Abstract
Enhanced intrinsic neuronal excitability of hippocampal pyramidal neurons via reductions in the postburst afterhyperpolarization (AHP) has been hypothesized to be a biomarker of successful learning. This is supported by considerable evidence that pharmacologic enhancement of neuronal excitability facilitates learning. However, it has yet to be demonstrated that pharmacologic reduction of neuronal excitability restricted to the hippocampus can retard acquisition of a hippocampus-dependent task. Thus, the present study was designed to address this latter point using a small conductance potassium (SK) channel activator NS309 focally applied to the dorsal hippocampus. SK channels are important contributors to intrinsic excitability, as measured by the medium postburst AHP. NS309 increased the medium AHP and reduced excitatory postsynaptic potential width of CA1 neurons in vitro. In vivo, NS309 reduced the spontaneous firing rate of CA1 pyramidal neurons and impaired trace eyeblink conditioning in rats. Conversely, trace eyeblink conditioning reduced levels of SK2 channel mRNA and protein in the hippocampus. Therefore, the present findings indicate that modulation of SK channels is an important cellular mechanism for associative learning and further support postburst AHP reductions in hippocampal pyramidal neurons as a biomarker of successful learning.
Keywords: afterhyperpolarization, intrinsic excitability, NS309, trace eyeblink conditioning
the learning-related modulation of all three phases of the postburst afterhyperpolarization (AHP: fast, medium, and slow) in principal neurons has been demonstrated to be a biomarker of successful learning in numerous tasks (Disterhoft and Oh 2006; Matthews et al. 2008; Saar and Barkai 2003; Santini et al. 2008). Hippocampus-dependent trace eyeblink conditioning has been the most extensively used learning paradigm to demonstrate the intrinsic excitability change (via reductions in the postburst AHP) in both CA1 and CA3 pyramidal neurons following successful learning (Moyer et al. 1996; Thompson et al. 1996). Furthermore, compounds that reduce the postburst AHP in CA1 pyramidal neurons have been shown to facilitate acquisition of various hippocampus-dependent tasks. One such compound is apamin, a specific blocker of small conductance potassium (SK2) channels that underlies the medium AHP in CA1 pyramidal neurons (Bond et al. 2004; Faber and Sah 2007; Kaczorowski et al. 2007; Kramar et al. 2004; Oh et al. 2000; Stocker et al. 1999; but see Gu et al. 2005, 2008). However, it has yet to be demonstrated that a specific pharmacologically induced increase in any phase of the postburst AHP localized to the dorsal CA1 region, shown to be the primary subregion of the hippocampus to change during trace eyeblink conditioning (Weible et al. 2006), can prevent or retard successful acquisition of a hippocampus-dependent task. Therefore, we used NS309, an SK channel activator that has been demonstrated to specifically enhance the apamin-sensitive postburst AHP (Pedarzani et al. 2005), to carefully examine the impact of a focally increased medium AHP in the dorsal hippocampal CA1 region on acquisition of hippocampus-dependent, trace eyeblink conditioning.
MATERIALS AND METHODS
Subjects used in the study were young adult (3–4 mo) male F1 F344XBN rats (Harlan, Indianapolis, IN) that were housed in groups of 2 to 3 with unrestricted access to food and water on a 14/10 h light/dark cycle. Rats were handled and housed in accordance with standards established by the Institutional Animal Care and Use Committee of Northwestern University and the USDA.
In vitro hippocampal brain slice recordings.
Behaviorally naïve rats were anesthetized with isoflurane and decapitated. Dorsal hippocampi placed in ice-cold artificial cerebrospinal fluid (aCSF: consisting of [in mM] 124 NaCl, 1.25 NaH2PO4, 2.5 KCl, 26 NaHCO3, 25 glucose, 2.4 CaCl2, and 2.0 MgSO4, saturated with 95% O2-5% CO2) were sliced into 300-μm-thick sections using a Leica VT1000s vibratome. Afterward, the slices were allowed to equilibrate at room temperature for at least 1 h before being used for recordings.
CA1 pyramidal neurons were visually identified using video DIC-IR optics on an upright Axioskop microscope and patched with pipettes filled with an internal solution containing (in mM): 120 KMeSO4, 10 KCl, 10 HEPES, 10 phosphocreatine sodium salt, 4 ATP magnesium salt, 0.4 GTP sodium salt, and 0.05% neurobiotin with pH corrected to 7.4 with KOH and osmolarity of 285 ± 5 mOsm. Whole cell recordings were collected at 10 kHz for the AHP and accommodation and 20 kHz for all other measures using a Dagan BVC-700 amplifier, digitized, and interfaced to a PC with an Axon Digidata 1322A analog to digital converter, and acquired and analyzed using pCLAMP 9.2. The current-clamp recordings were acquired with the neuron held at −65 mV and with the perfusate maintained at 32°C. Series resistance was monitored throughout recording and neurons with more than a 20% change were discarded. The AHP was elicited with five 2-ms, 2.0-nA current pulses at 50 Hz via the recording electrode. Accommodation was elicited with a 1-s current pulse that evoked five action potentials in the first 100 ms. Synaptic responses were elicited with a bipolar stimulating electrode (FHC, Bowdoin, ME) placed in the stratum radiatum, powered with a Digitimer DS2 isolated stimulator.
In vivo CA1 hippocampal recordings.
Surgeries to implant a cannula for drug infusion and, in close spatial proximity, a four-tetrode array for unit recordings in the left dorsal CA1 region were performed using previously published methods (Matthews and Disterhoft 2009) and after the rats were anesthetized with a ketamine and xylazine (0.87 mg/kg and 0.13 mg/kg, respectively; ip) mixture, supplemented as necessary during the surgery. The cannula was implanted at 4.8 mm posterior, 3.0 mm lateral from bregma, and 1.9 mm ventral from pia. The injection needle, when positioned in the cannula, protruded 0.1 mm below the tip of the cannula. The depth of penetration for the custom-made four-tetrode array (3.3 mm posterior, 1.7 mm lateral from bregma) was determined with auditory monitoring of neuronal activity that ensured placement in the CA1 pyramidal neuron layer. A tetrode was made with four formvar-coated nichrome wires (25-μm diameter, bare; 37-μm diameter coated) that were bonded at one end with epoxylite and exhibited an average impedance of 0.5–1.5 MΩ. After appropriate positioning, the electrode array and cannula were fixed in place with dental acrylic. Rats were given 1 wk to recover from surgery before experimentation.
