Abstract
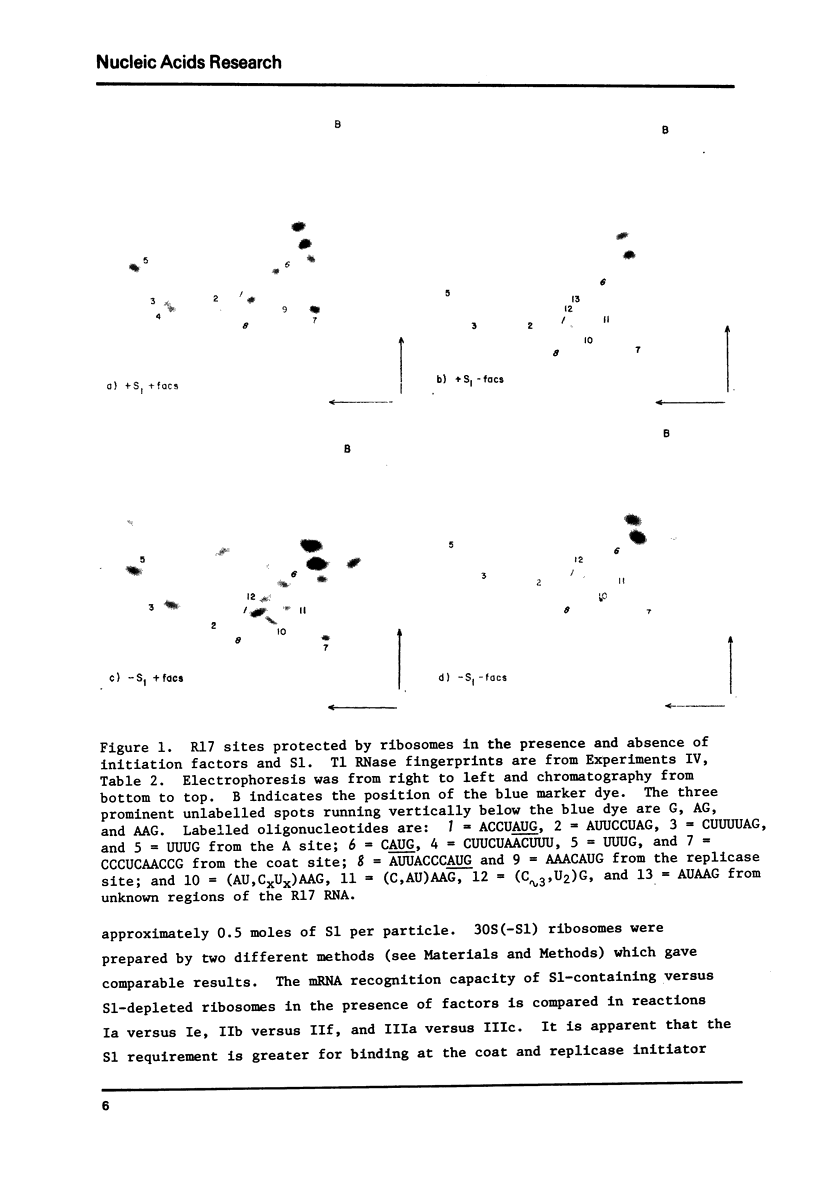
The initiation specificity of washed E. coli ribosomes in the presence and absence of purified initiation factors and/or S1 protein has been examined in protection experiments using 32P-labelled R17 RNA. We find that the three bacteriophage initiator regions do not exhibit equal requirements for either of these components during initiation complex formation. Specifically, both factors and S1 stimulate ribosome binding to the beginnings of the coat and replicase cistrons to a greater extent than they promote recognition of the A protein initiation site. The differential effects are therefore inversely correlated with the degree of mRNA-16S rRNA complementarity exhibited by the three initiator regions. We also observe that S1 suppresses ribosome binding to spurious sites in the R17 RNA.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear D. G., Ng R., Van Derveer D., Johnson N. P., Thomas G., Schleich T., Noller H. F. Alteration of polynucleotide secondary structure by ribosomal protein S1. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1824–1828. doi: 10.1073/pnas.73.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Arentzen R., Voorma H. O. The mechanism of action of inititation factor F1 from Escherichia coli. Biochim Biophys Acta. 1972 May 10;269(2):304–310. doi: 10.1016/0005-2787(72)90440-6. [DOI] [PubMed] [Google Scholar]
- Bollen A., Heimark R. L., Cozzone A., Traut R. R., Hershey J. W. Cross-linking of initiation factor IF-2 to Escherichia coli 30 S ribosomal proteins with dimethylsuberimidate. J Biol Chem. 1975 Jun 10;250(11):4310–4314. [PubMed] [Google Scholar]
- Carmichael G. G. Isolation of bacterial and phage proteins by homopolymer RNA-cellulose chromatography. J Biol Chem. 1975 Aug 10;250(15):6160–6167. [PubMed] [Google Scholar]
- Czernilofsky A. P., Kurland C. G., Stöffler G. 30S ribosomal proteins associated with the 3'-terminus of 16S RNA. FEBS Lett. 1975 Oct 15;58(1):281–284. doi: 10.1016/0014-5793(75)80279-1. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Dondon J., Godefroy-Colburn T., Graffe M., Grunberg-Manago M. IF-3 requirements for initiation complex formation with synthetic messengers in E. coli system. FEBS Lett. 1974 Sep 1;45(1):82–87. doi: 10.1016/0014-5793(74)80816-1. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Ebel J. P. Sequence analysis of the 3'-T1 oligonucleotide of 16S ribosomal RNA from Escherichia coli. FEBS Lett. 1974 Dec 1;49(1):47–48. doi: 10.1016/0014-5793(74)80628-9. [DOI] [PubMed] [Google Scholar]
- Fiser I., Scheit K. H., Stöffler G., Kuechler E. Identification of protein S 1 at the messenger RNA binding site of the Escherichia coli ribosome. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1112–1118. doi: 10.1016/0006-291x(74)90427-6. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Steitz J. A. Cistron specificity of 30S ribosomes heterologously reconstituted with components from Escherichia coli and Bacillus stearothermophilus. Biochemistry. 1974 May 7;13(10):2123–2129. doi: 10.1021/bi00707a020. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Hawley D. A., Slobin L. I., Wahba A. J. The mechanism of action of initiation factor 3 in protein synthesis. II. Association of the 30S ribosomal protein S12 with IF-3. Biochem Biophys Res Commun. 1974 Nov 27;61(2):544–550. doi: 10.1016/0006-291x(74)90991-7. [DOI] [PubMed] [Google Scholar]
