Abstract
The development of intrapulmonary shunts with increased cardiac output during exercise in healthy humans has been reported in several recent studies, but mechanisms governing their recruitment remain unclear. Dobutamine and dopamine are inotropes commonly used to augment cardiac output; however, both can increase venous admixture/shunt fraction (Qs/Qt). It is possible that, as with exercise, intrapulmonary shunts are recruited with increased cardiac output during dobutamine and/or dopamine infusion that may contribute to the observed increase in Qs/Qt. The purpose of this study was to examine how dobutamine and dopamine affect intrapulmonary shunt and gas exchange. Nine resting healthy subjects received serial infusions of dobutamine and dopamine at incremental doses under normoxic and hyperoxic (inspired O2 fraction = 1.0) conditions. At each step, alveolar-to-arterial Po2 difference (A-aDo2) and Qs/Qt were calculated from arterial blood gas samples, intrapulmonary shunt was evaluated using contrast echocardiography, and cardiac output was calculated by Doppler echocardiography. Both dobutamine and dopamine increased cardiac output and Qs/Qt. Intrapulmonary shunt developed in most subjects with both drugs and paralleled the increase in Qs/Qt. A-aDo2 was unchanged due to a concurrent rise in mixed venous oxygen content. Hyperoxia consistently eliminated intrapulmonary shunt. These findings contribute to our present understanding of the mechanisms governing recruitment of these intrapulmonary shunts as well as their impact on gas exchange. In addition, given the deleterious effect on Qs/Qt and the risk of neurological complications with intrapulmonary shunts, these findings could have important implications for use of dobutamine and dopamine in the clinical setting.
Keywords: inotropes, shunt, gas exchange
the recruitment of intrapulmonary (I-P) shunts during exercise has been a topic of recent interest (4, 19–21, 41, 42). Studies using both contrast echocardiography(4, 20, 41, 42) and 99mTc macroaggregated albumin (19) suggest that exercise opens previously closed large-diameter vessels (i.e., I-P shunt vessels) within the pulmonary vasculature, which would allow particles that would otherwise be trapped in the normal pulmonary capillaries to bypass the capillary network. Morphological studies documenting I-P shunts in previously healthy human cadavers (43, 44), the passage of microspheres through healthy human lungs (22), and the demonstration of similar inducible shunts in healthy exercising dogs (40) support the human exercise data. The anatomical basis and physiological significance of these I-P shunts, especially with regard to their impact on gas exchange, remain unclear (9, 10, 17, 18), as do the mechanisms governing their recruitment. Both Stickland et al. and La Gerche et al. demonstrated an association between I-P shunt recruitment and cardiac output during exercise (15, 42), but it is unclear whether the increase in cardiac output per se results in I-P shunt recruitment or whether the I-P shunts are an adaptive mechanism to decrease right ventricular afterload, thus allowing for greater augmentation of cardiac output (42).
Dobutamine and dopamine are inotropes commonly used to increase cardiac output, but both agents can have a detrimental effect on pulmonary gas exchange. In critically ill human subjects, both inotropes have been shown to increase venous admixture/shunt fraction (Qs/Qt) (28, 31) and impair ventilation-perfusion (VA/Q) matching, whereas an increase in true right-to-left shunt (i.e., VA/Q = 0), as demonstrated by the retention of inert gas, has been observed with dopamine (31). Of note, these studies were typically conducted on patients in intensive care, and, therefore, the effect of these inotropes on humans with healthy cardiopulmonary function is unclear. Given the association between I-P shunt recruitment and cardiac output during incremental exercise, it is possible that I-P shunts are similarly recruited with increases in cardiac output due to dobutamine and/or dopamine infusion negatively affecting gas exchange.
The purpose of this study was to investigate the effects of dobutamine and dopamine on intrapulmonary shunts as assessed by contrast echocardiography in healthy humans. It was hypothesized that both drugs would result in the recruitment of I-P shunt, with a corresponding impairment in gas exchange, measured by the alveolar to arterial Po2 difference (A-aDo2) and shunt fraction (Qs/Qt).
METHODS
The study received approval from the University of Alberta Health Research Ethics Board, and all participants provided written, informed consent to participate.
Subjects
Eleven healthy subjects without evidence of active cardiopulmonary disease on history, physical exam, pulmonary function tests, and screening cardiopulmonary exercise tests were enrolled. Two subjects with a significant intrapulmonary shunt (shunt score > 1; see below) on screening contrast echocardiogram were excluded. The final sample included nine subjects (6 men; 25–36 yr old) with mean (SD) baseline characteristics: forced expiratory volume in first second (FEV1) of 4.2 (0.8) liters [104 (13)% predicted], FEV1/FVC of 78 (12), lung diffusion capacity for carbon monoxide (DlCO) (seated) of 35 (6) ml·mmHg−1·min−1 [112 (12)% predicted], and maximum oxygen consumption of 52 (11) ml·kg−1·min−1.
Experimental Trial
Subject preparation.
A standard intravenous (IV) catheter (Smiths Medical Canada, Markham, ON, Canada) was inserted into an antecubital vein and attached via 6-in. extension tubing to two three-way stopcocks. The proximal stopcock was used for inotrope infusion, the distal stopcock for agitation/injection of saline contrast for contrast echocardiography. An 18-gauge angiocatheter (Becton-Dickinson, Mississauga, ON, Canada) was then inserted into the ipsilateral radial artery using local anesthesia (1% lidocaine HCl, AstraZeneca, Mississauga, ON, Canada). Patency of the arterial catheters was maintained with a pressurized flush system of normal saline.
