Abstract
Previous research has described kinetic characteristics of treadmill steps in very stable steppers, in cross-sectional designs. In this study we examined, longitudinally, muscle activation patterns during treadmill stepping, without practice, in 12 healthy infants at 1, 6, and 12 mo of age. We assessed lateral gastrocnemius, tibialis anterior, rectus femoris, and biceps femoris as infants stepped on a treadmill during twelve 20-s trials. Infants showed clear changes in kinematics, such as increased step frequency, increased heel contact at touch down, and more flat-footed contact at midstance. Electromyographic data showed high variability in muscle states (combinations), with high prevalence of all muscles active initially, reducing with age. Agonist-antagonist muscle coactivation also decreased as age increased. Probability analyses showed that across step cycles, the likelihood a muscle was on at any point tended to be <50%; lateral gastrocnemius was the exception, showing an adultlike pattern of probability across ages. In summary, over time, healthy infants produce a wide variety of muscle activation combinations and timings when generating stepping patterns on a treadmill, even if some levels of muscle control arose with time. However, the kinematic stability improved much more clearly than the underlying kinetic strategies. We conclude that although innate control of limb movement improves as infants grow, explore, and acquire functional movement, stepping on a treadmill is a novel and unpracticed one. Hence, developing stable underlying neural activations will only arise as functional practice ensues, similarly to that observed for other functional movements in infancy.
Keywords: electromyography, interlimb coordination
across the first year postbirth, healthy infants tend to perform steps when supported upright on a motorized treadmill (Thelen 1985; Thelen and Ulrich 1991; Yang et al. 1998). Kinematic analyses of these steps show quite clearly that significant changes occur over these 12 mo in infants' leg movement patterns and interlimb coordination (Groenen et al. 2010; Thelen and Ulrich 1991; Vereijken and Thelen 1997), as is true of all other motor behaviors when assessed over such a wide developmental time frame in infancy (e.g., Adolph et al. 1998; Freedland and Bertenthal 1994; Haehl et al. 2000; Konczak and Dichgans 1997; Lynch et al. 2008).
There are periods of time over the first year when most healthy babies step infrequently when supported in the treadmill context, months 1 through 2 or 3, followed by another couple of months during which infants show a gradual improvement in proportion of time during a test trial during which they produce steps (Teulier et al. 2009; Thelen and Ulrich 1991). Of those steps produced, ∼56% over the first 3 mo show the limbs engaged in alternation. The remainder includes parallel steps, in which legs initiate the swing phase at the same time, and single steps, ones that are not overlapped by a step performed by the other leg (Groenen et al. 2010; Teulier et al. 2009; Thelen and Ulrich, 1991). The percentage of alternation increases readily after the first few months to ∼87% by 7 mo (Teulier et al. 2009; Thelen and Ulrich 1991). Interlimb phase lags for alternation, as well, although demonstrating a mean of 180° out of phase, have a very high standard deviation and range from 20 to 80% in their coupling (Musselman and Yang 2008; Teulier et al. 2009). The treadmill context with partial body weight support has proven useful to test theoretical questions about postnatal development of motor control, but it also presents an important opportunity for monitoring the neuromotor control of very young infants at risk for motor disabilities. Assessment can begin with the neonate and continue through the onset of independent walking. Perturbations can be introduced to test the stability of the system. Also, many aspects of typical development have been characterized. Thus the potential to identify delays or aberrant trajectories of neuromotor control, as well as the impact of therapeutic intervention, to facilitate gait early in life can be monitored via attempts to elicit stepping in this context. Missing, however, from previous studies have been longitudinal studies of the activity of thigh and shank muscles during infants' treadmill steps. Only through longitudinal work can the changes in behavior at different points in time and their variability be confidently attributed to development, rather than differences among samples selected for the specific study at each age. Infants with motor disabilities generally produce much greater variability in performance around their group means and in their developmental trajectories than do healthy infants (Deffeyes et al. 2009; Ulrich and Ulrich 1995; van Haastert et al. 2006). Thus closer examination of the underlying control of treadmill stepping, reflected in electromyographic (EMG) activity, across the range of behavioral responses (good steppers and poor steppers) for a randomly selected sample of healthy infants will enhance our understanding of normal variations across infancy.
Our present goal was to examine, longitudinally, the underlying activation patterns of 4 primary gait muscles of both legs in 12 infants with typical development, from 1 mo postbirth through 12 mo.
METHODS
Participants
Participants were 12 infants (7 female) who were tested longitudinally for their responses to being supported upright on a motorized treadmill without any practice stepping on a treadmill between testing sessions (Teulier et al. 2009). Inclusion criteria were no known physical or cognitive disabilities and gestational age ≥36 wk [group mean (SD) = 39.5 (0.74) wk] when entering the study at 1 mo of age. Families were recruited via flyers posted throughout the local community.
Infants who were part of this research study had an overall decrease in ponderal index from 1 mo [26.77 (2.23)] to 12 mo [24.44 (2.49)] [6 mo = 27.21 (2.17)], similar to the results reported by Lande et al. (2005). In addition, these infants began to walk independently (defined as 3–5 steps without support) at 12.48 (1.52) mo.
Procedures
All testing occurred in the Developmental Neuromotor Control Laboratory, School of Kinesiology, at the University of Michigan. When families arrived at the laboratory, we explained procedures and asked parents to sign a consent form approved by the University of Michigan Institutional Review Board. Parents also completed a history survey. Infants were tested at 1, 3, 6, 9, and 12 mo of age.
To prepare infants for testing, we removed their clothing, shoes, and diaper and placed reflective markers (8-mm diameter at ages 1 and 3 mo; 18-mm diameter at ages 6, 9, and 12 mo) bilaterally on the iliac crest, greater trochanter, knee joint, lateral malleolus, and ventral surface of the third metatarsophalangeal joint. After cleaning the skin with an alcohol pad, we placed preamplified bipolar surface EMG electrodes (rectangular patch of 5 × 2.5 cm with electrodes placed 0.63 cm apart, electrode conductive surface diameter of 5 mm) over the muscle bellies of the lateral gastrocnemius (LG), tibialis anterior (TA), rectus femoris (RF), and biceps femoris (BF) muscles of the right leg for set 1 trials and moved them to the left leg for set 2 trials. To minimize the movement of wires and interference, a research assistant held the EMG cables well above the treadmill during each trial. Finally, an online screening of EMG values was visualized before each trial to check for clarity of the EMG burst(s) when the baby was moving and to reduce the chance that cross talk was recorded.
We used a custom-made infant-sized motorized treadmill, 18 × 42 × 82 cm (height × width × length) with a smooth belt surface (30 cm wide). The treadmill was placed on a large table 73 × 118 × 190 cm with 3 Peak Motus cameras placed on each side of the table to monitor joint marker positions at 60 Hz. A 60-Hz digital video camera was positioned on one side and perpendicular to the table to videotape leg movements for data capture verification purposes (for more details, refer to Teulier et al. 2009). The EMG data were collected at a sampling rate of 1,200 Hz using Therapeutics Unlimited Model 67 system (containing a built-in 40-Hz high-pass filter) and using the Peak Motus real-time system to digitize the data. All video camera, motion capture system, and EMG data were synchronized.
