Abstract
Odor signals are transmitted to the olfactory bulb by olfactory nerve (ON) synapses onto mitral/tufted cells (MCs) and external tufted cells (ETCs). ETCs, in turn, provide feedforward excitatory input to MCs. MC and ETCs are also regulated by inhibition: intraglomerular and interglomerular inhibitory circuits act at MC and ETC apical dendrites; granule cells (GCs) inhibit MC lateral dendrites via the MC→GC→MC circuit. We investigated the contribution of intraglomerular inhibition to MC and ETCs responses to ON input. ON input evokes initial excitation followed by early, strongly summating inhibitory postsynaptic currents (IPSCs) in MCs; this is followed by prolonged, intermittent IPSCs. The N-methyl-d-aspartate receptor antagonist dl-amino-5-phosphovaleric acid, known to suppress GABA release by GCs, reduced late IPSCs but had no effect on early IPSCs. In contrast, selective intraglomerular block of GABAA receptors eliminated all early IPSCs and caused a 5-fold increase in ON-evoked MC spiking and a 10-fold increase in response duration. ETCs also receive intraglomerular inhibition; blockade of inhibition doubled ETC spike responses. By reducing ETC excitatory drive and directly inhibiting MCs, intraglomerular inhibition is a key factor shaping the strength and temporal structure of MC responses to sensory input. Sensory input generates an intraglomerular excitation-inhibition sequence that limits MC spike output to a brief temporal window. Glomerular circuits may dynamically regulate this input-output window to optimize MC encoding across sniff-sampled inputs.
Keywords: interneurons, microcircuits, olfactory, γ-aminobutyric acid
odor information is initially processed in glomeruli where the olfactory nerve (ON) synapses onto apical dendritic tufts of principal output neurons, mitral (and tufted) cells (MCs). ON inputs also monosynaptically target external tufted cells (ETCs) (Hayar et al. 2004a), which provide feedforward excitatory input to MCs (Hayar et al. 2004b; De Saint Jan et al. 2009; Gire and Schoppa 2009; Najac et al. 2011; Gire et al. 2012) and, in addition, excite glomerular interneurons, including GABAergic periglomerular (PG) cells and short axon cells (Price and Powell 1970a; Pinching and Powell 1971a; Murphy et al. 2005; Hayar et al. 2004a, 2004b; Shao et al. 2009). Short axon cells form interglomerular circuits that inhibit neighboring glomeruli (Aungst et al. 2003; Shirley et al. 2010). MCs extend long lateral dendrites deep to the glomerular layer. These lateral dendrites form reciprocal dendrodendritic synapses with GABAergic granule cells (GCs), forming the MC-GC-MC circuit. ETC dendrites are restricted to the glomerulus; thus, they are not inhibited by GCs.
The MC-GC-MC circuit has been classically considered the primary source inhibitory regulation of MCs (Phillips et al. 1963; Shepherd 1963). MCs release glutamate onto GCs, which release GABA onto the same or other MCs to generate recurrent and lateral inhibition, respectively (Schoppa et al. 1998; Isaacson and Strowbridge 1998; Isaacson and Murphy 2001). However, PG inhibitory neurons in the glomeruli are at least as numerous as GCs (Parrish-Aungst et al. 2007). The functional impact of intraglomerular inhibition on ETCs and MCs is poorly understood but is potentially important, as glomerular circuits operate at the initial stage of olfactory input and influence how sensory signals generate MC output to all downstream networks. The present results show that intraglomerular circuits provide rapid, strong ON-evoked inhibition to both MCs and ETCs. ETCs provide feedforward excitation to MCs; thus, intraglomerular circuits may regulate MC spiking directly by postsynaptic inhibition and indirectly by reduced feedforward excitation.
ETCs generate spontaneous spike bursts (Hayar et al. 2004a; Liu and Shipley 2008a) that monosynaptically excite about two-thirds of PG cells. This results in tonic GABA release, which presynaptically inhibits ON terminals via GABAB receptors to regulate the gain of sensory input (Aroniadou-Anderjaska et al. 2000; Pirez and Wachowiak 2008; Shao et al. 2009). Spontaneous intraglomerular GABA release from PG cells might also activate postsynaptic GABAA receptors to tonically inhibit MCs and ETCs. The present findings demonstrate that intraglomerular circuits provide both ON-evoked and tonic postsynaptic inhibition to regulate MCs and ETCs. Tonic inhibition of ETCs reduces feedforward ETC→MC excitation to indirectly regulate spontaneous spiking in MCs.
Combined, ON-evoked and tonic intraglomerular inhibition regulate both the magnitude and temporal structure of glomerular output.
METHODS
All experiments were performed on olfactory bulb slices obtained from male C57/BL6 mice (Charles River, age: 6–8 wk old). Animals were anesthetized with saturated vapor isoflurane and decapitated, and the olfactory bulbs were surgically removed and immediately placed in 4°C oxygenated sucrose-artificial CSF (aCSF) containing (in mM) 26 NaHCO3, 1 NaH2PO4, 3 KCl, 5 MgSO4, 0.5 CaCl2, 10 glucose, and 248 sucrose equilibrated with 95% O2-5% CO2 (pH 7.38). Horizontal slices (400 μm thick) were cut with a Leica VT1000 vibratome. Slices were incubated in oxygenated aCSF [containing (in mM) 124 NaCl, 26 NaHCO3, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, and 10 glucose equilibrated with 95% O2-5% CO2 (pH 7.38)] at 30°C for 20–30 min and then at room temperature (22°C) in aCSF for at least 1 h before being used. For recording, individual slices were transferred to a recording chamber and perfused with aCSF (as above) at a rate of 2.5 ml/min maintained at a constant 30°C (Bipolar Temperature Controller, Norfolk, VA). MCs were observed with a ×40 water-immersion objective using an Olympus BX51W upright microscope equipped for near-infrared differential interference contrast optics (Olympus Optical). All experimental procedures were carried out in accordance with protocols submitted to and approved by the Institutional Animal Care and Use Committee of the University of Maryland. All drugs used in this study were purchased from Tocris (Ellisville, MO), including dl-2-amino-5-phosphonovaleric acid (APV), gabazine (SR-95531 hydrobromide), and N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium chloride (QX-314). All other chemicals were purchased from Sigma (St. Louis, MO).
Whole cell (current and voltage) patch-clamp recordings were performed as previously described (Hayar et al. 2001, 2004b, 2005; Heyward et al. 2001; Aungst et al. 2003; Shao et al. 2009). Briefly, recording pipettes were made from thick-wall borosilicate glass with filament (inner diameter: 0.75 mm, Sutter Instrument, Novato, CA) pulled on a P-97 Flaming-Brown puller (Sutter). For current clamp, the internal solution contained (in mM) 120 K-gluconate, 20 KCl, 10 HEPES, 2 MgCl2, 2 Mg2ATP, 0.2 Na3GTP, and 0.1 BAPTA with 0.02% lucifer yellow (pH 7.3 adjusted with KOH, osmolarity: 287–295 mosM). For voltage clamp, the internal solution contained (in mM) 120 CsMeSO4, 10 QX-314, 10 HEPES, 1 MgCl2, 2.5 Mg2ATP, 0.2 Na3GTP, 0.1 BAPTA, and 10 phosphocreatin with 0.02% lucifer yellow (pH 7.3 adjusted with CsOH, osmolarity: 287–295 mosM). The final pipette tip resistance was 5.0–5.5 MΩ, and seal resistance was routinely >4 GΩ. All data were acquired with pCLAMP 9 software using a MultiClamp 700A amplifier digitized with a DigiData 1322A analog-to-digital board (Axon Instruments) and low-pass filtered online at 2 kHz (voltage clamp, sampling rate: 5 kHz) and 10 kHz (current clamp, sampling rate: 40 kHz). Constant-current electrical stimulation (170-μs duration, PG4000A Digital Stimulator and SIU91 stimulus isolator, Cygnus Technology) was applied with dual-barrel glass microelectrodes (Borosilicate Theta, 5- to 10-μm tip pulled on a P-97 Flaming-Brown puller, filled with aCSF) positioned in the ON layer slightly anterior to the recording site (ON bundles course generally anterior to posterior across the bulb).
