Abstract
Airway remodeling is an important pathophysiological mechanism in a variety of chronic airway diseases. Historically investigators have had to use invasive techniques such as histological examination of excised tissue to study airway wall structure. The last several years has seen a proliferation of relatively noninvasive techniques to assess the airway branching pattern, wall thickness, and more recently, airway wall tissue components. These methods include computed tomography, magnetic resonance imaging, and optical coherence tomography. These new imaging technologies have become popular because to understand the physiology of lung disease it is important we understand the underlying anatomy. However, these new approaches are not standardized or available in all centers so a review of their validity and clinical utility is appropriate. This review documents how investigators are working hard to correct for inconsistencies between techniques so that they become more accepted and utilized in clinical settings. These new imaging techniques are very likely to play a frontline role in the study of lung disease and will, hopefully, allow clinicians and investigators to better understand disease pathogenesis and to design and assess new therapeutic interventions.
Keywords: radiology and other imaging, COPD, asthma, emphysema
asthma and chronic obstructive pulmonary disease (COPD) are the most prevalent lung diseases and together make a substantial contribution to global morbidity and mortality (5, 7, 18, 40). These disorders are based on an inflammatory/immune and repair process that changes the composition, quantity, and organization of the cellular and molecular constituents of lung tissue, and these changes tend to thicken the wall and narrow the lumen of the small conducting airways (30). This airway remodeling coupled with smooth muscle contraction, loss of lung elastic recoil, and mucus plugging of the lumen leads to airway obstruction and reduces maximal expiratory flow. However, measurement of maximal expiratory flow cannot distinguish the relative contribution of the obstructive changes in the conducting airways from those of emphysema in an individual person. To date, the gold standard for the assessment of airway remodeling has been quantitative histological examination (9, 29). Technical improvements in the spatial resolution of computed tomography (CT) have made it theoretically possible to examine medium-sized airways accurately. Furthermore, other imaging techniques such as optical coherence tomography (OCT) and magnetic resonance imaging (MRI) using hyperpolarized noble gas are providing information on airways that have not been available to this point. There is now more information being gathered on airway structure than ever before and it is important to determine how useful these data are. Has airway imaging provided insight into the prevalence, pathogenesis, phenotypic dissection and/or treatment of subjects who have lung disease? Many of these topics were recently reviewed (10–13, 28, 54, 61, 73, 74, 76) so we have chosen to focus this review on some technical aspects of the use of these airway imaging modalities as applied to the study of human airways in health and disease. Although an efficient, available, and useful algorithm to measure airway wall dimensions is not yet available, we propose that it is coming and will be much more than a gimmick!
The desire to acquire precise measurements of airway dimensions in vivo is driven by the desire to make meaningful measurements of airway wall remodeling in COPD and asthma. In COPD the impetus is the recognition that airflow limitations are caused by at least two pathophysiological processes that lead to distinct phenotypes. Although the inflammatory immune processes observed in the lung parenchyma and conducting airways appear similar (29), the remodeling of the parenchyma is characterized by the disappearance of tissue due to emphysematous destruction of the gas exchanging surface of the lung, whereas the lesions in the small conducting airway result from an increase in airway wall tissue. Airway wall thickening is due to an increased volume of epithelium and smooth muscle and greater deposition of connective tissue including collagen in the lamina propria and adventitial compartments of the airway wall. It is also known that emphysematous destruction of the lung parenchyma causes airflow limitation due to loss of elasticity of the lung. The ability of an imaging technique to determine the relative contributions of airway remodeling and emphysematous destruction to functional parameters would have significant clinical value. In particular, it would be possible to monitor the efficacy of treatments specifically directed toward either or both of the parenchymal and small airway components of COPD.
Assessment of airway dimensions is also a potentially useful phenotype in asthma. The severity of lung dysfunction in asthmatic subjects has been shown to be related to central airway wall thickness measured on CT (52) and improvement of lung function after administration of inhaled steroids was related to reduced wall thickness (53). An accurate, noninvasive measure of the extent of airway wall remodeling might be a useful additional parameter in the clinical assessment of asthmatics and could inform the need for more or less intensive anti-inflammatory therapy.
COMPUTED TOMOGRAPHY OF AIRWAYS
The measurement of airways using CT has its roots in histology. Airways are measured in cross section, and while this was originally performed manually, it quickly became obvious that due to measurement errors associated with the display features of the CT images as well as inter- and intra-observer variation, automated airway analysis was the desired method (44, 55). In the intervening years there have been numerous studies designed to develop and implement airway analysis algorithms (31, 49, 50, 58, 67) but to date there is no consensus on the most appropriate method because all algorithms seem to come with their own set of problems.
Technical issues and accuracy.
To be useful clinically the measurement of airway dimensions has to be accurate and provide an absolute or relative estimate of the true airway anatomy. Testing the accuracy of a CT measurement requires a gold standard. Although the ability of CT to estimate the macro or microscopic extent of emphysema has been studied extensively (14, 21–23, 37, 47), there are fewer studies comparing airway measurements to a gold standard. For airways a number of investigators have validated their automated methods using phantoms (4, 27, 31, 45, 56, 58, 59, 63, 75), which are usually plastic tubes of variable density often imbedded in a lower density material to simulate airways within the lung parenchyma. The accuracy of the CT algorithms as judged against the phantoms has generally been excellent; however, the use of smooth-edged artificial airways is an unrealistic recreation of true airways. The tubes do not have the subtle mucosal folding that is characteristic of airways, there is no liquid lining layer, and they are usually positioned exactly perpendicular to the plane of the CT image, whereas most airways will be sectioned somewhat obliquely to the CT image plane. A more robust gold standard would be actual human airways within inflated lung measured by classical stereological methods on histological sections. Nakano et al. (51) related the CT dimensions of airways measured on resected lungs and lobes to the dimensions of the membranous bronchioles measured histologically on the same lungs. They found that the degree of airway wall thickness of the airways large enough to measure on CT [>2 mm internal diameter, as defined using previous results showing that the error in the airway wall measurements became very large using this focal spot size and reconstruction algorithm (50)] was significantly related to the dimensions of the smaller bronchioles measured histologically. However, they did not attempt to match airways, and the relative wall area of the airways measured by CT was greater than that measured histologically, suggesting that a systematic error may have been present.