We held infants under the axillary regions so they were upright with feet resting on the treadmill belt for twelve 20-s trials. Trials were presented in two sets of six speeds. Trials 1 and 12 were baseline trials where the treadmill belt was stationary. During trials 2–6 and 7–11, speed increased from 0.068 to 0.22 m/s in increments of 0.038 m/s. Between sets, and as needed during testing, infants were given rest breaks. The data presented in this study are part of a larger study looking at the developmental trajectory of stepping in infants with spina bifida. The cohort of infants presented in this study was used as a control group (see Teulier et al. 2009). Speeds were manipulated in that study to see if the speeds known to stimulate infants with typical development (Thelen and Ulrich 1991) were stimulating differently infants with spina bifida.
After treadmill testing, we measured infants' total body weight and length, leg length (greater trochanter to lateral malleolus), thigh length (greater trochanter to lateral knee joint), and thigh and shank circumferences. We also administered the motor subscale of the Bayley Scales of Infant Development II (Bayley 1993) to assess concurrent levels of functional motor skill.
Data Reduction
For the purposes of the questions addressed in this work, we focus only on treadmill step parameters and patterns of muscle activity during alternating strides and not on speed adaptation. Because EMG signals were quite variable all along the first year, we decided to only look at those variables at 1, 6, and 12 mo to increase the power of the analysis and to clarify the reading of the results by focusing on three ages separated by acquisition of motor milestones. For additional details regarding overall step frequency, step type, and nonstepping behaviors by this cohort of infants, refer to Teulier et al. (2009).
Identification of stride events and characteristics.
Three trained behavior coders identified the occurrence of alternating steps by viewing, frame by frame, the recorded digital videos. They recorded the time (frame) when the following step events occurred: toe-off, touchdown, and end of stance.
For alternating steps, coders identified the part of the infant's foot that contacted the treadmill belt at touchdown and midstance. Foot contact was coded as toe, flat, heel, lateral, or medial. From this information, we then calculated the percentage of each foot posture that was used for touchdown and midstance. In addition, we coded each infant's leg posture at midswing and midstance during alternating stepping. Their legs were coded as high flexion (if the knee or hip flexion exceeded 90°) or low flexion. From this information, we calculated the percentage of leg flexion demonstrated by each infant when performing alternating steps. Training required each coder to practice with training tapes and, when tested for accuracy, to obtain a coefficient of agreement of 0.85 (interobserver reliability coefficient, κ) to five sets of data from five different infants validated previously by experts in our laboratory.
Description of the step cycle.
To account for the variability in absolute duration of stride cycles, we normalized raw gait cycle data by transforming the original data points to a 1,000-point distribution using a cubic spline interpolation. Stance (ST) and swing (SW) phase durations were calculated as a proportion of the gait cycle.
EMG data reduction.
Because the number of alternating strides produced by infants is often low across the first 6 mo postbirth, we used several steps in the process of extracting strides to analyze. To ensure reliability of the data, we extracted only steps located in a series of alternating steps, which reduced the analysis to only two to three consecutive strides per leg for each infant at each age. For a stride to be included, the entire cycle had to be completed within the 20-s trial and data for all four muscles had to be free from artifact and high levels of noise. We prioritized strides that occurred at the middle belt speed (0.144 m/s) because in our previous work we found this to be the optimal speed across the first year (Teulier et al. 2009). We followed by selecting strides occurring at 0.106 and 0.182 m/s if strides were not taken at 0.144 m/s. Last, if an infant produced no strides at these middle speeds but stepped at the slowest (0.068 m/s) or fastest (0.22 m/s) speeds, we included them for analyses to be as representative as possible of all infants.
To process EMG data, we first applied a band-pass filter with cutoff frequencies set at 75 and 300 Hz (Spencer et al. 2000; Spencer and Thelen 2000). The low-end frequency reduced electrical noise associated with wire sway and biological artifacts, whereas the high cutoff eliminated extraneous tissue noise at the electrode site. Next, we rectified the data, eliminated any high-frequency components added in the rectification procedure by using boxcar averaging with a window size of seven samples, and converted the EMG signal to an on-off designation.
The goal of using an objective series of steps to determine when increases in muscle activation occurred was to operationalize the procedures used, eliminating subjectivity in conclusions. To determine on-off activity, we began by moving a 50-ms window, frame by frame, across each EMG signal. If the average EMG activity within a window exceeded a minimum threshold (noise), the center value of that window was considered “on.” To determine the noise threshold, we computed frequency histograms of amplitudes for each EMG signal for each trial. In addition, we normalized the frequency histograms to the modal amplitude for each trial. We used a cutoff value of 0.15 of the normalized modal frequency to differentiate EMG on-off activity (Spencer et al. 2000; Spencer and Thelen 2000). Finally, the duration of “on” activity was summed across small segments of activity if the period of inactivity between segments was <50 ms.
Muscle activation patterns extracted were thus analyzed using three methods. First, we used a muscle state analysis to determine for each frame within a stride which muscles were active, that is, the “state” of EMG activity as the stride unfolded (Spencer et al. 2000; Spencer and Thelen 2000). Because we recorded EMG for 4 muscles, there were 16 possible combinations ranging from all “off” to all “on.” In addition, this method allowed us to determine the duration and frequency of occurrence for each state, reflecting which muscles were active and for how much of the normalized stride cycle.
Second, we calculated the percentage of coactivation between agonist-antagonist muscle pairs during the stride cycle as
This variable does not address amplitude of muscle activation, only the presence of it (on or off).
Third, to determine whether there was an increased likelihood of individual muscles being active or not at specific times across the stride cycle for all infants, we calculated muscle activation probability across infants. A probability value of 1 meant that the muscle was always on at that point in the cycle for all infants. A probability of 0.5 meant that the muscle was on at that point for 50% of the stride cycles produced by infants. Probabilities presented reflect the pooling of all on values for each time point during the cycle for all infants, divided by the total number of cycles included. To provide a reference to what a stable, rhythmical stepping pattern with low variability would look like, in contrast to the infants, we also looked at the probability of muscular activation for 4 typical adults who walked on a treadmill at 1.40 m/s for 2 trials of 1 min.
Data Analyses
For our statistical analyses, we used SPSS (version 14; IBM, Somers, NY). We used mixed-model regression analysis to determine the influence of age and phase (ST, SW) on parametric variables. Use of this method allows for random patterns of missing cells and thus is well suited for analysis of longitudinal data where missing data points typically occur. In this statistical model, random effects were specified as infant and infant × age interactions to control for within-participant effects.
Because the EMG was recorded unilaterally, we wanted to identify whether there was any effect of leg on the muscle activation characteristics. We tested the leg effect and leg × age interaction for each of our analysis, and no effect was found on any muscles. Therefore, leg was removed as a factor to obtain the best fitted statistical model.