Experimental protocol.
The entire protocol was performed with the subjects at rest in the supine position. Baseline control data were obtained after the subject had been resting for ≥5 min. O2 (100%) was then administered for 2 min using a non-rebreathing valve (Hans-Rudolph, 2700, Shawnee, KS), with data collection repeated 1 min into hyperoxia. Following a return to room air, the first inotrope infusion (dobutamine or dopamine) was initiated. The order of drug administration was randomly determined. Incremental inotrope doses were administered using an automatic IV infusion pump (Alaris, San Diego, CA): dopamine HCL (Hospira, Lake Forest, IL) at 2, 6, and 10 μg·kg−1·min−1, and dobutamine (Sandoz Canada, Quebec, Canada) at 2.5, 5, and 10 μg·kg−1·min−1. After a 4-min wash-in period on the lowest dose to account for IV dead space, inotropes were infused for 5 min at each stage before data collection to ensure a steady state. Following data collection at each stage on room air, subjects breathed 100% O2 for 2 min with measurements repeated 1 min into the hyperoxia. Hyperoxia was then discontinued, and the dose of drug was increased. After the first inotrope infusion was completed, a 20-min washout period was given before the second infusion was initiated, since both drugs have a reported half-life of <10 min (2).
Cardiorespiratory and Body Temperature Measures
Respiratory gas-exchange data were collected using a metabolic measurement system (Encore229 Vmax, SensorMedics, Yorba Linda, CA). DlCO was determined at baseline and on the highest dose of each inotrope using the single-breath breath-holding method (24) (Encore V62J Autobox, SensorMedics). Arterial blood samples were drawn from the arterial catheter and analyzed immediately (ABL800 FLEX Radiometer, Loveland, CO) with correction for temperature, measured by a core temperature pill (VitalSense, Bend, OR) ingested at the start of the trial. Alveolar Po2 (PaO2) was calculated using the alveolar gas equation {PaO2 = [FiO2 × (PB − PH2O) − (PaCO2/RQ)] + FiO2 × PaCO2 × (1 − R)/R} with water vapor pressure corrected for temperature. Standard formulas were used to calculate alveolar to arterial Po2 difference (A-aDo2) and shunt fraction (Qs/Qt). Of note, to account for any change in alveolar ventilation affecting Qs/Qt, end-capillary oxygen content (CcO2) for each condition was calculated assuming end-capillary Po2 was equivalent to PaO2 at that condition. Central venous oxygen content (CvO2) was calculated using the Fick equation [V̇o2 = Q × (CaO2 − CvO2)] with measured values for oxygen consumption (V̇o2) and arterial oxygen content (CaO2), and calculated values for cardiac output (Q).
Echocardiograms were performed by one experienced sonographer (Vivid 7, GE) and recorded onto DVDs that were later analyzed in triplicate by a cardiologist who was blinded to experimental conditions. Stroke volume (SV) was calculated using the average of five consecutive measured velocity time integrals (VTI) in the left ventricular (LV) outflow tract (LVOT): SV = LVOT × VTI. Q was then calculated as the product of SV and heart rate. To estimate LV systolic function, fractional shortening (FS) was calculated using LV end-diastolic (LVED) and end-systolic (LVES) dimensions obtained from the parasternal long axis view [FS = (LVED-LVES)/LVED × 100]. Pulmonary artery systolic pressure (PASP) was estimated by adding the trans-tricuspid pressure gradient (TR gradient) and the right atrial pressure (RAP). TR gradient was estimated from the tricuspid regurgitation velocity (TR gradient = 4 × tricuspid regurgitation velocity2) and right atrial pressure (RAP) using the inspiratory collapse of the inferior vena cava (IVC) (29). In cases where the tricuspid regurgitation jet was suboptimal agitated saline contrast was injected through the antecubital IV catheter to enhance the signal.
Contrast Echocardiography
The agitated saline contrast echocardiography technique was used to detect intracardiac and intrapulmonary shunt. Standard procedures were employed for the injection of solution (48). Briefly, 10 ml of saline was combined with 0.5 ml of air, and the solution was forcefully agitated through a three-way stopcock between two syringes to form fine suspended bubbles, which are generally much larger than the pulmonary capillaries (48). The solution was injected through the antecubital intravenous catheter, while the sonographer imaged all four chambers of the heart. Intracardiac shunt was determined by contrast appearance in the LV in <5 cardiac cycles; LV contrast after ≥5 cardiac cycles suggests I-P shunt (48).
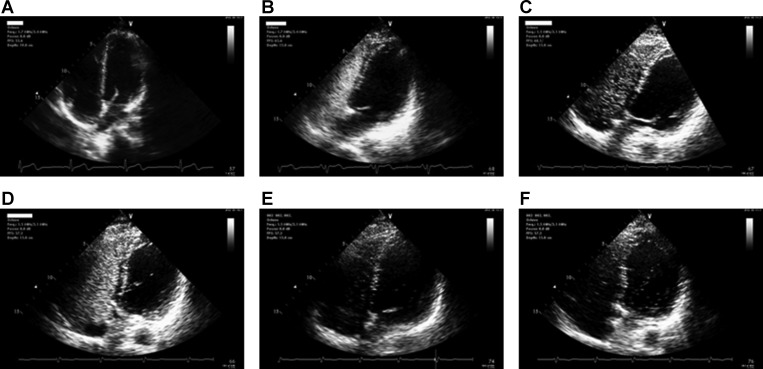
Representative saline contrast echocardiograms performed on a subject during incremental dobutamine infusion are shown in Fig. 1. I-P shunt was qualitatively scored based on a modification of a previously described scoring system (21). No contrast bubbles in the left ventricle in any frame received a score of 0, ≤3 bubbles a score of 1, 4–12 bubbles a score of 2, and >12 bubbles a score of 3. For the purpose of our study, we considered a shunt score of >1 “significant” shunt, consistent with studies showing small I-P shunt (1–4 bubbles) in up to 25% of healthy subjects, compared with only 3% with moderate or larger shunt (≥5 bubbles) (49). High intra-observer reliability of contrast echocardiography during high cardiac output conditions has been previously demonstrated (42), and periodic reviews of the images by the cardiologist in the present study confirmed consistent scoring.