Although this is primarily a review of published results, we have chosen to include some original data that address the issue of the validity of the absolute measurements of airway dimensions as measured by CT. We recently completed a study to compare CT estimates with histology. These analyses were performed on six patients who required either lobectomy or pneumonectomy to remove a peripheral, nonobstructing bronchogenic carcinoma. All of these patients had agreed to participate in an ongoing study of lung structure and function that has been fully described elsewhere (72) and has been approved by the ethical review boards of both St. Paul's Hospital and the University of British Columbia.
As part of their clinical care, six subjects received a noncontrast, helical CT examination on either a General Electric LightSpeed Plus (n = 2), LightSpeed Ultra (n = 2), or a Siemens Sensation 16 (n = 2). CT examinations were acquired at 120 kVp and 160.8 (± 1.3) mAs with the subjects supine while breath holding at suspended full inspiration. The CT images were reconstructed using a high spatial frequency kernel (i.e., “Bone” on General Electric or “B60f” on Siemens scanners), which has been previously employed (50), and contiguous thin sections (i.e., 1.25 mm on General Electric or 1.0 mm on Siemens scanners). Pixels dimensions ranged from 0.57 to 0.69 mm using a 512×512 matrix.
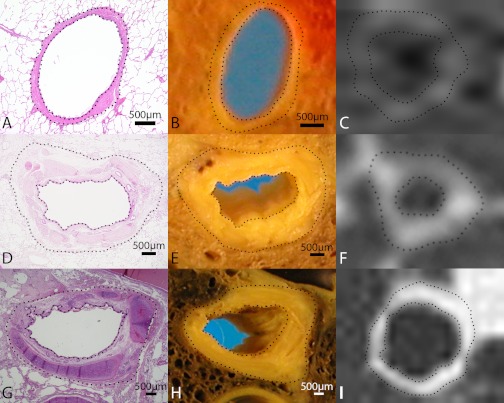
Following surgery, the resected specimens were transferred directly from the operating room to the laboratory where they were inflated with fixative (at 25 cm of water pressure) and sectioned in the axial plane. The cut surfaces of these slices were matched to the corresponding CT image using airways and blood vessels as anatomical landmarks. The matched airways on the cut surface of the lung slice were excised with some surrounding lung parenchyma and an image of the airway was captured using a high resolution digital camera (Nikon Coolpix, Nikon) (Fig. 1, B, E, and H) and the tissue blocks containing the airway were subsequently processed for histology. The microscopic digital images of the airways (Fig. 1, A, D, and G) were analyzed using the computer software Image Pro Plus 4.0. (Media Cybernetics, Silver Spring, MD). The CT image of the corresponding airways is shown in Fig. 1, C, F, and I.
Fig. 1.
Three separate airways (A–C, E–F, G–I) that have been compared using hematoxylin and eosin-stained histology (A, D, G), gross pathology (B, E, H), and computed tomography (CT; C, F, I). Airway A–C is larger than airway E–F, which is larger than airway G–I. Airways were identified and matched by comparing the cut surface of gross pathology lung slices to the CT images and matching for as many anatomic landmarks as possible. Dotted lines on the images show where the measurements of lumen area and wall area were made. Dotted line on the CT image was produced using the CT analysis algorithm (full width at half maximum). Scale bar represents 0.5 mm. Wall area on the CT appears blurred because of magnification and the display parameters (window width and level) that were chosen but is also indicative of the lesser spatial resolution of the CT images.
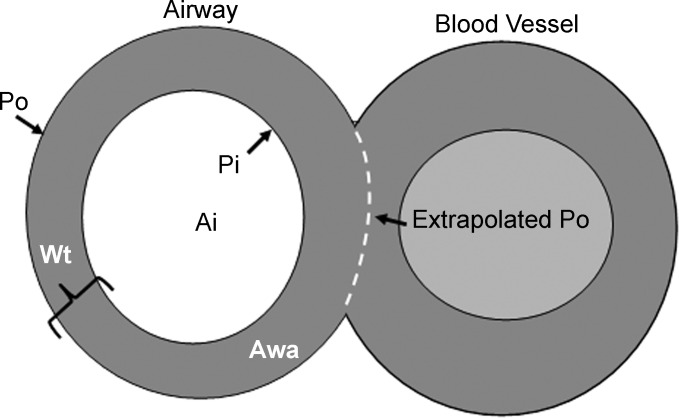
Parameters measured on the microscopic images and CT images are shown in Fig. 2 and included: 1) Ai (internal area in mm2): the area of the lumen of the airway measured by tracing the internal perimeter (Pi) of the lumen; 2) Awa (airway wall) the area enclosed by the outer (adventitial) border of the airway minus the luminal area (Ai).
Fig. 2.
A schematic of a cross-sectioned airway and blood vessel showing the measurements that were made. Pi, internal perimeter; Po, external perimeter; Ai, luminal area; Aaw, airway wall area. If an airway abutted a blood vessel the thickness of the airway wall was maintained over the area of contact as illustrated by the dotted line.
Airway measurements on CT (Fig. 1C) were obtained using EmphylxJ a previously described custom computer algorithm (50).
A total of 60 airways from 6 lung or lobar specimens were measured on microscopic images and could be matched with the same airways on CT images. Histological measurements of the airway lumen area averaged 6.4 ± 4.4 mm2 (median 5.5 mm2) and ranged from 0.6 to 23.4 mm2, which corresponds to a mean lumen diameter of 2.7 ± 1.0 mm (median 2.6 mm, range 0.8 to 5.5 mm). The corresponding value for Ai measured on the macroscopic images was 6.4 ± 4.4 mm2 (median 5.4 mm2, range 0.5 to 20.6 mm2), which corresponds to a mean lumen diameter of 2.7 ± 1.0 mm (median 2.6 mm, range 0.8 to 5.4 mm). The mean value for airway wall area measured histologically was 8.4 ± 8.2 mm2 (median 5.4 mm2, range 1.0 to 42.8) whereas the corresponding value for wall area on the gross images was 6.0 ± 5.2 (median 4.5 mm, range 0.7 to 24.6).