NS309 concentration to be used for the behavioral training study was identified by finding the concentration that significantly reduced the spontaneous firing rate of CA1 pyramidal neurons. Prior to the drug infusion and unit recordings, the rats were given one 60-min habituation session to the sound- and light-attenuated recording chamber (30.5 cm wide, 38.1 cm long), where they were allowed free movement in the chamber while being attached to a tether that connected the electrode array to the unit recording apparatus. After habituation, once daily unit recording sessions were conducted in the following manner: 10-min baseline, 5-min drug infusion, and 55-min postinfusion period. The rats were given 1.0 μL of one of four different drug concentrations (10 μM NS309, 100 μM NS309, and 1 mM NS309 or dimethyl sulfoxide [DMSO]) administered at a rate of 0.2 μL/min. Multiple drug concentrations were tested on the same rat in a blind pseudorandom counterbalanced manner in recording sessions separated by at least 24 h. Although it is not precisely known how long NS309 remains in the system in vivo, baseline neuronal firing rate did not reveal any significant difference across recording days (data not shown).
Single-neuron recording data were recorded using the Cheetah-32 system (Neuralynx, Bozeman, MT). Neuronal activity was passed to a headstage amplifier (HS-27) via a customized adapter, buffered with the HS-27, filtered (600–6,000 Hz) and amplified (20,000×). Following each recording session, individual neurons were isolated offline using Neuralynx spike-sort software based on firing rate and waveform characteristics. Criteria for inclusion in the analysis were: 1) exhibit a mean firing rate of greater than zero during any of the 5-min collection periods over the 70-min recording session, 2) exhibit a signal to noise ratio >2.5:1, and 3) have an average firing rate <6 Hz and a mean spike width (peak to valley) >0.3 ms to be characterized as a CA1 pyramidal neuron (McEchron and Disterhoft 1997; Ranck 1973). In all, 234 neurons met our criteria for the final analysis (1 mM, n = 50; 100 μM, n = 61; 10 μM, n = 42; DMSO, n = 81). At the conclusion of the recording sessions, a DC current (10 μA, 5-s duration) was passed through a single channel of the tetrode to verify the tetrode placement using standard histologic methods (McEchron and Disterhoft 1997).
Behavioral pharmacology.
Surgeries to implant the guide cannulae for drug infusion and a plastic connector strip for eyeblink conditioning were performed after the rats were anesthetized with isoflurane and placed in a stereotaxic apparatus. Guide cannulae, made of 26-gauge stainless steel tubing, were bilaterally implanted in the dorsal hippocampus (3.6 mm posterior and 2.0 mm lateral from bregma, 1.9 mm below the dura) and cemented in place with dental acrylic. A previous report using the same stereotaxic coordinates demonstrated that the diffusion of 1 μL of ibotenic acid injected into the dorsal CA1 region via guide cannulae was limited to the dorsal hippocampus (Matthews and Disterhoft 2009). Although not identical in structure, the spread of NS309 should be similar to that observed with ibotenic acid and be limited to the dorsal hippocampus. A plastic connector strip, which contained a wire to ground to the skull and two additional wires passed subcutaneously through the upper eyelid of the right eye to record the electromyographic activity of the orbicularis oculi muscle, was cemented between the cannulae. Rats were allowed 1 wk to recover before training began.
Trace eyeblink conditioning was conducted once a day for 10 consecutive days after the rats were habituated to the light- and sound-attenuated conditioning chamber and to the tether attaching the plastic connector strip and the cannulae that allows free movement in the training chamber. One microliter of 100 μM NS309 or DMSO was infused over 5 min bilaterally into each hippocampus using two 2-μL Hamilton syringes with 32-gauge stainless steel injection needles placed in the guide cannula, with its tip terminating 0.1 mm below the end of the cannula and a Stoelting dual infusion syringe pump (Stoelting, Wood Dale, IL). Trace eyeblink conditioning began approximately 30 min after drug infusion. During the training session, rats were presented with two stimuli: an 8-kHz, 85-dB, 250-ms tone and a 100-ms, 4-psi air puff to the cornea. Conditioned animals received 30 pairs of the tone and the airpuff separated by a 250-ms stimulus-free trace interval, a trace duration that has been demonstrated to make this task hippocampus dependent (Weiss et al. 1999). Pseudoconditioned animals also received 30 airpuffs and 30 tones per session, but the two were explicitly unpaired. The data acquisition, analysis, and storage were all performed using custom software written in LabVIEW (National Instruments, Austin, TX). Learning was measured as the percentage of correct responses, which are eyelid closures during the last 200 ms of the trace interval.
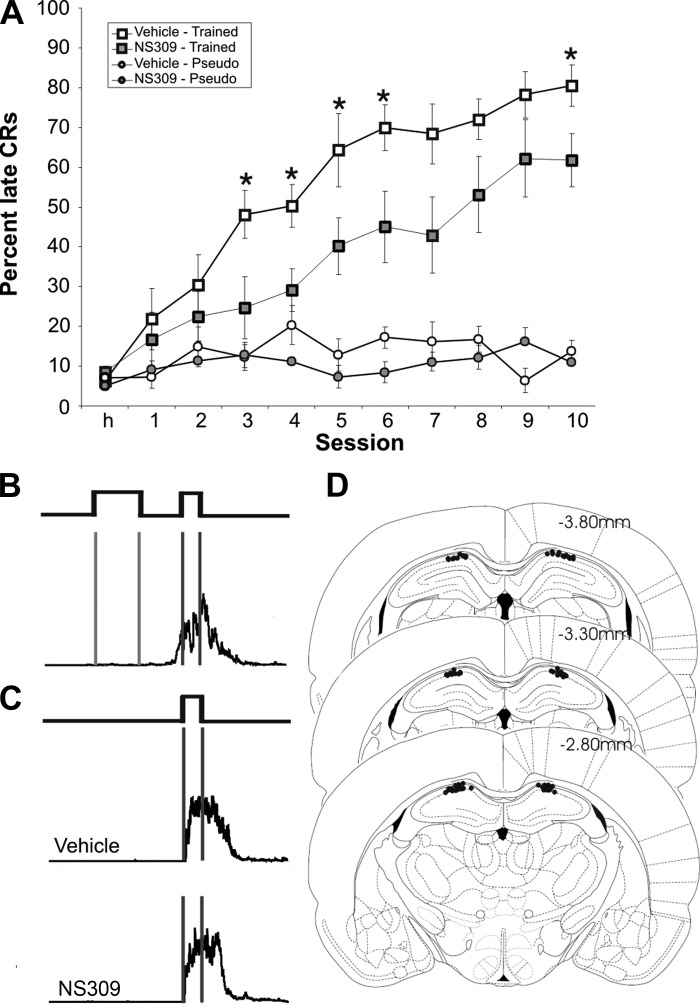
Cannula placement was verified at the conclusion of the training sessions. Anesthetized rats were transcardially perfused with 0.9% saline and 10% formalin. The brains were removed, frozen, and sliced into 80-μm coronal slices. Slices were stained with cresyl violet. Animals with incorrect cannula placement or excessive tissue damage were excluded from the study (n = 2) (see Fig. 3).
