Summary
Programmed death-1 (PD-1) is a cell surface molecule that regulates the adaptive immune response. Engagement of PD-1 by its ligands PD-L1 or PD-L2 transduces a signal that inhibits T-cell proliferation, cytokine production, and cytolytic function. While a great deal is known concerning the biological roles PD-1 plays in regulating the primary immune response and in T-cell exhaustion, comparatively little is known regarding how PD-1 ligation alters signaling pathways. PD-1 ligation is known to inhibit membrane-proximal T-cell signaling events, while ligation of the related inhibitory molecule cytotoxic T lymphocyte antigen-4 appears to target more downstream signaling pathways. A major obstacle to an in-depth understanding of PD-1 signaling is the lack of physiologic models in which to study signal transduction. This review focuses on: 1) signaling pathways altered by PD-1 ligation, 2) factors recruited upon PD-1 phosphorylation, and 3) exploring the hypothesis that PD-1 ligation induces distinct signals during various stages of immune-cell differentiation. Lastly, we describe models to dissect the function of the PD-1 cytoplasmic tail using primary cells in the absence of agonist antibodies.
Keywords: SHP-1, SHP-2, metabolism
Introduction
Programmed death-1 (PD-1) is a 288 amino acid protein expressed in B and T cells as well as myeloid-derived cells. PD-1 was initially cloned as a molecule overexpressed in apoptotic cells (1). Over the past 15 years it has become clear that PD-1’s primary function is to attenuate the immune response. PD-1 binds PD-L1 (B7-H1) and PD-L2 (B7-DC). While PD-L2 expression is limited to professional antigen presenting cells (APCs), PD-L1 has a much broader tissue distribution, and interactions between PD-L1 and PD-1 are thought to maintain peripheral tolerance (2). The identification of B7-1 (CD80) as an additional binding partner for PD-L1 revealed new ways in which the B7:CD28 family regulates T-cell activation and tolerance (3). The lymphocytic choriomeningitis virus (LCMV) model has provided a great deal of insight into PD-1 function. Infection of mice with LCMV Armstrong, or its closely related variant LCMV clone 13 initially generate similar LCMV-specific primary immune responses (4). However, differences in the T-cell responses to the respective LCMV strains can be identified as early as eight days post infection. PD-1 expression on LCMV-specific T cells isolated from mice infected with wildtype LCMV diminishes, and the resultant LCMV-specific memory pool retains polyfunctional capacity [the ability to coexpress tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) after antigen recognition] for greater than 80 days post infection. In contrast, LCMV-specific T cells isolated from LCMV clone 13-infected animals retain high levels of PD-1 expression and over time, they become mono-functional (only producing TNF-α or IFN-γ in response to antigen). By 80 days post infection, they are largely non-functional and are referred to as exhausted T cells (4). Blocking PD-1 and PD-L1 interactions restores some function to LCMV-specific T cells in clone 13 LCMV-infected mice and LCMV viral load is significantly reduced (5), indicating that PD-1 plays an important role in mediating the exhausted phenotype. An important but sometimes overlooked finding of the Barber et al paper (5) is that mice lacking PD-L1 die from immune-mediated pathology when infected with LCMV clone 13, indicating that T-cell exhaustion can serve as a mechanism to protect the host from a non-resolving immune response. The LCMV model has been further exploited to reveal additional functions of PD-1 within the immune system. Using an elegant bone marrow chimera system that introduced LCMV-restricted antigens under conditions that should induce tolerance, Probst et al. (6) observed that in the absence of PD-1, priming was observed instead of tolerance, supporting the view that PD-1 plays a role in maintaining peripheral tolerance.
In contrast to cytotoxic T lymphocyte antigen-4 (CTLA-4) deficiency, which leads to a massive lymphoproliferative disorder within weeks of birth (7), PD-1 deficiency leads to species-specific autoimmunity that occurs much later in life (8). More recently, it has been shown that the loss of PD-1 exacerbates the consequences of other genetic lesions that promote autoimmunity (9–11). For instance, loss of PD-1 accelerates the onset and frequency of type-I diabetes in non-obese diabetic (NOD) mice (11). Furthermore, blockade of PD-1 and PD-L1 interactions in a transplantation model leads to accelerated graft rejection (12). Thus, under normal conditions, PD-1 expression establishes a threshold of activation that must be overcome before initiation of an immune response. However, when confronted with persistent antigen expression, as in chronic viral infections and tumors, the role PD-1 plays in the immune system changes from gate keeper to veto signal. As briefly described above and in much greater detail in recent reviews (13–22), PD-1 plays a critical role in maintaining tolerance and shutting down ineffective immune responses when examined from a ‘glass is half full’ point of view. When examined from a ‘glass is half empty’ point of view, PD-1 interferes with vaccination and contributes to T-cell dysfunction in chronic viral infections and cancer. In any case, PD-1 is a powerful modulator of the immune system. What is unclear is exactly how PD-1 mediates these effects. A better understanding of PD-1 mediated signal transduction pathways will broaden the number of therapeutic targets and perhaps reveal novel means of modulating the immune system. This review focuses on PD-1-mediated signaling and discusses what is currently known about PD-1-mediated signal transduction, the issues that have hampered progress in understanding PD-1 function, and new approaches that will hopefully shed light on how PD-1 ligation controls immune-cell function. Finally, I explore the possibility that PD-1 signaling is influenced by the T-cell differentiation state.