MCs were selected for analysis only when they contained an intact apical dendrite with a visible intraglomerular tuft and exhibited membrane bistability in spontaneous activity. Membrane bistability, an intrinsic property of MCs, is sensitive to mechanical damage in slice preparations. MCs close to the cut surface of a slice rarely exhibit membrane bistability, whereas MCs deeper than 50 μm are typically bistable; MCs can lose bistabilty with time in slices but never regain this property (Heyward 2001). Thus, membrane bistability is indicative of MC 'health,“ and experiments were performed only on bistable MCs.
ETCs were initially reported in Golgi anatomy studies (Cajal 1911; Macrides and Schneider 1982; Pinching and Powell 1971a, 1971b). Recently, ETCs were rigorously characterized by correlating their morphological and electrophysiological properties (Hayar et al. 2004; Antal et al. 2006). Based on these studies, we defined ETCs by the following criteria: 1) spontaneous intrinsic burst firing that persists even when fast synaptic transmitter receptors are blocked (Hayar et al. 2004, Liu and Shipley 2008), 2) a ”pear-shaped“ cell body (10- to 15-μm diameter) located in the deep half of the glomerular layer, 3) an apical dendrite with extensively ramified tuft confined to the glomerulus, and 4) the absence of lateral dendrites in the external plexiform layer (EPL). A second type of tufted cell (∼30%) has lateral dendrites in the EPL and requires depolarizing current to exhibit bursting (Antal et al. 2006). We recognize cells with these features (Hayar et al. 2004) but term them ”superficial tufted cells“ to avoid confusion. The present study was restricted to ETCs.
Microinjection of drugs into a target glomerulus was performed using 30-psi pneumatic pressure applied for 2 ms (via a picospritzer, General Valve). Injection pipettes were made from thick-wall borosilicate glass with filament (inner diameter: 0.75 mm) pulled on a vertical pipette puller (6-μm tip opening). These parameters were calibrated to deliver an injection volume of ∼20 nl.
Inhibitory postsynaptic currents (IPSCs) were detected on the basis of amplitude and area by having at least a 3:1 signal-to-noise ratio as measured with Minianalysis software (Synaptosoft). In current-clamp recordings, action potentials were detected in pCLAMP and analyzed by Neuroexplorer (NEX Technologies). Comparisons of IPSC/inhibitory postsynaptic potentials evoked by ON stimulation were performed on frequency, amplitude, and area under the curve (charge), as measured by Minianlysis software. IPSCs were detected for each of the multiple sweeps (6–10 sweeps), and the detected IPSCs were averaged from across those sweeps to calculate a peristimulus time histograms (PSTH) using a custom Excel spreadsheet for each cell. Population PSTHs were calculated by normalizing each cell's response at each time point to the first bin after ON stimulation in aCSF and averaging each time point and treatment condition across multiple cells to generate the population response. Spontaneous IPSCs were analyzed from a minimum of 2 min of recording and >1,000 IPSCs. Unless otherwise stated, data are represented as means ± SE, with n indicating the number of cells examined. Statistical tests were performed on raw non-normalized data using t-tests, one-way ANOVA, or multivariate ANOVA (MANOVA; following the general linear model and post hoc with the Bonferroni test) to test for statistical significance.
RESULTS
Intraglomerular circuits inhibit MCs and ETCs.
GABAergic synapses from GCs onto the lateral dendrites of MCs regulate olfactory bulb output. However, the glomerular layer comprises approximately the same volume as the GC layer, and the number and density of neurons, most of which are inhibitory, is slightly greater in the glomerular layer than the GC layer, suggesting that intraglomerular inhibition may potently regulate MC activity (Parrish-Aungst et al. 2007). Inhibitory interneurons in the glomerular layer consist of GABAergic PG cells, which primarily innervate a single glomerulus, forming intraglomerular circuits, and dopaminergic/GABAergic short axon cells, which project across multiple glomeruli, forming interglomerular circuits (Aungst et al. 2003; Kiyokage et al. 2010). Dopaminergic/GABAergic short axon cells comprise only ∼10% of glomerular interneurons (Parrish-Aungst et al. 2007; Kiyokage et al. 2010). Thus, the major potential source of intraglomerular inhibition is GABAergic PG cells, but little is known of their functional impact on MCs and ETCs.
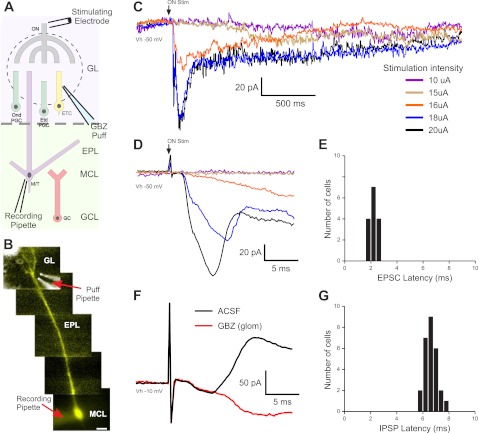
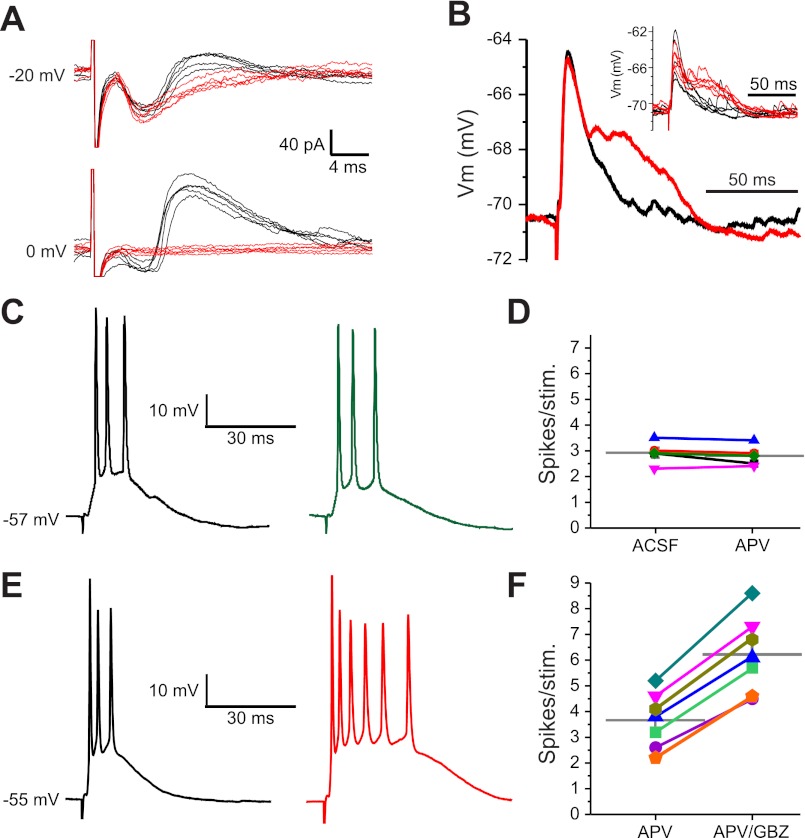
First, we examined the timing of excitatory and inhibitory currents evoked by sub- and perithreshold ON stimulation in MCs (Fig. 1, A and B). MCs were voltage clamped (−50-mV holding potential) with QX-314 and Cs2+ in the internal solution; Lucifer yellow was infused to visualize the MC apical dendrite in its target glomerulus (Fig. 1, A and B). A narrow range of minimal current (20.7 ± 0.7μA, n = 15) sufficed to evoke excitatory postsynaptic currents (EPSCs) in >95% of trials. MCs responded with an initial excitatory deflection consistent with monosynaptic ON→MC input (latency: 2.19 ± 0.07 ms, jitter: 194 ± 16 μs, n = 15; Fig. 1, C–E) (De Saint Jan et al. 2009; Najac et al. 2011). This was augmented by recurrent excitatory input that generates a prolonged inward current, the long-lasting depolarization (LLD; Fig. 1C) (Carlson et al. 2000; Schoppa and Westbrook 2001; Gire and Schoppa 2008; Urban and Sakmann 2002). As stimulus intensity was decreased, the short latency current dropped out, but in five of nine cells, a longer-latency (4.52 ± 0.64 ms), higher-jitter (1,455 ± 239 μs, n = 5), slow inward current persisted (Fig. 1D). This suggests that at stimulus intensities subthreshold for a given MC, sufficient ETCs in the same glomerulus are engaged to generate longer-latency recurrent excitation in MCs (De Saint Jan et al. 2009; Gire and Schoppa 2009; Najac et al. 2011).