- Held W. A., Gette W. R., Nomura M. Role of 16S ribosomal ribonucleic acid and the 30S ribosomal protein S12 in the initiation of natural messenger ribonucleic acid translation. Biochemistry. 1974 May 7;13(10):2115–2122. doi: 10.1021/bi00707a019. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Szer W. Replacement of ribosomal protein S1 by interference factor ialpha in ribosomal binding of phage Ms2 RNA. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4708–4712. doi: 10.1073/pnas.71.12.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille M. B., Miller M. J., Iwasaki K., Wahba A. J. Translation of the genetic message. VI. The role of ribosomal subunits in binding of formylmethionyl-tRNA and its reaction with puromycin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1652–1654. doi: 10.1073/pnas.58.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Pollack Y., Petre J. Physical and functional homology between ribosomal protein S1 and interference factor i. Eur J Biochem. 1974 Jun 1;45(1):109–117. doi: 10.1111/j.1432-1033.1974.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Isono K., Isono S. Lack of ribosomal protein S1 in Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1976 Mar;73(3):767–770. doi: 10.1073/pnas.73.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono S., Isono K. Role of ribosomal protein S1 in portein synthesis: effects of its addition to Bacillus stearothermophilus cell-free system. Eur J Biochem. 1975 Aug 1;56(1):15–22. doi: 10.1111/j.1432-1033.1975.tb02202.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Sabol S., Wahba A. J., Ochoa S. Translation of the genetic message. VII. Role of initiation factors in formation of the chain initiation complex with Escherichia coli ribosomes. Arch Biochem Biophys. 1968 May;125(2):542–547. doi: 10.1016/0003-9861(68)90612-7. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Initiation of protein synthesis. Binding of messenger RNA. J Biol Chem. 1975 Aug 10;250(15):5742–5748. [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Translational repression of a viral messenger RNA by a host protein. J Biol Chem. 1975 Aug 10;250(15):5749–5755. [PubMed] [Google Scholar]
- Jeppesen P. G., Steitz J. A., Gesteland R. F., Spahr P. F. Gene order in the bacteriophage R17 RNA: 5'-a protein-coat protein-synthetase-3'. Nature. 1970 Apr 18;226(5242):230–237. doi: 10.1038/226230a0. [DOI] [PubMed] [Google Scholar]
- Kenner R. A. A protein-nucleic acid crosslink in 30S ribosomes. Biochem Biophys Res Commun. 1973 Apr 16;51(4):932–938. doi: 10.1016/0006-291x(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Dewey K. F., Hershey J. W., Thach R. E. Guanosine 5'-triphosphatase activity of initiation factor f2. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1066–1070. doi: 10.1073/pnas.61.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog S., Coleman J. E. The catalytic mechanism of carbonic anhydrase. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2505–2508. doi: 10.1073/pnas.70.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Independent initiation of translation of two bacteriophage f2 proteins. Biochem Biophys Res Commun. 1969 Sep 24;37(1):127–136. doi: 10.1016/0006-291x(69)90890-0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Robertson H. D. Regulation of in vitro translation of bacteriophage f2 RNA. Cold Spring Harb Symp Quant Biol. 1969;34:655–673. doi: 10.1101/sqb.1969.034.01.076. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Niveleau A., Wahba A. J. Inhibition of synthetic and natural messenger translation. I. Purification and properties of a protein isolated from Escherichia coli MRE 600 ribosomes. J Biol Chem. 1974 Jun 25;249(12):3803–3807. [PubMed] [Google Scholar]
- Miller M. J., Wahba A. J. Chain initiation factor 2. Purification and properties of two species from Escherichia coli MRE 600. J Biol Chem. 1973 Feb 10;248(3):1084–1090. [PubMed] [Google Scholar]