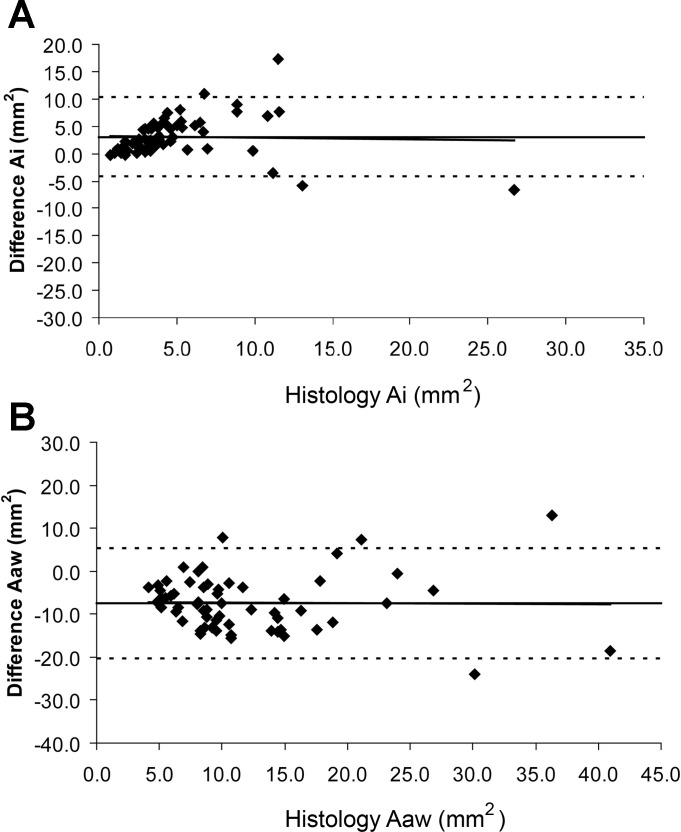
Figure 3A shows a Bland and Altman plot for the difference between the luminal area (Ai) measured histologically vs. Ai measured with the computer algorithm. These data show that the algorithm underestimates the lumen area across airways of all sizes compared with the measurements obtained using histology (corrected for shrinkage). Figure 3B shows a Bland and Altman plot for the difference between the airway wall area (Awa) measured histologically vs. Awa measured using CT. These data show that the algorithm overestimates airway wall area for all airway sizes. When the data are expressed as relative error the discrepancy between CT and histology becomes greater in smaller airways (not shown).
Fig. 3.
A: Bland and Altman plot of the mean airway lumen area (Ai in mm2) measured histologically vs. the difference between the CT measurements and the histological measurements (Ai CT − Ai micro in mm2). B: Bland and Altman plot of the mean airway wall area (Aaw − mm2) measured histologically vs. the difference between CT and histology measurements (Awa CT − Awa micro mm2) in B. Horizontal solid line represents the mean difference between CT and histology while the interrupted line indicates plus and minus two standard deviations of the difference. Line of best fit for the data is also included and is almost identical to the mean difference line.
Despite these errors the relationships between the histological and CT measurements were linear and significant: CT Ai mm2 = −0.31 mm2 + 0.59 mm2 histology Ai (R2 = 0.47). CT Awa mm2 = 10.4 mm2 + 0.60 mm2 histology Awa (R2 = 0.44). Thus one measurement was related to the other but the intercepts and slopes were considerably different from 0 and 1.
There are multiple potential reasons why CT measurements may not accurately reflect morphologic measurements. 1) The long axes of the airways are often oblique to the acquisition and reconstruction plane of the CT images, which distorts the airway lumen and wall dimensions due to the volume averaging that occurs because of the thickness of the CT slices (49). 2) Folding of the airway mucosa also creates difficulty in estimating Ai and Aaw for several reasons. First, each fold can be appreciated and traced when measured using histology, allowing the area between the folds to be assigned to the lumen. In contrast, these areas of lumen will probably be assigned to the wall in the CT analysis because the folds are well below the resolution of the CT scanner. In addition, in vivo these mucosal folds may actually be filled with surface lining fluid thereby effectively reducing the “real” lumen of the airway and making it closer to the lumen area measured using CT. Unfortunately, this fluid may be removed in laboratory processing resulting in the overestimation of the lumen area by histology. 3) Furthermore airway smooth muscle relaxation occurring either postoperatively or during fixation could cause a true increase in Ai measured histologically. 4) Technical factors related to the characteristics of the CT including the focal spot size of the CT scanner, the slice thickness, point-spread function of the scanner, partial volume averaging, the location of the airway within the field of view and the reconstruction kernel used to generate the image may affect the edge detection of the measurement algorithms. All of these factors can play a role in how well the airway is measured. Some of these parameters, such as airway algorithms that take into account the point-spread function of the CT scanner or where the airway is located within the field of view, may be minimized with time and more research. However, other parameters such as the focal spot size of the scanner and slice thickness are physical limitations of the CT scanner and may represent a limit that clinical CT scanners cannot pass. 5) Finally, because the matching of the airway on CT and macroscopic lung images was done using a visual comparison of the cut surface of lung slices and the CT scans, it is likely that some of the discrepancy between CT and histological measurements is due to a failure to precisely match the airways in terms of exact location and orientation with respect to the cut surface of the lung or the CT image, One such example could be Fig. 1B.
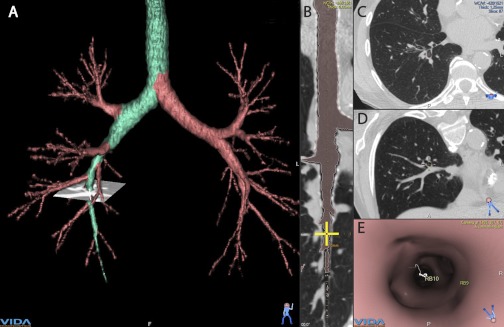
The errors in measurement of airway dimensions related to obliquely sectioned airways and volume averaging is theoretically mitigated by newer multi-detector CT scanners that reconstruct images using submillimeter slice thickness. Many of the new CT measurement algorithms use these new CT scans to realign the airway along the axis of the airway so that it is always cut in cross section, thereby minimizing volume averaging errors (Fig. 4). Of course while very appealing, this approach relies on the quality of the CT voxels, i.e., slice thickness and overlap between images.
Fig. 4.