PD-1: CD28 family on the outside, sialic acid binding immunoglobulin (Ig)-like lectins (Siglec) family on the inside
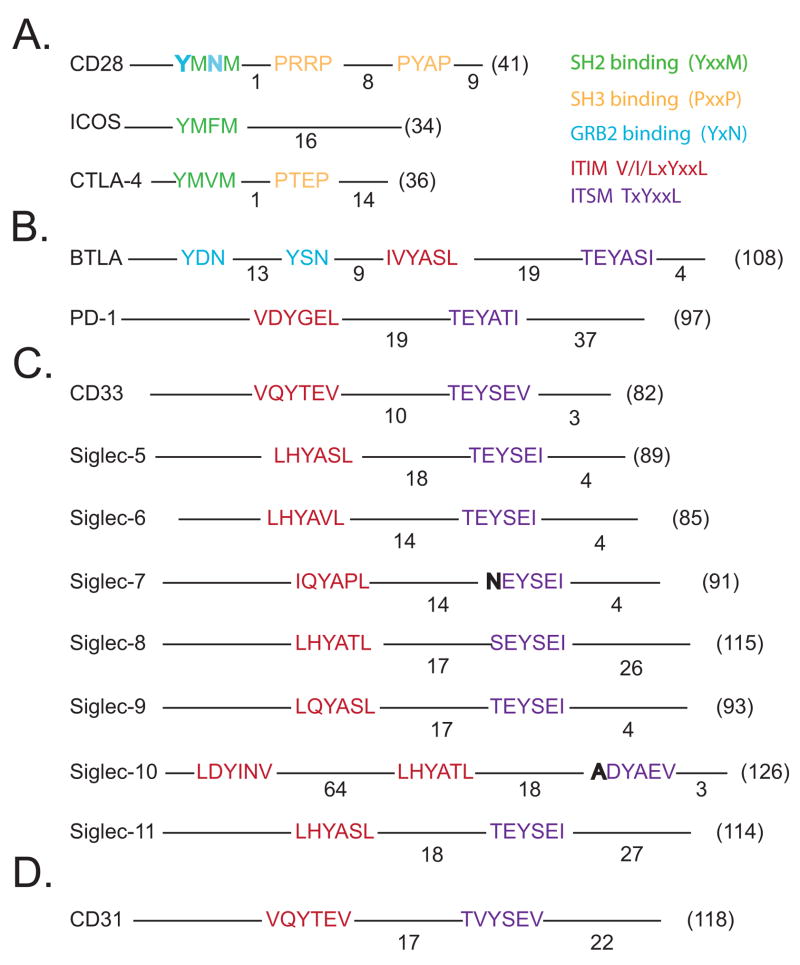
PD-1 consists of a single N-terminal IgV-like domain, an approximately 20 amino acid stalk separating the IgV domain from the plasma membrane, a transmembrane domain, and a cytoplasmic tail containing tyrosine-based signaling motifs. CD28, inducible costimulator (ICOS) and CTLA-4 all share this general design and modulate T-cell activity, so PD-1 was initially viewed as a member of the CD28 family. However, a close comparison of PD-1 with CD28, ICOS and CTLA-4 reveals many differences, especially from a signaling perspective, calling into question the authenticity of PD-1’s place in the CD28 family. B- and T-lymphocyte attenuator (BTLA), the most recently described member of the CD28 family (23), shares many features with PD-1 and may also be considered a CD28 family outlier. CD28, ICOS and CTLA-4 are genomic neighbors (human chromosome 2q33). In contrast, PD-1 is located on a different region of chromosome 2 (2q37), while BTLA is located on a different chromosome altogether (3q13). CD28, ICOS and CTLA-4 are well conserved in mammals. Comparison of the cytoplasmic tails of murine and human CD28, ICOS and CTLA-4 reveals 79%, 77%, and 100% identity, respectively, whereas the human PD-1 and BTLA cytoplasmic tails only share 59% and 54% identity with their murine homologues. Moreover, distinct mouse strains have different BTLA isoforms. For example, in some strains, two growth factor receptor-bound protein 2 (Grb2) (YXN) signaling motifs are present within the cytoplasmic tail, an arrangement seen in human BTLA; while in other strains, only one GRB2 domain is present (24). These observations suggest that PD-1 and BTLA are still under evolutionary pressure and the mechanism by which they signal is still being refined. Furthermore, the core CD28 family members (CD28, ICOS and CTLA-4) exist as dimers on the cell surface whereas PD-1 and BTLA exist as monomers (25–27). The differences in signaling events initiated by monomeric versus dimeric molecules have not been investigated thoroughly. A monomeric version of CD28 bound CD80 less well and costimulated T cells less efficiently than the wildtype dimer, suggesting that the dimeric nature of CD28 contributes to its function (28). However, we have observed that monomeric or dimeric forms of a chimeric molecule consisting of the murine CD28 extracellular domain fused to the human PD-1 cytoplasmic tail function equivalently (29).
CD28 family members, including family members whose heritage is in dispute, have no intrinsic enzymatic activity; rather, their tails serve as adaptors for signaling molecules recruited to the membrane following phosphorylation of tyrosine residues present within the tails. Thus, the sequence and presumably the position of the various tyrosine-based motifs ultimately determine how CD28 family members alter a T cell’s response to antigen. Human CD28, ICOS and CTLA-4 all have Src homology 2 (SH2)-binding (YxxM) motifs located in the center of their cytoplasmic tails (Fig. 1A). CD28 has two SH3-binding domains (PxxP), CTLA-4 has only one, and ICOS completely lacks an SH3-binding domain. PD-1 and BTLA, on the other hand, contain neither SH2 nor SH3 binding motifs (Fig. 1B). Similarly, CD28 has one Grb2-binding site (YxN) and BTLA has two, but Grb2-binding motifs are absent from the tails of all other family members. Furthermore, whether the motifs within the BTLA cytoplasmic tail actually recruit Grb2 or related molecules, or whether they are even important for BTLA function, is unclear (30, 31). In contrast, the importance of the Grb2 site for CD28 signaling is well documented (32–36).
Fig. 1. Schematic diagram showing the cytoplasmic tail of CD28 and Siglec family members.
(A) Dimeric members of the CD28 family. (B) Monomeric and Siglec-like members of the CD28 family. (C) Members of the Siglec family. (D) Example of non-CD28, non Siglec member with a single ITIM and ITSM.
PD-1 and BTLA do share a similar arrangement of tyrosine-containing immunoreceptor motifs not found in other CD28 family members: an immunoreceptor tyrosine-based inhibition motif (ITIM), defined as V/I/LxYxxL, followed by an immunoreceptor tyrosine-based switch motif (ITSM), defined as TxYxxL (Fig. 1B). Interestingly, the CD33-related Siglec family shares precisely this arrangement (Fig. 1C). Members of the CD33-Siglec family are expressed on the surface of the majority of immune cells as well as on cells in the central nervous system (37). Like PD-1 and BTLA, Siglec family members are thought to deliver a negative signal to counterbalance immunoreceptor tyrosine activation motif (ITAM)-positive signaling. Moreover, like PD-1 and BTLA, the Siglec family is evolving rapidly within mammalian species. Our closest evolutionary relative, the common chimpanzee (Pan troglodytes) expresses Siglec family members on T cells, whereas humans do not. Siglec expression limits chimpanzee T-cell expansion and is thought to contribute to the differential severity of T-cell-mediated diseases, such as acquired immunodeficiency syndrome and chronic active hepatitis, between humans and chimpanzees (38). The spacing between the ITSMs and ITIMs also appears to be conserved between the CD33-like Siglec family members and PD-1, suggesting that this spacing also may be important for function. Thus, the cytoplasmic tails of PD-1 and BTLA have much more in common with the Siglec family than the CD28 family, and signal transduction comparisons with the Siglec family will likely lead to greater insight into how PD-1 and BTLA function to limit T-cell expansion. Other molecules such as CD31 (Fig. 1D) also have a single ITIM and ITSM within their cytoplasmic tail so it will interesting to see if the mechanism of signaling is similar in all of these ITIM- and ITSM-containing receptors.
