Abstract
Inhibitory interneurons play a critical role in the generation of gamma (20–50 Hz) oscillations, either by forming mutually inhibitory networks or as part of recurrent networks with pyramidal cells. A key property of fast spiking interneurons is their ability to generate brief spikes and high-frequency spike trains with little accommodation. However, the role of their firing properties in network oscillations has not been tested in vivo. Studies in hippocampus in vitro have shown that high-frequency spike doublets in interneurons play a key role in the long-range synchronization of gamma oscillations with little phase lag despite long axonal conduction delays. We generated a knockout (KO) mouse lacking Kv3.2 potassium channel subunits, where infragranular inhibitory interneurons lose the ability both to sustain high-frequency firing and reliably generate high-frequency spike doublets. We recorded cortical local field potentials in anesthetized and awake, restrained mice. Spontaneous activity of the KO and the wild-type (WT) showed similar content of gamma and slow (0.1–15 Hz) frequencies, but the KO showed a significantly larger decay of synchronization of gamma oscillations with distance. Coronal cuts in the cortex of WT mice decreased synchronization to values similar to the intact KO. The synchronization of the slow oscillation showed little decay with distance in both mice and was largely reduced after coronal cuts. Our results show that the firing properties of inhibitory interneurons are critical for long-range synchronization of gamma oscillations, and emphasize that intrinsic electrophysiological properties of single cells may play a key role in the spatiotemporal characteristics of network activity.
Keywords: fast spiking interneurons, in vivo, spike doublets, parvalbumin, infragranular
the synchronization of gamma oscillations (20–80 Hz) has been proposed as a key element underlying information representation in nervous systems (Fries 2009; Laurent and Davidowitz 1994; Llinas et al. 1998; Llinas and Ribary 1998; Murthy and Fetz 1992; Perez-Orive et al. 2002; Ribary et al. 1991; Singer 1999; Vinck et al. 2010; Wehr and Laurent 1996; Womelsdorf et al. 2007). Gamma oscillations in the mammalian brain also appear spontaneously as part of the background activity during states associated with cell depolarization such as waking, rapid-eye movement sleep (Llinas and Ribary 1993), and in intracellular recordings during the depolarizing phases of the slow oscillation, which characterize sleep and deep anesthesia (Steriade et al. 1996).
Gamma oscillations in local cortical networks depend critically on the time constant of the GABAA IPSP (Volman et al. 2011; Wang and Buzsáki 1996; Whittington et al. 1995), and synchronization is provided by local axonal divergence, which has been recently shown to be massive and nonspecific within local populations both for parvalbumin- (Packer and Yuste 2011) and somatostatin- (Fino and Yuste 2011) positive interneurons. Less is known about the mechanisms of synchronization across neuronal populations, particularly because the conduction velocity of pyramidal cell axons imposes long time delays on the interaction between populations. Computer modeling (Bibbig et al. 2002; Traub et al. 1996b) and experimental (Whittington et al. 1995) results from hippocampal networks showed that long-range synchronization of gamma oscillations can be achieved by introducing a compensatory delay in the local population when inhibitory interneurons fire an extra spike (spike doublet) at each cycle of the oscillation. We have shown that the ability to reliably generate high-frequency spike doublets is impaired in a subpopulation of GABAergic interneurons in infragranular cortical layers of knockout (KO) mice lacking Kv3.2 potassium channel subunits (Lau et al. 2000). Voltage-gated K+ channels of the Kv3 subfamily are activated at very depolarized membrane potentials (positive to −20 mV), have very fast deactivation rates, and are, therefore, critical for the fast spiking phenotype of cortical interneurons (Erisir et al. 1999; Rudy et al. 1999; Rudy and McBain 2001). In addition a subpopulation, ∼30%, of infragranular somatostatin-positive interneurons also express Kv3.2 channels (Chow et al. 1999), although their role in the electrophysiological properties of these interneurons is not known. Here we show that Kv3.2 KO mice show significantly reduced synchronization of gamma oscillations over distance compared with their wild-type (WT) littermates. Our results suggest that the electrophysiological phenotype of fast spiking neurons is critical for the spatiotemporal pattern of gamma oscillations in neocortex.
MATERIALS AND METHODS
Local field potential recordings in anesthetized mice.
All experiments were approved by the University of Pennsylvania Animal Care and Use Committee. Mice were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (20 mg/kg). Depth of anesthesia was determined by heart rate and continuous monitoring of the local field potential (LFP) for high-amplitude low-frequency waves and maintained with supplemental doses of the same anesthetic. Mice were placed into a rodent stereotaxic apparatus (David Kopf, Tujunga, CA), and a craniotomy was performed over the right parietal cortex. Bipolar electrodes were fashioned from two tungsten electrodes (∼1 MΩ; FHC, Bowdoinham, MN) affixed with dental cement with tips separated vertically by 0.8 mm and horizontally by ∼100 μm. Four bipolar electrodes with constant interelectrode distance of 0.7 mm were lowered perpendicular to the surface and along the anterior posterior axis of the cortex (bregma: −0.1 to −2.2; Lat: 3.5; see Fig. 4) under visual inspection with a microscope so that the superficial electrode rested in the pial surface, and consequently the deep electrode was located in the infragranular layers. LFP recordings were then made in bipolar configuration (surface-depth) with the polarity adjusted to reflect that of the depth, i.e., neuronal depolarization corresponds to negative waves. Signals were filtered and amplified with an AC amplifier (FHC, Bowdoinham, MA) with a headstage and capacity compensation. Electrical stimulation was delivered through a low-impedance bipolar electrode with an isolated pulse stimulator (A-M Systems). Coronal cuts were made with a scalpel blade held by a coarse Kopf stereotaxic tower. Data were sampled at 1 kHz with an InstruNet (GW systems) AD card and analyzed with Igor (Wavemetrics) with customized routines.