This figure shows a screen capture of an analysis of the airway tree using a multi-detector row CT scan and commercially available CT analysis software. These CT scans can be used to segment the airway tree and analyze the airway dimensions at any location. A 3-dimensional reconstruction of the airway tree is shown in A with the airway path of interest highlighted in green. B: longitudinal section of a reformatted airway path from A. Yellow cross on the airway in B is at the location of the small “CT view” on A. C: transverse original CT image at the level of the yellow cross in B. D: reformatted CT scan image at the cross section to the airway centerline. E: internal view of the airway lumen at the level of the yellow cross. Measurements of airway dimensions are automated for each segment of the airway tree at the mid-point between 2 branch points. (Images created using the Apollo image analysis software from VIDA Diagnostics, Coralville, IA.)
Despite the dataset above and published results (31, 50) showing that CT overestimates the absolute values for airway wall area and underestimates luminal area, these results show that CT is useful for assessing the relative dimensions of the airways with the caveat that the same algorithm is used in any comparisons. Although the errors may be systematic when using one algorithm there is evidence that the size of the over- and underestimations differ with different algorithms (50). This forms a major drawback to airway research because there is no consensus about the appropriate algorithm to use limiting comparison of results between studies where different algorithms are employed (10). It is for these reasons that a recent COPD Foundation workshop held a consensus session on the use of CT in COPD (71) and the Radiological Society of North America has formed a COPD and Asthma technical committee as part of their quantitative imaging biomarkers alliance (QIBA) (http://www.rsna.org/QIBA.aspx). If CT methods are going to be used in the study of airways there must be protocols and standards that are rigorously followed.
Use of CT airway algorithms to measure airways.
Irrespective of the lack of consensus regarding the most appropriate airway algorithm, investigators have used CT to measure airway dimensions in subjects with COPD and asthma. In one of the earliest studies, Nakano et al. (48) assessed the apical segmental bronchus of the right upper lobe (RB1) in 114 smokers and showed that there was a significant correlation between the WA% (percentage of wall area to lumen area + wall area) and abnormal lung function, which was independent of the contribution of emphysematous changes in the lung. These and additional similar studies have clearly shown that airway and parenchymal abnormalities as measured on CT both relate to abnormal lung physiology and that one or other of the abnormalities may predominate in individual subjects. For example, in a large family-based study Patel and colleagues (57) confirmed that airway wall abnormalities and emphysema contributed independently to decline in FEV1 among smokers and also found that the predominant pathology showed familial concordance suggesting that specific pathogenetic mechanism are heritable. Related individuals who smoked were more likely to show concordance of airway or parenchymal disease than unrelated individuals.
Despite the relative lack of studies comparing CT measurements of airway dimensions with an anatomical gold standard there are many studies in which airway CT has been compared with symptoms and lung function. Interestingly these studies have shown that airway measurements have different relationships to specific symptoms and function tests than do measurements of emphysema. For example, symptoms usually associated with the “bronchitic” phenotype of COPD, including cough, wheeze, MRC-bronchitis score, and dyspnea have been found to be more strongly related to airway measurements of the medium or large airways than are measures of emphysema (24, 38). Recently Martinez et al. (39) examined the relationships between quantitative computed tomography (QCT) parameters of emphysema, airway wall remodeling, and airway narrowing to composite clinical and physiological indices of COPD such as the BODE index (body mass index, airflow obstruction, dyspnea, and exercise capacity) and a measurement of health status assessed using the St. George's Respiratory Questionnaire (SGRQ). Not surprisingly these QCT estimates of pathological changes were related to measures of clinical impact. More interestingly, the authors found that there were differences in the strength of the associations between measures of emphysema and airway disease and the composite indices. Measures of emphysema were more closely related to the BODE index, whereas the airway wall abnormalities were better predictors of the health status as measured by the SGRQ.
Most of the studies mentioned previously have relied on measuring random airways within the lung that happen to be cross-sectioned on the CT images. Although this approach was necessary using the standard high-resolution CT technique (axial images, gaps between images) CT scanner technology has improved dramatically over the last decade and multi-detector row CT scanners can now acquire images of the entire bronchial tree at or near submillimeter resolution. These scanners allow the acquisition of volumetric datasets so that the airway tree can be segmented, often as far as the 8th generation. This approach is thought to be superior to the random sampling technique because now one can select specific airways within the bronchial tree for measurement and for comparison between individuals (Fig. 4). In fact, data suggest that measurements of the 5th and 6th generation of the airway tree show a stronger association with lung function than do the measurements of more central airways (16, 27).
Even at this resolution, the airways are still not the small airways that are responsible for the airflow limitation. However, the functional consequences of remodeling of the even smaller airways can be measured by estimating gas trapping on expiratory CT scans. This can be quantified by measuring the percentage of lung voxels with x-ray attenuation values less than −856 HU on an expiratory scan. The threshold value of −856 HU (79) was chosen because previous work suggests that this value conforms to maximal lung inflation near total lung capacity and no lung units should remain this inflated on expiration. There are compelling data that gas-trapping measured by this technique is sensitive to airflow abnormalities and does correlate with clinical symptoms in severe asthmatics and to response to therapy (8, 80). There are also data that show that gas-trapping on expiratory CT can help identify and separate subjects with airflow limitation within a general population of smokers (46). Although the examination of expiratory CT scans is an interesting method to test for small airway disease, it is still only a surrogate measure at present, and more data are needed to assess the general applicability.
Although it is well accepted that the site of airflow limitation in COPD is the small airways, a new hypothesis has recently been proposed for airflow limitation by McDonough and Hogg (42). These investigators counted the total number and size of airways that could be visualized on multi-row detector CT scans in subjects with COPD compared with normal. They observed far fewer airways in those with severe airflow obstruction. Although this result might come about because the airways were too small to be detected in the obstructed individuals they also did microCT on small samples of diseased lung tissue where the resolution was sufficient to detect all visible airways (16 μm). On the microCT images they found a striking reduction in the number of terminal bronchioles as well as severe narrowing. Additionally they found that the reduction in terminal bronchiolar number was present even in areas of the lung without emphysema, suggesting that there is a significant loss of small airways in smokers preceding the development of emphysema and that the remodeling that is observed occurs only in the survivor airways (42). This is a very unique hypothesis because it shows that the destructive process that occurs in the lung parenchyma only micrometers away from the airway can also affect the airways themselves. Other data by Diaz et al. have shown that subjects with more advanced emphysema also have a reduction in the number of airways within generations 5–8 and that the loss of these airways was an independent predictor of the BODE score (17).