PD-1 signaling keeps T cells on a diet
While T-cell receptor (TCR) engagement orchestrates the changes in the T-cell gene expression profile, which enable a T cell to transform from perhaps the most quiescent cell in the body to an actively expanding immune effector (39–40), TCR engagement does not instruct T cells to uptake the necessary fuel (glucose) required for cell division and effector function (41). Costimulation, by activating phosphatidylinositol 3-kinase (PI3K) and its downstream target Akt, leads to increased expression of glucose transporters on the plasma membrane and general upregulation of glycolytic enzyme activity (42). Many receptors that promote cell growth and division, including CD28 and ICOS, bind the regulatory subunit of PI3K upon activation. This binding recruits the catalytic subunit of PI3K to the membrane, resulting in PI3K enzymatic activity. In the case of CD28 and ICOS, recruitment and activation of PI3K appears to be essential for their costimulatory activity (43). Thus, blocking PI3K activation would efficiently prevent T-cell activation because it would deny the cell the resources required for division, effector function, and differentiation.
To determine whether PD-1 engagement altered the CD28-mediated activation of PI3K, we assessed the ability of immunoprecipitated receptors to phosphorylate exogenous phosphatidylinositol. Primary human CD4+ T cells were preactivated with phytohemagglutinin (PHA) and IL-2 for three days to upregulate cell-surface expression of CTLA-4 and PD-1. CTLA-4 was included as a control, to determine if PI3K targeting was unique to PD-1-signal transduction. These preactivated T cells were stimulated with magnetic beads coated with anti-CD3, anti-human CD28, and anti-major histocompatibility complex (MHC) class I antibodies (CD3/h28/MHC I-coated beads), and then the same antibody-coated magnetic beads were used to immunoprecipitate signaling complexes associated with the respective cytoplasmic tails from cell lysates. CD28 costimulation resulted in a strong induction of PI3K activity detected in immunoprecipitates in agreement with previous reports (44). Stimulation of cells with CD3/h28/CTLA-4-coated beads resulted in precipitation of similar amounts of PI3K activity, indicating that CTLA-4 ligation does not inhibit PI3K signaling (Fig. 2) (45). In contrast, PD-1 engagement blocked the induction of PI3K activity. Since PI3K is required to activate Akt, the PD-1-mediated inhibition of PI3K activation is likely to be the major reason for the lack of Akt activation in cells following PD-1 ligation (45). Of interest, CTLA-4 engagement does interfere with glucose metabolism by inhibiting Akt phosphorylation through a protein phosphatase 2A-dependent mechanism (45). This observation suggests that targeting the ability of T cells to import glucose is a common and effective strategy for controlling T-cell activation.
Fig. 2. CTLA-4 and PD-1 target distinct signaling molecules.
Both PD-1 and CTLA-4 signaling inhibit Akt activation; however PD-1 ligation inhibits a more upstream membrane proximal step by blocking PI3K activation. In contrast, signaling by CTLA-4 preserves PI3K activity allowing expression of certain genes such as Bcl-xL, but inhibits Akt directly by activation of the phosphatase PP2A.
Even though PD-1 and CTLA-4 ligation efficiently block T-cell responses, the fact that they target distinct signaling molecules has potentially important implications. Perhaps the most significant implication is that agents that target PD-1 and CTLA-4 signaling should be synergistic. Additionally, cells undergoing PD-1 blockade will have a distinct phenotype from those undergoing CTLA-4 blockade. A comparison of the gene expression profiles of CD4+ T cells following CTLA-4 or PD-1 ligation reveals that PD-1-mediated signaling blocks T-cell activation more effectively than CTLA-4-mediated signaling. These data were quantified by measuring the number of transcripts whose expression levels were altered substantially (an increase or decrease of greater than fivefold) 24h after stimulation, relative to their levels in unstimulated T cells. We determined that levels of 517 transcripts were altered by CD3/CD28/CTLA-4 ligation, whereaslevels of only 128 transcripts were altered by CD3/CD28/PD-1 stimulation (45). To place these data in context, we compared them with data we previously generated comparing gene expression profiles of CD3/MHCI-, CD3/CD28-, and CD3/CD28/CTLA-4-stimulated cells (39). In that instance, the levels of 447 transcripts were altered at least fivefold by CD3/CD28/CTLA-4 stimulation, thus indicating that the results of these identically performed studies are directly comparable. CD3/MHC I stimulation resulted in altered levels of 238 transcripts; while after CD3/CD28 costimulation, expression levels of 1,427 transcripts were altered by this fivefold threshold. Therefore, CTLA-4 engagement reduced the number of transcripts that were regulated fivefold or more by CD3/CD28 costimulation by approximately 67%, while CD3/CD28/PD-1 engagement reduced the number of regulated transcripts by approximately 90%. PD-1 appears to be a more effective, or at least a more global, negative regulator of T-cell activation. Of note, the cell-survival gene Bcl-xL was upregulated following ligation of CTLA-4 (46), but not PD-1 (45), suggesting that PD-1 stimulation may render T cells more susceptible to apoptosis. The importance of targeting Akt and glucose metabolism by negative regulators of T-cell activation is underscored by the observations that IL-2 can override the effects of PD-1 ligation (47). IL-2 signaling triggers Akt activation via signal transducer and activator of transcription 5 signaling (48) and thus can bypass the blockade of TCR/costimulatory-mediated Akt activation.
A single mutation renders PD-1 non-functional
To identify the domains within the PD-1 cytoplasmic tail that are essential for inhibitory activity, we established a model to study PD-1 signaling in primary T cells by fusing the extracellular domain of murine CD28 (mCD28) to the human PD-1 cytoplasmic tail. This fusion permitted us to distinguish between introduced and endogenous PD-1 cytoplasmic tails. Stimulation of T cells expressing this chimeric signaling construct with an anti-mCD28 antibody inhibited the IL-2 production, Akt phosphorylation, and T-cell proliferation associated with costimulation with anti-human CD3 and CD28 (29). Therefore, this model faithfully recapitulated PD-1’s T-cell inhibitory functions. Next, we measured the contribution of the PD-1 ITIM and ITSM to this inhibition. We generated chimeric signaling constructs containing tyrosine to phenylalanine point mutations disrupting the ITIM (Y223F), ITSM (Y248F), or both (Y223F/Y248F). These constructs were introduced into primary human CD4+ T cells via lentiviral vector-mediated transduction, and the transduced cells were maintained in culture until their cell volume (size) declined to near resting T-cell levels. Each cell population was then restimulated with magnetic beads coated with CD3/MHC I, CD3/h28/MHC I, CD3/h28/mCD28 or CD3/h28/PD-1 antibodies, and Akt phosphorylation was monitored by western blot. No Akt phosphorylation was observed in unstimulated cells, but a modest induction of Akt-P was observed in anti-CD3/MHC I-restimulated cells. Phosphorylation of Akt was augmented considerably by the presence of anti-CD28 during restimulation. However, Akt phosphorylation was substantially reduced in cells restimulated with CD3/h28/PD-1 coated beads, indicating that engagement of the endogenous PD-1 receptor suppressed Akt phosphorylation in all transduced populations. This pattern of Akt phosphorylation was observed in all four cell populations, allowing us to evaluate the effect of the various PD-1 cytoplasmic-tail mutants in a side by side manner. Cells transduced with the mCD28/PD-1 wildtype construct and subsequently restimulated with CD3/h28/mCD28 coated beads contained substantially reduced levels of Akt phosphorylation compared to the same cells restimulated with CD3/h28/MHC class I-coated beads, validating this model for structure/function analyses of the PD-1 cytoplasmic tail. Cells transduced with mCD28/PD-1 Y223F behaved in a similar manner to those transduced with the mCD28/PD-1 wildtype construct, i.e. they contained diminished levels of Akt-P, indicating that the ITIM is not required to inhibit Akt phosphorylation. In contrast, in cells transduced with the mCD28/PD-1 Y248F construct or cells transduced with the double mCD28/PD-1 Y223F/Y248F construct, restimulation with CD3/h28/mCD28-coated beads resulted in high levels of phosphorylated Akt. These results indicate that factors binding to the ITSM within the PD-1 cytoplasmic tail mediate PD-1 suppression of PI3K/Akt activation and T-cell expansion. The requirement for an intact ITSM for PD-1 function was initially shown using an FcγRIIB extracellular domain in a murine B-cell line (49), suggesting that this motif is required in multiple species and cell types.