Fig. 1.
Representative saline contrast echocardiograms on a 35-yr-old subject at baseline and during incremental drug (dobutamine) infusion. Top: echocardiograms at baseline. A: precontrast. B: <5 cardiac cycles. C: >5 cardiac cycles. No intracardiac/pulmonary shunt was visualized. Bottom: echocardiograms during drug infusion. D: 2.5 μg·kg−1·min−1 (shunt score =1). E: 5 μg·kg−1·min−1 (shunt score = 2). F: 10 μg·kg−1·min−1 (shunt score = 3).
Statistical Analysis
For all inferential analyses, the probability of type I error was set at 0.05. Group data for each variable are expressed as means ± SE. Data were analyzed with a two-way repeated-measures ANOVA. Where main effects were found, Fisher's least significant difference post hoc tests were used.
RESULTS
Dobutamine
Cardiac response.
Grouped hemodynamic responses are presented in Table 1 and Figs. 2 and 3. Dobutamine increased Q and PASP above baseline at doses of ≥5 μg·kg−1·min−1. SV increased at all stages of dobutamine infusion, whereas a significant increase in heart rate and fractional shortening was seen only at the highest dose.
Table 1.
Mean hemodynamic responses at baseline and during drug infusion (n = 9)
| Dobutamine, μg · kg−1 · min−1 |
Dopamine, μg · kg−1 · min−1 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dobutamine Baseline | 2.5 | 5 | 10 | Dopamine Baseline | 2 | 6 | 10 | |
| Cardiac output, liters/min | 6.02 ± 0.46 | 6.72 ± 0.46 | 7.66 ± 0.62* | 8.95 ± 0.73* | 5.71 ± 0.28 | 5.91 ± 0.52 | 6.49 ± 0.97 | 7.45 ± 0.90* |
| Stroke volume, ml | 100 ± 9 | 111 ± 10* | 121 ± 10* | 128 ± 12* | 95 ± 6 | 96 ± 8 | 100 ± 10 | 110 ± 10* |
| Heart rate, beats/min | 62 ± 2 | 62 ± 2 | 64 ± 3 | 71 ± 3* | 60 ± 3 | 62 ± 2 | 64 ± 3 | 68 ± 3 |
| Fractional shortening | 36 ± 2 | 38 ± 2 | 42 ± 2 | 49 ± 3* | 42 ± 3 | 42 ± 3 | 38 ± 2 | 42 ± 3 |
| Mean arterial pressure, mmHg | 88 ± 3 | 88 ± 3 | 93 ± 3* | 94 ± 3* | 85 ± 2 | 83 ± 2 | 85 ± 3 | 90 ± 2* |
Values are means ± SE.
Significant difference vs. baseline (no drug) (P < 0.05).
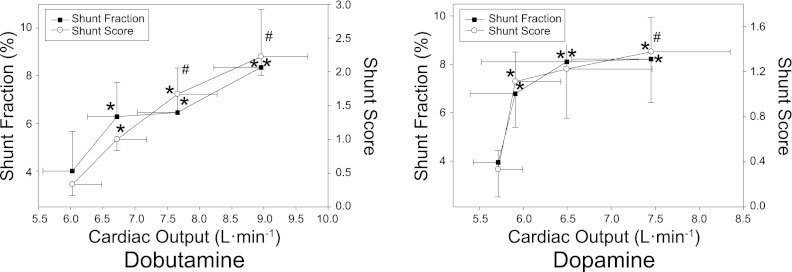
Fig. 2.
Means ± SE physiological shunt fraction (Qs/Qt) and intrapulmonary shunt as assessed by contrast echocardiography (shunt score) in relation to cardiac output at baseline and during incremental dobutamine (left) or dopamine (right) infusion. *Significant difference vs. baseline for shunt fraction/shunt score (P < 0.05). #Significant difference vs. baseline for cardiac output (P < 0.05).
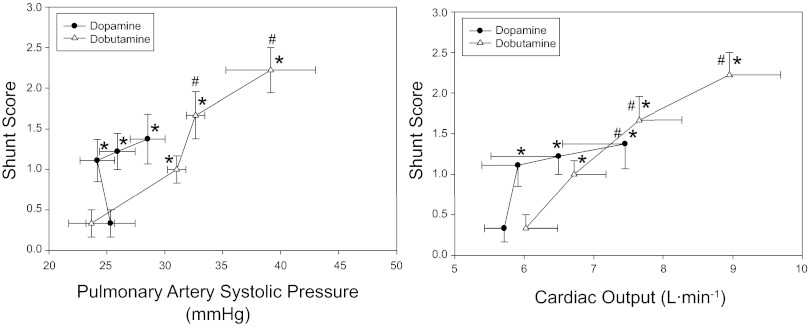
Fig. 3.
Means ± SE intrapulmonary shunt score in relation to pulmonary artery systolic pressure (left) and cardiac output (right) at baseline and during inotrope infusion. *Significant difference vs. baseline for shunt score (P < 0.05). #Significant difference vs. baseline for PASP/cardiac output (P < 0.05).
