Abstract
Exercise training induces multiple adaptations within skeletal muscle that may improve local O2 delivery-utilization matching (i.e., Po2mv). We tested the hypothesis that increased nitric oxide (NO) function is intrinsic to improved muscle Po2mv kinetics from rest to contractions after exercise training. Healthy young Sprague-Dawley rats were assigned to sedentary (n = 18) or progressive treadmill exercise training (n = 10; 5 days/wk, 6–8 wk, final workload of 60 min/day at 35 m/min, −14% grade) groups. Po2mv was measured via phosphorescence quenching in the spinotrapezius muscle at rest and during 1-Hz twitch contractions under control (Krebs-Henseleit solution), sodium nitroprusside (SNP, NO donor; 300 μM), and NG-nitro-l-arginine methyl ester (l-NAME, nonspecific NO synthase blockade; 1.5 mM) superfusion conditions. Exercise-trained rats had greater peak oxygen uptake (V̇o2peak) than their sedentary counterparts (81 ± 1 vs. 72 ± 2 ml·kg−1·min−1, respectively; P < 0.05). Exercise-trained rats had significantly slower Po2mv fall throughout contractions (τ1; time constant for the first component) during control (sedentary: 8.1 ± 0.6; trained: 15.2 ± 2.8 s). Compared with control, SNP slowed τ1 to a greater extent in sedentary rats (sedentary: 38.7 ± 5.6; trained: 26.8 ± 4.1 s; P > 0.05) whereas l-NAME abolished the differences in τ1 between sedentary and trained rats (sedentary: 12.0 ± 1.7; trained: 11.2 ± 1.4 s; P < 0.05). Our results indicate that endurance exercise training leads to greater muscle microvascular oxygenation across the metabolic transient following the onset of contractions (i.e., slower Po2mv kinetics) partly via increased NO-mediated function, which likely constitutes an important mechanism for training-induced metabolic adaptations.
Keywords: endurance exercise training, kinetics, microvascular partial pressure of oxygen, oxygen delivery, oxygen uptake, skeletal muscle microcirculation
endurance exercise training induces multiple structural and functional adaptations that enhance the capacities for skeletal muscle O2 delivery and utilization (Q̇o2 and V̇o2, respectively; Refs. 37, 58, 71). At any given submaximal contractile activity, this cluster of adaptations reduces the level of metabolic perturbations (e.g., changes in ADP, PCr, and Cr concentrations) required to drive V̇o2 and improves the coupling between energy utilization and muscle mitochondrial ATP production (70). These properties reduce the rate of glycolysis and reliance on finite energy sources and increase exercise tolerance (37).
Microcirculatory adaptations to training are particularly important considering that the greatest resistance to O2 flux into skeletal muscle fibers resides primarily in the short distance between the red blood cell and the adjacent subsarcolemmal space (23, 29). As dictated by Fick's law of diffusion, the O2 pressure within the microvasculature (i.e., muscle Po2mv) constitutes the exclusive driving force for blood-myocyte O2 transfer. The time course of skeletal muscle Po2mv during transitions in metabolic demand is determined by the dynamic matching between Q̇o2 and V̇o2 (i.e., Q̇o2/V̇o2 ratio) (8). Therefore, because alterations in muscle Po2mv have a direct impact on oxidative metabolism and contractile performance (36, 68), increased Po2mv during contractions likely contributes to the beneficial effects of exercise training on muscle function.
Substantial evidence indicates that increased nitric oxide (NO) function is a key factor improving skeletal muscle hemodynamic and metabolic control following exercise training (28, 53). Moreover, previous reports from our laboratory indicate that alterations in NO levels impact profoundly muscle Po2mv during transitions in metabolic demand in health and disease (24, 25, 31) and suggest that increased NO-mediated function could underlie, at least in part, enhanced muscle microvascular oxygenation in the trained state.
The purpose of the present study was to determine the effects of endurance exercise training on muscle Po2mv and whether augmented NO-mediated function contributes mechanistically to potential increases in Po2mv from rest to contractions in rat skeletal muscle in situ after training. Based on the potential enhancement of NO-mediated function following endurance exercise training (28, 53), the hypotheses were tested that 1) exercise training would elevate muscle Po2mv and slow Po2mv kinetics (i.e., resulting in higher Po2mv across the on-contraction transient); 2) increased NO levels (via the NO donor sodium nitroprusside; SNP) would elevate Po2mv and slow Po2mv kinetics to a greater extent in sedentary compared with trained rats; and 3) reduced NO levels (nonspecific NO synthase blockade with NG-nitro-l-arginine methyl ester, l-NAME) would lower Po2mv and speed Po2mv kinetics to a greater extent in trained compared with sedentary rats during the transition from rest to contractions.
MATERIALS AND METHODS
Animal selection and care.
A total of 28 male Sprague-Dawley rats (4–5 mo old; Charles Rivers Laboratories, Boston, MA) were used to investigate the effects of exercise training on, and the NO contribution to, skeletal muscle microvascular oxygenation. Additional rats were used in supplementary experiments to 1) evaluate the reproducibility and demonstrate the lack of an ordering effect of the protocol (n = 7); and 2) assess potential cyanide-induced impairment of skeletal muscle function with the present SNP superfusion protocol (n = 9). All experimental procedures followed guidelines established by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Kansas State University. Rats were maintained on a 12:12-h light-dark cycle with food and water provided ad libitum. Before initiation of the experimental protocol, rats were familiarized with downhill running on a custom-built motor-driven treadmill over the course of a 1-wk period (5–10 min/day at a speed of 20 m/min and −14% grade). After the familiarization phase, rats were assigned randomly to either sedentary (n = 18) or endurance exercise trained (n = 10) groups. Sedentary control rats were confined to cage activities whereas trained rats ran 5 days/wk for 6–8 wk on the declined treadmill (−14% grade). All rats underwent the same training program, in which treadmill running duration and speed were increased progressively from 10 min at 25 m/min to 60 min at 35 m/min. This final workload was kept for at least 3–4 wk. Previous work from our laboratory has demonstrated that downhill treadmill running recruits the rat spinotrapezius muscle (41, 57) and constitutes an effective model for exercise training programs (30).
Peak oxygen uptake measurements.
Upon completion of the training program, peak oxygen uptake (V̇o2peak) was measured in sedentary and trained rats during a downhill (−14% grade) running test performed in a metabolic chamber placed on the treadmill. As described in detail previously (16, 30), the speed was set initially to 25 m/min for 2–3 min and then increased progressively in a ramplike fashion by ∼5–10 m/min until the rat was unable to keep pace with the treadmill belt or no further elevations in V̇o2 were observed despite continued increases in treadmill speed. At this point of the test V̇o2peak was measured and recorded. Alterations in gait (e.g., lowering of the hindlimbs, dropping of the tail, and elevation of the snout) normally occurred immediately prior to termination of the test. Six of the 28 rats tested had to repeat their maximal tests on a separate day (i.e., ≥24 h of recovery) due to failure to achieve the predetermined criteria. Gas measurements were performed in real time via an inline O2 analyzer (model S-3A/I; AEI Technologies; Pittsburgh, PA). The analyzer was calibrated before and after each maximal exercise test with precision-mixed gases that spanned the expected range of gas concentrations based on previous studies. We have reported recently highly reproducible V̇o2peak measurements using the aforementioned techniques and protocol (16).