In addition, to determine whether the data reduction principles we followed affected the mean speed from which cycles were selected, we conducted a mixed-model one-way ANOVA with repeated measures across age. Results showed that speed did not vary significantly across ages [F(2,49) = 113, P = 0.329]. Differences across months were a maximum of 0.8 mm/s. This small difference could arguably have a minimal effect on our mean values for muscle activation duration and cycle duration. All other gait cycle variables were normalized to a proportion of the gait cycle.
RESULTS
Stride Characteristics
To provide an overview of what the overt behavior looked like as infants stepped, we begin by describing stride characteristics and their change over time. We used three one-way mixed-model ANOVAs to assess the relation between age and cycle duration, proportion of time spent in stance during the stride cycle, and percentage of the cycle during which the leg was highly flexed. Absolute cycle duration [F(2,21) = 8.707, P = 0.003] and proportion of time spent in stance during the stride cycle increased with age [F(2,21) = 9.27, P < 0.001], whereas the percentage of time spent with the leg highly flexed decreased [F(2,21) = 9.27, P < 0.001] (see Table 1 for post hoc results). On average, the stance phase comprised 67.7% of the gait cycle, with an increase from 58.2% at 1 mo to 73.3% at 12 mo.
Table 1.
Description and statistics for stride parameters by age
| 1 mo | 6 mo | 12 mo | |
|---|---|---|---|
| Cycle duration, s | 1.72 (0.62)† | 1.95 (0.32)† | 2.12 (0.43) |
| Stance phase, %cycle | 58.2 (14.0) | 66.8 (7.0)* | 73.3 (6.1)* |
| Leg flexed in swing, % | 73.3 (18.1) | 60.4 (21.8) | 45.5 (36.1)* |
| Interlimb phase lag, % | 50.8 (15.0) | 50.4 (14.7) | 51.6 (10.8) |
| Foot contact at touchdown, % | |||
| Heel | 5.7 (1.0) | 10.2 (1.8)* | 13.2 (2.6)* |
| Flat | 23.0 (4.0) | 31.4 (5) | 40.5 (6.2) |
| Toe | 47.4 (4.6) | 50.4 (7.0) | 40.5 (7.3) |
| Medial/lateral | 23.8 (4.0) | 7.8 (2.7)* | 5.7 (1.3)* |
| Foot contact at midstance, % | |||
| Flat | 21.6 (5.5)† | 33.7 (7.5)† | 70.4 (5.5) |
| Toe | 73.5 (5.2)† | 63.9 (8.5)† | 27.1 (5.8) |
Significantly different from 1 mo.
Significantly different from 12 mo.
To examine the part of the foot making contact with the treadmill at touchdown and at midstance, we used two one-way multivariate ANOVAs (MANOVAs) with repeated measures on age. Dependent variables for the first MANOVA (for touchdown) were percentages of heel contact, flat-footed contact, toe contact, and medial or lateral contact. For midstance, only flat-footed and toe contact were entered in the analysis, because they represented >95% of the occurrences. We obtained a significant age effect [Wilks λ = 0.242, F(8,34) = 4.392, P = 0.001] for foot contact at touchdown. Post hoc ANOVA results revealed a significant decrease for medial/lateral contact at touchdown with increasing age [F(2,20) = 18.5, P < 0.001] as well as a significant increase in heel contact [F(2,20) = 4.2, P = 0.029]. For contact at midstance, an age effect was obtained [Wilks λ = 0.287, F(8,21) = 8.21, P < 0.001]. Post hoc ANOVAs revealed a significant decrease in toe contact with increasing age [F(2,20) = 20.18, P < 0.001] as well as a significant increase in flat-footed contact [F(2,20) = 17.5, P < 0.001] at midstance.
Muscle Activation Characteristics
In our first analysis of muscle activity, we examined simple characteristics, including burst duration and frequency within a stride cycle. We used separate one-way mixed-model ANOVAs for each muscle to determine the relation between of age and the mean muscle burst duration and mean number of muscle bursts of activity per stride cycle. The absolute duration of muscle activation showed a significant decrease from 0.75 s at 1 mo to 0.33 s at 12 mo for the RF [F(2,24) = 8.50, P = 0.002]. Follow-up univariate analyses showed a significant difference between ages 1 and 6 mo (P = 0.003) and between 1 and 12 mo (P = 0.005). For frequency of muscle activations, none of the ANOVAs were significant, but we noted a strong trend for the TA [F(2,18) = 3.05, P = 0.072], LG [F(2,19) = 3.460, P = 0.053], and RF [F(2,17) = 3.37, P = 0.058] to increase the number of muscle activations within a cycle as age increased.
Muscle Activity Patterns
Sixteen muscle states.
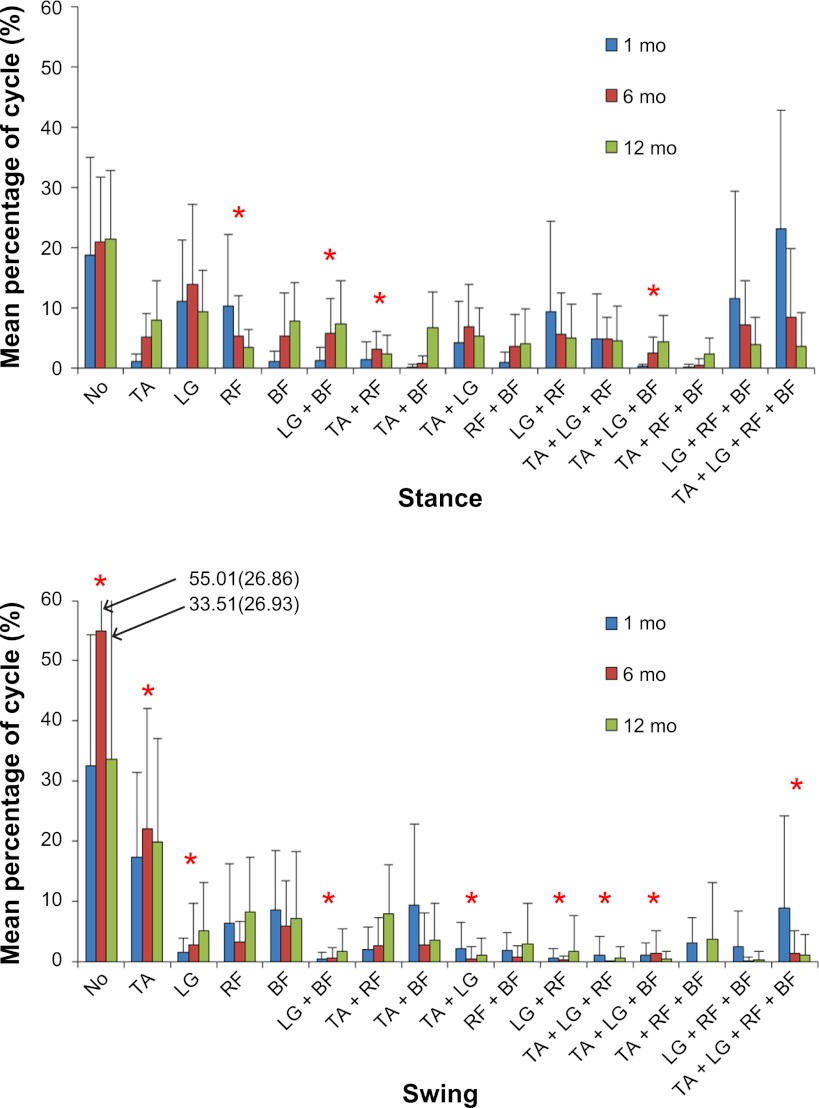
To look at how each muscle was activated in relation to other thigh and shank muscles, we examined their state, or combinations, across the cycle. For each possible muscle state, we ran a mixed-model 3 (age) × 2 (phase: ST and SW) ANOVA on the percentage of time the muscle state was present. Figure 1 illustrates the distribution of all states at each time point. Data in Fig. 1 show that infants spent more time with none of their four muscles activated during the SW phase than during the ST phase [F(1,52) = 22.2, P < 0.001] at all ages (P = 0.094). Also at all ages, the TA was activated significantly more often during SW than during ST [F(1,72) = 26.1, P < 0.001]. The reverse occurred for LG, which was significantly more active during ST than during SW [F(1,43) = 25.8, P < 0.001]. For RF, we found an age × phase interaction [F(2,46) = 3.6, P = 0.034]. Inspection of the means revealed a decrease in RF activation during ST with increasing age, but not in SW. No statistical differences occurred for BF or the muscle combinations of RF + BF and TA + BF. For the muscle state combinations of TA + LG, LG + RF, and LG + BF, infants showed greater frequency during ST than during SW [F(1,46) = 21.7, P < 0.001; F(1,41) = 13, P = 0.0001; and F(1,48) = 16.9, P < 0.001, respectively]. LG + BF also showed a trend for increased presence with increasing age [F(2,19) = 3.4, P = 0.053]. For TA + RF, an age effect [F(2,72) = 3.43, P = 0.038] and an interaction effect were found [F(2,72) = 3.13, P = 0.049], reflecting an increase during SW, but not ST, as infants got older.
