Abstract
NMDA receptor (NMDAR) activation requires coincident binding of the excitatory neurotransmitter glutamate and a coagonist, either glycine or d-serine. Changes in NMDAR currents during neural transmission are typically attributed to glutamate release against a steady background of coagonist, excluding the possibility of coagonist release. AMPA receptor (AMPAR) stimulation evokes d-serine release, but it is unknown whether this is a physiological phenomenon capable of influencing synaptic responses. In this study, we utilized the intact retina to determine whether light-evoked synaptic activity in retinal ganglion cells (RGCs) is shaped by a dynamic pool of coagonist. The application of AMPAR antagonist abolished light-evoked NMDAR currents, which were rescued by adding coagonist to the bath. When NMDA was globally applied to RGCs via bath or picospritzing, the coagonist occupancy was also dependent on AMPARs but to a lesser extent than that observed during light responses, suggesting a difference in extrasynaptic coagonist regulation. By saturating the glutamate binding site of NMDARs, we were able to detect released coagonist reaching RGCs during light-evoked responses. Mutant mice lacking the d-serine-synthesizing enzyme serine racemase were deficient in coagonist release. Coagonist release in wild-type retinas was notably greater in ON than in OFF responses and depended on AMPARs. These findings suggest activity-dependent modulation of coagonist availability, particularly d-serine, and may add an extra dimension to NMDAR coincidence detection in the retina.
Keywords: extrasynaptic receptors, glycine
n-methyl-d-aspartate receptors (NMDARs) serve as molecular coincidence detectors, requiring sufficient depolarization for the removal of an external Mg2+ block and glutamate binding to the GluN2 subunit. Early studies showed that ion channel gating of NMDARs also requires binding of a coagonist, either glycine or d-serine, to the GluN1 subunit (Johnson and Ascher 1987; Kleckner and Dingledine 1988; Schell 2004). It was initially thought that high levels of ambient glycine in the nervous system were sufficient to fully occupy the coagonist binding sites. However, numerous studies throughout the nervous system have since demonstrated that the coagonist sites are not saturated (Chen et al. 2003; Martina et al. 2003; Stevens et al. 2003), raising the possibility that changes in coagonist levels could determine the number of NMDARs available during excitatory transmission.
The discovery that d-serine is present in the brain (Hashimoto et al. 1992) and that bath application of d-serine-degrading enzymes reduces NMDAR currents (Mothet et al. 2000; Stevens et al. 2003) added further complexity to understanding coagonist regulation and function. d-Serine and its synthesizing enzyme serine racemase have been found in glia (Schell et al. 1995) and more recently in neurons (Kartvelishvily et al. 2006). Biochemical measurements of extracellular d-serine from glial (Schell et al. 1995; Mothet et al. 2005) and neuronal (Kartvelishvily et al. 2006) cultures have shown that AMPA receptor (AMPAR) stimulation evokes d-serine release. d-Serine levels may increase after AMPAR stimulation by activating serine racemase via the glutamate receptor interacting protein (GRIP) (Kim et al. 2005). Although AMPAR stimulation is sufficient for d-serine release, it is unknown whether this mechanism is utilized during synaptic transmission. There is electrophysiological evidence for coagonist release acting on postsynaptic NMDARs during evoked potentials in hippocampus (Li et al. 2009), cerebellar slices (Billups and Attwell 2003), and spinal cord (Ahmadi et al. 2003), but these studies did not differentiate between glycine and d-serine.
The retina is an ideal tissue for studying the physiological mechanisms of coagonist release because glutamate release from ON bipolar cells is mediated by mGluR6 receptors, leaving ON responses in ganglion cells intact under a variety of pharmacological conditions. In rat retinal slice preparations, pharmacological activation of ON bipolar cells evokes coagonist release capable of acting on retinal ganglion cell (RGC) NMDARs (Kalbaugh et al. 2009). However, from these studies it was unclear whether the coagonist release observed was glycine or d-serine. d-Serine is present in Müller cells and astrocytes of the retina and is essential for the activation of the NMDARs involved in light responses (Gustafson et al. 2007; Stevens et al. 2003). Experiments in mice have revealed that AMPAR stimulation causes release of d-serine from retinal glia (Sullivan and Miller 2010), although the mechanism of this release and whether or not it happens during light stimulation have not been established.
In the present study we detected light-evoked coagonist release in the isolated mouse retina that contributed to RGC NMDAR currents. We exploited a serine racemase knockout (SRKO) mouse in which d-serine levels are significantly reduced (Basu et al. 2009; Sullivan et al. 2011) to aid in identifying the coagonist. Light-evoked coagonist release was significantly reduced in the SRKO RGC, suggesting that the release measured was d-serine. Light-evoked coagonist release was significantly reduced by applying the AMPAR antagonist NBQX. When we applied NMDA to the bath or directly pressure-ejected NMDA onto RGCs, we observed that NQBX also reduced coagonist occupancy, although this effect was substantially smaller than that observed for light-evoked responses. NMDA-evoked currents in SRKO RGCs were similar in magnitude to wild type (wt), despite the fact that SRKO mice have little or no coagonist contribution to light responses (Sullivan et al. 2011). On the basis of these overall findings, we conclude that AMPAR-mediated d-serine release during light responses serves as the coagonist for the RGC NMDARs activated by synaptic glutamate, whereas another coagonist, presumably glycine, acts on nonsynaptic NMDARs that are revealed by global NMDA application. A model describing our interpretation of these experiments is presented in discussion (see Fig. 4).