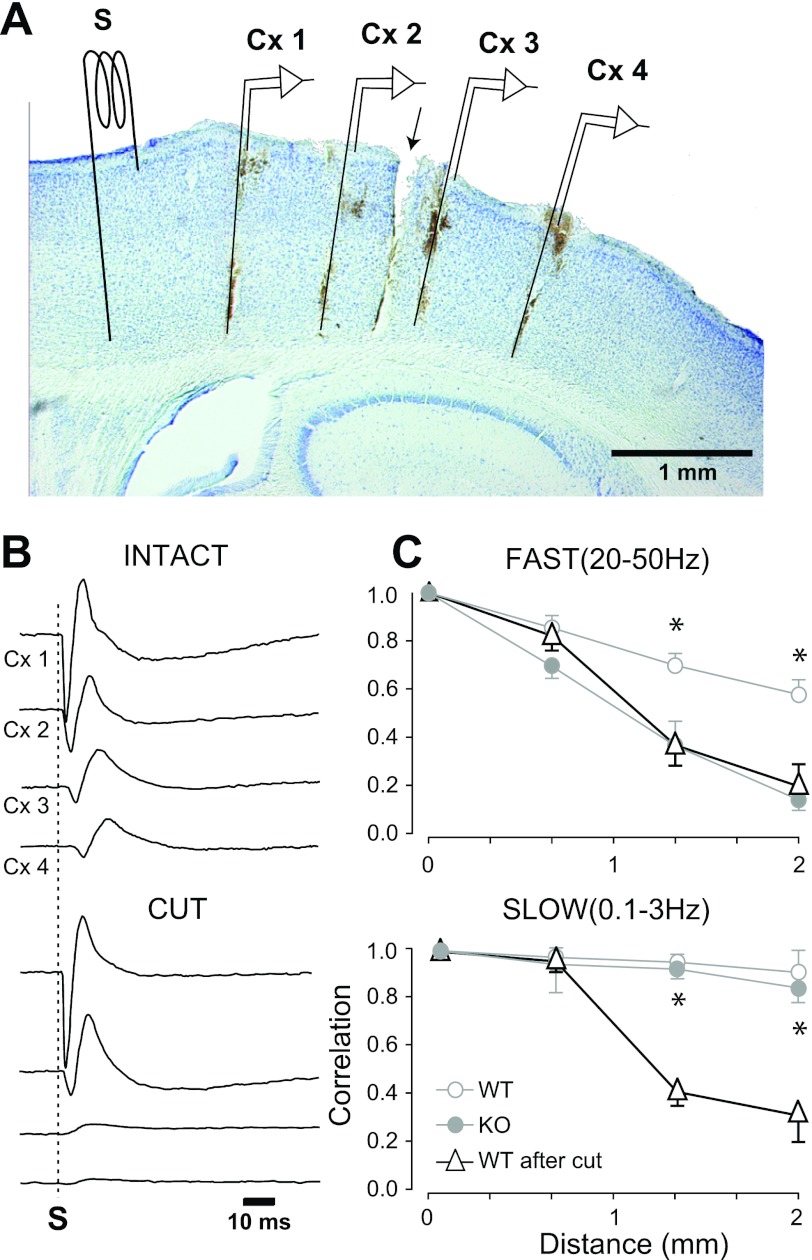
Fig. 4.
Experimental arrangement of recording and stimulating electrodes and the effects of coronal cuts. A: Nissl-stained sagittal section showing vertical tracts left by the bipolar recording electrodes and indicated by the details labeled Cx1-Cx4. Position of the stimulating electrode (S), anterior to Cx1, is indicated. Because it was not in the same plane as the recording electrodes, the tract is not visible. B: average (n = 10) evoked potentials from the same mouse in the control (INTACT) situation showing absence of response across the cut in Cx3 and Cx4 (CUT). C: plots of the average from all animals of the peak crosscorrelation after the cut (dark lines and triangles) for fast (top plot) and slow (bottom plot) activity. For comparison, the values of the WT and KO (from Fig. 3) are plotted in light gray. *Significant differences (t-test) in WT mice before and after transcortical cuts.
LFP recordings in the non-anesthetized restrained mice.