SHP-1 and/or SHP-2: that is the question
To identify signaling proteins who’s binding to the PD-1 cytoplasmic tail correlates with their ability to suppress T-cell activation, we introduced mCD28/PD-1 wildtype, mCD28/PD-1 Y223F, mCD28/PD-1 Y248F, and mCD28/PD-1 Y223F/Y248F chimeric receptors into primary human CD4+ T cells. Following stimulation with pervanadate to maximally phosphorylate the tyrosines within the PD-1 cytoplasmic tail, the chimeric receptors were immunoprecipitated and associated molecules were identified by western blot using a phosphotyrosine-specific antibody. We found that in addition to the phosphorylated chimeric receptor, which migrates at ~50kDa, only one other band, corresponding to an ~70kDa tyrosine-phosphorylated protein(s), was immunoprecipitated by the mCD28 antibody from cells transduced with the mCD28/PD-1 WT construct. This complex was still present in precipitates from cells expressing a PD-1 cytoplasmic tail with a mutant ITIM, but was severely reduced in cells expressing the mCD28/PD-1 Y248F. This complex was absent in cells transduced with the double mutant mCD28/PD-1 Y223F Y2248F construct, indicating that the formation of this complex was dependent upon the PD-1 ITSM. Also, the formation of this ~70 kDa PD-1-associated complex correlated with the ability of PD-1 ligation to regulate T-cell expansion and IL-2 production, making it a strong candidate for a factor(s) mediating PD-1 signaling. Others have found that SHP-2 is recruited to the PD-1 cytoplasmic tail in a B-cell line (49) and in Jurkat T cells (50). To evaluate whether this might also be the case in primary human T cells, we probed for SHP-2 and demonstrated that at least one component of this 70kD complex is SHP-2. SHP-1 also interacts with PD-1 in a modified yeast two-hybrid screen (51), making it another potential candidate for interaction with the PD-1 cytoplasmic tail. To determine whether SHP-1 was recruited to the PD-1 cytoplasmic tail in primary human CD4+ T cells, we probed for SHP-1 in these same immunoprecipitates and observed that, like SHP-2, SHP-1 was also recruited to the PD-1 cytoplasmic tail. Structural mapping demonstrated that SHP-1 also bound the PD-1 ITSM, suggesting that the requirements for SHP-1 and SHP-2 binding to the PD-1 cytoplasmic tail overlap. In contrast, using an analogous system in a B-cell tumor line, Okazaki et al. (52) did not detect SHP-1 binding to PD-1, indicating that PD-1 signaling may differ between B and T cells and/or primary and transformed cells.
SHP-1 and SHP-2 are structurally related protein tyrosine phosphatases (PTPs) that each contain two N-terminal SH2 domains, a classic PTP domain and a C-terminal tail that harbor sites for tyrosine phosphorylation (53, 54). A SH2 domain is an ~100 amino acid sequence that binds phosphorylated tyrosines. The specificity of each SH2 domain is largely determined by the three amino acids upstream of the tyrosine (55). The enzymatic activity of SHP proteins is negatively regulated by their own SH2-binding domain. When SHP proteins encounter an SH2-binding motif on a cytoplasmic tail, this inhibition is removed, and the attached SHP molecules are free to dephosphorylate nearby sequences recognized by their PTP domain (55). While many receptors (including PD-1) recruit both SHP-1 and SHP-2, there are clearly differences in their in vivo function and there is little evidence for functional redundancy. Thus, the cis factors that recruit SHP-1 and SHP-2 to a receptor are likely to be similar, but the downstream targets of SHP-1 and SHP-2 are likely to be different (54).
All of the data gathered to date indicate that SHP-1 functions as a negative regulator of cell activation. Mice deficient in SHP-1 expression (the naturally occurring motheaten phenotype has been studied in detail) are characterized by spotty hair loss and abnormalities in phagocytic leukocytes, which mediate inflammatory pathophysiology resulting in the motheaten mouse’s death 2–3 weeks after birth (56). SHP-1 expression is largely confined to cells of hematopoietic lineage (57). Thymocytes from SHP-1 deficient mice have prolonged phosphorylation of the TCR/CD3 complex, increased activation of Lck, Fyn, and other proximal TCR-signaling components, and they are hyper-responsive to TCR signaling (58–60). The role of SHP-2 within T cells is much less clear. SHP-2 is ubiquitously expressed and SHP-2 deficiency is embryonic lethal in mice (25). In most cases, SHP-2 plays a positive role in cell activation. SHP-2 can act as an adapter to recruit IRS to the insulin receptor (61) and Grb2 to both the platelet-derived growth factor receptor (62) and erythropoietin receptor (63). SHP-2 can also dephosphorylate and hence inactivate multiple negative regulators of cell activation. In particular, SHP-2 appears necessary for optimal induction of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) pathway as a phosphatase-dead mutant of SHP-2 interferes with epidermal growth factor, insulin and hepatocyte growth factor receptor signaling (63–65). While much of the research on SHP-2 focuses on how it enhances cell activation, there is evidence that SHP-2 activation is involved in negative regulation of growth hormone and IL-6 signaling (66, 67) and natural killer (NK)-cell activation (68), suggesting that SHP-2 is also a viable candidate to mediate PD-1 immunosuppressive functions. Most proteins that bind SHP-1 and SHP-2 contain multiple ITIMs. Whether these motifs work cooperatively or independently to recruit these phosphatases is currently unknown. To date, it has proven difficult to determine whether SHP-1, SHP-2, or both SHP-1 and SHP-2 (or neither) are required for PD-1 function. Since SHP-1 and SHP-2 bind the same sequence within the PD-1 cytoplasmic tail, it is not possible to preferentially inhibit binding of either SHP. Targeting SHP-1 and SHP-2 expression via knockout mice, small interfering RNA (siRNA), or small molecule inhibitors is possible, but the difficulty then is to determine whether any observed phenotype is a direct result of loss of PD-1 activity or a secondary effect resulting from the disabling of another inhibitory receptor. There are over 100 receptors that contain ITIM and/or ITSM sequences (69) and loss of SHP-1 and/or SHP-2 will also affect these signaling proteins. For instance, as described above, mature T cells from SHP-1-deficient (motheaten) animals are viable but are also hyperresponsive. Thus, the inability of PD-1 engagement to block motheaten mouse T-cell proliferation could indicate that SHP-1 is required PD-1 function, or alternatively, it