CLINICAL APPLICATIONS OF CT?
The studies reviewed in the previous section suggest that it may be time to incorporate CT measurements of airway remodeling into the clinical assessment and management of COPD. CT can allow the contributions of airway and parenchymal changes to be determined, and these measures relate differently to symptoms and to pulmonary function. Recently Han and colleagues (25) studied the relationship between radiologic emphysema, airway disease, and acute exacerbations of COPD. They showed that, irrespective of the level of airflow limitation, assessed using spirometry, increases in the airway wall thickness and the extent of emphysema (in subjects with severe emphysema) both predicted increased risk of acute exacerbations (25). The results of this investigation may allow clinicians and clinical investigators to identify who is at greatest risk for an acute exacerbation of COPD and to select patients in whom to maximize preventive therapies in the outpatient setting. One additional practical application of quantitative CT imaging is the recent trial of bronchoscopically placed one-way valves to achieve minimally invasive volume reduction. These studies have shown that valve placement can result in an overall decrease in lung volume (15), and in other studies CT has been used to demonstrate that those subjects with incomplete interlobar fissures have the lowest chance of procedural benefit likely due to collateral ventilation (62). Despite these promising leads there is, as yet, no clear vision of how quantitative assessments of parenchymal, airway, and vascular disease can guide the clinical care of patients with COPD. True integration into clinical care will depend on two major developments; the acceptance of a standard method to measure airway wall remodeling and clear evidence that the management of individual patients who have, or are at risk for, COPD should differ based on this information. Tempering enthusiasm for the clinical use of CT as a management tool is the increasing awareness of the risks associated with the radiation exposure necessary for CT acquisition. Although the estimates of the associations between the dose of radiation and risk of cancer vary and remain highly controversial (35, 64, 65, 69), there are consistent data showing that medical radiation, and CT in particular, is the second largest source of ionizing radiation after natural background radiation (1, 6). Therefore, all studies recommend using the ALARA principle (as low as reasonably achievable) when exposing subjects to medical radiation.
MAGNETIC RESONANCE IMAGING
A recent novel approach to airway imaging has been the use of MRI to assess airway function. Although conventional proton MRI is readily available in most radiology departments, a number of fundamental challenges have limited its usefulness for lung imaging [reviewed here (19, 74)]. However, recent attention has turned to imaging following the inhalation of hyperpolarized helium or xenon gas because it is possible to observe and quantify the regions of the lung that receive gas during a normal breath hold and to use these measurements to quantify airway function within that region (32, 41, 66, 70). By using this approach, the volume or percentage of the lung that does not receive hyperpolarized gas is quantified as the ventilation defect volume/percentage. These ventilation defects can be due to airway obstruction or emphysematous destruction (41), but when combined with another MRI measurement, the apparent diffusion coefficient, measurements of alveolar size can be derived and used to separate the two processes (41, 60, 78). Another advantage to MRI is that imaging can be performed during an inhalation and exhalation (wash-in or wash-out) maneuver that may provide dynamic information on lung function. Additionally, because there is no-ionizing radiation associated with MRI this technique is ideal for longitudinal studies to follow disease progression and or response to an intervention. However, there are numerous drawbacks to this technique. First, this is a very technically demanding technique and is, currently, limited to only a few select centers in the world. Second, most of the research in this field has used hyperpolarized helium, but there is a finite amount of this gas available and the cost has risen substantially over the last few years leading many centers to begin to use xenon. Finally, there is still a lot of basic research that needs to be completed in this field to understand the data that are being produced. For example, what is the exact cause of the ventilation defects? It seems obvious that when gas does not reach a region of the lung there is some barrier to ventilation, but how much of that is related to airways and how much is caused by the lung parenchyma is still unknown. However, although the clinical potential of this approach is still to be determined, the available data do suggest that these measurements can provide useful information on disease mechanisms in asthma and COPD, and this technique may provide a new method to assess lung structure and, most importantly, function.
OPTICAL COHERENCE TOMOGRAPHY
There are many optical techniques for measuring airways, including confocal microscopy, video bronchoscopy, and OCT. Many of these have been reviewed recently (54), and in the current review we have chosen to focus on just one of these, OCT. OCT is a new and exciting technique that has some interesting applications to the study of airways. Initially OCT was developed for ophthalmic applications in the early 1990s and is now used in other organ systems including the heart to assess vessel structure and atherosclerotic plaques. Recently investigators have begun applying OCT to studies of airway wall structure because the relatively noninvasive nature of the technique allows the acquisition of extremely high-resolution images of the bronchial walls. OCT is ideal for airway assessment because although the technique is analogous to B-type ultrasound, OCT uses a low coherence near-infrared light such as that from a 1,300-nm superluminescent diode source instead of sound and, therefore, does not require a transducing medium. The basic physics and technical aspects of OCT and the different types of OCT including the new frequency domain OCT are beyond the scope of this review but have been described elsewhere (12, 13, 33, 54). Briefly, imaging is achieved by passing a small fiberobtic cable (1.3 mm total diameter) into the distal airways through the biopsy port of a standard bronchoscope. Images are acquired by a rotating probe that directs (and scans) the airway wall in a circumferential manner building up two- and three-dimensional images of the wall microstructure (Fig. 5). The near-infrared light will penetrate the airway wall to depths of up to 2–3 mm, and because the composition and density of the different layers of tissue (e.g., lumen, epithelium, and extracellular matrix), have different optical refractive properties, they produce distinct image patterns on OCT. For example, collagen and elastin have very strong back-scattering properties compared with the epithelial layer; thus, extracellular matrix appears brighter on OCT than the epithelial layer (Fig. 5). Furthermore the spatial resolution of OCT approaches 10 μm in vitro and 20–30 μm in vivo (16, 26), giving it a distinct spatial resolution advantage over CT and MRI but also has advantages over confocal microscopy in that it can penetrate tissue three times deeper, does not require contact with the tissue surface, and is less susceptible to motion artifacts from cardiac pulsation and respiratory movements (20). The size of the probe (1.3 mm total diameter) makes it ideal for imaging of small airways but less ideal for larger airways as the light does not penetrate well in airways with diameters larger than 2 cm. This is a great advantage over CT imaging because images of the airway wall at site of airflow limitation can be obtained. The main disadvantage of OCT is that it requires bronchoscopy. However, OCT is safe and generally well tolerated, and the light source has not been associated with any significant health hazards.
