Abstract
GABA depolarizes and excites central neurons during early development, becoming inhibitory and hyperpolarizing with maturation. This “developmental shift” occurs abruptly, reflecting a decrease in intracellular Cl− concentration ([Cl−]i) and a hyperpolarizing shift in Cl− equilibrium potential due to upregulation of the K+-Cl− cotransporter KCC2b, a neuron-specific Cl− extruder. In contrast, primary afferent neurons (PANs) are depolarized by GABA throughout adulthood because of expression of NKCC1, a Na+-K+-2Cl− cotransporter that accumulates Cl− above equilibrium. The GABAA-mediated depolarization of PANs determines presynaptic inhibition in the spinal cord, a key mechanism gating somatosensory information. Little is known about developmental changes in Cl− transporter expression and Cl− homeostasis in PANs. Whether NKCC1 is expressed in PANs of all phenotypes or is restricted to subpopulations (e.g., nociceptors) is debatable. Likewise, whether PANs express KCC2s is controversial. We investigated NKCC1 and K+-Cl− cotransporter expression in rat and mouse dorsal root ganglion (DRG) neurons with molecular methods. Using fluorescence imaging microscopy, we measured [Cl−]i in acutely dissociated rat DRG neurons (P0–P21) loaded with N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide and classified with phenotypic markers. DRG neurons of all sizes express two NKCC1 mRNAs, one full-length and a shorter splice variant lacking exon 21. Immunolabeling with validated antibodies revealed ubiquitous expression of NKCC1 in DRG neurons irrespective of postnatal age and phenotype. As maturation progresses [Cl−]i decreases gradually, persisting above equilibrium in >95% mature neurons. DRG neurons express mRNAs for KCC1, KCC3s, and KCC4, but not for KCC2s. Mechanisms underlying PANs' developmental changes in Cl− homeostasis are discussed and compared with those of central neurons.
Keywords: intracellular chloride regulation, primary sensory neurons, NKCC1, KCC1, KCC2, KCC3, KCC4
gaba depolarizes and excites central nervous system (CNS) neurons during early development and becomes hyperpolarizing and inhibitory with maturation. This transition from depolarizing to hyperpolarizing occurs relatively abruptly during development, and it is referred to as a “developmental switch” (Ben-Ari 2002) or, more appropriately, as a “developmental shift” (Blaesse et al. 2009). The Cl− gradient providing the driving force for the depolarizing Cl− efflux in embryonic and early postnatal CNS neurons is generated by isoform 1 of the Na+-K+-2Cl− cotransporter (NKCC1), a transport protein that mediates active Cl− uptake across the plasma membrane, maintaining the intracellular Cl− concentration ([Cl−]i) above electrochemical equilibrium (Achilles et al. 2007; Clayton et al. 1998; Plotkin et al. 1997b; Sun and Murali 1999). The hyperpolarizing shift in EGABA, the reversal potential of the anion current through GABAA channels (Owens et al. 1996; Yamada et al. 2004), is caused by a concomitant decrease in [Cl−]i due to increased functional expression of isoform 2 of the K+-Cl− cotransporter (KCC2), which actively extrudes Cl− in neurons, outweighing NKCC1 activity (Payne et al. 1996; Rivera et al. 1999; Stein et al. 2004; Stil et al. 2009). The hypothesis that the hyperpolarizing shift in EGABA reflects a decrease in [Cl−]i during development is supported by direct noninvasive measurements of [Cl−]i at various postnatal ages in CNS cortical neurons (Berglund et al. 2006; Marandi et al. 2002) and retinal neurons (Zhang et al. 2006).
Unlike CNS neurons, most primary afferent neurons (PANs) are depolarized by GABA throughout adulthood (Desarmenien et al. 1984; Labrakakis et al. 2003). GABA depolarizations in PANs are also mediated by Cl− efflux via GABAA receptor channels (Gallagher et al. 1978; Hattori et al. 1984; Valeyev et al. 1999). The depolarization is possible because, unlike adult CNS neurons, adult PANs maintain their [Cl−]i above electrochemical equilibrium, generating an outward Cl− electrical gradient across their plasma membrane. This Cl− gradient is maintained throughout the entire cell surface, including the soma and the central and peripheral processes, as shown by direct [Cl−]i measurements in their cell bodies in dorsal root ganglia (DRGs) (Alvarez-Leefmans et al. 1988; Rocha-Gonzalez et al. 2008) and indirectly by testing the depolarizing action of GABA in their central and peripheral processes (Ault and Hildebrand 1994; Bhisitkul et al. 1987; Carlton et al. 1999; Curtis and Lodge 1982; reviewed in Alvarez-Leefmans 2009; Rudomin and Schmidt 1999; Willis 2006). The Cl− gradient in PANs is of high functional significance; for instance, at the peripheral endings it may contribute to excitation of nociceptors by chemical mediators of pain, released upon tissue damage (Alvarez-Leefmans 2009; Frings 2009; Granados-Soto et al. 2005), whereas at the central terminals it makes possible the primary afferent depolarization (PAD) produced by GABA released from spinal interneurons. PAD underlies presynaptic inhibition, a ubiquitous phenomenon in the spinal cord and a pivotal mechanism in the gating of somatosensory information (Rudomin and Schmidt 1999; Willis 2006).
Functional studies established that the outward Cl− gradient in PANs is generated and maintained predominantly by a Na+-K+-2Cl− cotransporter (Alvarez-Leefmans et al. 1988; Rocha-Gonzalez et al. 2008), identified as NKCC1 by molecular methods (Kanaka et al. 2001; Plotkin et al. 1997a; Sung et al. 2000). However, PANs exhibit a high degree of phenotypic heterogeneity reflecting their functional specialization for various sensory modalities (Lawson 2005). The question arises as to whether expression of NKCC1 and [Cl−]i depend on DRG neuron cell type and/or postnatal development. Recent studies suggested that NKCC1 mRNA is expressed only in small- and medium-diameter neurons, many of which are nociceptors, and that NKCC1 protein does not reside in the cell bodies but only in central and peripheral terminals of PANs (Price et al. 2006, 2009). Moreover, whether KCC2 is expressed in DRG neurons and produces a decline in [Cl−]i with postnatal development is controversial. One report concluded that the mean [Cl−]i in mature DRG neurons is lower than in newborn animals (Gilbert et al. 2007) and attributed the decline to activation of KCC2. This was interpreted as a “developmental transition of chloride homeostasis” during postnatal development of DRG neurons, which parallels the developmental “chloride switch” in the CNS. This agrees with one functional study suggesting expression of a K+-Cl− cotransport mechanism in mouse DRG neurons (Sung et al. 2000) and with two reports showing that DRG neurons express both KCC2 transcript and protein (Gilbert et al. 2007; Lu et al. 1999). In contrast to these observations, earlier studies reported barely detectable levels of KCC2 transcripts in DRG cells (Rivera et al. 1999). Subsequent work confirmed and extended this observation by showing that KCC2 mRNA and protein were undetectable in DRG neurons (Coull et al. 2003; Kanaka et al. 2001; Morales-Aza et al. 2004; Toyoda et al. 2005).
To resolve these controversies, we investigated the expression of NKCC1 in DRG neurons of different phenotypes, using in situ hybridization (ISH) and immunolabeling with antibodies of validated specificity for NKCC1. We also measured [Cl−]i during postnatal development in single DRG neurons classified according to their size and other phenotypic markers. We searched for transcripts for all known KCCs, including both variants of KCC2 (Uvarov et al. 2007), and for KCC2 protein expression in DRG with RT-PCR and immunolabeling. The results show that virtually all DRG neurons, irrespective of their phenotype, express NKCC1 protein and mRNAs for two NKCC1 variants and exhibit higher than passive [Cl−]i. Direct measurements of [Cl−]i in single DRG neurons at various postnatal ages revealed that during postnatal development there is a downward drift in [Cl−]i but not an abrupt transition, i.e., a “chloride shift” like that observed in CNS neurons. The changes observed in Cl− homeostasis during maturation cannot be attributed to KCC2 since both KCC2 transcripts and proteins were absent in DRG neurons. Our findings suggest that NKCC1 maintains higher than equilibrium [Cl−]i in mature primary sensory neurons irrespective of their phenotype or postnatal age, and that Cl− uptake is unopposed by Cl− extrusion mediated by KCC2 as occurs in CNS neurons. The present results are consistent with the widespread GABA-mediated depolarization of primary afferents underlying presynaptic inhibition in the spinal cord.
MATERIALS AND METHODS
The use and handling of all animals (rats and mice) included in this study were approved by the Wright State University Laboratory Animal Care and Use Committee and were in accordance with guidelines provided by the National Institutes of Health.
Immunofluorescence Microscopy
To study the pattern of NKCC1 immunoreactivity in adult and newborn (P1) DRG neurons we used Sprague-Dawley rats. For validation of specificity of the NKCC1 antibodies we compared the pattern of immunofluorescence of tissues from homozygous transgenic mice in which gene Slc12a2 (encoding NKCC1) was disrupted (NKCC1−/−) with homologous tissues obtained from wild-type (WT) mice. We used four NKCC1−/− mice and as controls four WT (C57/Black) mice (NKCC1+/+). Initially we used the strain of NKCC1−/− fully described in previous reports (Delpire et al. 1999; Sung et al. 2000). In later phases of this work we used one NKCC1−/− and one NKCC1+/+ mouse on a 129 Black Swiss mixed background from a colony raised at Wright State University Animal Care Facility that was started with breeding pairs kindly donated by Professor Gary Shull (University of Cincinnati). These transgenic mice as well as the genotyping procedures are described in the original paper from Shull's group (Flagella et al. 1999). NKCC1 immunoreactivity in both WT mouse strains was indistinguishable. In neither type of NKCC1−/− mice was NKCC1 immunoreactivity detected in DRG (see below) or in tissues with high expression of NKCC1 protein (kidney and choroid plexus).
All animals were deeply anesthetized with pentobarbital sodium (50 mg/kg) or Euthasol and perfused transcardially with 200–300 ml of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.3. The spinal cords with attached spinal ganglia and other tissues (kidney and choroidal epithelium) were extracted and subjected to different postfixation protocols; we found that NKCC1 immunoreactivity is sensitive to the level of fixation. Therefore, tissues were either postfixed for 2–4 h in the same fixative solution (high fixation) or not postfixed (low fixation). Whether postfixed or not, tissues were stored at 4°C in 0.1 M PBS with 15% sucrose until used. As positive controls we used rat and mouse choroid plexus and rat kidney outer medulla, two tissues in which expression of NKCC1 is well established (Ginns et al. 1996; Piechotta et al. 2002; Wu et al. 1998).
Cryostat sections (20 μm thick) were obtained from all tissues (spinal cord, choroid plexus, DRG, and kidney) and collected on gelatinized slides. After wash (3 times for 5 min each) in 0.01 M PBS-0.1% Triton X-100 (PBS-T; pH 7.4), they were blocked with 10% normal donkey or horse serum for 0.5–2 h and incubated overnight at 4°C with the corresponding primary antibodies diluted in PBS-T. In some cases, prior to wash in PBS-T the sections were treated for 5 min in 0.01 M PBS-1% SDS; SDS enhances NKCC1 immunoreactivity with some antibodies (reviewed in Alvarez-Leefmans 2009). For detection of NKCC1 we used an affinity-purified polyclonal antibody raised in rabbits against a fusion protein fragment encompassing amino acids 938–1011 of the carboxy terminus (CT) of mouse NKCC1 (Kaplan et al. 1996). We refer to this antibody as Kaplan-CT. It recognizes both NKCC1 variants, short (NKCC1-S) and long (NKCC1-L), when tested with maltose binding fusion proteins with or without exon 21. The Kaplan-CT NKCC1 antibody was used at a dilution of 1:100, 1:250, or 1:500 in PBS-T, followed by donkey anti-rabbit secondary antibodies coupled to cyanine 3 (Cy3) and diluted 1:50 (Jackson ImmunoResearch, West Grove, PA). In double-labeling experiments this NKCC1 primary antibody was combined with mouse anti-NeuN (dilution 1:500; Chemicon, Temecula, CA), an antibody used as a pan-neuronal marker (Mullen et al. 1992). NeuN-immunoreactive sites were revealed with species-specific FITC-conjugated secondary antibodies raised in donkey (Jackson ImmunoResearch) and diluted 1:50 in PBS-T. The sections were washed in PBS, coverslipped in Vectashield (Vector Labs), and analyzed in either a BX60 Olympus epifluorescence microscope or an FX Olympus confocal microscope.
Besides Kaplan-CT, we probed two other anti-NKCC1 antibodies (α-wCT and α-wNT) that were generously provided by Dr. R. James Turner (Molecular Physiology and Therapeutics Branch, National Institute of Dental and Craniofacial Research, Bethesda, MD). The first of these antibodies (α-wCT) was raised in rabbits against a 6xHIS fusion protein encompassing amino acids 750–1203 of the CT of rat NKCC1 (Kurihara et al. 1999). The second antibody (α-wNT) was also raised in rabbits against a 6xHIS fusion protein encompassing amino acids 3–202 of the amino terminus (NT) of rat NKCC1. Both of these antibodies at dilutions of 1:2,000 were validated against NKCC1−/− tissues and gave results that were indistinguishable from those obtained with the Kaplan-CT. A limited screening was also done with commercial anti-NKCC1 antibodies (sc-21547 and sc-21545). The corresponding epitopes of these antibodies were not disclosed by the manufacturer (Santa Cruz Biotechnology). According to the manufacturer's information sc-21547 is “an affinity purified goat polyclonal antibody raised against a peptide mapping within an internal region of NKCC1 of human origin.” This antibody failed to give specific immunoreactivity in mouse DRG, and therefore it was discarded. The second antibody (sc-21545) is, according to the manufacturer, “an affinity purified goat polyclonal antibody raised against a peptide mapping near the N-terminus of NKCC1 of human origin.” This antibody gave a pattern of immunoreactivity similar to that obtained with Kaplan-CT, α-wCT, and α-wNT, but with a dilution of 1:200. Since the epitopes of these commercial antibodies have not been disclosed, the results were not included in this report.
For detection of KCC2 we used an affinity-purified polyclonal antibody (1:500) raised in rabbit against a fusion protein corresponding to residues 932–1043 of rat KCC2 (Upstate, Temecula, CA). The amino acid sequence recognized by this antibody is the same in both KCC2 variants, i.e., KCC2a and KCC2b (Payne 2009). The secondary antibody used to visualize KCC2 was donkey anti-rabbit conjugated to Cy3 (dilution 1:50). The specificity of this antibody has been validated (Stil et al. 2011). The labeling we observed in spinal neurons with this antibody was indistinguishable from that reported in the literature (Stil et al. 2009, 2011).
Identification of Transcripts of Cation-Chloride Cotransporters by RT-PCR
Total RNA extraction and reverse transcription.