An initial surgery under deep barbiturate anesthesia, and in the stereotaxic position, was initially performed to install a head mount made of dental cement with a center opening large enough for the bipolar electrode array. Embedded in the head mount were bolts that allowed posterior positioning of the animals in the stereotaxic apparatus without pain or pressure. Two silver-ball electrodes were fixed into the supraorbital cavity with dental cement to record the electrooculogram (EOG). Two Teflon-insulated wires were inserted in the neck muscles to monitor electromyographic (EMG) activity. The coarse electroencephalogram (EEG) was recorded with two stainless steel screws anchored to the bone overlying the right frontal and parietotemporal regions; these screws were also critical for the anchoring of the dental cement. Bicillin (im once a day for 3 days) and buprenorphine (0.03 mg/kg im every 12 h for 24 h, but animals were checked every 8 h) were administered postoperatively. The animals were allowed to recover for 7–10 days. Posteriorly, animals were slowly habituated to be in the stereotaxic apparatus with the head fixed and the body comfortably restrained inside a cotton bag over a heating pad. In between experimental sessions, the animals were allowed to sleep, eat, and drink ad libitum. During the recording sessions, the EEG, EOG, and EMG signals were used to distinguish behavioral states of vigilance. Each mouse was recorded for 3 or 4 sessions of ∼1 h each and then euthanized with a lethal injection of pentobarbital.
Data analysis.
We calculated the power spectrum from consecutive 2-min windows from each unfiltered bipolar LFP. From each power spectrum, we obtained the total power as the area under the curve between 0.1 and 15 Hz (low frequencies) and between 20 and 50 Hz (high frequencies).
Auto and crosscorrelation analysis was performed on 0.5-s epochs of gamma-rich LFP, after digitally filtering the LFP between 20 and 50 Hz using custom made routines in IGOR (Wavemetrics). To measure the effect of distance on correlation, we used the peak of the pairwise crosscorrelogram between progressively increasing distant pairs of bipolar electrodes.
From the electrically evoked responses, we measured the latency to onset as the time between stimulus and the departure from baseline of the averaged (n = 20 repetitions) evoked response.
For statistical comparisons of the four means, we used Student's t-tests (cx1–2, cx1–3, cx1–4 between WT and KO, or in the WT across the coronal cut). In addition, in the case of the coronal cut, we also compared cx3–4 on the other side of the cut with cx1–2. Given the simplicity of the statistical comparison, we did not use ANOVA.
Histology.
At the end of the experiments, animals were perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer and stored overnight in the same solution. Sections 100-μm thick were cut and stained with Nissl to reveal electrode tracks and the coronal cuts.
RESULTS
Spontaneous activity patterns and their frequency content.
To compare the spatiotemporal properties of gamma oscillations between WT and KO mice, we applied correlation analysis to spontaneous LFPs recorded from the parietal cortex of ketamine-xylazine anesthetized (n = 24, 12 WT and 12 KO), as well as from non-anesthetized, awake and restrained (n = 8, 4 WT and 4 KO) mice. Cortical LFPs were recorded with arrays of four equidistant bipolar electrodes with a distance of 2 mm in the anterior-posterior plane and inserted under visual inspection to assure the correct placement of the superficial electrode on the pial surface (see materials and methods). Bipolar recordings minimize contamination by volume conduction from neighboring cortical areas, and, most importantly, from the hippocampus, which lies immediately underneath and generates powerful spontaneous gamma oscillations (Penttonen et al. 1998; Soltesz and Deschenes 1993; Traub et al. 1996a). However, bipolar recordings represent the average activity mainly restricted to the vertical column of cortex between the surface and the depth electrodes and therefore do not localize the activity recorded to any particular layer.
Under anesthesia, the spontaneous cortical LFP from both mouse genotypes (Fig. 1A) showed a slow oscillation (0.8–0.9 Hz) similar to that described previously in cats (Amzica and Steriade 1995; Contreras and Steriade 1995; Steriade et al. 1993b) and humans during slow wave sleep (Achermann and Borbely 1997; Molle et al. 2002; Steriade et al. 1993b). Each cycle of the oscillation, as recorded in the LFP, consisted of a high-amplitude negative phase (polarity set to match that of the depth electrode) followed by long-lasting (0.2–1 s) positive phase. The negative phases of the oscillation reflect cell depolarization (also called upstates) and are crowned by smaller-amplitude gamma oscillations (Contreras and Steriade 1995; Steriade and Amzica 1996). Gamma oscillations do not occur during the positive phase of the oscillation (also called downstates), which reflect cell hyperpolarization (Contreras and Steriade 1995; Sanchez-Vives and McCormick 2000; Steriade et al. 1993b).