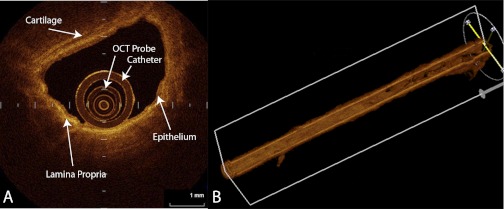
Fig. 5.
Optical coherence tomography (OCT) images of a medium-sized airway in cross-section (A) and reconstructed 3-dimensional airway obtained by a “pull back technique” where the OCT probe is retracted 5 cm up the airway during scanning (B). OCT probe and surrounding catheter can be seen in lumen of A. A also shows the bright contrast pattern of the lamina propria and the less contrast of the epithelial layer and the cartilage. The pull back technique produces images of an airway path while the OCT probe is automatically retracted 5 cm. Images are obtained in a “helical” method and can be reconstructed into the 3-dimensional image of the airway path. (Images courtesy of Dr. Keishi Ohtani, BC Cancer Research Centre, Vancouver, BC, and Department of Surgery, Tokyo Medical University, Tokyo, Japan).
The use of OCT imaging of airways to assess airflow limitation is limited, but preliminary data are promising (26, 34, 68). In a proof-of-principle study in assessing airway remodeling of COPD, we reported a strong correlation between CT and OCT measurements of lumen and wall area (16). However, what we also found was that although there was a correlation between FEV1% predicted and both CT and OCT measured wall area the slope of the relationship was much steeper using OCT than using CT, suggesting greater sensitivity of OCT in detecting changes in wall measurements that relate to FEV1 (16).
Another type of OCT imaging that has been described is anatomical OCT (aOCT), which uses an extended range approach specialized for scanning the lumen of large structures such as the upper and central airways. This technique can produce images that are similar to CT images but with much greater resolution. The disadvantage of aOCT is that although it produces surface images, the spatial resolution of the subsurface structures is limited. Therefore, aOCT is used to image the upper airways and pharyngeal area in conditions such as obstructive sleep apnea to examine changes in airway shape and size during the respiratory cycle during sleep Williamson and colleagues (77) recently reported on the use of aOCT to study obstructive lung disease. They used aOCT to compare the elastic properties of the central airways in patients who had COPD, asthma, bronchiectasis with subjects without airway disease. They found that the airway lumen area was lower in subjects with asthma and that the relationship between transpulmonary pressure and lumen area was altered in COPD (77). These data indicate that aOCT can be used to assess changes in the elastic properties of the central airways of subjects with chronic airflow obstruction.
Finally, aOCT images can be reconstructed into three-dimensional images (43) to allow geometric computational flow modeling of the pharynx during the inspiratory phase of the respiratory cycle (36). Although aOCT does show some promise to be able to measure size and shape dynamically and repeatedly over extended periods, aOCT systems are still experimental and only exist in research laboratories.
OCT is an exciting new technique but there are still a lot of questions to be answered about its utility. For example, because OCT is a dynamic in vivo imaging modality, standards for proper measurements of airway dimensions need to be developed. Deep inspiration increases the size of airway lumen (and decreases the airway wall thickness), whereas full expiration decreases the luminal size. Thus it is imperative that the measurements be standardized or normalized to a particular phase of the respiratory cycle. Additionally, more validation of the measurements compared with histology must be performed to determine the accuracy of the technique. Furthermore, variation in bronchial anatomy and heterogeneity of disease activity may make it challenging to accurately and consistently measure airways or to be positive that images are being obtained from the same airway during subsequent imaging procedures. Therefore, variation in airway measurements within and between subjects must be quantified before this approach can be used clinically. However, that being said the exquisite resolution of this technique makes it very appealing to study interventions on airway dimensions. It may be that this is the best technique for assessing changes in airway wall structure in short-term clinical trials.
SUMMARY
A proper understanding of airway structure is vital for the understanding of airway function and theoretically could be useful in the clinical management of individual patients. Historically the only way to measure the airway wall was to prepare histological sections, but the advent of new noninvasive imaging techniques means that anatomic and structural information can be acquired without the need for histology. These new techniques, including CT, MRI, and OCT provide unique tools to be able to assess airway structure. However, none of these techniques are without problems and limitations and it may seem that these airway tools are more gimmicks than useful tools. On the other hand, it may also be that a careful understanding of how these tools are used will still provide useful data that have not been available to date. It may be that CT can and should be used to initially assess subjects with airway disease, but OCT should be used to provide the detail of the airway wall that is beyond the resolution of CT. Furthermore, the functional aspects of MRI provide a unique way to assess how the airway structure relates to lung function that has not been available until now. Therefore, noninvasive airway imaging is a useful and helpful clinical research tool and should be used in the study of lung diseases responsible for chronic airflow limitation and the response to intervention.
GRANTS
Dr. Coxson was supported by a Roberta R. Miller Fellowship in Thoracic Imaging from the British Columbia Lung Association and was also supported, in part, by the University of Pittsburgh COPD SCCOR and NIH 1P50 HL084948 and R01 HL085096 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, to the University of Pittsburgh.
DISCLOSURES
HC received $11,000 in 2007–1012 for serving on an advisory board for GSK. In addition HC has been the coinvestigator on two multi-center studies sponsored by GSK and has received travel expenses to attend meetings related to the project. HC has a contract service agreement with Spiration Inc. to quantify the CT scans in subjects with very severe COPD. There is no financial relationship between any industry and the current study.
AUTHOR CONTRIBUTIONS
Author contributions: P.D.P., T.N., and H.O.C. conception and design of research; P.D.P., T.N., and H.O.C. performed experiments; P.D.P., T.N., and H.O.C. analyzed data; P.D.P., T.N., and H.O.C. interpreted results of experiments; P.D.P., T.N., and H.O.C. prepared figures; P.D.P., T.N., and H.O.C. drafted manuscript; P.D.P., T.N., and H.O.C. edited and revised manuscript; P.D.P., T.N., and H.O.C. approved final version of manuscript.