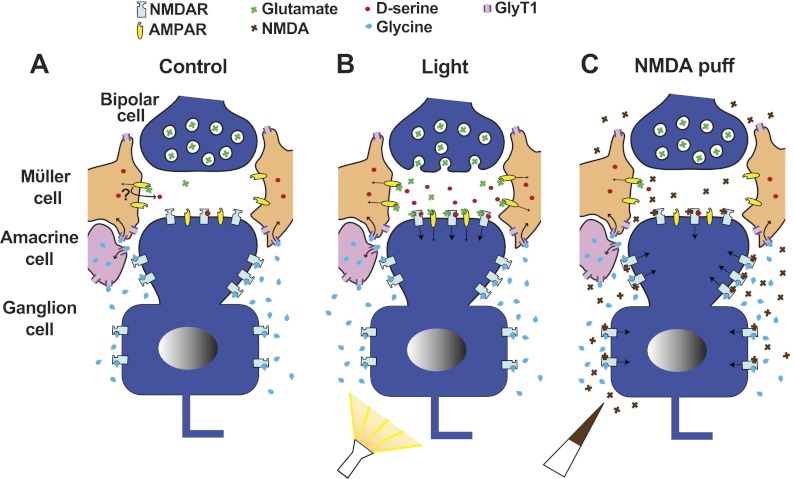
Fig. 4.
Proposed model illustrating the regulation of coagonist in the inner plexiform layer. Müller cell processes surrounding the bipolar-ganglion cell synapse contribute d-serine at RGC synaptic sites, while glycine transporters (GlyT1), expressed in Müller and amacrine cells, restrict glycine to extrasynaptic sites. NMDAR activation requires glutamate and a coagonist. Gated NMDA and AMPARs indicated by arrow. A: in the absence of light stimulation, moderate activation of Müller cell AMPARs by ambient glutamate causes the release of d-serine through an unknown mechanism. B: light-evoked glutamate release from bipolar cell terminals activates additional AMPARs, evoking currents in RGCs and also causing further d-serine release from Müller cells. d-Serine recruits additional synaptic NMDARs for activation via glutamate. C: pressure-ejected or bath-applied NMDA largely activates extrasynaptic NMDARs occupied by glycine, while synaptic activation is limited by low levels of d-serine.
MATERIALS AND METHODS
Retina preparation.
Experiments were performed in strict accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Adult C57BL/6 mice were killed by an overdose of Nembutal (0.1 ml of 50 mg/ml ip) followed by pneumothorax. Eyes were enucleated and placed in bicarbonate Ringer solution (in mM: 111 NaCl, 3 KCl, 2 CaCl2, 1 MgSO4, 32 NaHCO3, 0.5 NaH2PO4, and 15 dextrose; bubbled with 95% O2-5% CO2) for surgical isolation of the retina. After vitreous removal, retinas were treated with an enzyme solution containing collagenase (120 U/ml) and hyaluronidase (465 U/ml). Retinas were flattened over a glass coverslip of a perfusion chamber and secured with a platinum ring crossed with nylon threading. The chamber was perfused continuously with bicarbonate Ringer solution bubbled with 95% O2-5% CO2 at a flow rate of ∼2 ml/min. RGCs were identified by IR-DIC prior to patching and, after recordings, by fluorescently imaging cells patch-loaded with Alexa 594 (0.1 mg/ml).
Light-evoked inward currents.
Patch pipettes (3–8 MΩ) contained (in mM) 128 KCH3SO4, 5 NaCH3SO4, 2 MgCl2, 5 EGTA, 5 HEPES, 1 glutathione, 2 ATP-Mg2+, and 0.2 GTP (3Na). RGCs were voltage clamped at the estimated chloride reversal potential (−65 mV). The bathing medium contained 0 Mg2+, 10 μM strychnine, and 1 μM TTX. For each recorded sweep, retinas were exposed to a single flash of light (∼100-μm-diameter spot, 600 lux, 600-ms duration, 10-s interstimulus interval) generated with a digital projector controlled by custom software (Vision Egg). A trace averaged over eight repeated responses was used for data analysis.
NMDA pressure ejection.
Whole cell recordings were made at −65 mV. Conditions were identical to those for measuring light-evoked inward currents, except that the bathing solution also contained TPMPA (50 μM) and picrotoxinin (50 μM). Pipettes (3–5 MΩ) were loaded with oxygenated (95% O2-5% CO2) bicarbonate Ringer solution containing 10 mM NMDA. RGCs were identified as ON, OFF, or ON-OFF depending on their response to flashes of light. The puff-pipette was positioned near the ganglion cell layer, upstream (with respect to the direction of perfusion flow) of the patched cell, and ejections were made at 10 psi every 45 s. Ejection times were adjusted (40–80 ms) to obtain the maximum peak inward current. Responses to two consecutive puffs of NMDA were averaged for data analysis. All other drugs were applied by switching the perfusion medium.
Measuring light-evoked coagonist release.
Outward currents were measured by clamping RGCs at +40 mV. The light stimulation protocol was the same as that for measuring inward currents. The extracellular solution was identical to that for recording inward currents with the addition of Mg2+ (1 mM), TPMPA (50 μM), and picrotoxinin (50 μM). Recordings were made with 3- to 7-MΩ pipettes containing the standard intracellular solution, except that KCH3SO4 was substituted with 108 mM CsCH3SO4 and 20 mM TEA-Cl. The light-evoked outward current was measured, after which 100 μM NMDA was added to the bathing medium, which evoked a baseline increase in steady-state outward current. Once the baseline stabilized, light responses were recorded. This was followed by addition of 100 μM d-serine plus 100 μM NMDA to the bathing medium. Once the steady-state shift stabilized, the light-evoked response was recorded again. The average response in NMDA plus d-serine was subtracted from the average response in NMDA alone, to derive a resultant trace that represented the time course and magnitude of coagonist release. For data analysis, the integrated peak area (charge) of the resultant trace was normalized to the initial light-evoked current. Resultant traces were averaged to generate Fig. 3B. All traces used for subtraction were averages of eight light responses in the specified condition. To calculate the percent potentiation of bath-applied d-serine, the baseline shift when switching from NMDA to NMDA plus d-serine was divided by the baseline shift going from control to NMDA.