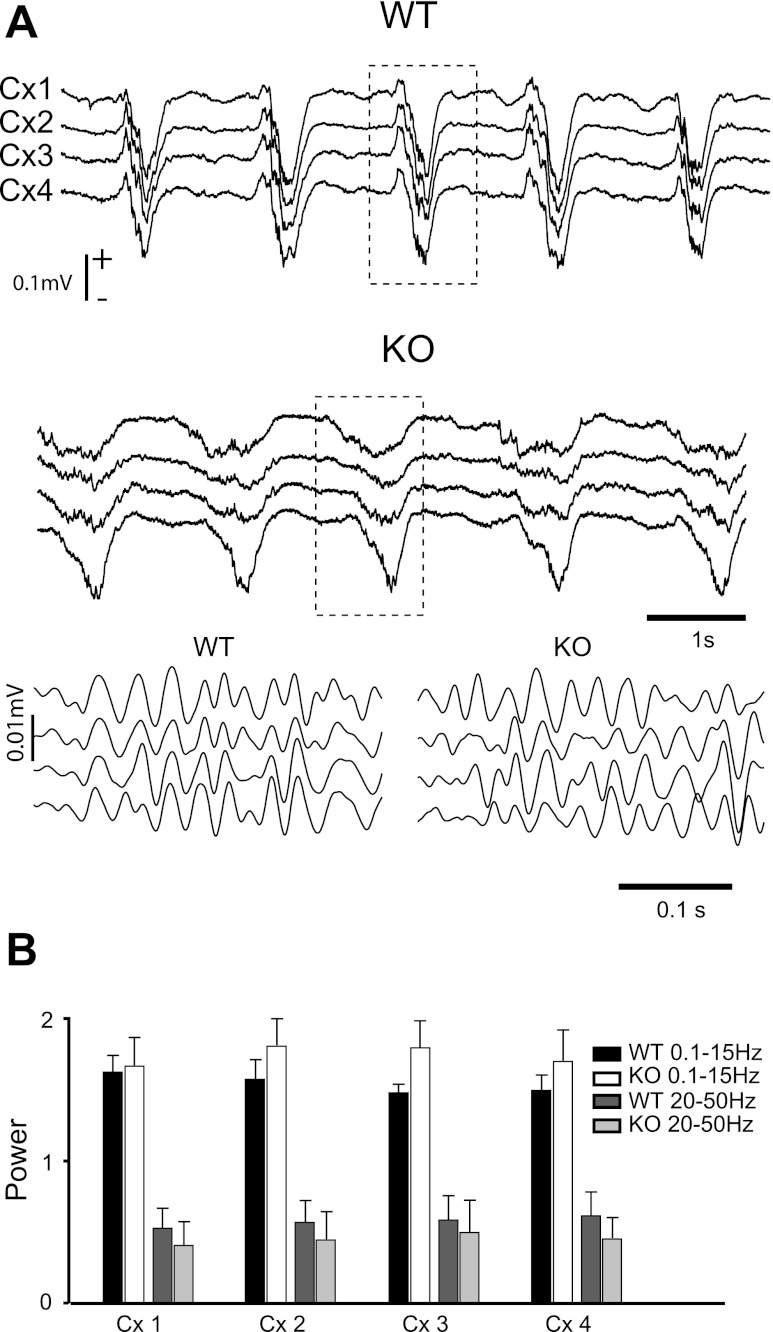
Fig. 1.
Wild-type (WT) and Kv3.2 knockout (KO) mice under ketamine-xylazine anesthesia have similar amounts of fast (20–50 Hz) and slow (0.1–15 Hz) oscillations in the spontaneous cortical local field potentials (LFP). A: LFPs (Cx1-Cx4) show spontaneous slow oscillation with a mean frequency of 1.3 Hz. Gamma oscillations crowned the negative phase of the LFPs as shown by the filtered (20–50 Hz) traces (details 1 and 2, amplified from the sections indicated by dotted boxes). B: histograms represent total power between 0.1 and 15 Hz and between 20 and 50 Hz calculated from 2-min periods of stable spontaneous LFPs (n = 5 epochs, n = 10 mice; 5 KO and 5 WT).
To verify that artifacts were not introduced in the correlation analysis by large differences in the spectral composition of the spontaneous LFPs between the two mouse types or among the different electrodes, we calculated the power spectrum over 2-min epochs (n = 5) of unfiltered data (n = 10 mice, 5 KO and 5 WT) and measured the total power (area under the curve) between 0.1 and 15 Hz, which includes the slow and spindle oscillations, and between 20 and 50 Hz, which is the range of gamma oscillations that we used for analysis. The total power in both frequency bands was not different (pairwise comparisons of means, Student's t-test, P > 0.05) among recording electrodes (Fig. 1B, Cx1-Cx4) or between KO and WT. Identical results were obtained in non-anesthetized animals.
The decay of correlation with distance.
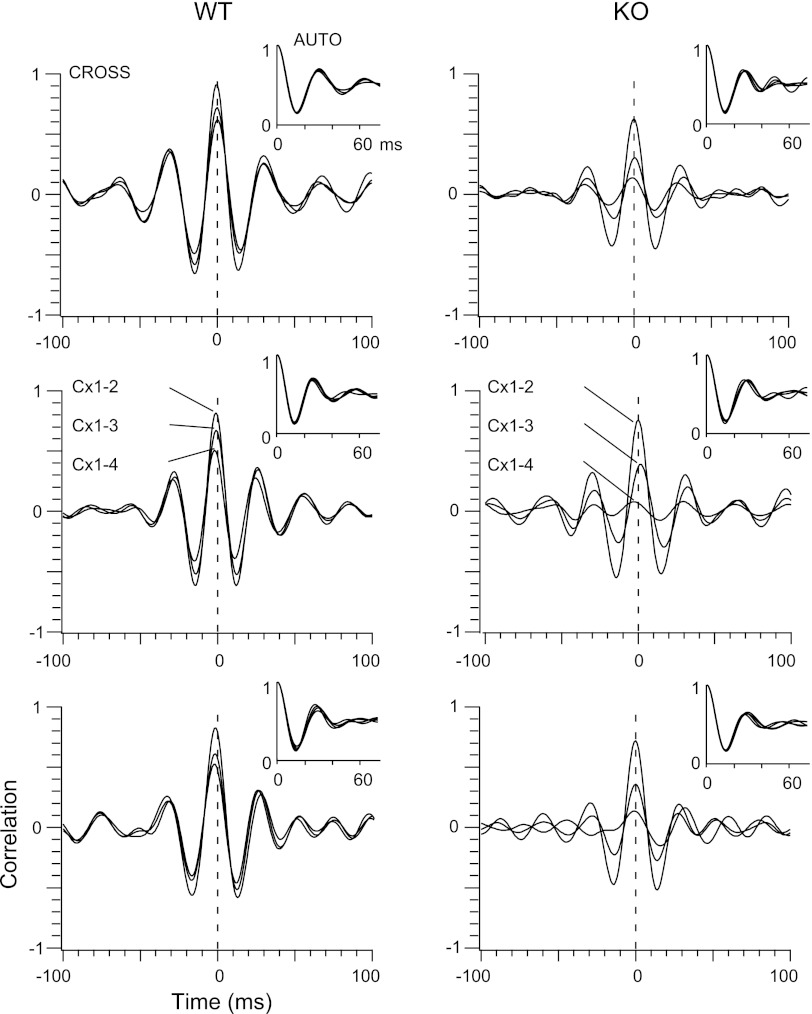
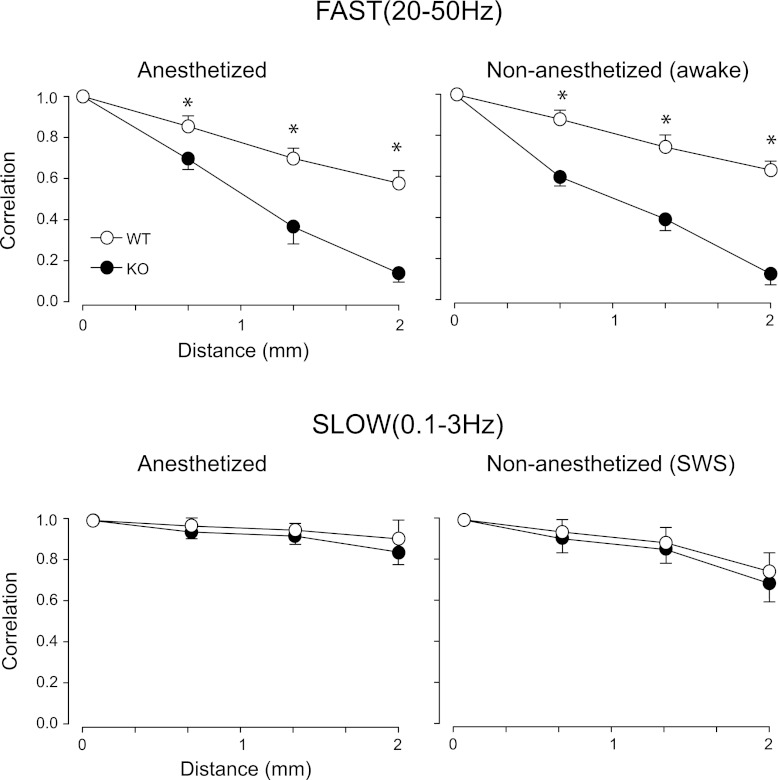
Since the amplitude of the gamma oscillations is small compared with the slow waves, we digitally filtered the LFP between 20 and 50 Hz (Fig. 1A) prior to performing correlation analysis. Visual inspection of the filtered data already revealed less coherence among electrodes in KO compared with WT (Fig. 1A). Since gamma oscillations appear only transiently during the negative phases (upstates) of the slow oscillations in the LFPs (Fig. 1A, see also Steriade et al. 1996), we selected for analysis epochs of 0.5-s duration (n = 20 per mouse) that showed robust gamma oscillations in all four electrodes as determined by spectral and autocorrelation analysis (Fig. 2). For each epoch, we calculated the crosscorrelograms between Cx1 and each of the other three electrodes at progressively