Pulmonary gas exchange and physiological shunt.
Group gas exchange and mixed venous data are shown in Table 2. Gas exchange, as assessed by A-aDo2, did not change with dobutamine infusion. There was a small but significant increase in V̇o2 from baseline at 5 and 10 μg·kg−1·min−1. Since there was a greater proportional increase in Q relative to V̇o2 at 10 μg·kg−1·min−1, calculated CvO2 was increased from baseline. Qs/Qt was increased from baseline at all stages of dobutamine infusion (Fig. 2).
Table 2.
Mean gas exchange and mixed venous data at baseline and during drug infusion (n = 9)
| Dobutamine, μg · kg−1 · min−1 |
Dopamine, μg · kg−1 · min−1 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dobutamine Baseline | 2.5 | 5 | 10 | Dopamine Baseline | 2 | 6 | 10 | |
| PaO2, Torr | 85 ± 2 | 87 ± 1 | 90 ± 2 | 92 ± 2* | 84 ± 2 | 80 ± 2 | 76 ± 2* | 85 ± 3 |
| SaO2, % | 97 ± 0.3 | 97 ± 0.3 | 97 ± 0.2* | 98 ± 0.3* | 96 ± 0.3 | 96 ± 0.3 | 95 ± 0.3* | 97 ± 0.3 |
| PaCO2, Torr | 37 ± 0.7 | 37 ± 1 | 36 ± 31 | 34 ± 1* | 38 ± 1 | 39 ± 1* | 40 ± 0.7* | 38 ± 1.7 |
| A-aDO2, Torr | 4.6 ± 0.9 | 6.0 ± 0.7 | 4.7 ± 0.3 | 5.2 ± 1.3 | 4.9 ± 1 | 6.7 ± 1.5 | 7.6 ± 1.5* | 6.3 ± 1.6 |
| DLCO, ml · min−1 · mmHg−1 | 40 ± 2 | N/A | N/A | 38 ± 2 | 40 ± 2 | N/A | N/A | 38 ± 3 |
| V̇o2, l/min | 0.32 ± 0.01 | 0.34 ± 0.01 | 0.38 ± 0.02* | 0.37 ± 0.01* | 0.33 ± 0.02 | 0.34 ± 0.02 | 0.36 ± 0.02 | 0.35 ± 0.02 |
| CvO2, ml O2/dl | 12.1 ± 0.8 | 12.4 ± 0.6 | 12.6 ± 0.6 | 13.7 ± 0.6* | 11.7 ± 0.5 | 11.1 ± 0.7 | 10.8 ± 0.6 | 12.7 ± 0.7* |
Values are means ± SE.
Significant difference vs. baseline (P < 0.05). PaO2 and PaCO2, partial pressure of oxygen and carbon dioxide, respectively, in arterial blood; SaO2, arterial blood oxygen saturation; A-aDO2, alveolar to arterial Po2 difference; DLCO, diffusion capacity for carbon monoxide; CvO2, central venous oxygen content. CvO2 was calculated rather than measured, as outlined above.
I-P shunt.
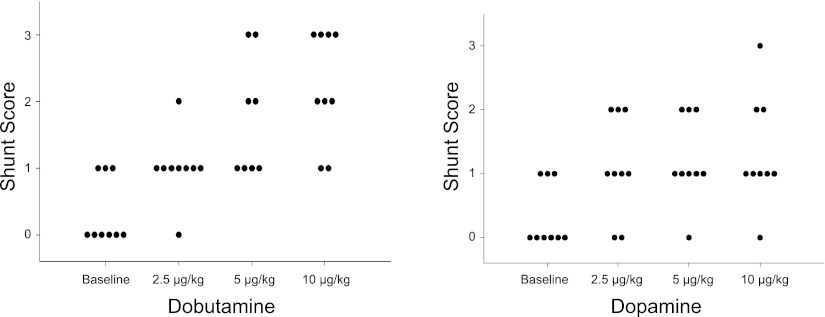
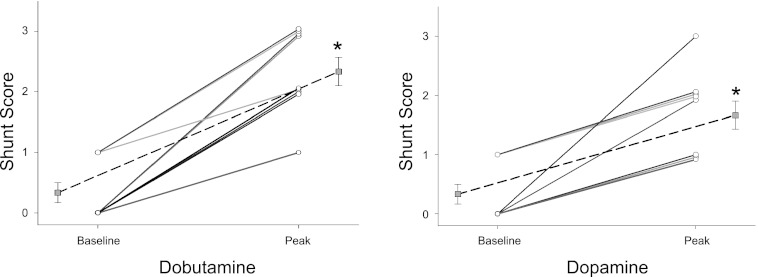
The effect of incremental dobutamine on individual I-P shunt, and peak individual shunt score during dobutamine infusion are shown in Figs. 4 and 5, respectively. An increase in shunt score was seen in all subjects, with eight of nine subjects demonstrating significant shunt (Fig. 5). Mean shunt score was increased from baseline at all stages of dobutamine infusion (Figs. 2 and 3); grouped data indicate that the increase in shunt score parallels the increase in Q, PASP, and Qs/Qt (Figs. 2 and 3).
Fig. 4.
The effect of incremental drug infusion on intrapulmonary shunt score. Left: dobutamine. Right: dopamine.
Fig. 5.
The effect of incremental drug infusion on peak individual shunt score. Left: dobutamine. Right: dopamine. Dotted lines represent mean shunt score. *Significant difference vs. baseline (P < 0.05).