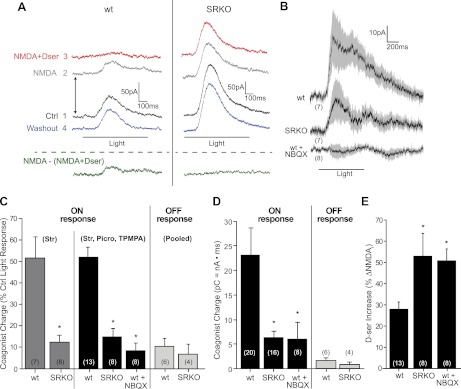
Fig. 3.
Light-evoked coagonist release is diminished in SRKOs and blocked by NBQX. RGC responses were recorded at +40 mV. Recordings were made in 1 mM Mg2+, 1 μM TTX, 10 μM strychnine, 50 μM TPMPA, and 50 μM picrotoxinin. TPMPA and picrotoxinin were excluded where specified. A: raw trace showing the effects of bath-applied NMDA (100 μM) or NMDA + d-serine (100 μM) on light-evoked ON responses. Numbers indicate the order of drug application. Traces are offset to show the baseline shift in current caused by the different pharmacological conditions (arrows). The green trace, representing coagonist release, was obtained by subtracting the NMDA + d-serine trace from the NMDA trace. B: average coagonist release traces (SE shown in gray) for ON cells compared between wt and SRKO RGCs in control conditions and wt RGCs in the continuous presence of NBQX. C: charge carried by coagonist release in the resultant trace normalized to the charge of the initial light response. ON responses were significantly larger in wt than SRKO either when only strychnine (Str) was present or when TPMPA and picrotoxinin (Picro) were also present. Coagonist release was lower for both genotypes during OFF responses compared with ON responses. No difference between genotypes was observed for OFF responses (data pooled for just strychnine and strychnine, TPMPA, and picrotoxinin). NBQX significantly reduced coagonist release during ON responses in wt. D: total charge (picocoulombs) carried by coagonist release. Pooled data with and without GABAergic block. E: % increase in the baseline shift (see A) from NMDA after adding NMDA + d-serine. d-Serine-induced potentiation of NMDAR currents (ΔNMDAi) was larger in SRKO than in wt. In the continuous presence of NBQX, d-serine potentiation was enhanced in wt. Number of cells recorded from (n) indicated in parentheses. *P < 0.05 compared with wt in control conditions.
Statistics.
All comparisons between groups were made with Student's one-tailed t-test, with n as the number of cells recorded from. Z-tests were used when the null hypothesis stated no change from a fixed value. All data are expressed as means ± SE, with significance defined as P < 0.05.
RESULTS
Blocking AMPARs Reduces Coagonist Availability During Light-Evoked RGC Responses
To determine whether AMPARs influence coagonist levels during light responses, we first measured excitatory ON responses from the RGCs of isolated retinas and determined their sensitivity to NBQX. OFF responses were excluded from analysis because OFF bipolar cell excitation is driven in part by AMPARs, whereas ON bipolar cell activity is mediated by mGluR6. RGCs were clamped at the calculated chloride reversal potential (−65 mV), and light-evoked inward currents were measured in the presence of TTX and strychnine, with Mg2+ absent to favor NMDAR currents. Photoreceptors and bipolar cells are nonthresholded; therefore synaptic transmission to RGCs was conserved in the presence of TTX. Under these control conditions, d-serine application led to a slight but significant enhancement in the peak amplitude of light responses [122.8 ± 12.8% control (ctrl), n = 11; P < 0.05] (Fig. 1, C and D), showing that NMDAR coagonist sites were not saturated.
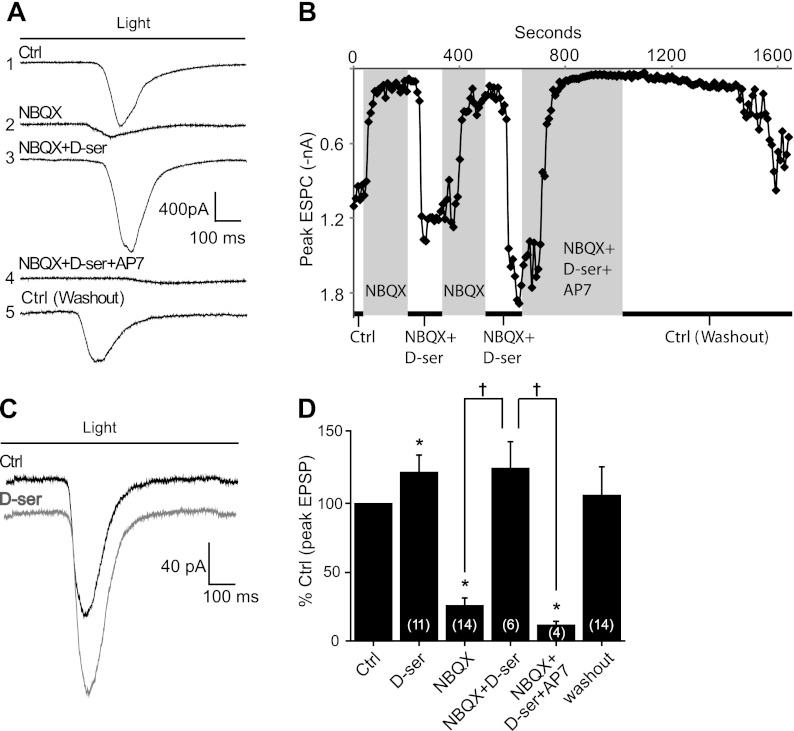
Fig. 1.