Fig. 1.
Latencies of mitral cell (MC) excitatory and inhibitory responses to olfactory nerve (ON) stimulation. A: schematic of the experimental setup indicating the location of the pipette used to puff gabazine (GBZ). GL, glomerular layer; ONd PGC, ON-driven periglomerular cell (PGC); ETd PGC, external tufted cell (ETC)-driven PGC; EPL, external plexiform layer; MCL, MC layer; GCL, granule cell (GC) layer. B: MC showing a Lucifer yellow (LY) dye-filled apical dendrite, superimposed with a bright field image of the GBZ puff pipette. C: MC responses to ON stimulation with increasing stimulation strength [holding potential (Vh): −50 mV, average of 6–8 sweeps]. In this cell, stimulation intensities below 16 μA elicited no response (purple trace: 10 μA, brown trace: 15 μA); at 16 μA, the cell responded with a slow excitatory ramp into a long-lasting depolarization (LLD; red trace). At 18 μA or greater, a short-latency excitatory postsynaptic current (EPSC) and LLD occurred (blue trace: 18 μA, black trace: 20 μA). D: expanded traces from A showing the latency and onset of ON-evoked MC responses. MCs held at −50 mV responded to suprathreshold ON stimulation with a short-latency EPSC (downward deflection) followed by an inhibitory current (upward deflection). E: histogram showing EPSC latency to ON stimulation in 15 MCs. F: in artificial cerebrospinal fluid (aCSF; black trace, average of 10 sweeps), MCs held at −10 mV showed only the prominent upward-deflection inhibitory postsynaptic current (IPSC). Microinjection of 100 μM GBZ into the glomerulus containing the MC apical dendrite abolished the IPSC (red trace, average of 10 sweeps). G: histogram of MC IPSC latencies to ON stimulation in aCSF (n = 27, solid bars).
To assess latencies of inhibitory inputs, we recorded ON-evoked IPSCs in MCs voltage clamped at −10 mV (n = 27). At this holding potential, IPSCs generate outward currents clearly distinguishable from inward EPSCs. The mean IPSC onset latency was 6.62 ± 0.09 ms (jitter: 432 ± 49 μs; Fig. 1, F and G). To determine if intraglomerular circuits contribute to this relatively short-latency outward current, we blocked intraglomerular GABAA receptors by micropuffing gabazine into the glomerulus containing the apical tuft of the recorded MC. Intraglomerular block of GABAA receptors eliminated early onset ON-evoked IPSPs (Fig. 1F).
This early onset outward current was due to a compound IPSC that peaked at 11.8 ± 3.5 ms, with 90% of the IPSC integrated area occurring in the first 19.3 ± 2.4 ms (160.3 ± 24.8 pA·ms, n = 7). This fit with a two-parameter exponential predicting a return to baseline at 37.7 ms (curve fit regression R = 0.99). This early compound IPSC was followed by longer-latency, intermittent IPSCs, which generated a smaller integrated area (16.4 ± 2.2 pA·ms, n = 7). The duration of the late IPSC train was 806 ± 133 ms (calculated from the time at which the IPSC integrated area in a rolling 30-ms window returned to 2 SD of baseline, n = 7). Thus, we operationally distinguished two poststimulus time windows for further analysis of inhibition: 0–20 ms, comprising ”early“ IPSCs, and 21–800 ms, containing ”late“ IPSCs.
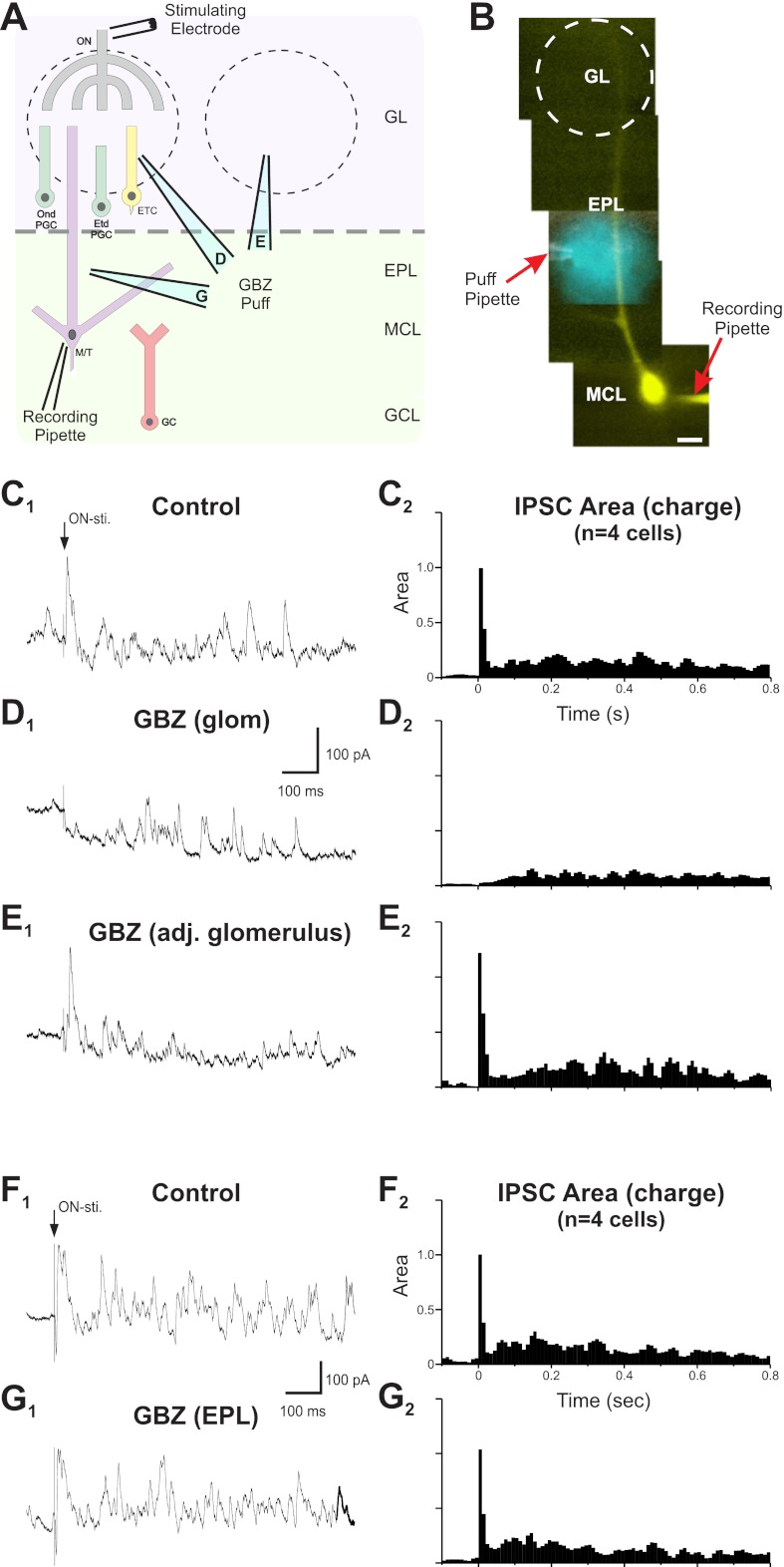
To investigate the contribution of intraglomerular circuits to early and late ON-evoked IPSCs, we puffed gabazine into the glomerulus containing the apical tuft of the recorded MC (Fig. 2). Intraglomerular GABAA receptor blockade abolished virtually all of the early IPSCs (97 ± 2% reduction in the early IPSC area, P < 0.00001 by MANOVA, n = 4; Fig. 2D) and reduced late IPSCs by 44 ± 9% (P < 0.05 by MANOVA, n = 4). To assess how well gabazine was restricted to the targeted glomerulus, we puffed equal amounts of the drug into an adjacent glomerulus or into the EPL immediately below the target glomerulus. Puffing gabazine into an adjacent glomerulus had no effect on early or late IPSCs (P = 0.9 for early IPSCs and P = 0.4 for late IPSCs by MANOVA, n = 4; Fig. 2E). Gabazine puffed into the EPL below the target glomerulus had no effect on early IPSCs (P = 0.9) but slightly attenuated late IPSCs (11 ± 4% reduction in the late IPSC area, P < 0.05 by MANOVA, n = 4; Fig. 2, F and G). Early IPSCs were eliminated only when gabazine was applied to the glomerulus containing the apical dendrite of the recorded MC.