increasing distances up to 2 mm. The auto and crosscorrelograms from all epochs (n = 20) in each animal were then averaged. Figure 2 shows the averaged correlograms from three anesthetized animals of each type. The averaged autocorrelograms (Fig. 2) from both mouse types were modulated at frequencies between 30 and 34 Hz. This is in agreement with the similarity in total spectral power within 20–50 Hz between KO and WT as reported above (Fig. 1B) and indicates that the mechanisms of generation and local synchronization of spontaneous gamma oscillations are not affected by changes in the electrophysiological properties of cortical GABAergic interneurons caused by the absence of the Kv3.2 channel. In contrast, crosscorrelation analysis (Fig. 2) showed a marked difference between KO and WT. We measured the amplitude at the peak of the crosscorrelograms between Cx1 and the three other electrodes (Fig. 2) and averaged the values for all mice of each type (Fig. 3). While in both mouse types the peak of the averaged crosscorrelograms decreased monotonically with increasing distance (Fig. 3), the decay was significantly larger in the KO, i.e., at all distances, the peak of the average crosscorrelogram was of significantly smaller amplitude in the KO as follows: in the anesthetized animals (n = 24, 12 WT and 12 KO; Fig. 3) the amplitude at the peak of the crosscorrelogram between Cx1 and Cx2 (∼0.7-mm distance) was 0.85 ± 0.03 (means ± SE) for the WT and 0.68 ± 0.05 for the KO (P < 0.05), and decreased to 0.55 ± 0.03 for the WT and 0.14 ± 0.04 for the KO between electrodes Cx1 and Cx4 (2-mm distance; Cx1–3, P < 0.005; Cx1–4, P < 0.0001). We repeated the correlation analysis in non-anesthetized animals (n = 8, 4 KO and 4 WT; Fig. 3) for periods of quiet wakefulness during which the animals had their eyes open and the cortical LFPs showed low-amplitude, high-frequency activity characteristic of the waking state. Average autocorrelograms (n = 20 epochs of 0.5 s per animal) were similar among electrodes and between WT and KO, and were modulated at a frequency of ∼30 Hz. The decrease in the crosscorrelation peak amplitude over distance in the non-anesthetized animals was comparable to the anesthetized, and was significantly larger in KO compared with the WT for Cx1–3 (WT = 0.74 ± 0.02; KO = 0.36 ± 0.03, P < 0.05) and Cx1–4 (WT = 0.61 ± 0.03; KO = 0.14 ± 0.04, P < 0.05).