could indicate that PD-1 can only block T-cell activation in T cells with much lower thresholds for activation. Similarly, while SHP-1 binds to FcγRIIB, it has been demonstrated that this binding is not required for FcγRIIB inhibitory activity (70). Thus, assays linking factor recruitment and activation with biological readouts are required to determine the role of the SHPs in PD-1-mediated signal transduction. Moreover, current models of SHP-1 and SHP-2 binding and activation envision individual SH2 domains binding discrete phosphorylated ITIMs. We and others have shown that PD-1 is quite functional when it contains just a single tyrosine molecule (29, 49). Likewise, BTLA retains full inhibitory function when just one of its four tyrosine residues is present (31). These data force us to consider other models. The NK inhibitory receptor 2DL4 (CD158d) contains only one ITIM in its cytoplasmic tail and it is able to effectively block NK responses (68). This receptor recruits SHP-2, but not SHP-1, to its cytoplasmic tail, reinforcing the finding that SHP-1 requires two phosphotyrosine motifs for efficient binding and activity (71). This observation would seem to support the notion that PD-1 does indeed recruit SHP-2. On the other hand, the ITSM is required for SHP-1 but not SHP-2 recruitment in Siglec cytoplasmic tails, consistent with the notion that SHP-1 may be the main effector in PD-1-mediated signaling (72). These uncertainties suggest the existence of additional levels of complexity in PD-1 signaling. For example, the ITSM in CD150 recruits SHP-2 upon receptor engagement. However, in the presence of the small adaptor molecules SH2 domain protein 1A (SH2D1A) or Ewing’s sarcoma/ Friend leukemia virus integration 1-activated transcript 2 (Eat2) (73), the ITSM recruits SHIP to CD150, and hence, it is able to ‘switch’ which molecules it recruits based on the presence or absence of SH2D1A. In contrast to the CD150 ITSM, the PD-1 ITSM appears to be unable to bind SH2D1A, suggesting that there is heterogeneity in the mechanism by which ITSMs signal. However, other heretofore uncharacterized adaptor molecules may also play critical roles in binding of cofactors to the ITSM within the PD-1 cytoplasmic tail.
It should be stressed that the presence of an ITIM and/or ITSM does not signify that SHP-1 and/or SHP-2 are recruited under physiologic conditions. As stated above, there are no published findings in which SHP-1 and/or SHP-2 are recruited to the PD-1 cytoplasmic tail under physiologic conditions. SH2-containing inositol phosphatase (Ship) also binds to ITIMs and can inhibit PI3K activity (74). We did not observe Ship recruitment upon pervanadate treatment (J. M. Chemnitz and J. L. Riley, unpublished data) but perhaps under physiologic stimuli it is recruited to the PD-1 cytoplasmic tail. Another candidate is C-terminal Src kinase (Csk) (75). Leukocyte-associated Ig-like receptor-1 (LAIR) can recruit SHP-1 and SHP-2 (51, 76, 77) but in cells that naturally lack both SHP-1 and SHP-2 this receptor still has inhibitory activity (78). By using a yeast tri-hybrid system, phosphorylated LAIR was found to bind Csk, suggesting that it plays a role in LAIR-mediated inhibition (78). ILT2 (CD85j) is another receptor that recruits Csk to an ITIM (79). Thus, SHP-1 and SHP-2 are only two of a wide array of signaling molecules that could potentially bind to the PD-1 tail. Satisfactory resolution of these questions will require the development of better models.
Cell-specific effects of PD-1 signaling?
Resting T cells from healthy individuals express barely detectable levels of PD-1 on their cell surface. However, when PD-1 is engaged on these cells, the earliest events in T-cell activation are blocked, including PI3K activation (29, 45). This suggests that PD-1 is a highly effective negative regulator of T-cell activation even when modestly expressed on the T-cell surface, cautioning against correlations between expression levels and PD-1 function. Yet, as described above, PD-1 is significantly upregulated in exhausted T cells, and interfering with PD-L1 and PD-1 interactions does restore some aspects of T-cell function in exhausted T cells (5, 80–82). Whether higher expression levels of PD-1 lead to the triggering of distinct signaling pathways or whether additional PD-1 engagement leads to ‘more of the same’ signaling is currently unknown. Given the differences in the outcome of PD-1 signaling on naive cells (prevention of T-cell activation) and exhausted T cells (progressive loss of effector functions) it is interesting to entertain the idea that distinct PD-1-mediated signaling pathways could be triggered in distinct cell types. SHP-1 was recently shown to be a digital regulator of T-cell activation (83). That is, modest changes in its expression level were able to influence whether a particular T cell responded to antigen. Thus, one prediction/implication of the model proposed by Feinerman et al. (83) is that as a T cell differentiates from naive to effector to memory and/or exhausted phenotypes, the relative level of SHP-1 may change. For instance, SHP-1 may be relatively high in naive T cells but lower in effector and memory cells and it is interesting to speculate about SHP-1 expression levels in exhausted cells. Perhaps in naive T cells SHP-1 is able to outcompete SHP-2 for binding to the PD-1 cytoplasmic tail, and this binding results in attenuation of T-cell activation. Thus, even though there is less PD-1 on the cell surface, the overall effect of PD-1 ligation on T-cell activation would be more severe because SHP-1 is an efficient inhibitor of T-cell activation. As the T cell differentiates into an exhausted T cell, perhaps the relative ratio of SHP-1 and SHP-2 changes, or the elevated expression of PD-1 on the T-cell surface allows more recruitment of SHP-2. The consequences of SHP-2 signaling may trigger chromatin changes so that the promoter regions of IL-2, TNF-α, and IFN-γ become progressively less accessible to transcription factors. This change in the relative recruitment could explain the distinct outcomes of PD-1 ligation in naive and exhausted T cells. Likewise, excess PD-1 and other inhibitory receptors could provide more targets than SHP-1 or SHP-2 can bind, perhaps allowing other signaling molecules to bind and trigger distinct signaling pathways. Similarly, distinct outcomes of PD-1 ligation may occur in B cells (49), myeloid cells, and T regulatory cells. Once more physiologic models of PD-1 signal transduction are available, it should be relatively straightforward to formally test these hypotheses.