- Miller M. J., Wahba A. J. Inhibition of synthetic and natural messenger translation. II. Specificity and mechanism of action of a protein isolated from Escherichia coli MRE 600 ribosomes. J Biol Chem. 1974 Jun 25;249(12):3808–3813. [PubMed] [Google Scholar]
- Moore P. B. Reaction of N-ethyl maleimide with the ribosomes of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):169–184. doi: 10.1016/0022-2836(71)90456-6. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Herr W. Nucleotide sequence of the 3' terminus of E. coli 16S ribosomal RNA. Mol Biol Rep. 1974 Dec;1(8):437–439. doi: 10.1007/BF00360668. [DOI] [PubMed] [Google Scholar]
- Scheps R., Wax R., Revel M. Reactivation in vitro of inactive ribosomes from stationary phase Escherichia coli. Biochim Biophys Acta. 1971 Feb 25;232(1):140–150. doi: 10.1016/0005-2787(71)90498-9. [DOI] [PubMed] [Google Scholar]
- Schiff N., Miller M. J., Wahba A. J. Purification and properties of chain initiation factor 3 from T4-infected and uninfected Escherichia coli MRE 600. Stimulation of translation of synthetic and natural messengers. J Biol Chem. 1974 Jun 25;249(12):3797–3802. [PubMed] [Google Scholar]
- Senear A. W., Steitz J. A. Site-specific interaction of Qbeta host factor and ribosomal protein S1 with Qbeta and R17 bacteriophage RNAs. J Biol Chem. 1976 Apr 10;251(7):1902–1912. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Steitz J. A. The 3' terminal oligonucleotide of E. coli 16S ribosomal RNA: the sequence in both wild-type and RNase iii- cells is complementary to the polypurine tracts common to mRNA initiator regions. Nucleic Acids Res. 1975 Jun;2(6):787–798. doi: 10.1093/nar/2.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Specific recognition of non-initiator regions in RNA bacteriophage messengers by ribosomes of Bacillus stearothermophilus. J Mol Biol. 1973 Jan;73(1):1–16. doi: 10.1016/0022-2836(73)90155-1. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Leffler S. Ribosomal protein S1 and polypeptide chain initiation in bacteria. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2325–2329. doi: 10.1073/pnas.72.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Leffler S. Interaction of Escherichia coli 30S ribosomal subunits with MS2 phage RNA in the absence of initiation factors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3611–3615. doi: 10.1073/pnas.71.9.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Aviram M., Kanarek A., Weiss A. Polyuridylic acid binding and translating by Escherichia coli ribosomes: stimulation by protein I, inhibition by aurintricarboxylic acid. Biochim Biophys Acta. 1972 Oct 27;281(3):381–392. doi: 10.1016/0005-2787(72)90452-2. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. I. Purification and physical chemical properties of the enzyme amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:417–426. doi: 10.1016/0006-3002(60)90194-3. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Iwasaki K., Miller M. J., Sabol S., Sillero M. A., Vasquez C. Initiation of protein synthesis in Escherichia coli. II. Role of the initiation factors in polypeptide synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:291–299. doi: 10.1101/sqb.1969.034.01.035. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J. Chain initiation factors from Escherichia coli. Methods Enzymol. 1974;30:3–18. doi: 10.1016/0076-6879(74)30003-1. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Yoshida M., Rudland P. S. Ribosomal binding of bacteriophage RNA with different components of initiation factor F3. J Mol Biol. 1972 Jul 28;68(3):465–481. doi: 10.1016/0022-2836(72)90100-3. [DOI] [PubMed] [Google Scholar]
- van Duin J., van Knippenberg P. H. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J Mol Biol. 1974 Mar 25;84(1):185–195. doi: 10.1016/0022-2836(74)90221-6. [DOI] [PubMed] [Google Scholar]