Blocking AMPA receptors (AMPARs) with NBQX reduces coagonist availability during light-evoked retinal ganglion cell (RGC) responses: voltage-clamp recordings of RGC excitatory postsynaptic currents (EPSCs) evoked by the onset of light. Cells were held at −65 mV, and recordings were made in 0 Mg2+, 1 μM TTX, and 10 μM strychnine. Duration of light stimulus is indicated by bar in A and C. A: light-evoked current of a sample RGC; average of 8 ON responses. NBQX (10 μM) diminished ON responses, and this current was rescued by d-serine (d-ser). Rescued currents were blocked by 50 μM AP7. Inward currents returned to control (Ctrl) values after washout of NBQX, d-serine, and AP7. Numbers on left indicate the order of drug application for this recording. B: demonstration of the time course of drug washin and washout in a single RGC (different from A). Each point (sampled every 10 s) represents the peak EPSC evoked by light at the corresponding time. Time spent in each pharmacological condition is indicated by white and gray columns. Rescue of the NBQX block by d-serine was confirmed twice in this cell. C: addition of 100 μM d-serine modestly potentiated ON responses in the absence of NBQX. D: average peak excitatory postsynaptic potential (EPSP) amplitude in specified condition across RGCs normalized to control light responses (%Ctrl). Number of cells recorded from (n) indicated in parentheses. *P < 0.05 compared with control; †P < 0.05 compared with NBQX + d-serine group.
Figure 1 summarizes the effects of NBQX on light-evoked whole cell currents in wt RGCs. ON responses were substantially reduced by 10 μM NBQX to 25.5 ± 5.9% of control light response (n = 14; P < 0.01) (Fig. 1, A and D). In the same mouse breed and under identical pharmacological conditions it was shown by blocking NMDARs that less than ∼30% of RGC light-evoked currents are carried by AMPARs (Sullivan et al. 2011). Therefore, the reduction in light-evoked currents by NBQX was markedly larger than would be expected if it were exclusively blocking RGC AMPARs. To test whether the exaggerated block of excitatory RGC currents by NBQX was due to reduced coagonist, we applied d-serine (100 μM) to the bathing medium. d-Serine coapplied with NBQX rescued light-evoked ON responses to 125.7 ± 19.67% of control light response without NBQX (n = 6; P < 0.005 for NBQX vs. NBQX + d-serine) (Fig. 1, A and D). No significant difference was detected between the control and NBQX + d-serine conditions, suggesting that NBQX did not substantially alter glutamate release. Coapplication of the NMDAR antagonist AP7 (50 μM) with NBQX and d-serine virtually eliminated the current rescued by d-serine (11.3 ± 3.0% ctrl, n = 4; P < 0.005) (Fig. 1, A and D), which returned when the drugs were removed from the bath. Figure 1B illustrates the course of a complete experiment carried out in a single ganglion cell as a plot of the peak light-evoked current over time. Here, the exaggerated block by NBQX and the rescue of light-evoked currents by d-serine was demonstrated twice in the same cell. The rescued current was blocked by AP7 and slowly recovered after drug washout. These findings suggest that the rescue of light responses in the presence of NBQX by d-serine was through its action on NMDARs. The near-complete block of light responses when NBQX and AP7 were combined is consistent with previous studies demonstrating that RGC excitatory currents are primarily carried by NMDA and AMPARs (Yu and Miller 1996). Collectively, these observations illustrate that the exaggerated block of light-evoked responses in ganglion cells by NBQX was in part due to the reduction of coagonist availability during synaptic responses. For simplicity, we refer to the RGC NMDARs active during light responses as “synaptic,” although there is evidence that extrasynaptic receptors are also activated under certain conditions (Zhang and Diamond 2006).
The Coagonist of Extrasynaptic NMDARs is Less Dependent on AMPARs
It was unclear whether NBQX was reducing ambient coagonist levels set by tonic AMPAR activity, resulting in a steady background, or if activation of AMPARs during light stimulation was required for phasic coagonist release. To test the first possibility (tonic release), we measured the effects of NBQX on currents evoked by pressure-ejecting NMDA in the ganglion cell layer adjacent to the recorded cell as shown in Fig. 2. Ejection times were adjusted until the response saturated to ensure that NMDA reached the dendrites (see materials and methods). Puff-evoked currents were abolished by bath application of the NMDAR antagonist AP7 (Fig. 2A), confirming agonist specificity. In contrast to light-evoked responses (Fig. 1), no significant decrease in puff-evoked NMDAR currents was observed when NBQX was bath applied to the retina (ctrl = −262.6 ± 41.8 pA, n = 11; NBQX = −271.6 ± 44.7 pA, n = 11; P = 0.89) (Fig. 2, B and C). These findings confirm that the block of light-evoked NMDAR currents by NBQX was not due to any nonspecific action of NBQX on NMDARs and suggest that the effects of NBQX on puff-evoked NMDAR currents were far less pronounced compared with those observed for the light-evoked currents. We considered two major possibilities to account for these findings: 1) The AMPARs involved in providing synaptic NMDARs with coagonist during light responses were not active under basal conditions, or 2) NBQX reduced synaptic coagonist levels, but this effect was masked in our NMDA puff-evoked responses by the activation of extrasynaptic NMDARs that do not depend on AMPARs as their source of coagonist.