REFERENCES
- 1. Aldrich JE, Bilawich AM, Mayo JR. Radiation doses to patients receiving computed tomography examinations in British Columbia. Can Assoc Radiol J 57: 79–85, 2006 [PubMed] [Google Scholar]
- 2. Armstrong JJ, Leigh MS, Sampson DD, Walsh JH, Hillman DR, Eastwood PR. Quantitative upper airway imaging with anatomic optical coherence tomography. Am J Respir Crit Care Med 173: 226–233, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong JJ, Leigh MS, Walton ID, Zvyagin AV, Alexandrov SA, Schwer S, Sampson DD. In vivo size and shape measurement of the human upper airway using endoscopic long-range optical coherence tomography. Optics Express 2003: 1817–1826, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bankier AA, Fleischmann D, Mallek R, Windisch A, Winkelbauer FW, Kontrus M, Havelec L, Herold CJ, Hubsch P. Bronchial wall thickness: Appropriate window settings for thin-section CT and radiologic-anatomic correlation. Radiology 199: 831–836, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 31: 143–178, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 357: 2277–2284, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis 12: 703–708, 2008 [PubMed] [Google Scholar]
- 8. Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 135: 48–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 147: 405–410, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Coxson HO. Quantitative computed tomography assessment of airway wall dimensions: current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc 5: 940–945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coxson HO, Eastwood PR, Williamson JP, Sin DD. Phenotyping airway disease with optical coherence tomography. Respirology 16: 34–43, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Coxson HO, Lam S. Quantitative assessment of the airway wall using computed tomography and optical coherence tomography. Proc Am Thorac Soc 6: 439–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180: 588–597, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Coxson HO, Mayo JR, Behzad H, Moore BJ, Verburgt LM, Staples CA, Pare PD, Hogg JC. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol 79: 1525–1530, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, Muller NL, Cogswell S, Dillard DH, Finger CL, Springmeyer SC. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J 32: 1443–1450, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Coxson HO, Quiney B, Sin DD, McWilliams AM, Mayo JR, Lam S. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med 177: 1201–1206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diaz AA, Valim C, Yamashiro T, Estepar RS, Ross JC, Matsuoka S, Bartholmai B, Hatabu H, Silverman EK, Washko GR. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest 138: 880–887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fabbri LM, Hurd SS. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J 22: 1–2, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Fain S, Schiebler ML, McCormack DG, Parraga G. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: review of current and emerging translational methods and applications. J Magn Reson Imaging 32: 1398–1408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol 21: 1361–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 152: 653–657, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 154: 187–192, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Gould GA, MacNee W, McLean A, Warren PM, Redpath A, Best JJ, Lamb D. CT measurements of lung density in life can quantitate distal airspace enlargement—an essential defining feature of human emphysema. Am Rev Respir Dis 137: 380–392, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med 181: 353–359, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA, Anzueto AR, Make BJ, Hokanson JE, Crapo JD, Silverman EK, Martinez FJ, Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D'Aco K, Sciurba FC, Hatabu H, Rosas IO. Chronic obstructive pulmonary disease exacerbations in the COPD Gene Study: associated radiologic phenotypes. Lung volumes and emphysema in smokers with interstitial lung abnormalities. Radiology 261: 274–282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanna N, Saltzman D, Mukai D, Chen Z, Sasse S, Milliken J, Guo S, Jung W, Colt H, Brenner M. Two-dimensional and 3-dimensional optical coherence tomographic imaging of the airway, lung, and pleura. J Thorac Cardiovasc Surg 129: 615–622, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 1309–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 519–532, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004 [DOI] [PubMed] [Google Scholar]
- 30. James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 30: 134–155, 2007 [DOI] [PubMed] [Google Scholar]
- 31. King GG, Muller NL, Whittall KP, Xiang QS, Pare PD. An analysis algorithm for measuring airway lumen and wall areas from high-resolution computed tomographic data. Am J Respir Crit Care Med 161: 574–580, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Kirby M, Mathew L, Heydarian M, Etemad-Rezai R, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: quantification of bronchodilator effects by using hyperpolarized He MR imaging. Radiology 261: 283–292, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Lam S, Standish B, Baldwin C, McWilliams A, leRiche J, Gazdar A, Vitkin AI, Yang V, Ikeda N, MacAulay C. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res 14: 2006–2011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lam S, Standish B, Baldwin C, McWilliams A, leRiche J, Gazdar A, Vitkin AI, Yang V, Ikeda N, MacAulay C. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res 14: 2006–2011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 251: 6–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lucey A, King A, Tetlow G, Wang J, Armstrong J, Leigh M, Paduch A, Walsh J, Sampson D, Eastwood P, Hillman D. Measurement, reconstruction and flow-field computation of the human pharynx with application to sleep apnea. IEEE Trans Biomed Eng 57: 2535–2548, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector Row CT—Comparison with macroscopic and microscopic morphometry. Radiology 238: 1036–1043, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, MacNee W. Airway dimensions in COPD: relationships with clinical variables. Resp Med 104: 1683–1690, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Martinez CH, Chen YH, Westgate PM, Liu LX, Murray S, Curtis JL, Make BJ, Kazerooni EA, Lynch DA, Marchetti N, Washko GR, Martinez FJ, Han MK, COPDGene Investigators Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax 67: 399–406, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469–478, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Mathew L, Kirby M, Etemad-Rezai R, Wheatley A, McCormack DG, Parraga G. Hyperpolarized (3)He magnetic resonance imaging: preliminary evaluation of phenotyping potential in chronic obstructive pulmonary disease. Eur J Radiol 79: 140–146, 2011 [DOI] [PubMed] [Google Scholar]
- 42. McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Pare PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 365: 1567–1575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLaughlin RA, Williamson JP, Phillips MJ, Armstrong JJ, Becker S, Hillman DR, Eastwood PR, Sampson DD. Applying anatomical optical coherence tomography to quantitative 3D imaging of the lower airway. Opt Express 16: 17521–17529, 2008 [DOI] [PubMed] [Google Scholar]