Surgical preparation.
On the day of data collection, rats were anesthetized initially with a 5% isoflurane-O2 mixture and subsequently maintained on 2–3% isoflurane-O2 (Butler Animal Health Supply, Dublin, OH). The left carotid and caudal (tail) arteries were cannulated (PE-10 connected to PE-50; Intra-Medic Tubing, Clay Adams Brand, Sparks, MD) for continuous monitoring of mean arterial pressure (MAP; Digi-Med BPA model 200, Louisville, KY) and infusion of the phosphorescent probe palladium meso-tetra-(4-carboxyphenyl)porphyrin dendrimer (R2; 15 mg/kg; Oxygen Enterprises, Philadelphia, PA). Blood from the tail catheter was sampled at the end of each experimental protocol for determination of arterial blood gases, pH, and systemic hematocrit (Nova Stat Profile M, Waltham, MA). Anesthetized rats were placed on a heating pad to maintain core temperature, measured via rectal probe, at ∼37–38°C.
Following catheter placement procedures, isoflurane inhalation was discontinued progressively and rats were kept under anesthesia with intra-arterial pentobarbital sodium throughout the experiment. The level of anesthesia was monitored frequently via the toe-pinch and blink reflexes and supplemented as necessary. Overlying skin and fascia from the middorsal region of the rat were reflected carefully to expose the right spinotrapezius muscle. The spinotrapezius was moistened constantly during the surgical preparation via superfusion of Krebs-Henseleit (K-H) bicarbonate-buffered solution (4.7 mM KCl, 2.0 mM CaCl2, 2.4 mM MgSO4, 131 mM NaCl, and 22 mM NaHCO3; pH = 7.4; equilibrated with 5% CO2 and 95% N2 at ∼38°C). Surrounding tissue was covered with Saran wrap (Dow Brands, Indianapolis, IN). Stainless steel electrodes were sutured to the rostral (cathode) and caudal (anode) regions of the spinotrapezius for electrically induced contractions. Previous reports from our laboratory demonstrate that these surgical procedures do not impact the microvascular integrity and responsiveness of the spinotrapezius muscle (3).
Experimental protocol.
Three separate contraction bouts were performed under control (5 ml K-H), SNP (NO donor; 5 ml of a 300 μM solution), and l-NAME (non-isoform-specific NO synthase inhibitor; 5 ml of a 1.5 mM solution) superfusion conditions. Drugs were purchased from Sigma-Aldrich (St. Louis, MO), and concentrations were chosen based on previous studies in our laboratory (24, 25, 31). All solutions were maintained at ∼38°C. The dose of SNP was titrated to elicit consistent alterations in Po2mv without compromising systemic hemodynamics (i.e., a decrease in MAP below 70 mmHg at any time; Refs. 10, 25). Preliminary experiments indicate that significantly greater hypotensive responses and increases in resting Po2mv are evoked by progressively higher SNP doses (up to 1,200 μM; unpublished data). To prevent SNP photodecomposition and potential cyanide release (11, 13), SNP solutions were protected from light sources by covering containers and syringes with aluminum foil and, as a mandate for Po2mv measurements using phosphorescence quenching, performing our experiments in a dark room. While superfusion order was randomized between control and SNP conditions, l-NAME was always the last treatment because of its relatively long half-life. The spinotrapezius was superfused with each solution (average flow rate of ∼1.5 ml/min) for a total time of 3 min, followed by a 2- to 3-min incubation period to allow resting muscle Po2mv to stabilize. Subsequently, electrical stimulation (1 Hz, 6–7 V, 2-ms pulse duration) of the muscle was evoked via a Grass stimulator (model s48, Quincy, MA) for 3 min. The muscle was then allowed to recover for ∼25 min before the next condition was initiated (stimulation parameters were held constant). During the recovery period following the SNP trial, the muscle was superfused at an average flow rate of ∼1.5 ml/min with K-H to wash out SNP. At the end of each experiment, rats were euthanized with intra-arterial pentobarbital sodium overdose (∼50 mg/kg).
Supplementary experiments.
The spinotrapezius preparation exhibits reproducible Po2mv parameters during transitions in metabolic demand evoked by 1-Hz twitch contractions when a minimum of 20 min of recovery is allowed between contraction bouts (31, 34). A >30 min period between consecutive contractions (i.e., 3-min off-transition, ∼25 min recovery, 3 min superfusion, 2- to 3-min incubation) was employed herein to prevent any priming and drug-ordering effects that could confound the experimental interpretation of the Po2mv responses to muscle contractions (9, 25). Accordingly, supplementary time control experiments (n = 7) revealed reproducible Po2mv profiles from three contraction bouts separated by ∼30 min (within-animal coefficient of variation: 13 ± 2% for baseline, steady-state, and all primary component kinetics parameters) with no ordering effect (P > 0.05 for baseline, steady-state, and all kinetics parameters).
Considerable controversy exists surrounding potential cyanide generation from SNP (11, 13, 27) despite widespread usage of this NO donor in both clinical and research arenas (human and animal models). A major source of this controversy may lie within the methods utilized for assaying blood cyanide concentrations and the importance of photodecomposition in generating cyanide from SNP (during both SNP infusion and cyanide measurement; Refs. 11, 13). As described above, in the present study SNP solutions were protected from light sources by covering containers and syringes with aluminum foil and performing experiments in a dark room. Nonetheless, supplementary experiments were conducted to examine potential cyanide-induced impairment of skeletal muscle function with the present SNP superfusion protocol. Three contraction bouts were conducted in the following superfusion order: control 1 (K-H), SNP (300 μM), control 2 (K-H). Recovery time and washout procedures were identical to those described above. Spinotrapezius muscle Po2mv was measured at rest and throughout contractions (n = 9). Spinotrapezius muscle blood flow (Q̇m) and oxygen utilization (V̇o2) were determined at rest and during the contraction steady state via radiolabeled microspheres and direct Fick calculation (as described in detail previously; Ref. 34), respectively (n = 4). In each condition, the stimulated right and nonstimulated left spinotrapezius muscles represented the contracting and resting Q̇m and V̇o2 measurements, respectively.
During Q̇m measurements, the tail artery catheter was connected to a 1-ml syringe, and blood withdrawal was initiated at a constant rate of 0.25 ml/min via a Harvard pump (model 907). Differentially radiolabeled microspheres (46Sc and 85Sr, 15-μm diameter; Perkin Elmer Life and Analytical Sciences) were injected in random order into the aortic arch via the carotid artery catheter during the contracting steady state (i.e., ∼3 min after onset of muscle contractions). Upon completion of the experiment, the right and left spinotrapezius muscles and kidneys were dissected, removed, and weighed immediately after euthanasia. The thorax was opened, and placement of the catheter into the aortic arch was confirmed. Tissue radioactivity was determined on a gamma scintillation counter (Packard Auto Gamma Spectrometer, Cobra model 5003), and Q̇m was determined by the reference method (39) and expressed as milliliters per minute per 100 g of tissue. Adequate mixing of the microspheres was verified for each injection by demonstrating a <15% difference in Q̇m between the right and left kidneys.
