Abstract
Background There is often controversy regarding the optimal management for patients with facial nerve schwannomas (FNSs) of the cerebellopontine angle (CPA).
Methods The clinical and radiological outcomes in 14 patients with CPA FNS were retrospectively reviewed.
Results Patients underwent resection with anatomic nerve preservation (n = 3), facial-hypoglossal nerve anastomosis (n = 4), gamma knife radiosurgery (GKS) (n = 6), or observation (n = 1). A total of 83% of tumors that underwent GKS were stable or decreased in size. No patient who underwent resection showed evidence of tumor recurrence; the tumor under observation remained unchanged with normal facial function at the time of the last follow-up. Facial function was decreased in 57%, stable in 14%, and improved in 29% of those who underwent microsurgery. A total of 67% of patients who underwent GKS had stable facial function. Serviceable hearing was maintained in 50% of patients in the GKS group and 67% of the tumor resection group. Mean and median follow-up was 48 and 43 months, respectively (range, 12 to 95 months).
Conclusion Observation should be the primary management when encountered with FNS of the CPA in those with good neurologic function. Microsurgery or radiosurgery may be used in those with poor facial function or tumor progression.
Keywords: facial nerve, schwannoma, cerebellopontine angle, vestibular schwannoma
Introduction
Facial nerve schwannomas (FNSs) are slow-growing benign tumors that can occur along any segment of the facial nerve. Symptoms can be variable depending on the size and location of the tumor, but commonly include facial paresis, hearing loss, and vestibular symptoms. Cerebellopontine angle (CPA) facial schwannomas are infrequent, accounting for less than 20% of all FNS. However, there are growing numbers of cases in the literature suggesting the tumor may not be as rare as once thought. The variable radiological patterns and clinical presentation of these tumors pose added diagnostic and therapeutic challenges, as it can mimic other tumors in this region, namely, vestibular schwannomas.
There has been an evolution in the management of FNS in the last several years. Over time, observation has emerged as the primary approach in those with normal facial function. Growing tumors can be managed with resection with or without reanimation and/or stereotactic radiosurgery, although the timing and approach to these tumors in the literature has been somewhat controversial. Our experience and a treatment algorithm for CPA FNS utilizing observation, microsurgery, and radiosurgery are described.
Methods
From June 1998 to July 2011, 14 patients (10 women and 4 men) underwent evaluation for CPA FNSs. All patients were managed by the senior authors (C.L.W.D. and M.J.L.). The median age at diagnosis was 45 years (range 25 to 79). Nine patients had a period of observation before treatment, four patients were treated at the time of the primary evaluation due to unusual imaging characteristics and progressive neurologic symptoms, and one patient remained under observation at the last clinical follow-up. The nine patients who were followed conservatively had a mean and median time to treatment of 60 and 48 months, respectively (range 12 to 132 months). A total of 13 patients underwent treatment of their tumor by the following modalities: surgical resection with anatomic nerve preservation (n = 3), surgical resection followed by facial-hypoglossal nerve anastomosis for facial reanimation (n = 4), and gamma knife stereotactic radiosurgery (GKS) (n = 6). Pre- and posttreatment facial nerve assessment using the House–Brackmann (HB) score1 was available in all patients at the last clinical follow-up and pre- and posttreatment hearing outcome using the American Academy of Otolaryngology-Head and Neck Surgery scale2 (AAO-HNS) was available in 10 patients. The mean and median time to follow-up was 48 and 43 months, respectively (range 12 to 95 months).
Results
Patients presented with clinical facial nerve dysfunction (n = 7), hearing loss (n = 5), or brainstem symptoms (n = 2) at the time of treatment. The mean time to treatment was 60 months. Modalities for treatment included observation, microsurgery with or without facial nerve reanimation, and GKS. Patient characteristics are shown in Table 1.