Hyperoxia.
Hyperoxia consistently eliminated significant I-P shunt at all stages of dobutamine infusion (mean shunt score of <1). Q was decreased across all doses by an average of 901 ml·min−1 (P < 0.01) with hyperoxia. No consistent change was seen in PASP with hyperoxia.
Dopamine
Cardiac response.
Grouped hemodynamic responses are presented in Table 1 and Figs. 2 and 3. A significant increase in Q and SV from baseline was observed only at the highest dose of dopamine (10 μg·kg−1·min−1), whereas no change was seen in heart rate or fractional shortening. PASP did not change significantly with dopamine infusion (Fig. 3).
Pulmonary gas exchange and physiological shunt.
Gas exchange, as assessed by A-aDo2, did not change with dopamine infusion (Table 2). Low dose dopamine (2 μg·kg−1·min−1) resulted in hypoventilation (increased PaCO2, decreased PaO2), an effect that was not seen at the highest dose. CvO2 increased from baseline at the highest dose of dopamine. An increase in Qs/Qt from baseline was seen at all stages of dopamine infusion (Fig. 2).
I-P shunt.
The effects of dopamine infusion on individual I-P shunt and peak individual shunt score are shown in Figs. 4 and 5, respectively. An increase in I-P shunt score occurred in all subjects during dopamine infusion, with five of nine subjects developing significant I-P shunt (Fig. 5). Mean shunt score was increased from baseline at all stages of dopamine infusion and paralleled the increase in Qs/Qt (Fig. 2). Of note, two subjects developed significant shunt with low-dose dopamine (2–6 μg·kg−1·min−1) that was not seen at higher doses (10 μg·kg−1·min−1). In addition, low-dose dopamine (2–6 μg·kg−1·min−1) caused a significant increase in mean shunt score without a corresponding increase in Q or PASP (Fig. 3).
Hyperoxia.
Hyperoxia consistently eliminated significant I-P shunt in all subjects (mean shunt score = 0). Q was decreased by an average of 426 ml·min−1 (P = 0.03) across all doses with hyperoxia. No change in PASP was observed with hyperoxia.
DISCUSSION
This study examined the effects of dobutamine and dopamine on gas exchange, Qs/Qt, and I-P shunt. The majority of subjects developed significant I-P shunt, as assessed by agitated saline contrast echocardiography during inotrope infusion with a corresponding increase in Qs/Qt. These results suggest that the recruitment of I-P shunts with increasing cardiac output may be contributing to the increase in Qs/Qt commonly observed with inotropic agents.
The increase in Qs/Qt during drug infusion in this study is in keeping with previous studies in patients demonstrating an increase in shunt fraction with increased Q during both dopamine (12, 14, 31) and dobutamine (28, 31) infusions. The correlation between Qs/Qt and Q has also been observed in pigs (34) and in dogs when Q was altered by both pharmacological and nonpharmacological means (23, 38). Although it is generally accepted that Qs/Qt may worsen with increased Q during inotrope infusion, the effects of dopamine and dobutamine on Qs/Qt and I-P shunt in healthy humans with normal cardiopulmonary function have not been previously examined.
The development of I-P shunt with increased Q in this study is consistent with previous studies demonstrating I-P shunt recruitment during conditions associated with increased Q, such as exercise (4, 40–42) or hypoxia (20). In this study, the hemodynamic responses and pattern of I-P shunt recruitment (and correspondingly the Qs/Qt response) differed between the two inotropes. Dobutamine had a more pronounced effect on both Q and PASP, and the increase in mean shunt score and Qs/Qt appeared to parallel the increase in Q and PASP (Figs. 2 and 3). In contrast, mean shunt score and Qs/Qt increased during dopamine infusion without a significant change in PASP, and low-dose dopamine (2–6 μg·kg−1·min−1) caused an increase in mean shunt score and Qs/Qt with little change in Q. The divergent response observed with dobutamine vs. dopamine suggests that I-P shunt recruitment may be occurring via different mechanisms with these two inotropes.
Mechanism of I-P Shunt Recruitment
The ability of the pulmonary vasculature to limit right ventricular afterload despite large increases in Q has been attributed to a drop in pulmonary vascular resistance (PVR) through the classic flow-dependent mechanisms of capillary distension (6) and recruitment (46). The association between I-P shunt, Qs/Qt, and increased Q in the present and previous studies suggests a similar flow-dependent mechanism in I-P shunt recruitment. Several investigators, however, have suggested that dobutamine and/or dopamine may have direct vasodilatory effects on the pulmonary vasculature that could also contribute to the development of I-P shunt. Dobutamine has a variable effect on PVR in humans (28, 36, 50), but when pulmonary blood flow is kept constant during dobutamine infusion, PVR does not appear to change (16). The effect of dopamine on PVR has also been variable in human (12, 14, 28, 31, 36, 50) and animal (5, 16, 27) studies. However, unlike dobutamine, which stimulates mainly β1-adrenergic receptors (2), the physiological effect of dopamine is dose dependent: low doses of dopamine act mainly on dopaminergic (vasodilatory) receptors, whereas moderate to high doses affect β1- and α-adrenergic receptors (2). Dopamine receptors have been identified within the pulmonary vasculature (32), and dopaminergic stimulation causes pulmonary arterial relaxation that is attenuated by dopamine antagonists (11, 30). In the present study, the linear relationship between mean I-P shunt score, Qs/Qt, and Q during dobutamine infusion supports a flow-dependent mechanism of shunt recruitment, similar to normal pulmonary capillary recruitment. In contrast, low-dose dopamine increased mean shunt score and Qs/Qt without changing Q, and two subjects developed significant I-P shunt with low-dose dopamine that was not seen at higher doses. This suggests that dopamine may be affecting I-P shunt through flow-independent means, possibly via dopaminergic receptors within the pulmonary circulation that would be activated by low-dose dopamine. Of note, increased levels of circulating dopamine have been observed during exercise in humans (8); it is possible, therefore, that dopamine-mediated pulmonary vasodilation is contributing to the recruitment of previously closed I-P shunt vessels during exercise, supporting the theory that these vessels are anatomically distinct from the normal pulmonary capillary network. Further studies are needed to determine the role of dopaminergic receptors in I-P shunt recruitment.