Fig. 2.
Examples of correlation analysis of gamma oscillations in WT and KO mice. Shown are average autocorrelations (AUTO) and crosscorrelations (CROSS) from 3 examples of WT (left) and KO (right) mice. Autocorrelograms are 4 superimposed traces from each of the 4 electrodes. Crosscorrelograms are 3 superimposed traces corresponding to the relationship between the electrode Cx1 and each of the 3 other electrodes (Cx1–2, Cx1–3, and Cx1–4).
Fig. 3.
Crosscorrelation as a function of distance. The value at the peak of the crosscorrelograms calculated from LFP activity filtered for fast (top plots) and slow (bottom plots) frequency was average for all WT (open circles) and all KO (filled circles) mice. Left column shows the analysis for ketamine-xylazine-anesthetized mice, whereas right column shows analysis for non-anesthetized chronic mice. *Significant differences (t-test) between WT and KO mice.
Slow oscillations.
To rule out nonspecific, global deficits on neocortical synchronization in the KO, we tested whether slow oscillations, which are known to be synchronized over large cortical and thalamic territories (Amzica and Steriade 1995; Contreras and Steriade 1995; Steriade et al. 1993a), showed similar crosscorrelation values as a function of distance in KO and WT mice. The peak amplitude of the crosscorrelograms obtained from cortical LFPs filtered between 0.1 and 3 Hz (Fig. 3) decreased less over distance than those from gamma oscillations. More importantly, the decay of correlation of the slow oscillation was not different between WT and KO mice both in anesthetized (Fig. 3, WT = 0.97 ± 0.01 for Cx1-Cx2; 0.90 ± 0.03 for Cx1-Cx4; KO = 0.94 ± 0.01 for Cx1-Cx2; 0.83 ± 0.02 for Cx1-Cx4) and non-anesthetized animals (Fig. 3, WT = 0.94 ± 0.03 for Cx1-Cx2; 0.73 ± 0.05 for Cx1-Cx4; KO = 0.90 ± 0.02 for Cx1-Cx2; 0.70 ± 0.02 for Cx1-Cx4).
The role of intracortical connections.
We wanted to determine the contribution of rostrocaudal intracortical connections to the correlation values measured here. This is important because, although Kv3.2 channels are not present in excitatory cortical neurons, they are present in thalamocortical terminals (Moreno et al. 1995), and their absence could also affect synchronization relying on cortico-thalamocortical loops. In addition, corticocortical horizontal connections have been shown to play a critical role in long-range synchronization of fast and slow oscillations in cats (Destexhe et al. 1999; Steriade et al. 1996). We made deep coronal cuts in WT mice (n = 4) between electrodes Cx2 and Cx3. Cuts were 3–5 mm wide (mediolateral axis) and ∼1 mm deep, resulting in a complete cut from layers 1 through 6, but not of the underlying white matter (Fig. 4A). The peak amplitude of the average crosscorrelation across the cut (Cx1–3 and Cx1–4) decreased significantly with respect to the intact cortex (Cx1–3, P < 0.05; Cx1–4, P < 0.05). More importantly, the reduced peak values across the cut were almost identical to those of the intact KO (Fig. 4C). As expected, the decay in correlation with distance was similar on both sides of the cut (Cx1–2 vs. Cx3–4; P > 0.05). This result indicates that long-range synchronization of gamma oscillations strongly relies on corticocortical connections running within the gray matter and suggests that the synchronizing role of infragranular interneurons is ultimately exerted via these connections. To demonstrate that areas across the coronal cut were functionally disconnected, we placed a stimulating electrode ∼0.5 mm rostral to the array, and measured the amplitude of evoked potentials from all electrodes. Single electrical stimuli (100 μs, 0.2–0.5 mA) gave rise to a biphasic negative-positive potential. In the example illustrated in Fig. 4B, the evoked response had a latency to onset of 1.8 ms in Cx1 and 10.2 ms in Cx4, indicating a rostrocaudal propagation velocity of 238 mm/s in the intact animal (mean = 229 ± 19 mm/s; n = 4), which is compatible with measurements from slices in vitro (Contreras and Llinas 2001). As shown in Fig. 4B, the evoked potentials from the proximal side of the cut (Cx1 and Cx2) remained intact, while across the cut (Cx3 and Cx4) evoked potentials were virtually eliminated.
In agreement with studies in cats (Amzica and Steriade 1995), coronal cuts significantly decreased the correlation values across the cut compared with controls (Fig. 4, Cx1–3 = 0.38 ± 0.04, P < 0.005; Cx1–4 = 0.28 ± 0.09, P < 0.005). Again, as expected, the correlation between electrodes on the same side of the cut was similar (Cx1–2 and Cx3–4; P > 0.05).