Total RNA from newborn or adult rat tissues was obtained by using the RNeasy RNA extraction kit (no. 74104) according to the manufacturer's instructions (Qiagen, Valencia, CA). First-strand cDNA synthesis was initiated with 2–4 μg of DNAse (no. 18047019, Invitrogen, Carlsbad, CA)-treated total RNA and 50 ng of random hexamers (no. 48190011, Invitrogen), 0.5 mM deoxyribonucleotide triphosphates (dNTPs, no. 77100, USB, Cleveland, OH), 20 mM dithiothreitol (DTT, no. 15508013, Invitrogen), 40 U of RNase OUT (no. 10777019, Invitrogen), and 200 U of SuperScript III reverse transcriptase (no. 18080044, Invitrogen). The mixture was incubated at 50°C for 50 min. After reverse transcription, reverse transcriptase was inactivated at 85°C for 15 min.
PCR amplification of rat NKCC1 and KCC transcripts.
NKCC1 and KCC mRNAs were PCR-amplified from 2–4 μl of the cDNA synthesis reaction following an enhanced sensitivity method described elsewhere (Di Fulvio and Gomez-Cambronero 2005). In brief, the PCR reaction was performed with 0.25 U of Pfx polymerase (no. 11708013, Invitrogen), 0.4 mM of dNTP, 2 mM MgSO4, and 50 pmol of gene-specific primers in a final volume of 50 μl. The PCR was carried out in a thermocycler (MyCycler, Bio-Rad) as follows: denaturation at 94°C for 2 min, followed by 40 cycles of 45 s at 94°C, annealing for 45 s at 55–65°C, and extension for 1 min at 68°C, depending on the primers used.
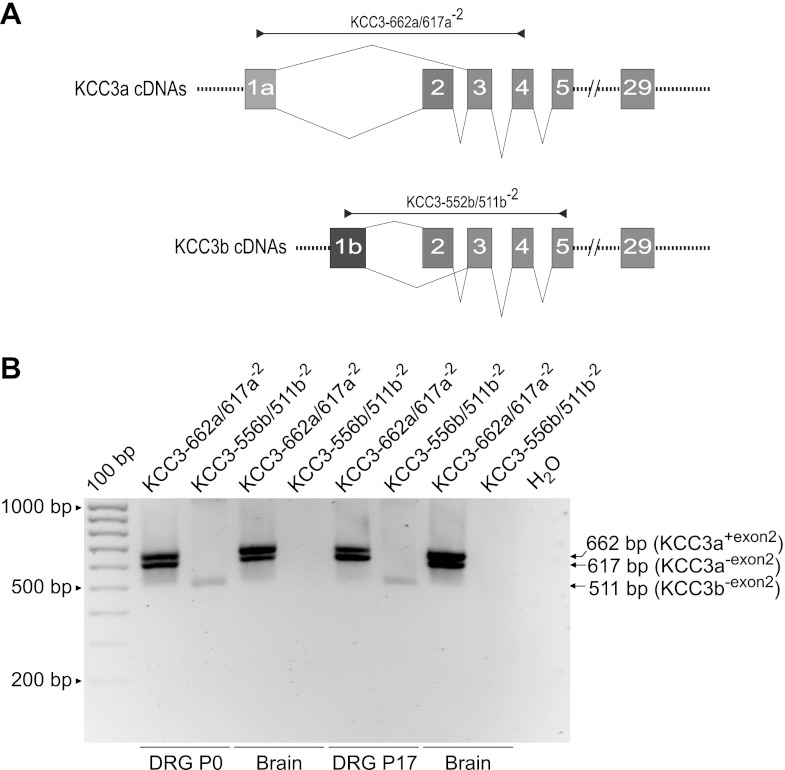
PCR primers designed to amplify specific isoforms and variants of rat NKCC1, KCC1, KCC2, KCC3, and KCC4 transcripts were constructed by using cDNA or RefSeq nucleotide sequences as templates. The sense and antisense sequences of all the primer sets used in this study are referred to by the transcript to be amplified followed by a number that indicates the predicted length in base pairs (bp) of the corresponding RT-PCR products (Table 1). NKCC1 primer sets were designed by using the rat NKCC1-L cDNA sequence as template (GenBank accession no. AF051561; Moore-Hoon and Turner 1998). The specific primer sets were as follows: rNKCC1-511 for detecting both NKCC1 splice variants, i.e., rNKCC1-S and rNKCC1-L (511 bp); rNKCC1-404-L specifically detects rNKCC1-L (404 bp); and rNKCC1-502L/453S, designed for simultaneous detection of rNKCC1-L (502 bp) and rNKCC1-S (453 bp).The primer sets for rat KCC mRNA amplification were as follows: rKCC1-520, constructed with rat KCC1 cDNA sequence (GenBank accession no. U55815; Gillen et al. 1996) and designed to amplify all KCC1 transcripts (520 bp); KCC2a-468, constructed with rKCC2a cDNA (GenBank accession no. EF641113; Uvarov et al. 2007) and designed to specifically amplify the “a” isoform of KCC2 (468 bp); and KCC2b-484, constructed with rKCC2b cDNA (GenBank accession no. U55816; Payne et al. 1996) and designed to specifically amplify the “b” isoform of KCC2 (484 bp). Because full-length rat KCC3 cDNAs are not available in GenBank, the design of primer sets for amplification of rKCC3 transcripts was based on rat predicted sequences (XM_001066756, GeneID: 691209, locus LOC691209) and known mouse/human KCC3 cDNAs encoding for KCC3 splice variants. The KCC3-547 primer set was designed to detect all predicted KCC3 splice variants KCC3a and KCC3b (Mercado et al. 2005). The KCC3-662a/617a−2 primer set specifically detected the “a” variants of KCC3 with or without exon 2 (662 bp or 617 bp, respectively). The KCC3-556b/511b−2 primer set was specific for the “b” variants of KCC3 with or without exon 2 (556 bp or 511 bp, respectively). The design of the KCC4 primer set KCC4-665 was also based on predicted rat KCC4 cDNA sequences (XM_001071999, Gene ID: 308069).
Table 1.
PCR primer sequences and nomenclature
| Primer Set | Sense | Antisense |
|---|---|---|
| rNKCC1-511 | CTGCCGAAAGTAAAGGAGTTGTA | ATGGCCAGAAGAAGAATCACC |
| rNKCC1-404L | ATGAGGAAGAGGATGGCAAGACTTCAACTC | GTATGGCTCAATCATGTCATCAAAAGCGAC |
| rNKCC1-502L/453S | TGTGGTCATTCGCCTAAAGGAAGGACTGGATATATC | GGAGAAGTCTATTCGGAATTTACTGAGTAA |
| rKCC1-520 | GACTATGACAACCTCGAGGGGCTCAGTTGGGTGGAC | TCATGAAGTAAGAGCCACCAGCTGGAACCACACCAT |
| KCC2a-468 | AGGTTCACGGTCACCTCGCT | GACCATGCAGAAGGACTCCA |
| KCC2b-484 | ATGCTCAACAACCTGACGGACTGCGAGGACGGCGAT | CAGCAGGCACAACACCATTGGTTGCAATTGCGCTCA |
| KCC3-547 | CCTGGGTAGTGGGAACAGCTGGAATCCTTCAGGCCTTT | GAGCAAATGTCAAGGTGTCTTGATGACAGG |
| KCC3-662a/617a−2 | ATGCATCCACCAGAAGCCACCACCAAGATGTCCTCA | AAGGCCTGAAGGATTCCAGCTGTTCCCACTACCCAGG |
| KCC3-556b/511b−2 | ATGCCACATTTTACTGTGACCAAGGTAGAA | AGATGGCAGTTAACATTGTACAGCAGCAGC |
| KCC4-665 | ATGCCCACGAACTTTACGGTGGTGCCGGTG | TCGATGGTTCCCAGGATGTACATGGCGCCT |
All RT-PCR primers were designed to anneal in exons or exon/exon boundaries of rat NKCC/KCC genes with the Vector NTI Suite programs (Invitrogen) and were synthesized from Integrated DNA Technologies (Coralville, IA). All RT-PCR products of predicted sizes were purified from dNTPs/single-stranded DNA with ExoSAPIt (no. 78250, USB) and identified by direct sequencing in both directions (Agencourt Bioscience, Beverly, MA).
In Situ Hybridization of Mouse NKCC1 in DRG Neurons
Animals and tissue preparation.
Three male C57BL mice were used for ISH experiments. The mice were deeply anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and perfused transcardially with 50 ml of vascular rinse solution (0.01 M PBS, pH 7.4), followed by 150 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The spinal cord and DRG were dissected out, postfixed for 1–2 h, and cryoprotected at 4°C with 30% sucrose in 0.01 M PBS for a minimum of 48 h. Tissues were stored at −80°C until further use.
Design and preparation of oligodeoxynucleotide probes.
Oligonucleotide antisense probes and their corresponding sense probes were designed based on mouse NKCC1 gene (Slc12a2, GenBank accession no. NM_009194). Probe design and nomenclature are illustrated at the bottom of Fig. 1. The probe mNKCC1-S was designed to specifically detect the short mNKCC1 spliced variant lacking exon 21. Specifically, this antisense probe (32 bp) was designed to target mRNA transcribed by exon 20 (13 bp, 3021–3033) and exon 22 (19 bp, 3082–3100), without cross-reaction with exon 21 mRNA. The antisense sequence was 5′-TAG GGC CTT TGG ATT CTT TCT GTG TAA TGG AT-3′. The probe mNKCC1-L was designed to specifically detect exon 21. Thus this probe detected only the long (L) NKCC1 variant i.e., the full-length mNKCC1. This antisense probe (33 bp) was designed to react with mRNA transcribed by exon 21 (3038–3070). The antisense sequence was 5′-GCT GAG TTG GAG TCT TGC CAT CCT CTT CCT CAT-3′. The probe mNKCC1-L+S was designed to detect both long (L) and (S) variants. This antisense probe (38 bp) was designed to target mRNA transcribed by exon 2 (943–980). The antisense sequence was 5′-ACA CCC TTG ATC CAG CCA AAC TTT ACA ACT CCT TTA CT-3′. The corresponding sense (control) probes for each of the above antisense probes were as follows: sense mNKCC1-S (32 bp): 5′-ATC CAT TAC ACA GAA AGA ATC CAA AGG CCC TA-3′; sense mNKCC1-L (33 bp): 5′-ATG AGG AAG AGG ATG GCA AGA CTC CAA CTC AGC-3′; NKCC1-L/S (38 bp): 5′-AGT AAA GGA GTT GTA AAG TTT GGC TGG ATC AAG GGT GT-3′. Sense probes were used as negative controls. The probes were 3′ end-labeled with DNA terminal deoxynucleotidyl transferase (Roche Diagnostics, Mannheim, Germany) with [a-35S]dATP (1,000–1,500 Ci/mmol). The labeled probes were purified by chromatographic separation using Mini Quick DNA columns (Boehringer Mannheim).
Fig. 1.
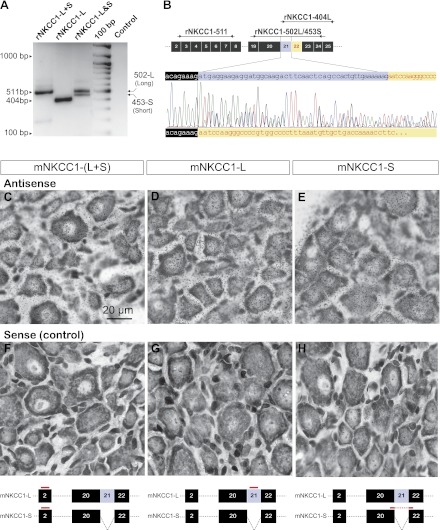
Expression of NKCC1 transcripts in rat and mouse dorsal root ganglion (DRG) by RT-PCR and in situ hybridization (ISH). A: detection of NKCC1 mRNAs by RT-PCR of total RNA from rat DRG (P17) using the primers designed as shown in B. Rat DRG cells express both full (L)- and short (S)-length NKCC1 variants, denoted rNKCC1-L and rNKCC1-S, respectively. The band rNKCC1-L+S (511 bp) was detected with rNKCC1-511, a nonvariant specific primer that amplified both L and S NKCC1 variants. The band rNKCC1-L (404 bp) was detected with primer rNKCC1-404L that specifically amplified rNKCC1-L. The 2 bands labeled rNKCC1-L&S correspond to rNKCC1-L (502 bp) and rNKCC1-S (453 bp) and were amplified with primer rNKCC1-502L/453S designed for simultaneous detection of both variants. Control lane was water instead of RNA. B, top: diagram of PCR-amplified regions of rNKCC1 mRNA encompassing exons 2–8 and 19–25 (boxed numbers). Exon 21 is spliced out in rNKCC1-S variant. Arrowheads indicate sense and antisense primers. The primers and the regions amplified by each of them are rNKCC1-511, rNKCC1-404L, and rNKCC1-502L/453S. All RT-PCR products were sequenced. Bottom: cDNA sequence analysis chromatogram of the RT-PCR product rNKCC1-502-L/453-S. Both bands (502-L and 453-S in A) corresponding to the rNKCC1-L&S RT-PCR fragments were sequenced simultaneously in a single reaction pass. The sense coding strand sequence of rNKCC1-502L in the region of interest (exons 20–22) is indicated at top of the chromatogram, and the sequence of rNKCC1-453S is shown at bottom of the chromatogram. Color codes of chromatogram: adenine (a, green), cytosine (c, blue), guanine (g, black), thymine (t, red). C–H: ISH of adult mouse (P60) DRG showing that L and S NKCC1 variants (mNKCC1-L and mNKCC1-S) are ubiquitously expressed in the cell bodies of DRG neurons of all sizes. Diagram at bottom illustrates the regions of mNKCC1 mRNA identified by each hybridization probe. The antisense probes (C–E) were designed to detect both L and S variants (mNKCC1-L+S), the full-length variant mNKCC1-L, and the short spliced variant lacking exon 21 (mNKCC1-S). The corresponding sense probes were the negative controls (F–H). Scale bar in C (20 μm) applies to all micrographs.
In situ hybridization.
Serial sections (10 μm thick) were cut on a cryostat and thaw-mounted onto Gold Seal Micro Slides. The slide-mounted sections were rinsed in 0.01 M PBS buffer three times (5 min each) and then washed for 30 min in 2× SSC (1× SSC contains 0.15 M sodium chloride and 0.015 M sodium citrate). Hybridization was performed by incubating the sections for 18 h at 40°C with hybridization buffer (50% deionized formamide, 4× SSC, 10% dextran sulfate, 1× Denhardt solution, 5 mM dithiothreitol, 250 μg/ml sperm DNA, 250 μg/ml yeast tRNA) containing [35S]dATP-labeled probes (0.3–0.5 × 106 cpm/100 μl). After hybridization, the sections were washed with hybridization buffer in 2× SSC for 10 min, three times (10 min each) with 2× SSC-50% formamide at 40°C, and three times (20 min each) with 1× SSC at 50°C. The sections were dried and placed in a film cassette with a 14C Standard slide for 3 days at room temperature.
Autoradiography.
