] [Google Scholar]
- 20.Morita A, Shin M, Sekhar L N, Kirino T. Endoscopic microneurosurgery: usefulness and cost-effectiveness in the consecutive experience of 210 patients. Neurosurgery. 2008;62(Suppl 02):607–613. doi: 10.1227/01.neu.0000316264.59596.c5. [DOI] [PubMed] [Google Scholar]
- 21.Cappabianca P, Decq P, Schroeder H W. Future of endoscopy in neurosurgery. Surg Neurol. 2007;67(5):496–498. doi: 10.1016/j.surneu.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 22.Prevedello D M, Doglietto F, Jane J A Jr, Jagannathan J, Han J, Laws E R Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107(1):206–213. doi: 10.3171/JNS-07/07/0206. [DOI] [PubMed] [Google Scholar]
- 23.Schaberg M R, Anand V K, Schwartz T H, Cobb W. Microscopic versus endoscopic transnasal pituitary surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18(1):8–14. doi: 10.1097/MOO.0b013e328334db5b. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mefty O, Pravdenkova S, Gragnaniello C. A technical note on endonasal combined microscopic endoscopic with free head navigation technique of removal of pituitary adenomas. Neurosurg Rev. 2010;33(2):243–248, discussion 248–249. doi: 10.1007/s10143-010-0241-1. [DOI] [PubMed] [Google Scholar]
- 25.Roth J, Singh A, Nyquist G. et al. Three-dimensional and 2-dimensional endoscopic exposure of midline cranial base targets using expanded endonasal and transcranial approaches. Neurosurgery. 2009;65(6):1116–1128, discussion 1128–1130. doi: 10.1227/01.NEU.0000360340.85186.7A. [DOI] [PubMed] [Google Scholar]
- 26.Couldwell W T. Endoscope or microscope? J Neurosurg. 2007;106(4):730–731, author reply 731. doi: 10.3171/jns.2007.106.4.730. [DOI] [PubMed] [Google Scholar]