Improved models to study PD-1 signaling
Transfected, transformed cell lines have been the workhouse of signaling studies because they are easy: easy to culture, easy to transfect, and easy to share among different laboratories. However, within the last several years it has become apparent that Jurkat and other T cell lines have many drawbacks for studying costimulatory pathways (84). In a gene array study, we found that within the first two hours of stimulation, Jurkat T cells upregulated 93 transcripts when stimulated with anti-CD3 antibody, and 148 transcripts when stimulated with anti-CD3 and anti-CD28 (39). In contrast, primary human CD4+ T cells upregulated a similar number of transcripts after anti-CD3 antibody stimulation (145), but after anti-CD3 and anti-CD28 antibody costimulation, we observed that 909 transcripts were upregulated during the first two hours of activation. Clearly, Jurkat T cells are impaired in their ability to receive costimulatory signals. Use of transformed cell lines is even more perilous when studying negative regulators of T-cell activation. Part of the explanation of why Jurkat T cells are less responsive to CD28 costimulation than primary cells is that Jurkat T cells are always in cell cycle. Since many of the transcripts altered by CD28 costimulation help T cells progress past G0 in the cell cycle, these changes are not observed in cells that are never in G0. Furthermore, transformed cell lines like Jurkat are defective in signaling molecules such as phosphatase and tensin homolog (PTEN) and Ship that oppose uncontrolled growth (85,86). We and many others have shown that the main targets of negative regulators of T-cell activation are cell growth and metabolism (14, 16, 42, 87). Thus, if a cell is not responsive to signals that limit proliferation and metabolism, then it is extraordinarily limited as a model for studying negative regulators such as PD-1. For these reasons, using primary cells for signaling studies is essential for understanding the function of PD-1 and other negative regulators of T-cell activation.
To date, all studies identifying factors recruited to the PD-1 cytoplasmic tail have been performed using pervanadate to phosphorylate the PD-1 cytoplasmic tail. Pervanadate, by inhibiting phosphatase activity, is perhaps the most effective way to recruit phospho-specific signalling molecules to cytoplasmic tails, but it is highly non-physiologic. In a study examining factors recruited to the closely related BTLA cytoplasmic tail, we found that both SHP-1 and SHP-2 bound after pervanadate treatment (31). However, when we used a more physiologic stimulus, magnetic beads coated with anti-CD3, anti-CD28 and anti-BTLA antibody, we observed only SHP-1 recruitment. Additionally, mutations that abrogated SHP-1 recruitment did not impair BTLA function (31). These studies called into question the role, if any, of SHP-1 and SHP-2 in BTLA function, and ended our use of pervanadate as a means to initiate signal transduction cascades in primary cells. The use of antibody coated beads to engage CD3, CD28, and PD-1 is more physiologic than pervanadate, and has the added benefit that these beads are magnetic and contain no protein that could interfere with T-cell signal transduction assays, facilitating their use in immunoprecipitation studies (88). However, the use of antibodies instead of natural ligands results in non-physiologic engagement of the TCRs, forcing one to titrate the amount of antibody added (especially anti-CD3 antibody) in order to observe the effects of PD-1 ligation (29). In vivo, dendritic cells (DCs) are the best APCs for expansion of antigen-specific T cells (89). However, they have several limitations that preclude their use in signaling studies. They are not self-renewing, and they are difficult to culture reproducibly. Furthermore, DCs express so many costimulatory and coinhibitory molecules that reductionist approaches focusing on the contribution of individual signaling molecules are difficult, if not impossible. Thus, a self-renewing, fully defined artificial APC (aAPC) that circumvents these issues would be useful. We chose the human erythromyeloid chronic myelogenous leukemia cell line K562 as a scaffold for these aAPCs, because they do not express human leukocyte antigen (HLA) proteins that would promote allogeneic responses, but they do express intracellular adhesion molecule (ICAM, CD54) and lymphocyte function antigen-3 (LFA-3, CD58), both of which promote interactions with T cells (90). Additionally, K562 cells are highly susceptible to lentiviral vector-mediated transduction, permitting the simultaneous introduction of multiple vectors and thus the rapid generation of ‘customized’ aAPCs (91).
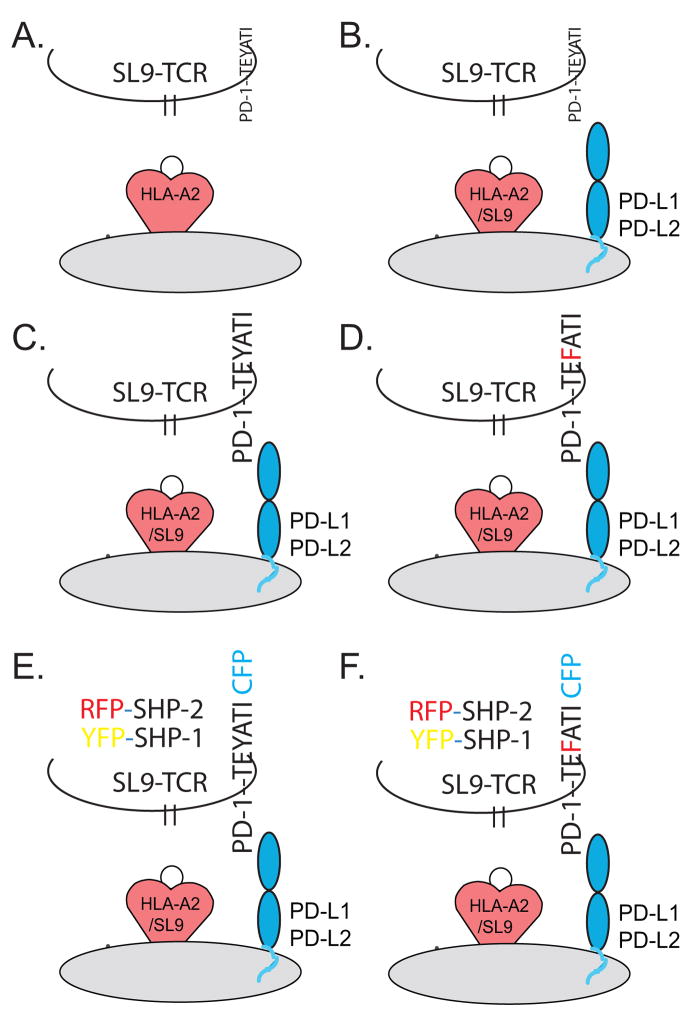
aAPCs can provide the natural ligands for costimulatory and coinhibitory receptors and they can present antigen via MHC expression. However, to achieve physiologic T-cell stimulation, signal 1 must be delivered by the TCR-binding peptide-MHC (pMHC) in an antigen-specific manner. T cell clones can be used, but these cells often lose their effector functions. Moreover, T cell clones are likely to be less responsive to negative signals and thus may not be optimal models. A more attractive option would be to use freshly isolated T cells modified to express TCRs that confer MHC-restricted antigen specificity. We and others recently reported the generation of human CD8+ T cells transduced with TCRs recognizing the HLA-A2 restricted human immunodeficiency virus (HIV)GAG-specific epitope SL9 (amino acids 77 to 85; SLYNTVATL)(92, 93). Thus, polyclonal human T cells can be converted to monoclonal transgenic T cells; enabling antigen-specific activation of sufficient numbers of human T cells (10–40 million) for the performance of biochemical studies. The use of the picornavirus-like 2A ‘self-cleaving’ peptide facilitates signal transduction studies in primary cells, because multiple gene products can be introduced using a single vector (94). In particular, the use of the 2A sequence permits coordinate expression of TCRα and TCRβ-chain genes using a single vector and additional genes can be linked using additional 2A sequences, facilitating more complex signal transduction assays (95). Fig. 3 illustrates how antibody-free signal transduction assays can be performed using primary human T cells. We have previously demonstrated that CD8+ T cells transduced with SL9-specific TCRs proliferate and produce cytokines in response to K562 cells transduced with HLA-A2 and HIV-1GAG (K.A2.GAG) (92). This response is dependent upon both the expression of HLA-A2 and HIV-1GAG in the aAPC. With this basic framework in hand, one can modify both the aAPC and T cell to dissect PD-1 signal transduction pathways. Importantly, introduction of PD-L1 into the aAPC interferes with this response (Z. Wei and J. L. Riley, unpublished data), validating this system as a means to study PD-1 signal transduction (Fig. 3A, B). Moreover, additional costimulatory and coinhibitory molecules could be introduced to determine how these additional signals alter the PD-1 signal. Additionally, the