The sections were exposed to a Fuji film phosphor-imaging system (Fuji FLA-5100), and positive slides were coated with Kodak NBT-2 emulsion (Kodak) diluted 1:1 with water and placed at 4°C in a tightly sealed dark box for 3 wk. The sections were developed in D-19 developer (Kodak) for 3 min, fixed with photographic fixer (F-24) for 7 min, washed with tap water for 15 min, counterstained with cresyl violet, dehydrated with ethanol (70–100%) and xylene, and coverslipped with DPX. The sections were analyzed by brightfield illumination on a BX60 Olympus microscope coupled to a SPOT II digital color camera.
Isolation, Preparation, and IB4 Labeling of Dorsal Root Ganglion Neurons for Measurement of Intracellular Cl− Concentration
DRG neurons were isolated from Sprague-Dawley rats (P0–P1 to P21) following the methods described in detail in a previous paper (Rocha-Gonzalez et al. 2008). In brief, after decapitation the spinal ganglia were dissected in Dulbecco's PBS without Ca2+ and Mg2+ (GIBCO-Invitrogen). The ganglia were collected at room temperature in a 15-ml sterile conical tube containing 10 ml of Hanks' balanced salt solution (HBSS) without Ca2+ and Mg2+ (GIBCO-Invitrogen) and centrifuged at 500 rpm (∼22 g) during 5 min. The superatant was aspirated, and the ganglia were resuspended in 3 ml of DRG growth medium (see below). The latter was aspirated and replaced by 3 ml of the collagenase-containing solution described previously. The ganglia were incubated for 30 min at 37°C.
The composition of the DRG growth medium was the same as described previously (Rocha-Gonzalez et al. 2008) except that in the present study we added 100 μl of 5-fluoro-2′-deoxyuridine (50 μM) and 100 μl of uridine (50 μM), both from Sigma-Aldrich (Burkey et al. 2004). At the conclusion of collagenase incubation the ganglia were centrifuged (∼22 g, 5 min) and the supernatant aspirated. The pellet was resuspended in 3 ml of DRG growth medium. Cells were dissociated by mechanical trituration through a 20-gauge 1.5 needle (∼3 gentle passes through the needle) and plated by adding ∼0.3 ml of the suspension on 25-mm sterile coverslips as described previously (Rocha-Gonzalez et al. 2008). The cell suspension was left undiluted for 20 min to allow initial cell attachment to coverslips. Then, 1.7 ml of fresh DRG growth medium was added to each dish to a final volume of 2 ml. Cells were incubated at 37°C in a 5% CO2-95% air atmosphere and used for experiments from 12 to a maximum of 24 h after plating.
Each coverslip with attached cells was mounted in an imaging chamber (RC-21BRW, Warner Instruments, Hamden CT) and placed on the stage of an epifluorescence inverted microscope (Olympus IX-81, Olympus America, Center Valley, PA) equipped with a fluor oil-immersion lens (Olympus, ×40, NA 1.35) and differential interference contrast (DIC) optics. The fluorescent dye N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) was used to measure [Cl−]i in DRG neurons (see below). Prior to MQAE loading, neurons were visualized with DIC optics and labeled with FITC-conjugated isolectin B4 (IB4) from the plant Griffonia simplicifolia (Vector Laboratories, no. FL-1201). The final concentration of IB4-FITC in the imaging chamber was 20 μg/ml. Labeling was done for 10–15 min at room temperature. DRG neurons were image-captured with DIC and then with the appropriate set of filters, to visualize IB4-FITC with epifluorescence (excitation 487 ± 5 nm, emission 535 ± 13 nm). Images were digitally stored for subsequent analysis. DIC images were used for off-line measurement of the cross-sectional areas (CSAs) of the cell bodies, whereas FITC images were used to determine which cells were labeled with IB4. IB4 labeling did not alter MQAE fluorescence or Cl− fluxes in DRG neurons. It is also known that electrophysiological properties of these neurons are not affected by IB4 labeling (Liu et al. 2004; Stucky and Lewin 1999).
Saline Solutions
The control isosmotic (ISO) solution contained (in mM) 123 NaCl, 3.5 KCl, 1.0 CaCl2, 1.25 MgCl2, 5 HEPES, and 10 glucose. The pH was adjusted to 7.3 with NaOH. Hence, the total [Cl−] of this solution was 131 mM and the total [Na+] was 125.5 mM. The osmolality was adjusted with sucrose to 290 ± 2 mosmol/kgH2O. The HEPES concentration was 5 mM in all solutions, including those used for calibration (see below), because this acid quenches MQAE fluorescence (Kaneko et al. 2002). This concentration of HEPES had sufficient buffering capacity with minimal dye quenching.
The Cl−-free ISO solution (0 Cl−) was made by replacing Cl− with gluconate in the ISO control solution on a mole-for-mole basis. The Na+-free ISO solution (0 Na+) was prepared by mole-for-mole replacement of Na+ with N-methyl-d- glucamine and the pH adjusted with HCl. The other components of the solutions were exactly the same as those in the control ISO solution. The final pH of all the solutions was adjusted to 7.3. The osmolality of all these solutions was 290 ± 2 mosmol/kgH2O, the same as that of the ISO solution.
In one series of experiments [Cl−]i was measured in cells bathed in solutions buffered with CO2/HCO3− (room temperature). The CO2/HCO3− control ISO solution contained (in mM) 105.2 NaCl, 3.0 KCl, 1.0 CaCl2, 1.25 MgCl2, 24.8 NaHCO3, 2 NaH2PO4, 5 HEPES, and 10 glucose. Hence the total [Cl−] of this solution was 112.7 mM (rounded to 113 mM for calculation of Cl− equilibrium potentials) and the total [Na+] was 132 mM. The osmolality was adjusted with sucrose to 290 ± 2 mosmol/kgH2O. The solution was bubbled with 5% CO2, and the pH at 25°C was 7.3. The Cl−-free ISO solution (0 Cl−) was made by replacing Cl− with gluconate in the ISO control solution on a mole-for-mole basis.
Measurement of Intracellular [Cl−] in MQAE-Loaded Cells
The fluorescent dye MQAE (Verkman et al. 1989) was used to measure [Cl−]i in single DRG neurons following the methods detailed in a previous study (Rocha-Gonzalez et al. 2008). After washout of FITC-IB4, DRG neurons were loaded with MQAE by incubation in the imaging chamber with 5 mM MQAE (Invitrogen-Molecular Probes) diluted in DRG growth medium for 1–2 h (25°C). The dye-loading solution was washed out with ISO control solution, and the cells were equilibrated for at least 10–15 min before initial fluorescence measurements were made. Experimental solutions were perfused at a flow rate of 6 ml/min by means of two electronic valve systems (VC-6; Warner Instruments, Hamden, CT). The fluid volume of the chamber (300–400 μl) was exchanged with a half-time of <5 s. The temperature of the chamber was 25–26°C; cell viability increased at this temperature compared with 37°C. Basal [Cl−]i measured at 37°C was not different from that at 25°C (Rocha-Gonzalez et al. 2008). For all thermodynamic calculations in the present study we used 25°C.
Basic details of the imaging setup are described elsewhere (Alvarez-Leefmans et al. 2006; Rocha-Gonzalez et al. 2008). In brief, cell images were captured with a cooled digital-CCD camera (ORCA 2-ER C4742-95, Hamamatsu, Hamamatsu City, Japan) using MetaFluor imaging software (Molecular Devices, Sunnyvale, CA). Recordings of MQAE emitted fluorescence at 460 ± 25 nm were made from a digital circular region placed at the image plane of each DRG cell body (see Fig. 4A). The circular regions had 30 pixels in diameter (1 μm = 3.15 pixels). The dye was excited at 350 ± 5 nm with a monochromator (Optoscan, Cairn Research, Faversham, UK) in which both input and output slits were set to the same bandwidth. The excitation light source was a 75-W xenon arc lamp. The excitation light passed through a liquid light guide before entering the microscope optical path. A filter cube containing a dichroic mirror (400 nm) and a 460 ± 25 nm emission filter (Chroma Technology, Rockingham, VT) was positioned underneath the objective lens in the filter holder of the microscope. Cells were exposed to excitation light pulses (10–30 ms), and MQAE emission signals were sampled at a frequency of 0.1 Hz.
Fig. 4.
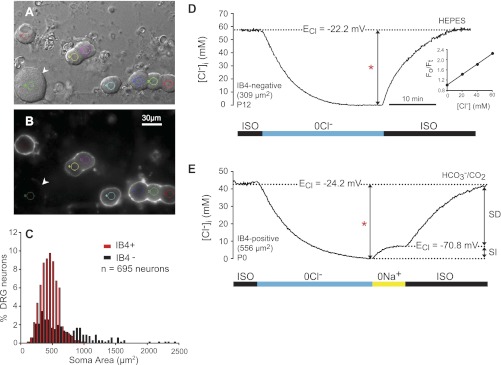
Intracellular Cl− concentration ([Cl−]i) in DRG neurons of identified phenotype. A: differential interference contrast (DIC) image of acutely dissociated rat DRG neurons (P12). B: isolectin B4 (IB4) fluorescent labeling (FITC) of the cells in A. IB4 appears as a white halo around the surface of the cells that bind it. IB4+ cells are of small size [soma cross-sectional area (CSA) ∼450 μm2]. The only large neuron (1,506 μm2) in the field (arrowhead) is IB4−. Small colored circles in A and B delimit the digital pinhole areas from which MQAE fluorescence was collected. C: frequency distribution of dissociated DRG neurons according to cell size (soma area) and IB4 labeling (n = 695). Red bars represent IB4+ cells and black bars IB4− cells. All IB4+ cells are small or medium size, whereas IB4− cells are small or medium to large size (note skewed tail toward large sizes). D: typical intracellular Cl− transient in response to isosmotic removal and readdition of external Cl− used to measure basal [Cl−]i and Cl− equilibrium potential (ECl) in single-identified DRG neurons loaded with MQAE. The neuron was initially equilibrated in isosmotic control solution (ISO). On removal of external Cl− (0 Cl−), the cell was depleted of Cl−. On restoration of external Cl− (131 mM), [Cl−]i recovered to initial values. All the solutions in this group of cells were buffered with HEPES. The basal [Cl−]i (measured as the difference indicated by the bracketed arrow labeled with a red asterisk) in this neuron (IB4−, 309 μm2 CSA, P12) was 60 mM, and ECl = −22.2 mV. Inset: calibration plot of relative MQAE-emitted fluorescence (Fo/Ft) as a function of [Cl−] in the calibration solutions. The slope of this relation is the Stern-Volmer constant (Ksv in Eq. 1), which in this case was 19.2 M−1. E: changes in [Cl−]i following isosmotic removal and readdition of external Cl−, in the absence and presence of external Na+. All the solutions were buffered with CO2/HCO3−. On removal of external Cl− (0 Cl−), the cell (IB4+, CSA 556 μm2, P0) was depleted of Cl−. On restoration of external Cl− (113 mM) in the absence of external Na+ (0 Na+) there was a small increase in [Cl−]i that reached steady state at the expected electrochemical equilibrium (ECl = −70.8 mV; [Cl−]i = 7.2). This is the sodium-independent (SI) component of Cl− accumulation. On exposure to the ISO control solution, [Cl−]i recovered to initial values (44 mM, measured as indicated by red asterisk; ECl = 24.2 mV). The later recovery is the sodium-dependent (SD) component of Cl− accumulation. Scale bar in B applies to A.
Net Cl− efflux and influx and basal [Cl−]i were determined as described previously (Rocha-Gonzalez et al. 2008). In brief, cells were equilibrated in control ISO solution and then exposed to the 0 Cl− ISO solution until they were depleted of Cl−. Cells were assumed to be depleted of Cl− when the MQAE fluorescence signal recorded in the 0 Cl− solution reached a new steady state. The fluorescence signal when the apparent [Cl−]i = 0 mM was taken as F0 (see Eq. 1 below), an assumption that was validated upon dye calibration as explained below. The perfusion solution was then changed to one containing a physiological [Cl−] ([Cl−]o), i.e., ISO control, until [Cl−]i recovered to its initial value.
MQAE signal drift was corrected off-line by best fitting either a straight line or a monoexponential function to the data points of the whole transient (Rocha-Gonzalez et al. 2008). Irrespective of the function used, the correlation coefficient of the fit was >0.98; otherwise, cells were discarded for further analysis. The MQAE fluorescence signals recorded from each individual cell were transformed into [Cl−]i with the following equation derived from the Stern-Volmer relation:
| (1) |
where F0 is the steady-state fluorescence from each cell measured in 0 Cl− ISO solution (i.e., fluorescence in the virtual absence of external and internal free Cl−); Ft is the fluorescence recorded with respect to time during the experimental transients or that measured in the calibration solutions (i.e., fluorescence in the presence of Cl−), and Ksv is the Stern-Volmer quenching constant of MQAE for Cl−. The value of Ksv was calculated for each cell following the four-point calibration procedure outlined below. The average Ksv for intracellular calibration of MQAE was 16.1 ± 0.3 M−1 (mean ± SE; n = 227 cells), which is similar to that previously determined for rat DRG neurons (Rocha-Gonzalez et al. 2008). Furthermore, this value falls within the range of 9–25 M−1 reported for other cell types (Kaneko et al. 2004; Maglova et al. 1998; Marandi et al. 2002). We found that calibration plots can be reliably used when Ksv > 10 M−1 (see below).
MQAE fluorescence was calibrated against [Cl−]i by use of the “double ionophore” technique (Koncz and Daugirdas 1994; Krapf et al. 1988). Accordingly, at the end of each experiment cells were exposed to a series of calibration solutions containing the ionophores tributyltin (a Cl−/OH− exchanger; Sigma-Aldrich) and nigericin (a K+/H+ exchanger; Sigma-Aldrich). Under steady-state conditions, [Cl−]o and [Cl−]i were assumed to be equal. To this end, cells were exposed to 5 ml of each calibration solution for 3–6 min. The calibration solutions contained (in mM) 0.01 tributyltin, 0.005 nigericin, 10 glucose, 5 HEPES, 120 K+, and variable NO3− and Cl−. In these solutions [Cl−] was varied from 0 to 60 mM, keeping the sum [NO3−] + [Cl−] = 120 mM. The osmolality was adjusted with sucrose to 290 mosmol/kgH2O and the pH to 7.3. The first calibration solution to which the cells were exposed was that containing 120 mM KNO3 (0 Cl−). Given that NO3− in the range between 0 and 100 mM does not produce quenching of MQAE fluorescence (Kaneko et al. 2002), the fluorescence of each cell in the 120 mM KNO3 solution was taken as a the MQAE fluorescence in 0 Cl− and is denoted as F0(cal). In a previous study (Rocha-Gonzalez et al. 2008), it was found that in cells equilibrated with the 0 Cl− ISO solution, which were assumed to be depleted of intracellular free Cl−, the difference between the absolute value of the fluorescence (F0) and F0(cal) was negligible (1.2 ± 0.2 mM, n = 29). This validated the assumption that F0, the steady-state MQAE fluorescence recorded in 0 Cl− ISO solution, signals [Cl−]i ≈ 0 mM. Thus the error in the [Cl−]i arising from the difference F0 − F0(cal) is approximately ±1 mM. The 120 mM KNO3 (0 Cl−) solution was followed by those containing 20, 40, and 60 mM Cl−. Finally, the cells were exposed to a solution containing KSCN (150 mM) to quench the MQAE fluorescence. The KSCN-quenched fluorescence was taken as the background (Fb). Under these conditions, Fb was measured and subtracted point by point from the whole experimental transient and from the fluorescence readings in the calibration solutions. The MQAE fluorescence signals were transformed into [Cl−]i with Eq. 1.