Table 1. Patient Characteristics.
| Patient | Age | Side | Procedure | Hearing PreOp/PostOp | Graft | GKS | HB PreOp/PostOp |
|---|---|---|---|---|---|---|---|
| 1 | 44/F | R | Retrosigmoid (resection aborted) | Class A/unavailable | Y | 1/1 | |
| 2 | 68/F | R | None | Class D/class D | Y | 1/1 | |
| 3 | 54/F | L | None | Class A/class A | Y | 2/2 | |
| 4 | 66/F | R | Retrosigmoid (resection aborted) | Class B/class D | Y | 1/1 | |
| 5 | 41/M | L | Tympanomastoidectomy (tumor extended from middle ear to IAC; resection aborted) | Class B/unavailable | Y | 2/6 | |
| 6 | 79/F | L | Retrosigmoid (resection aborted) | Unavailable/unavailable | Y | 1/6 | |
| 7 | 43/M | R | Middle fossa approach | Class A/class A | N | N | 2/3 |
| 8 | 39/F | L | Middle fossa approach | Class A/class A | N | N | 1/3 |
| 9 | 51/M | R | Retrosigmoid | Unavailable/unavailable | N | N | 1/3 |
| 10 | 46/M | R | Translabyrinthine | Class D/class D | Y (7/12) | N | 6/4 |
| 11 | 25/F | R | Translabyrinthine | Class D/class D | Y (7/12) | N | 3/3 |
| 12 | 61/F | L | Translabyrinthine | Class D/class D | Y (7/12) | N | 6/4 |
| 13 | 25/F | L | Retrosigmoid | Class A/class D | Y (7/12) | N | 1/3 |
| 14 | 37/F | R | Translabyrinthine (resection aborted and no treatment done; patient still under observation) | Class C/class D (no treatment done) | − | N | 1/1 (no treatment done) |
GKS, gamma knife radiosurgery; HB, House–Brackmann; IAC, internal acoustic canal; PostOp, postoperative; PreOp, preoperative.
Diagnosis
The tumor was determined to be a FNS by radiographic characteristics in two patients (14%) with characteristic pathologic enhancement and enlargement along the labyrinthine segment of the facial nerve and geniculate ganglion as well as a CPA component (e.g., Fig. 1). The tumor was noted to arise from the facial nerve at the time of surgery in 12 patients (86%) with vestibular schwannoma being the most common preoperative diagnosis.
Figure 1.
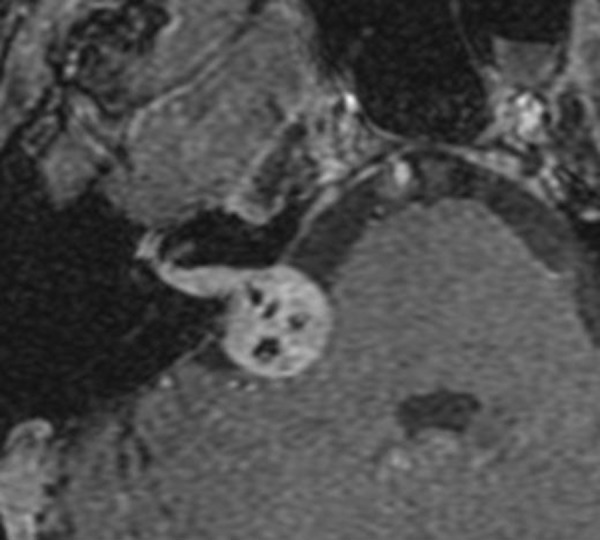
Axial contrast-enhanced T1-weighted image showing enhancement in the internal acoustic canal, labyrinthine segment and geniculate ganglion with a cerebellopontine angle component.
Microsurgery
The surgical approach in those found to have FNS intraoperatively included retrosigmoid craniotomy (n = 5), tympanomastoidectomy (n = 1) (this was done as an exploration of the middle ear space as the tumor extended from the middle ear to the internal auditory canal and surgery was terminated upon seeing it was a FNS. Patient was subsequently followed for 9 years before GKS at the time of tumor progression), translabyrinthine (n = 4), and middle fossa craniotomy (n = 2). The translabyrinthine approach was chosen in those who did not have useful hearing (AAO-HNS Class C or D).
In 5 of the 12 patients who underwent surgery, the procedure was aborted upon realizing that the tumor was arising from the seventh nerve and no resection was performed. In the other seven patients who underwent surgery, three patients had tumor resection with nerve preservation (patients 7 to 9), and four patients underwent tumor resection followed by facial-hypoglossal anastomosis due to the large size and progressive symptoms from the tumor (patients 10 to 13). No patients who underwent resection with or without reanimation showed evidence of tumor recurrence during the follow-up period.