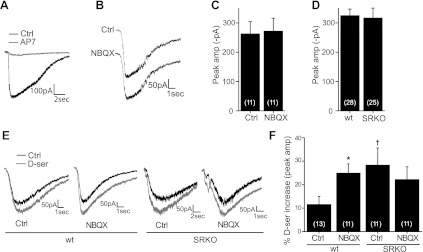
Fig. 2.
Effects of NBQX on coagonist availability during NMDA application: inward currents evoked by picospritzing 10 mM NMDA (40- to 80-ms duration) near the ganglion cell soma. Cells were held at −65 mV, and recordings were made in 0 Mg2+, 1 μM TTX, 10 μM strychnine, 50 μM TPMPA, and 50 μM picrotoxinin. Puffs initiated 300 ms into traces. A: raw trace showing that puff-evoked inward currents are blocked by bath-applied AP7. B: bath-applied NBQX did not alter puff-evoked NMDA receptor (NMDAR) currents. C: there was no significant decrease in the average puff-evoked peak inward current by NBQX. D: no significant difference in the average puff-evoked peak inward current was detected between wild-type (wt) and serine racemase knockout (SRKO) RGCs. E: raw traces showing the potentiation of NMDAR currents by d-serine in wt and SRKO RGCs, with or without NBQX in the bathing medium. F: summary of data in E. In wt RGCs, bath-applied d-serine significantly potentiated NMDA currents in ctrl conditions, but in the presence of NBQX this potentiation was larger. In SRKO RGCs, d-serine potentiated NMDA currents greater than in wt RGCs. Unlike wt, there was no further potentiation by d-serine in the presence of NBQX. Number of cells recorded from (n) indicated in parentheses. *P < 0.05 between conditions within genotype; †P < 0.05 between genotypes under same conditions.
SRKO mice display a marked reduction in retinal d-serine, and their RGCs consequently have virtually no NMDAR contribution to light-evoked responses (Sullivan et al. 2011), suggesting a critical role for d-serine in the activation of synaptic NMDARs. However, there are also NMDARs present on RGC dendrites distant from synaptic sites (Zhang and Diamond 2006) and on cell bodies (Fletcher et al. 2000). Given that the reduction in coagonist occupancy by NBQX observed when NMDA was bath- or puff-applied was less than that observed for light responses, it is possible that the receptors activated by global NMDA application were primarily occupied by coagonist originating from a different source, such as ambient glycine. To determine the contribution of d-serine to ambient coagonist levels, we measured puff-evoked NMDAR currents in SRKO RGCs. No difference (P = 0.47) was detected in puff-evoked inward currents between wt (−326.7 ± 20.7 pA, n = 28) and SRKO RGCs (−316.5 ± 32.8 pA, n = 25) when a mix of ON, OFF, and ON/OFF cells was sampled (Fig. 2D). When we compared different RGC types separately, a marginally significant decrease was only detected in the ON RGCs of SRKOs (wt = −322.8 ± 34.5 pA, n = 11; SRKO = −212.7 ± 32.5 pA, n = 7; P < 0.05). Overall these findings imply that, unlike synaptic NMDARs, extrasynaptic RGC NMDARs are primarily occupied by a coagonist other than d-serine.
To more closely examine the effects of NBQX on coagonist availability during puff-evoked NMDAR currents, we measured the potentiating effects of bath-applied d-serine on these responses. In control conditions, d-serine significantly potentiated NMDA-evoked currents in wt retinas by 11.5 ± 3.4% (n = 13, P < 0.005) (Fig. 2, E and F). In the presence of NBQX, wt ganglion cells were potentiated significantly more by d-serine than when NBQX was excluded from the medium (NBQX = 24.9 ± 4.0% increase, n = 11; P < 0.01 between ctrl and NBQX), although this effect was less pronounced than that observed for light-evoked currents (Fig. 1). SRKO RGCs were more potentiated by d-serine than wt RGCs in control conditions (SRKO = 28.27 ± 7.4% increase, n = 11; P < 0.05 between genotypes), suggesting some role for d-serine in mediating puff-evoked responses. In contrast to wt RGCs, coagonist potentiation in SRKO RGCs was similar in the presence of NBQX to control conditions (22.14 ± 5.5% increase, n = 11; P = 0.26 compared with SRKO control without NBQX) (Fig. 2, E and F). Because NBQX did not affect SRKO RGCs, its effect on wt coagonist occupancy appears to be via a reduction in d-serine.
AMPAR-Dependent Light-Evoked d-Serine Release
NBQX moderately reduced steady-state ambient coagonist levels (Fig. 2), but this did not exclude the possibility that it also reduced light-evoked coagonist release. Previous studies have shown that AMPAR activation can evoke d-serine release from the isolated retina (Sullivan and Miller 2010). We carried out additional experiments to explore the possibility that AMPARs mediate the light-evoked release of coagonist. This evaluation employed an experimental strategy previously used to detect coagonist release (Kalbaugh et al. 2009). Retinas were bathed in a cocktail of inhibitory antagonists, and light-evoked outward currents were measured from RGCs voltage clamped at +40 mV as shown in Fig. 3A. Initially, we included GABAC antagonist (TPMPA) and GABAA antagonist (picrotoxinin) in addition to strychnine (glycine receptor antagonist) to limit the contribution of inhibitory outward currents. To mask the changes in NMDAR currents caused by glutamate release and to ensure that any released coagonist would activate NMDARs, we saturated the glutamate binding site of NMDARs by bath-applying 100 μM NMDA. The bathing solution also contained 1 mM Mg2+ to limit the global effects of NMDA perfusion.