In the experiments reported in the present study only cells fulfilling the following criteria for cell viability and MQAE performance were chosen for analysis: 1) calibration plot yielding Ksv ≥ 10 M−1; 2) correlation coefficient of calibration plot ≥ 0.99; 3) MQAE fluorescence in each calibration solution reached steady state; 4) MQAE fluorescence signal reached steady state in the ISO control solution and in the ISO Cl−-free solution (0 Cl−). Statistical analysis was carried out with Student's t-test. Differences were considered significant when P < 0.05. Data are expressed as means ± SE unless otherwise specified. Analysis of variance (ANOVA) was used for multiple comparisons.
RESULTS
Expression of NKCC1 Transcripts in Rat and Mouse DRG
Full-length NKCC1 cDNAs have been cloned from murine tissues (Di Fulvio and Alvarez-Leefmans 2009). These cDNAs encoding NKCC1 were cloned from mouse inner medullary collecting duct kidney cell lines (Delpire et al. 1994) and from rat parotid glands (Moore-Hoon and Turner 1998). They are here referred to as mNKCC1-L and rNKCC1-L, respectively, where the “L” stands for “long variant.” Partial molecular characterization of a shorter NKCC1 splice variant lacking exon 21 was performed in mouse brain tissue (Randall et al. 1997). Here we show that a similar splicing event also occurs in rNKCC1. These short (S) variants are referred to here as mNKCC1-S and rNKCC1-S for mouse and rat, respectively. RT-PCR analysis of total RNA from rat DRG reveals that both the full-length (rNKCC1-L) and the short-length (rNKCC1-S) variants are expressed in DRG (Fig. 1, A and B). RT-PCR analysis of total RNA from mouse DRG yielded a similar result (not shown). Nucleotide sequence analysis revealed that mNKCC1-S and rNKCC1-S are 100% identical.
To determine which DRG cells express NKCC1 mRNA for each variant (L and S), ISH was done in mouse DRG. Accordingly, three antisense probes denoted as mNKCC1-S, mNKCC1-L, and mNKCC1-L+S were designed (see Fig. 1, bottom) and used to detect 1) the short spliced variant lacking exon 21 (mNKCC1-S), 2) the full-length variant mNKCC1-L, and 3) both L and S variants (mNKCC1-L+S). In addition, three sense probes were designed and used as the corresponding negative controls (Fig. 1, F–H). The results showed that both variants are expressed in all DRG neurons irrespective of their size (Fig. 1, C–E).
NKCC1 Immunolabeling in Adult and Newborn Rat DRG Neurons
Validation of NKCC1 antibody specificity.
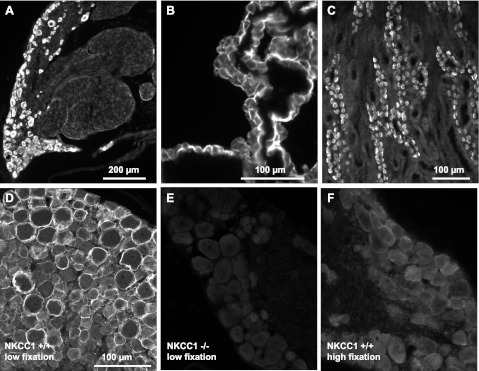
Sections of rat and mouse DRGs probed with the anti-NKCC1 polyclonal antibody, here referred to as Kaplan-CT (see materials and methods), revealed widespread NKCC1 immunoreactivity (IR) in virtually all the cell bodies of rat DRG neurons (Figs. 2A and 3). As positive control we used rat tissues where NKCC1 expression is undisputed, such as choroid plexus epithelial cells (Piechotta et al. 2002) and α-intercalated cells in the outer medullary collecting duct of kidney (Ginns et al. 1996), as shown in Fig. 2, B and C, respectively. Interestingly, the intensity of NKCC1-IR was strongly influenced by the level of fixation (compare Fig. 2D with Fig. 2F). The highest levels of NKCC1-IR were observed in tissues processed with no postfixation (see materials and methods for details).
Fig. 2.
Validation of NKCC1 antibody specificity. A–C: the polyclonal antibody (Kaplan-CT), raised in rabbit against amino acids 938–1011 of the carboxy terminus of mNKCC1, selectively labels all neurons in rat DRG (A) and also selectively labels rat tissues known to express NKCC1 such as the apical membrane of rat choroid plexus epithelial cells (B) and the basolateral membrane of α-intercalated cells of the outer medullary collecting duct of rat kidney (C). D: NKCC1 immunofluorescence of DRG neurons from wild-type mouse (NKCC1+/+) obtained with the Kaplan-CT antibody. Note that the antibody preferentially labels the surface of neurons and that the most intense immunofluorescence frequently surrounds the cells of larger diameter. This NKCC1 immunoreactivity (IR) is typically obtained when the tissues are prepared in “low fixation” conditions (i.e., no postfixation following perfusion-fixation with 4% paraformaldehyde). NKCC1-IR is totally absent in DRGs from NKCC1−/− mice (E) and is very significantly decreased in NKCC1+/+ DRGs postfixed in 4% paraformaldehyde for ≥2 h after perfusion-fixation (F, “high fixation”). This suggests that NKCC1 epitope is sensitive to fixation protocols (compare D, low postfixation, with F, high fixation). Scale bar in D applies to E and F.
Fig. 3.
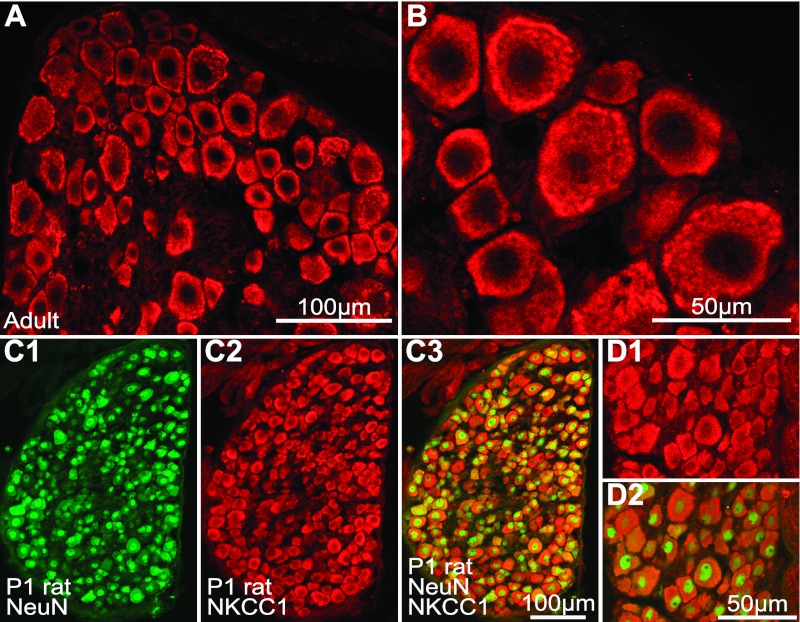
NKCC1 immunolabeling in adult and newborn rat DRG neurons. A and B: adult rat DRG section at low (A) and high (B) magnification showing NKCC1-IR (Cy3, red, Kaplan-CT antibody) in large and small neurons. NKCC1-IR was localized toward the cell periphery as in mouse DRG (Fig. 2D) but was also observed in the cytosol. C: newborn (P1) rat DRG section dual-immunolabeled for NeuN (FITC, green, C1) and NKCC1 (Cy3, red, C2). C1: NeuN immunofluorescence alone. C2: NKCC1 immunofluorescence alone. C3: superimposition of NeuN and NKCC1 immunofluorescence. D1: high magnification of NKCC immunofluorescence shown in C2. D2: merged NKCC1 and NeuN immunofluorescence. NeuN-IR is higher in the cell nucleus; NKCC1-IR is observed in all DRG neurons, and it is characteristically distributed throughout the cytosol in newborn rat.
The most stringent test of antibody specificity is to immunolabel tissues with and without the antigen of interest (Saper and Sawchenko 2003). Accordingly, the NKCC1 antibodies were validated by comparing the IR of DRG neurons from NKCC1+/+ mice with that of NKCC1−/−. NKCC1-IR was observed in NKCC1+/+ DRG neurons (Fig. 2D) but was absent in DRG from NKCC1−/− mice treated with the same postfixation regime and identical immunostaining conditions (Fig. 2E). Similar results to those obtained for the NKCC1 Kaplan-CT antibody were observed with two other antibodies against NKCC1: α-wCT and α-wNT (not shown; see materials and methods). None of the available antibodies against NKCC1 discriminates between the L and S variants.
Distribution of NKCC1 immunoreactivity.
NKCC1-IR was evident not only in adult rat DRG neurons (Fig. 3, A and B) but also in those of newborn (P1) rats (Fig. 3C2). In both adult and neonates, NKCC1-IR was found in virtually all DRG neurons irrespective of their size, an observation that is consistent with the expression pattern of NKCC1 mRNA revealed by ISH. The ubiquitous expression of NKCC1 protein in all DRG neurons was confirmed in double-immunolabeling experiments using the pan-neuronal marker NeuN (Fig. 3, C and D). At high magnification NKCC1-IR in adult rat DRG neurons was found preferentially localized toward the cell periphery, although IR was also present in the cytosol (Fig. 3B). In neonate rats, NKCC1-IR was found to be distributed more homogeneously throughout the cytoplasm of DRG neuron's cell bodies (Fig. 3D1).
Intracellular [Cl−] in DRG Neurons of Identified Phenotype
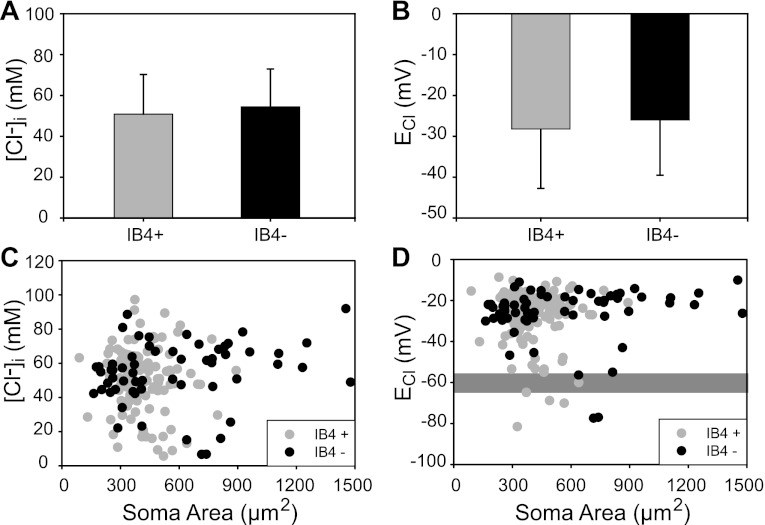
Primary sensory neurons exhibit great phenotypic diversity (e.g., size, IB4-binding capacity, peptide content). This diversity reflects their functional specialization as conduits for various sensory modalities (e.g., proprioception, pain); there is a tight correlation between their size and neurochemical phenotypes and the type of sensory receptor they innervate (Lawson 2005; Willis and Coggeshall 2004). As shown above, ISH and immunolabeling with validated antibodies revealed that both mRNA and NKCC1 protein are expressed in all DRG neurons irrespective of their size. The functional counterpart of this NKCC1-protein expression pattern suggests that [Cl−]i may be higher than equilibrium in all DRG neurons irrespective of their phenotype. To test this hypothesis, we measured [Cl−]i in dissociated rat DRG neurons classified according to size (estimated as soma CSA) and labeling with IB4, a lectin that binds to the surface of small (<400 μm2)- and medium (400–600 μm2)-size nonpeptidergic nociceptive neurons. The soma CSA of each neuron in a given field was measured with DIC images (Fig. 4A), and the same cells were then imaged with the appropriate set of filters to determine which were labeled with IB4 (Fig. 4B).
To compare the phenotypic characteristics of dissociated DRG neurons with those studied in fixed tissues in situ, we plotted the frequency distribution of a population of 695 dissociated DRG neurons according to cell size (soma CSA in μm2) and IB4 labeling (Fig. 4C). The frequency distribution of the dissociated DRG cell population that was labeled with IB4 (i.e., IB4+ cells) is shown with red bars, whereas that of the IB4 negative cells (IB4−) is shown with black bars. The population of dissociated IB4+ DRG neurons had mean CSA of 420 ± 141 μm2 (SD, n = 457). Considering that our measurements were done in live unfixed cells, these values fall reasonably well within the size range found in fixed tissues in situ for rat IB4+ cells (Fang et al. 2006; Gerke and Plenderleith 2001). The mean CSA of dissociated IB4− neurons was 629 ± 416 μm2 (SD, n = 238), and they ranged in CSA from 87 μm2 to 2,366 μm2 (black bars in Fig. 4C). These values also fall within the range found in situ (Lawson 2005).
When studied in situ and classified by their soma CSA, DRG neurons lead to bimodal distributions representing two overlapping populations of cells: small to medium and large (Lawson 2005). This bimodal distribution was preserved in the cells dissociated from DRG used in the experiments described here (Fig. 4C). Small- and medium-size IB4− cells are mostly peptidergic nociceptors. Overlapping with this population are the small- and medium-size IB4+ cells known to be nonpeptidergic nociceptors (Silverman and Kruger 1990; reviewed in Alvarez and Fyffe 2000) The second population is composed of large (mostly >800 μm2) IB4− cells known to innervate low-threshold cutaneous mechanoreceptors, Golgi tendon organs, and muscle spindles (Julius and Basbaum 2001; Lawson 2005). Thus our results indicate that all major DRG neuron populations present in situ were represented in the dissociated cells in vitro.
To measure [Cl−]i in DRG neurons identified by CSA and IB4 labeling, cells were loaded with MQAE. After a stable baseline fluorescence reading was obtained, the cells were depleted of Cl− by exposure to a Cl−-free solution (0 Cl−) isosmotic with the control solution (ISO). [Cl−]i was measured as the difference between the baseline and 0 Cl−, as indicated by the bracketed arrows labeled with a red asterisk in the sample traces shown in Fig. 4, D and E. Upon readmission of the Cl−-containing control solution, cells reaccumulated Cl− to their initial levels. Knowing [Cl−]i and [Cl−]o, it was possible to calculate ECl, the Cl− equilibrium potential, from the Nernst equation:
| (2) |
where z (= −1), R, T, and F have their usual meaning.