GKS
At the time of tumor progression, six patients (43%) underwent stereotactic radiosurgery using the gamma knife. Of these patients, two were diagnosed by radiographic criteria, and four patients were previously noted to have tumors arising from the facial nerve at the time of surgery where no resection was performed. The mean margin dose to the 50% isodose line was 13 Gy (range 12 to 14 Gy) and the mean max dose was 26 Gy (range 24 to 28 Gy). Of the six patients, five (83%) had either stable or smaller tumor volume at the last radiographic follow-up. One patient showed cystic degeneration and tumor enlargement 42 months after GKS (patient 6). This patient developed concomitant complete facial paresis (HB 6) during this time and was lost to further follow-up due to unrelated systemic illness. Another patient (patient 5) developed sudden onset geniculate neuralgia and complete facial paresis (HB 6) within 1 week of GKS. The geniculate neuralgia resolved with steroids, however, the facial weakness is currently being observed for improvement.
Facial Nerve Outcome
The mean and median follow-up for all patients was 48 and 43 months, respectively (range 12 to 95 months). Facial nerve outcomes are shown in Table 1. Of the three patients who underwent surgical resection without facial reanimation, a decrease in facial function was seen in all patients at the last clinical follow-up, deteriorating from HB 2 to 3 (n = 1) and HB 1 to 3 (n = 2). Among those that had resection along with facial-hypoglossal anastomosis, there was a decrease in facial function (from HB 1 to 3) in one patient, no change in facial function (stable HB 3) in one patient, and an improvement in facial function (from HB 6 to 3) in two patients. Collectively, in the patients that underwent surgical resection of their tumor, there was a decrease in facial function in 57%, no change in facial function in 14% and an improvement in facial function in 29%. All six patients who underwent GKS had a pretreatment HB of 1 to 2 and this remained unchanged in four patients (67%). Two of the patients who underwent GKS (33%) had complete facial paresis (HB 6); one within the first week after treatment (patient 5) and another ~42 months after treatment following tumor enlargement and cystic degeneration (patient 6).
Hearing Outcome
Hearing was graded using the AAO-HNS scale, and both pre- and posttreatment outcomes were available in 10 patients (71%). Hearing outcomes are shown in Table 1. Of the patients with available hearing data, only five patients (50%) had serviceable hearing preoperatively (AAO-HNS Class A or B). Among those with pretreatment serviceable hearing, 50% maintained this in the GKS group and 67% of those who underwent tumor resection maintained useful hearing in the follow-up period.
Discussion
There are several reports in the literature outlining institutional outcomes for the management of FNS. Those occurring in the CPA account for less than 20% of all FNS and ~1% of all CPA tumors.3,4,5,6,7,8 These patients represent a unique subset of CPA tumors as their clinical course can be indistinguishable from those with vestibular schwannoma.
While the presenting symptoms of patients can be variable, a meta-analysis of 427 patients with FNS showed facial weakness (63%) and hearing loss (51%) to be the most common.9 In the latter study, 76 patients (17.8%) had tumors confined to the CPA. In our series, 50% of patients presented with facial weakness, and 36% presented with hearing loss. Although this parallels the distribution of FNS in general, studies have noted a tendency for FNS confined to the CPA/internal acoustic canal to present with a higher rate of hearing loss and much lower rate of facial paresis (similar to vestibular schwannomas). Nonetheless, recurrent episodes of transient facial weakness and hemifacial spasm seemed to be more frequent with FNS in the CPA as compared with vestibular schwannomas.9,10,11,12,13
Normal facial function has been reported in 27 to 54% of cases.14,15,16 However, a careful history may uncover subtle transient episodes of facial dysfunction. Patients presenting with new onset progressive or recurrent facial nerve dysfunction with or without hearing loss should be evaluated for a facial nerve tumor. All patients should undergo audiologic evaluation and if possible electrophysiologic testing may be helpful in the evaluation of these tumors.3,17,18
Magnetic resonance imaging (MRI) remains the modality of choice when evaluating CPA lesions, including FNSs. Enhancement of the geniculate ganglion as well as enhancing enlargement along portions of the facial nerve can be clues suggestive of a FNS. Additionally, high-resolution computed tomography (CT) imaging can be helpful to assess enlargement of the facial canal or bony erosion around the geniculate ganglion/otic capsule. Collectively, the presence of an enhancing lesion on MRI along with bony destruction on CT is most helpful. Nonetheless, even when there are precise clinical symptoms coupled with suggestive imaging, some of these tumors may be indistinguishable from other pathologies of the CPA thereby resulting in exploration. In our series, 86% of patients were found to have a FNS at the time of surgery while only two patients (14%) were diagnosed based on clinical and radiographic features alone.