Bath-applied NMDA caused an increase in the baseline outward current recorded from RGCs, as depicted by the trace offset in Fig. 3A. With the glutamate binding site saturated, a residual outward light-evoked current persisted. To determine whether any residual current was due to coagonist release, we then saturated the NMDAR coagonist site in addition to the glutamate binding site (NMDA + d-serine) so any released coagonist would not cause a change in current. Bath-applied d-serine (100 μM) resulted in a further baseline increase (Fig. 3A) and a reduction in the change from baseline current induced by light. Subtracting the light-evoked current obtained in the presence of NMDA alone from the current in the presence of both NMDA and d-serine allowed us to quantify the current carried by coagonist changes in the resultant trace (Fig. 3A). Averaging the subtracted resultant traces from multiple ON cells showed a clear trend of light-evoked coagonist release (Fig. 3B). The charge induced by coagonist release in wt retinas was 52.1 ± 4.7% (n = 13) of the charge measured in control light responses, without NMDA added.
To test whether the coagonist release we measured in wt retinas was d-serine, we repeated these experiments in SRKO mice. The charge transfer due to coagonist release in SRKO ON responses was substantially lower than wt ON responses (14.9 ± 3.9% ctrl, n = 8; P < 0.005 compared with wt) (Fig. 3, B and C), implying that the released coagonist measured was predominantly d-serine while also validating our method for measuring coagonist release. A nearly identical result was observed when TPMPA and picrotoxinin were excluded from the bathing medium (strychnine only; same pharmacology used in Fig. 1), suggesting that release did not depend on the absence of GABAA or GABAC inhibition (wt = 52.2 ± 10.0% ctrl, n = 7; SRKO = 12.3 ± 3.3% ctrl, n = 8; P < 0.01 compared with wt) (Fig. 3C). Coagonist release during wt ON responses was blocked when these experiments were repeated in the continuous presence of NBQX (8.5 ± 3.6% ctrl, n = 8; P < 0.005 compared with wt) (Fig. 3, B and C), consistent with our findings that NBQX reduced coagonist availability during ON responses (Fig. 1). Coagonist release was minimal in OFF responses and showed no significant difference between SRKO (6.7 ± 4.6% ctrl, n = 4) and wt (13.6 ± 2.5% ctrl, n = 6; P = 0.56 between genotypes) animals (Fig. 3C). Comparing the average charge of coagonist release without normalizing to the initial light response displayed a similar trend (Fig. 3D), namely, release in wt cells was substantially greater than that observed in SRKO cells [wt = 23.0 ± 5.7 picocoulombs (pC), n = 20; SRKO = 6.3 ± 1.3 pC, n = 16; P < 0.01] and blocked by NBQX (6.0 ± 3.6 pC, n = 8; P < 0.01 compared with ctrl). OFF responses were substantially smaller (wt = 1.5 ± 0.6 pC, n = 6; SRKO = 0.7 ± 0.6 pC, n = 4; P < 0.001 compared with ON response in same genotype) and did not show a significant difference between the genotypes (P = 0.2) (Fig. 3, C and D).
Figure 3E summarizes the change in current observed when d-serine was added along with NMDA in wt and SRKO RGCs under the different pharmacological conditions. The relative potentiation of the currents measured in NMDA alone by the coapplication of NMDA and d-serine to the bath was comparable to our observations with puff-applied NMDA. Specifically, d-serine significantly potentiated NMDAR currents in wt (28.0 ± 3.4% increase) and SRKO (52.8 ± 10.7% increase), but to a greater extent in SRKO (P < 0.05 between genotypes) (Fig. 3E). In wt RGCs, NBQX enhanced the potentiating effects of d-serine (50.6 ± 5.6% increase; P < 0.01 between ctrl and NBQX), suggesting that NBQX reduces the baseline level of coagonist that is present in the absence of light stimulation (also shown in Fig. 2), in addition to preventing light-evoked coagonist release.
DISCUSSION
Our study in the retina demonstrates that AMPAR-dependent release of coagonist is a feature of light-evoked responses. Using a SRKO mouse, we provide evidence that the released coagonist acting on RGC NMDARs during ON responses was d-serine. Light-evoked NMDAR currents in wt retinas were nearly abolished by NBQX but could be rescued by adding d-serine (Fig. 1), demonstrating that AMPARs are critical in providing synaptic NMDARs with coagonist. In wt mice, the potentiation of puff responses by d-serine was exaggerated in the presence of NBQX, suggesting that NBQX reduced basal levels of coagonist. This effect was absent in SRKO mice (Fig. 2), implying that NBQX acted by reducing basal levels of d-serine in wt RGCs. The reduction in coagonist occupancy by block of AMPARs observed during puff responses was much smaller than for light-evoked responses. Thus the regulation of NMDAR coagonist may be different at the synaptic sites active during light stimulation versus the extrasynaptic sites reached by puff-applied NMDA. NMDA puff responses in SRKO retinas showed lower coagonist site occupancy, suggesting that a component of basal coagonist levels at nonsynaptic sites is dependent on d-serine. However, because puff-evoked NMDAR responses were still fairly robust in SRKO compared with wt retinas, it seems likely that an alternative to d-serine plays a significant role at nonsynaptic NMDAR coagonist sites. Overall these findings illustrate a complex system of coagonist regulation in which synaptic NMDARs receive d-serine from AMPAR-dependent coagonist release while extrasynaptic NMDARs are regulated primarily by relatively stable levels of a different coagonist, perhaps glycine.