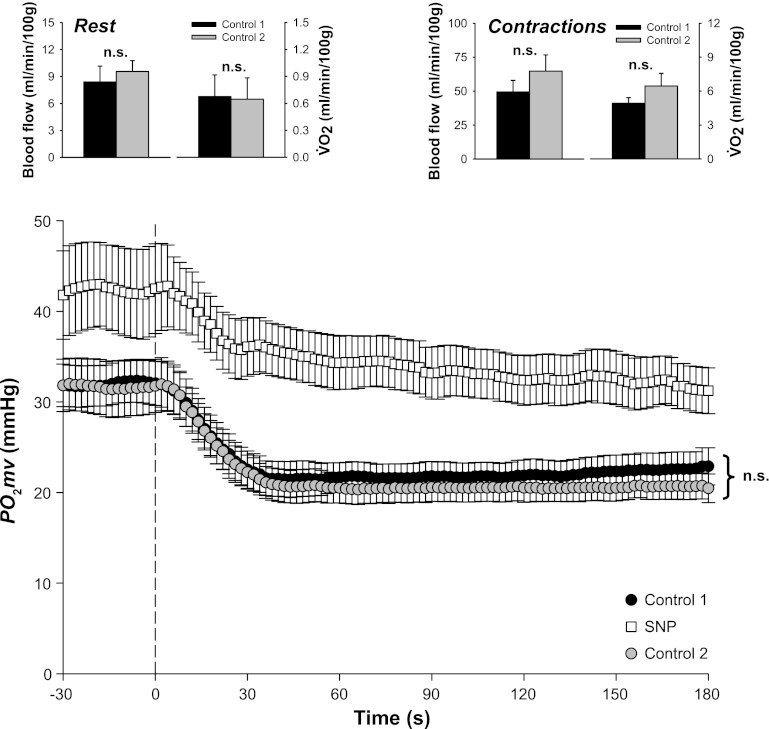
Spinotrapezius muscle V̇o2 was estimated from Po2mv and Q̇m measurements as described in detail previously (34). Briefly, arterial O2 concentration (CaO2) was calculated from arterial blood samples whereas venous O2 concentration (CvO2) was calculated from the resting or contracting steady-state Po2mv using the rat O2 dissociation curve (Hill coefficient of 2.6), the measured hemoglobin (Hb) concentration, a P50 of 38 mmHg, and an O2-carrying capacity of 1.34 ml O2/g Hb (1). Resting and contracting steady-state spinotrapezius Q̇m values were then used to calculate V̇o2 using the Fick equation [i.e., V̇o2 = Q̇m(CaO2 − CvO2)]. As illustrated in Fig. 1, results from these experiments suggest that mitochondrial and vascular control were not impaired following the SNP condition.
Fig. 1.
Results from experiments performed to examine potential cyanide-induced impairment of skeletal muscle function with the current sodium nitroprusside (SNP) superfusion protocol. Three contraction bouts were conducted in the following superfusion order: control 1 (Krebs-Henseleit), SNP (300 μM), control 2 (Krebs-Henseleit). Spinotrapezius O2 pressure within the microvasculature (muscle Po2mv) was measured at rest and throughout contractions (n = 9). Spinotrapezius muscle blood flow (Q̇m) and oxygen utilization (V̇o2) were determined at rest and during the contraction steady state via radiolabeled microspheres and direct Fick calculation, respectively (n = 4). Top panels: spinotrapezius Q̇m and V̇o2 at rest and during the contraction steady state during the first and third bouts (i.e., control 1 and 2, respectively). Bottom panel: spinotrapezius Po2mv at rest and following the onset of contractions under all 3 conditions (control 1, SNP, and control 2). Time zero denotes the onset of contractions. That resting and contracting spinotrapezius muscle V̇o2 did not differ between the first and third bouts (i.e., control 1 vs. control 2; P > 0.05) suggests preserved mitochondrial function post-SNP condition. The possibility of prolonged and/or irreversible vasodilation following the SNP condition is not supported based on similar resting and contraction steady-state Q̇m between the first and third bouts (i.e., control 1 vs. control 2; P > 0.05). Similar Po2mv profiles during the first and third bouts (i.e., control 1 vs. control 2; P > 0.05 for all kinetics parameters) are also consistent with the notion that mitochondrial and vascular control were not impaired following the SNP condition (i.e., second bout). ns, not significantly different.
Muscle Po2mv measurement.
Po2mv was measured by phosphorescence quenching using a Frequency Domain Phosphorometer (PMOD 5000; Oxygen Enterprises, Philadelphia, PA). The principles of the phosphorescence quenching method have been described in detail previously (8). Briefly, this method applies the Stern-Volmer relationship (63), which describes quantitatively the O2 dependence of the phosphorescent probe (R2) via the following equation:
where kQ is the quenching constant and τ and τ° are the phosphorescence lifetimes in the absence of O2 and the ambient O2 concentration, respectively. The phosphor R2 (τ° = 601 μs and kQ = 409 mmHg−1·s−1 at pH = 7.4 and temperature ∼38°C) (50) was infused ∼15 min before initiation of muscle contractions. The R2 probe binds to albumin and is distributed uniformly in the plasma, therefore providing a signal corresponding to the volume-weighted O2 pressure in the microvascular compartment (mainly the Po2 within the capillaries, which volumetrically represents the major intramuscular space; Ref. 59). The negative charge of the R2 probe also facilitates its restriction to the muscle intravascular space (60). The common end of the bifurcated light guide was positioned 2–4 mm superficial to the dorsal surface of the exposed spinotrapezius muscle. The phosphorometer modulates sinusoidal excitation frequencies between 100 Hz and 20 kHz and allows phosphorescence lifetime measurements from 10 μs to ∼2.5 ms. The excitation light (524 nm) was focused on a randomly selected area of ∼2 mm diameter of exposed muscle and has a penetration depth of ∼500 μm. Po2mv was recorded at 2-s intervals throughout the duration of the experimental protocol (i.e., superfusion, incubation, electrical stimulation, and recovery periods).
Movement of the light guide and/or animal was avoided so as to monitor the same sampling site during experiments. However, alteration of the Po2mv measurement plane (e.g., deep sighs, accidental splash of the light guide during superfusion) during muscle contractions precluded kinetic curve fitting in some instances. Thus, Po2mv results from the present study are presented from animals under the following conditions: sedentary control (n = 18); sedentary SNP (n = 14); sedentary l-NAME (n = 14); trained control (n = 10); trained SNP (n = 9); and trained l-NAME (n = 10).
Po2mv kinetics analysis.
The kinetics of Po2mv were described by nonlinear regression analysis using the Marquardt-Levenberg algorithm (SigmaPlot 11.2; Systat software, San Jose, CA) for the onset of contractions. Transient Po2mv responses were fit with either a one- or two-component model (7, 8) as follows.
For one-component model:
and for two-component model:
where Po2mv(t) is the Po2mv at a given time t; Po2mv(BL) corresponds to the precontracting resting Po2mv; Δ1 and Δ2 are the amplitudes for the first and second components, respectively; TD1 and TD2 are the independent time delays for each component; and τ1 and τ2 are the time constants (i.e., time to achieve 63% of the response) for each component. Goodness of fit was determined using three criteria: 1) the coefficient of determination; 2) the sum of squared residuals; and 3) visual inspection.