Fig. 1.
Muscle activity patterns for 16 muscle states by stance (top) or swing phase (bottom). TA, tibialis anterior; LG, lateral gastrocnemius; RF, rectus femoris; BF, biceps femoris. Histogram values are means ± SD. *Significant age or interaction effects.
The occurrence of states in which three muscles were active concurrently increased, typically, during ST, for TA + LG + RF [F(1,45) = 28.1, P < 0.049], LG + RF + BF [F(1,72) = 13.03, P = 0.001], and TA + LG + BF [F(1,52) = 4.7, P = 0.033]. For TA + LG + BF, we also found an interaction effect, reflecting an increase in appearance with age during ST but not SW [F(2,52) = 4.6, P = 0.014]. No significant effects were detected for TA + RF + BF. Interestingly, the muscle state in which all four muscles were activated occurred more frequently in ST than in SW [F(1,47) = 11.9, P = 0.001] and more often at 1 mo than at older ages [F(2,12) = 6.9, P = 0.01].
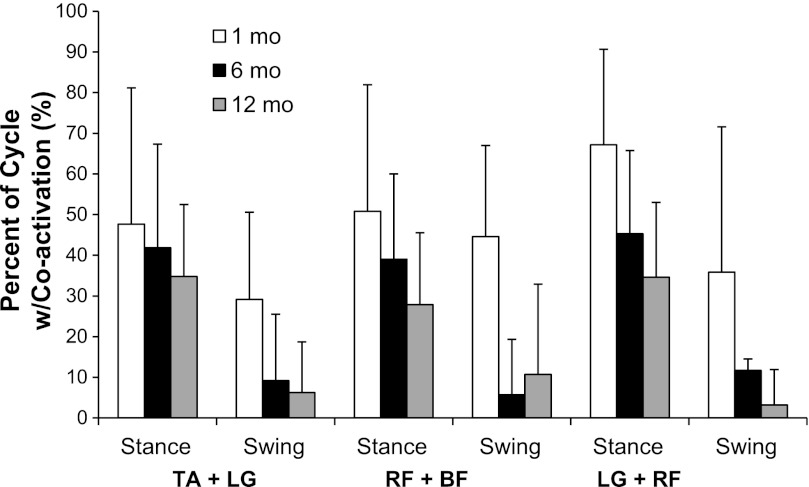
Coactivation muscle patterns.
Figure 2 presents coactivation percentages and shows a decrease, with age, for all agonist-antagonist pairs [TA + LG: F(2,22) = 3.77, P = 0.035; RF + BF: F(2,19) = 9.05, P = 0.002; and LG + RF: F(2,25) = 8.3, P = 0.002]. In addition, all co-contractions were more prevalent during ST than during SW [TA + LG: F(1,65) = 44.6, P < 0.001; RF + BF: F(1,16) = 18.05, P = 0.001; and LG + RF: F(1,12) = 70.5, P < 0.001].
Fig. 2.
Percentage of stride cycle during which agonist-antagonist muscles were simultaneously coactivated. Histogram values are means ± SD.
Probability of muscle activity.
Figure 3 presents the probability that a particular muscle was active, at each point across the stride cycle, collapsed over all babies. Graphs show data for infants at 1, 6, and 12 mo as well as for 4 young adults. Readily apparent is the fact that only the LG shows a probability value that reached or exceeded 50%. The distribution of probabilities (e.g., their trajectories of rising and falling across the stride cycle) differs significantly for the RF, TA, and BF, with low probability at all ages.
Fig. 3.
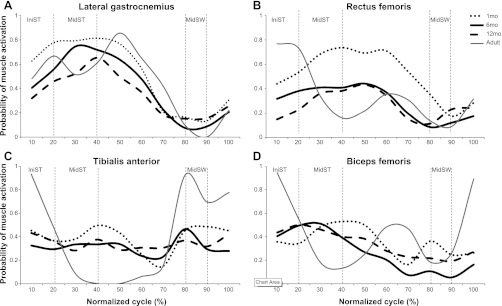
Probability of muscle activity across stride cycle, by age, in LG (A), RF (B), TA (C), and BF muscle (D). X-axis values represent the normalized cycle (0–100%). IniST, initial stance; MidST, midstance; MidSW, midswing.
To test the significance of changes in probability data over age, we identified three phases during which, based on walking in adult skilled performers, one might anticipate contrasting levels of muscle activity across the stride cycle. Within the stance phase, we looked at initial stance (IniST; around 0–20% of the gait cycle) and midstance (MidST; around 20–40% of the cycle). IniST usually represents a peak of muscular activity, whereas MidST represents a decrease in muscular activity (only LG is expected to be highly active). Finally, midswing (MidSW; around 80–90% of the cycle) was chosen to contrast ST and SW when TA is expected to reach peak activity. We then used a 3 (age) × 3 (phase) mixed-model ANOVA for each muscle to determine if significant differences emerged as a function of events within the cycle or with age.