Figure 4 presents a model that summarizes the implications derived from the present study. Coagonist regulation is depicted in the inner plexiform layer of the retina where bipolar cells make connections with amacrine and ganglion cells. Müller cell processes in the inner plexiform are active during light stimulation and influence ganglion cell activity (Newman 2004). We have modeled the d-serine release sites within Müller cells because AMPAR-dependent d-serine release depends on glia (Sullivan and Miller 2010). In the absence of light stimulation, a basal release of glutamate minimally activates Müller cell AMPARs, causing a small amount of d-serine release (Fig. 4A). In periods of light stimulation, increased release of glutamate from bipolar cells further activates Müller cell AMPARs, heightening the release of d-serine, which recruits additional ganglion cell NMDARs to be activated by glutamate. Glycine uptake via GlyT1, expressed predominantly by amacrine cells (Pow and Hendrickson 1999) but possibly also by Müller cells (Gadea et al. 1999), prevents ambient glycine from reaching synaptic NMDARs (Reed et al. 2009; Stevens et al. 2010), allowing d-serine to be the primary coagonist contributing to light responses (Stevens et al. 2003; Sullivan et al. 2011) (Fig. 4B). Since amacrine cell processes do not extend down into the ganglion cell layer, we propose that extrasynaptic regions of the ganglion cell, such as the soma and proximal dendrites, are less stringently regulated by GlyT1, allowing glycine to achieve levels that are sufficient to activate the coagonist sites of NMDARs. These extrasynaptic receptors are therefore activated when NMDA is puff- or bath-applied to ganglion cells (Fig. 4C).
In a previous report, NBQX dramatically reduced the NMDAR currents in RGCs evoked by the pharmacological activation of ON bipolar cells, which can be rescued by exogenous d-serine. These pharmacologically evoked currents lasted several orders of magnitude longer than a typical light response, raising the question of whether the coagonist release observed was a physiological phenomenon (Kalbaugh et al. 2009). Our findings show that NBQX also reduced the coagonist contribution to light-evoked ON responses in RGCs. The potentiation of NMDA puff responses by d-serine was also increased in NBQX, suggesting a reduction in coagonist occupancy but to a lesser extent than that observed in response to light. In fact, NBQX did not noticeably alter puff-evoked NMDAR currents (Fig. 2C). These findings imply that synaptic NMDARs depend on AMPAR activity for coagonist supply, while extrasynaptic coagonist is primarily regulated via an unknown mechanism.
Enzymatic degradation of extracellular d-serine (Gustafson et al. 2007) or knocking out serine racemase (Sullivan et al. 2011) abolishes RGC NMDAR currents during ON and OFF light responses. However, much to our surprise, we found that RGC responses to direct NMDA application were relatively normal in SRKO mice, with only a slightly augmented potentiation by coagonist application compared with wt. Similarly, it has been shown that enzymatic removal of extracellular d-serine only reduces NMDA puff-evoked currents (Stevens et al. 2003) and NMDA-induced Ca2+ responses to bath-applied NMDA by roughly 40% (Daniels and Baldridge 2010). Our findings are consistent with this apparent difference in the degree of d-serine dependence observed during light responses versus direct application of NMDA and are reconciled by a model with heterogeneous distribution of coagonist in the retina. Specifically, the synaptic NMDARs involved in RGC light responses require d-serine for activation, while extrasynaptic receptors, which are revealed by puff-applied NMDA, are not occupied by d-serine but perhaps by glycine (Fig. 4C).
Direct measurement of global glycine concentrations in the retina (Reed et al. 2009; Sullivan and Miller 2010) shows that glycine reaches levels high enough to saturate NMDARs (McBain et al. 1989). The glycine transporter GlyT1 is critical in limiting these high concentrations of glycine from saturating the RGC NMDARs involved in light responses (Reed et al. 2009; Stevens et al. 2010). In contrast, glycine might be regulated less stringently away from synaptic sites, serving as a coagonist to extrasynaptic NMDARs. Consistent with this concept, GlyT1 expression is relatively high in the inner plexiform layer, where bipolar cells synapse onto RGCs, but is absent in the ganglion cell layer (Pow and Hendrickson 1999), where NMDARs are also expressed (Haverkamp and Wassle 2004). Exploiting the differences in the coagonist utilized by different NMDAR populations in the central nervous system might serve to target extrasynaptic NMDA receptors, which are suspect in excitotoxic cell death (Hardingham et al. 2002) and Alzheimer's disease (Bordji et al. 2010).
We found evidence for light-evoked coagonist release acting on RGC NMDARs during ON responses (Fig. 3). Coagonist release was abolished by blocking AMPARs, implying that the reduced coagonist availability we observed after the application of NBQX (Fig. 1) was due to blocking phasic coagonist release, as opposed to merely reducing ambient coagonist levels set by tonic AMPAR activity in the absence of light. Work in hippocampus (Li et al. 2009) and cerebellum (Billups and Attwell 2003) has provided evidence for activity-dependent coagonist release acting on NMDARs, but it was unclear whether this coagonist was d-serine or glycine. A previous report in retina showed that the application of a d-serine-degrading enzyme (d-amino acid oxidase) did not influence pharmacologically evoked coagonist release, leaving glycine as the primary suspect (Kalbaugh et al. 2009). Indeed, RGCs receive inhibitory glycinergic input from amacrine cells, which depend on AMPARs for activation. On the other hand, AMPAR activation evokes d-serine release from isolated retinas (Sullivan and Miller 2010). We used SRKO mice to test whether the coagonist released during ON responses was d-serine and found that it was dramatically reduced, suggesting a predominant role for d-serine release in RGC responses (Fig. 4B). It is possible that the different form of stimulation used in our study (light) versus that used previously (pharmacological activation of ON bipolar cells; Kalbaugh et al. 2009) accounts for the apparent discrepancy in the coagonist released.