The mean response time (MRT; Ref. 51) was used to describe the overall dynamics of the Po2mv response:
where TD1 and τ1 are defined above. The MRT analysis was limited to the first component of the Po2mv response given that inclusion of an emergent second component underestimates the actual speed of Po2mv fall following the onset of contractions (33, 34).
Citrate synthase activity measurement.
The activity of the mitochondrial enzyme citrate synthase (a marker of oxidative capacity) from the spinotrapezius and select individual hindlimb muscles or muscle parts (soleus, red gastrocnemius, mixed gastrocnemius, and plantaris) was measured in duplicate from muscle homogenates by a modification of the method described by Srere (66). Upon termination of the experimental protocol and euthanasia, the muscles were removed, dissected free of connective tissue, and weighed. Citrate synthase activity was measured spectrophotometrically (Spectramax M5 microplate, Molecular Devices, Sunnyvale, CA) in 300-μl aliquots at 30°C.
Statistical analyses.
Data comparison was performed using unpaired Student's t-test, Mann-Whitney rank-sum test, or two-way repeated-measures ANOVA where appropriate. F-statistics were calculated using Type III (adjusted) sums of squares due to the unbalanced nature of the data. Student-Newman-Keuls post hoc tests were utilized to determine where the differences were located. A one-tailed test was performed when a priori directional hypotheses were tested (24, 25). The level of significance was set at P < 0.05. Results are presented as means ± SE.
RESULTS
Body mass and spinotrapezius muscle mass were not different between sedentary (462 ± 12 and 0.43 ± 0.1 g, respectively) and exercise-trained (480 ± 9 and 0.44 ± 0.1 g, respectively) rats after the training program was completed (P > 0.05 for both). There were no differences in arterial O2 saturation (sedentary: 91.0 ± 1.7; trained: 93.6 ± 1.7%), Po2 (sedentary: 91.9 ± 3.3; trained: 94.2 ± 3.6 mmHg), Pco2 (sedentary: 37.3 ± 1.7; trained: 35.5 ± 0.5 mmHg), pH (sedentary: 7.40 ± 0.01; trained: 7.42 ± 0.01), and systemic hematocrit (sedentary: 34.9 ± 0.8%; trained: 37.2 ± 0.7%) when comparing sedentary and trained rats (P > 0.05 for all).
Exercise-trained rats evidenced higher V̇o2peak (sedentary: 72 ± 2; trained: 81 ± 1 ml·kg−1·min−1; P < 0.05) than sedentary rats. Citrate synthase activity was higher in the soleus (sedentary: 17.5 ± 0.5; trained: 20.9 ± 1.5 μmol·g−1·min−1) and red gastrocnemius (sedentary: 24.2 ± 0.5; trained: 30.2 ± 1.1 μmol·g−1·min−1) muscles from trained rats (P < 0.05 for both). There was a tendency for greater citrate synthase activity in the mixed gastrocnemius (sedentary: 15.9 ± 1.1; trained: 18.7 ± 1.9; μmol·g−1·min−1; P = 0.064) and plantaris (sedentary: 13.5 ± 0.8; trained: 16.3 ± 1.8 μmol·g−1·min−1; P = 0.058) muscles from trained rats. Unexpectedly, citrate synthase activity from the spinotrapezius was not different between sedentary and trained rats (13.4 ± 0.7 and 12.9 ± 0.7 μmol·g−1·min−1, respectively; P > 0.05).
Effects of exercise training on muscle Po2mv.
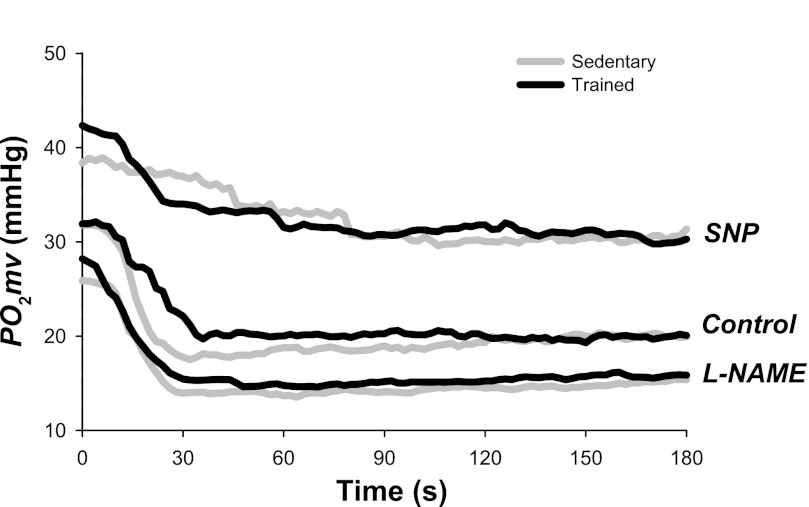
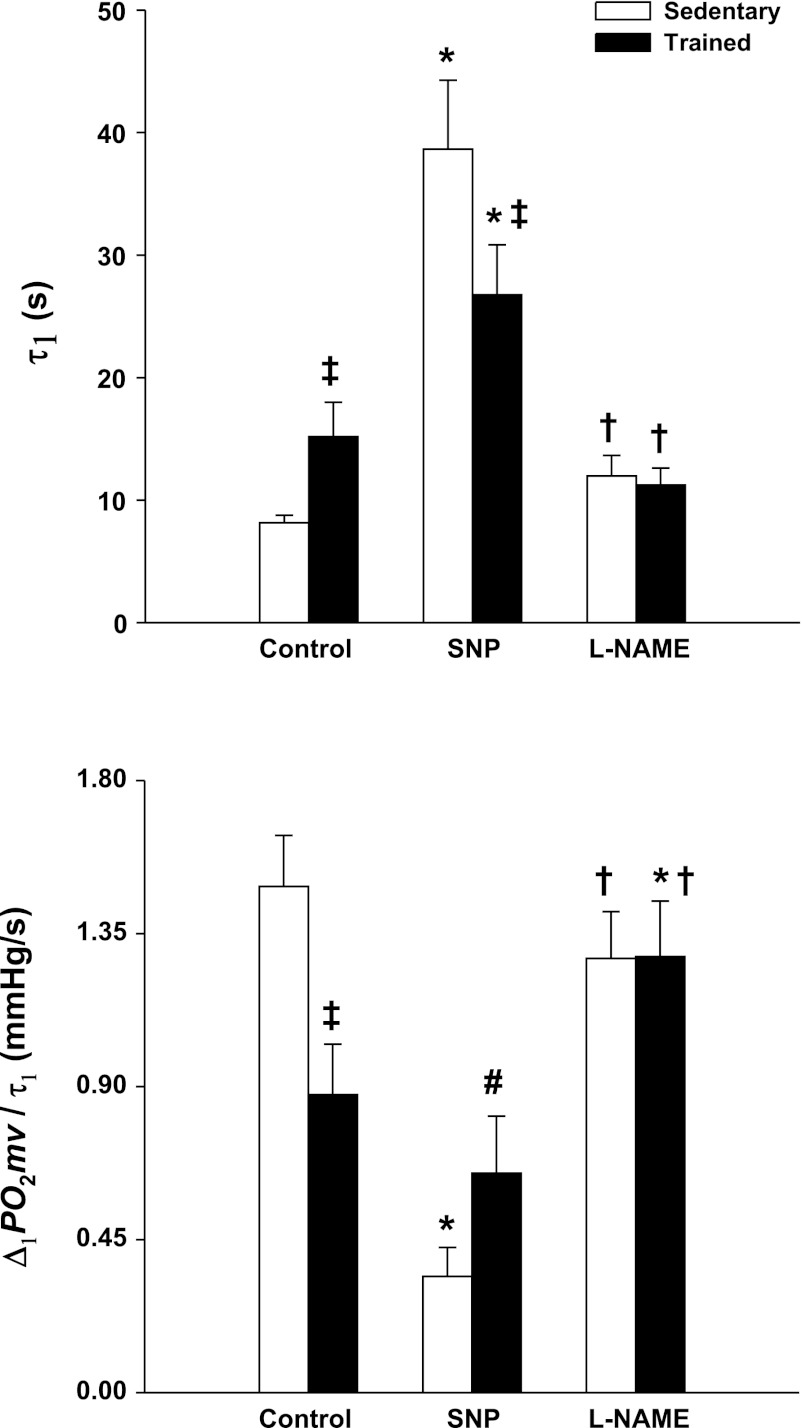
MAP was not different in sedentary compared with exercise-trained rats either before or after K-H superfusion (Table 1; P > 0.05). Although Po2mv values at rest [Po2mv(BL)] and during the contracting steady-state [Po2mv(SS)] were not different between groups (P > 0.05 for both), exercise training induced significant differences in the time course of Po2mv at the onset of contractions under the control condition (Figs. 2 and 3, Table 2). Specifically, the speed of Po2mv fall during contractions (as assessed by the time constant for the first component and relative rate of Po2mv fall; τ1 and Δ1Po2mv/τ1, respectively) was markedly slowed in trained compared with sedentary rats (Fig. 3). No significant differences in the time delay for the first component (TD1; P > 0.05) were observed whereas the mean response time (MRT) tended to be longer in trained rats (P = 0.13; Table 2).