Results show that LG (Fig. 3A) decreased probability of activation with age [F(2,142) = 3.9, P = 0.02] and demonstrated significant differences in level of activation across subphases of the gait cycle [F(1,134) = 67.1, P < 0.001]. Mean values suggest a higher probability of activation during MidST than either MidSW or IniST (P < 0.001 for both), but the probability of activation during IniST was higher than during MidSW (P < 0.001). Compared with the probability of muscle activation for adults, the probability of activation for the LG in infants follows a similar pattern despite the tendency for infants to activate their LG earlier in the stride cycle.
The probability of activation for RF (Fig. 3B) showed an age effect [F(2,10) = 6.7, P = 0.013], phase effect [F(2,98) = 23.4, P < 0.001], and interaction effect [F(4,98) = 3.8, P = 0.006]. Comparison of the means shows a reduction in probability of activation during ST with increasing age, but the probability of muscle activation remained similar across ages during the SW phase. The probability of activation for the BF in infants across the first year of life does not exceed 50%, showing a lack of clear, rhythmical activity across step as usually can be seen in adults. Similarly, the probability of activation of BF (Fig. 3D) showed a phase effect [F(2,104) = 14.5, P < 0.001] but no age (P = 0.648) or interaction effects (P = 0.331). Mean values showed higher probabilities during MidST and IniST than during MidSW (P < 0.001). Overall, however, the probability of activating BF, like that of RF, was weak in infants.
For the TA (Fig. 3C), age, phase, and interaction effects were not significant. Overall, TA decreased its probability of being activated over the first year postbirth, and no differences among subphases were detected in terms of the probability of activation. Figure 3C also shows that adults have a very strong probability of activating TA during MidSW and IniST as they prepare to and initiate foot contact with the floor. This pattern of probability for activation of the TA for adults is clearly not observed in infants at any age across the first year of life, even though by 12 mo all were cruising and some were walking.
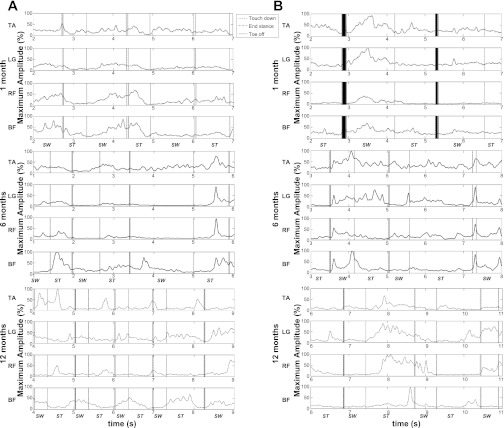
Examples of EMG traces for individual infants at 1, 6, and 12 mo.
Because EMG data are often presented as ensemble averages, which are not sensible when variability is very high, and because we chose to focus on ways to quantify objectively aspects of the EMG activity produced by infants, we add here examples of the smoothed and rectified data for two infants across all three ages to illustrate the quality of their muscle activity (Fig. 4). We specifically chose one infant who might be classified as a poor stepper (Fig. 4A), who produced only three to five consecutive alternating steps maximum at 1 and 6 mo but who stepped consistently by 12 mo, and a good stepper (Fig. 4B), an infant who produced a minimum of eight consecutive alternating steps at each age. Neither infant was unusual; each simply reflects two common overt behavior types. Each muscle trace shows two to three strides and illustrates the enormous variability individuals demonstrated, whether consistent or not in behavioral outcome, in the ways in which they marshaled active and passive forces to produce the step pattern. As the Fig. 4 shows, rhythmic activations were not evident, nor were consistent firing patterns among muscles to produce one stride, relative to the next, at any age.
Fig. 4.
Smoothed, rectified electromyograms (EMG) from 2 infants. A: EMG traces from an infant who produced alternating steps consistently at all ages. B: EMG traces from an infant who took fewer consecutive, alternating steps at 1 and 6 mo. Y-axis values represent the percentage of maximal amplitude for that muscle. Maximum amplitude was identified for each infant, at each test session for each muscle, as the highest peak value across all steps produced by that infant. ST, stance phase; SW, swing phase.
DISCUSSION
Our goal in this study was to examine, longitudinally, the muscle activity underlying step patterns of infants, when they were supported upright on a treadmill, regardless of how stable or unstable their step frequencies were, to understand the variety of muscle patterns healthy infants demonstrate across this first year postbirth. We chose to examine the four major muscles of the thigh and shank, often referred to as core gait muscles in highly skilled walkers, because of the clear kinematic similarity between treadmill steps and steps produced during walking. Overall, our results show that although kinematic behavior improved in terms of the consistency with which infants produced steps, their reduction in overall leg flexion, and improved foot placement patterns, the underlying muscle activation patterns that contributed to these overt trajectories were generally quite inconsistent. We believe our data also suggest a refinement in control of the major muscles of the leg, but because this behavior (stepping) is not one infants practice or use throughout most of this time period, each time they are tested they responded with exploratory behavior that, we argue, reflects a goal of overcoming the instability of having their feet moved backward, beyond their center of mass, rather than a desire to “step” or walk.
Lack of Clear Patterns of Muscle Organization Across First Year
Lack of muscle organization across the first year postbirth was illustrated by several objective and quantitative measures of activity. The muscle state analysis illustrates clearly that many combinations of muscle activation were produced across the cycle. Infants spent a considerable amount of cycle time with no muscles active, an efficient response during swing but that also occurred, on average, 20% of the time during stance. At 1 mo, all four muscles often activated concurrently; during stance, it was as likely that all muscles were active as that none of them was active. Across combinations of muscle activity, infants seemed to be somewhat randomly activating the options available to them, with small fluctuations in combinations (some increasing and others decreasing) over time. Probability data further illustrate that for three of the four muscles, the likelihood at any point in the cycle that the muscle was on, across cycles and infants, was consistently well below 50%. Only for the RF at 1 mo and LG did this differ, and, in fact, the LG demonstrated a pattern of probability approaching that of skilled adult walkers.
The lack of overall organization at the muscular level is in opposition with the improvement seen at the kinematic level. This cohort of infants was stepping almost continuously on the treadmill by 12 mo of age (0.8 steps/s), and the percentage of alternative stepping increased from 55% at 1 mo to 95% at 12 mo of age (Teulier et al. 2009), with more foot control and leg control (reduced high flexion during swing/more heel to flat transition). These findings are consistent with Chang et al. (2006, 2009) and the results of Ivanenko et al. (2005) in toddlers learning to walk overground with or without balance support. Stability first arose at the kinematic level and then at the nervous and muscular level, with the plasticity of the nervous and muscular systems being so enormous that a significant amount of experience/practice to achieve consistency was required.
Other functional motor skills that have been studied longitudinally show similar EMG results. For the development of skill in reaching or seated posture, muscle activity is highly variable initially, even when the outcome is successful. Only with weeks to months of goal-directed practice do rhythmic and stable muscle activity patterns emerge (de Graaf-Peters et al. 2007). Perhaps the clearest picture of infants' muscle activity across the first year is represented by the muscle traces shown in Fig. 4 for two individual infants, a good stepper and a poor stepper. By simply looking at the traces themselves, it would be difficult to guess which infant was more likely to step a lot and which one not. Each demonstrated normal variability in active force production and no obvious pattern of improvement, even by 12 mo of age.