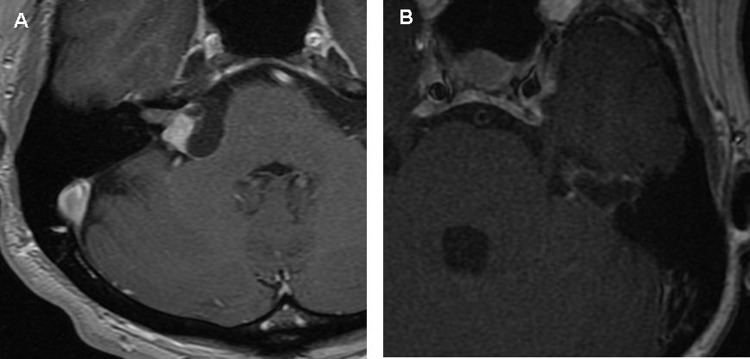
When encountered with a FNS of the CPA whether through surgical discovery or through clinical/radiologic parameters, the timing and modality of treatment can be challenging. Over the years, the management of these tumors has evolved to allow for maximal long-term facial nerve preservation and hearing preservation in those with useful hearing. Complete tumor resection with interposition graft usually results in nerve recovery no greater than HB 3. In our series, three patients underwent tumor removal with preservation of nerve continuity and all of these tumors were relatively small. While some centers have advocated nerve preservation surgery by teasing the tumor off the facial nerve as a method to preserve normal facial function,3,4,9,19,20,21 this has not been our experience. In the three patients of our series who underwent tumor removal with preservation of nerve continuity, the final facial nerve outcome decreased in all patients to HB 3 from HB 1 to 2. Nadeau and Sataloff reviewed 11 patients in which fascicle preservation surgery was performed on FNS involving the posterior fossa; of these, 45% had HB 1 to 2, 36% had HB 3, and 18% had HB 5 facial function at their last clinical follow-up.3 Rodrigues et al reported two patients who presented with enlarging cystic FNS of the CPA who underwent retrosigmoid craniotomies for what were thought to be vestibular schwannomas.22 Both patients underwent cyst decompression with preservation of facial function (HB 1 to 2) however 1 patient required a repeat decompression 1 year following the procedure and the second patient had follow-up less than 1 year. The two patients in our series, for which resection with preservation of nerve continuity was performed, had cystic FNS (patients 8 and 9, Fig. 2a, b). Both patients underwent retrosigmoid craniotomy, however in addition to decompression of the cyst, meticulous removal of the cyst wall was also undertaken which may explain their decrease in facial function (HB 1 to 3). However, neither patient had any recurrence at almost 2 years follow-up.
Figure 2.
Axial contrast-enhanced T1-weighted image showing cystic facial nerve schwannoma in patient 8 (A) and patient 9 (B).
Correlating with previously reported results following interposition grafting, in all patients in our series who underwent tumor resection followed by facial-hypoglossal anastomosis, the final facial nerve outcome was HB 3. Overall, microsurgery with preservation of nerve continuity as well as reanimation are reasonable options when microsurgery is used as primary treatment, however, proper counseling and patient selection should be employed. In cases in which a clear plane cannot be identified or there is gross involvement of the motor fibers of the facial nerve, resection followed by nerve grafting appears to be the best option.