Fig. 3.
NS309 (100 μM) significantly impaired trace eyeblink conditioning. A: rats were divided into four groups: vehicle trained (n = 9), NS309 trained (n = 10), vehicle pseudo (n = 5), NS309 pseudo (n = 6). Both control and NS309 animals learned the paradigm, but compared with controls, animals receiving NS309 infusions were significantly impaired on days 3–6 and 10 (*P < 0.05). NS309 had no effect on pseudoconditioning. B, top: conditioned animals received a 250-ms tone followed by a 250-ms stimulus-free trace interval and then a 100-ms puff of air to the eye. Bottom: late conditioned responses (CRs) were measured as eyelid closures during and after the tone (two vertical gray lines) in the 200 ms before the airpuff (two vertical black lines). C: eyeblinks from pseudoconditioned animals in response to the airpuff alone were not affected by NS309 (top, vehicle; bottom, 100 μM NS309; repeated-measures ANOVA, P = 0.43), indicating that hippocampally infused NS309 had no effect on the sensory input or motor ability of the animal to blink. D: cannula placement was verified in cresyl violet—stained slices. Those rats in which the cannula tracks did not terminate at or closely above the pyramidal band of CA1 were excluded from the study.
Molecular analyses of SK2 channel.
Microarray analysis was conducted using previously published methods (Kroes et al. 2007). The genes comprising the in-house prepared rat CNS microarray (1,178 cloned rat CNS genes, representing 90% of the major gene ontologic categories) were spotted in quadruplicate onto slides. Microarrays were performed in triplicate using hippocampal mRNA isolated 24 h after the last training session from the left hippocampi of individual animals. Normalized microarray data were analyzed by significant analysis of microarray (SAM) with a stringent false discovery rate cutoff of 1%, followed by ontologic data mining using GoMiner (Zeeberg et al. 2003) and χ2 analysis to identify enriched pathways, as described in Burgdorf et al. (2011).
Quantitative real-time PCR analysis (qRT-PCR) was conducted as previously described (Burgdorf et al. 2011; Kroes et al. 2006) in a blind manner on dorsal hippocampus of individual rats that were trace eyeblink conditioned (n = 7), pseudoconditioned (n = 6), or naïve controls (n = 6). The sequences of the qRT-PCR primers used in the study were as follows: KCNN2 (NM_019314), forward 5′-AGT AAG GAA GCA TCA ACGG-3′ and reverse 5′-GTA TTC GCT TGG TCA TTC AG-3′; B2M (NM_012512), forward 5′-CCG TGA TCT TTC TGG TGC TT-3′ and reverse 5′-AAG TTG GGC TTC CCA TTC TC-3′.
Western blot analysis was conducted using hippocampal tissue from another group of trained rats (trace conditioned, n = 6; pseudoconditioned, n = 3; naïve, n = 4). Membranes were prepared as described elsewhere (N'Gouemo et al. 2009). In brief, tissue was Dounce homogenized in ice-cold TE buffer (10 mM Tris-HCl (7.4), 1 mM EDTA, 0.5% protease inhibitor cocktail (P8340, Sigma Chemical, St. Louis, MO), 1% phosphatase inhibitor cocktail 2 and 3 (P0044 and P5726, Sigma). Samples were centrifuged at 25,000g for 15 min at 4°C, the supernatant was decanted, and pellets were resuspended in boiling lysis buffer (10 mM Tris-HCl [7.4], 1% SDS, 0.5% protease inhibitor cocktail [Sigma], 1% phosphatase inhibitor cocktail 2 and 3 [P0044 and P5726, Sigma]). Samples were boiled for 10 min, centrifuged at 25,000g for 10 min, and the supernatant was aliquoted and stored at −80°C until assay. Total protein content was determined by the BCA assay (Pierce, Rockford, IL), and samples were analyzed by SDS-PAGE. Samples (45 μg) were electrophoresed through 8% gels (Hoeffer), transferred onto PVDF membranes (Millipore, Billerica, MA) and blocked in 0.2% nonfat dry milk (NFDM), 1% BSA in TBS containing 0.05% Tween-20 (TBS-T) for 1 h at 25°C. Membranes were probed with one of two SK2 antibodies raised against two independent regions of the full-length SK2 protein (APC-028, 1:500; Alomone Labs, Jerusalem, Israel, aa 542–559; AV35094, Sigma, 1:500, aa 444–493) in 0.2% NFDM, 1% BSA TBS overnight at 4°C, followed by a 1-h incubation at 25°C with a horseradish peroxidase (HRP) conjugated secondary antibody (sc-2313, 1:5,000, Santa Cruz Biotechnology, Santa Cruz, CA) in 0.2% NFDM, 1% BSA TBS-T. Western blots were visualized by enhanced chemiluminescence (Immun-Star HRP, Bio-Rad, Hercules, CA) and developed on film (BioMax, Kodak, Carestream Health, Edison, NJ). SK2-immunoreactive bands were quantified by ImageJ (NIH, Bethesda, MD). The blots used for SK2 protein quantification were stripped and reprobed with beta-actin as a protein loading control. Beta-actin protein levels were not altered in the naïve, trace, or pseudoconditioned animals [F(2,9) = 0.16, P > 0.05]. SK2 antibody from both Sigma and Alomone recognized a 64-kD band corresponding to the full-length SK2 protein in rat and mouse whole brain membrane preparations. In addition, both antibodies revealed that there was no difference observed in SK2 protein levels in the control tissue (both antibodies: Fisher PLSD values of P > 0.05 pseudoconditioned vs. naïve). Thus, the results of both antibodies were combined and used for analysis.
Statistics.
Repeated-measures ANOVAs, ANOVAs, and t-tests were performed, where appropriate, using StatView to analyze the in vitro and in vivo electrophysiology, Western blots, and behavior data.
RESULTS
NS309 reduces the excitability of CA1 pyramidal neurons and reduces the width of the EPSP in vitro.