Given the variability of timing and duration of individual “gait” muscles, it seems reasonable to point out that there are many other muscles we did not monitor that likely contributed to the net flexor and extensor torques responsible for the kinematic flexions and extensions created. Even when none of the muscles we monitored showed activity, combinations of deeper muscles may have been at work. Without a stepping “goal” and significant practice intentionally producing this goal, settling into a stable and efficient force production pattern may not emerge. Yang and Gorassini (2006), in their review of studies comparing availability of a spinal pattern generator for stepping in primates and other mammals of the type defined by Grillner et al. (1981), concluded that “there is a general sense that the pattern generator is either not as important for human walking or not as easy to activate or both.”
On the surface, at least, the variability we observed in early muscle activity during stepping seems to contrast with that reported recently by Dominici et al. (2011). These researchers studied the EMG activity during stepping in 3-day-old neonates and walking in toddlers, using mathematical techniques to extract commonalities. They concluded, and we agree in the abstract, that neonates' leg behaviors show more extension than flexion in stance and more flexion than extension during swing, and that by 12 mo, muscle activation patterns show greater differentiation among muscles. However, they also concluded that both age groups show sinusoidal muscle activity across strides at both ages, which we did not observe. Differences in our data may reflect age differences or the support surfaces (ours was moving and theirs was stationary) or the fact that their conclusions were based on a compression of data that masks the underlying variability.
Refinement of Control in Gait Muscles, More Generally
What is clear is that all of these infants were practicing some functional motor skills across this first year and, thus, might be assumed to have improved their level of lower limb control. By 6 mo, all were sitting alone and some were crawling, and by 12 mo, all were cruising, with some beginning to walk. Furthermore, the quality of their limb postures (less flexion, more extension) and foot placements (less lateral and toe contact at touchdown, more flat-footed stance) suggests better foot control, and perhaps increased muscle strength. An overall improvement in their ability to generate or inhibit activity in their lower limb muscles was observed in everyday life, as they intentionally got into seated postures, moved toward objects of interest, and avoided collisions and change directions. This refinement in their use of muscle force and adapting to novel contexts is apparent here in their reduction in coactivation of agonist and antagonist muscles, across ages, during supported treadmill stepping and was also reported on stepping on a firm surface (Okamoto et al. 2003). Reduction in coactivation occurred most dramatically during swing, from about 40% to an average of, 10%. Although reduced, coactivation during stance remained at ∼30%, even at 12 mo. Although cross talk cannot be ruled out with absolute certainty when working with infants, the low average correlation we obtained for filtered and smoothed data of r2 = 0.13 (SD 0.13) for the LG-TA pairs and r2 = 0.17 (SD 0.13) for the RF-BF pair, suggests that the high level of coactivation we report in this study across the different ages is not significantly affected by cross talk. Moreover, the electrode conducting surface of 5 mm used in this study is typical from what has been reported previously in the literature to properly pick motor activity in infants in the same age range (Hadders-Algra et al. 1992; Lamb and Yang 2000; Okamoto et al. 2003). Coactivation is one way a system can increase stiffness, which has been argued to be a response elicited by humans during gait when they perceive their stability is threatened (Holt et al. 2006; Ulrich et al. 2004). Standing on the moving belt of a treadmill, even with researchers holding their trunks, is likely to feel like a very unstable posture to infants, thus demanding more coactivity than they will ultimately use as skilled walkers overground.
Probability graphs, as well, showed a reduction in mean values with age, especially from 1 mo compared with 6 and 12 mo. This is particularly easily seen in the RF muscle in stance, which also showed a significant reduction in burst duration with age. This reduced activation probability likely also reflects the reduction in coactivation and the capacity to activate muscle groups in isolation and more “at will.” Our muscle state data (Fig. 1) indicate that infants increased, generally, the frequency with which single muscles were active, or active in pairs, rather than activating all muscles concurrently. Studies on spontaneous kicking also show that infants refine their intralimb coordination with age (Jeng et al. 2002; Thelen et al. 1981) by decoupling the hip, knee, and ankle joints. This decoupling can also be seen in our data (reduced burst duration and coactivation, increased frequency) and shows that the development of stepping is parallel to the development of basic leg control.
Similarly, researchers working with other species have shown that spinal and supraspinal reorganization occurring during the neonatal period induce a reduction of sensitivity of the motor units in other species with age (Bradley and Smith 1988; Cazalets et al. 1990). Hadders-Algra et al. (1992) hypothesized that the same process engendered a reduction in duration of muscle activation in infants' spontaneous kicking.
Although the level of EMG activity decreased with age (burst duration, coactivation, probabilities across the stride cycle), infants nevertheless showed a scale up in activity at an appropriate time in the stride cycle. For most muscles, the peak of activity/probability of being active occurred well into stance. This suggests that their behavior reflects weight acceptance and efforts to support their bodies in upright position. Significant increases in muscle activation at touchdown have been reported many times for infant stepping (Forssberg 1985; Ivanenko et al. 2005; Okamoto et al. 2003; Yang et al. 2005) and were primarily associated with hypersensitivity of the segmental reflex (Forssberg 1985; Okamoto et al. 2003). Our data, in line with the findings of Ivanenko et al. (2005), suggest several arguments against reflexive muscle activity, certainly at the initiation of stance. First, the inconsistency of the activity at touchdown suggests that if contact transmits input to reflex receptors, they are not consistently activated. Second, the muscle burst activity we observed seems to persist longer than a reflex response would be expected to do so. For example, our results show that the range of mean burst duration varied from between 0.29 and 0.60 s (95% confidence interval) for the BF to 0.60 to 0.92 s for the RF at 1 mo of age. These durations are clearly greater than the 10- to 15-ms reflex response that has been recorded in adults (Cheng et al. 1995; Gottlieb et al. 1979) or infants (Myklebust 1990; Myklebust and Gottlieb 1993; Myklebust et al. 1986; O'Sullivan et al. 1991; Teulier et al. 2011). Third, the responses at initial stance, as shown in Fig. 3, tended to be lower in probability than ones later in stance.
Also, of all four gait muscles, one seemed to reflect a better control, with higher level of probability and a pattern quite similar in overall pattern to that of skilled adult stepper: the LG. We hypothesize that the capacity to increase activation of this muscle in stance as early as 1 mo may be because infants discover and exploit plantarflexion (pushing) against the uterine wall to aid in repositioning prebirth. This hypothesis is supported by two recent findings. The first showed that in rats, experience in utero contributes to the development of coordinated motor behavior before birth that is maintained after birth (Robinson 2005). Second, Musselman and Yang (2008) found that postbirth, in humans, rhythmical and coordinated interlimb movements were transferable to different leg movements. In fact, they showed that infants who were able to practice alternating or synchronous weight-bearing movements were able to transfer those interlimb coordinations into spontaneous kicking, leading these researchers to hypothesize that interlimb coordinations might share similar neural substrates. In utero, when babies push against the uterine wall and effect valued outcomes (e.g., repositioning), synaptic linkages among relevant neurons and motor units contributing to the kick/leg extension and the sensory receptors activated upon contact with the wall as movement occurs are strengthened. These same neural substrates may be utilized when the baby's foot strikes the surface at touchdown, eliciting the biarticular capacity of the LG o both stabilize the knee and produce antigravity forces at the ankle. Later, creeping and crawling for locomotion and exploration may enhance not only strength but also adaptability to varied contexts. This may account for the fact that peak activation of the LG shifts toward that of skilled walkers, that is, later in stance, with age (see Fig. 3). That this is the most stable occurrence among the four gait muscles does not mean it is stable across infants and consecutive strides. As the profiles of two of our infants show, many cycles still fail to show LG bursts in stance, but the likelihood seems to increases by 12 mo.