- 44. McNamara AE, Muller NL, Okazawa M, Arntorp J, Wiggs BR, Pare PD. Airway narrowing in excised canine lung measured by high-resolution computed tomography. J Appl Physiol 73: 307–316, 1992 [DOI] [PubMed] [Google Scholar]
- 45. McNitt-Gray MF, Goldin JG, Johnson TD, Tashkin DP, Aberle DR. Development and testing of image-processing methods for the quantitative assessment of airway hyperresponsiveness from high-resolution CT images. J Comput Assist Tomogr 21: 939–947, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Mets OM, Buckens CF, Zanen P, Isgum I, van Ginneken B, Prokop M, Gietema HA, Lammers JW, Vliegenthart R, Oudkerk M, van Klaveren RJ, de Koning HJ, Mali WP, de Jong PA. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA 306: 1775–1781, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask.” An objective method to quantitate emphysema using computed tomography. Chest 94: 782–787, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 162: 1102–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 162: 1102–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Nakano Y, Whittall KP, Kalloger SE, Coxson HO, Pare PD. Development and validation of human airway analysis algorithm using multidetector row CT. Proc SPIE 4683: 460–469, 2002 [Google Scholar]
- 51. Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 171: 142–146, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med 162: 1518–1523, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Niimi A, Matsumoto H, Amitani R, Nakano Y, Sakai H, Takemura M, Ueda T, Chin K, Itoh H, Ingenito EP, Mishima M. Effect of short-term treatment with inhaled corticosteroid on airway wall thickening in asthma. Am J Med 116: 725–731, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Ohtani K, Lee AM, Lam S. Frontiers in bronchoscopic imaging. Respirology 17: 261–269, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Okazawa M, Muller NL, McNamara AE, Child S, Verburgt L, Pare PD. Human airway narrowing measured using high resolution computed tomography. Am J Respir Crit Care Med 154: 1557–1562, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Palagyi K, Tschirren J, Hoffman EA, Sonka M. Quantitative analysis of pulmonary airway tree structures. Comput Biol Med 36: 974–996, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Patel BD, Coxson HO, Pillai SG, Agusti AG, Calverley PM, Donner CF, Make BJ, Muller NL, Rennard SI, Vestbo J, Wouters EF, Hiorns MP, Nakano Y, Camp PG, Nasute Fauerbach PV, Screaton NJ, Campbell EJ, Anderson WH, Pare PD, Levy RD, Lake SL, Silverman EK, Lomas DA. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178: 500–505, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Reinhardt JM, D'Souza ND, Hoffman EA. Accurate measurement of intrathoracic airways. IEEE Trans Med Imaging 16: 820–827, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Saba OI, Hoffman EA, Reinhardt JM. Maximizing quantitative accuracy of lung airway lumen and wall measures obtained from X-ray CT imaging. J Appl Physiol 95: 1063–1075, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP., 3rd Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes—initial experience. Radiology 222: 252–260, 2002 [DOI] [PubMed] [Google Scholar]
- 61. San Jose Estepar R, Reilly JJ, Silverman EK, Washko GR. Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc 5: 905–909, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G, Group VSR. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 363: 1233–1244 [DOI] [PubMed] [Google Scholar]
- 63. Seneterre E, Paganin F, Bruel JM, Michel FB, Bousquet J. Measurement of the internal size of bronchi using high resolution computed tomography (HRCT). Eur Respir J 7: 596–600, 1994 [DOI] [PubMed] [Google Scholar]
- 64. Smith-Bindman R. Is computed tomography safe? N Engl J Med 363: 1–4, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de Gonzalez A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169: 2078–2086, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tooker AC, Hong KS, McKinstry EL, Costello P, Jolesz FA, Albert MS. Distal airways in humans: dynamic hyperpolarized 3He MR imaging—feasibility. Radiology 227: 575–579, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Tschirren J, Hoffman EA, McLennan G, Sonka M. Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE Trans Med Imaging 24: 1529–1539, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsuboi M, Hayashi A, Ikeda N, Honda H, Kato Y, Ichinose S, Kato H. Optical coherence tomography in the diagnosis of bronchial lesions. Lung Cancer 49: 387–394, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 251: 13–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tzeng YS, Hoffman E, Cook-Granroth J, Gereige J, Mansour J, Washko G, Cho M, Stepp E, Lutchen K, Albert M. Investigation of hyperpolarized 3He magnetic resonance imaging utility in examining human airway diameter behavior in asthma through comparison with high-resolution computed tomography. Acad Radiol 15: 799–808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walsh JW, Wanner A. Purpose of the conference. Proc Am Thorac Soc 5: 873, 2008 [Google Scholar]
- 72. Wang IM, Stepaniants S, Boie Y, Mortimer JR, Kennedy B, Elliott M, Hayashi S, Loy L, Coulter S, Cervino S, Harris J, Thornton M, Raubertas R, Roberts C, Hogg JC, Crackower M, O'Neill G, Pare PD. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med 177: 402–411, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Washko GR. Diagnostic imaging in COPD. Semin Respir Crit Care Med 31: 276–285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Washko GR, Parraga G, Coxson HO. Quantitative pulmonary imaging using computed tomography and magnetic resonance imaging. Respirology 17: 432–444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Webb WR, Gamsu G, Wall SD, Cann CE, Proctor E. CT of a bronchial phantom: factors affecting appearance and size measurements. Invest Radiol 19: 394–398, 1984 [DOI] [PubMed] [Google Scholar]
- 76. Williamson JP, James AL, Phillips MJ, Sampson DD, Hillman DR, Eastwood PR. Quantifying tracheobronchial tree dimensions: methods, limitations and emerging techniques. Eur Respir J 34: 42–55, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Williamson JP, McLaughlin RA, Noffsinger WJ, James AL, Baker VA, Curatolo A, Armstrong JJ, Regli A, Shepherd KL, Marks GB, Sampson DD, Hillman DR, Eastwood PR. Elastic properties of the central airways in obstructive lung diseases measured using anatomical optical coherence tomography. Am J Respir Crit Care Med 183: 612–619, 2011 [DOI] [PubMed] [Google Scholar]
- 78. Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 56: 1293–1300, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yuan R, Hogg JC, Pare PD, Sin DD, Wong JC, Nakano Y, McWilliams AM, Lam S, Coxson HO. Prediction of the rate of decline in FEV(1) in smokers using quantitative computed tomography. Thorax 64: 944–949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zeidler MR, Kleerup EC, Goldin JG, Kim HJ, Truong DA, Simmons MD, Sayre JW, Liu W, Elashoff R, Tashkin DP. Montelukast improves regional air-trapping due to small airways obstruction in asthma. Eur Respir J 27: 307–315, 2006 [DOI] [PubMed] [Google Scholar]