Fig. 2.
ON stimulation engages intraglomerular circuits to generate short-latency, summating IPSCs in MCs. A: schematic of the experimental setup indicating different locations of pipettes used to puff GBZ. B: a LY-filled MC superimposed with a bright-field image showing the puff pipette used to deliver GBZ or fluorescent dye into the EPL to assess the spread of the puff. C1, D1, and E1: voltage-clamp recordings (Vh = −10 mV; internal solution contained QX-314, Cs2+, and LY; bath contains aCSF) of a MC response to ON stimulation. C2, D2, and E2: normalized population peristimulus time histograms (PSTHs) of the IPSC integrated area averaged from four cells. C: control. In aCSF, ON stimulation evoked an initial EPSC followed by a rapid onset barrage of IPSCs and then lower-frequency IPSCs. D: Glomerular injection of 100 μM GBZ abolished early IPSCs and attenuated late IPSCs. E: Injection of GBZ into an adjacent glomerulus had no influence on early or late IPSCs. F1 and G1: voltage-clamp recordings in a separate experiment (Vh = −10 mV; internal solution contained QX-314, Cs2+, and LY; bath contained aCSF) of a MC response to ON stimulation. F2 and G2: normalized population PSTHs of the IPSC integrated area averaged from four cells. F: control. In aCSF, ON stimulation evoked an initial EPSC followed by a rapid onset barrage of IPSCs and then lower-frequency intermittent IPSCs identical to the previous control cells. G: GBZ injected into the EPL. Early IPSCs were not influenced by the injection of GBZ into the EPL below the glomerulus containing the dendrite of the recorded MC. Late IPSCs were slightly attenuated (11% reduction).
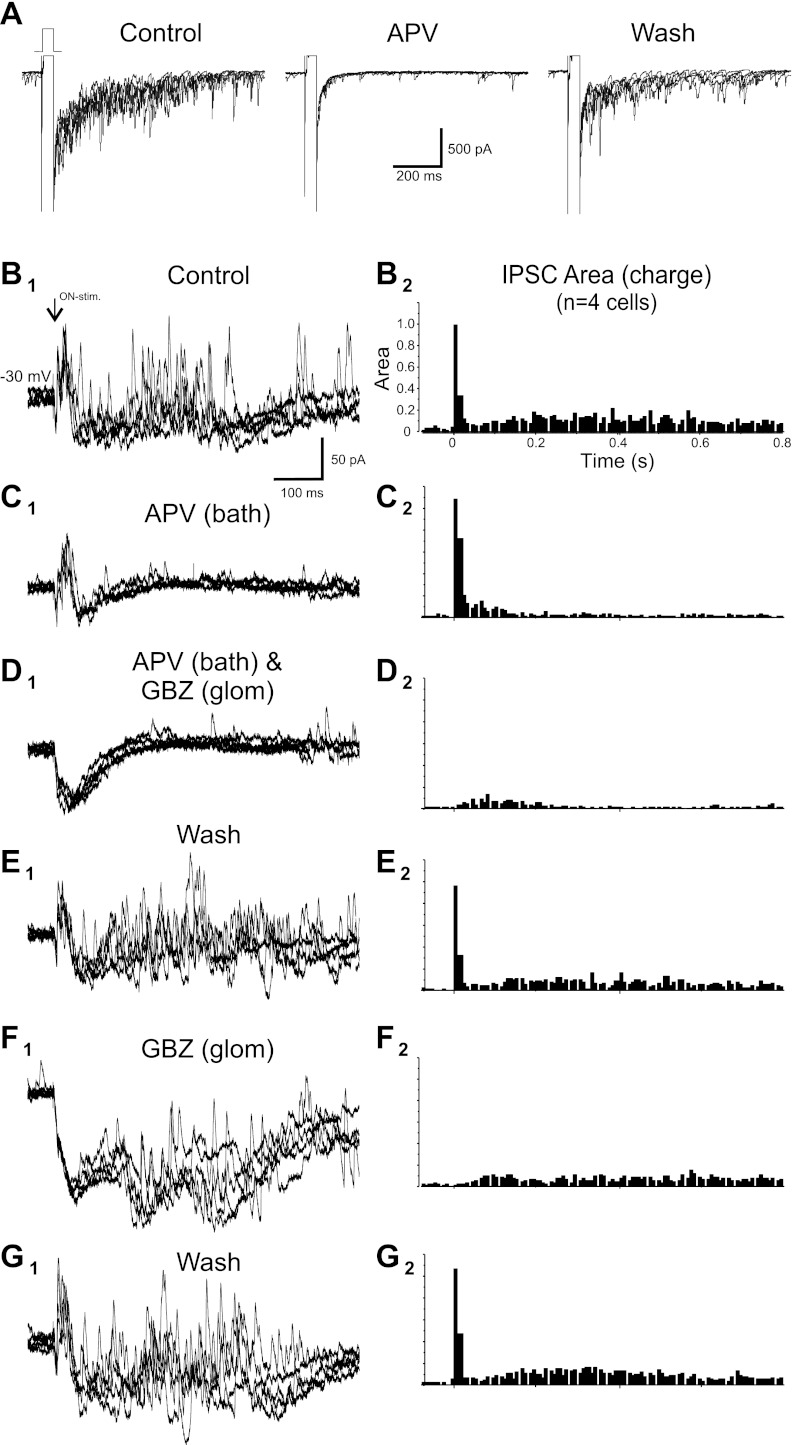
These results indicate that essentially all early IPSCs and approximately half of the late IPSCs evoked by ON stimulation derive from intraglomerular circuits. We reasoned that most of the remaining late IPSCs reflect GC feedback as they were partially attenuated by gabazine puffed into the EPL. It was not feasible to puff sufficient gabazine into the EPL to block all GC-derived IPSCs without the potential for drug spread into the glomeruli, so we used another strategy to suppress GC input to MCs. In rat bulb slices, GABA release from the GC→MC synapse is dependent on Ca2+ influx via activation of the N-methyl-d-aspartate (NMDA) receptor at the MC→GC synapse (Isaacson and Strowbridge 1998; Schoppa et al. 1998; Chen et al. 2000); the NMDA receptor antagonist APV eliminates most MC-evoked IPSCs from GCs. In contrast, APV has little impact on GABA release from PG cells (Hayar et al. 2004b, 2005). We first validated the NMDA receptor dependency of the MC→GC synapse in mouse bulb slices. MCs were step depolarized from −70 to 0 mV in the presence of TTX to evoke recurrent IPSCs. When APV (50 μM) was added to the bath, 79 ± 10% of recurrent IPSCs were eliminated (P < 0.005 by t-test, n = 4; Fig. 3A). The addition of gabazine blocked all IPSCs (not shown).
Fig. 3.
Intraglomerular and GC inhibition of MCs. A, left: voltage-clamp recording of a MC (internal solution contained high chloride; bath contained 1 μM TTX). Voltage steps from −70 to 0 mV evoked extensive feedback IPSCs. Middle, addition of dl-amino-5-phosphovaleric acid (APV) to the bath abolished most step depolarization-evoked IPSCs. Right, washout of APV restored evoked IPSCs. B1, C1, D1, E1, F1, and G1: separate experiments with voltage-clamp recording of a MC (Vh = −30 mV; internal solution contained QX-314, Cs2+, and LY; bath solution contained aCSF only) showing responses to ON stimulation (5 superimposed sweeps). B2, C2, D2, E2, F2, and G2: normalized population PSTHs for the IPSC integrated area averaged from multiple cells. B: control. In aCSF, ON stimulation evoked an initial EPSC followed by a rapid onset early barrage of IPSCs and longer-latency, lower-frequency IPSCs. C: Bath application of 50 μM APV attenuated late IPSCs with no change of early IPSCs. D: with APV still in the bath, intraglomerular injection of 100 μM GBZ abolished early IPSCs. E: washout of APV and GBZ restored both early and late IPSCs. F: glomerular injection of 100 μM GBZ abolished early IPSCs and reduced late IPSCs by ∼45%. G: washout of GBZ restored all IPSCs.