DISCUSSION
Our results show that: 1) The presence of the Kv3.2 channel in cortical inhibitory interneurons is critical for the long-range synchronization of gamma oscillations. This conclusion is supported by the decrease (∼70%) in the synchronization of gamma oscillations within 2 mm in the KO compared with the WT mouse. 2) The presence of the Kv3.2 channel is not critical for the generation or local synchronization of gamma oscillations in cortical networks. This conclusion is supported by the similar spectral composition of gamma oscillations in KO compared with WT in the LFPs from all electrodes. 3) The amount of synchronization of gamma oscillations for which the presence of the Kv3.2 channel is critical is mediated by intracortical horizontal connections. This conclusion is supported by the similarity in correlation values after coronal cuts in the WT compared with the intact KO. 4) Long-range horizontal connections are functionally intact in the KO. This conclusion is supported by the similar synchronization of slow oscillations over distance between the WT and the KO, a process known to depend almost entirely on horizontal corticocortical connections (Amzica and Steriade 1995). 5) The fast spiking phenotype of the Kv3.2 expressing inhibitory interneurons is not necessary for the generation or long-range synchronization of the slow oscillation.
Our results are in agreement with previous analysis of the spatiotemporal properties of spontaneous slow and gamma oscillations in cats (Destexhe et al. 1999; Steriade et al. 1996), showing a much higher spatial correlation for slow than for gamma oscillations, as well as a dramatic reduction in correlation of the slow oscillation after a coronal cut.
Our results are compatible with the model proposed by Traub et al. (1996b) in which doublets of spikes (∼5-ms interval) fired by interneurons are required for long-range synchronization of gamma oscillations, but not for their generation or local synchronization. The second spike in the doublet generates an extra delay in the local population that compensates for conduction delays. The doublet interval is modulated by the phase relationships between the activity of distant and local excitatory populations and can thus serve as a temporal error signal (Ermentrout and Kopell 1998). The model by Traub et al. (1996b) is radically different from models based on the adjustment of conduction velocity according to distance to match various delays (Knoblauch and Palm 2002). The sustained firing of high-frequency doublets with relatively constant interspike intervals by infragranular cortical interneurons depends in part on the presence of the voltage-gated K+ channel Kv3.2 (Erisir et al. 1999; Lau et al. 2000). This channel allows for a rapid repolarization of the action potential (Erisir et al. 1999; Lau et al. 2000), necessary for the generation of spike doublets.
Our correlation analysis showed that phase lags among the peaks of the crosscorrelograms, both from single 20-s epochs (not shown) and after averaging (see Fig. 2), were shorter than 4 ms and were independent of distance. Such delays cannot be due to the action of direct horizontal long-range connections since conduction velocity in the rostrocaudal direction, measured from the latency of the evoked potentials to electrical stimulation, was estimated at ∼0.25 m/s. Such conduction velocities would yield phase lags among the peaks of the crosscorrelograms no shorter than 8 ms.
The value of the peak of the crosscorrelograms, either across the cut in the WT mouse or between Cx1 and Cx 4 in the KO, was never zero. The remaining correlation with distance may be due to other long-range corticocortical connections (for example callosal) or most probably to cortico-thalamocortical loops, which have been shown to play a critical role in cortico-cortical synchronization of gamma oscillations in animal experiments (Barth and MacDonald 1996; Steriade and Amzica 1996) and in humans (Ribary et al. 1991). We did not see a stronger participation of thalamocortical loops, probably because of the short distances studied (2 mm).
In cortex, potassium channels with Kv3.2 subunits are expressed in a subpopulation of parvalbumin expressing inhibitory interneurons and in ∼30% of somatostatin expressing interneurons, both types located in infragranular layers (Chow et al. 1999). Since they cannot be studied pharmacologically, the role of these channels in determining interneuron's electrophysiological properties was studied by generating a KO mouse (the same mouse used in this study). In vitro studies from the Kv3.2 KO (Lau et al. 2000) showed that FS neurons in infragranular layers, but not in supragranular layers, were impaired in their ability to generate trains of high-frequency spikes without accommodation or doublets of spikes in rapid succession, which supposedly led to their higher susceptibility for seizures with no detectable alteration in behavior (Lau et al. 2000). These firing patterns studied in vitro remain to be corroborated in vivo.