GKS has emerged as very viable treatment option for FNS.19,23,24,25,26,27 In the review by Wilkinson et al of 43 patients with FNS who underwent stereotactic radiosurgery, 26% had improvement, 72% were unchanged, and 2% had worsened facial function.19 With regard to tumor size, there was 93% tumor control rate, while 7% of patients had an increase in tumor size following GKS. All our patients who underwent GKS had normal to near-normal (HB 1 to 2) facial function preoperatively. This was preserved in 4/6 patients (67%). One patient had tumor enlargement following cystic degeneration almost 2 years following treatment resulting in worsened facial function, and another patient had facial weakness a week following treatment likely secondary to posttreatment edema, requiring steroids. Cystic degeneration following GKS for FNS has also been described by Litre et al and Wilkinson et al, and the patients required additional treatment with microsurgery.19,24 Cystic degeneration has also been described for vestibular schwannomas.28,29 Studies in vestibular schwannomas have shown the time to cystic degeneration from the time of radiosurgery to range from 6 months to over 10 years.28,30 GKS remains an excellent treatment option for those with relatively preserved facial function who show signs of tumor progression, allowing the chance to avoid surgery with good tumor control and preservation of facial function. However, continued follow-up in patients following GKS is imperative.
Hearing outcome following treatment for FNS has not been reliably discussed in the literature, likely given the fact that most patients with FNS of the CPA have no useful hearing at the time of treatment. In our series, among those with useful hearing preoperatively, 50% maintained this in the GKS group and 67% maintained useful hearing in the surgical resection group during the follow-up period.
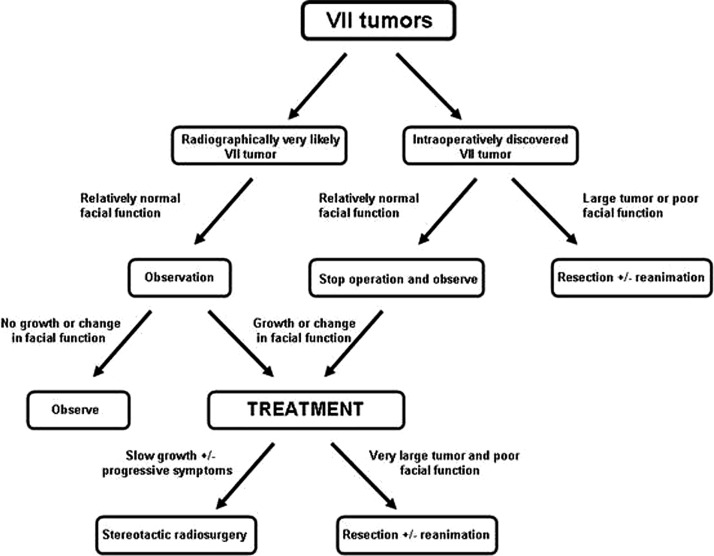
For FNSs of the CPA treated at our institution, we have used the algorithm shown in Fig. 3. Patients who are found to have FNS radiographically should be followed conservatively with serial imaging until there is evidence of tumor growth or change in neurologic function. In patients without significant facial function compromise (HB 1 to 3) and without evidence of brainstem compression or significant neurologic symptoms, a conservative course may be pursued utilizing annual MRI, audiograms, and electrodiagnostic testing. Many patients who have been managed conservatively have shown very little evidence of tumor growth and have maintained intact facial nerve function for up to 10 years.31
Figure 3.
Schematic for management of facial nerve schwannoma of the cerebellopontine angle.
Given the sometimes indistinguishable characteristics to vestibular schwannomas both clinically and radiographically, many patients may undergo open surgery and are subsequently found to have FNS intraoperatively. In such circumstances, the surgery may be aborted and the tumors followed with serial imaging if there is not pre-existing significant brainstem compression as the initial indication for the operation. When there is evidence of tumor progression or worsening of facial function, patients may be treated with stereotactic radiosurgery or open resection depending on the size of the tumor and severity of symptoms. Those patients without useful hearing were generally treated with a translabyrinthine approach. In patients with enlarging cystic components or clear planes between the facial nerve and tumor, particularly in smaller tumors, tumor resection may be performed with preservation of facial nerve continuity. For those with cystic FNS, while nerve continuity may be preserved, we believe the cyst lining should be removed when possible to prevent further recurrence requiring repeat surgery. In those with gross involvement of the facial nerve, nerve resection followed by facial-hypoglossal reconstruction should be employed. Unfortunately, we have not found adequate proximal facial nerve stumps to allow direct cable grafting for reanimation. While other groups have described preservation of normal facial function following “fascicle-sparing” FNS resection, which has not been our experience. All patients who underwent microsurgery for FNS either with or without facial reanimation did not achieve facial nerve function beyond HB 3.