Development of Flexor-Extensor Synergies
When considering the overall profile of EMG activity, our results also show a lack of clear alteration between flexor and extensor muscle activity that others have reported (Berger et al. 1984; Leonard et al. 1991). In fact, at 1 mo, flexors and extensors spent 50% of the stance phase being coactivated and 20% not being activated at all. More interestingly, our longitudinal data seem to show that even for infants who master the alternation pattern at an interlimb coordination level (95% of alternating steps), underlying alternation between flexor and extensors at the muscular level is still questionable. In fact, even though we observed a decrease in co-contraction, the TA and LG are still coactivating 30% of the time at 12 mo of age, and the very weak probability of muscle activation for the TA across points in the cycle reinforces the plasticity of the neuromuscular system in infants, allowing them to find an enormous number of solutions to create their kinematic movements, but the lack of experience in this specific context does not allow them to stabilize to any one solution.
Over the first year of life it seems that lower limb control emerges from the repeated perception and action cycles (spontaneous kicking/crawling in various environments) based on the availability of a complex neural network undergoing dynamic structural reorganizations, sensitive to practice and experience. Because infants in this study did not practice stepping on a treadmill between visits to the laboratory, we argue that our results reflect an improvement in adapting to an unusual environment threatening the infant's stability by better controlling lower limb motion, rather than a clear development of a goal-orientated stepping pattern. Nevertheless, we believe that with practice, the development of a more rhythmical neuromuscular pattern on the treadmill will emerge, unfolding in a quite similar manner to those seen in toddlers learning to walk (Chang et al. 2006, 2009; Ivanenko et al. 2005). Markham and Greenough (2004) stated that “morphological plasticity in the brain occurring in response to an increase in the complexity of the environment appears to reflect brain substrates of adaptation to the demands and opportunities provided by experience, including both relatively typical forms of learning and memory and adjustments associated with fundamental processes such as sensory, motor and cognitive processing.” We hypothesize that the same ubiquitous role of experience should not be seen differently when looking at the acquisition of a functional motor skill such as walking.
Conclusion
Our goal in this work was to examine, longitudinally, the patterns of muscle activation produced by healthy infants, over the first year of life, when stepping while supported upright on a treadmill. Furthermore, we intentionally included infants regardless of whether they responded with many steps or few, because this range is typical in healthy infants. To compare the neuromuscular development of atypically developing infants with that of healthy ones, this diversity in sample is critical. Interestingly, this continuum of ability in our sample did not affect the underlying and main conclusion: across these ages, step cycles were created by infants by a wide variety of muscle combinations and timing of their activations. Nevertheless, some levels of muscle control were evident, such as stable probability of gastrocnemius activity and reduction in coactivation with age. However, kinematic stability improved much more clearly over the first year than did the kinetic strategies that underlie this overtly patterned and stable (by 12 mo) behavior. Thus we conclude that although innate control of their limb movements improves as infants grow, explore, and acquire functional movements, the treadmill context is a novel and unpracticed one. As observed for other functional skills of infancy, underlying neural activation of muscles used to walk (on or off the treadmill) will resolve into more stable patterns as functional practice ensues.
GRANTS
Funding for this study was provided by National Institute of Child Health and Human Development Grant R01 HD047567 (to B. D. Ulrich).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.T. and B.D.U. performed experiments; C.T. and J.K.S. analyzed data; C.T., J.K.S., and B.D.U. interpreted results of experiments; C.T. and J.K.S. prepared figures; C.T., J.K.S., and B.D.U. drafted manuscript; C.T., J.K.S., K.M., and B.D.U. edited and revised manuscript; C.T., J.K.S., K.M., and B.D.U. approved final version of manuscript; B.D.U. conception and design of research.
ACKNOWLEDGMENTS
We thank the participants and their families for taking part in this research study. In addition, we thank the physicians and staff, especially those of the University of Michigan Hospital and the Children's Hospital of Michigan.
REFERENCES
- Adolph KE, Vereijken B, Denny M. Learning to crawl. Child Dev 69: 1299–1312, 1998 [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development II (2nd ed.). San Antonio, TX: Pearson, 1993 [Google Scholar]
- Berger W, Altenmueller E, Dietz V. Normal and impaired development of children's gait. Hum Neurobiol 3: 163–170, 1984 [PubMed] [Google Scholar]
- Bradley NS, Smith JL. Neuromuscular patterns of stereotypic hindlimb behaviors in the first two postnatal months. III. Scratching and the paw-shake response in kittens. Brain Res 466: 69–82, 1988 [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Grillner P, Menard I, Cremieux J, Clarac F. Two types of motor rhythm induced by NMDA and amines in an in vitro spinal cord preparation of neonatal rat. Neurosci Lett 111: 116–121, 1990 [DOI] [PubMed] [Google Scholar]
- Chang CL, Kubo M, Buzzi U, Ulrich B. Early changes in muscle activation patterns of toddlers during walking. Infant Behav Dev 29: 175–188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Kubo M, Ulrich BD. Emergence of neuromuscular patterns during walking in toddlers with typical development and with Down syndrome. Hum Mov Sci 28: 283–296, 2009 [DOI] [PubMed] [Google Scholar]
- Cheng J, Brooke JD, Staines WR, Misiaszek JE, Hoare J. Long-lasting conditioning of the human soleus H reflex following quadriceps tendon tap. Brain Res 681: 197–200, 1995 [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Bakker H, van Eykern LA, Otten B, Hadders-Algra M. Postural adjustments and reaching in 4- and 6-month-old infants: an EMG and kinematical study. Exp Brain Res 181: 647–656, 2007 [DOI] [PubMed] [Google Scholar]
- Deffeyes JE, Harbourne RT, Kyvelidou A, Stuberg WA, Stergiou N. Nonlinear analysis of sitting postural sway indicate developmental delay in infants. Clin Biomech (Bristol, Avon) 24: 564–570, 2009 [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondì V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 334: 997–999, 2011 [DOI] [PubMed] [Google Scholar]
- Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res 57: 480–493, 1985 [DOI] [PubMed] [Google Scholar]