Consistent with the findings of Lovering et al. (21), hyperoxia consistently eliminated significant I-P shunt. As in previous studies (7), Q decreased with hyperoxia due to the increase in CaO2 allowing for a lower Q to maintain the same oxygen delivery. Although the reduction in Q could in part explain the decrease in I-P shunt, significant shunt was seen at a similar cardiac output under normoxic conditions, suggesting that hyperoxia is affecting I-P shunt through additional flow-independent mechanisms. The means by which oxygen tension alters I-P shunt recruitment remains unclear and warrants further investigation.
Implications
The physiological significance of these inducible I-P shunts and their impact on gas exchange remains highly controversial (9, 10, 17, 18). In the present study, a detrimental effect on gas exchange was suggested by the parallel increase in Qs/Qt and mean I-P shunt score; however, ventilation and perfusion were not measured, and thus the extent to which VA/Q mismatch affected Qs/Qt cannot be determined. Increased cardiac output during inotrope infusion can result in increased flow to poorly ventilated regions (low VA/Q) (25, 31), which alone could explain the increase in Qs/Qt observed during dobutamine and dopamine infusion. However, in contrast to earlier work, this study examined the effects of dobutamine and dopamine on Qs/Qt in healthy subjects in whom low VA/Q regions would be expected to be minimal and demonstrated a parallel increase in Qs/Qt and I-P shunt. Furthermore, previous exercise studies in healthy subjects have suggested a relationship between I-P shunt recruitment and worsening A-aDo2 (20, 42), lending support to the theory that the I-P shunts are contributing to impaired gas exchange. Of note, although in the present study a parallel increase in Qs/Qt and mean shunt score was observed, A-aDo2 did not increase. The lack of effect on A-aDo2 can be explained by the increase in CvO2 secondary to increased cardiac output with dobutamine and dopamine infusion and thus a reduction in the “entrance difference” (i.e., PaO2 − PvO2), which by itself would decrease A-aDo2 (35). Alternately, or in addition, rather than functioning as true right-to-left shunts (VA/Q = 0), these vessels may participate in limited gas exchange (39).
Whether pure venous admixture (true right-to-left shunt) occurs with these drugs remains unclear. Russell et al. (34) showed an increase in Qs/Qt with both dobutamine and dopamine in pigs ventilated with 100% O2. Importantly, 100% O2 would abolish the effect of diffusion limitation and low VA/Q lung units on Qs/Qt, indicating that the increase in Qs/Qt is instead the result of right-to-left shunt. Furthermore, an increase in true shunt (VA/Q = 0), as measured by inert gas techniques, has been seen with dopamine infusion in critically ill patients (31). Combined, these observations would suggest that pure right-to-left shunt may develop with these drugs; however, further studies in healthy subjects using concurrent inert gas techniques are needed.
In addition to gas exchange, the pulmonary vasculature plays an important role as a biological sieve to prevent various venous emboli from entering the systemic circulation. As an example, patients with known pulmonary arteriovenous malformations are at increased risk of paradoxical thrombotic and septic emboli (37). ICU patients, who often receive inotropes as part of clinical care, are also at increased risk of neurological complications, including ischemic stroke (1) and intracerebral infection (13). Although a specific relationship to inotrope use has not been studied in these patients, the recruitment of I-P shunt with dobutamine and/or dopamine could have important repercussions on the efficacy of the lung as a biological filter.
Methodological Considerations
Agitated saline contrast echocardiography is a standard technique used to assess intracardiac and intrapulmonary shunt with high intra- and interobserver reliability both at rest and during exercise (15, 42). However, it has several major limitations that have been addressed at length in previous studies (4, 20, 42). Although contrast grading scales have been proposed for research purposes (15, 21), the technique is not fully quantitative. Because the exact size of the contrast bubbles is unknown, small bubbles passing through normal pulmonary capillaries could appear in the LV; however, this is unlikely given that bubbles of <10 μm in diameter survive <200 ms in static blood (26) and in nonstatic conditions would dissolve faster due to increased fluid pressure (45) and flow velocity (51). Even during intense exercise, mean pulmonary capillary transit time does not fall below 450 ms (47), making it highly unlikely that bubbles small enough to pass through normal pulmonary capillaries would survive long enough to be visualized in the LV during inotrope infusion. Physiological capillary distension due to increased perfusion pressure could allow larger bubbles to traverse the pulmonary circulation. However, Glazier et al. (6) demonstrated that, even with perfusion pressures up to 100 cmH2O, maximum capillary diameter did not exceed 13 μm, making this an unlikely explanation for the appearance of LV contrast. Increased perfusion pressures could also cause deformation of larger contrast bubbles that would allow for their passage through normal pulmonary capillaries, but Roelandt demonstrated that, even with injection through a firmly wedged Swan-Ganz catheter, an injection pressure of 300 Torr, much higher than any physiological PASP, was needed to observe contrast in the LV (33). As noted above, we saw I-P shunt with low-dose dopamine without a corresponding increase in PASP or Q, thus arguing against the appearance of LV contrast as a result of increased perfusion pressure causing either capillary distension or bubble deformation.