Table 1.
Mean arterial pressure (expressed in mmHg) pre- and postsuperfusion of Krebs-Henseleit (control), SNP, and l-NAME in sedentary and exercise-trained rats
| Control |
SNP |
l-NAME |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Sedentary | 123 ± 4 | 124 ± 4 | 125 ± 5 | 111 ± 4* | 128 ± 4 | 132 ± 5 |
| Trained | 132 ± 3 | 133 ± 4 | 134 ± 5 | 95 ± 6*† | 134 ± 4 | 137 ± 4 |
Values are means ± SE. Results are presented from animals under the following conditions: sedentary control (n = 18), sedentary sodium nitroprusside (SNP) (n = 14), sedentary NG-nitro-l-arginine methyl ester (l-NAME) (n = 14), trained control (n = 10), trained SNP (n = 9), and trained l-NAME (n = 10). Significantly different from:
all other conditions within group;
sedentary post-SNP superfusion.
Fig. 2.
Spinotrapezius muscle Po2mv response from representative sedentary and exercise-trained rats under control, SNP, and NG-nitro-l-arginine methyl ester (l-NAME) conditions. Time zero denotes the onset of contractions. Note that exercise training slowed the Po2mv fall during contractions (τ1, time constant for the first component) under the control condition. SNP slowed τ1 to a greater extent in sedentary rats, whereas l-NAME abolished the differences in τ1 between sedentary and trained rats (see text for details).
Fig. 3.
Spinotrapezius muscle Po2mv kinetics (top panel: τ1, time constant for the first component; bottom panel: Δ1Po2mv/τ1; relative rate of Po2mv fall) in sedentary and exercise-trained rats under control, SNP, and l-NAME conditions. Significantly different from *control within group; †SNP within group; ‡sedentary within superfusion condition. #P = 0.12 vs. sedentary SNP.
Table 2.
Spinotrapezius muscle Po2mv kinetics following the onset of contractions under control, SNP, and l-NAME conditions in sedentary and exercise-trained rats
| Control |
SNP |
l-NAME |
||||
|---|---|---|---|---|---|---|
| Sedentary | Trained | Sedentary | Trained | Sedentary | Trained | |
| Po2mv(BL), mmHg | 29.2 ± 1.3 | 27.8 ± 1.2 | 38.7 ± 2.3* | 42.9 ± 3.5* | 25.5 ± 1.7† | 26.4 ± 1.5† |
| Δ1Po2mv, mmHg | 11.4 ± 1.2 | 10.6 ± 0.8 | 9.3 ± 1.5 | 15.6 ± 3.3‡ | 13.0 ± 1.0 | 13.0 ± 1.2 |
| Δ2Po2mv, mmHg | 2.3 ± 0.3 | 2.5 ± 0.3 | 2.3 ± 0.4 | 2.8 ± 1.3 | ||
| ΔTotalPo2mv, mmHg | 9.8 ± 1.2 | 8.6 ± 0.8 | 9.3 ± 1.5 | 15.6 ± 3.3*‡ | 11.2 ± 0.9 | 12.1 ± 1.1 |
| Po2mv(SS), mmHg | 19.4 ± 1.0 | 19.2 ± 1.5 | 29.4 ± 1.5* | 27.3 ± 1.4* | 14.3 ± 0.8*† | 14.2 ± 0.9*† |
| TD1, s | 10.2 ± 0.7 | 10.3 ± 1.6 | 8.3 ± 1.6 | 11.8 ± 3.6 | 6.2 ± 0.5* | 6.5 ± 0.9*† |
| TD2, s | 55.1 ± 9.5 | 53.4 ± 12.4 | 55.0 ± 6.8 | 41.3 ± 7.2 | ||
| τ2, s | 39.5 ± 7.8 | 32.3 ± 10.1 | 55.5 ± 8.9 | 51.9 ± 18.9 | ||
| MRT, s | 18.3 ± 0.9 | 25.5 ± 3.3# | 47.0 ± 5.8* | 38.6 ± 5.2*‡ | 18.1 ± 1.7† | 17.7 ± 1.6† |
Values are means ± SE. Po2mv, microvascular Po2; Po2mv(BL), resting Po2mv; Δ1Po2mv, amplitude of the first component; Δ2Po2mv, amplitude of the second component; ΔTotalPo2mv, overall amplitude regardless of one- or two-component model fit; Po2mv(SS), contracting steady-state Po2mv; TD1, time delay for the first component; TD2, time delay for the second component; τ2, time constant for the second component, MRT, mean response time. The time constant for the first component (τ1) and relative rate of Po2mv fall (Δ1Po2mv /τ1) are shown in Fig. 3. The one-component exponential model was used to analyze the Po2mv kinetics in the following conditions: sedentary control (6/18), sedentary SNP (14/14), sedentary l-NAME (3/14), trained control (2/10), trained SNP (9/9), and trained l-NAME (7/10). Significantly different from:
control within group;
SNP within group;
sedentary within superfusion condition.
P = 0.13 vs. sedentary control.
Effects of altered NO on muscle Po2mv in sedentary and exercise-trained rats.