With GABA release at GC-MC synapses suppressed by bath application of APV (50 μM), ON-evoked early IPSCs in MCs were completely unaffected (7.5 ± 1.4% increase, not significant by MANOVA, n = 4; Fig. 3C), but the late IPSCs were attenuated 55 ± 9% (P < 0.05 by MANOVA, n = 4). This indicates that the MC-GC-MC circuit contributes little, if any, to the early barrage of IPSCs but contributes approximately half of late IPSCs. Lack of APV attenuation of early IPSCs was consistent with reports that PG cell GABA release is relatively independent of NMDA receptor activation (Hayar et al. 2004b, 2005). When gabazine was puffed into the glomerulus containing the apical tuft of the recorded MC with APV still in the bath, early IPSCs were abolished (96 ± 4% reduction, P < 0.0001 by MANOVA, n = 4; Fig. 3D) and late IPSCs suppressed an additional 51 ± 11% from APV alone (P < 0.05 by MANOVA, n = 4; Fig. 3D). After the washout of all drugs (Fig. 3E), a second intraglomerular microinjection of gabazine still abolished early IPSCs (Fig. 3F) and reduced late IPSCs by ∼50%, as described above (Fig. 1).
These results demonstrate that early ON-evoked IPSCs derive from intraglomerular circuits. Late IPSCs derive from both intraglomerular and MC→GC→MC circuits. It is important to acknowledge, however, that the relative strengths of inhibition contributed by these two sources cannot be assessed in slice preparations. A major input to GCs consists of excitatory synapses from the olfactory cortex. These inputs generate feedback and possibly tonic inhibition of MCs in vivo, but these connections are severed in slices. In addition, the lateral dendrites of MCs, which are targeted by GC synapses, extend for hundreds of micrometers, and some are inevitably truncated in slices. However, while reduced in slices, GC inputs to lateral dendrites remain and can be activated by direct depolarization of MCs (Fig. 3A). APV strongly suppresses these recurrent IPSCs but has no effect on ON-evoked early IPSCs. Thus, while the relative magnitudes of sensory-evoked inhibition from intraglomerular and GC→MC circuits cannot be inferred from these slice experiments, their dynamics are clearly different: 1) intraglomerular circuits generate rapid onset inhibition that is relatively insensitive to NMDA receptor block but entirely eliminated by local intraglomerular GABAA receptor block and 2) inhibition from GCs is slower onset and highly sensitive to NMDA receptor block. Thus, MCs are differentially regulated by fast intraglomerular inhibitory inputs that target the apical dendrite and slower GC inputs that synapse on the lateral dendrites.
Intraglomerular inhibition regulates MCs and ETCs.
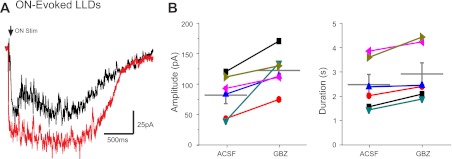
The preceding results show that sensory input generates an excitation-inhibition sequence in MCs and that rapid onset inhibition derives mainly from intraglomerular circuits. Early IPSCs target the intraglomerular dendritic tuft of the MC, which is the site of LLD generation (Carlson et al. 2000; Schoppa and Westbrook 2001; Gire and Schoppa 2008; Urban and Sakmann 2002). Thus, we reasoned that intraglomerular inhibition should attenuate LLDs, and, indeed, when it was blocked by intraglomerular gabazine, both the amplitude and duration of ON-evoked LLDs increased (amplitude: 82.3 ± 13.9 vs. 122.8 ± 12.9 pA with gabazine block, P < 0.01 by t-test, and duration: 2.5 ± 0.4 vs. 2.9 ± 0.5 s with gabazine block, P < 0.05 by t-test, n = 6; Fig. 4). Thus, intraglomerular inhibition attenuates ON-evoked LLD amplitude by 33%, duration by 15%, and integrated area (charge) by 42%.
Fig. 4.
Intraglomerular inhibition reduces MC responses to ON stimulation. A: ON-evoked MC LLDs in control (aCSF, black trace, average of 10 sweeps) and after glomerular injection of 100 μM GBZ (red trace, average of 10 sweeps) into the glomerulus containing the MC apical dendrite. B: plot of ON stimulation-evoked LLD amplitude and duration in aCSF and GBZ.
Intraglomerular GABAergic synapses also target ETCs, and ETCs provide feedforward excitation to MCs, contributing to LLD (Carlson et al. 2000; Schoppa and Westbrook 2001; De Saint et al. 2009; Gire and Schoppa 2009; Najac et al. 2011). Thus, intraglomerular inhibition could attenuate LLDs by reducing the output of ETCs, by postsynaptically inhibiting MCs, or both. The previous results show that intraglomerular circuits generate rapid postsynaptic inhibition in MCs. Next, we assessed their actions on ETCs. First, we examined the timing of excitatory and inhibitory currents evoked by perithreshold ON stimulation in ETCs voltage clamped at −20 to 0 mV (internal Cs2+). ON stimulation evoked a short-latency (2.12 ± 0.18 ms), low-jitter (83 ± 10 μs) EPSC (n = 6). This was consistent with previous studies showing monosynaptic excitatory ON input to ETCs (Hayar et al. 2004a, 2004b). The EPSC was followed by a compound IPSC with an onset latency of 6.4 ± 0.5 ms and a jitter of 695 ± 79 μs (Fig. 5A). Bath application of gabazine blocked all spontaneous and ON-evoked IPSCs in ETCs. As ETCs do not have extraglomerular dendrites, they are not targeted by GC synapses. Indeed, when we examined the effect of APV alone, which would block MC excitation of GCs, there were no changes in ON-evoked ETC spiking (2.9 ± 0.2 spikes/stimulus in aCSF vs. 2.8 ± 0.2 spikes/stimulus in APV, not significant by t-test, n = 5; Fig. 5, C and D) or spontaneous ETC activity (3.7 ± 1.0 Hz bursting in aCSF vs. 3.4 ± 0.8 Hz bursting in APV, not significant by t-test, n = 5). Thus, we interpret ON-evoked IPSCs to reflect di- or plurisynaptic inhibitory input via either ON→PG→ET circuits, ON→ET→PG→ET circuits, or both (Shao et al. 2009). GABAergic deep short axon cells send projections to the glomeruli, but these neurons target GCs and PGs not ETCs (Eyre et al. 2008). Taken together, these results indicate that ON excitation of ETCs is regulated by rapid intraglomerular feedback and/or feedforward inhibition by PG cells.
Fig. 5.
Intraglomerular inhibition of ETCs. A: ETC responses to ON stimulation held at −20 or 0 mV in aCSF (black traces) or GBZ (red traces). ETCs held at −20 mV responded to suprathreshold ON stimulation with a short-latency EPSC (downward deflection) followed by an inhibitory current (upward deflection). ETCs held at 0 mV showed only the prominent upward-deflection IPSC. B: average of five excitatory postsynaptic potentials (EPSPs) in an ETC held at −70 mV after ON stimulation in aCSF (black trace) or GBZ (red trace). Vm, membrane voltage. Inset: individual sweeps. C: current-clamp recording in an ETC after ON stimulation in control (aCSF, black trace) and after the addition of 50 μM APV (green trace). D: plot of individual ETC spikes/stimulation in control and after the addition of APV. The gray horizontal bar shows the population mean. E: current-clamp recording in an ETC after ON stimulation in control (aCSF containing 50 μM APV, black trace) and after the addition of 100 μM GBZ (red trace). F: plot of individual ETC spikes/stimulation in control and after the addition of GBZ. The gray horizontal bars show population means.
Intraglomerular inhibition should reduce ETC excitability. Figure 5B shows excitatory postsynaptic potentials evoked by ON input in an ETC held below threshold for spiking. When intraglomerular inhibition was blocked, the monosynaptic excitatory postsynaptic potentials were followed by recurrent excitatory inputs. This indicates that inhibition reduces ON-evoked recurrent excitation and suggests that it could limit ETC spike generation. As shown in Fig. 5, C and D, ON stimulation evoked an average of 3.7 ± 0.4 spikes/stimulus. After pharmacological block of intraglomerular inhibition, the same input generated an average of 6.2 ± 0.6 spikes/stimulus (P < 0.0001 by t-test, n = 7; Fig. 5, E and F). Thus, intraglomerular inhibition reduced ETC spike responses to sensory input by nearly one-half. As ETCs provide feedforward excitatory drive to MCs, intraglomerular inhibition would limit ON-evoked ETC excitation of MCs. This is consistent with the finding that block of inhibition by an intraglomerular puff of gabazine increased the amplitude and duration of MC LLDs. Thus, intraglomerular inhibition reduces MC LLDs directly by postsynaptic inhibition of MCs and indirectly by reducing ETC excitatory drive.