Finally, another limitation of this study lies in the inherent difficulty of estimating PASP from noninvasive measures (3); ideally, a Swan-Ganz catheter would be inserted to provide the most accurate measurement of pulmonary vascular pressures.
In conclusion, both dobutamine and dopamine resulted in the development of significant I-P shunt in the majority of normal subjects with a parallel increase in shunt fraction. The pattern of I-P shunt recruitment during dobutamine vs. dopamine infusion, as well as the effect of hyperoxia, suggests that both hemodynamic and non-hemodynamic mechanisms may be contributing to shunt recruitment. These findings add to our understanding of the various mechanisms controlling the recruitment of these inducible I-P shunts and the impact of these shunt vessels on gas exchange. As well, they further illuminate the effects of dobutamine and dopamine in humans and offer a possible explanation for the increase in Qs/Qt commonly observed during inotrope infusion. Given the effect on Qs/Qt as well as the increased risk of neurological complications with I-P shunt, these findings could also have important implications for use of these inotropes in critically ill patients.
GRANTS
Funding for this study was provided by the Natural Science and Engineering Council of Canada (NSERC). M. K. Stickland was supported by a Canadian Institutes of Health Research New Investigator Award and Heart and Stroke Foundation of Canada New Investigator Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.B., S.V.D., M.B., M.S., R.W., and M.K.S. conception and design of research; T.B., S.V.D., M.B., and M.K.S. performed experiments; T.B., S.V.D., M.B., and M.K.S. analyzed data; T.B., S.V.D., M.B., and M.K.S. interpreted results of experiments; T.B. and M.K.S. prepared figures; T.B., S.V.D., M.B., M.S., R.W., and M.K.S. drafted manuscript; T.B., S.V.D., M.B., M.S., R.W., and M.K.S. edited and revised manuscript; T.B., S.V.D., M.B., M.S., R.W., and M.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of Ed James and David Pawluski and the Pulmonary Function Lab at the University of Alberta Hospital, Tracey Clare, Lisa Jelaine Zandbeek, Michael Nelson, Roman Rashkovetsky, Siri Holm, and the numerous volunteers who participated as subjects in this study.
REFERENCES
- 1. Bleck TP, Smith MC, Pierrelouis SJC, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illnesses. Crit Care Med 21: 98–103, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Brunton l Lazo J, Parker K. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 2006 [Google Scholar]
- 3. Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol 9: 549–554, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol 97: 797–805, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Furman WR, Summer WR, Kennedy TP, Sylvester JT. Comparison of the effects of dobutamine, dopamine, and isoproterenol on hypoxic pulmonary vasoconstriction in the pig. Crit Care Med 10: 371–374, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Glazier JB, Hughes JM, Maloney JE, West JB. Measurements of capillary dimensions and blood volume in rapidly frozen lungs. J Appl Physiol 26: 65–76, 1969 [DOI] [PubMed] [Google Scholar]
- 7. Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during exercise at sea level. J Appl Physiol 60: 1590–1598, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Hopkins SR, Bogaard HJ, Niizeki K, Yamaya Y, Ziegler MG, Wagner PD. Beta-adrenergic or parasympathetic inhibition, heart rate and cardiac output during normoxic and acute hypoxic exercise in humans. J Physiol 550: 605–616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hopkins SR, Olfert IM, Wagner PD. Last Word on Point:Counterpoint: Exercise-induced intrapulmonary shunting is imaginary vs. real. J Appl Physiol 107: 1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopkins SR, Olfert IM, Wagner PD. Point: Exercise-induced intrapulmonary shunting is imaginary. J Appl Physiol 107: 993–994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoshino Y, Obara H, Iwai S. Relaxant effect of dopamine on isolated rabbit pulmonary artery. Life Sci 39: 2525–2531, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Huckauf H, Ramdohr B, Schroder R. Dopamine induced hypoxemia in patients with left heart failure. Int J Clin Pharmacol Biopharm 14: 217–224, 1976 [PubMed] [Google Scholar]
- 13. Jackson AC, Gilbert JJ, Young GB, Bolton CF. The encephalopathy of sepsis. Canadian J Neurol Sci 12: 303–307, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Jardin F, Gurdjian F, Desfonds P, Margairaz A. Effect of dopamine on intrapulmonary shunt fraction and oxygen transport in severe sepsis with circulatory and respiratory failure. Crit Care Med 7: 273–277, 1979 [DOI] [PubMed] [Google Scholar]
- 15. La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbuchel H, Prior DL. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 109: 1307–1317, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Lejeune P, Leeman M, Deloof T, Naeije R. Pulmonary hemodynamic response to dopamine and dobutamine in hyperoxic and in hypoxic dogs. Anesthesiology 66: 49–54, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Lovering AT, Eldridge MW, Stickland MK. Counterpoint: Exercise-induced intrapulmonary shunting is real. J Appl Physiol 107: 994–997, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lovering AT, Eldridge MW, Stickland MK. Last Word on Point:Counterpoint: Exercise-induced intrapulmonary shunting is imaginary vs. real. J Appl Physiol 107: 1003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lovering AT, Haverkamp HC, Romer LM, Hokanson JS, Eldridge MW. Transpulmonary passage of 99mTc macroaggregated albumin in healthy humans at rest and during maximal exercise. J Appl Physiol 106: 1986–1992, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol 104: 1418–1425, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Lovering AT, Stickland MK, Amann M, Murphy JC, O'Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol 586: 4559–4565, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-microm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol 292: H1777–H1781, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Lynch JP, Mhyre JG, Dantzker DR. Influence of cardiac output on intrapulmonary shunt. J Appl Physiol 46: 315–321, 1979 [DOI] [PubMed] [Google Scholar]