Since the Kv3.2 expressing interneurons (both parvalbumin and somatostatin expressing) are located in infragranular layers, it remains to be determined whether these interneurons participate in the synchronization of gamma oscillations via indirect effects on supragranular layers and the long-range connections within, or whether their synchronizing role is entirely meditated by effects on infragranular layers. Our LFP recordings were made in the bipolar configuration (surface-depth), and, therefore, they do not provide information regarding the localization of oscillations in the vertical axis of the cortex. Plenty of evidence exists for cross layer inhibition of infragranular inhibitory interneurons (Thomson 2010; Thomson and Lamy 2007). Furthermore, it was recently shown that both types of neurons (and including those in supragranular layers) have profuse and nonspecific divergent connections in their local populations (Fino and Yuste 2011; Packer and Yuste 2011), providing a strong substrate for the generation of gamma oscillations in local circuits. Finally, inhibitory interneurons in infragranular layers receive facilitatory synapses from neighboring pyramidal cells (West et al. 2006) providing other network elements that can strongly generate and/or distribute gamma oscillations. An additional network element with key contributions to the generation and local synchronization of gamma oscillation is the presence of electrical coupling (Galarreta and Hestrin 1999; Gibson et al. 1999; Traub et al. 2003). Indeed, gap junctions between axons of pyramidal cells or dendrites of interneurons contribute importantly to the power of gamma oscillations in local networks (Buhl et al. 2003; Traub et al. 2001; Traub et al. 2003). The role of gap-junctions was clearly illustrated by the disruption of gamma oscillations in connexin 36-deficinet mice in which electrical signaling is impaired (Buhl et al. 2003; Hormuzdi et al. 2001). The lack of significant differences in gamma power between KO and WT in our results suggests, however, that the lack of Kv3.2 does not affect the dynamic contribution of electrical coupling to the generation of gamma oscillations in local networks. Our results point to a critical role of Kv3.2 specifically, and inhibitory interneurons generally, in determining long-range synchronization of gamma oscillations. These results thus represent a strong argument supporting the idea that the intrinsic electrophysiological properties of single cells are important for the organization of large network behavior (Llinas 1988).
GRANTS
This work was supported by The Klingenstein Fund, The American Sleep Medicine Foundation to D. Contreras, and by National Institute of Neurological Disorders and Stroke Grant NS-30989 to B. Rudy and Training Grant NS-07457 to M. Harvey.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.H., D.L., B.R., and D.C. conception and design of research; M.H., D.L., and E.C. performed experiments; M.H., D.L., and E.C. analyzed data; M.H., D.L., E.C., B.R., and D.C. interpreted results of experiments; M.H., D.L., E.C., and D.C. prepared figures; M.H., D.L., B.R., and D.C. drafted manuscript; M.H., D.L., E.C., B.R., and D.C. edited and revised manuscript; M.H., D.L., B.R., and D.C. approved final version of manuscript.
REFERENCES
- Achermann P, Borbely AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81: 213–222, 1997 [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol 73: 20–38, 1995 [DOI] [PubMed] [Google Scholar]
- Barth DS, MacDonald KD. Thalamic modulation of high-frequency oscillating potentials in auditory cortex. Nature 383: 78–81, 1996 [DOI] [PubMed] [Google Scholar]
- Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol 88: 1634–1654, 2002 [DOI] [PubMed] [Google Scholar]
- Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci 23: 1013–1018, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, Lau D, Welker E, Rudy B. K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci 19: 9332–9345, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Llinas R. Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J Neurosci 21: 9403–9413, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15: 604–622, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci 19: 4595–4608, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol 82: 2476–2489, 1999 [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci USA 95: 1259–1264, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron 69: 1188–1203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009 [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402: 72–75, 1999 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron 31: 487–495, 2001 [DOI] [PubMed] [Google Scholar]
- Knoblauch A, Palm G. Scene segmentation by spike synchronization in reciprocally connected visual areas. II. Global assemblies and synchronization on larger space and time scales. Biol Cybern 87: 168–184, 2002 [DOI] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera E, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci 20: 9071–9085, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Davidowitz H. Encoding of olfactory information with oscillating neural assemblies. Science 265: 1872–1875, 1994 [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA 90: 2078–2081, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1841–1849, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242: 1654–1664, 1988 [DOI] [PubMed] [Google Scholar]
- Llinas RR, Ribary U. Temporal conjunction in thalamocortical transactions. Adv Neurol 77: 95–102; discussion 102–103, 1998 [PubMed] [Google Scholar]
- Molle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci 22: 10941–10947, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Kentros C, Bueno E, Weiser M, Hernandez A, Vega-Saenz de Miera E, Ponce A, Thornhill W, Rudy B. Thalamocortical projections have a K+ channel that is phosphorylated and modulated by cAMP-dependent protein kinase. J Neurosci 15: 5486–5501, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA 89: 5670–5674, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31: 13260–13271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci 10: 718–728, 1998 [DOI] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science 297: 359–365, 2002 [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Mogilner A, Llinas R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA 88: 11037–11041, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E. Contributions of Kv3 channels to neuronal excitability. Ann NY Acad Sci 868: 304–343, 1999 [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24: 517–526, 2001 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000 [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24: 49–65, 111–125, 1999 [DOI] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol 70: 97–116, 1993 [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci USA 93: 2533–2538, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci 16: 392–417, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci 13: 3284–3299, 1993a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat 4: 13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci 1: 19–42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci 21: 9478–9486, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, LeBeau FE, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci USA 100: 1370–1374, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol 493: 471–484, 1996a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383: 621–624, 1996b [DOI] [PubMed] [Google Scholar]
- Vinck M, Lima B, Womelsdorf T, Oostenveld R, Singer W, Neuenschwander S, Fries P. Gamma-phase shifting in awake monkey visual cortex. J Neurosci 30: 1250–1257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 31: 18137–18148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16: 6402–6413, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature 384: 162–166, 1996 [DOI] [PubMed] [Google Scholar]
- West DC, Mercer A, Kirchhecker S, Morris OT, Thomson AM. Layer 6 cortico-thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex 16: 200–211, 2006 [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373: 612–615, 1995 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science 316: 1609–1612, 2007 [DOI] [PubMed] [Google Scholar]