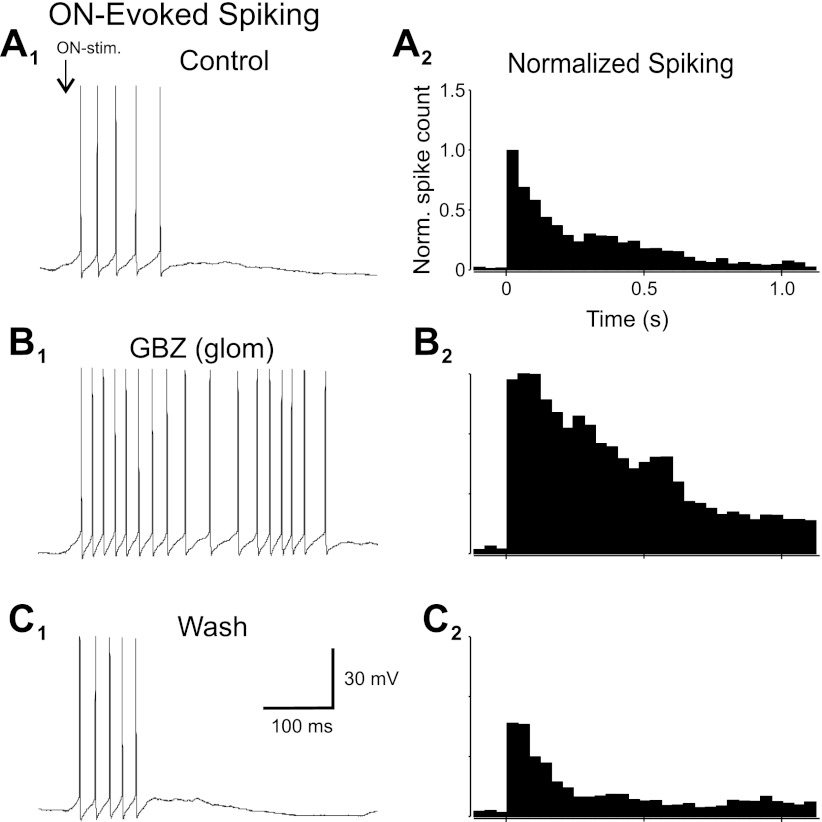
These actions of intraglomerular inhibition should limit MC spike generation. MCs responded to perithreshold ON stimulation with a burst of spikes (Fig. 6A). When gabazine was puffed into the glomerulus containing the apical tuft of the recorded MC (n = 10; Fig. 6B), the ON-evoked spike response increased nearly six-fold (582 ± 219%, P < 0.002 by t-test, n = 10; Fig. 6B). This was due in part to a 175 ± 53% increase in spiking the first 100 ms after stimulation (P < 0.05 by t-test). However, the major factor in the overall approximately sixfold increase was a prolongation of the time course of elevated spiking. The ON-evoked MC spike response decayed with a time constant of 93.7 ms (single exponential, 0.95 correlation coefficient), but when intraglomerular inhibition was blocked, the time constant increased nearly 10-fold to 904.2 ms (correlation coefficient: 0.94, P < 0.001). Thus, by reducing ETC excitatory drive and increasing postsynaptic inhibition of MC apical dendrites, intraglomerular inhibition significantly shapes both the strength and the temporal structure of the MC responses to sensory input.
Fig. 6.
Intraglomerular inhibition reduces MC spike responses to ON stimulation. A1, B1, and C1: MC spiking in response to ON stimulation. A2, B2, and C2: normalized population PSTHs. A: control. In aCSF, ON stimulation evoked a short-latency burst of spikes riding on an LLD. B: glomerular injection of 100 μM GBZ dramatically increased spike output. C: washout of GBZ restored normal spike patterns.
Glomerular circuits tonically inhibit MCs.
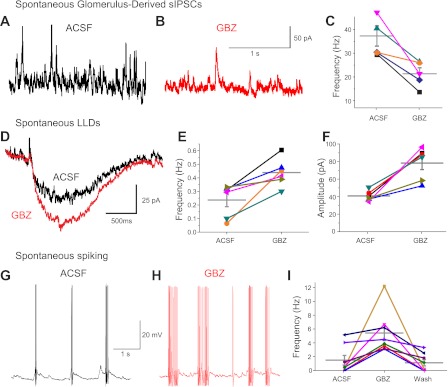
ETCs spontaneously generate rhythmic, spike bursting, which provides recurring bursts of monosynaptic excitation of PG cells (Hayar et al. 2004a; Shao et al. 2009). This causes intraglomerular GABA release, which activates GABAB receptors on ON terminals to produce tonic presynaptic inhibition of sensory input (Shao et al. 2009; Aroniadou-Anderjaska et al. 2000; Pirez and Wachowiak 2008). Thus, we reasoned that ETC-driven GABA release from PG cells should produce tonic postsynaptic inhibition of MCs. To test this, spontaneous IPSCs were recorded in MCs before and after gabazine was puffed into the glomerulus containing its apical dendrite. Intraglomerular microinjection of gabazine reduced the frequency of spontaneous IPSCs by 42% (38.0 ± 8.6 Hz in control vs. 22.3 ± 5.8 Hz with gabazine puff, n = 5, P < 0.05 by t-test; Fig. 7, A–C) and the amplitude by 23% from 24.0 ± 2.8 to 18.5 ± 1.5 pA (n = 5, P < 0.05 by t-test). The decrease in IPSC amplitudes may reflect larger average amplitudes of PG- versus GC-mediated IPSCs in MCs. Upon washout, frequency returned to 97 ± 19% and amplitude returned to 92 ± 12% of control. Thus, in slices, half of all spontaneous IPSCs in MCs are of glomerular origin. This is consistent with spontaneous IPSCs in MCs reduced by 54% in slices with the glomerular layer cut away (Dong et al. 2007).
Fig. 7.
Tonic intraglomerular inhibition regulates spontaneous MC activity. A–C: glomerulus-derived spontaneous MC IPSCs. A: spontaneous (s)IPSCs in aCSF. B: glomerular injection of 100 μM GBZ reduced MC sIPSCs (Vh = −10 mV). C: plot of sIPSC frequency (in Hz) of individual MCs in aCSF and GBZ. The horizontal gray bars show population means. D–F: spontaneous MC LLDs. D: spontaneous LLD in aCSF (black trace, average of 21 spontaneous LLDs aligned to 50% rise time) and after intraglomerular injection of GBZ (red trace, average of 32 spontaneous LLDs aligned to 50% rise time). E and F: plot of spontaneous LLD frequency (in Hz) and amplitude in aCSF and GBZ. G–I: glomerular injection of GBZ increased spontaneous action potentials. G: spontaneous action potentials in aCSF. H: intraglomerular injection of GBZ increased the number of MC action potentials. I: plot of individual MC spike frequency in aCSF, in GBZ, and after the washout of GBZ. The gray horizontal bars show population means.
Tonic intraglomerular inhibition should regulate MC excitability. If true, then blocking it should increase spontaneous LLDs. Indeed, intraglomerular microinjection of gabazine increased both the amplitude and frequency of spontaneous LLDs (amplitude: 40.8 ± 2.3 pA in control vs. 78.2 ± 7.4 pA with gabazine puff, P < 0.01 by t-test, and frequency: 0.23 ± 0.05 Hz in control vs. 0.44 ± 0.04 Hz with gabazine, P < 0.05 by t-test, n = 6; Fig. 7, D–F), but there was no effect on duration (1.82 ± 0.12 s in control vs. 1.83 ± 0.13 s with gabazine puff). This suggests that duration may be governed by excitatory mechanisms such as the long-lasting NMDA currents present in MCs (Ennis et al. 1996). The increased amplitude and frequency of spontaneous LLDs caused by intraglomerular GABAA receptors should increase spontaneous spike generation in MCs. Intraglomerular gabazine puff caused an approximately threefold increase in spontaneous spiking (1.6 ± 0.6 Hz in control vs. 4.6 ± 1.1 Hz with gabazine, washout: 1.4 ± 0.6 Hz, P < 0.05 by t-test, n = 10; Fig. 7, G–I). ETC spontaneous spike bursts are generated by intrinsic conductances (Liu and Shipley 2008). Block of all intraglomerular fast synaptic transmission had no impact on ETC bursts per second, but selective block of GABAA receptors caused an ∼20% increase in spikes per burst (Hayar and Ennis 2007). Thus, part of the increased MC excitability after intraglomerular gabazine puff may be due to increased spontaneous ETC drive on MCs in addition to the ∼50% reduction in postsynaptic inhibition. These results show that intraglomerular circuits exert tonic inhibition that attenuates LLDs and strongly limits spontaneous spiking in MCs.