potential contribution of TCR affinity to PD-1-mediated signal transduction can be measured by using TCRs with varying affinities for pMHC (92, 96). To determine if PD-1-expression levels alter the composition of signaling complexes recruited to the PD-1 cytoplasmic tail, PD-1 chimeric receptors under the control of promoters of varying strength can be transduced into the T cell (Fig. 3C, D). After mixing the transduced T cells with aAPCs, PD-1 can be immunoprecipitated so that the complexes binding its cytoplasmic can be examined by western blot. Likewise, fluorescent versions of SHP proteins could be introduced, as well as a PD-1 molecule that has cyan fluorescent protein attached to its cytoplasmic tail. With these molecules, microscopy-based assays such as fluorescence resonance energy transfer (FRET) could be employed to determine whether SHP-1 and/or SHP-2 are recruited upon PD-1 engagement (Fig. 3E, F). Similar models can be employed to study murine T cells. Given the more profound effects of PD-1 blockade observed in murine models when compared to those observed in human models (15), it would be interesting to perform side-by-side studies to determine if distinct species-specific PD-1 signaling complexes are recruited.
Fig. 3. Models to study PD-1 signaling transduction in primary T cells in the absence of antibodies.
(A) T cells transduced with SL9-specific TCRs recognize and respond to base aAPC expressing HLA-A2 and HIV-1GAG. (B) Introduction of PD-L1 to the aAPC diminishes antigen-specific responses. (C) Introduction of PD-1 by transduction permits the side by side study of T cells with varying levels of PD-1. (D) Use of labeled signaling molecules permits microscope-based localization and functional studies.
Conclusions
PD-1 was initially described as a molecule preferentially expressed in dying cells (1). Despite a few reports claiming its ligand PD-L1 (B7-H1) was a costimulatory molecule (97), the initial studies involving PD-1 function and signaling were relatively straightforward, especially when compared to the other members of the CD28 family (98). However, the striking overexpression of PD-1 on resting exhausted T cells and the restoration of immune function in exhausted T cells upon PD-1 blockade (5) forever changed PD-1’s perception as the easy child of the CD28 family. These findings significantly increased the importance of understanding how PD-1 functions in normal cells and ushered in a whole new set of questions including: does PD-1 signal differently in naive, effector, memory or exhausted T cells; can alterations in PD-1 signaling prevent differentiation or T-cell exhaustion; and how does PD-1 engagement affect the generation of T-cell effector functions? A full understanding of PD-1 signal transduction will shed important light on the molecular mechanisms behind T-cell exhaustion and will potentially reveal ways to overcome T-cell exhaustion to enable clearance of chronic infection and tumors.
Acknowledgments
I would like to thank my long time collaborator Dr. Richard Carroll for helpful suggestions; current and former Riley/June lab members especially Drs. Richard Parry and Jens Chemnitz for making important contributions to study of PD-1 signaling transduction, and members of PD-1 PO1 group (AI080192) for helpful suggestions and insight. Support for these studies was provided by the Abramson Family Research Cancer Institute and the NIH (AI080192, AI057838, and CA113783).
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 6.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol. 2007;179:7466–7477. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Jiang F, Honjo T, Okazaki T. PD-1 deficiency reveals various tissue-specific autoimmunity by H-2b and dose-dependent requirement of H-2g7 for diabetes in NOD mice. Proceedings of the National Academy of Sciences. 2008;105:3533–3538. doi: 10.1073/pnas.0710951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Jr, Sayegh MH, Najafian N. Analysis of the Role of Negative T Cell Costimulatory Pathways in CD4 and CD8 T Cell-Mediated Alloimmune Responses In Vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 13.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 15.Riley JL, June CH. The road to recovery: translating PD-1 biology into clinical benefit. Trends Immunol. 2007;28:48–50. doi: 10.1016/j.it.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 17.Martinic MM, von Herrath MG. Novel strategies to eliminate persistent viral infections. Trends Immunol. 2008;29:116–124. doi: 10.1016/j.it.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 20.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 22.Grakoui A, John WE, Hanson HL, Walker C, Ahmed R. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J Hepatol. 2006;45:468–472. doi: 10.1016/j.jhep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 24.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 25.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 26.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Lazar-Molnar E, Almo SC, Nathenson SG. The interchain disulfide linkage is not a prerequisite but enhances CD28 costimulatory function. Cell Immunol. 2006;244:125–129. doi: 10.1016/j.cellimm.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 30.Gavrieli M, Murphy KM. Association of Grb-2 and PI3K p85 with phosphotyrosile peptides derived from BTLA. Biochem Biophys Res Commun. 2006;345:1440–1445. doi: 10.1016/j.bbrc.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 31.Chemnitz JM, Lanfranco AR, Braunstein I, Riley JL. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J Immunol. 2006;176:6603–6614. doi: 10.4049/jimmunol.176.11.6603. [DOI] [PubMed] [Google Scholar]
- 32.Ellis JH, Ashman C, Burden MN, Kilpatrick KE, Morse MA, Hamblin PA. GRID: a novel Grb-2-related adapter protein that interacts with the activated T cell costimulatory receptor CD28. J Immunol. 2000;164:5805–5814. doi: 10.4049/jimmunol.164.11.5805. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe R, Harada Y, Takeda K, Takahashi J, Ohnuki K, Ogawa S, Ohgai D, Kaibara N, Koiwai O, Tanabe K, Toma H, Sugamura K, Abe R. Grb2 and Gads exhibit different interactions with CD28 and play distinct roles in CD28-mediated costimulation. J Immunol. 2006;177:1085–1091. doi: 10.4049/jimmunol.177.2.1085. [DOI] [PubMed] [Google Scholar]
- 34.Okkenhaug K, Rottapel R. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J Biol Chem. 1998;273:21194–21202. doi: 10.1074/jbc.273.33.21194. [DOI] [PubMed] [Google Scholar]
- 35.Kim HH, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J Biol Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- 36.Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci U S A. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 42.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 43.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 44.Ward SG, Westwick J, Hall ND, Sansom DM. Ligation of CD28 receptor by B7 induces formation of D-3 phosphoinositides in T lymphocytes independently of T cell receptor/CD3 activation. Eur J Immunol. 1993;23:2572–2577. doi: 10.1002/eji.1830231029. [DOI] [PubMed] [Google Scholar]