When the cell population in which [Cl−]i was measured (n = 176) was divided according to IB4 labeling, the [Cl−]i values (mean ± SD) were 51 ± 19 mM in IB4+ cells (n = 117) and 54 ± 18 mM in IB4− cells (n = 59). The difference was not statistically significant (Fig. 5A). The corresponding ECl for each population were −28 ± 13 mV for IB4+ cells and −26 mV ± 13 mV for IB4− cells (Fig. 5B). As expected from the measurements of [Cl−]i, there was no statistically significant difference in ECl values between the two populations. Analysis of scatterplots of [Cl−]i or ECl as a function of soma area in the same population of IB4+ and IB4− neurons revealed that there was no correlation between these variables and cell size (Fig. 5, C and D). These results indicate that basal [Cl−]i and ECl are independent of DRG cell phenotype.
Fig. 5.
Intracellular [Cl−] and ECl are independent of DRG cell phenotype. A: basal [Cl−]i in IB4+ and IB4− DRG neurons. Average basal [Cl−]i was 51 ± 19 mM (n = 117 cells; mean ± SD) in IB4+ neurons and 54 ± 18 mM (n = 59 cells) in IB4− neurons. The difference between means was not significant (P = 0.27). B: ECl in IB4+ (−28 ± 13 mV) and IB4− (−26 ± 13 mV) cells (mean ± SD). The difference between means was not significant (P = 0.44). C: scatterplot of [Cl−]i and soma area in IB4+ and IB4− DRG neurons. There was no correlation between [Cl−]i and soma area (r = 0.06). D: scatterplot of ECl and soma area in IB4+ and IB4− DRG neurons. The gray shaded rectangular area represents the reported average range of resting membrane potential (Em; −55 to −65 mV). ECl was less negative than Em in most cells (95.5%); ECl was equal to Em in ∼2% of the cells; and ECl was more negative than Em in only ∼3% of the cells. (HEPES-buffered solutions, [Cl−]o = 131 mM; error bars in A and B represent SD).
Another conclusion that can be drawn from this analysis is that in most DRG neurons ECl was more depolarized (less negative) than the resting membrane potential (Em), as can be observed in Fig. 5D, in which the gray shaded area indicates the average range of Em for DRG neurons i.e., −55 to −65 mV (Rocha-Gonzalez et al. 2008). Specifically, ECl was less negative than Em (−55 mV) in 95.5% of the whole population of neurons that fulfilled the stringent criteria for cell viability and dye performance outlined in materials and methods. In only ∼2% of the cells ECl values fell within −55 to −65 mV, and in ∼3% ECl was more negative than −65 mV. These observations suggest that Cl− was passively distributed across the DRG neuronal membrane in only 2% and lower than equilibrium in only 3% of the cells, whereas it is maintained well above electrochemical equilibrium in most (95.5%) DRG neurons.
Net Cl− Fluxes in DRG Neurons of Identified Phenotype
The results described so far show that [Cl−]i and ECl are independent of neuron phenotype. The question arises as to whether the rate of cell Cl− loss and accumulation per unit area of membrane correlates with cell phenotype. This is an important issue; net Cl− fluxes could vary with cell phenotype, reflecting differences in the density and/or kinetics of Cl− transporters expressed in their plasma membrane. To investigate this issue we measured the net Cl− fluxes per unit membrane area upon transient exposure to 0 Cl− ISO solution in cells classified according with their size and IB4-binding properties following the same experimental design shown in Fig. 4D. The results are shown in Table 2.
Table 2.
Net Cl− fluxes in DRG neurons of different phenotype
| n | JCl out, pmol/cm2s | JCl in, pmol/cm2s | P Value | |
|---|---|---|---|---|
| IB4+ | 117 | −31.1 ± 17.6 | 48.7 ± 28.12 | <0.0001 |
| SE = 1.63 | SE = 2.61 | |||
| IB4− | 59 | −48.4 ± 42.6 | 61.6 ± 44.3 | <0.05 |
| SE = 5.6 | SE = 5.86 | |||
| P value | <0.05 | <0.05 | ||
| Whole population | 176 | −36.9 ± 29.6 | 53 ± 35 | <0.0001 |
| SE = 2.24 | SE = 2.6 |
DRG, dorsal root ganglion; IB4, isolectin B4; JCl out, net Cl− efflux; JCl in, net Cl− influx.
Consistent with previous observations (Rocha-Gonzalez et al. 2008), the net Cl− influx (JCl in) in the whole population of neurons (n = 176) was significantly larger (P < 0.0001) than the net Cl− efflux (JCl out), irrespective of cell type. Interestingly, the net Cl− fluxes of IB4− cells were significantly larger (P < 0.05) than those of IB4+ cells, suggesting that IB4− cells have a higher density of Cl− transporters and/or a population of transporters with faster kinetics. The question arises as to whether there are differences in net Cl− fluxes within the population of IB4− cells; as mentioned above, this population of neurons comprises peptidergic nociceptors having CSAs of <600 μm2 and large IB4− cells (>600 μm2), most of which innervate proprioceptors. Thus for further analysis we divided the population of IB4− cells (n = 59) into small-medium (CSA < 600 μm2; n = 33) and large (CSA > 600 μm2, n = 26). The mean JCl in in large IB4− neurons was 83 ± 10 pmol/cm2s, whereas that in the small-medium size population was 45 ± 5 pmol/cm2s. The difference was statistically significant (P < 0.005), indicating that JCl in of large IB4− cells was larger (almost double) than that measured in the small-medium size population. The mean JCl out of large IB4− cells was −53 ± 7 pmol/cm2s whereas that in the small-medium size population was −44.6 ± 8.8 pmol/cm2s, but the difference was not statistically significant.
Intracellular [Cl−] in DRG Neurons Superfused with Solutions Buffered with CO2/HCO3−
The measurements of [Cl−]i described above were obtained in cells superfused with HEPES-buffered solutions. Given that DRG neurons may also express HCO3−-coupled Cl− transport mechanisms in addition to NKCC1, we repeated the measurements in cells in which the bathing solutions were buffered with CO2/HCO3−. The mean [Cl−]i and ECl of DRG neurons superfused with CO2/HCO3− solutions were 44.5 ± 15.6 mM and −26 ± 11 mV, respectively (n = 101). The corresponding values for [Cl−]i and ECl in HEPES-buffered solutions were 52 ± 19 mM and −27 ± 13 mV (n = 176). Although the mean [Cl−]i was ∼7.5 mM lower in CO2/HCO3−-buffered solutions, the difference in the value of ECl was not statistically significant (P = 0.45). The difference between the means of [Cl−]i is related to the fact that the [Cl−]o in the CO2/HCO3−-buffered solutions was 18 mM lower than in the HEPES-buffered solutions. As for the mean ECl values measured in HEPES- and CO2/HCO3−-buffered solutions, these were similar, indicating that there was no difference in the electrochemical driving force for Cl− under the two experimental conditions.
When the cell population in which [Cl−]i was measured in CO2/HCO3− solutions (n = 101) was divided according to IB4 labeling properties, the [Cl−]i values were 44 ± 14 mM in IB4+ cells (n = 67) and 46 ± 18 mM in IB4− cells (n = 34). There was no statistical difference in basal [Cl−]i between the IB4+ and IB4− cell populations. The corresponding ECl for each population were −26 ± 11 mV for IB4+ cells and −26 mV ± 12 mV for IB4− cells. When the cells were divided according to size, there were no differences in basal [Cl−]i or ECl between small-medium and large populations. Altogether, the above results indicate that basal [Cl−]i and ECl are independent of cell phenotype (soma size or IB4-labeling characteristics) or whether cells are superfused with HEPES- or CO2/HCO3−-buffered solutions.
Consistent with previous observations made in neurons equilibrated with HEPES-buffered solutions (Rocha-Gonzalez et al. 2008), DRG neurons superfused with CO2/HCO3−-buffered solutions also exhibited Na+-dependent and Na+-independent components of Cl− reaccumulation following Cl− depletion denoted as SD (sodium-dependent component) and SI (sodium-independent component), respectively (Fig. 4E). The SI component reflects a passive Cl− leak conductance triggered by a rise in intracellular [Ca2+] upon exposure to the 0 Na+ solution, whereas the SD reflects active accumulation of Cl− brought up primarily by NKCC1 (Alvarez-Leefmans 2009; Rocha-Gonzalez et al. 2008).
In these experiments Cl− reaccumulation in Cl−-depleted cells was studied by restoring the control [Cl−]o first in the absence of external Na+ (by isosmotic replacement of Na+ with glucamine) for 15 to 30 min, followed by the control ISO solution. Both the Na+-free and ISO solutions were buffered with CO2/HCO3−. The SI component reached a maximum at [Cl−]i = 7.6 ± 0.4 mM (SD 3.8, n = 101 cells) and remained stable throughout the duration of exposure to the Na+-free solution. The [Cl−]i at which the SI component stabilized corresponded to an ECl of −72.6 ± 1.35 mV (SD 13.5, n = 101 cells). In neurons buffered with HEPES the SI component reached a maximum at [Cl−]i = 10.5 ± 1.2 mM (SD 7, n = 37 cells) and remained stable throughout the duration of exposure to the Na+-free solution. The [Cl−]i at which the SI component stabilized corresponded to an ECl of −70.6 ± 3.6 mV (SD 21.5, n = 37 cells). These values were not statistically different from those measured with solutions buffered with CO2/HCO3−. The results show that Cl− reaccumulation via Na+-dependent and Na+-independent mechanisms is similar in HEPES- and CO2/HCO3−-buffered solutions.
Intracellular [Cl−] in DRG Neurons at Different Postnatal Ages
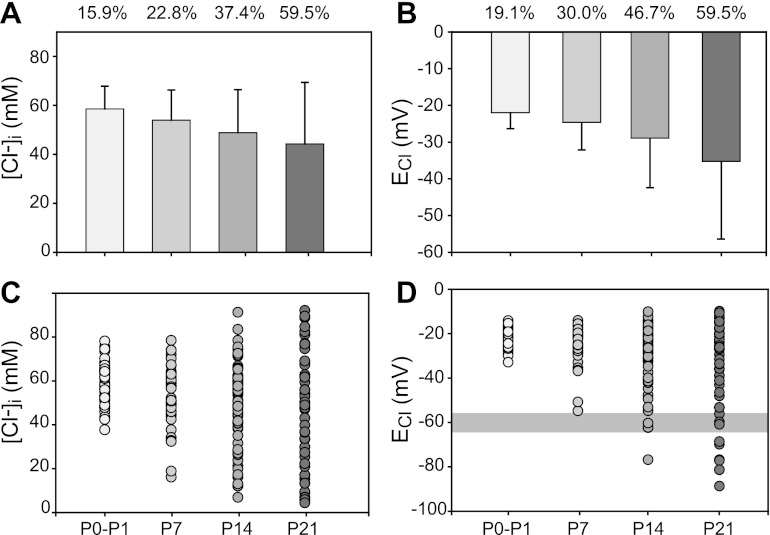
Cortical neurons undergo a postnatal “shift” in [Cl−]i that results in a hyperpolarizing shift in ECl occurring between P7 and P10 (Berglund et al. 2006; Marandi et al. 2002), whereas most DRG neurons maintain [Cl−]i well above electrochemical equilibrium in the adult (Rocha-Gonzalez et al. 2008). These findings raise the question of whether DRG neurons undergo changes in intracellular Cl− homeostasis during postnatal development. To address this question, [Cl−]i and ECl were measured in DRG neurons (n = 227) classified according to postnatal age (P0–P1, P6–P7, P13–P15, and P20–P22). The results show that [Cl−]i is maintained above electrochemical equilibrium in most DRG neurons irrespective of postnatal date (Fig. 6). However, as postnatal maturation progresses there is a downward drift in [Cl−]i and, consequently, a hyperpolarizing drift in ECl. These changes are accompanied by an increase in variability in [Cl−]i and ECl, as indicated by the increase in the corresponding coefficients of variation (Fig. 6, A and B).
Fig. 6.
Changes in [Cl−]i and ECl in rat DRG neurons at different postnatal ages. A: mean [Cl−]i at different postnatal ages from P0–P1 through P21. The numbers of neurons (n) for each group were 46 for P0–P1, 57 for P6–P7, 69 for P13–P15, and 55 for P0–P22. The corresponding mean [Cl−]i (±SE) for each group was (in mM) 58.6 ± 1.4, 54.0 ± 1.6, 49.9 ± 2.3, and 43.8 ± 3.5. B: mean ECl calculated for the data in A. corresponding mean ECl values (±SE) for each group were (in mV) −22.0 ± 0.6, −24.6 ± 0.9, −29.9 ± 1.6, and −35.2 ± 2.9. Numbers at top of A and B are the corresponding coefficient of variation expressed as %. The coefficient of variation for each mean population was calculated as (SD/mean) × 100. All error bars represent SD. C: scatterplot of [Cl−]i values. D: scatterplot of ECl values. Shaded gray area corresponds to reported Em range (−55 to −65 mV). Note the increased variability of [Cl−]i and ECl with postnatal age. All measurements were done in neurons equilibrated with HEPES-buffered ISO solution.
Statistical analysis (ANOVA) of measured [Cl−]i in DRG neurons from animals at different postnatal ages showed that mean [Cl−]i at P20–P22 was significantly lower (P < 0.001) compared with that at P0–P1. At P6–P7 [Cl−]i was also significantly lower (P < 0.05) than at P0–P1. However, the differences in mean [Cl−]i between P6–P7 and P13–P15 and between P13–P15 and P20–P22 were not significant (P > 0.1). Similar results were obtained in neurons buffered with CO2/HCO3− solutions. These results indicate that the most significant changes occurred between P0–P1 and P6–P7. The lack of statistical significance between P6–P7 and P13–P15 and between P13–P15 and P20–P22 is likely to be due to the progressive increase in variability as postnatal maturation progresses (Fig. 6).
The gradual decrease in [Cl−]i during postnatal maturation could be due to a gradual increase in functional expression of an active Cl− extrusion mechanisms, e.g., a K+-Cl− cotransporter. To test this hypothesis, we measured the steady state [Cl−]i reached during the SI component of Cl− accumulation following the same protocol illustrated in Fig. 4E. In the absence of external Na+, Cl− accumulation cannot occur via NKCC1 or any transport pathway driven by the energy stored in the inward Na+ gradient. If there was a developmentally upregulated Cl− extrusion mechanism, its functional expression should become evident in 0 Na+ because there would be no NKCC1 offsetting its action. Furthermore, if Na+-independent Cl− extrusion increased as maturation progresses, one would expect a decrease in the steady-state [Cl−]i measured in the absence of external Na+. Table 3 shows the steady-state [Cl−]i and ECl measured at various postnatal ages in the absence of external Na+. Analysis of the data shown in Table 3 revealed that there were no statistically significant differences in [Cl−]i and ECl measured at different postnatal ages in the absence of external Na+. Nevertheless, there was a tendency for ECl to become more hyperpolarized as maturation progressed.
Table 3.