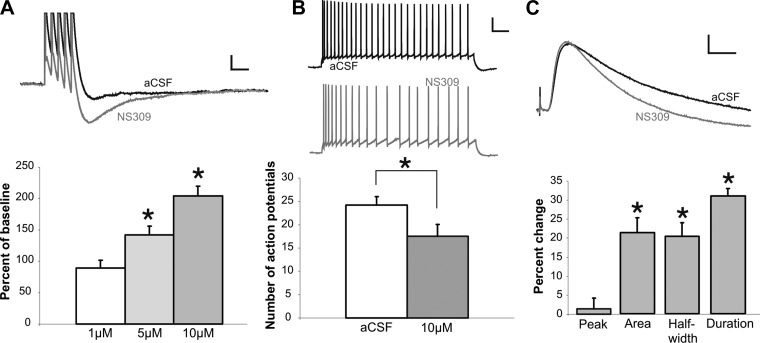
Before conducting the behavioral experiments, we first replicated the observations reported by Pedarzani and colleagues (2005) that bath application of NS309 increased the apamin-sensitive medium postburst AHP of CA1 pyramidal neurons in vitro. NS309 significantly enhanced the peak of the postburst AHP at 5 and 10 μM (Fig. 1A: paired t-test, values of P < 0.05) without affecting the slow component of the postburst AHP (P = 0.94) or the basic membrane properties of the neuron (Table 1). Consistent with previous reports (Pedarzani et al. 2005), 10 μM NS309 also increased spike frequency accommodation (Fig. 1B: paired t-test, P < 0.01).
Fig. 1.
NS309 reduces the excitability of CA1 pyramidal neurons and decreases the width of the excitatory postsynaptic potential (EPSP). A: repeated-measures ANOVA revealed a significant impact of NS309 bath application on the postburst afterhyperpolarization (AHP) measures [F(1,8) = 3.78, P < 0.005]. Planned post hoc analysis further revealed that the peak amplitude of the postburst AHP was significantly enlarged by the bath application of 5 μM (n = 6) and 10 μM (n = 9) NS309, whereas 1 μM NS309 had no effect (n = 5). The AHP was evoked with five action potentials at 50 Hz. Action potentials are truncated for clarity. Scale bar: 2 mV, 100 ms. B: NS309 (10 μM) significantly increased accommodation (n = 6). Accommodation was measured as the number of action potentials elicited in 1 s by a depolarizing current step, which evokes five action potentials in the first 100 ms. Scale bar: 20 mV, 100 ms. C: in addition to its effect on the AHP and accommodation, NS309 (10 μM) significantly reduced the area, duration, and halfwidth of the EPSP (n = 6). EPSPs were evoked with a stimulating electrode, which was placed in the stratum radiatum with the stimulus intensity adjusted so that the amplitude of the EPSP was the same before and after bath application of NS309 or dimethyl sulfoxide (DMSO). Scale bar: 2 mV, 100 ms. Data are presented as mean ± SE. *P < 0.05.
Table 1.
Bath application of NS309 did not significantly affect the basic membrane properties of CA1 pyramidal neurons ex vivo
| aCSF | NS309 | |
|---|---|---|
| Vrest (mV) | −65.1 ± 0.9 | −64.8 ± 1.2 |
| Input resistance (MΩ) | 88.6 ± 2.3 | 83.5 ± 4.3 |
| Sag (mV) | 3.22 ± 0.29 | 3.46 ± 0.42 |
| sAHP (mV) | −1.77 ± 0.25 | −1.61 ± 0.39 |
| AP thresh (mV) | −50.1 ± 1.5 | −51.5 ± 1.9 |
| AP height (mV) | 94.2 ± 4.3 | 94.1 ± 2.7 |
| AP half-width (ms) | 1.14 ± 0.06 | 1.05 ± 0.06 |
Values are means ± SE. Vrest is the resting membrane potential immediately after breaking into the cell. Input resistance is calculated as the slope of the current—voltage (I–V) curve. Sag is the difference between the peak and steady-state hyperpolarization in response to a −250 pA, 800-ms current injection. The sAHP was measured 1 s after the end of the last action potential in a five-spike train. Action potentials were elicited by a long depolarizing pulse sufficient to evoke a single action potential in 100 ms. Threshold was measured where the first derivative of the upslope of the trace equals 20 mV/ms. Height is the difference between the baseline and maximal depolarization. Half-width is width of the action potential at the midpoint between the maximal depolarization and the threshold. N = 9.
In addition to increasing the medium postburst AHP, NS309 could also influence the synaptically evoked excitatory postsynaptic potentials (EPSPs), since SK channel activation reduces the EPSP width by shunting current near the synapse (Ngo-Anh et al. 2005). Thus, we also tested whether 10 μM NS309 affected the EPSP. For this test, the isolated stimulator output was controlled to evoke an EPSP with a peak height of 7 mV before and after drug application so that NS309's impact on the EPSP could be examined independent of its potential action on the EPSP amplitude. NS309 significantly reduced the EPSP area, halfwidth, and duration (Fig. 1C: paired t-tests: area, P = 0.005; duration, P < 0.01; half width, P < 0.05).
The intrinsic neuronal excitability measures were assessed after 100 μM NS309 bath application in two neurons to determine if further reduction in intrinsic neuronal excitability could be obtained with a higher concentration of NS309. The postburst AHP measures after 100 μM NS309 application were 197 ± 15% (n = 2) of the baseline measurements. The number of action potentials evoked during accommodation measures was reduced by five action potentials (pre-NS309: 29 ± 2; post-NS309: 24 ± 2; n = 2). The EPSP duration was reduced by 33.5 ± 5.5% (n = 2). These results with 100 μM NS309 indicate that a ceiling effect on the intrinsic excitability was achieved with 10 μM NS309. Thus, the SK channel activator NS309 reduced the excitability of CA1 pyramidal neurons by increasing the medium postburst AHP and by increasing the calcium-dependent outward current at the synapses.
NS309 reduces the spontaneous firing rate of CA1 principal cells in vivo.