- 45.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair PJ, Riley JL, Levine BL, Lee KP, Craighead N, Francomano T, Perfetto SJ, Gray GS, Carreno BM, June CH. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-X(L) induction. J Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- 47.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Lockyer HM, Tran E, Nelson BH. STAT5 Is Essential for Akt/p70S6 Kinase Activity during IL-2-Induced Lymphocyte Proliferation. J Immunol. 2007;179:5301–5308. doi: 10.4049/jimmunol.179.8.5301. [DOI] [PubMed] [Google Scholar]
- 49.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 51.Sathish JG, Johnson KG, Fuller KJ, LeRoy FG, Meyaard L, Sims MJ, Matthews RJ. Constitutive association of SHP-1 with leukocyte-associated Ig-like receptor-1 in human T cells. J Immunol. 2001;166:1763–1770. doi: 10.4049/jimmunol.166.3.1763. [DOI] [PubMed] [Google Scholar]
- 52.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 54.Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 55.Tsui F, Martin A, Wang J, Tsui H. Investigations into the regulation and function of the SH2 domain-containing protein-tyrosine phosphatase, SHP-1. Immunologic Research. 2006;35:127–136. doi: 10.1385/IR:35:1:127. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 57.Ulyanova T, Blasioli J, Thomas ML. Regulation of cell signaling by the protein tyrosine phosphatases, CD45 and SHP-1. Immunol Res. 1997;16:101–113. doi: 10.1007/BF02786326. [DOI] [PubMed] [Google Scholar]
- 58.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 59.Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenz U, Ravichandran KS, Burakoff SJ, Neel BG. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci U S A. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kharitonenkov A, Schnekenburger J, Chen Z, Knyazev P, Ali S, Zwick E, White M, Ullrich A. Adapter function of protein-tyrosine phosphatase 1D in insulin receptor/insulin receptor substrate-1 interaction. J Biol Chem. 1995;270:29189–29193. doi: 10.1074/jbc.270.49.29189. [DOI] [PubMed] [Google Scholar]
- 62.Tauchi T, Feng GS, Shen R, Hoatlin M, Bagby GC, Jr, Kabat D, Lu L, Broxmeyer HE. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J Biol Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 63.Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett AM, Hausdorff SF, O’Reilly AM, Freeman RM, Neel BG. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stofega MR, Herrington J, Billestrup N, Carter-Su C. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol Endocrinol. 2000;14:1338–1350. doi: 10.1210/mend.14.9.0513. [DOI] [PubMed] [Google Scholar]
- 67.Lehmann U, Schmitz J, Weissenbach M, Sobota RM, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki A, Schneider-Mergener J, Yoshimura A, Neel BG, Heinrich PC, Schaper F. SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130. J Biol Chem. 2003;278:661–671. doi: 10.1074/jbc.M210552200. [DOI] [PubMed] [Google Scholar]
- 68.Yusa S, Catina TL, Campbell KS. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol. 2002;168:5047–5057. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 69.Staub E, Rosenthal A, Hinzmann B. Systematic identification of immunoreceptor tyrosine-based inhibitory motifs in the human proteome. Cell Signal. 2004;16:435–456. doi: 10.1016/j.cellsig.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 70.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 71.Fry AM, Lanier LL, Weiss A. Phosphotyrosines in the killer cell inhibitory receptor motif of NKB1 are required for negative signaling and for association with protein tyrosine phosphatase 1C. J Exp Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) Can Mediate Inhibitory Signaling in the Absence of Immunoreceptor Tyrosine-based Inhibitory Motif Phosphorylation. J Biol Chem. 2005;280:19843–19851. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 73.Roncagalli R, Taylor JE, Zhang S, Shi X, Chen R, Cruz-Munoz ME, Yin L, Latour S, Veillette A. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat Immunol. 2005;6:1002–1010. doi: 10.1038/ni1242. [DOI] [PubMed] [Google Scholar]
- 74.Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide Lipid Phosphatases: Natural Regulators of Phosphoinositide 3-Kinase Signaling in T Lymphocytes. J Biol Chem. 2008;283:2465–2469. doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- 75.Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- 76.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 77.Verbrugge A, Ruiter TT, Clevers H, Meyaard L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int Immunol. 2003;15:1349–1358. doi: 10.1093/intimm/dxg134. [DOI] [PubMed] [Google Scholar]
- 78.Verbrugge A, Rijkers ES, de RT, Meyaard L. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur J Immunol. 2006;36:190–198. doi: 10.1002/eji.200535226. [DOI] [PubMed] [Google Scholar]
- 79.Sayos J, Martinez-Barriocanal A, Kitzig F, Bellon T, Lopez-Botet M. Recruitment of C-terminal Src kinase by the leukocyte inhibitory receptor CD85j. Biochemical and Biophysical Research Communications. 2004;324:640–647. doi: 10.1016/j.bbrc.2004.09.097. [DOI] [PubMed] [Google Scholar]
- 80.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 81.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 82.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feinerman O, Veiga J, Dorfman JR, Germain RN, tan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321:1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 85.Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freeburn RW, Wright KL, Burgess SJ, Astoul E, Cantrell DA, Ward SG. Evidence That SHIP-1 Contributes to Phosphatidylinositol 3,4,5-Trisphosphate Metabolism in T Lymphocytes and Can Regulate Novel Phosphoinositide 3-Kinase Effectors. J Immunol. 2002;169:5441–5450. doi: 10.4049/jimmunol.169.10.5441. [DOI] [PubMed] [Google Scholar]
- 87.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 88.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 89.Young JW, Merad M, Hart DNJ. Dendritic Cells in Transplantation and Immune-Based Therapies. Biology of Blood and Marrow Transplantation. 2007;13:23–32. doi: 10.1016/j.bbmt.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 90.Thomas AK, Maus MV, Shalaby WS, June CH, Riley JL. A cell-based artificial antigen-presenting cell coated with anti-CD3 and CD28 antibodies enables rapid expansion and long-term growth of CD4 T lymphocytes. Clin Immunol. 2002;105:259–272. doi: 10.1006/clim.2002.5277. [DOI] [PubMed] [Google Scholar]
- 91.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joseph A, Zheng JH, Follenzi A, Dilorenzo T, Sango K, Hyman J, Chen K, Piechocka-Trocha A, Brander C, Hooijberg E, Vignali DA, Walker BD, Goldstein H. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82:3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang J, Qian JJ, Yi S, Harding TC, Tu GH, Vanroey M, Jooss K. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 95.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 96.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 97.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 98.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]