The d-serine release we observed during ON responses was relatively rapid and overlapped with excitatory glutamatergic input, raising interesting questions about the cell type and mechanisms of release. d-Serine is present in retinal glia (Stevens et al. 2003) and possibly neurons (Takayasu et al. 2008), but AMPA-induced d-serine release from isolated retinas acts independently of neural activity and depends on glial cell function (Sullivan and Miller 2010). Rapid vesicular release of neurotransmitter has been demonstrated in glia (Araque et al. 2000), but it is unclear how effectors downstream of AMPAR activation could elevate Ca2+ levels fast enough in glia, unless the Ca2+ originated from the AMPARs themselves. Indeed, AMPA-induced d-serine release in retina depends on Ca2+-permeable AMPARs (Sullivan and Miller 2010). Further work is required to determine the mechanism of d-serine release, but our work emphasizes that coagonist release should be considered as one of the coinciding factors required for NMDAR activation during excitatory transmission in the retina.
GRANTS
This work was funded by National Eye Institute Grant EY-03014. Support for S. J. Sullivan was provided by T32 EY-07133.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.S. and R.F.M. conception and design of research; S.J.S. performed experiments; S.J.S. analyzed data; S.J.S. and R.F.M. interpreted results of experiments; S.J.S. prepared figures; S.J.S. drafted manuscript; S.J.S. and R.F.M. edited and revised manuscript; S.J.S. and R.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank lab members Manuel Esguerra and Eric Gustafson for valuable technical assistance and discussions. We thank Derek Miller for editorial assistance. We also thank Joseph T. Coyle (Harvard Medical School) for kindly providing us with the SRKO mice used in these studies.
REFERENCES
- Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science 300: 2094–2097, 2003 [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci 20: 666–673, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 14: 719–727, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups D, Attwell D. Active release of glycine or d-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. Eur J Neurosci 18: 2975–2980, 2003 [DOI] [PubMed] [Google Scholar]
- Bordji K, Becerril-Ortega J, Nicole O, Buisson A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-β production. J Neurosci 30: 15927–15942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine transporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol 89: 691–703, 2003 [DOI] [PubMed] [Google Scholar]
- Daniels BA, Baldridge WH. d-Serine enhancement of NMDA receptor-mediated calcium increases in rat retinal ganglion cells. J Neurochem 112: 1180–1189, 2010 [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol 420: 98–112, 2000 [PubMed] [Google Scholar]
- Gadea A, Lopez E, Lopez-Colome AM. Characterization of glycine transport in cultured Muller glial cells from the retina. Glia 26: 273–279, 1999 [DOI] [PubMed] [Google Scholar]
- Gustafson EC, Stevens ER, Wolosker H, Miller RF. Endogenous d-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol 98: 122–130, 2007 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414, 2002 [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. The presence of free d-serine in rat brain. FEBS Lett 296: 33–36, 1992 [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol 468: 251–263, 2004 [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531, 1987 [DOI] [PubMed] [Google Scholar]
- Kalbaugh TL, Zhang J, Diamond JS. Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J Neurosci 29: 1469–1479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem 281: 14151–14162, 2006 [DOI] [PubMed] [Google Scholar]
- Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA 102: 2105–2110, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241: 835–837, 1988 [DOI] [PubMed] [Google Scholar]
- Li Y, Krupa B, Kang JS, Bolshakov VY, Liu G. Glycine site of NMDA receptor serves as a spatiotemporal detector of synaptic activity patterns. J Neurophysiol 102: 578–589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Krasteniakov NV, Bergeron R. d-Serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol 548: 411–423, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Kleckner NW, Wyrick S, Dingledine R. Structural requirements for activation of the glycine coagonist site of N-methyl-d-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol 36: 556–565, 1989 [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 97: 4926–4931, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA 102: 5606–5611, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. A dialogue between glia and neurons in the retina: modulation of neuronal excitability. Neuron Glia Biol 1: 245–252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci 16: 231–239, 1999 [DOI] [PubMed] [Google Scholar]
- Reed BT, Sullivan SJ, Tsai G, Coyle JT, Esguerra M, Miller RF. The glycine transporter GlyT1 controls N-methyl-d-aspartic acid receptor coagonist occupancy in the mouse retina. Eur J Neurosci 30: 2308–2317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ. The N-methyl d-aspartate receptor glycine site and d-serine metabolism: an evolutionary perspective. Philos Trans R Soc Lond B Biol Sci 359: 943–964, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92: 3948–3952, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. d-Serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA 100: 6789–6794, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Gustafson EC, Miller RF. Glycine transport accounts for the differential role of glycine vs. d-serine at NMDA receptor coagonist sites in the salamander retina. Eur J Neurosci 31: 808–816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ, Esguerra M, Wickham RJ, Romero GE, Coyle JT, Miller RF. Serine racemase deletion abolishes light-evoked NMDA receptor currents in retinal ganglion cells. J Physiol 589: 5997–6006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ, Miller RF. AMPA receptor mediated d-serine release from retinal glial cells. J Neurochem 115: 1681–1689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu N, Yoshikawa M, Watanabe M, Tsukamoto H, Suzuki T, Kobayashi H, Noda S. The serine racemase mRNA is expressed in both neurons and glial cells of the rat retina. Arch Histol Cytol 71: 123–129, 2008 [DOI] [PubMed] [Google Scholar]
- Yu W, Miller RF. The mechanism by which NBQX enhances NMDA currents in retinal ganglion cells. Brain Res 709: 184–196, 1996 [DOI] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol 498: 810–820, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]