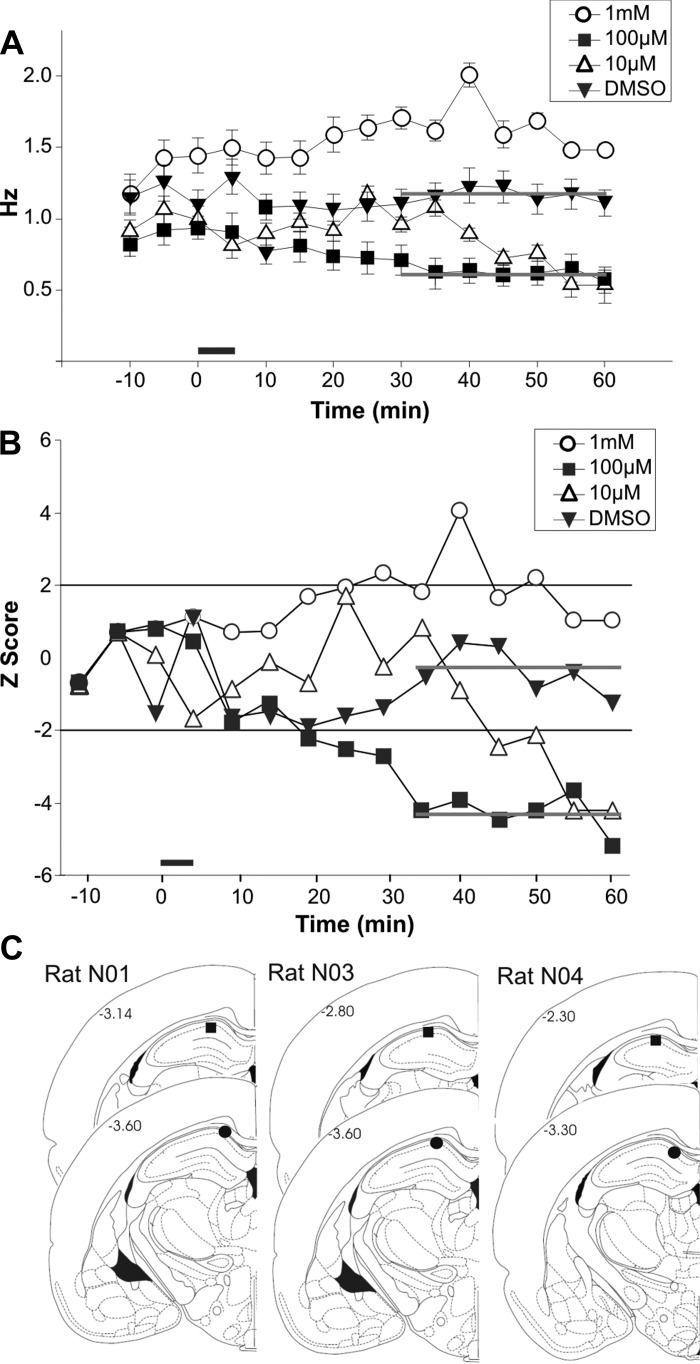
Given that the detailed kinetics of NS309 action may differ in the in vivo from the in vitro situation, we wanted to find the most effective dose to use in the awake rat for subsequent behavioral experiments. Thus, a guide cannula and an electrode array were implanted unilaterally into dorsal CA1, approximately 2.0 mm apart, and cemented into place (see Fig. 2C). After recovery, conscious, freely moving rats received once-daily infusion of 10 μM, 100 μM, or 1 mM of NS309 or vehicle control (DMSO). Neuronal activity was monitored with a four-tetrode extracellular microelectrode array for a 10-min baseline period, a 5-min infusion of 1 μL of the drug, and a 55-min observation period. No statistical differences were observed in the baseline periods between the drugs or between days [F(2,156) = 0.26, P > 0.75].
Fig. 2.
NS309 (100 μM) maximally suppresses spontaneous firing rate in vivo. A: the activity of individual neurons was isolated. Activity was monitored during a 10-min baseline, 5-min infusion (black bar), and 55-min postinfusion period. DMSO vehicle injections had no effect on firing rate. The gray lines indicate the average firing rate during the time period when behavioral training was done for the 100 μM concentration of NS309 and the DMSO control injected in the subsequent behavioral experiments. A total of 234 neurons from three rats reached criterion for final analysis (1 mM, n = 50; 100 μM, n = 61; 10 μM, n = 42; DMSO, n = 81). B: the 100 μM dose robustly and significantly suppressed the firing rate for the duration of the recordings, and thus was used for the behavioral pharmacologic studies. C: tetrode bundle placement was identified by passing a current (DC, 10 μA, 5-s duration) through a single channel of one tetrode. Tetrode placement is indicated by a square; cannula placement is indicated by a circle.
Statistical analysis of the effect of the different drug concentrations on pyramidal cell firing rate demonstrated a significant interaction of drug concentration by time [F(8,77) = 2.46; P < 0.05]. Post hoc analyses demonstrated that compared with DMSO controls, 100 μM NS309 significantly reduced CA1 spontaneous firing 20 min following drug infusion (see Fig. 2). NS309 (10 μM) also reduced firing rate for a few time points; however, not as robustly as the 100 μM dose. Post hoc analyses demonstrated that 1 mM NS309 significantly elevated spontaneous firing rate after drug infusion compared with DMSO. This suggests a classic U-shaped dose—response curve, and that at higher doses NS309 may have nonspecific effects. DMSO infusion had no significant effect on spontaneous firing rate. It is important to note that the concentration of NS309 seen by the recorded hippocampal neurons is lower than the 1 μL of a given concentration of NS309 infused directly into the dorsal CA1 hippocampus after local diffusion. A previous report using the same stereotaxic coordinates demonstrated that the diffusion of 1 μL of ibotenic acid injected into the dorsal CA1 region via guide cannulae resulted in a spread of approximately 0.17 cm in radius, which was limited to the dorsal hippocampus (Matthews and Disterhoft 2009). If a spherical volume of diffusion is assumed, then a compound will diffuse and occupy about 0.015 cm3 (or ∼15 μL) of space. Thus, 1 μL of 100 μM NS309 delivered into 15 μL of space would result in a net of 6–7 μM (100 μM in 16-μL volume) of NS309 for a period of time before NS309 is removed (by clearance or breakdown) from the space. This calculation assumes that the nearly 15 μL of brain space is similar to water and NS309 would have free movement to equilibrate; but brain space is not the same as water, so the actual NS309 concentration after about 30 min of diffusion is considerably less than the original 100 μM but somewhat greater than 6–7 μM. Regardless, 100 μM produced the largest and most uniform decrease in firing rate when injected in vivo, so this dose of NS309 was selected for subsequent behavioral studies.
NS309 impairs learning of trace eyeblink conditioning.
Rats were habituated to the injection and training paradigm for one session. For the next 10 consecutive days, rats were once daily given an infusion of NS309 through cannulae bilaterally implanted into CA1 approximately 30 min prior to conditioning (see Fig. 3D) then trace eyeblink conditioned. Trace-conditioned rats received 30 trials of paired tones and airpuffs separated by a 250-ms stimulus-free trace interval, whereas pseudoconditioned rats received the same stimuli but tones and airpuffs were explicitly unpaired. Eyelid closures during the last 200 ms of the trace interval were conditioned responses.