Steady-state [Cl−]i and ECl in absence of external Na+ in DRG neurons at various postnatal ages
| P0–P1 (n = 44) | P6–P7 (n = 27) | P13–P15 (n = 20) | P20–P22 (n = 10) | |
|---|---|---|---|---|
| [Cl−]i, mM | 7.3 ± 0.4 | 9.0 ± 0.8 | 6.5 ± 0.8 | 7.0 ± 2.2 |
| ECl, mV | −72.0 ± 1.5 | −67.3 ± 2.2 | −78.0 ± 3.9 | −78.8 ± 6.3 |
Values are means ± SE for n neurons. Measurements were done in neurons equilibrated with CO2/HCO3−-buffered 0 Na+ ISO solution. [Cl−]i, intracellular Cl− concentration; ECl, Cl− equilibrium potential.
Changes in [Cl−]i and ECl with Postnatal Age of DRG Neurons Are Not Related to KCC2 Expression
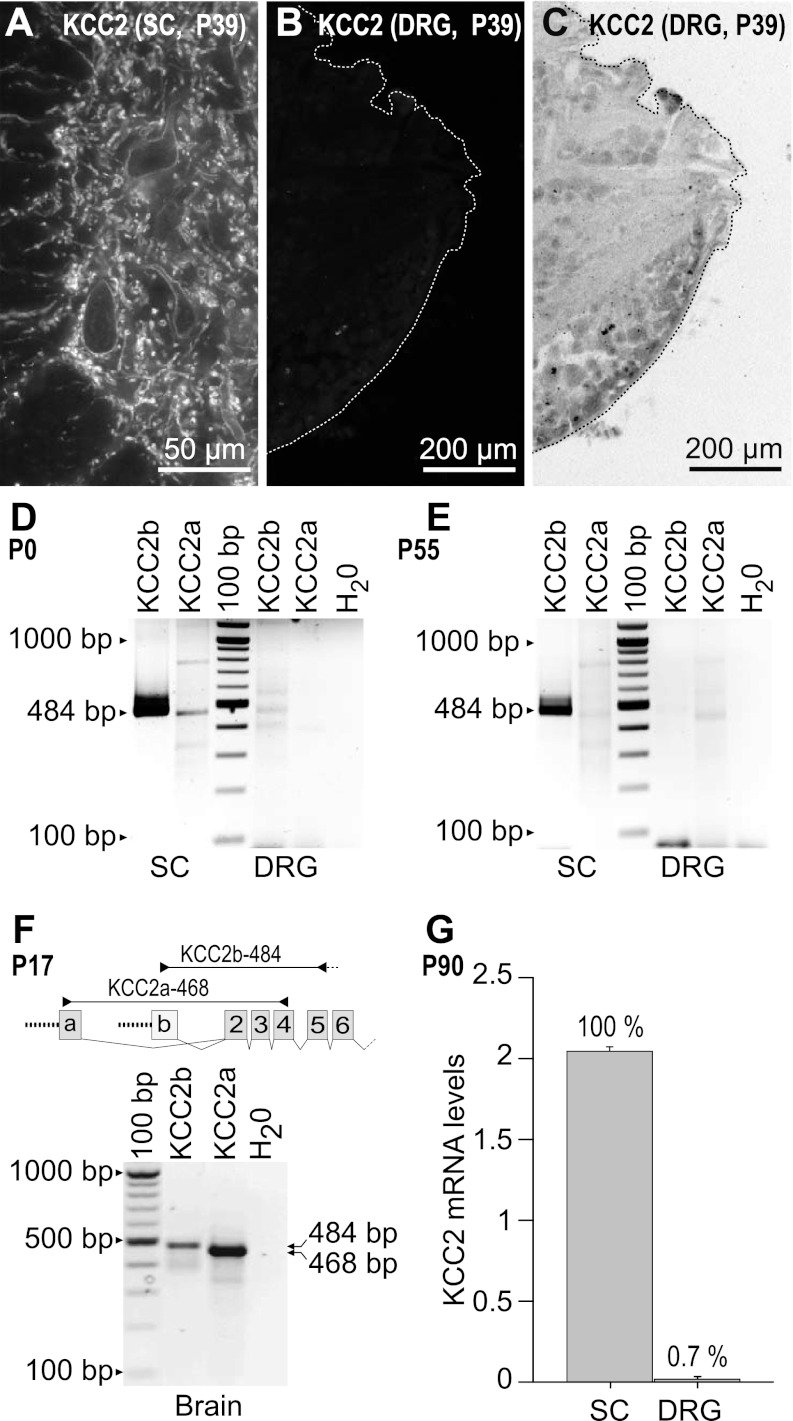
The above results suggest that the changes in [Cl−]i during postnatal maturation measured in control ISO solutions cannot be attributed to a developmentally regulated increase in expression of a Na+-independent Cl− extrusion mechanism like KCC2, which is responsible for the hyperpolarizing shift in ECl in central neurons (Rivera et al. 1999; Stein et al. 2004). To further explore this issue, we looked for KCC2 expression in DRG neurons. KCC2 exhibits amino-terminal heterogeneity due to the use of alternative promoters and alternative first exons (Uvarov et al. 2007). Exon 1a encodes 40 amino-terminal amino acid residues giving rise to KCC2a, and exon 1b encodes 17 amino acids giving rise to KCC2b, the originally described variant (Payne et al. 1996). There is no information about the expression of KCC2 variants in DRG. This is important because both variants are expressed in CNS neurons but they differ in their level and time of expression during development. In mouse brain, KCC2b is steeply upregulated during postnatal development whereas KCC2a is not (Uvarov et al. 2007). Whether or not DRG neurons express KCC2 protein is at present controversial, as discussed in the introduction of this report. In contrast, KCC2 protein expression in both ventral and dorsal horns of spinal cord of rat and mice is well documented (Coull et al. 2003; Delpy et al. 2008; Stil et al. 2009). Using an antibody that recognizes both KCC2 variants (see materials and methods), we confirmed that KCC2-IR was expressed abundantly on the somato-dendritic membranes of motoneurons and interneurons throughout the dorsal and ventral horns of the adult rat spinal cord (Fig. 7A). In contrast, there was no detectable KCC2 protein expression in DRG sections coimmunostained simultaneously on the same slides (Fig. 7B). Consistent with this finding is the RT-PCR analysis shown in Fig. 7, D and E, in which KCC2 transcript expression in newborn (P0) spinal cord and DRG is compared with KCC2 transcript expression in adult rat (P55). The results show that irrespective of postnatal age, KCC2b is highly expressed in spinal cord but is undetectable or barely detectable in DRG. KCC2a is only expressed at low levels in the newborn spinal cord and not in DRG. As positive control for both KCC2a and KCC2b we used rat brain (Fig. 7F), where both splice variants are known to be expressed (Uvarov et al. 2007). The negligible level of expression of KCC2 mRNAs in DRG compared with spinal cord is supported by database analysis from mouse gene chip (LeDoux et al. 2006), as shown in Fig. 7G.
Fig. 7.
Absence of KCC2 protein and transcript expression in rat DRG. A: KCC2-IR in a section of the spinal cord (SC) ventral horn of an adult (P39) rat used as positive control. KCC2-IR appears in the membrane region, and it is best visualized in the cell bodies. The smaller processes showing KCC2-IR are dendrites. B: KCC2-IR was absent in DRG sections coimmunostained with SC sections on the same slides. C: negative, inverted image of B showing localization of the DRG neurons. KCC2 immunolabeling was done with an affinity-purified polyclonal antibody (1:500) raised in rabbit against an amino-terminal His-tag fusion protein corresponding to residues 932–1043 of rat KCC2 (Upstate). The amino acid sequence recognized by this antibody is the same for both KCC2 variants. The secondary antibody used to visualize KCC2 was donkey anti-rabbit Cy3 (1:50). D and E: RT-PCR of KCC2 transcripts. Total RNA from DRG and SC from newborn [P0 (D)] and adult [P55 (E)] rats was reverse-transcribed and subjected to PCR using the KCC2a- and KCC2b-specific primer sets illustrated in F (KCC2a-468 and KCC2b-484). As RT-PCR negative control, nuclease-free water (H2O) was used instead of total RNA. Bands of expected size corresponding to KCC2b (484 bp) and/or KCC2a (468 bp) were detected only in SC of newborn and adult rats. The faint bands observed in the KCC2b line of DRG most likely represent nonspecific amplification products of the RT-PCR reaction. F: KCC2a and KCC2b expression in rat brain (P17, positive control). G: database analysis of expression levels of KCC2 transcripts detected in the cDNA array-based platform GPL1261 (Affymetrix GeneChip Mouse Genome 430 2.0 Array) posted in GeoProfile record GDS2209 (www.ncbi.nlm.nih.gov/sites/entrez?db=geo). Histogram shows transcript levels in SC and DRG in arbitrary units. Numbers above bars are expression levels of KCC2 transcripts as relative % with respect to SC (100%). C is at the same magnification as B.
Expression of KCC Transcripts Other than KCC2 in DRG Neurons
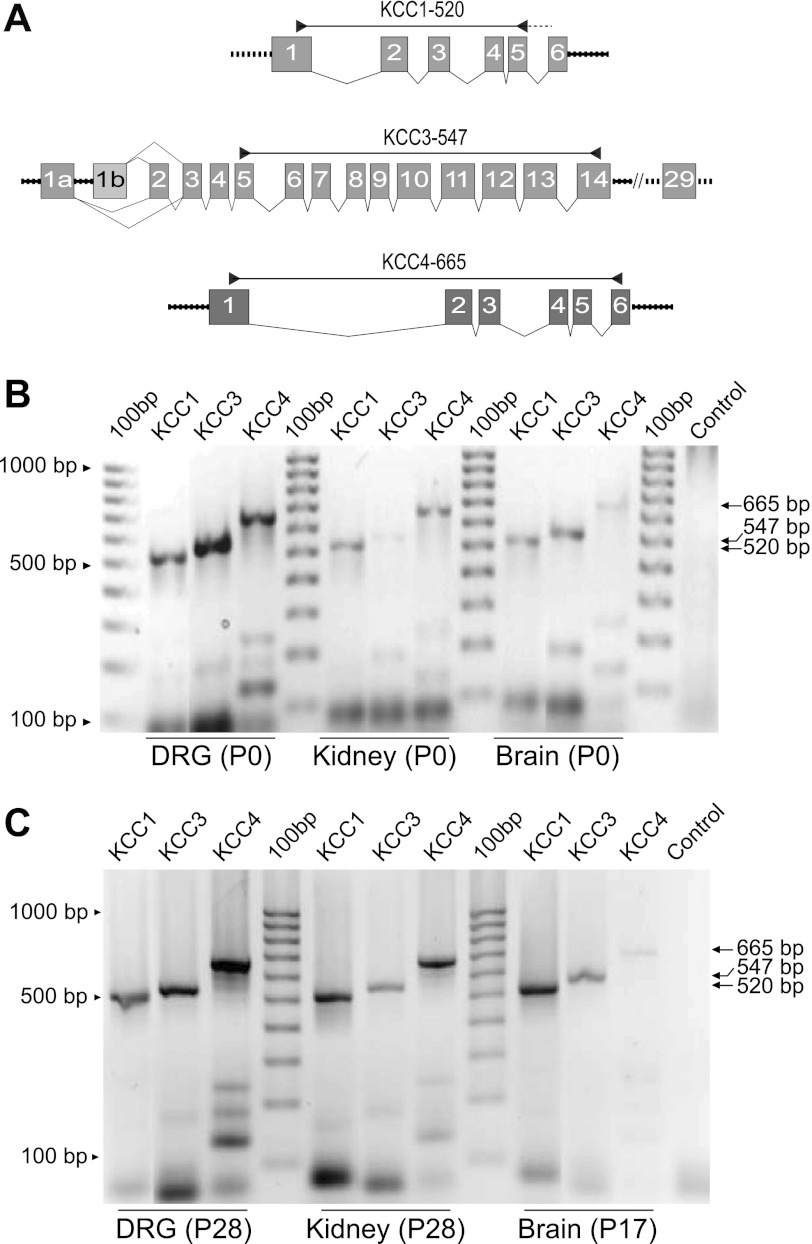
Although DRG neurons did not express KCC2a or KCC2b, we detected the transcripts for other K+-Cl− cotransporter isoforms, namely KCC1s, KCC3s, and KCC4, in DRG from both newborn (P0) and young adult (P28) rats (Fig. 8).
Fig. 8.
Expression of KCC1, KCC3, and KCC4 mRNAs in neonate and adult rat DRG. A: schematic diagram of the primers designed to detect KCCs other than KCC2 in rat DRG, kidney, and brain tissues. The KCC1 primer set (KCC1-520) is predicted to amplify all KCC1 transcripts from the mRNA region encompassing the 3′-end of exon 1 and 3′/5′ boundaries of exons 5 and 6. The KCC3-547 primer set amplifies all predicted KCC3 isoforms and splice variants, and the primer KCC4-665 amplifies predicted rat KCC4 cDNA sequences (see materials and methods for details). B and C: expression of KCCs in rat DRG, kidney, and brain in neonate (P0, B) and adult (P28 and P17, C) rat. Control lane in B and C was water instead of RNA.
The primer sets shown in Fig. 8A were designed to amplify all known isoforms and splice variants of rat KCC1 (KCC1-520), KCC3s (KCC3-547), and KCC4 (KCC4-665). As positive controls of mRNA expression of KCCs we used total RNA from kidney and brain tissues (whole brain without cerebellum), where expression of KCCs has been demonstrated by others (Boettger et al. 2002; Gillen et al. 1996; Karadsheh et al. 2004; Mercado et al. 2005). KCC1s and KCC3s were expressed in DRG, brain, and kidney in the newborn (P0) and young adult (P17 and P28) rats. KCC4 was barely detectable in brain both at P0 and P17 but was abundant in kidney and DRG.
As discussed below, of all these KCC transcripts (i.e., 1, 3, and 4) only KCC3 might have the potential to work as a Cl− extrusion mechanism under basal isosmotic conditions, depending on its state of phosphorylation (Rinehart et al. 2009). Therefore, we analyzed further the expression of KCC3. The mammalian KCC3 gene has the potential to produce several alternative splice variants (Mercado et al. 2005). There is no information about the expression of these splice variants in mammalian DRG. The main KCC3 splice variants result from the election of two alternative promoters located upstream of two different first coding exons [exon 1a (KCC3a) and exon 1b (KCC3b)]. Additionally, exclusion of exon 2 in KCC3a or KCC3b generates KCC3a−2 and KCC3b−2 variants, respectively. As shown in Fig. 9, KCC3a, KCC3a−2, and KCC3b−2 transcripts were detected in DRG from both neonatal (P0) and young adult (P17) rats. Brain tissues expressed KCC3a and KCC3a−2. However, neither KCC3b nor KCC3b−2 was detected in brain.
Fig. 9.
Expression of KCC3 splice variants in neonate and adult rat DRG. A: schematic diagram of the primers designed to detect KCC3 isoforms and splice variants in rat tissues. The primer KCC3-662a/617a−2 was designed to selectively detect the “a” variants of KCC3, with or without exon 2 (662 bp or 617 bp, respectively). The primer set KCC3-556b/511b−2 was designed to specifically detect the “b” variants of KCC3 with or without exon 2 (556 bp or 511 bp, respectively). B: KCC3 transcript expression in neonate (P0) and juvenile (P17) rat DRG and brain. Note that DRG expresses at least 3 KCC3 splice variants.