DISCUSSION
In the neocortex, excitatory “principle” neurons outnumber inhibitory interneurons by ∼4–5:1. In the olfactory bulb, this is dramatically reversed, with 5–10:1 inhibitory to principle neurons (Parrish-Aungst et al. 2007). Olfactory bulb inhibitory neurons comprise two major classes: 1) GCs form serial and reciprocal synapses with the lateral dendrites of the principle output neurons, MCs, and 2) glomerular interneurons form synapses with the apical dendrites of MCs and with a glomerular excitatory neuron, the ETC. While much is known about the functional organization of the MC-GC-MC circuit, our knowledge of inhibition generated by glomerular circuits is limited.
Glomerular circuits transform sensory input into output signals transmitted by MCs to downstream olfactory networks, including the MC-GC-MC circuit. Sensory inputs monosynaptically excite MCs and ETCs, and ETCs provide parallel feedforward excitation that amplifies sensory drive on and promotes synchronous activation of MCs (Hayar et al. 2004b; De Saint Jan et al. 2009; Gire and Schoppa 2009; Najac et al. 2011; Gire et al. 2012). There are two types of glomerular inhibitory neurons: 1) GABAergic PG cells, which innervate a single glomerulus; and 2) dopaminergic/GABAergic short axon cells, which innervate multiple glomeruli. As short axon cells comprise only ∼10% of glomerular interneurons (Aungst et al. 2003; Kiyokage et al. 2010; Parrish-Aungst et al. 2007), intraglomerular inhibition derives mainly from PG cells. The present experiments in slices demonstrated that in addition to exciting MCs and ETCs, perithreshold input activates intraglomerular inhibitory circuits. These circuits provide fast feedforward and feedback GABAergic inhibition of MCs and ETCs. Spontaneous activity in these intraglomerular circuits generates tonic inhibition of MCs.
Intraglomerular inhibition regulates MC and ETC excitability.
Sensory input generates LLD in MCs but only a brief burst of action potentials during the early phase of the LLD, suggesting that spiking is curtailed by inhibition. Indeed, ON-evoked excitatory currents are followed by inhibition with two distinct temporal phases: an early, summating barrage of IPSCs and later intermittent IPSCs. Intraglomerular block of GABAA receptors selectively and completely abolishes the early inhibition. Selective suppression of GABA release from GCs reduces late but not early IPSCs. Thus, intraglomerular circuits provide rapid inhibition of MCs.
Intraglomerular inhibition potently controls MC and ETC responses to ON input. When this inhibition is selectively blocked by restricted intraglomerular puffs of a GABAA receptor antagonist, the MC spike response to ON stimulation increased ∼6-fold, but the most pronounced effect was a nearly 10-fold increase in the duration of the spike response. In addition, glomerular GABAA antagonists nearly doubled ON-evoked ETC spiking. Thus, intraglomerular inhibition exerts a strong brake upon sensory activation of MCs and ETCs. Because ETCs provide feedforward excitation of MCs, intraglomerular inhibition of ETCs should impact feedforward excitatory generation of LLDs in MCs. Indeed, intraglomerular block of GABAA receptors significantly increased LLD currents, consistent with the generation and regulation of LLDs by intraglomerular circuits (Carlson et al. 2000; Schoppa and Westbrook 2001; Gire and Schoppa 2008; Urban and Sakmann, 2002). Thus, by both reducing ETC feedforward excitation and increasing postsynaptic inhibition of MCs, intraglomerular inhibition potently shapes the strength and temporal structure of glomerular output responses to sensory input.
Intraglomerular circuits also generate tonic inhibition of MCs. This derives from spontaneous busting of ETCs, which monosynaptically excite two-thirds of all PG cells, causing tonic GABA release (Hayar et al. 2004; Shao et al. 2009). Spontaneous activity of MCs and other classes of tufted cells may also contribute excitatory drive PG cells to tonically release GABA. Tonic intraglomerular GABA release produces presynaptic inhibition that reduces ON transmitter release by ∼30% in slices (Shao et al. 2009) and intact animals (Pirez and Wachowiak 2008). Thus tonic presynaptic inhibition can regulate the gain of sensory input. Sustained intraglomerular GABA release also activates GABAA receptors on MCs to cause tonic postsynaptic inhibition. This limits spontaneous spiking and may regulate the threshold for sensory-evoked spike generation. Together, tonic pre- and postsynaptic intraglomerular inhibition regulates the gain and threshold of the glomerulus input-output function.
Two-thirds of PG cells are most efficiently activated by the ON→ET→PG circuit (Shao et al. 2009). The latency and synaptic jitter of ON-evoked intraglomerular IPSCs in MCs and ETCs is consistent with generation by this plurisynaptic circuit. Moreover, this circuit generates bursts of PG cell output consistent with the compound ON-evoked IPSCs observed in MCs and ETCs. A contribution from the ON→PG circuit cannot be ruled out based on latency data alone, however, and the predicted rapid feedforward IPSC from this circuit might superimpose with the ON-evoked EPSC and be difficult to identify with our approaches.
In addition to regulating magnitude and temporal structure of MC spiking, intraglomerular inhibition may suppress MC responses to weak sensory input. ETCs are activated by weaker ON inputs than MCs (Gire and Schoppa 2009; De Saint Jan et al. 2009; Najac et al. 2011; Gire et al. 2012). Weak sensory activation of ETCs would excite PG cells and increase GABA release above tonic levels. This should increase postsynaptic inhibition of MCs and reduce their sensitivity to weak sensory signals. Thus, intraglomerular circuits could provide fast, feedforward inhibition that could enhance contrast between glomeruli receiving weak and strong sensory signals, a mechanism that would not require spatially ordered chemotopic input to different glomeruli (Cleland and Sethupathy 2006; Cleland 2010).
The MC-GC-MC circuit mediates recurrent and lateral inhibition of MCs and deep tufted cells. There is evidence that GC inputs regularize MC spike timing (David et al. 2008; Giridhar et al. 2011; Schoppa 2006). MCs project heavily to the olfactory cortex, which sends strong feedback excitation to GCs (Balu et al. 2007; Strowbridge 2009). These feedback synapses support paired-pulse facilitation and spike-dependent plasticity in MCs (Balu et al. 2007; Gao and Strowbridge 2009). This cortical feedback might enhance the effectiveness of inhibition of MCs during repetitive activation when odors are sampled by sniffing or play a role in olfactory learning (Doucette et al. 2011).
Glomerulus input-output function.
Each glomerulus receives signals from olfactory sensory neurons that express the same odorant receptor; different glomeruli receive input from olfactory sensory neurons sensitive to different odorants. Thus, odor quality is determined, in part at least, by a combinatorial code computed across spatial and temporal patterns of glomerular activity.
The glomerulus input-output function must ensure that sensory signals are processed such that downstream circuits can decode information across glomeruli and across time. In mammals, odors are sampled by repetitive sniffing. MCs faithfully encode sensory signals across the dynamic range of active sniffing up to 10 Hz (Carey and Wachowiak, 2011; Smear et al. 2011). Interestingly, while the rise time of the MC spike response envelope to odor stimulation is longer than the rise time after synchronous electrical stimulation in slices, the decay times of spiking responses are surprisingly similar (∼100 ms) in vivo and in vitro. In vivo, as sniff frequencies increase, MC response rise and decay times are shortened (Carey and Wachowiak 2011). This sharpens temporal response patterns to maintain fidelity with the time-varying input.