Daily infusion of NS309 significantly impaired trace eyeblink conditioning compared with controls (see Fig. 3A). Repeated-measures ANOVA demonstrated a main effect of training [F(1,26) = 58.2, P < 0.001] and drug [F(1,26) = 4.9, P < 0.05]. NS309 had a significant effect on trace eyeblink conditioning [F(1,17) = 6.8, P < 0.05], but had no effect on pseudoconditioning [F(1,9) = 2.6, P > 0.10]. Because there was a significant effect of NS309 on the level of acquisition in the trace-conditioned rats, planned comparisons were done using unpaired t-tests on the individual days for the trained animals. NS309-treated rats performed at a significantly lower rate than the DMSO control-treated rats on days 3–6 and 10 (P < 0.05). Although NS309-treated rats were significantly impaired, they were able to learn the task [F(1,9) = 20.3, P < 0.0001], albeit at a slower rate and to a lower level. It is important to emphasize that NS309 had no noticeable effect on the amplitude of unconditioned eyeblinks from pseudoconditioned animals (repeated-measures ANOVA, P = 0.43; see Fig. 3C), indicating that NS309 was acting on associative ability and not the sensorimotor capacity of the rats to produce eyeblinks per se.
SK2 mRNA and protein levels are reduced after learning.
The behavioral data strongly suggested that modulation of apamin-sensitive SK2 channels is an essential component for successful learning and for the learning-related postburst AHP reduction in hippocampal pyramidal neurons. Thus, we conducted molecular assays to verify that learning changes the apamin-sensitive SK2 channels.
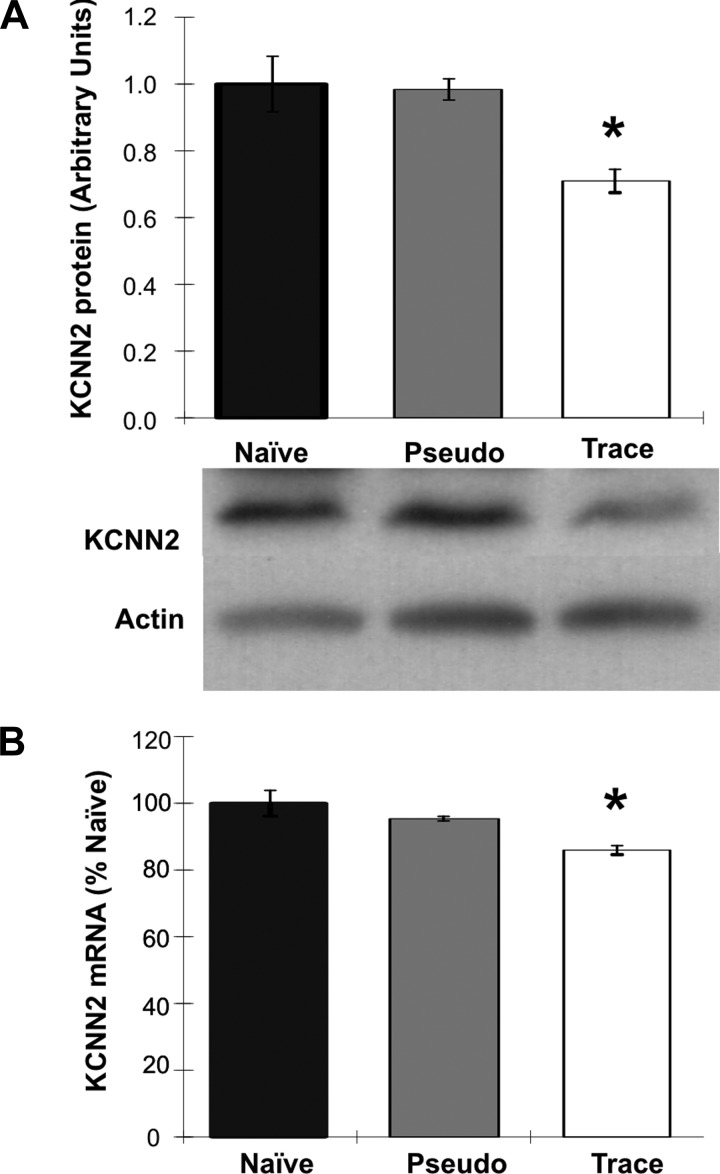
Rats were trace eyeblink conditioned, pseudoconditioned, or left naïve, as described earlier. One day after training, the hippocampi were extracted and used for microarray, qRT-PCR, and/or Western blot analyses to measure mRNA and protein levels. The microarray analysis using SAM and GoMiner revealed that 102 total genes were significantly differentially expressed by learning the trace eyeblink conditioning task (see Supplemental Table S1). Of particular interest in our current study, the KCNN2 gene was significantly downregulated in trace-conditioned rats. This latter result was verified by qRT-PCR analysis that showed a significant reduction of KCNN2 mRNA levels in the hippocampus from trained compared with pseudoconditioned and naïve animals [Fig. 4B: F(2,15) = 9.4, P < 0.005; Fisher PLSD post hoc, values of P < 0.01]. Finally, the Western blot analysis revealed a significant 30% reduction in SK2 levels in trace eyeblink conditioned rats [Fig. 4A: F(2,9) = 9.5 P < 0.01; Fisher PLSD post hoc test, values of P < 0.01], and no significant difference in the control rats (P > 0.05 pseudoconditioned vs. naïve). Thus, the molecular analyses clearly demonstrate that the apamin-sensitive SK2 expression is reduced following successful learning.
Fig. 4.
Trace eyeblink conditioning decreases KCNN2 mRNA and protein levels in the hippocampus. A: KCNN2 protein levels (arbitrary units) as measured by Western analysis. B: KCNN2 mRNA levels normalized to beta 2-microglobulin as measured by qRT-PCR in the hippocampus of rats receiving trace eyeblink conditioning, pseudo eyeblink conditioning, or no training (naïve). n = 3–7 per group, *P < 0.01. Fisher PLSD post hoc test comparing trace vs. pseudo or trace vs. naïve. Data are presented as mean ± SE.
DISCUSSION
The results from the present study are the first to demonstrate that direct modulation of the medium postburst AHP restricted to the dorsal CA1 region can influence the acquisition of a hippocampus-dependent task. Direct infusion of the SK channel activator NS309 into the dorsal CA1 region caused a significant reduction in spontaneous firing rate of the pyramidal neurons and also significantly slowed the acquisition rate and magnitude on the hippocampus-dependent trace eyeblink conditioning task. Single-neuron recording studies have shown that the dorsal CA1 region of the hippocampus shows the most substantial functional changes during trace eyeblink conditioning (Weible et al. 2006). The importance of learning-related reductions of apamin-sensitive SK2 channels was verified through the use of microarray, qRT-PCR, and Western blotting techniques. Importantly, these findings strengthen our hypothesis that the increase in intrinsic hippocampal pyramidal neuronal excitability, via reductions in the postburst AHP, is a key cellular biomarker of successful learning on a hippocampus-dependent task.