SNP superfusion decreased MAP to a greater degree in trained compared with sedentary rats (Table 1; P < 0.05). Relative to the control condition, SNP increased Po2mv(BL) in both sedentary and trained rats (Table 2; P < 0.05). Although SNP increased the overall amplitude of Po2mv fall during contractions (ΔTotalPo2mv) only in trained rats, there were no differences in Po2mv(SS) between sedentary and trained rats (Table 2). Both groups exhibited greater Po2mv(SS) with SNP compared with the control condition (P < 0.05; Table 2). As illustrated in Fig. 2, SNP had strikingly distinct effects on the Po2mv time course following the onset of contractions in sedentary and trained rats. Albeit no differences between groups were found in TD1 with SNP (P > 0.05), τ1 and MRT were increased to a greater extent in sedentary compared with trained rats (P < 0.05; Table 2 and Fig. 3). Analysis of Δ1Po2mv/τ1 indicates that SNP slowed the relative rate of Po2mv fall during contractions in sedentary but not trained rats (Fig. 3; P < 0.05). Additionally, there was a tendency for Δ1Po2mv/τ1 to be slower in sedentary compared with trained rats during the SNP condition (Fig. 3; P = 0.12).
l-NAME superfusion did not change MAP in either sedentary or exercise-trained rats (Table 1; P > 0.05). Although l-NAME did not significantly modify Po2mv(BL) and ΔTotalPo2mv, both sedentary and trained rats had lower Po2mv(SS) compared with their control conditions (Table 2; P < 0.05). The effects of NO synthase inhibition with l-NAME were such that the spinotrapezius muscle Po2mv profile from rest to contractions in trained rats was similar to that of sedentary rats (Figs. 2 and 3, Table 2). Relative to the control condition, l-NAME significantly speeded TD1 in both sedentary and trained rats (Table 2). Notably, l-NAME abolished the differences in τ1, Δ1Po2mv/τ1, and MRT between sedentary and trained rats evident during the control condition (P > 0.05 for all; Table 2 and Fig. 3). Moreover, l-NAME speeded Δ1Po2mv/τ1 in trained rats to similar values found in sedentary rats (Fig. 3).
DISCUSSION
The present study demonstrates that endurance exercise training improves significantly the microvascular oxygenation profile (i.e., slowed Po2mv kinetics and therefore enhanced Po2mv) across the metabolic transient following the onset of contractions in the spinotrapezius muscle of healthy young rats. Compared with the control condition, increased NO with SNP slowed the Po2mv fall throughout contractions (τ1) to a greater extent in sedentary rats whereas decreased NO with l-NAME abolished the differences in τ1 between sedentary and trained rats. These results suggest that the enhanced driving force for blood-myocyte O2 flux during contractions with exercise training is mediated, at least in part, via increased NO-mediated function.
Exercise training and muscle microvascular oxygenation.
As stated above, muscle Po2mv kinetics is dictated by the dynamic Q̇o2/V̇o2 matching within the microvascular space (8). Slowed Po2mv kinetics in trained rats (Figs. 2 and 3, Table 2) therefore suggest that the rate of adjustment in Q̇o2 during contractions was relatively faster than that of V̇o2 compared with sedentary rats (5, 21, 26), such that fractional O2 extraction was reduced up until the steady state is achieved. The unexpected lack of change in citrate synthase activity found herein with training further supports this notion (see discussion below). Enhanced Q̇o2 across the rest-contractions transient with training is important to support potential augmented mitochondrial function (37, 70) and/or attenuate regional Q̇o2/V̇o2 mismatch (45), both of which might be linked mechanistically to faster muscle V̇o2 kinetics and improved exercise tolerance. With respect to the capacity to extract O2 it is important to note that there is an interdependence between the diffusive and conductive O2 transport components (62):
where Do2 is the effective muscle O2 diffusing capacity, β corresponds to the slope of the O2 dissociation curve in the physiologically relevant range, and Q̇m is muscle blood flow. Although Do2 represents a lumped parameter that includes the impediments to blood-myocyte O2 transfer and is determined by a complex interaction between structural and functional factors, it appears that Do2 is dictated largely by capillary hematocrit and the volume density of red blood cell flowing capillaries (23, 29, 58, 62). As β is unlikely to be affected appreciably by exercise training, alterations in O2 extraction will depend on the Do2/Q̇m ratio (62). The significance of the abovementioned relationship is that it provides information regarding alterations in diffusive and conductive components of O2 transport. Given that exercise training is known to improve both Do2 (4, 54, 62) and Q̇m kinetics (65), it can be surmised that trained rats had a relatively greater increase in microvascular Q̇m than in Do2 (i.e., lower Do2/Q̇m ratio, which dictates reduced fractional O2 extraction) across the rest-contractions transient. This analysis suggests that adaptations in conductive (mainly red blood cell flux; fRBC) rather than diffusive capillary mechanisms with exercise training are of relatively greater importance in setting enhanced muscle microvascular oxygenation during metabolic transients as measured herein (Fig. 2 and Table 2). Importantly, the resultant slowed Po2mv kinetics and reduced fractional O2 extraction act to increase the pressure head for O2 diffusion at a time when V̇o2 is rising at its fastest rate and would therefore be expected to improve muscle O2 supply and oxidative function (5, 8, 36, 44, 68).
Effects of altered NO on Po2mv kinetics.
NO and its derivatives modulate multiple physiological processes including muscle hemodynamic and metabolic control (12, 67). More specifically, NO contributes to the increase in muscle Q̇o2 during contractions mainly via endothelium-dependent vasodilation (28, 32, 35, 53) and to the inertia of oxidative metabolism (i.e., finite V̇o2 kinetics) via inhibition of mitochondrial respiration (40, 43). Accordingly, alterations in NO levels modulate the dynamic Q̇o2/V̇o2 matching during metabolic transitions in health and disease (24, 25, 31). The lack of change in citrate synthase activity with training in the present investigation supports that SNP and l-NAME treatments had similar effects on V̇o2 dynamics of sedentary and trained rats. Consequently, this implies that differences in the Po2mv profiles between sedentary and trained rats with SNP and l-NAME resulted primarily from alterations in Q̇o2.
The main effects of altered NO with either SNP or l-NAME were seen during the contraction transient (Figs. 2 and 3, Table 2). Specifically, SNP slowed τ1 to a greater extent in sedentary rats whereas l-NAME abolished the differences in τ1 between sedentary and trained rats that were evident in the control condition (Fig. 3). These results suggest that exercise training increases the contribution of NO to the dynamic Q̇o2/V̇o2 matching and enhances the capacity for O2 flux across metabolic transients in healthy skeletal muscle.
Primary mechanisms for slowed Po2mv kinetics with training.
From the above it becomes apparent that the principal mechanisms improving microvascular oxygenation with exercise training likely involve enhanced NO-mediated regulation of fRBC. Potential candidates include 1) enhanced endothelial-mediated vasodilation (28, 53); and/or 2) enhanced attenuation of sympathetic vasoconstriction (i.e., functional sympatholysis; Ref. 69); and/or 3) training-induced alterations in Q̇m distribution (2, 35) that could attenuate spatial heterogeneities in contracting muscle microvascular oxygenation (45).