DISCUSSION
The major findings of the present study are that NKCC1 is expressed in virtually all DRG neurons, from P0 to adult, irrespective of their size and other phenotypic indicators. Furthermore, unlike CNS neurons, DRG neurons do not express KCC2, the neuronal Cl− extruder, and do not exhibit a “developmental shift” in [Cl−]i. The most parsimonious explanation for the latter is that active Cl− uptake mediated by NKCC1 is not offset by coexistent active Cl− extrusion that otherwise would be mediated by KCC2. In fact, the present results agree with the widely held view that the “developmental shift” in [Cl−]i in CNS neurons is mediated by the emergence of functional KCC2 activity. Nevertheless, DRG neurons undergo developmental changes in intracellular Cl− homeostasis, although these changes are quite different from those exhibited by central neurons; as postnatal maturation progresses, [Cl−]i decreases smoothly and becomes more variable, although it persists above electrochemical equilibrium in most (∼95%) mature DRG neurons. More specifically, the developmental change in [Cl−]i in CNS neurons is relatively abrupt; it is characterized by a transition of ECl from depolarizing to hyperpolarizing that in most cases is due to increased functional activity of KCC2. In contrast, the developmental change in [Cl−]i observed in DRG neurons is gradual, does not exhibit transitions, follows a different time course, and is not due to an increase in KCC2 activity. Thus we refer to this change as a developmental “drift” in Cl− homeostasis to distinguish it from the developmental “shift” in Cl− homeostasis observed in many CNS neurons. The possible mechanisms underlying this “developmental drift” in [Cl−]i and the associated increase in [Cl−]i and ECl variability are discussed below.
Rat and Mouse DRG Express Two NKCC1 Transcripts
RT-PCR revealed that rat DRG cells express two NKCC1 transcripts, one corresponding to the full-length NKCC1 (rNKCC1-L) and the other to a shorter-length splice variant (rNKCC1-S) resulting from exon 21 exclusion (Fig. 1, A and B). So far only two NKCC1-L cDNAs have been cloned and characterized from mouse and rat tissues: mNKCC1 (Delpire et al. 1994) and rNKCC1 (Moore-Hoon and Turner 1998). NKCC1-S was partially cloned from mouse tissues, where it was found to be expressed predominantly in the brain (Randall et al. 1997). Our results show that a similar splicing event occurs in rNKCC1. To identify the DRG cellular elements in which these two NKCC1 mRNAs are expressed, we used ISH. The results showed that both splice variants are expressed in all DRG neurons irrespective of their size (Fig. 1, C–H), suggesting that these variants are expressed in primary sensory neurons of all phenotypes. Neither of the two NKCC1 short splice variants lacking exon 21 (rNKCC1-S and mNKCC1-S) has been fully cloned, and their function is unknown. Interestingly, exon 21 encodes a 16-amino acid peptide located in the carboxy terminus of the cotransporter protein; its absence predicts the loss of a protein kinase A (PKA) consensus site of the cotransporter protein (Randall et al. 1997). This suggests that when both variants are coexpressed in a neuron they may be subjected to different regulation. Absence of exon 21 does not appear to affect NKCC1 transport function, as suggested from experiments in MDCK cells transfected with constructs of NKCC1-S (Carmosino et al. 2008). As far as we know, NKCC1 phosphorylation affecting cotransport activity in DRG neurons is carried out by the kinases SPAK and OSR1 at specific threonine residues located in the amino terminus of the cotransporter (Geng et al. 2009). However, it is unknown whether PKA phosphorylation plays a direct role in NKCC1 in DRG neurons. Interestingly, the sequence encoded by exon 21 also contains a conserved dileucine motif that appears to be involved in sorting of NKCC1 in polarized epithelial cells (Carmosino et al. 2008). It has been proposed that dileucine motifs function as dendritic targeting signals for a variety of proteins expressed in CNS neurons (Rivera et al. 2003). In primary sensory neurons exon 21 may endow these cells with the capacity to target NKCC1 to different plasma membrane domains (e.g., cell body and central and peripheral endings). Existing NKCC1 antibodies do not discriminate between NKCC1 splice variants, so it is not possible, at this stage, to determine their exact cellular localization.
Widespread Expression of NKCC1 in DRG Neurons of Different Phenotype
Using confocal immunofluorescence and three validated antibodies directed against different epitopes of rat NKCC1 (Kurihara et al. 1999) and mouse NKCC1 (Kaplan et al. 1996), we show that NKCC1 is ubiquitously expressed in all rat DRG neurons, irrespective of their phenotype (soma CSA) or postnatal age. In neonate (P1) rats NKCC1 appears homogeneously distributed throughout the cell body (cytoplasm and membrane region), whereas in adult neurons it is more abundant in the membrane region, although it is also expressed in the cytoplasm. The antibodies used were validated against NKCC1−/− mouse tissues, and as positive controls we used rat tissues where NKCC1 expression is undisputed, such as choroid plexus epithelial cells (Piechotta et al. 2002) and α-intercalated cells in the outer medullary collecting duct of kidney (Ginns et al. 1996). Previous immunolabeling studies showed NKCC1 expression in most DRG neurons (Alvarez-Leefmans et al. 2001; Gilbert et al. 2007). However, unlike the present study, the specificity of the antibodies was not validated against NKCC1−/− tissues. More recent studies proposed that NKCC1 mRNA is predominantly expressed in small- and medium-diameter neurons and that the NKCC1 protein does not reside in the cell bodies but in central and peripheral terminals (Price et al. 2006, 2009). The present results are in sharp contrast with the findings reported by Price and coworkers. The explanation for this discrepancy remains to be determined. It is worth noting that NKCC1-IR is strongly influenced by the level of fixation, and this could result in detection failure under certain conditions. This would explain why these authors found NKCC1 transcript in a subpopulation of small DRG neuronal cell bodies with ISH but failed to demonstrate the expression of the protein. Moreover, we detected the transcripts for both NKCC1 variants in all DRG neurons, including the largest ones, and found that NKCC1-IR levels frequently appeared higher around the periphery of larger cells in both rat and mouse tissues (Figs. 2D and 3B). These observations could correlate with the significantly larger net Cl− influx measured in large cells compared with small cells. Therefore, we must conclude that if there are any differences in NKCC1 expression among phenotypes, NKCC1 should be more highly expressed in the large cells, and certainly not absent. This agrees with the fact that GABA-mediated presynaptic depolarization in the spinal cord is very prominent in cutaneous mechanoreceptors and muscle proprioceptor afferent fibers (Rudomin and Schmidt 1999), both originated in the large DRG neuronal population (Lawson 2005).
Intracellular [Cl−] Is Above Electrochemical Equilibrium in Most DRG Neurons Irrespective of Their Phenotype and Postnatal Age
In agreement with the ubiquitous expression of NKCC1 transcripts and protein in DRG neurons, the present results show that [Cl−]i is above electrochemical equilibrium in >95% of DRG neurons irrespective of their phenotype (cell size and IB4 labeling characteristics) or postnatal age (Figs. 5 and 6). This is consistent with earlier work showing that the vast majority of adult rat DRG neurons are depolarized by GABA irrespective of their conduction velocity (Desarmenien et al. 1984) and that [Cl−]i is maintained above electrochemical equilibrium in DRG neurons of various sizes (Gilbert et al. 2007; Rocha-Gonzalez et al. 2008). This is also in agreement with the finding that intraspinal terminals of various types of adult afferent fibers are depolarized by GABA, a depolarization that has long been postulated to be crucial for presynaptic inhibition of these synaptic terminals in the spinal cord (reviewed in Alvarez-Leefmans 2009).
DRG Neurons Undergo a “Developmental Drift” in Intracellular [Cl−] and Do Not Express the Neuronal Cl− Extruder KCC2
CNS neurons undergo a well-characterized hyperpolarizing shift in ECl during postnatal development due to a decrease in [Cl−]i. This shift in [Cl−]i during neuronal development occurs relatively abruptly (Berglund et al. 2006; Marandi et al. 2002) and results in GABAergic transmission switching from depolarizing to hyperpolarizing. In parallel with the developmental changes in polarity of GABA-mediated currents in CNS neurons, there is an increase in expression of functional KCC2 (Clayton et al. 1998; Payne 2009; Rivera et al. 1999; Stein et al. 2004; Stil et al. 2009). We found that the developmental changes in Cl− homeostasis exhibited by DRG neurons are very different from those in CNS neurons: the decrease in [Cl−]i during postnatal maturation of DRG neurons does not occur abruptly but gradually, and in mature DRG neurons [Cl−]i is maintained above electrochemical equilibrium in >95% of cells. Furthermore, in sharp contrast with CNS neurons, the observed changes in Cl− homeostasis cannot be attributed to a developmental increase in KCC2b expression; DRG neurons express neither KCC2a nor KCC2b mRNA nor KCC2 protein (Fig. 7). The lack of expression of KCC2 explains why, unlike CNS neurons, DRG neurons do not exhibit a “developmental shift” in Cl− homeostasis. The gradual decrease in [Cl−]i could reflect a developmental increase in expression of leak pathways (Cl− channels). However, the resting Cl− conductance of DRG neurons is negligibly small; they lack the ClC-2 inward-rectifying Cl− channels expressed in other neurons (reviewed in Alvarez-Leefmans 2009). However, DRG neurons express Ca2+-activated Cl− channels (reviewed in Duran and Hartzell 2011). Whether these channels are active under basal conditions and if their expression changes during development remains to be elucidated.
Another interesting feature of DRG neurons found in the present study is that the gradual decrease in [Cl−]i during postnatal development is accompanied by a progressive increase in variability of [Cl−]i and ECl (Fig. 6). The origin of this variability is unknown. It may reflect developmental changes in NKCC1 regulatory kinases such as OSR1, SPAK, and WNKs, known to be expressed in DRG neurons (Geng et al. 2009). The concerted action of some of these kinases phosphorylates and activates NKCC1 (Delpire and Austin 2010). Thus the variability in [Cl−]i and ECl may reflect various degrees of NKCC1 phosphorylation.
Expression of K+-Cl− Cotransporters Other Than KCC2 in DRG Neurons
DRG neurons from both newborn and adult rats express several transcripts for KCC1, KCC3, and KCC4 (Fig. 8). Protein expression of KCC3 and KCC4 but not KCC1 has been demonstrated in DRG and trigeminal ganglion neurons (Byun and Delpire 2007; Karadsheh et al. 2004). Little is known about the function of these KCCs in primary sensory neurons with the possible exception of KCC3. Mutations in the gene SLC12A6 (solute carrier family 12A, member 6), which encodes KCC3, result in severe sensorimotor neuropathy in humans (Howard et al. 2002). Moreover, full KCC3−/− mice exhibit axonal and Schwann cell swelling of sciatic nerves that ultimately results in neurodegeneration (Byun and Delpire 2007). This suggests that KCC3 is involved in cell water volume maintenance in sensorimotor axons, and that this mechanism is critical for peripheral nerve integrity and function. In other cell types KCC1, 3, and 4 have been implicated in transepithelial ion transport and cell volume regulation (Gamba 2005). However, unlike KCC2 and KCC3, KCC1 and KCC4 do not exhibit constitutive activity in isosmotic media; they are activated upon osmotic cell swelling (Mercado et al. 2000), and thus it is unlikely that they participate in intracellular Cl− homeostasis in DRG neurons in isosmotic physiological conditions. KCC4 has been recently proposed to function as a plasma membrane scaffold protein with ezrin in the assembly of cytoskeletal complexes involved in cellular invasive migration (Chen et al. 2009). The complex of KCC4 and ezrin may be important for regulating the integrity of cytoskeleton and maintaining cell adhesion needed for migration, which may be relevant during primary sensory axon growth and regeneration (Hellal et al. 2011). Clearly, more work is needed to elucidate the functional significance of KCC1 and KCC4 in DRG neurons.
The expression of KCC3s in DRG neurons deserves further comment. Like KCC1 and KCC4, KCC3 is activated by osmotic swelling, but unlike KCC1 and KCC4, it can also exhibit constitutive activity in isosmotic media depending on its degree of phosphorylation (Rinehart et al. 2009). Dephosphorylation renders KCC3 constitutively active in isosmotic media. Furthermore, like KCC2b, KCC3 protein increases with postnatal development in rat brain, although the cellular elements in which this increase in expression occurs have not been determined (Pearson et al. 2001). Whether or not this developmental increase in KCC3 protein expression occurs in DRG neurons needs to be established. With fluorescence ISH and immunolabeling it has been shown that KCC3a is mainly expressed in adult mouse cortical neurons and, to a much lesser extent, in some glial cells, whereas KCC3b is not expressed in mouse brain (Shekarabi et al. 2011). We confirm and extend these observations by showing that brain tissues from neonatal (P0) and young adult (P17) rats expressed KCC3a and KCC3a−2, but neither KCC3b nor KCC3b−2 could be detected. However, we detected KCC3a, KCC3a−2, and KCC3b−2 transcripts in DRG from both neonatal (P0) and young adult (P17) rats. The KCC3b−2 band was faint but clear and consistent (Fig. 9). The cellular elements in which these splice variants are expressed remain to be elucidated. Developmental changes in functional expression of KCC3 in DRG could also contribute to the observed postnatal decrease in [Cl−]i and its increased variability. Future studies are needed to determine whether KCC3 increases functional expression with development of DRG neurons. Given that NKCC1 and KCCs are reciprocally regulated by various kinases, an increase in phosphorylation of NKCC1 could occur concomitantly with a decrease in activity of KCC3, adding a new level of regulation of intracellular Cl− in primary sensory neurons that could impact the gating of sensory signaling. For instance, depolarizing shifts in ECl like those thought to be required for transforming PAD into dorsal root reflexes causing neurogenic pain and inflammation (reviewed in Alvarez-Leefmans 2009) could occur by phosphorylation of NKCC1 and/or dephosphorylation of KCC3s.
GRANTS
This work was partially supported by National Institute of Neurological Disorders and Stroke Grant NS-29227 and Boonshoft School of Medicine Grant RIF 226119 to F. J. Alvarez-Leefmans.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M., T.G.-M., M.D., Y.C., E.D., F.J.A., and F.J.A.-L. performed experiments; S.M., T.G.-M., M.D., F.J.A., and F.J.A.-L. analyzed data; S.M., M.D., F.J.A., and F.J.A.-L. interpreted results of experiments; S.M., T.G.-M., M.D., Y.C., E.D., F.J.A., and F.J.A.-L. approved final version of manuscript; T.G.-M., M.D., F.J.A., and F.J.A.-L. prepared figures; M.D., Y.C., F.J.A., and F.J.A.-L. conception and design of research; F.J.A. and F.J.A.-L. edited and revised manuscript; F.J.A.-L. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to Karin Flues and Gisela Llobet from the Biomedical Sciences Exchange Program for their help with some of the immunolabeling experiments and to Wenfeng Zhang for help with the ISH experiments. The immunolocalization experiments were performed in the laboratory of F. J. Alvarez; all other experiments were performed in the laboratory of F. J. Alvarez-Leefmans.