The theory that intrinsic excitability must first be altered for learning to take place (Disterhoft and Oh 2006) is supported by the literature on aging animals. Aging is accompanied by a decrease in intrinsic excitability as measured by an increase in the AHP (Landfield and Pitler 1984). More specifically, many aged animals are learning impaired, and those that are impaired have larger medium and slow postburst AHPs than those that are able to learn associative (Moyer et al. 2000) and spatial (Tombaugh et al. 2005) hippocampus-dependent tasks. If the AHP in aged animals is pharmacologically manipulated to be equal to that from young animals, their learning also improves to levels comparable to that from young animals. This has been demonstrated with different compounds acting through several independent pathways. The cholinesterase inhibitors metrifonate and galantamine, as well as the M1 muscarinic agonist CI-1017 and the L-type calcium channel antagonist nimodipine, all act to increase intrinsic excitability in CA1 pyramidal neurons from aged animals by reducing the AHP and accommodation (Moyer et al. 1992; Norris et al. 1998; Oh et al. 1999, 2006; Weiss et al. 2000). In addition, all of these compounds improved the performance of aged rabbits in trace eyeblink conditioning (Deyo et al. 1989; Kronforst-Collins et al. 1997; Weible et al. 2004; Weiss et al. 2000). Importantly, the effect of NS309 on learning shows that the opposite of these aging studies is also true: decreasing intrinsic excitability in young animals causes them to be learning impaired. Since NS309 held the hippocampal neurons of young animals in a less excitable state and made them “aged” with regard to the size of their medium AHP, this study supports the theory that intrinsic excitability must be altered for learning to take place.
Increasing SK channel activity with NS309 increased the medium (not slow) postburst AHP, reduced in vivo CA1 neuron firing rate, and significantly impaired learning, but did not block it all together. NS309's effect was largest on the acquisition phase, days 3–6 (see Fig. 4). This supports the view that SK channels are important for the encoding phase of learning (Vick et al. 2010). NS309 had less effect once the animals began to learn the task, as seen by the fact that there was no difference between NS309 and vehicle control groups on days 7–9 (see Fig. 4). Based on our data, animals receiving NS309 likely would have eventually reached the same level of performance as control animals had we trained the rats for more days. However, if we were able to truly mimic normal aging by increasing both the medium and the slow postburst AHP, as observed in CA1 pyramidal neurons from aged animals (Matthews et al. 2009; Moyer et al. 2000), then learning may have been prevented in these cognitively intact young adult rats just as that observed in normal aging-impaired subjects. Thus, the present findings also highlight how the slow postburst AHP, in combination with the medium AHP, might serve as the potential cellular mechanism that determines successful learning.
Changes in synaptic transmission, in addition to the intrinsic excitability changes, in the CA1 region, may also underlie successful learning. The apamin-sensitive SK channel conductance in dendritic spines of CA1 pyramidal neurons has been shown to modulate excitatory synaptic transmission via their close association with N-methyl-d-aspartate (NMDA) receptors (Bloodgood and Sabatini 2007; Lin et al. 2008; Ngo-Anh et al. 2005). Blockade of SK channels with apamin enhances the EPSPs (Lin et al. 2008; Ngo-Anh et al. 2005) and facilitates long-term potentiation of excitatory synaptic transmission (Behnisch and Reymann 1998; Stackman et al. 2002), whereas, the SK channel activator NS309 reduced the EPSP width and area (Fig. 1C). Increases in multisynaptic boutons (Geinisman et al. 2001) and a modest increase in excitatory postsynaptic potential (EPSP) measured in vivo (Gruart et al. 2006) have been observed in the CA1 region following trace eyeblink conditioning. Ex vivo, a modest EPSP increase in CA1 region was observed immediately (1 h), but not 24 h, after successful learning the trace eyeblink conditioning task (Power et al. 1997). This lack of a sustained learning-related EPSP increase ex vivo in the CA1 region is due in part to a corresponding increase in inhibitory transmission that may mask the EPSP enhancement following successful learning (McKay and Disterhoft 2010). Thus, the present learning impairment observed with NS309 treatment may also be due, in part, to the reduced NMDA-dependent synaptic plasticity following an increase in the apamin-sensitive SK conductance in dendritic spines.
Previous reports using the SK channel agonists 1-EBIO and CyPPA, applied systemically, demonstrated impaired encoding of object memory, but no effect on fear conditioning (Vick et al. 2010). However, the systemically applied 1-EBIO and CyPPA caused a decline in motor behavior and may have confounded the results. To prevent activation of SK and IK channels in the periphery, we injected NS309 directly into the hippocampus. NS309 had no noticeable effect on eyeblinks from pseudoconditioned animals and did not influence the rats' reflexive eyeblink response (the unconditioned response) (Fig. 3C), suggesting potential motor impairments were not an issue in the current experiments.
The current experiments were designed to test our hypothesis that modulation of the postburst AHP in the hippocampus is a key factor for successful learning. This does not preclude the potential negative impact of any other pharmacologic compounds that may dampen the neural activity of hippocampal pyramidal neurons, such as the M-channel activator, retigabine. Our previous work clearly suggests that learning changes basal firing rates of CA1 pyramidal neurons in vivo and increases intrinsic excitability of hippocampal pyramidal neurons by reducing the fast, medium, and slow postburst afterhyperpolarization ex vivo (reviewed in Disterhoft and Oh 2006, 2007). However, learning the trace eyeblink conditioning task did not change the KCNQ-mediated M-current in CA1 pyramidal neurons (Kuo et al. 2008). In addition, although there are numerous pharmacologic compounds that would reduce the slow postburst AHP, normal aging is the only source for enlarging the slow AHP in hippocampal pyramidal neurons (Disterhoft and Oh 2007). Thus, we have focused and designed our present experiments to systematically evaluate the potential impact of modulating the medium, SK channel—mediated postburst AHP in young adult rats.
Our present findings also add to the growing body of work that demonstrates the importance of learning-related SK channel alteration in other brain regions and after learning other associative tasks. In the olfactory cortex, SK2 but not SK3 mRNA levels are reduced after learning an odor-discrimination task (Brosh et al. 2007). In situ hybridization using radiolabeled apamin and SK2 mRNA showed a decrease in binding sites in CA1 and CA3 following spatial learning (Mpari et al. 2010). Together, these studies provide convincing evidence that fewer SK channels are made and inserted in the membrane after successful learning. The diversity of tasks after which this observation is made suggests SKs are universally involved in the molecular basis for learning.
Calcium-dependent potassium channels play an important role in learning and memory (Disterhoft and Oh 2006; Hammond et al. 2006). We focused on SK channels as one member of this family with well-characterized and specific agonists, antagonists, and antibodies. NS309 reduced in vivo CA1 pyramidal neuron firing rate and the medium postburst AHP and EPSPs recorded in vitro, and impaired acquisition of the early phase of trace eyeblink conditioning by nearly 50%. The present findings provide strong support for the hypothesis that proper function and modulation of SK channels are important for acquiring associative learning tasks, further strengthening our hypothesis that postburst AHP reductions in hippocampal pyramidal neurons are a biomarker of successful learning.