- Freedland RL, Bertenthal BI. Developmental changes in interlimb coordination: transition to hands-and-knees crawling. Psychol Sci 5: 26–32, 1994 [Google Scholar]
- Gottlieb GL, Agarwal GC. Response to sudden torques about ankle in man: myotatic reflex. J Neurophysiol 42: 91–106, 1979 [DOI] [PubMed] [Google Scholar]
- Grillner S, McClellan A, Sigvardt K, Wallen P, Wilen M. Activation of NMDA-receptors elicits “fictive locomotion” in lamprey spinal cord in vitro. Acta Physiol Scand 113: 549–551, 1981 [DOI] [PubMed] [Google Scholar]
- Groenen AATK, Kruijsen AJA, Mulvey GM, Ulrich BD. Constraints on early movement: tykes, togs, and technology. Infant Behav Dev 33: 16–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M, Van Eykern LA, Klip-Van den Nieuwendijk AW, Prechtl HF. Developmental course of general movements in early infancy. II. EMG correlates. Early Hum Dev 28: 231–251, 1992 [DOI] [PubMed] [Google Scholar]
- Haehl V, Vardaxis V, Ulrich BD. Learning to cruise: Bernstein's theory applied to skill acquisition during infancy. Hum Mov Sci 19: 685–715, 2000 [Google Scholar]
- Holt KG, Saltzman E, Ho CL, Kubo M, Ulrich BD. Discovery of the pendulum and spring dynamics in the early stages of walking. J Mot Behav 38: 206–218, 2006 [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Lacquaniti F. Kinematics in newly walking toddlers does not depend upon postural stability. J Neurophysiol 94: 754–763, 2005 [DOI] [PubMed] [Google Scholar]
- Jeng SF, Chen LC, Tsou KI. Kinematic analysis of kicking movements in preterm infants with very low birth weight and full-term infants. Phys Ther 82: 148–559, 2002 [PubMed] [Google Scholar]
- Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Exp Brain Res 117: 346–354, 1997 [DOI] [PubMed] [Google Scholar]
- Lamb T, Yang JF. Could different directions of infant stepping be controlled by the same locomotor central pattern generator? J Neurophysiol 83: 2814–2824, 2000 [DOI] [PubMed] [Google Scholar]
- Lande B, Andersen LF, Henriksen T, Baerug A, Johannson L, Trygg KU, Bjørneboe GE, Veierød MB. Relations between high ponderal index at birth, feeding practices and body mass index in infancy. Eur J Clin Nutr 59: 1241–1249, 2005 [DOI] [PubMed] [Google Scholar]
- Leonard CT, Hirschfeld H, Moritani T, Forssberg H. Myotatic reflex development in normal children and children with cerebral palsy. Exp Neurol 111: 379–382, 1991 [DOI] [PubMed] [Google Scholar]
- Lynch A, Lee HM, Bhat A, Galloway JC. No stable arm preference during the pre-reaching period: a comparison of right and left hand kinematics with and without a toy present. Dev Psychobiol 50: 390–398, 2008 [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 1: 351–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman KE, Yang JF. Interlimb coordination in rhythmic leg movements: spontaneous and training-induced manifestations in human infants. J Neurophysiol 100: 2225–2234, 2008 [DOI] [PubMed] [Google Scholar]
- Myklebust BM. A review of myotatic reflexes and the development of motor control and gait in infants and children: a special communication. Phys Ther 70: 188–203, 1990 [DOI] [PubMed] [Google Scholar]
- Myklebust BM, Gottlieb GL. Development of the stretch reflex in the newborn: reciprocal excitation and reflex irradiation. Child Dev 64: 1036–1045, 1993 [PubMed] [Google Scholar]
- Mykelbust BM, Gottlieb GL, Agarwal GC. Stretch reflexes of the normal infant. Dev Med Child Neurol 28: 440–449, 1986 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Okamoto K, Andrew PD. Electromyographic developmental changes in one individual from newborn stepping to mature walking. Gait Posture 17: 18–27, 2003 [DOI] [PubMed] [Google Scholar]
- O'Sullivan MC, Eyre JA, Miller S. Radiation of phasic stretch reflex in biceps brachii to muscles of the arm in man and its restriction during development. J Physiol 439: 529–543, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR. Conjugate limb coordination after experience with an interlimb yoke: evidence for motor learning in the rat fetus. Dev Psychobiol 47: 328–344, 2005 [DOI] [PubMed] [Google Scholar]
- Spencer JP, Thelen E. Spatially specific changes in infants' muscle coactivity as they learn to reach. Infancy 1: 275–302, 2000 [DOI] [PubMed] [Google Scholar]
- Spencer J, Vereijken B, Diedrich FJ, Thelen E. Posture and emergence of manual skills. Dev Sci 3: 216–233, 2000 [Google Scholar]
- Teulier C, Smith BA, Kubo M, Chang CL, Moerchen V, Murazko K, Ulrich BD. Stepping responses of infants with myelomeningocele when supported on a motorized treadmill. Phys Ther 89: 60–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulier C, Ulrich BD, Martin B. Functioning of peripheral Ia pathways in infants with typical development: responses in antagonist muscle pairs. Exp Brain Res 208: 581–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Developmental origins of motor coordination: leg movements in human infants. Dev Psychobiol 18: 1–22, 1985 [DOI] [PubMed] [Google Scholar]
- Thelen E, Bradshaw G, Ward JA. Spontaneous kicking in month-old infants: manifestation of a human central locomotor program. Behav Neural Biol 32: 45–53, 1981 [DOI] [PubMed] [Google Scholar]
- Thelen E, Cooke DW. Relationship between newborn stepping and later walking: a new interpretation. Dev Med Child Neurol 29: 380–393, 1987 [DOI] [PubMed] [Google Scholar]
- Thelen E, Ulrich BD. Hidden skills: a dynamic systems analysis of treadmill stepping during the first year. Monogr Soc Res Child Dev 56: 104, 1991 [PubMed] [Google Scholar]
- Ulrich BD, Haehl V, Buzzi UH, Kubo M, Holt KG. Modeling dynamic resource utilization in populations with unique constraints: preadolescents with and without Down syndrome. Hum Mov Sci 23: 133–156, 2004 [DOI] [PubMed] [Google Scholar]
- Ulrich BD, Schneider K, Jensen JL, Zernicke RF, Thelen E. Adaptive dynamics of the leg movement patterns of human infants. 2. Treadmill stepping in infants and adults. J Mot Behav 26: 313–324, 1994 [DOI] [PubMed] [Google Scholar]
- Ulrich BD, Ulrich DA. Spontaneous leg movements of infants with Down syndrome and nondisabled infants. Child Dev 66: 1844–1855, 1995 [PubMed] [Google Scholar]
- Van Haastert IC, de Vries LS, Helders PJM, Jongmans MJ. Early gross motor development of preterm infants according to the Alberta Infant Motor Scale. J Pediatr 149: 617–622, 2006 [DOI] [PubMed] [Google Scholar]
- Vereijken B, Thelen E. Training infant treadmill stepping: the role of individual pattern stability. Dev Psychobiol 30: 89–102, 1997 [DOI] [PubMed] [Google Scholar]
- Yang JF, Gorassini M. Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist 12: 379–390, 2006 [DOI] [PubMed] [Google Scholar]
- Yang JF, Lamont EV, Pang MYC. Split-belt treadmill stepping in infants suggests autonomous pattern generators for the left and right leg in humans. J Neurosci 25: 6869–6876, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. J Physiol 507: 927–937, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]