Intraglomerular excitatory and inhibitory circuits are well suited to regulate the temporal patterning of MC responses. Spontaneous activity in the ET→PG circuit generates tonic inhibition of MCs, limiting spike generation to background odorants. Weak sensory input should increase tonic postsynaptic inhibition of MCs and raise the threshold for spike initiation. Stronger sensory input produces monosynaptic excitation of MCs augmented by excitatory spike bursts from the ON→ET→MC circuit. This generates a LLD that overcomes tonic inhibition and drives the MC to threshold for action potentials. Excitation is rapidly and potently opposed by inhibition generated by intraglomerular circuits. The time interval between sensory-evoked excitation and intraglomerular inhibition creates a brief window for action potential generation in MCs. The relative strengths of excitation and inhibition, and the timing of these excitatory-inhibitory sequences, determine how MC spiking is gated. Thus, fast feedforward intraglomerular inhibition may be important for gating MC spike output, although more difficult odor discriminations involving longer time courses in vivo may be impacted by late IPSCs from GCs (Abraham et al. 2010).
When ON input frequency increases, as at higher sniffing rates, more ETCs are entrained (Hayar et al. 2004b; Wachowiak and Shipley 2006), increasing synchronous excitatory drive to MCs. This should reduce the MC response rise time. ETC entrainment also increases excitatory drive on the ET→PG→MC circuit, resulting in strong, summating postsynaptic inhibition, which shortens the decay time of MC spike responses. Indeed, when we blocked intraglomerular inhibition, the spiking decay in MCs was ∼10 times longer, indicating that intraglomerular inhibition normally strongly limits the duration of the MC spike response. Thus, the net effect of increased input frequency may be to preserve MC response patterns across changes in sniff frequency by reducing both response rise and decay times. Indeed, a recent in vivo study has shown that increased sniff frequencies were associated with decreased rise and decay times in MC spike responses (Carey and Wachowiak 2011). Such changes in the temporal structure of the MC response may impact processing of olfactory information by downstream olfactory circuits. Cury and Uchida (2010) reported that substantial odor encoding occurs in the first ∼100–150 ms of the sniff cycle. The frequency-dependent response window generated by intraglomerular circuits may constrain MCs to encode odor signals early in each sniff cycle.
Although intraglomerular inhibition reduces LLD amplitude, the LLD persists for up to a second. However, spike responses do not summate across sniff cycles in vivo (Carey and Wachowiak 2011). Why don't excitatory inputs summate with previous LLDs to increase MC responses to subsequent sniffs? One possibility is that MC spike output engages cortical feedback excitation of GCs to inhibit MCs and reduce their sensitivity until the next sniff. Cortical feedback inhibition proportional to MC output strength could operate conjointly with intraglomerular and recurrent GC inhibition to maintain optimal sensitivity to sensory input across changes in sniff frequency and odorant concentration.
GRANTS
This work was supported by National Institutes of Health Grants DCCD-005676 and DCCD-19015.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S. and S.L. performed experiments; Z.S., S.L., and M.T.S. analyzed data; Z.S., A.C.P., S.L., and M.T.S. interpreted results of experiments; Z.S., S.L., and M.T.S. prepared figures; Z.S. and M.T.S. drafted manuscript; A.C.P. and M.T.S. conception and design of research; A.C.P. and M.T.S. edited and revised manuscript; A.C.P. and M.T.S. approved final version of manuscript.
REFERENCES
- Abraham NM, Egger V, Shimshek DR, Renden R, Fukunaga I, Sprengel R, Seeburg PH, Klugmann M, Margrie TW, Schaefer AT, Kuner T. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron 11: 399–411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M, Eyre M, Finklea B, Nusser Z. External tufted cells in the main olfactory bulb form two distinct subpopulations. Eur J Neurosci 24: 1124–1136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Dendrodendritic recurrent excitation in mitral cells of the rat olfactory bulb. J Neurophysiol 82: 489–494, 1999 [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic, and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABAB heteroreceptors. J Neurophysiol 84: 1194–1203, 2000 [DOI] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003 [DOI] [PubMed] [Google Scholar]
- Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci 27: 5621–5632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20: 2011–2021, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Wachowiak M. Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci 31: 10615–10626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron 25: 625–633, 2000 [DOI] [PubMed] [Google Scholar]
- Cleland TA. Early transformations in odor representation. Trends Neurosci 33: 130–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci 7: 7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron 68: 570–585, 2010 [DOI] [PubMed] [Google Scholar]
- David F, Linster C, Cleland TA. Lateral dendritic shunt inhibition can regularize mitral cell spike patterning. J Comput Neurosci 25: 25–38, 2008 [DOI] [PubMed] [Google Scholar]
- De Saint Jan JD, Hirnet D, Westbrook GL, Charpak S. External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29: 2043–2052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Hayar A, Ennis M. Activation of group I metabotropic glutamate receptors on main olfactory bulb granule cells and periglomerular cells enhances synaptic inhibition of mitral cells. J Neurosci 27: 5654–5663, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron 69: 1176–1187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zimmer LA, Shipley MT. Olfactory nerve stimulation activates rat mitral cells via NMDA and non-NMDA receptors in vitro. Neuroreport 7: 989–992, 1996 [DOI] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci 12: 731–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Long-term enhancement of synchronized oscillations by adrenergic receptor activation in the olfactory bulb. J Neurophysiol 99: 2021–2025, 2008 [DOI] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29: 13454–13464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R, Schoppa NE. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J Neurosci 32:2964–2975, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giridhar S, Doiron B, Urban NN. Timescale-dependent shaping of correlation by olfactory bulb lateral inhibition. Proc Natl Acad Sci USA 108: 5843–5848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of α1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol 86: 2173–2182, 2001 [DOI] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24: 6676–6685, 2004a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency, and are entrained by patterned olfactory input. J Neurosci 24: 1190–1199, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward P, Ennis M, Keller A, Shipley MT. Membrane bistability in olfactory bulb mitral cells. J Neurosci 21: 5311–5320, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnup SV, Hayar A, Shipley MT, Kurnikova MG. Spontaneous field potentials in the glomeruli of the olfactory bulb: the leading role of juxtaglomerular cells. Neuroscience 142: 203–221, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. J Neurosci 30: 1185–1196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron 23: 377–384, 1999 [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca2+-activated K+ channels. Neuron 31: 1027–1034, 2001 [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron 20: 749–761, 1998 [DOI] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Intrinsic conductances actively shape excitatory, and inhibitory postsynaptic responses in olfactory bulb external tufted cells. J Neurosci 28: 10311–10322, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Schneider SP. Laminar organization of mitral, and tufted cells in the main olfactory bulb of the adult hamster. J Comp Neurol 208: 419–430, 1982 [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci 8: 354–364, 2005 [DOI] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P, Charpak S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31: 8722–8729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol 501: 825–836, 2007 [DOI] [PubMed] [Google Scholar]
- Phillips CG, Powell TP, Shepherd GM. Responses of mitral cells to stimulation of the lateral olfactory tract in the rabbit. J Physiol 168: 65–88, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuron types of the glomerular layer of the olfactory bulb. J Cell Sci 9: 305–345, 1971a [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci 9: 347–377, 1971b [DOI] [PubMed] [Google Scholar]
- Pirez N, Wachowiak M. In vivo modulation of sensory input to the olfactory bulb by tonic, and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci 28: 6360–6371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral, and short axon cells of the olfactory bulb. J Cell Sci 7:631–651, 1970a [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP. The synaptology of the granule cells of the olfactory bulb. J Cell Sci 7: 125–155, 1970b [DOI] [PubMed] [Google Scholar]
- Schoppa NE. AMPA/kainate receptors drive rapid output and precise synchrony in olfactory bulb granule cells. J Neurosci 26: 12996–3006, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci 18: 6790–6802, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron 31: 639–651, 2001 [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic, and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101: 1988–2001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. Responses of mitral cells to olfactory nerve volleys in the rabbit. J Physiol 168: 89–100, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley CH, Coddington EJ, Heyward PM. All-or-none population bursts temporally constrain surround inhibition between mouse olfactory glomeruli. Brain Res Bull 201081: 406–415, 2010 [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O'Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature 479: 397–400, 2011 [DOI] [PubMed] [Google Scholar]
- Strowbridge BW. Role of cortical feedback in regulating inhibitory microcircuits. Ann NY Acad Sci 1170: 270–4, 2009 [DOI] [PubMed] [Google Scholar]
- Urban NN, Sakmann B. Reciprocal intraglomerular excitation, and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol 542: 355–367, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol 17: 411–423, 2006 [DOI] [PubMed] [Google Scholar]