GRANTS
This work was supported by National Institutes of Health Grants R37 AG-008796 and R01 AG-017139 (to J.F.D.); T32 AG-020506 (to B.M.M.); R01 MH-094835 (to J.B.); R01 NS-059879 (to C.W.); and R01 NS-038880 (to J.P.A.); and The Ralph and Miriam Falk Trust (Chicago, IL) (to J.R.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.M.M., M.M.O., and J.F.D. conception and design of research; B.M.M., M.M.O., R.G., J.B., R.A.K., and C.W. performed experiments; B.M.M., M.M.O., R.G., J.B., R.A.K., C.W., and J.F.D. analyzed data; B.M.M., M.M.O., R.G., J.B., R.A.K., C.W., J.P.A., J.R.M., and J.F.D. interpreted results of experiments; B.M.M., R.G., J.B., R.A.K., and C.W. prepared figures; B.M.M., M.M.O., and J.F.D. drafted manuscript; B.M.M., M.M.O., J.B., R.A.K., C.W., J.P.A., J.R.M., and J.F.D. edited and revised manuscript; B.M.M., M.M.O., R.G., J.B., R.A.K., C.W., J.P.A., J.R.M., and J.F.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Wei-Chung Wang, Amy Gamelli, John Linardakis, and Janitza Montalvo Ortiz for assistance in the behavioral experiments; Felix Nunez for collecting preliminary molecular data; and Mary Schmidt for technical assistance.
REFERENCES
- Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett 253: 91–94, 1998 [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron 53: 249–260, 2007 [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24: 5301–5306, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh I, Rosenblum K, Barkai E. Learning-induced modulation of SK channels-mediated effect on synaptic transmission. Eur J Neurosci 26: 3253–3260, 2007 [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Weiss C, Oh MM, Disterhoft JF, Brudzynski SM, Panksepp J, Moskal JR. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience 192: 515–523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science 243: 809–811, 1989 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci 29: 587–599, 2006 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 6: 327–336, 2007 [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol 34: 1077–1083, 2007 [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci 21: 5568–5573, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci 26: 1077–1087, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Hu H, Vervaeke K, Storm JF. SK (KCa2) channels do not control somatic excitability in CA1 pyramidal neurons but can be activated by dendritic excitatory synapses and regulate their impact. J Neurophysiol 100: 2589–2604, 2008 [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566: 689–715, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci 26: 1844–1853, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol 578: 799–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci 24: 5151–5161, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes RA, Burgdorf J, Otto NJ, Panksepp J, Moskal JR. Social defeat, a paradigm of depression in rats that elicits 22-kHz vocalizations, preferentially activates the cholinergic signaling pathway in the periaqueductal gray. Behav Brain Res 182: 290–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: social dominance-submission gene expression patterns in rat neocortex. Neuroscience 137: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Moriearty PL, Schmidt B, Disterhoft JF. Metrifonate improves associative learning and retention in aging rabbits. Behav Neurosci 111: 1031–1040, 1997 [DOI] [PubMed] [Google Scholar]
- Kuo AG, Lee G, McKay BM, Disterhoft JF. Enhanced neuronal excitability in rat CA1 pyramidal neurons following trace eyeblink conditioning acquisition is not due to alterations in IM. Neurobiol Learn Mem 89: 125–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science 226: 1089–1092, 1984 [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral—CA1 synapses. Nat Neurosci 11: 170–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Disterhoft JF. Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learn Mem 16: 106–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci USA 105: 15154–15159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol 78: 1030–1044, 1997 [DOI] [PubMed] [Google Scholar]
- McKay BM, Disterhoft JF. Increase in intrinsic excitability of inhibitory interneurons in CA1 stratum oriens following associative learning. Program No. 199.13. 2011 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, 2011. Online [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci 20: 5476–5482, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol 68: 2100–2109, 1992 [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 16: 5536–5546, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpari B, Sreng L, Manrique C, Mourre C. KCa2 channels transiently downregulated during spatial learning and memory in rats. Hippocampus 20: 352–363, 2010 [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- N'Gouemo P, Yasuda RP, Faingold CL. Protein expression of small conductance calcium-activated potassium channels is altered in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res 1270: 107–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci 18: 3171–3179, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, McKay BM, Power JM, Disterhoft JF. Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci USA 106: 1620–1625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Power JM, Thompson LT, Disterhoft J. Apamin increases excitability of CA1 hippocampal pyramidal neurons. Neurosci Res Commun 27: 135–142, 2000 [Google Scholar]
- Oh MM, Power JM, Thompson LT, Moriearty PL, Disterhoft JF. Metrifonate increases neuronal excitability in CA1 pyramidal neurons from both young and aging rabbit hippocampus. J Neurosci 19: 1814–1823, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Wu WW, Power JM, Disterhoft JF. Galantamine increases excitability of CA1 hippocampal pyramidal neurons. Neuroscience 137: 113–123, 2006 [DOI] [PubMed] [Google Scholar]
- Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem 280: 41404–41411, 2005 [DOI] [PubMed] [Google Scholar]
- Power JM, Thompson LT, Moyer JR, Jr, Disterhoft JF. Enhanced synaptic transmission in CA1 hippocampus after eyeblink conditioning. J Neurophysiol 78: 1184–1187, 1997 [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol 41: 461–531, 1973 [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci 17: 2727–2734, 2003 [DOI] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28: 4028–4036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22: 10163–10171, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol 76: 1836–1849, 1996 [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25: 2609–2616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick KA, 4th, Guidi M, Stackman RW., Jr In vivo pharmacological manipulation of small conductance Ca(2+)-activated K(+) channels influences motor behavior, object memory and fear conditioning. Neuropharmacology 58: 650–659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Oh MM, Lee G, Disterhoft JF. Galantamine facilitates acquisition of hippocampus-dependent trace eyeblink conditioning in aged rabbits. Learn Mem 11: 108–115, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, O'Reilly JA, Weiss C, Disterhoft JF. Comparisons of dorsal and ventral hippocampus cornu ammonis region 1 pyramidal neuron activity during trace eye-blink conditioning in the rabbit. Neuroscience 141: 1123–1137, 2006 [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res 99: 123–132, 1999 [DOI] [PubMed] [Google Scholar]
- Weiss C, Preston AR, Oh MM, Schwarz RD, Welty D, Disterhoft JF. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J Neurosci 20: 783–790, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S, Bussey KJ, Riss J, Barrett JC, Weinstein JN. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol 4: R28, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]