- 24. Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735, 2005 [DOI] [PubMed] [Google Scholar]
- 25. McFarlane PA, Mortimer AJ, Ryder WA, Madgwick RG, Gardaz JP, Harrison BJ, Sykes MK. Effects of dopamine and dobutamine on the distribution of pulmonary blood flow during lobar ventilation hypoxia and lobar collapse in dogs. Eur J Clin Invest 15: 53–59, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Meltzer RS, Tickner EG, Popp RL. Why do the lungs clear ultrasonic contrast. Ultrasound Med Biol 6: 263–269, 1980 [DOI] [PubMed] [Google Scholar]
- 27. Mentzer RM, Alegre CA, Nolan SP. The effects of dopamine and isoproterenol on the pulmonary circulation. J Thorac Cardiovasc Surg 71: 807–814, 1976 [PubMed] [Google Scholar]
- 28. Molloy DW, Ducas J, Dobson K, Girling L, Prewitt RM. Hemodynamic management in clinical acute hypoxemic respiratory failure. Dopamine vs. dobutamine. Chest 89: 636–640, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Oh JK, Seward JB, Tajik AJ. The Echo Manual. New York: Lippincott Williams & Wilkins, 2006 [Google Scholar]
- 30. Polak MJ, Kennedy LA, Drummond WH. Manipulation of dopamine receptors alters hypoxic pulmonary vasoconstriction in isolated perfused rat lungs. Life Sci 51: 1317–1323, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Rennotte MT, Reynaert M, Clerbaux T, Willems E, Roeseleer J, Veriter C, Rodenstein D, Frans A. Effects of two inotropic drugs, dopamine and dobutamine, on pulmonary gas exchange in artificially ventilated patients. Int Care Med 15: 160–165, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Ricci A, Mignini F, Tomassoni D, Amenta F. Dopamine receptor subtypes in the human pulmonary arterial tree. Autonomic Autacoid Pharmacol 26: 361–369, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Roelandt J. Contrast echocardiography. Ultrasound Med Biol 8: 471–492, 1982 [DOI] [PubMed] [Google Scholar]
- 34. Russell WJ, James MF. The effects on arterial haemoglobin oxygen saturation and on shunt of increasing cardiac output with dopamine or dobutamine during one-lung ventilation. Anaesth Intensive Care 32: 644–648, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Scheid P, Piiper J. Diffusion. In: The Lung: Scientific Foundations (2nd ed.), edited by Crystal RG, West JB, Barnes PJ, Weibel ER. Philadelphia, PA: Lippincott-Raven, 1997, p. 1681–1690 [Google Scholar]
- 36. Shoemaker WC, Appel PL, Kram HB. Hemodynamic and oxygen transport effects of dobutamine in critically ill general surgical patients. Crit Care Med 14: 1032–1037, 1986 [DOI] [PubMed] [Google Scholar]
- 37. Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H, Kulinskaya E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 63: 259–266, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Smith G, Cheney FW, Jr, Winter PM. The effect of change in cardiac output on intrapulmonary shunting. Br J Anaesth 46: 337–342, 1974 [DOI] [PubMed] [Google Scholar]
- 39. Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev 34: 99–106, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med 176: 300–305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Effect of acute increases in pulmonary vascular pressures on exercise pulmonary gas exchange. J Appl Physiol 100: 1910–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol 561: 321–329, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tobin CE. Arteriovenous shunts in the peropheral pulmonary circulation in the human lung. Thorax 21: 197–204, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tobin CE, Zariquiey MO. Arteriovenous shunts in the human lung. Proc Soc Exper Biol Med Soc 75: 827–829, 1950 [DOI] [PubMed] [Google Scholar]
- 45. Tsujino T, Shima A. The behaviour of gas bubbles in blood subjected to an oscillating pressure. J Biomech 13: 407–416, 1980 [DOI] [PubMed] [Google Scholar]
- 46. Warrell DA, Evans JW, Clarke RO, Kingaby GP, West JB. Pattern of filling in the pulmonary capillary bed. J Appl Physiol 32: 346–356, 1972 [DOI] [PubMed] [Google Scholar]
- 47. Warren GL, Cureton KJ, Middendorf WF, Ray CA, Warren JA. Red blood cell pulmonary capillary transit time during exercise in athletes. Med Sci Sports Exerc 23: 1353–1361, 1991 [PubMed] [Google Scholar]
- 48. Weyman AE. Principles and Practice of Echocardiography. Philadelphia, PA: Lea & Febiger, 1994 [Google Scholar]
- 49. Woods TD, Harmann L, Purath T, Ramamurthy S, Subramanian S, Jackson S, Tarima S. Small- and moderate-size right-to-left shunts identified by saline contrast echocardiography are normal and unrelated to migraine headache. Chest 138: 264–269, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Worthley LI, Tyler P, Moran JL. A comparison of dopamine, dobutamine and isoproterenol in the treatment of shock. Int Care Med 11: 13–19, 1985 [DOI] [PubMed] [Google Scholar]
- 51. Yang WJ, Echigo R, Wotton DR, Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. II. Moving bubbles or liquids. J Biomechanics 4: 283–288, 1971 [DOI] [PubMed] [Google Scholar]