Although it must be acknowledged that aged and diseased states also present impairments in muscle Do2, it is interesting to note that these conditions are characterized by reduced NO-mediated function (20, 32, 56) and demonstrate opposite effects on capillary hemodynamics (i.e., impaired fRBC; Refs. 17, 61) and Q̇o2/V̇o2 matching (i.e., faster Po2mv kinetics; Refs. 6, 21) during contractions compared with healthy young individuals. In this context, endurance exercise training has profound clinical implications especially for aged and diseased populations as it constitutes a nonpharmacological therapeutic intervention capable of mitigating microcirculatory deficits.
Vascular control mechanisms (35, 48) and vascular adaptations to exercise training (2, 52) are known to vary according to muscle fiber type composition and oxidative capacity. Consequently, differences might exist in the relative contribution of NO to alterations in gas exchange properties of the microcirculation with training in muscles comprised of distinct fiber types. In this regard, it is noteworthy that the spinotrapezius possesses a mixed fiber type composition and oxidative capacity that resembles the human quadriceps (19, 49), therefore representing a useful analog of human locomotor muscle.
Experimental considerations.
Downhill treadmill running was used herein as a model of endurance exercise training given that this protocol recruits the rat spinotrapezius muscle (41, 57) and may promote training adaptations that include increased V̇o2peak, muscle citrate synthase activity, and resistance to fatigue (30). Accordingly, exercise training protocols that do not recruit the spinotrapezius (e.g., inclined treadmill running) do not induce changes in Po2mv kinetics in healthy young rats (55; cf. Refs. 46, 47). It is important to note that, in the present investigation, trained rats had ∼12% greater V̇o2peak as well as distinct MAP and Po2mv responses following SNP and l-NAME superfusion (Tables 1 and 2, Figs. 2 and 3) compared with sedentary rats, thus providing compelling evidence of a training effect. Furthermore, the markedly slowed Po2mv kinetics (Figs. 2 and 3, Table 2) is consistent with expected adaptations to training. While the lack of change in citrate synthase activity of the spinotrapezius muscle in trained rats is surprising, it suggests that adaptations in vascular control (i.e., improved fRBC as discussed above) likely facilitated the enhanced microvascular oxygenation seen herein during contractions in the trained state. In this sense, potential structural and/or functional vascular adaptations (i.e., ↑flow capacity; the potential for conductive delivery of blood to and from exchange vessels) that occurred independent of alterations in mitochondrial oxidative capacity in trained rats could enhance the dynamic Q̇o2/V̇o2 matching and improve the ability of the microcirculation to support skeletal muscle metabolism.
Eccentric exercise such as downhill running promotes muscle damage that impairs capillary hemodynamics and microvascular O2 transfer during subsequent contractile activity (42; see also Ref. 18). Interestingly, evidence from both human and animal studies indicates that muscle damage from a single bout of eccentric exercise is considerably reduced following repeated bouts as performed herein, and any damage from the first bout(s) would be expected to have ameliorated during the ∼2-mo training period (14, 64). Although unlikely, any potential deleterious effects of eccentric exercise on muscle function would therefore only underestimate the improvements in Po2mv kinetics with training.
Reduced driving pressure with SNP (Table 1) could constrain blood flow dynamics and influence Po2mv kinetics during metabolic transitions. However, previous studies from our laboratory (10) indicate that this effect is negligible when MAP is above ∼70 mmHg as herein.
Summary and conclusions.
Resolution of muscle Po2mv kinetics and their mechanistic bases in the exercise-trained state are essential to understand how muscle microcirculatory plasticity evokes improvements in contractile performance. The present novel findings in healthy skeletal muscle suggest that endurance exercise training enhances microvascular oxygenation during contractions (i.e., slowed Po2mv kinetics) partly via increased NO-mediated function. As mentioned above, enhanced Po2mv during metabolic transitions facilitates blood-myocyte O2 flux to support oxidative phosphorylation and consequently reduces the rate of anaerobic glycolysis and reliance on finite energy sources, all of which likely contribute to improved muscle contractile performance following exercise training (36, 37, 58, 68, 70). Important clinical implications arise from our results considering that aged and patient (e.g., chronic heart failure; CHF) populations are characterized by reduced NO signaling (20, 32, 56), impaired microvascular oxygenation (6, 21), and poor exercise capacity (15, 38). It is noteworthy that CHF patients, for instance, retain considerable plasticity within their skeletal muscle O2 transport system (both convective and diffusive components) in response to exercise training programs (22). Taken together, these observations suggest that exercise training is a powerful nonpharmacological strategy to improve NO-mediated function, thereby likely ameliorating muscle microvascular oxygenation deficits and exercise intolerance in aging and disease states.
GRANTS
This project was supported in part by a Fellowship from the Brazilian Ministry of Education/CAPES-Fulbright and a Doctoral Student Research Grant from the American College of Sports Medicine Foundation to D. M. Hirai; American Heart Association Heartland Affiliate Grant 0750090Z to T. I. Musch; and Kansas State University SMILE Grant and National Institutes of Health Grant HL-108328 to D. C. Poole.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M.H., S.W.C., T.I.M., and D.C.P. conception and design of research; D.M.H., S.W.C., S.K.F., C.T.H., D.J.M., B.J.B., T.I.M., and D.C.P. collected and analyzed data; D.M.H., S.W.C., T.I.M., and D.C.P. interpreted results of experiments; D.M.H. prepared figures; D.M.H. drafted manuscript; D.M.H., S.W.C., S.K.F., C.T.H., D.J.M., B.J.B., T.I.M., and D.C.P. edited and revised manuscript; D.M.H., S.W.C., S.K.F., C.T.H., D.J.M., B.J.B., T.I.M., and D.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. S. Hageman for invaluable technical assistance.