Present addresses: S. Mao, Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, MI, 48201; T. Garzon-Muvdi, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD 21231; F. J. Alvarez, Department of Physiology, Emory University, Atlanta, GA 30322.
REFERENCES
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Yamada J, Fukuda A, Luhmann HJ, Kilb W. Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl− cotransport in immature rat neocortical neurons. J Neurosci 27: 8616–8627, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Fyffe RE. Nociceptors for the 21st century. Curr Rev Pain 4: 451–458, 2000 [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ. Chloride transporters in presynaptic inhibition, pain and neurogenic inflammation. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System, edited by Alvarez-Leefmans FJ, Delpire E. Amsterdam: Academic-Elsevier, 2009, p. 439–470 [Google Scholar]
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol 406: 225–246, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Herrera-Perez JJ, Marquez MS, Blanco VM. Simultaneous measurement of water volume and pH in single cells using BCECF and fluorescence imaging microscopy. Biophys J 90: 608–618, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Leon-Olea M, Mendoza-Sotelo J, Alvarez FJ, Anton B, Garduno R. Immunolocalization of the Na+-K+-2Cl− cotransporter in peripheral nervous tissue of vertebrates. Neuroscience 104: 569–582, 2001 [DOI] [PubMed] [Google Scholar]
- Ault B, Hildebrand LM. GABAA receptor-mediated excitation of nociceptive afferents in the rat isolated spinal cord-tail preparation. Neuropharmacology 33: 109–114, 1994 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3: 728–739, 2002 [DOI] [PubMed] [Google Scholar]
- Berglund K, Schleich W, Krieger P, Loo LS, Wang D, Cant NB, Feng G, Augustine GJ, Kuner T. Imaging synaptic inhibition in transgenic mice expressing the chloride indicator, Clomeleon. Brain Cell Biol 35: 207–228, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisitkul RB, Villa JE, Kocsis JD. Axonal GABA receptors are selectively present on normal and regenerated sensory fibers in rat peripheral nerve. Exp Brain Res 66: 659–663, 1987 [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron 61: 820–838, 2009 [DOI] [PubMed] [Google Scholar]
- Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416: 874–878, 2002 [DOI] [PubMed] [Google Scholar]
- Burkey TH, Hingtgen CM, Vasko MR. Isolation and culture of sensory neurons from the dorsal-root ganglia of embryonic or adult rats. In: Pain Research: Methods and Protocols, edited by Luo DZ. Totowa, NJ: Humana, 2004, p. 189–202 [DOI] [PubMed] [Google Scholar]
- Byun N, Delpire E. Axonal and periaxonal swelling precede peripheral neurodegeneration in KCC3 knockout mice. Neurobiol Dis 28: 39–51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Coggeshall RE. Peripheral GABAA receptors: evidence for peripheral primary afferent depolarization. Neuroscience 93: 713–122, 1999 [DOI] [PubMed] [Google Scholar]
- Carmosino M, Gimenez I, Caplan M, Forbush B. Exon loss accounts for differential sorting of Na-K-Cl cotransporters in polarized epithelial cells. Mol Biol Cell 19: 4341–4351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Chou CY, Wilkins RJ, Ellory JC, Mount DB, Shen MR. Motor protein-dependent membrane trafficking of KCl cotransporter-4 is important for cancer cell invasion. Cancer Res 69: 8585–8593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl− cotransporter expression in rat neocortex. Brain Res Dev Brain Res 109: 281–292, 1998 [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424: 938–942, 2003 [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lodge D. The depolarization of feline ventral horn group Ia spinal afferent terminations by GABA. Exp Brain Res 46: 215–233, 1982 [DOI] [PubMed] [Google Scholar]
- Delpire E, Austin TM. Kinase regulation of Na+-K+-2Cl− cotransport in primary afferent neurons. J Physiol 588: 3365–3373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22: 192–195, 1999 [DOI] [PubMed] [Google Scholar]
- Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na+-K+-2Cl− cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem 269: 25677–25683, 1994 [PubMed] [Google Scholar]
- Delpy A, Allain AE, Meyrand P, Branchereau P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J Physiol 586: 1059–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarmenien M, Santangelo F, Loeffler JP, Feltz P. Comparative study of GABA-mediated depolarizations of lumbar Adelta and C primary afferent neurones of the rat. Exp Brain Res 54: 521–528, 1984 [DOI] [PubMed] [Google Scholar]
- Di Fulvio M, Alvarez-Leefmans FJ. The NKCC and NCC genes: an in silico view. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System (1st ed.), edited by Alvarez-Leefmans FJ, Delpire E. Amsterdam: Academic-Elsevier, 2009, p. 169–208 [Google Scholar]
- Di Fulvio M, Gomez-Cambronero J. Phospholipase D (PLD) gene expression in human neutrophils and HL-60 differentiation. J Leukoc Biol 77: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- Duran C, Hartzell HC. Physiological roles and diseases of Tmem16/Anoctamin proteins: are they all chloride channels? Acta Pharmacol Sin 32: 685–692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci 26: 7281–7292, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274: 26946–26955, 1999 [DOI] [PubMed] [Google Scholar]
- Frings S. Chloride-based signal amplification in olfactory sensory neurons. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System (1st ed.), edited by Alvarez-Leefmans FJ, Delpire E. Amsterdam: Academic-Elsevier, 2009, p. 413–424 [Google Scholar]
- Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol 275: 263–282, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- Geng Y, Hoke A, Delpire E. The Ste20 kinases Ste20-related proline-alanine-rich kinase and oxidative-stress response 1 regulate NKCC1 function in sensory neurons. J Biol Chem 284: 14020–14028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke MB, Plenderleith MB. Binding sites for the plant lectin Bandeiraea simplicifolia I-isolectin B4 are expressed by nociceptive primary sensory neurones. Brain Res 911: 101–104, 2001 [DOI] [PubMed] [Google Scholar]
- Gilbert D, Franjic-Wurtz C, Funk K, Gensch T, Frings S, Mohrlen F. Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int J Dev Neurosci 25: 479–489, 2007 [DOI] [PubMed] [Google Scholar]
- Gillen CM, Brill S, Payne JA, Forbush B., 3rd Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J Biol Chem 271: 16237–16244, 1996 [DOI] [PubMed] [Google Scholar]
- Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996 [DOI] [PubMed] [Google Scholar]
- Granados-Soto V, Arguelles CF, Alvarez-Leefmans FJ. Peripheral and central antinociceptive action of Na+-K+-2Cl− cotransporter blockers on formalin-induced nociception in rats. Pain 114: 231–238, 2005 [DOI] [PubMed] [Google Scholar]
- Hattori K, Akaike N, Oomura Y, Kuraoka S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am J Physiol Cell Physiol 246: C259–C265, 1984 [DOI] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331: 928–931, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J, Fan X, Song L, Riviere JB, Prevost C, Horst J, Simonati A, Lemcke B, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL, Jr, McDonald MP, Bouchard JP, Mathieu J, Delpire E, Rouleau GA. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet 32: 384–392, 2002 [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 104: 933–946, 2001 [DOI] [PubMed] [Google Scholar]
- Kaneko H, Putzier I, Frings S, Gensch T. Determination of intracellular chloride concentration in dorsal root ganglion neurons by fluorescence lifetime imaging. In: Calcium-Activated Chloride Channels, edited by Fuller CM. Boston: Elsevier/Academic, 2002, p. 167–189 [Google Scholar]
- Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci 24: 7931–7938, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadsheh MF, Byun N, Mount DB, Delpire E. Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience 123: 381–391, 2004 [DOI] [PubMed] [Google Scholar]
- Koncz C, Daugirdas JT. Use of MQAE for measurement of intracellular [Cl−] in cultured aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 267: H2114–H2123, 1994 [DOI] [PubMed] [Google Scholar]
- Krapf R, Berry CA, Verkman AS. Estimation of intracellular chloride activity in isolated perfused rabbit proximal convoluted tubules using a fluorescent indicator. Biophys J 53: 955–962, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara K, Moore-Hoon ML, Saitoh M, Turner RJ. Characterization of a phosphorylation event resulting in upregulation of the salivary Na+-K+-2Cl− cotransporter. Am J Physiol Cell Physiol 277: C1184–C1193, 1999 [DOI] [PubMed] [Google Scholar]
- Labrakakis C, Tong CK, Weissman T, Torsney C, MacDermott AB. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol 549: 131–142, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson S. The peripheral sensory nervous system: dorsal root ganglion neurons. In: Peripheral Neuropathy, edited by Dyck PJ, Thomas PK. Philadelphia, PA: Saunders, 2005, p. 163–202 [Google Scholar]
- LeDoux MS, Xu L, Xiao J, Ferrell B, Menkes DL, Homayouni R. Murine central and peripheral nervous system transcriptomes: comparative gene expression. Brain Res 1107: 24–41, 2006 [DOI] [PubMed] [Google Scholar]
- Liu M, Willmott NJ, Michael GJ, Priestley JV. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience 127: 659–672, 2004 [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol 39: 558–568, 1999 [PubMed] [Google Scholar]
- Maglova LM, Crowe WE, Altamirano AA, Russell JM. Human cytomegalovirus infection stimulates Cl−/HCO3− exchanger activity in human fibroblasts. Am J Physiol Cell Physiol 275: C515–C526, 1998 [DOI] [PubMed] [Google Scholar]
- Marandi N, Konnerth A, Garaschuk O. Two-photon chloride imaging in neurons of brain slices. Pflügers Arch 445: 357–365, 2002 [DOI] [PubMed] [Google Scholar]
- Mercado A, Song L, Vazquez N, Mount DB, Gamba G. Functional comparison of the K+-Cl− cotransporters KCC1 and KCC4. J Biol Chem 275: 30326–30334, 2000 [DOI] [PubMed] [Google Scholar]
- Mercado A, Vazquez N, Song L, Cortes R, Enck AH, Welch R, Delpire E, Gamba G, Mount DB. NH2-terminal heterogeneity in the KCC3 K+-Cl− cotransporter. Am J Physiol Renal Physiol 289: F1246–F1261, 2005 [DOI] [PubMed] [Google Scholar]
- Moore-Hoon ML, Turner RJ. Molecular and topological characterization of the rat parotid Na+-K+-2Cl− cotransporter 1. Biochim Biophys Acta 1373: 261–269, 1998 [DOI] [PubMed] [Google Scholar]
- Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis 17: 62–69, 2004 [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116: 201–211, 1992 [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 16: 6414–6423, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA. The potassium-chloride cotransporters: from cloning to structure and function. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: from Molecules to Diseases (1st ed.), edited by Alvarez-Leefmans FJ, Delpire E. Amsterdam: Academic-Elsevier, 2009, p. 333–356 [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem 271: 16245–16252, 1996 [DOI] [PubMed] [Google Scholar]
- Pearson MM, Lu J, Mount DB, Delpire E. Localization of the K+-Cl− cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience 103: 481–491, 2001 [DOI] [PubMed] [Google Scholar]
- Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002 [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol 272: C173–C183, 1997a [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol 33: 781–795, 1997b [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev 60: 149–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Hargreaves KM, Cervero F. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain Res 1112: 146–158, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J, Thorne T, Delpire E. Partial cloning and characterization of Slc12a2: the gene encoding the secretory Na+-K+-2Cl− cotransporter. Am J Physiol Cell Physiol 273: C1267–C1277, 1997 [DOI] [PubMed] [Google Scholar]
- Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138: 525–536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397: 251–255, 1999 [DOI] [PubMed] [Google Scholar]
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci 6: 243–250, 2003 [DOI] [PubMed] [Google Scholar]
- Rocha-Gonzalez HI, Mao S, Alvarez-Leefmans FJ. Na+,K+,2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. J Neurophysiol 100: 169–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999 [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol 465: 161–163, 2003 [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Salin-Cantegrel A, Laganiere J, Gaudet R, Dion P, Rouleau GA. Cellular expression of the K+-Cl− cotransporter KCC3 in the central nervous system of mouse. Brain Res 1374: 15–26, 2011 [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol 19: 789–801, 1990 [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol 468: 57–64, 2004 [DOI] [PubMed] [Google Scholar]
- Stil A, Jean-Xavier C, Liabeuf S, Brocard C, Delpire E, Vinay L, Viemari JC. Contribution of the potassium-chloride co-transporter KCC2 to the modulation of lumbar spinal networks in mice. Eur J Neurosci: 33: 1212–1222, 2011 [DOI] [PubMed] [Google Scholar]
- Stil A, Liabeuf S, Jean-Xavier C, Brocard C, Viemari JC, Vinay L. Developmental up-regulation of the potassium-chloride cotransporter type 2 in the rat lumbar spinal cord. Neuroscience 164: 809–821, 2009 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci 19: 6497–6505, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Murali SG. Na+-K+-2Cl− cotransporter in immature cortical neurons: a role in intracellular Cl− regulation. J Neurophysiol 81: 1939–1948, 1999 [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci 20: 7531–7538, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Yamada J, Ueno S, Okabe A, Kato H, Sato K, Hashimoto K, Fukuda A. Differential functional expression of cation-Cl− cotransporter mRNAs (KCC1, KCC2, and NKCC1) in rat trigeminal nervous system. Brain Res Mol Brain Res 133: 12–18, 2005 [DOI] [PubMed] [Google Scholar]
- Uvarov P, Ludwig A, Markkanen M, Pruunsild P, Kaila K, Delpire E, Timmusk T, Rivera C, Airaksinen MS. A novel N-terminal isoform of the neuron-specific K-Cl cotransporter KCC2. J Biol Chem 282: 30570–30576, 2007 [DOI] [PubMed] [Google Scholar]
- Valeyev AY, Hackman JC, Holohean AM, Wood PM, Katz JL, Davidoff RA. GABA-induced Cl− current in cultured embryonic human dorsal root ganglion neurons. J Neurophysiol 82: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- Verkman AS, Sellers MC, Chao AC, Leung T, Ketcham R. Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal Biochem 178: 355–361, 1989 [DOI] [PubMed] [Google Scholar]
- Willis W, Coggeshall R. Sensory mechanisms of the spinal cord. Primary Afferent Neurons and the Spinal Dorsal Horn (3rd ed.). New York: Kluwer Academic/Plenum, 2004, vol. 1 [Google Scholar]
- Willis WD. John Eccles' studies of spinal cord presynaptic inhibition. Prog Neurobiol 78: 189–214, 2006 [DOI] [PubMed] [Google Scholar]
- Wu Q, Delpire E, Hebert SC, Strange K. Functional demonstration of Na+-K+-2Cl− cotransporter activity in isolated, polarized choroid plexus cells. Am J Physiol Cell Physiol 275: C1565–C1572, 1998 [DOI] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol 557: 829–841, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol 95: 2404–2416, 2006 [DOI] [PubMed] [Google Scholar]