Conclusion
The management for FNS has evolved to a more conservative approach, providing long-term facial function preservation without the morbidity associated with microsurgery or stereotactic radiosurgery. In those with large tumors (>3 cm) who are symptomatic with signs of progression, microsurgical removal with nerve reconstruction remains the best approach. A unique subset of patients may have cystic features and clear plains allowing a “nerve sparing” approach; however, subtotal removal may increase the chance of recurrence. For those with tumor progression and small to mid-sized tumors, stereotactic radiosurgery is a safe and effective management strategy with good tumor control and functional preservation in select patients.
References
- 1.House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113(3):186–187. doi: 10.1016/S0194-5998(95)70103-6. [DOI] [PubMed] [Google Scholar]
- 3.Nadeau D P, Sataloff R T. Fascicle preservation surgery for facial nerve neuromas involving the posterior cranial fossa. Otol Neurotol. 2003;24(2):317–325. doi: 10.1097/00129492-200303000-00031. [DOI] [PubMed] [Google Scholar]
- 4.McMenomey S O, Glasscock M E, Minor L B, Jackson C G, Strasnick B. Facial nerve neuromas presenting as acoustic tumors. Am J Otol. 1994;15(3):307–312. [PubMed] [Google Scholar]
- 5.Selesnick S H, Jackler R K. Clinical manifestations and audiologic diagnosis of acoustic neuromas. Otolaryngol Clin North Am. 1992;25(3):521–551. [PubMed] [Google Scholar]
- 6.Brackmann D E, Bartels L J. Rare tumors of the cerebellopontine angle. Otolaryngol Head Neck Surg. 1980;88(5):555–559. doi: 10.1177/019459988008800508. [DOI] [PubMed] [Google Scholar]
- 7.Grey P L, Moffat D A, Hardy D G. Surgical results in unusual cerebellopontine angle tumours. Clin Otolaryngol Allied Sci. 1996;21(3):237–243. doi: 10.1111/j.1365-2273.1996.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 8.Moffat D A, Saunders J E, McElveen J T, McFerran D J, Hardy D G. Unusual cerebello-pontine angle tumours. J Laryngol Otol. 1993;107(12):1087–1098. doi: 10.1017/s0022215100125393. [DOI] [PubMed] [Google Scholar]
- 9.Sherman J D, Dagnew E, Pensak M L, Loveren H R van, Tew J M. Facial nerve neuromas: report of 10 cases and review of the literature. Neurosurgery. 2002;50(3):450–456. doi: 10.1097/00006123-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Fagan P A, Misra S N, Doust B. Facial neuroma of the cerebellopontine angle and the internal auditory canal. Laryngoscope. 1993;103(4 Pt 1):442–446. doi: 10.1002/lary.5541030414. [DOI] [PubMed] [Google Scholar]
- 11.Lee K S, Britton B H, Kelly D L. Schwannoma of the facial nerve in the cerebellopontine angle presenting with hearing loss. Surg Neurol. 1989;32(3):231–234. doi: 10.1016/0090-3019(89)90184-5. [DOI] [PubMed] [Google Scholar]
- 12.O'Donoghue G M, Brackmann D E, House J W, Jackler R K. Neuromas of the facial nerve. Am J Otol. 1989;10(1):49–54. [PubMed] [Google Scholar]
- 13.Symon L, Cheesman A D, Kawauchi M, Bordi L. Neuromas of the facial nerve: a report of 12 cases. Br J Neurosurg. 1993;7(1):13–22. doi: 10.3109/02688699308995051. [DOI] [PubMed] [Google Scholar]
- 14.Lassaletta L, Roda J M, Frutos R, Patrón M, Gavilán J. Facial nerve schwannoma of the cerebellopontine angle: a diagnostic challenge. Skull Base. 2002;12(4):203–207. doi: 10.1055/s-2002-35752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleh E, Achilli V, Naguib M. et al. Facial nerve neuromas: diagnosis and management. Am J Otol. 1995;16(4):521–526. [PubMed] [Google Scholar]