REFERENCES
- 1. Altman PL, Dittmer DS. Biology Data Book. Bethesda, MD: FASEB, 1974 [Google Scholar]
- 2. Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol 246: H59–H68, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol 279: H3131–H3137, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bebout DE, Hogan MC, Hempleman SC, Wagner PD. Effects of training and immobilization on V̇o2 and Do2 in dog gastrocnemius muscle in situ. J Appl Physiol 74: 1697–1703, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol 133: 229–239, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146: 259–268, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Behnke BJ, Kindig CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 283: H926–H932, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Behnke BJ, Kindig CA, Musch TI, Sexton WL, Poole DC. Effects of prior contractions on muscle microvascular oxygen pressure at onset of subsequent contractions. J Physiol 539: 927–934, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Behnke BJ, Padilla DJ, Ferreira LF, Delp MD, Musch TI, Poole DC. Effects of arterial hypotension on microvascular oxygen exchange in contracting skeletal muscle. J Appl Physiol 100: 1019–1026, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Bisset WI, Butler AR, Glidewell C, Reglinski J. Sodium nitroprusside and cyanide release: reasons for re-appraisal. Br J Anaesth 53: 1015–1018, 1981 [DOI] [PubMed] [Google Scholar]
- 12. Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta 1411: 351–369, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Butler AR, Glidewell C, McGinnis J, Bisset WI. Further investigations regarding the toxicity of sodium nitroprusside. Clin Chem 33: 490–492, 1987 [PubMed] [Google Scholar]
- 14. Byrnes WC, Clarkson PM, White JS, Hsieh SS, Frykman PN, Maughan RJ. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol 59: 710–715, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol 28: 1092–1102, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Copp SW, Davis RT, Poole DC, Musch TI. Reproducibility of endurance capacity and V̇o2peak in male Sprague-Dawley rats. J Appl Physiol 106: 1072–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvasc Res 77: 113–119, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Davies RC, Eston RG, Poole DC, Rowlands AV, DiMenna F, Wilkerson DP, Twist C, Jones AM. Effect of eccentric exercise-induced muscle damage on the dynamics of muscle oxygenation and pulmonary oxygen uptake. J Appl Physiol 105: 1413–1421, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Didion SP, Mayhan WG. Effect of chronic myocardial infarction on in vivo reactivity of skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 272: H2403–H2408, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Diederich ER, Behnke BJ, McDonough P, Kindig CA, Barstow TJ, Poole DC, Musch TI. Dynamics of microvascular oxygen partial pressure in contracting skeletal muscle of rats with chronic heart failure. Cardiovasc Res 56: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 58: 1353–1362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Federspiel WJ, Popel AS. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res 32: 164–189, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol (Oxf) 186: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Ferreira LF, Poole DC, Barstow TJ. Muscle blood flow-O2 uptake interaction and their relation to on-exercise dynamics of O2 exchange. Respir Physiol Neurobiol 147: 91–103, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Friederich JA, Butterworth JF. Sodium nitroprusside: twenty years and counting. Anesth Analg 81: 152–162, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Groebe K, Thews G. Calculated intra- and extracellular Po2 gradients in heavily working red muscle. Am J Physiol Heart Circ Physiol 259: H84–H92, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Hahn SA, Ferreira LF, Williams JB, Jansson KP, Behnke BJ, Musch TI, Poole DC. Downhill treadmill running trains the rat spinotrapezius muscle. J Appl Physiol 102: 412–416, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Hirai DM, Copp SW, Ferreira LF, Musch TI, Poole DC. Nitric oxide bioavailability modulates the dynamics of microvascular oxygen exchange during recovery from contractions. Acta Physiol (Oxf) 200: 159–169, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Hirai DM, Copp SW, Hageman KS, Poole DC, Musch TI. Aging alters the contribution of nitric oxide to regional muscle hemodynamic control at rest and during exercise in rats. J Appl Physiol 111: 989–998, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Hirai DM, Copp SW, Herspring KF, Ferreira LF, Poole DC, Musch TI. Aging impacts microvascular oxygen pressures during recovery from contractions in rat skeletal muscle. Respir Physiol Neurobiol 169: 315–322, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Hirai DM, Copp SW, Schwagerl PJ, Musch TI, Poole DC. Acute effects of hydrogen peroxide on skeletal muscle microvascular oxygenation from rest to contractions. J Appl Physiol 110: 1290–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol 77: 1288–1293, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD. Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73: 728–736, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984 [DOI] [PubMed] [Google Scholar]
- 38. Inbar O, Oren A, Scheinowitz M, Rotstein A, Dlin R, Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20- to 70-yr-old men. Med Sci Sports Exerc 26: 538–546, 1994 [PubMed] [Google Scholar]
- 39. Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol Heart Circ Physiol 239: H443–H449, 1980 [DOI] [PubMed] [Google Scholar]
- 40. Jones AM, Wilkerson DP, Koppo K, Wilmshurst S, Campbell IT. Inhibition of nitric oxide synthase by l-NAME speeds phase II pulmonary V̇o2 kinetics in the transition to moderate-intensity exercise in man. J Physiol 552: 265–272, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kano Y, Padilla D, Hageman KS, Poole DC, Musch TI. Downhill running: a model of exercise hyperemia in the rat spinotrapezius muscle. J Appl Physiol 97: 1138–1142, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Kano Y, Padilla DJ, Behnke BJ, Hageman KS, Musch TI, Poole DC. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J Appl Physiol 99: 1516–1522, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Kindig CA, McDonough P, Erickson HH, Poole DC. Nitric oxide synthase inhibition speeds oxygen uptake kinetics in horses during moderate domain running. Respir Physiol Neurobiol 132: 169–178, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol 92: 2513–2520, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Lash JM. Exercise training enhances adrenergic constriction and dilation in the rat spinotrapezius muscle. J Appl Physiol 85: 168–174, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol 72: 2052–2062, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Laughlin MH, Armstrong RB. Adrenoreceptor effects on rat muscle blood flow during treadmill exercise. J Appl Physiol 62: 1465–1472, 1987 [DOI] [PubMed] [Google Scholar]
- 49. Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280: R441–R447, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Lo LW, Vinogradov SA, Koch CJ, Wilson DF. A new, water soluble, phosphor for oxygen measurements in vivo. Adv Exp Med Biol 428: 651–656, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Macdonald M, Pedersen PK, Hughson RL. Acceleration of V̇o2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol 83: 1318–1325, 1997 [DOI] [PubMed] [Google Scholar]
- 52. McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol 98: 753–761, 2005 [DOI] [PubMed] [Google Scholar]
- 53. McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab 33: 173–178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McAllister RM, Terjung RL. Training-induced muscle adaptations: increased performance and oxygen consumption. J Appl Physiol 70: 1569–1574, 1991 [DOI] [PubMed] [Google Scholar]
- 55. McCullough DJ, Davis RT, 3rd, Dominguez JM, 2nd, Stabley JN, Bruells CS, Behnke BJ. Effects of aging and exercise training on spinotrapezius muscle microvascular PO2 dynamics and vasomotor control. J Appl Physiol 110: 695–704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Musch TI, Poole DC. Blood flow response to treadmill running in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 271: H2730–H2734, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Poole DC. Influence of exercise training on skeletal muscle oxygen delivery and utilization. In: The Lung: Scientific Foundations, edited by Crystal RG, West JB, Weibel ER, Barnes PJ. New York: Raven, 1997, p. 1957–1967 [Google Scholar]
- 59. Poole DC, Behnke BJ, McDonough P, McAllister RM, Wilson DF. Measurement of muscle microvascular oxygen pressures: compartmentalization of phosphorescent probe. Microcirculation 11: 317–326, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Poole DC, Wagner PD, Wilson DF. Diaphragm microvascular plasma Po2 measured in vivo. J Appl Physiol 79: 2050–2057, 1995 [DOI] [PubMed] [Google Scholar]
- 61. Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol 95: 1055–1062, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at V̇o2 max. J Appl Physiol 73: 1067–1076, 1992 [DOI] [PubMed] [Google Scholar]
- 63. Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241: 1649–1651, 1988 [DOI] [PubMed] [Google Scholar]
- 64. Schwane JA, Armstrong RB. Effect of training on skeletal muscle injury from downhill running in rats. J Appl Physiol 55: 969–975, 1983 [DOI] [PubMed] [Google Scholar]
- 65. Shoemaker JK, Phillips SM, Green HJ, Hughson RL. Faster femoral artery blood velocity kinetics at the onset of exercise following short-term training. Cardiovasc Res 31: 278–286, 1996 [PubMed] [Google Scholar]
- 66. Srere PA. Citrate synthase. In: Methods in Enzymology 13: 3–11, 1969 [Google Scholar]
- 67. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Stary CM, Hogan MC. Effect of varied extracellular Po2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tonkonogi M, Sahlin K. Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev 30: 129–137, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Whyte JJ, Laughlin MH. The effects of acute and chronic exercise on the vasculature. Acta Physiol (Oxf) 199: 441–450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]