- 16.Sataloff R T Frattali M A Myers D L Intracranial facial neuromas: total tumor removal with facial nerve preservation: a new surgical technique Ear Nose Throat J 1995744244–246., 248–256 [PubMed] [Google Scholar]
- 17.Lipkin A F, Coker N J, Jenkins H A, Alford B R. Intracranial and intratemporal facial neuroma. Otolaryngol Head Neck Surg. 1987;96(1):71–79. doi: 10.1177/019459988709600113. [DOI] [PubMed] [Google Scholar]
- 18.Neely J G, Neblett C R. Differential facial nerve function in tumors of the internal auditory meatus. Ann Otol Rhinol Laryngol. 1983;92(1 Pt 1):39–41. doi: 10.1177/000348948309200109. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson E P, Hoa M, Slattery W H. et al. Evolution in the management of facial nerve schwannoma. Laryngoscope. 2011;121(10):2065–2074. doi: 10.1002/lary.22141. [DOI] [PubMed] [Google Scholar]
- 20.Lee J D, Kim S H, Song M H, Lee H K, Lee W S. Management of facial nerve schwannoma in patients with favorable facial function. Laryngoscope. 2007;117(6):1063–1068. doi: 10.1097/MLG.0b013e31804b1a51. [DOI] [PubMed] [Google Scholar]
- 21.Fenton J E, Morrin M M, Smail M, Sterkers O, Sterkers J M. Bilateral facial nerve schwannomas. Eur Arch Otorhinolaryngol. 1999;256(3):133–135. doi: 10.1007/s004050050125. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues S J, Fagan P A, Biggs N D. Management of cystic facial neuromas: an alternative approach. Otol Neurotol. 2004;25(2):183–185. doi: 10.1097/00129492-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Kida Y, Yoshimoto M, Hasegawa T. Radiosurgery for facial schwannoma. J Neurosurg. 2007;106(1):24–29. doi: 10.3171/jns.2007.106.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Litre C F Gourg G P Tamura M et al. Gamma knife surgery for facial nerve schwannomas Neurosurgery 2007605853–859., discussion 853–859 [DOI] [PubMed] [Google Scholar]
- 25.Madhok R Kondziolka D Flickinger J C Lunsford L D Gamma knife radiosurgery for facial schwannomas Neurosurgery 20096461102–1105., discussion 1105 [DOI] [PubMed] [Google Scholar]
- 26.Nishioka K, Abo D, Aoyama H. et al. Stereotactic radiotherapy for intracranial nonacoustic schwannomas including facial nerve schwannoma. Int J Radiat Oncol Biol Phys. 2009;75(5):1415–1419. doi: 10.1016/j.ijrobp.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 27.Hillman T A, Chen D A, Fuhrer R. An alternative treatment for facial nerve tumors: short-term results of radiotherapy. Ear Nose Throat J. 2008;87(10):574–577. [PubMed] [Google Scholar]
- 28.Murakami K, Jokura H, Kawagishi J, Watanabe M, Tominaga T. Development of intratumoral cyst or extratumoral arachnoid cyst in intracranial schwannomas following gamma knife radiosurgery. Acta Neurochir (Wien) 2011;153(6):1201–1209. doi: 10.1007/s00701-011-0972-y. [DOI] [PubMed] [Google Scholar]
- 29.Piccirillo E, Wiet M R, Flanagan S. et al. Cystic vestibular schwannoma: classification, management, and facial nerve outcomes. Otol Neurotol. 2009;30(6):826–834. doi: 10.1097/MAO.0b013e3181b04e18. [DOI] [PubMed] [Google Scholar]
- 30.Pollock B E Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience Neurosurgery 2006582241–248., discussion 241–248 [DOI] [PubMed] [Google Scholar]
- 31.Liu R, Fagan P. Facial nerve schwannoma: surgical excision versus conservative management. Ann Otol Rhinol Laryngol. 2001;110(11):1025–1029. doi: 10.1177/000348940111001106. [DOI] [PubMed] [Google Scholar]